Synthesis of LDHs Based on Fly-Ash and Its Influence on the Flame Retardant Properties of EVA/LDHs Composites
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of LDHs
2.3. Preparation of the EVA Composites
2.4. Characterization
3. Results and Discussion
3.1. XRD Characterization of Materials
3.2. Performance Test of EVA/LDHs Composites
3.2.1. CCT of EVA/LDHs Composites
3.2.2. SDT of EVA/LDHs Composites
3.2.3. LOI and UL-94 Test of EVA/LDHs Composites
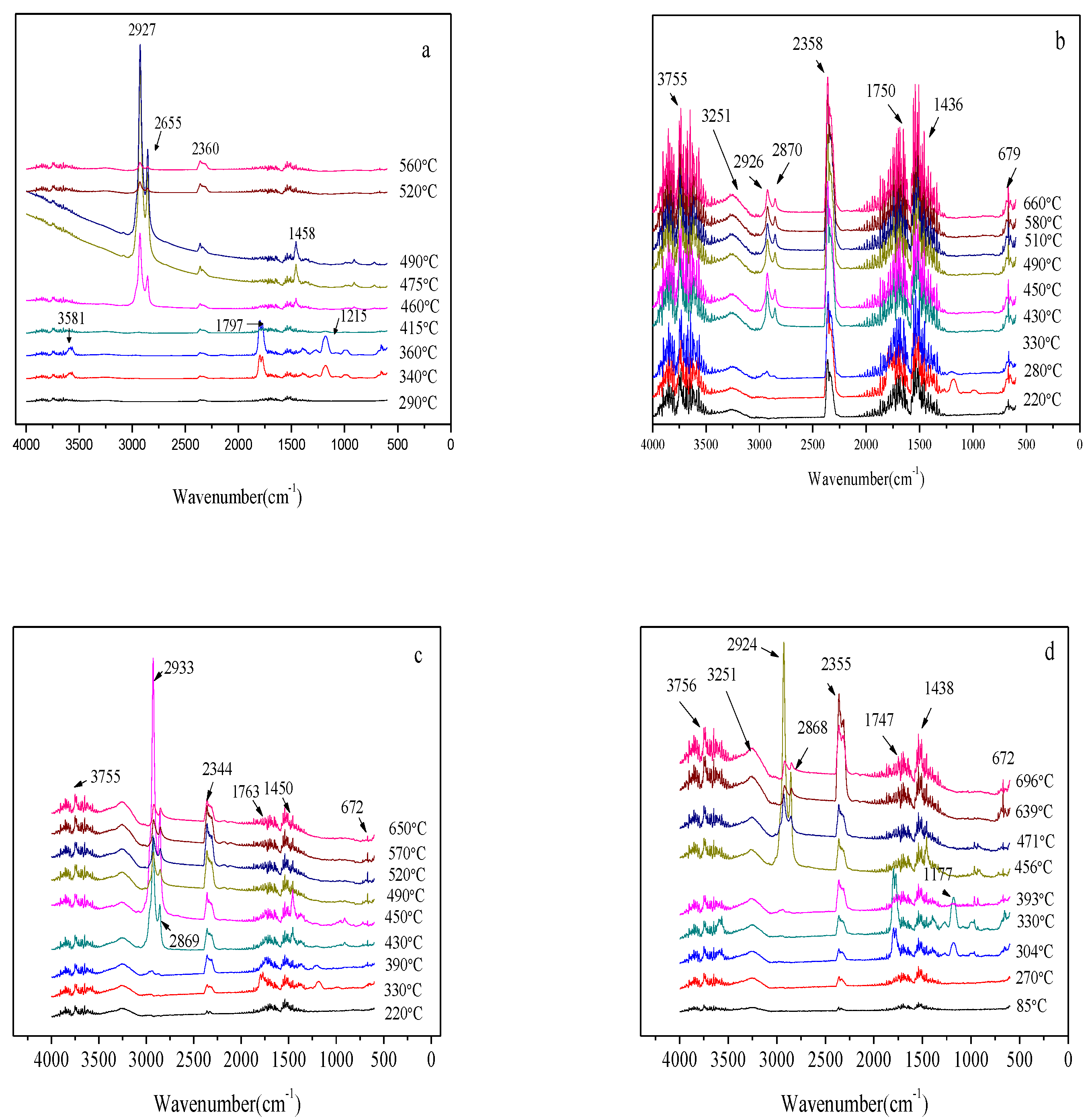
3.2.4. TG-FTIR Characterization of the EVA/LDHs Composites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guan, D.; Su, X.; Zhang, Q.; Peters, G.P.; Liu, Z.; Lei, Y.; He, K. The socioeconomic drivers of China’s primary PM2.5 emissions. Environ. Res. Lett. 2014, 9, 024010. [Google Scholar] [CrossRef] [Green Version]
- Żyrkowski, M.; Neto, R.C.; Santos, L.F.; Witkowski, K. Characterization of fly-ash cenospheres from coal-fired power plant unit. Fuel 2016, 174, 49–53. [Google Scholar] [CrossRef]
- Ducman, V.; Korat, L. Characterization of geopolymer fly-ash based foams obtained with the addition of Al powder or H2O2, as foaming agents. Mater. Charact. 2016, 113, 207–213. [Google Scholar] [CrossRef]
- Yadav, S.; Pandey, V.C.; Singh, L. Ecological restoration of fly-ash disposal areas: Challenges and opportunities. Land Degrad. Dev. 2021, 32, 4453–4471. [Google Scholar] [CrossRef]
- Lu, C.H.; Chen, J.C.; Chuang, K.H.; Wey, M.Y. The different properties of lightweight aggregates with the fly ashes of fluidized-bed and mechanical incinerators. Constr. Build. Mater. 2015, 101, 380–388. [Google Scholar] [CrossRef]
