Modification of DSSC Based on Polymer Composite Gel Electrolyte with Copper Oxide Nanochain by Shape Effect
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of CuO Nanofillers
2.3. Preparation of Polymer Composite Gel Electrolyte-Based P(VB-co-VA-co-VAc) (PCGE) Samples
2.4. Characterizations of CuO Nanofiller and PCGE Samples
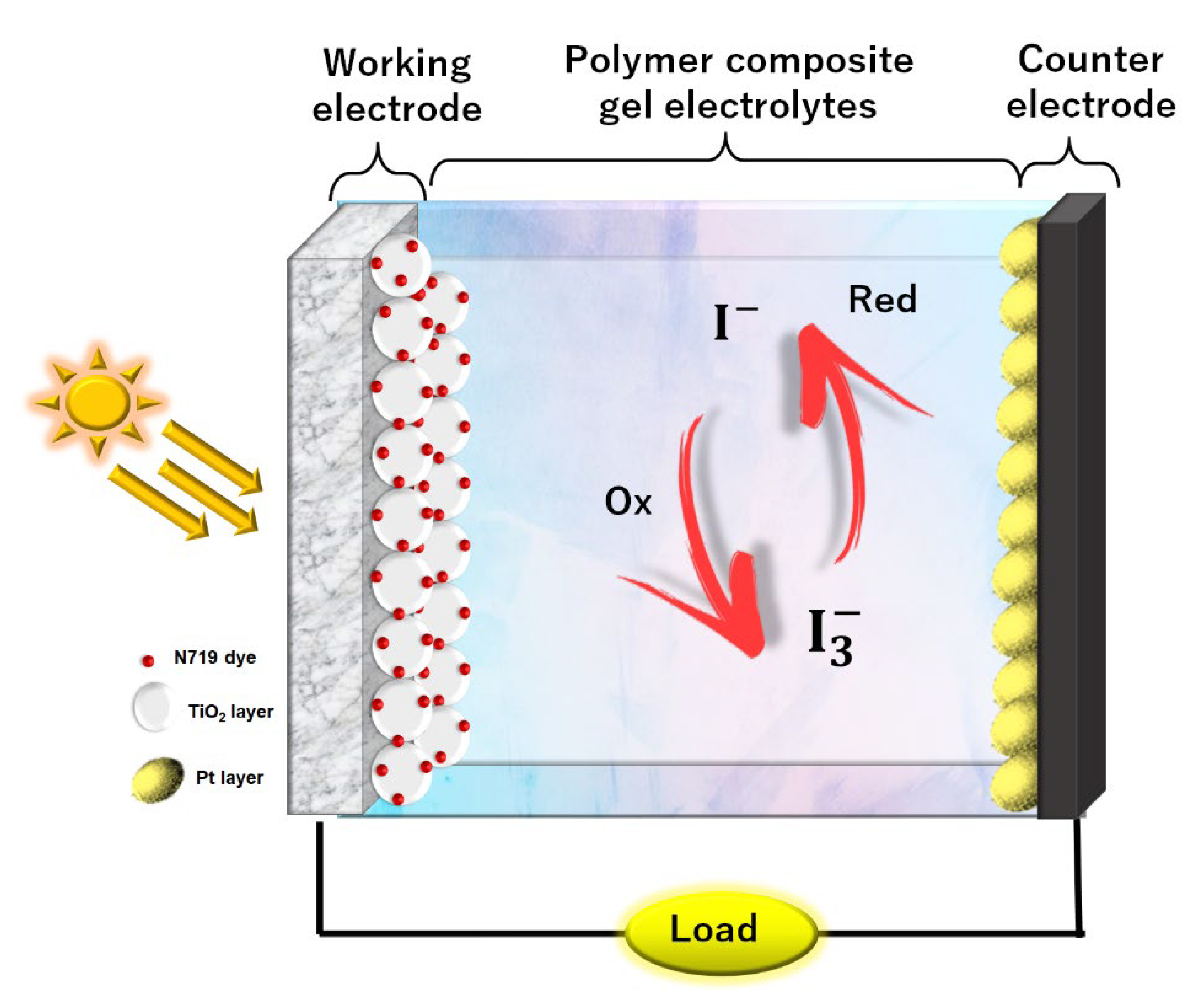
2.5. Fabrication of DSSC and Its Characterization
3. Results and Discussion
