Selective Electroless Copper Plating of Ink-Jet Printed Textiles Using a Copper-Silver Nanoparticle Catalyst
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Equipment
2.3. Electroless Copper Plating Procedure Using Cu–Ag NP Catalyst
2.3.1. Synthesis of Cu–Ag NP Catalyst
2.3.2. Printing of Cu–Ag NP Catalyst and Electroless Plating
2.4. Characterisation of the Samples
2.4.1. Characterisation of Cu–Ag NP Catalyst
2.4.2. Characterisation of Electroless Copper Coatings
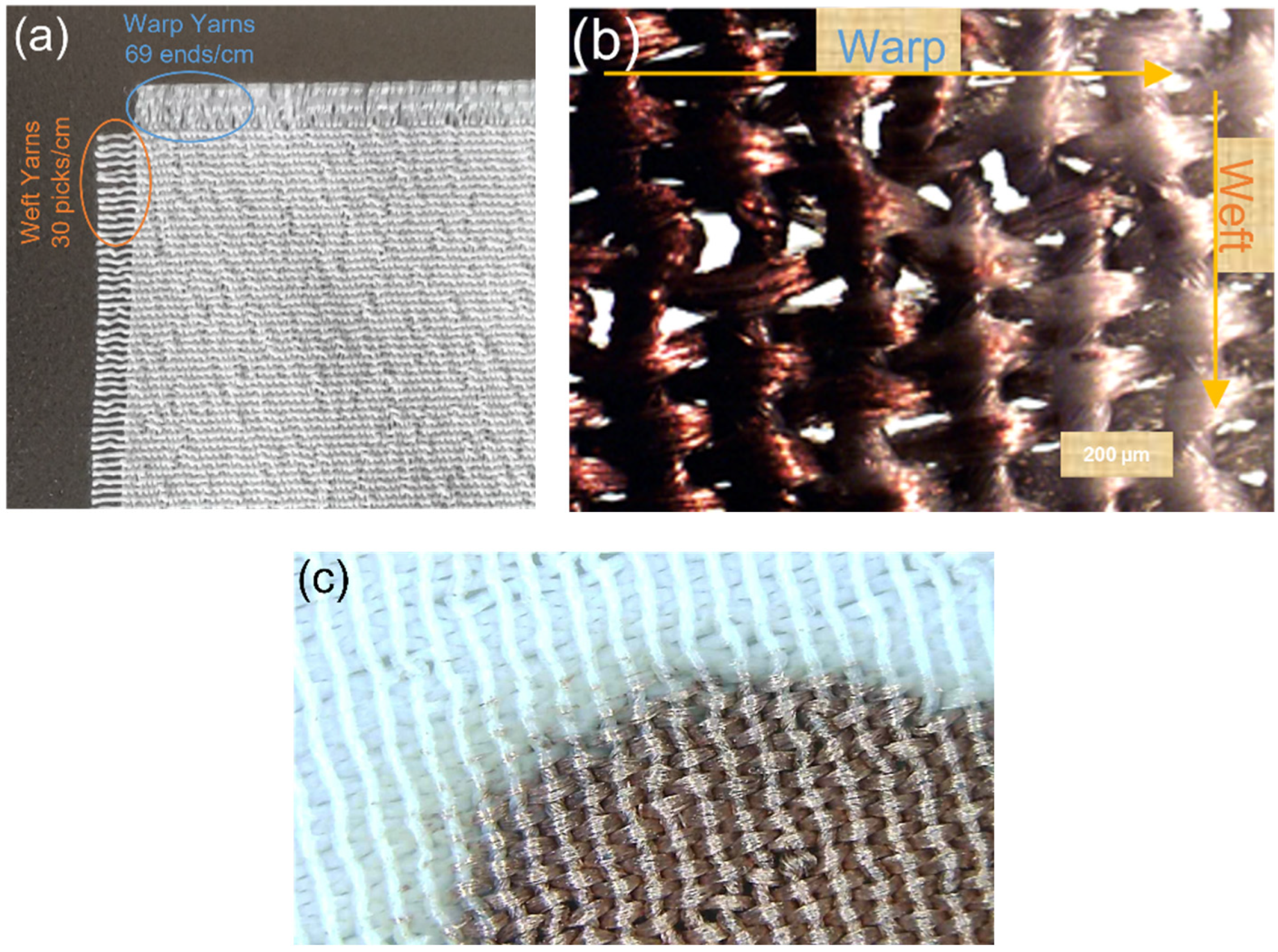
2.4.3. Textile Characterisations
3. Results and Discussion