- Zhu, Z.; Pu, S.; Zhang, J.; Wan, Y.; Song, W.; Wang, H. Water resistance and compressibility of silt solidified with lime and fly-ash mixtures. Environ. Earth Sci. 2021, 80, 1–14. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, P.; Li, J.; Chen, D. Stabilization and separation of heavy metals in incineration fly ash during the hydrothermal treatment process. J. Hazard. Mater. 2015, 299, 149–157. [Google Scholar] [CrossRef]
- Edenharter, A.; Breu, J. Applying the flame retardant LDH as a Trojan horse for molecular flame retardants. Appl. Clay Sci. 2015, 114, 603–608. [Google Scholar] [CrossRef]
- Li, C.; Wei, M.; Evans, D.G.; Duan, X. Recent advances for layered double hydroxides (LDHs) materials as catalysts applied in green aqueous media. Catal. Today 2015, 247, 163–169. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, Q.; Wang, J.; Huang, L.; Yan, X.; Zhang, X.; Xing, Z.; He, Q.; Guo, Z. Synthesis of highly efficient flame retardant high-density polyethylene nanocomposites with inorgano-layered double hydroxides as nanofiller using solvent mixing method. ACS Appl. Mater. Interfaces 2014, 6, 5094–5104. [Google Scholar] [CrossRef]
- Qi, L.; Liu, K.; Wang, R.; Li, J.; Zhang, Y.; Chen, L. Removal of chlorine ions from desulfurization wastewater by modified fly ash hydrotalcite. ACS Omega 2020, 5, 31665–31672. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Shui, Z.; Chen, W.; Li, Q.; Chen, G. Effect of MgO content of synthetic slag on the formation of Mg-Al LDHs and sulfate resistance of slag-fly ash-clinker binder. Constr. Build. Mater. 2016, 125, 766–774. [Google Scholar] [CrossRef]
- Volli, V.; Purkait, M.K. Preparation and characterization of hydrotalcite-like materials from flyash for transesterification. Clean Technol. Environ. Policy 2016, 18, 529–540. [Google Scholar] [CrossRef]