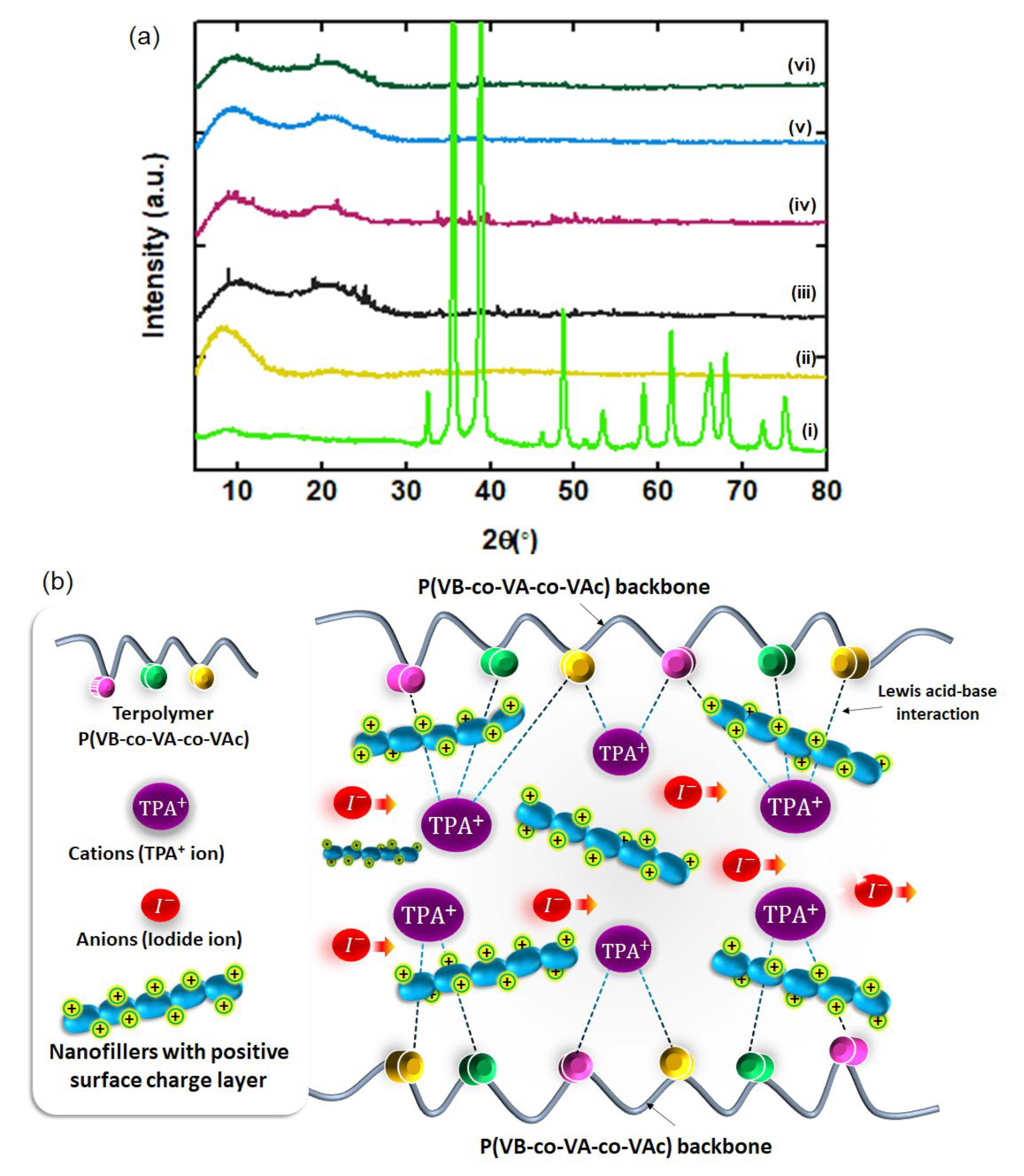
3.1. X-ray Diffraction (XRD)
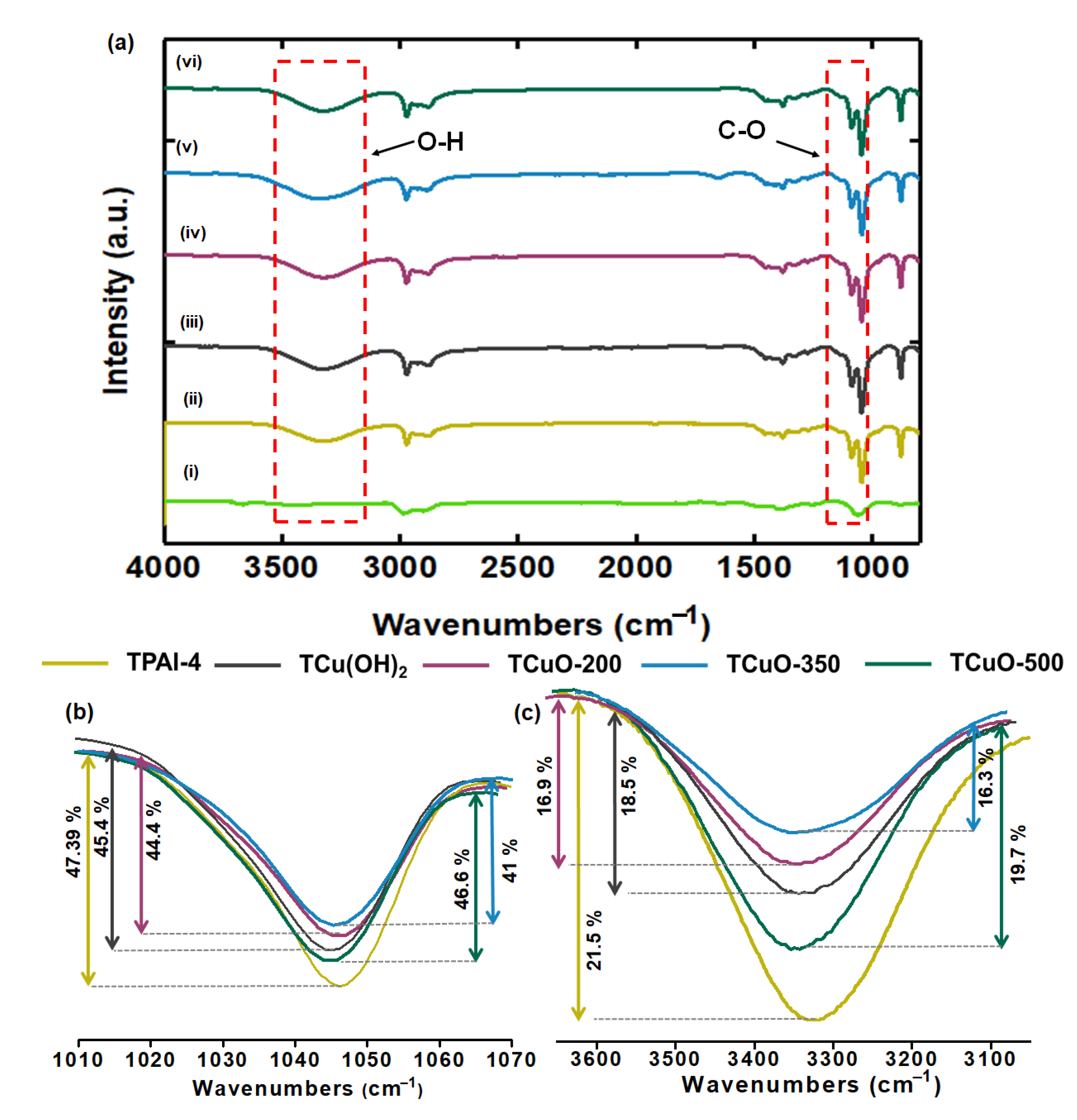
3.2. Fourier Transform Infrared Studies
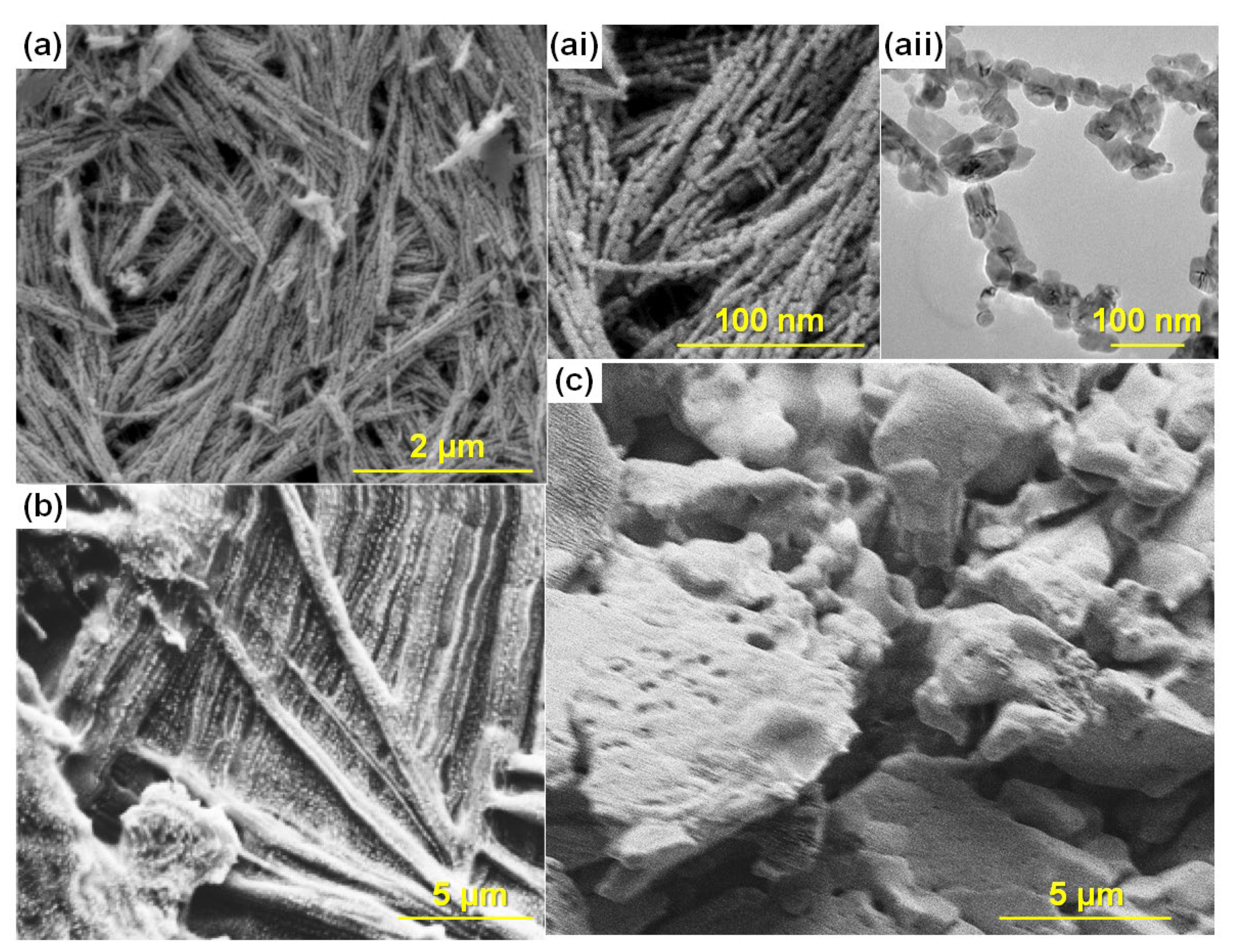
3.3. Morphology Studies
3.4. Electrochemical Impedance Studies
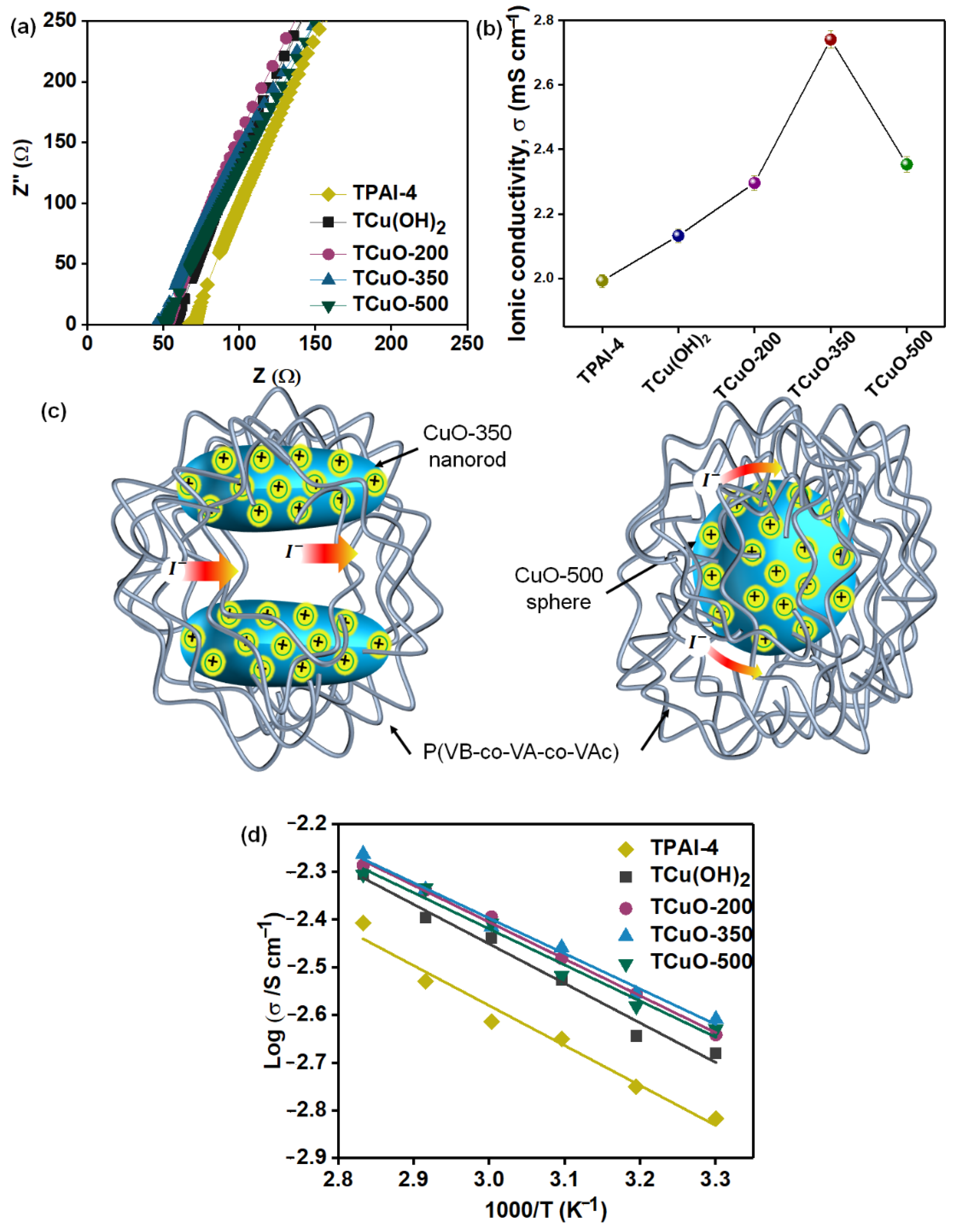
3.4.1. Ionic Conductivity Studies
3.4.2. Dielectric Properties Studies
3.4.3. Loss Tangent and Frequency Dependance Conductivity Studies
3.5. Linear Sweep Voltammetry Studies