3.1. Characterisation of Cu–Ag NP Catalyst
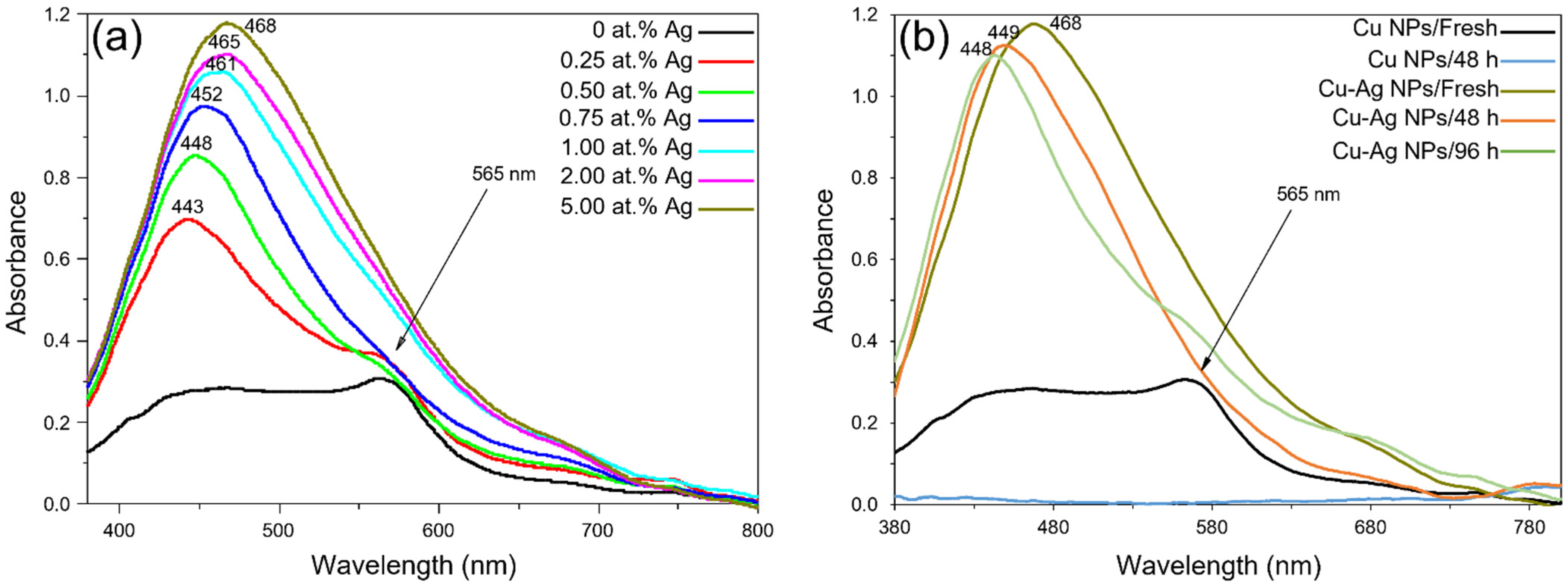
3.1.1. UV/Vis Spectroscopy Characterization
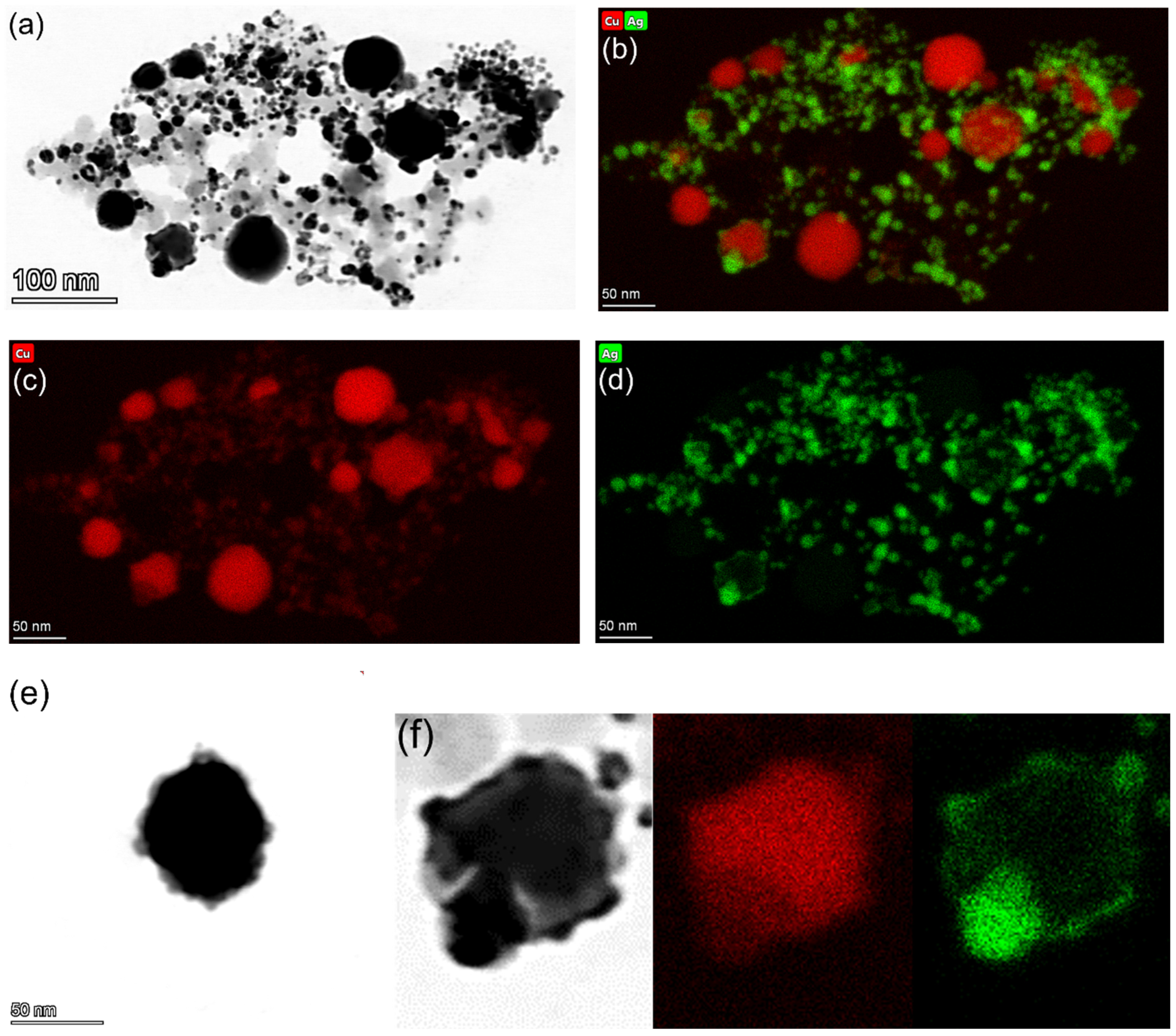
3.1.2. TEM Characterization