- Muriithi, G.N.; Petrik, L.F.; Doucet, F.J. Synthesis, characterisation and CO2 adsorption potential of NaA and NaX zeolites and hydrotalcite obtained from the same coal fly ash. J. CO2 Util. 2020, 36, 220–230. [Google Scholar] [CrossRef]
- Ruan, S.; Wang, T.; Guo, R.; Unluer, C. Assessment of the properties and environmental impact of carbonated reactive magnesia containing industrial waste. Thermochim. Acta 2021, 706, 179051. [Google Scholar] [CrossRef]
- Basu, D.; Das, A.; Wang, D.Y.; George, J.J.; Stöckelhuber, K.W.; Boldt, R.; Leuteritz, A.; Heinrich, G. Fire-safe and environmentally friendly nanocomposites based on layered double hydroxides and ethylene propylene diene elastomer. RSC Adv. 2016, 6, 26425–26436. [Google Scholar] [CrossRef]
- Chen, Y.; Zou, H.; Liang, M.; Cao, Y. Melting and crystallization behavior of partially miscible high density polyethylene/ethylene vinyl acetate copolymer (HDPE/EVA) blends. Thermochim. Acta 2014, 586, 1–8. [Google Scholar] [CrossRef]
- Wang, B.; Wang, X.; Tang, G.; Shi, Y.; Hu, W.; Lu, H.; Song, L.; Hu, Y. Preparation of silane precursor microencapsulated intumescent flame retardant and its enhancement on the properties of ethylene–vinyl acetate copolymer cable. Compos. Sci. Technol. 2012, 72, 1042–1048. [Google Scholar] [CrossRef]
- Jiao, C.M.; Wang, Z.Z.; Chen, X.L.; Hu, Y. Synthesis of a magnesium/aluminum/iron layered double hydroxide and its flammability characteristics in halogen—free, flame—retardant ethylene/vinyl acetate copolymer composites. J. Appl. Polym. Sci. 2008, 107, 2626–2631. [Google Scholar] [CrossRef]
- Qian, Y.; Li, S.; Chen, X. Synthesis and characterization of LDHs using Bayer red mud and its flame-retardant properties in EVA/LDHs composites. J. Mater. Cycles Waste Manag. 2015, 17, 646–654. [Google Scholar] [CrossRef]