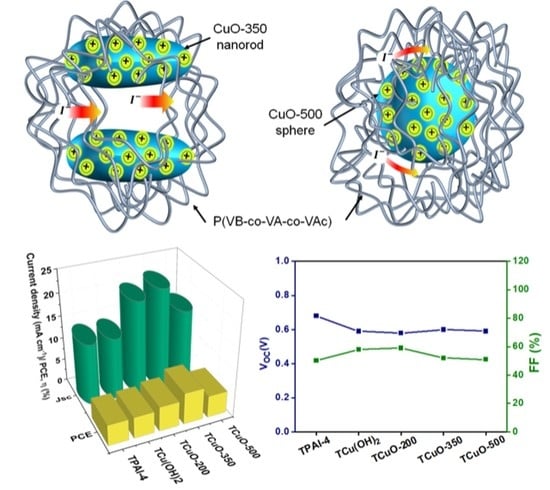
3.6. Photovoltaic Performance Studies

4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Energy Agency. IEA Pathway to Critical and Formidable Goal of Net-Zero Emissions by 2050 Is Narrow but Brings Huge Benefits, According to IEA Special Report. Available online: https://www.iea.org/news/pathway-to-critical-and-formidable-goal-of-net-zero-emissions-by-2050-is-narrow-but-brings-huge-benefits (accessed on 27 January 2022).