- Wang, C.; Chen, Y.; Shang, X.; Hou, X.; Li, H.; Guo, Z. Facile synthesis of Ca/Mg/Al/Fe layered double hydroxides using steelmaking slag as raw material. Mater. Lett. 2016, 173, 115–118. [Google Scholar] [CrossRef]
- Tseng, C.H.; Hsueh, H.B.; Chen, C.Y. Effect of reactive layered double hydroxides on the thermal and mechanical properties of LDHs/epoxy nanocomposites. Compos. Sci. Technol. 2007, 67, 2350–2362. [Google Scholar] [CrossRef]
- Li, L.; Qian, Y.; Zhang, H.; Han, H.; Qiao, P. Synthesis of LDHs using red mud and bittern and its influence on the flame retardant properties of EVA/LDHs composites. Polym. Polym. Compos. 2020, 28, 14–25. [Google Scholar] [CrossRef]
- Liu, C.; Yao, A.; Chen, K.; Shi, Y.; Feng, Y.; Zhang, P.; Yang, F.; Liu, M.; Chen, Z. MXene based core-shell flame retardant towards reducing fire hazards of thermoplastic polyurethane. Compos. Part B Eng. 2021, 226, 109363. [Google Scholar] [CrossRef]
- Liu, C.; Xu, K.; Shi, Y.; Wang, J.; Ma, S.; Feng, Y.; Lv, Y.; Yang, F.; Liu, M.; Song, P. Fire-Safe, Mechanically Strong and Tough Thermoplastic Polyurethane/MXene Nanocomposites with Exceptional Smoke Suppression. Mater. Today Phys. 2022, 22, 100607. [Google Scholar] [CrossRef]
- Mensah, R.A.; Xu, Q.; Asante-Okyere, S.; Jin, C.; Bentum-Micah, G. Correlation analysis of cone calorimetry and microscale combustion calorimetry experiments. J. Therm. Anal. Calorim. 2019, 136, 589–599. [Google Scholar] [CrossRef]