- Sharma, K.; Sharma, V.; Sharma, S.S. Dye-Sensitized Solar Cells: Fundamentals and Current Status. Nanoscale Res. Lett. 2018, 13, 381. [Google Scholar] [CrossRef] [PubMed]
- Bozhilov, V.; Kozhukharov, S.; Bubev, E.; Machkova, M.; Kozhukharov, V. Classification and Functional Characterization of the Basic Types of Photovoltaic Elements. In Proceedings of the Sofia Electrochemical Days 2012 (SED2012), Sofia, Bulgaria, 10–13 December 2012; Volume 11, p. 16. [Google Scholar]
- Wu, X.; Guo, J.; Ji, X.; Chen, G. Energy use in world economy from household-consumption-based perspective. Energy Policy 2019, 127, 287–298. [Google Scholar] [CrossRef]
- O’Regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Jiang, R.; Michaels, H.; Vlachopoulos, N.; Freitag, M. Beyond the Limitations of Dye-Sensitized Solar Cells. In Dye-Sensitized Solar Cells: Mathematical Modelling, and Materials Design and Optimization; Elsevier: Amsterdam, The Netherlands, 2019; pp. 285–323. ISBN 9780128145425. [Google Scholar]
- Li, P.; Yuan, S.; Tang, Q.; He, B. Robust conducting gel electrolytes for efficient quasi-solid-state dye-sensitized solar cells. Electrochim. Acta 2014, 137, 57–64. [Google Scholar] [CrossRef]
- Umek, P.; Seo, J.W.; Forro, L.; Cevc, P.; Jagličić, Z.; Škarabot, M.; Zorko, A.; Arčon, D. Synthesis and Magnetic Characterization of Cu(OH)2 Nanoribbons. AIP Conf. Proc. 2004, 723, 427–430. [Google Scholar] [CrossRef]
- Manafi, P.; Nazockdast, H.; Karimi, M.; Sadighi, M.; Magagnin, L. Microstructural Development and Rheological Study of a Nanocomposite Gel Polymer Electrolyte Based on Functionalized Graphene for Dye-Sensitized Solar Cells. Polymers 2020, 12, 1443. [Google Scholar] [CrossRef]
- Chowdhury, F.I.; Khalil, I.; Khandaker, M.U.; Rabbani, M.M.; Uddin, J.; Arof, A.K. Electrochemical and structural characterization of polyacrylonitrile (PAN)–based gel polymer electrolytes blended with tetrabutylammonium iodide for possible application in dye-sensitized solar cells. Ionics 2020, 26, 4737–4746. [Google Scholar] [CrossRef]
- Cameron, P.J.; Peter, L.M.; Zakeeruddin, S.M.; Grätzel, M. Electrochemical studies of the Co(III)/Co(II)(dbbip)2 redox couple as a mediator for dye-sensitized nanocrystalline solar cells. Coord. Chem. Rev. 2004, 248, 1447–1453. [Google Scholar] [CrossRef]
- Oskam, G.; Bergeron, B.V.; Meyer, G.J.; Searson, P.C. Pseudohalogens for Dye-Sensitized TiO2 Photoelectrochemical Cells. J. Phys. Chem. B 2001, 105, 6867–6873. [Google Scholar] [CrossRef]
- Wang, Z.-S.; Sayama, A.K.; Sugihara, H. Efficient Eosin Y Dye-Sensitized Solar Cell Containing Br-/Br3- Electrolyte. J. Phys. Chem. B 2005, 109, 22449–22455. [Google Scholar] [CrossRef]
- Yella, A.; Lee, H.-W.; Tsao, H.N.; Yi, C.; Chandiran, A.K.; Nazeeruddin, M.K.; Diau, E.W.-G.; Yeh, C.-Y.; Zakeeruddin, S.M.; Grätzel, M. Porphyrin-Sensitized Solar Cells with Cobalt (II/III)-Based Redox Electrolyte Exceed 12 Percent Efficiency. Science 2011, 334, 629–634. [Google Scholar] [CrossRef]
- Green, M.A.; Dunlop, E.D.; Hohl-Ebinger, J.; Yoshita, M.; Kopidakis, N.; Hao, X. Solar cell efficiency tables (version 56). Prog. Photovolt. Res. Appl. 2020, 28, 629–638. [Google Scholar] [CrossRef]
- Bandara, T.M.W.J.; Weerasinghe, A.M.J.S.; Dissanayake, M.A.K.L.; Senadeera, G.K.R.; Furlani, M.; Albinsson, I.; Mellander, B.E. Characterization of Poly (Vinylidene Fluoride-Co-Hexafluoropropylene) (PVdF-HFP) Nanofiber Membrane Based Quasi Solid Electrolytes and Their Application in a Dye Sensitized Solar Cell. Electrochim. Acta 2018, 266, 276–283. [Google Scholar] [CrossRef]
- Bousrez, G.; Renier, O.; Adranno, B.; Smetana, V.; Mudring, A.-V. Ionic Liquid-Based Dye-Sensitized Solar Cells—Insights into Electrolyte and Redox Mediator Design. ACS Sustain. Chem. Eng. 2021, 9, 8107–8114. [Google Scholar] [CrossRef]
- Liu, J.; Yang, X.; Cong, J.; Kloo, L.; Sun, L. Solvent-free ionic liquid electrolytes without elemental iodine for dye-sensitized solar cells. Phys. Chem. Chem. Phys. 2012, 14, 11592–11595. [Google Scholar] [CrossRef]
- Imperiyka, M.; Ahmad, A.; Hanifah, S.; Bella, F. A UV-prepared linear polymer electrolyte membrane for dye-sensitized solar cells. Phys. B Condens. Matter 2014, 450, 151–154. [Google Scholar] [CrossRef]
- Noor, M.; Buraidah, M.; Careem, M.; Majid, S.; Arof, A. An optimized poly(vinylidene fluoride-hexafluoropropylene)–NaI gel polymer electrolyte and its application in natural dye sensitized solar cells. Electrochim. Acta 2014, 121, 159–167. [Google Scholar] [CrossRef]
- Buraidah, M.H.; Shah, S.; Teo, L.P.; Chowdhury, F.I.; Careem, M.A.; Albinsson, I.; Mellander, B.-E.; Arof, A.K. High efficient dye sensitized solar cells using phthaloylchitosan based gel polymer electrolytes. Electrochim. Acta 2017, 245, 846–853. [Google Scholar] [CrossRef]
- Gonçalves, L.M.; Bermudez, V.D.Z.; Ribeiro, H.A.; Mendes, A.M. Dye-sensitized solar cells: A safe bet for the future. Energy Environ. Sci. 2008, 1, 655–667. [Google Scholar] [CrossRef] [Green Version]
- Kuppu, S.V.; Jeyaraman, A.R.; Guruviah, P.K.; Thambusamy, S. Preparation and characterizations of PMMA-PVDF based polymer composite electrolyte materials for dye sensitized solar cell. Curr. Appl. Phys. 2018, 18, 619–625. [Google Scholar] [CrossRef]
- Won, L.J.; Kim, J.H.; Thogiti, S. A Polymer Electrolyte for Dye-Sensitized Solar Cells Based on a Poly(Polyvinylidenefluoride-Co-Hexafluoropropylene)/Hydroxypropyl Methyl Cellulose Blend. Electron. Mater. Lett. 2018, 14, 342–347. [Google Scholar] [CrossRef]
- Pavithra, N.; Velayutham, D.; Sorrentino, A.; Anandan, S. Thiourea incorporated poly(ethylene oxide) as transparent gel polymer electrolyte for dye sensitized solar cell applications. J. Power Sources 2017, 353, 245–253. [Google Scholar] [CrossRef]
- Akhtar, M.S.; Park, J.-G.; Lee, H.-C.; Lee, S.-K.; Yang, O.-B. Carbon nanotubes–polyethylene oxide composite electrolyte for solid-state dye-sensitized solar cells. Electrochim. Acta 2010, 55, 2418–2423. [Google Scholar] [CrossRef]
- Aram, E.; Ehsani, M.; Khonakdar, H. Improvement of ionic conductivity and performance of quasi-solid-state dye sensitized solar cell using PEO/PMMA gel electrolyte. Thermochim. Acta 2015, 615, 61–67. [Google Scholar] [CrossRef]
- Yang, H.; Huang, M.; Wu, J.; Lan, Z.; Hao, S.; Lin, J. The polymer gel electrolyte based on poly(methyl methacrylate) and its application in quasi-solid-state dye-sensitized solar cells. Mater. Chem. Phys. 2008, 110, 38–42. [Google Scholar] [CrossRef]
- Kang, Y.-I.; Moon, J.H. In situ Poly(methyl methacrylate)/Graphene Composite Gel Electrolytes for Highly Stable Dye-Sensitized Solar Cells. ChemSusChem 2015, 8, 3799–3804. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.S.; Li, Z.Y.; Park, D.M.; Oh, D.W.; Kwak, D.-H.; Yang, O.-B. A new carbon nanotubes (CNTs)–poly acrylonitrile (PAN) composite electrolyte for solid state dye sensitized solar cells. Electrochim. Acta 2011, 56, 9973–9979. [Google Scholar] [CrossRef]
- Ng, H.; Ramesh, S.; Ramesh, K. Exploration on the P(VP-co-VAc) copolymer based gel polymer electrolytes doped with quaternary ammonium iodide salt for DSSC applications: Electrochemical behaviors and photovoltaic performances. Org. Electron. 2015, 22, 132–139. [Google Scholar] [CrossRef]
- Saidi, N.M.; Ng, H.M.; Omar, F.S.; Bashir, S.; Ramesh, K.; Ramesh, S. Polyacrylonitrile–poly(1-vinyl pyrrolidone-co-vinyl acetate) blend based gel polymer electrolytes incorporated with sodium iodide salt for dye-sensitized solar cell applications. J. Appl. Polym. Sci. 2019, 136, 47810. [Google Scholar] [CrossRef]
- Sundararajan, V.; Saidi, N.M.; Ramesh, S.; Ramesh, K.; Selvaraj, G.; Wilfred, C.D. Quasi solid-state dye-sensitized solar cell with P(MMA-co-MAA)-based polymer electrolytes. J. Solid State Electrochem. 2019, 23, 1179–1189. [Google Scholar] [CrossRef]
- Farhana, N.; Ramesh, S.; Ramesh, K. Efficiency enhancement of dye-sensitized solar cell based gel polymer electrolytes using Poly(vinyl butyral-co-vinyl alcohol-co-vinyl acetate)/tetrapropylammonium iodide. Mater. Sci. Semicond. Process. 2019, 91, 414–421. [Google Scholar] [CrossRef]
- Zebardastan, N.; Khanmirzaei, M.; Ramesh, S.; Ramesh, K. Novel poly(vinylidene fluoride-co-hexafluoro propylene)/polyethylene oxide based gel polymer electrolyte containing fumed silica (SiO2) nanofiller for high performance dye-sensitized solar cell. Electrochim. Acta 2016, 220, 573–580. [Google Scholar] [CrossRef]
- Venkatesan, S.; Lee, Y.-L. Nanofillers in the electrolytes of dye-sensitized solar cells—A short review. Coord. Chem. Rev. 2017, 353, 58–112. [Google Scholar] [CrossRef]
- Yang, Y.; Yi, P.; Zhou, C.; Cui, J.; Zheng, X.; Xiao, S.; Guo, X.; Wang, W. Magnetic field processed solid-state dye-sensitized solar cells with nickel oxide modified agarose electrolyte. J. Power Sources 2013, 243, 919–924. [Google Scholar] [CrossRef]
- Sethupathy, M.; Pandey, P.; Manisankar, P. Evaluation of photovoltaic efficiency of dye-sensitized solar cells fabricated with electrospun PVDF-PAN-Fe2O3 composite membrane. J. Appl. Polym. Sci. 2014, 131, 1–8. [Google Scholar] [CrossRef]
- Saidi, N.M.; Omar, F.S.; Numan, A.; Apperley, D.C.; Algaradah, M.M.; Kasi, R.; Avestro, A.-J.; Subramaniam, R.T. Enhancing the Efficiency of a Dye-Sensitized Solar Cell Based on a Metal Oxide Nanocomposite Gel Polymer Electrolyte. ACS Appl. Mater. Interfaces 2019, 11, 30185–30196. [Google Scholar] [CrossRef]
- Zebardastan, N.; Khanmirzaei, M.; Ramesh, S.; Ramesh, K. Performance enhancement of poly (vinylidene fluoride-co-hexafluoro propylene)/polyethylene oxide based nanocomposite polymer electrolyte with ZnO nanofiller for dye-sensitized solar cell. Org. Electron. 2017, 49, 292–299. [Google Scholar] [CrossRef]
- Saidi, N.M.; Farhana, N.; Ramesh, S.; Ramesh, K. Influence of different concentrations of 4-tert-butyl-pyridine in a gel polymer electrolyte towards improved performance of Dye-Sensitized Solar Cells (DSSC). Sol. Energy 2021, 216, 111–119. [Google Scholar] [CrossRef]
- Tiautit, N.; Puratane, C.; Panpinit, S.; Saengsuwan, S. Effect of SiO2 and TiO2 Nanoparticles on the Performance of Dye- Sensitized Solar Cells Using PVDF-HFP/PVA Gel Electrolytes. Energy Procedia 2014, 56, 378–385. [Google Scholar] [CrossRef] [Green Version]
- Tan, J.; Dun, M.; Li, L.; Zhao, J.; Li, X.; Hu, Y.; Huang, G.; Tan, W.; Huang, X. Self-template derived CuO nanowires assembled microspheres and its gas sensing properties. Sens. Actuators B Chem. 2017, 252, 1–8. [Google Scholar] [CrossRef]