- Liu, C.; Yang, D.; Sun, M.; Deng, G.; Jing, B.; Wang, K.; Shi, Y.; Fu, L.; Feng, Y.; Lv, Y.; et al. Phosphorous-Nitrogen flame retardants engineering MXene towards highly fire safe thermoplastic polyurethane. Compos. Commun. 2022, 29, 101055. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Y.; Peng, S.; Pan, B.; Lu, C.; Liu, H.; Ma, J.; Niu, Q. Fire property and charring behavior of high impact polystyrene containing expandable graphite and microencapsulated red phosphorus. Polym. Degrad. Stab. 2015, 121, 261–270. [Google Scholar] [CrossRef]
- Yi, J.; Yin, H.; Cai, X. Effects of common synergistic agents on intumescent flame retardant polypropylene with a novel charring agent. J. Therm. Anal. Calorim. 2013, 111, 725–734. [Google Scholar] [CrossRef]
- Li, J.; Prétrel, H.; Beji, T.; Merci, B. Influence of fire heat release rate (HRR) evolutions on fire-induced pressure variations in air-tight compartments. Fire Saf. J. 2021, 126, 103450. [Google Scholar] [CrossRef]
- Xie, R.; Qu, B. Synergistic effects of expandable graphite with some halogen-free flame retardants in polyolefin blends. Polym. Degrad. Stab. 2001, 71, 375–380. [Google Scholar] [CrossRef]
- Qian, Y.; Wei, P.; Jiang, P.; Li, Z.; Yan, Y.; Ji, K. Aluminated mesoporous silica as novel high-effective flame retardant in polylactide. Compos. Sci. Technol. 2013, 82, 1–7. [Google Scholar] [CrossRef]
- Liu, L.; Hu, J.; Zhuo, J.; Jiao, C.; Chen, X.; Li, S. Synergistic flame retardant effects between hollow glass microspheres and magnesium hydroxide in ethylene-vinyl acetate composites. Polym. Degrad. Stab. 2014, 104, 87–94. [Google Scholar] [CrossRef]
- Chen, X.; Jiang, Y.; Jiao, C. Smoke suppression properties of ferrite yellow on flame retardant thermoplastic polyurethane based on ammonium polyphosphate. J. Hazard. Mater. 2014, 266, 114–121. [Google Scholar] [CrossRef]
- Hu, W.; Yu, B.; Jiang, S.D.; Song, L.; Hu, Y.; Wang, B. Hyper-branched polymer grafting graphene oxide as an effective flame retardant and smoke suppressant for polystyrene. J. Hazard. Mater. 2015, 300, 58–66. [Google Scholar] [CrossRef]