- Xu, H.; Zeiger, B.W.; Suslick, K.S. Sonochemical synthesis of nanomaterials. Chem. Soc. Rev. 2013, 42, 2555–2567. [Google Scholar] [CrossRef] [Green Version]
- Mahbubul, I.M.; Chong, T.H.; Khaleduzzaman, S.S.; Shahrul, I.M.; Saidur, R.; Long, B.D.; Amalina, M.A. Effect of Ultrasonication Duration on Colloidal Structure and Viscosity of Alumina–Water Nanofluid. Ind. Eng. Chem. Res. 2014, 53, 6677–6684. [Google Scholar] [CrossRef]
- Afzal, A.; Nawfal, I.; Mahbubul, I.M.; Kumbar, S.S. An Overview on the Effect of Ultrasonication Duration on Different Properties of Nanofluids. J. Therm. Anal. Calorim. 2019, 135, 393–418. [Google Scholar] [CrossRef]
- Choudhary, S.; Bald, A.; Sengwa, R.J.; Chęcińska-Majak, D.; Klimaszewski, K. Effects of Ultrasonic Assisted Processing and Clay Nanofiller on Dielectric Properties and Lithium Ion Transport Mechanism of Poly(Methyl Methacrylate) Based Plasticized Polymer Electrolytes. J. Appl. Polym. Sci. 2015, 132, 18–27. [Google Scholar] [CrossRef]
- Ali, T.M.; Padmanathan, N.; Selladurai, S. Effect of nanofiller CeO2 on structural, conductivity, and dielectric behaviors of plasticized blend nanocomposite polymer electrolyte. Ionics 2015, 21, 829–840. [Google Scholar] [CrossRef]
- Shanti, R.; Bella, F.; Salim, Y.; Chee, S.; Ramesh, S.; Ramesh, K. Poly(methyl methacrylate-co-butyl acrylate-co-acrylic acid): Physico-chemical characterization and targeted dye sensitized solar cell application. Mater. Des. 2016, 108, 560–569. [Google Scholar] [CrossRef]
- Vijayakumar, E.; Subramania, A.; Fei, Z.; Dyson, P.J. High-performance dye-sensitized solar cell based on an electrospun poly(vinylidene fluoride-co-hexafluoropropylene)/cobalt sulfide nanocomposite membrane electrolyte. RSC Adv. 2015, 5, 52026–52032. [Google Scholar] [CrossRef]
- Bandara, T.M.W.J.; Karunathilaka, D.G.N.; Ratnasekera, J.L.; De Silva, L.A.; Herath, A.C.; Mellander, B.-E. Electrical and complex dielectric behaviour of composite polymer electrolyte based on PEO, alumina and tetrapropylammonium iodide. Ionics 2017, 23, 1711–1719. [Google Scholar] [CrossRef]
- Tan, C.; Omar, F.; Saidi, N.M.; Farhana, N.; Ramesh, S.; Ramesh, K. Optimization of poly(vinyl alcohol-co-ethylene)-based gel polymer electrolyte containing nickel phosphate nanoparticles for dye-sensitized solar cell application. Sol. Energy 2019, 178, 231–240. [Google Scholar] [CrossRef]
- Farhana, N.; Bashir, S.; Ramesh, S.; Ramesh, K. Augmentation of dye-sensitized solar cell photovoltaic conversion efficiency via incorporation of terpolymer Poly(vinyl butyral-co-vinyl alcohol-co-vinyl acetate) based gel polymer electrolytes. Polymer 2021, 223, 123713. [Google Scholar] [CrossRef]
- Lin, J.; Liu, Y.; Wang, Y.; Jia, H.; Chen, S.; Qi, J.; Qu, C.; Cao, J.; Fei, W.; Feng, J. Designed formation of NiO@C@Cu2O hybrid arrays as battery-like electrode with enhanced electrochemical performances. Ceram. Int. 2017, 43, 15410–15417. [Google Scholar] [CrossRef]
- Pramanik, A.; Maiti, S.; Mahanty, S. Metal hydroxides as a conversion electrode for lithium-ion batteries: A case study with a Cu(OH)2nanoflower array. J. Mater. Chem. A 2014, 2, 18515–18522. [Google Scholar] [CrossRef]
- Fu, W.; Han, W.; Zha, H.; Mei, J.; Li, Y.; Zhang, Z.; Xie, E. Nanostructured CuS networks composed of interconnected nanoparticles for asymmetric supercapacitors. Phys. Chem. Chem. Phys. 2016, 18, 24471–24476. [Google Scholar] [CrossRef]
- Gandhi, S.; Subramani, R.H.H.; Ramakrishnan, T.; Sivabalan, A.; Dhanalakshmi, V.; Nair, M.R.G.; Anbarasan, R. Ultrasound assisted one pot synthesis of nano-sized CuO and its nanocomposite with poly(vinyl alcohol). J. Mater. Sci. 2010, 45, 1688–1694. [Google Scholar] [CrossRef]
- Bandara, T.M.W.J.; Senavirathna, S.L.N.; Wickramasinghe, H.M.N.; Vignarooban, K.; De Silva, L.A.; Dissanayake, M.A.K.L.; Albinsson, I.; Mellander, B.-E. Binary counter ion effects and dielectric behavior of iodide ion conducting gel-polymer electrolytes for high-efficiency quasi-solid-state solar cells. Phys. Chem. Chem. Phys. 2020, 22, 12532–12543. [Google Scholar] [CrossRef]
- Chalkias, D.; Giannopoulos, D.; Kollia, E.; Petala, A.; Kostopoulos, V.; Papanicolaou, G. Preparation of polyvinylpyrrolidone-based polymer electrolytes and their application by in-situ gelation in dye-sensitized solar cells. Electrochim. Acta 2018, 271, 632–640. [Google Scholar] [CrossRef]
- Yusuf, S.N.F.; Azzahari, A.D.; Yahya, R.; Majid, S.R.; Careem, M.A.; Arof, A.K. From crab shell to solar cell: A gel polymer electrolyte based on N-phthaloylchitosan and its application in dye-sensitized solar cells. RSC Adv. 2016, 6, 27714–27724. [Google Scholar] [CrossRef]
- Tan, H.W.; Ramesh, S.; Liew, C.-W. Electrical, thermal, and structural studies on highly conducting additive-free biopolymer electrolytes for electric double-layer capacitor application. Ionics 2019, 25, 4861–4874. [Google Scholar] [CrossRef]
- Arya, A.; Sharma, A.L. Polymer Nanocomposites: Synthesis and Characterization. In Environmental Nanotechnology; Spinger: Cham, Switzerland, 2020; Volume 4, pp. 265–315. [Google Scholar]
- Chang, W.-C.; Sie, S.-Y.; Yu, W.-C.; Lin, L.-Y.; Yu, Y.-J. Preparation of Nano-composite Gel Electrolytes with Metal Oxide Additives for Dye-sensitized Solar Cells. Electrochim. Acta 2016, 212, 333–342. [Google Scholar] [CrossRef]
- Venkatesan, S.; Chen, Y.-Y.; Chien, C.-Y.; Tsai, M.-H.; Teng, H.; Lee, Y.-L. Quasi-solid-state composite electrolytes with Al2O3 and ZnO nanofillers for dye-sensitized solar cells. Electrochim. Acta 2020, 380, 137588. [Google Scholar] [CrossRef]
- Utpalla, P.; Sharma, S.; Prakash, J.; Bahadur, J.; Sahu, M.; Pujari, P. Free volume structure at interphase region of poly (ethylene oxide)-Al2O3 nanorods composites based solid polymer electrolyte and its direct correlation with Li ion conductivity. Solid State Ionics 2022, 375, 115840. [Google Scholar] [CrossRef]
- Suganya, N.; Jaisankar, V. A Study on the effect of dye and polymer nanocomposite electrolyte on the performance of natural dye sensitized solar cells. Mater. Today Proc. 2019, 14, 471–481. [Google Scholar] [CrossRef]
- Li, Q.; Ardebili, H. Atomistic investigation of the nanoparticle size and shape effects on ionic conductivity of solid polymer electrolytes. Solid State Ionics 2014, 268, 156–161. [Google Scholar] [CrossRef]
- Arya, A.; Saykar, N.G.; Sharma, A.L. Impact of shape (nanofiller vs. nanorod) of TiO2 nanoparticle on free-standing solid polymeric separator for energy storage/conversion devices. J. Appl. Polym. Sci. 2018, 136, 47361. [Google Scholar] [CrossRef]
- Do, N.S.T.; Schaetzl, D.M.; Dey, B.; Seabaugh, A.C.; Fullerton-Shirey, S.K. Influence of Fe2O3 Nanofiller Shape on the Conductivity and Thermal Properties of Solid Polymer Electrolytes: Nanorods versus Nanospheres. J. Phys. Chem. C 2012, 116, 21216–21223. [Google Scholar] [CrossRef]
- Mohamad, A.A.; Mohamed, N.S.; Yahya, M.Z.A.; Othman, R.; Ramesh, S.; Alias, Y.; Arof, A.K. Ionic Conductivity Studies of Poly(Vinyl Alcohol) Alkaline Solid Polymer Electrolyte and Its Use in Nickel-Zinc Cells. Solid State Ionics 2003, 156, 171–177. [Google Scholar] [CrossRef]
- Manafi, P.; Nazockdast, H.; Karimi, M.; Sadighi, M.; Magagnin, L. A study on the microstructural development of gel polymer electrolytes and different imidazolium-based ionic liquids for dye-sensitized solar cells. J. Power Sources 2021, 481, 228622. [Google Scholar] [CrossRef]
- Tan, C.Y.; Farhana, N.K.; Saidi, N.M.; Ramesh, S.; Ramesh, K. Conductivity, Dielectric Studies and Structural Properties of P(VA-Co-PE) and Its Application in Dye Sensitized Solar Cell. Org. Electron. Phys. Mater. Appl. 2018, 56, 116–124. [Google Scholar] [CrossRef]
- Aziz, S.B.; Abdullah, O.G.; Saeed, S.R.; Ahmed, H.M. Electrical and Dielectric Properties of Copper Ion Conducting Solid Polymer Electrolytes Based on Chitosan: CBH Model for Ion Transport Mechanism. Int. J. Electrochem. Sci. 2018, 13, 3812–3826. [Google Scholar] [CrossRef]
- Abdulwahid, R.T.; Aziz, S.B.; Kadir, M.F.Z. Insights into Ion Transport in Biodegradable Solid Polymer Blend Electrolyte Based on FTIR Analysis and Circuit Design. J. Phys. Chem. Solids 2022, 167, 110774. [Google Scholar] [CrossRef]
- Kumar, A.; Madaan, M.; Arya, A.; Tanwar, S.; Sharma, A.L. Ion transport, dielectric, and electrochemical properties of sodium ion-conducting polymer nanocomposite: Application in EDLC. J. Mater. Sci. Mater. Electron. 2020, 31, 10873–10888. [Google Scholar] [CrossRef]
- Selvalakshmi, S.; Mathavan, T.; Premalatha, M.; Selvasekarapandian, S. Study on NH4I composition effect in agar–agar-based biopolymer electrolyte. Ionics 2017, 23, 2791–2797. [Google Scholar] [CrossRef]
- Arya, A.; Sharma, A.L. Effect of salt concentration on dielectric properties of Li-ion conducting blend polymer electrolytes. J. Mater. Sci. Mater. Electron. 2018, 29, 17903–17920. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.-L.; Teng, H.; Lee, Y.-L. Preparation of highly efficient gel-state dye-sensitized solar cells using polymer gel electrolytes based on poly(acrylonitrile-co-vinyl acetate). J. Mater. Chem. 2011, 21, 628–632. [Google Scholar] [CrossRef]
- Lan, Z.; Wu, J.; Wang, D.; Hao, S.; Lin, J.; Huang, Y. Quasi-solid state dye-sensitized solar cells based on gel polymer electrolyte with poly(acrylonitrile-co-styrene)/NaI+I2. Sol. Energy 2006, 80, 1483–1488. [Google Scholar] [CrossRef]
- Ulvestad, A.; Reksten, A.H.; Andersen, H.F.; Carvalho, P.A.; Jensen, I.J.T.; Nagell, M.U.; Mæhlen, J.P.; Kirkengen, M.; Koposov, A.Y. Crystallinity of Silicon Nanoparticles: Direct Influence on the Electrochemical Performance of Lithium Ion Battery Anodes. ChemElectroChem 2020, 7, 4349–4353. [Google Scholar] [CrossRef]
- Croce, F.; Persi, L.; Scrosati, B.; Serraino-Fiory, F.; Plichta, E.; Hendrickson, M. Role of the ceramic fillers in enhancing the transport properties of composite polymer electrolytes. Electrochim. Acta 2001, 46, 2457–2461. [Google Scholar] [CrossRef]
- Jeong, N.C.; Lee, J.S.; Tae, E.L.; Lee, Y.J.; Yoon, K.B. Acidity Scale for Metal Oxides and Sanderson’s Electronegativities of Lanthanide Elements. Angew. Chem. Int. Ed. 2008, 47, 10128–10132. [Google Scholar] [CrossRef]