- Li, L.; Qian, Y.; Han, H.; Qiao, P.; Zhang, H. Effects of functional intercalation and surface modification on the flame retardant performance of EVA/LDHs composites. Polym. Polym. Compos. 2021, 29, 842–853. [Google Scholar] [CrossRef]
- Zhao, C.X.; Liu, Y.; Wang, D.Y.; Wang, D.L.; Wang, Y.Z. Synergistic effect of ammonium polyphosphate and layered double hydroxide on flame retardant properties of poly(vinyl alcohol). Polym. Degrad. Stab. 2008, 93, 1323–1331. [Google Scholar] [CrossRef]
- Zanetti, M.; Kashiwagi, T.; Falqui, L.; Camino, G. Cone calorimeter combustion and gasification studies of polymer layered silicate nanocomposites. Chem. Mater. 2002, 14, 881–887. [Google Scholar] [CrossRef]
- Weil, E.D.; Patel, N.G. Iron compounds in non-halogen flame-retardant polyamide systems. Polym. Degrad. Stab. 2003, 82, 291–296. [Google Scholar] [CrossRef]
- Gregoriou, V.G.; Kandilioti, G.; Bollas, S.T. Chain conformational transformations in syndiotactic polypropylene/layered silicate nanocomposites during mechanical elongation and thermal treatment. Polymer 2005, 46, 11340–11350. [Google Scholar] [CrossRef]
- Chen, Y.J.; Zhan, J.; Zhang, P.; Nie, S.B.; Lu, H.D.; Song, L.; Hu, Y. Preparation of intumescent flame retardant poly(butylene succinate) using fumed silica as synergistic agent. Ind. Eng. Chem. Res. 2010, 49, 8200–8208. [Google Scholar] [CrossRef]
- Xu, J.; Liu, C.; Qu, H.; Ma, H.; Jiao, Y.; Xie, J. Investigation on the thermal degradation of flexible poly (vinyl chloride) filled with ferrites as flame retardant and smoke suppressant using TGA–FTIR and TGA–MS. Polym. Degrad. Stab. 2013, 98, 1506–1514. [Google Scholar] [CrossRef]