- Chai, K.; Noor, I.; Aung, M.M.; Abdullah, L.C.; Kufian, M. Non-edible oil based polyurethane acrylate with tetrabutylammonium iodide gel polymer electrolytes for dye-sensitized solar cells. Sol. Energy 2020, 208, 457–468. [Google Scholar] [CrossRef]
- Vinoth, S.; Kanimozhi, G.; Narsimulu, D.; Kumar, H.; Srinadhu, E.; Satyanarayana, N. Ionic relaxation of electrospun nanocomposite polymer-blend quasi-solid electrolyte for high photovoltaic performance of Dye-sensitized solar cells. Mater. Chem. Phys. 2020, 250, 122945. [Google Scholar] [CrossRef]
- Yang, Y.; Cui, J.; Yi, P.; Zheng, X.; Guo, X.; Wang, W. Effects of nanoparticle additives on the properties of agarose polymer electrolytes. J. Power Sources 2014, 248, 988–993. [Google Scholar] [CrossRef]
- Goh, Z.; Saidi, N.M.; Farhana, N.; Bashir, S.; Iqbal, J.; Ramesh, K.; Ramesh, S.; Wageh, S.; Kalam, A. Sonochemically synthesized cobalt oxide nanoparticles as an additive for natural polymer iodide electrolyte based dye-sensitized solar cells. Sustain. Energy Technol. Assess. 2022, 49, 101746. [Google Scholar] [CrossRef]
- Chan, X.H.; Khanmirzaei, M.H.; Omar, F.S.; Ramesh, K.; Ramesh, S.; Ramesh, S. Enhanced Efficiency in Dye-Sensitized Solar Cell Based on Zinc Oxide-Modified Poly(Ethylene Oxide) Gel Electrolyte. Ionics 2018, 24, 1221–1226. [Google Scholar] [CrossRef]
- Lim, S.M.; Moon, J.; Choi, G.H.; Baek, U.C.; Lim, J.M.; Park, J.T.; Kim, J.H. Surface Carbon Shell-Functionalized ZrO2 as Nanofiller in Polymer Gel Electrolyte-Based Dye-Sensitized Solar Cells. Nanomaterials 2019, 9, 1418. [Google Scholar] [CrossRef] [Green Version]
- Yan, Y.; Jie, T.; Xin, J.; Qi, Q. Preparation and characterization of a microporous polymer electrolyte based on poly(vinylidene fluoride)/ionic-liquid-functionalized SiO2 for dye-sensitized solar cells. J. Appl. Polym. Sci. 2011, 121, 1566–1573. [Google Scholar] [CrossRef]
- Aziz, M.; Buraidah, M.; Careem, M.; Arof, A. PVA based gel polymer electrolytes with mixed iodide salts (K+I− and Bu4N+I−) for dye-sensitized solar cell application. Electrochim. Acta 2015, 182, 217–223. [Google Scholar] [CrossRef]
- Wang, X.; Kulkarni, S.A.; Ito, B.I.; Batabyal, S.K.; Nonomura, K.; Wong, C.C.; Grätzel, M.; Mhaisalkar, S.G.; Uchida, S. Nanoclay Gelation Approach toward Improved Dye-Sensitized Solar Cell Efficiencies: An Investigation of Charge Transport and Shift in the TiO2 Conduction Band. ACS Appl. Mater. Interfaces 2013, 5, 444–450. [Google Scholar] [CrossRef]
- Mohan, V.M.; Murakami, K. Dye Sensitized Solar Cell with Carbon Doped (PAN/PEG) Polymer Quasi-Solid Gel Electrolyte. J. Adv. Res. Phys. 2011, 2, 021112. [Google Scholar]

| Samples | Peak 1 | Peak 2 | ||||
|---|---|---|---|---|---|---|
| FHWM (⁰) | 2θ (⁰) | τ (Å) | FHWM (⁰) | 2θ (⁰) | τ (Å) | |
| TPAI-4 | 4.61 | 8.9 | 4.61 | 6.4 | 21.9 | 2.28 |
| TCu(OH)2 | 5.76 | 10.1 | 2.42 | 11.8 | 20.3 | 1.91 |
| TCuO-200 | 5.10 | 9.9 | 2.73 | 12.2 | 19.7 | 1.15 |
| TCuO-350 | 6.18 | 10.5 | 2.25 | 16.0 | 19.5 | 0.88 |
| TCuO-500 | 5.66 | 10.6 | 2.49 | 12.5 | 19.6 | 1.12 |
| PCGE Samples | Rb (Ω) | Ea (eV) | 10−3 S cm−1) |
|---|---|---|---|
| TPAI-4 | 64.5 | 0.166 | 1.58 |
| TCu(OH)2 | 58.4 | 0.164 | 1.99 |
| TCuO-200 | 54.3 | 0.153 | 2.14 |
| TCuO-350 | 45.5 | 0.147 | 2.51 |
| TCuO-500 | 52.9 | 0.150 | 2.24 |
| PCGE Samples | Jlim (mA cm−1) | (×10−6 cm2 s−1) |
|---|---|---|
| TPAI-4 | 1.22 | 5.94 |
| TCu(OH)2 | 1.34 | 6.74 |
| TCuO-200 | 1.93 | 9.70 |
| TCuO-350 | 2.48 | 12.5 |
| TCuO-500 | 1.49 | 7.49 |
| Electrolytes | Type of Nanofiller | η (%) | Reference |
|---|---|---|---|
| P(VB-co-VA-co-VAc)/TPAI/CuO-350 (TCuO-350) | CuO-350 (Nanorod) | 7.05 | In the present work |
| PVDF-HFP: PMMA/BMII/LiI | TiO2 (nanocrystalline) | 7.08 | [84] |
| Agarose/LiI/NMP | NiO | 2.02 | [85] |
| HPC/KI | Co3O4 (nanocuboid) | 5.80 | [86] |
| PEO/NaI | ZnO | 6.94 | [87] |
| PEG/LiI/MPII | Modified-ZrO2 | 5.60 | [88] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farhana, N.K.; Omar, F.S.; Mohamad Saidi, N.; Ling, G.Z.; Bashir, S.; Subramaniam, R.; Kasi, R.; Iqbal, J.; Wageh, S.; Algarni, H.; et al. Modification of DSSC Based on Polymer Composite Gel Electrolyte with Copper Oxide Nanochain by Shape Effect. Polymers 2022, 14, 3426. https://doi.org/10.3390/polym14163426
Farhana NK, Omar FS, Mohamad Saidi N, Ling GZ, Bashir S, Subramaniam R, Kasi R, Iqbal J, Wageh S, Algarni H, et al. Modification of DSSC Based on Polymer Composite Gel Electrolyte with Copper Oxide Nanochain by Shape Effect. Polymers. 2022; 14(16):3426. https://doi.org/10.3390/polym14163426
Chicago/Turabian StyleFarhana, Nur Khuzaimah, Fatin Saiha Omar, Norshahirah Mohamad Saidi, Goh Zhi Ling, Shahid Bashir, Ramesh Subramaniam, Ramesh Kasi, Javed Iqbal, Swelm Wageh, Hamed Algarni, and et al. 2022. "Modification of DSSC Based on Polymer Composite Gel Electrolyte with Copper Oxide Nanochain by Shape Effect" Polymers 14, no. 16: 3426. https://doi.org/10.3390/polym14163426
APA StyleFarhana, N. K., Omar, F. S., Mohamad Saidi, N., Ling, G. Z., Bashir, S., Subramaniam, R., Kasi, R., Iqbal, J., Wageh, S., Algarni, H., & Al-Sehemi, A. G. (2022). Modification of DSSC Based on Polymer Composite Gel Electrolyte with Copper Oxide Nanochain by Shape Effect. Polymers, 14(16), 3426. https://doi.org/10.3390/polym14163426