- Lee, J.H.; Zhang, W.; Ryu, H.J.; Choi, G.; Choi, J.Y.; Choy, J.H. Enhanced thermal stability and mechanical property of EVA nanocomposites upon addition of organo-intercalated LDH nanoparticles. Polymer 2019, 177, 274–281. [Google Scholar] [CrossRef]
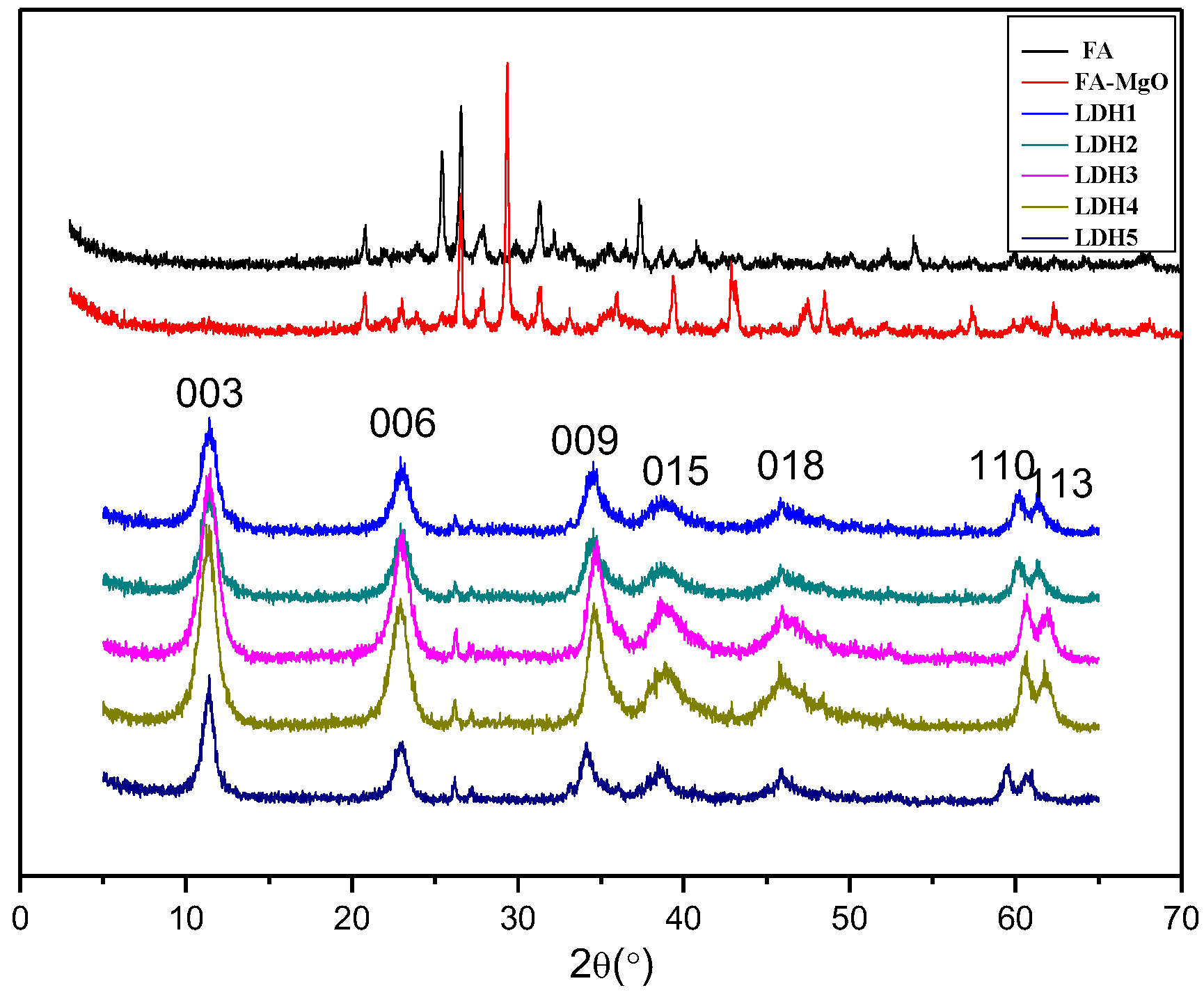






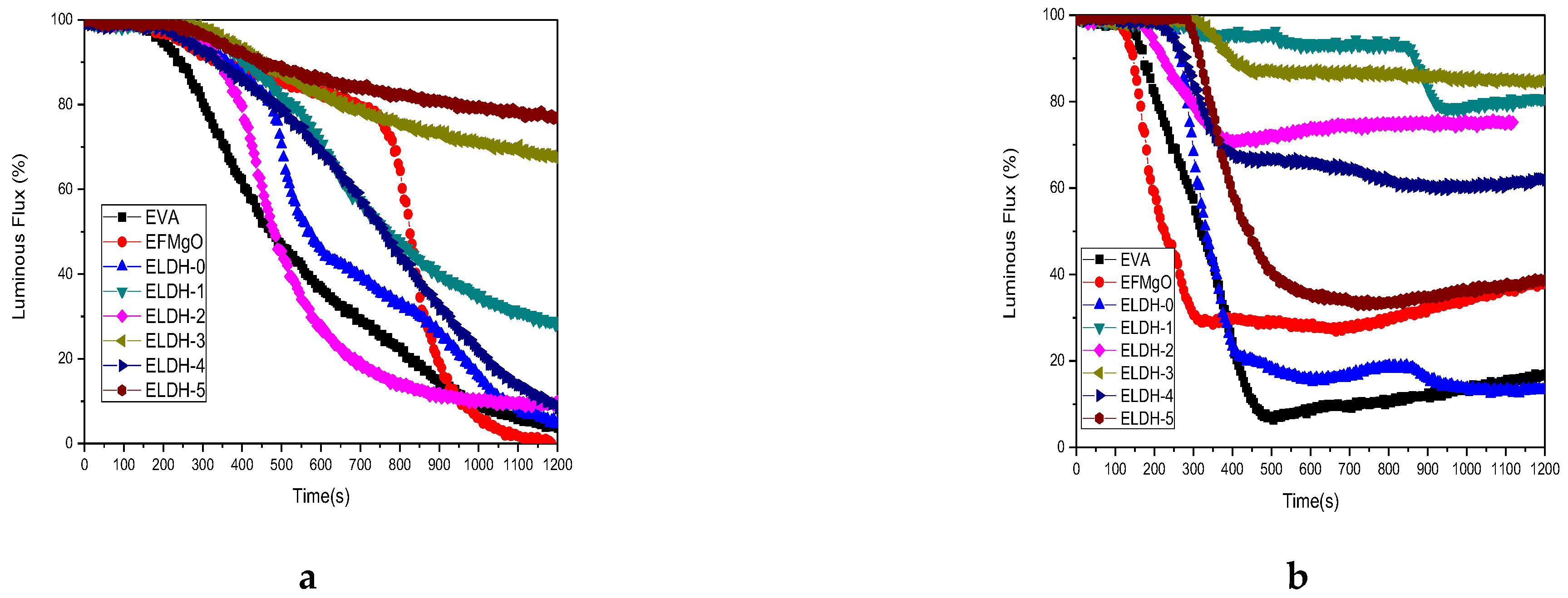
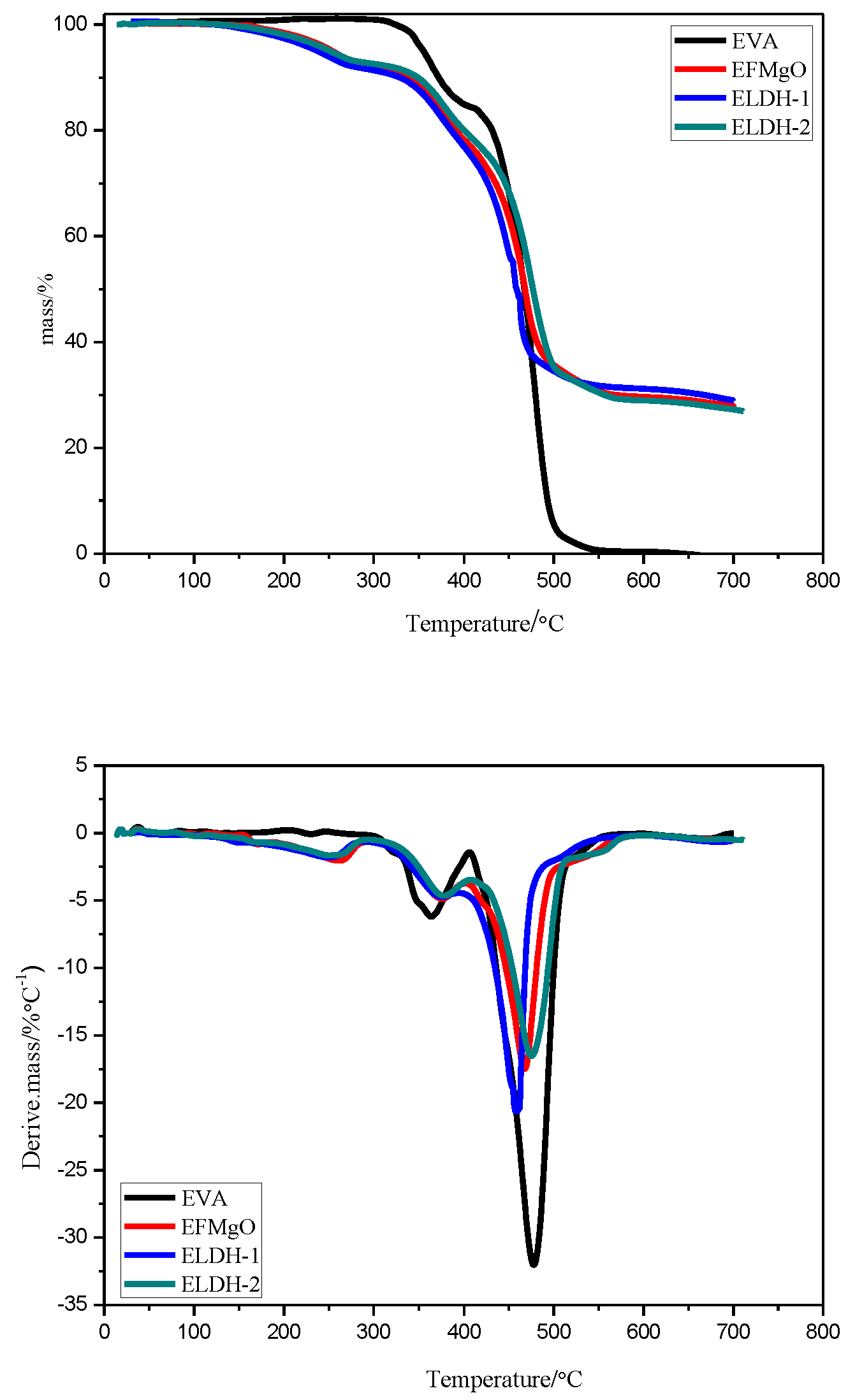
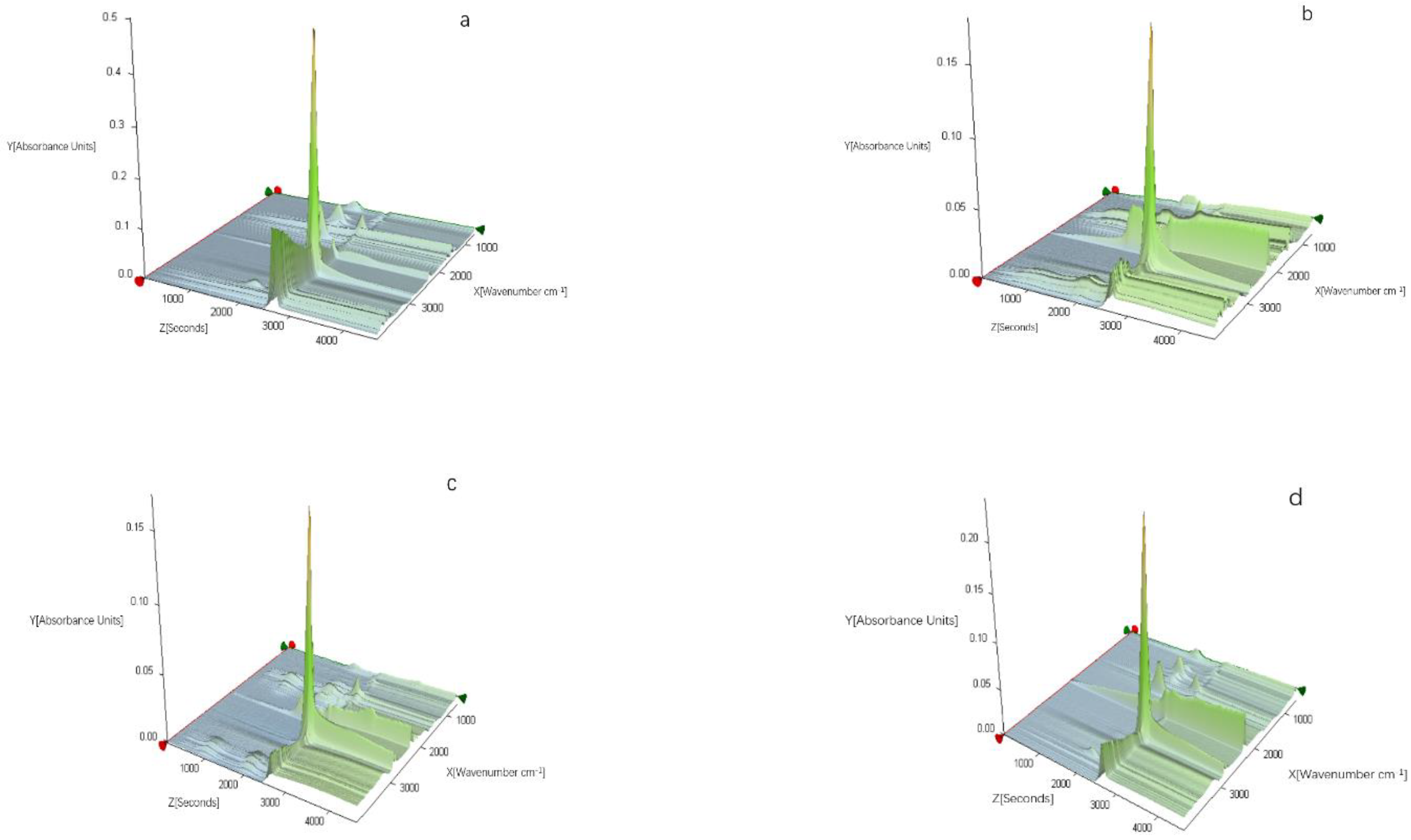
| Sample Code | LDH1 | LDH2 | LDH3 | LDH4 | LDH5 |
|---|---|---|---|---|---|
| d(003), nm | 0.770 | 0.773 | 0.789 | 0.784 | 0.769 |
| d(006), nm | 0.380 | 0.387 | 0.389 | 0.386 | 0.370 |
| d(009), nm | 0.244 | 0.246 | 0.247 | 0.246 | 0.241 |
| d(015), nm | 0.234 | 0.234 | 0.235 | 0.234 | 0.230 |
| d(110), nm | 0.150 | 0.150 | 0.152 | 0.152 | 0.149 |
| a, nm | 0.300 | 0.300 | 0.304 | 0.304 | 0.298 |
| c, nm | 2.310 | 2.319 | 2.367 | 2.352 | 2.307 |
| Sample Code | pHRR (Kw·m−2) | THR (MJ·m−2) | Time to pHRR (s) | Time to Flame Out (s) |
|---|---|---|---|---|
| EVA | 1717.9 ± 21.4 | 150.6 ± 0.52 | 195 ± 12 | 302 ± 12 |
| EFMgO | 331.9 ± 17.9 | 123.5 ± 0.46 | 410 ± 9 | 575 ± 15 |
| ELDH-0 | 360.6 ± 19.6 | 124.1 ± 0.33 | 195 ± 7 | 581 ± 21 |
| ELDH-1 | 178.4 ± 12.8 | 114.5 ± 0.35 | 120 ± 10 | 1218 ± 13 |
| ELDH-2 | 207.2 ± 18.9 | 126.5 ± 0.41 | 145 ± 14 | 1033 ± 18 |
| ELDH-3 | 208.9 ± 18.3 | 128.2 ± 0.28 | 230 ± 8 | 1142 ± 20 |
| ELDH-4 | 227.8 ± 20.6 | 120.4 ± 0.50 | 175 ± 12 | 900 ± 26 |
| ELDH-5 | 317.1 ± 23.5 | 119.1 ± 0.24 | 205 ± 12 | 806 ± 28 |
| Sample Code | LOI(%) | Rating | Dripping Behavior |
|---|---|---|---|
| EVA | 19.8 ± 0.1 | --- | No dripping |
| EFMgO | 21.5 ± 0.1 | V-2 | Dripping |
| ELDH-0 | 21.6 ± 0 | V-0 | Dripping |
| ELDH-1 | 28.5 ± 0.1 | V-0 | Dripping |
| ELDH-2 | 27.5 ± 0.1 | V-0 | Dripping |
| ELDH-3 | 27.2 ± 0.1 | V-0 | Dripping |
| ELDH-4 | 26.8 ± 0 | V-0 | Dripping |
| ELDH-5 | 26.5 ± 0.1 | V-1 | Dripping |
| Sample Code | T-5 (°C) | T-50 (°C) | T-max (°C) | ||
|---|---|---|---|---|---|
| Step 1 | Step 2 | Step 3 | |||
| EVA | 361.9 ± 12.4 | 473.7 ± 10.2 | 356.7 ± 5.9 | 481.3 ± 9.2 | --- |
| EFMgO | 255.2 ± 12.9 | 470.2 ± 9.6 | 261.8 ± 4.6 | 373.5 ± 7.6 | 470.2 ± 11.5 |
| ELDH-1 | 247.3 ± 14.8 | 452.6 ± 8.8 | 252.3 ± 5.2 | 373.1 ± 7.5 | 452.6 ± 10.6 |
| ELDH-2 | 251.6 ± 13.5 | 480.3 ± 12.4 | 252.8 ± 6.1 | 375.2 ± 8.3 | 480.3 ± 13.2 |
| Sample Code | Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|
| EVA | 11.8 ± 0.4 | 363.8 ± 22.4 |
| EFMgO | 9.2 ± 0.3 | 300.6 ± 19.2 |
| ELDH-0 | 9.7 ± 0.2 | 314.6 ± 19.6 |
| ELDH-1 | 10.1 ± 0.3 | 327.7 ± 18.6 |
| ELDH-2 | 9.9 ± 0.3 | 324.5 ± 18.0 |
| ELDH-3 | 9.4 ± 0.4 | 318.4 ± 20.3 |
| ELDH-4 | 9.3 ± 0.2 | 298.6 ± 19.8 |
| ELDH-5 | 9.2 ± 0.2 | 298.2 ± 16.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Zhu, X.-d.; Li, L.; Qian, Y.; Guo, Q.; Ma, J. Synthesis of LDHs Based on Fly-Ash and Its Influence on the Flame Retardant Properties of EVA/LDHs Composites. Polymers 2022, 14, 2549. https://doi.org/10.3390/polym14132549
Li S, Zhu X-d, Li L, Qian Y, Guo Q, Ma J. Synthesis of LDHs Based on Fly-Ash and Its Influence on the Flame Retardant Properties of EVA/LDHs Composites. Polymers. 2022; 14(13):2549. https://doi.org/10.3390/polym14132549
Chicago/Turabian StyleLi, Shaoquan, Xiao-dong Zhu, Long Li, Yi Qian, Qingjie Guo, and Jingjing Ma. 2022. "Synthesis of LDHs Based on Fly-Ash and Its Influence on the Flame Retardant Properties of EVA/LDHs Composites" Polymers 14, no. 13: 2549. https://doi.org/10.3390/polym14132549