3.2. Characterisation of Electroless Copper Coatings
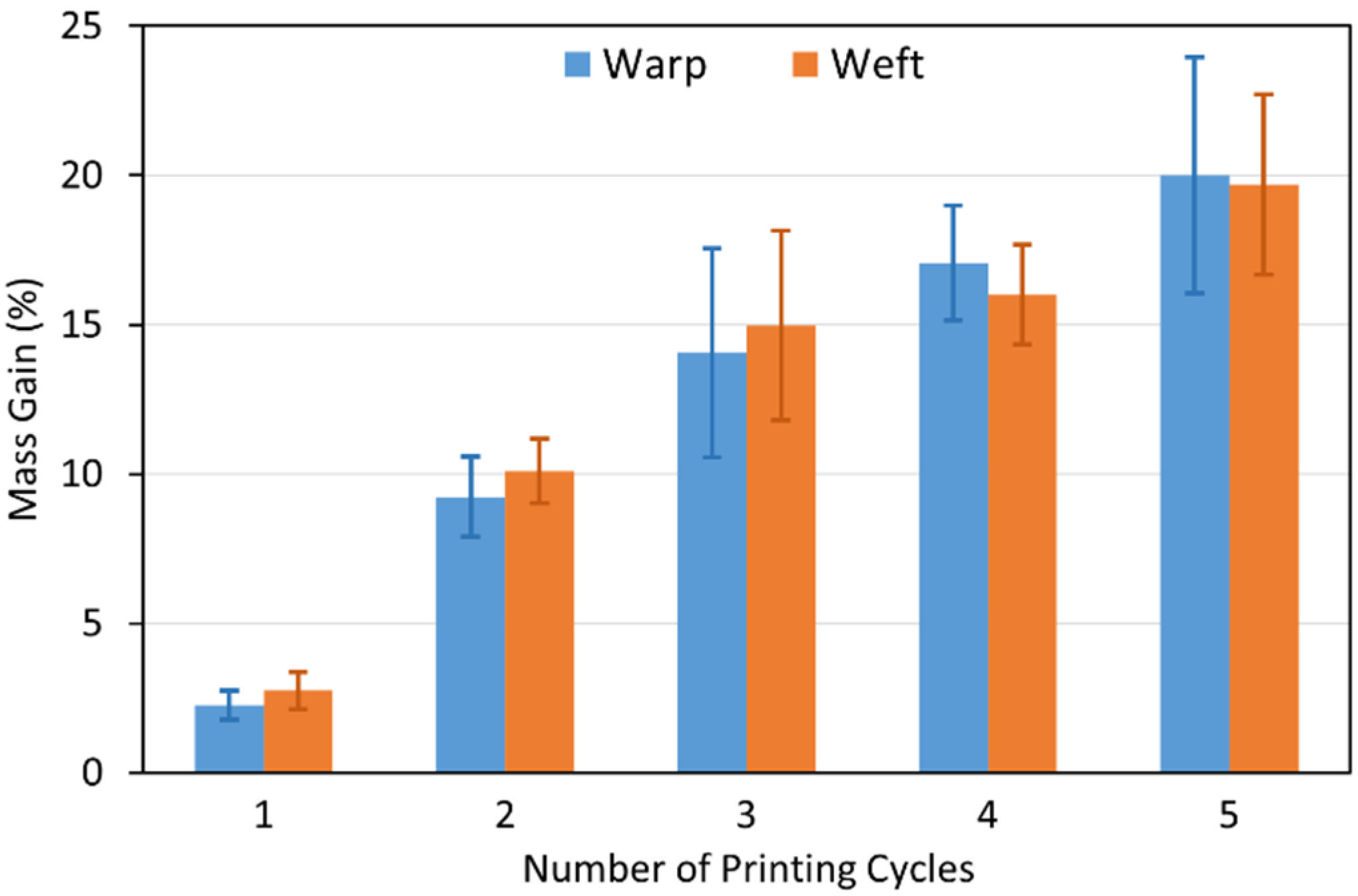
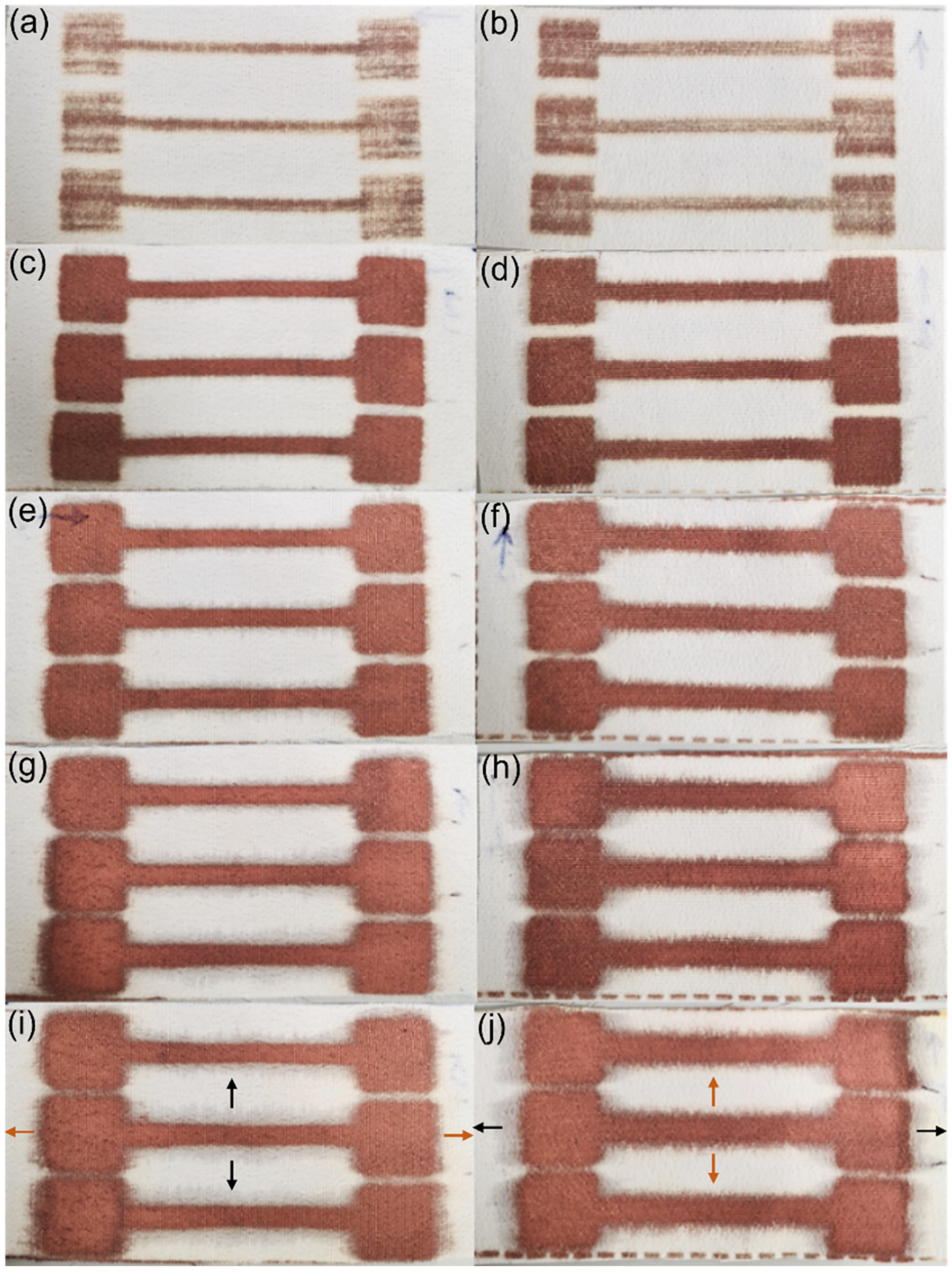
3.2.1. Visual Inspection and Copper Mass Gain Measurements
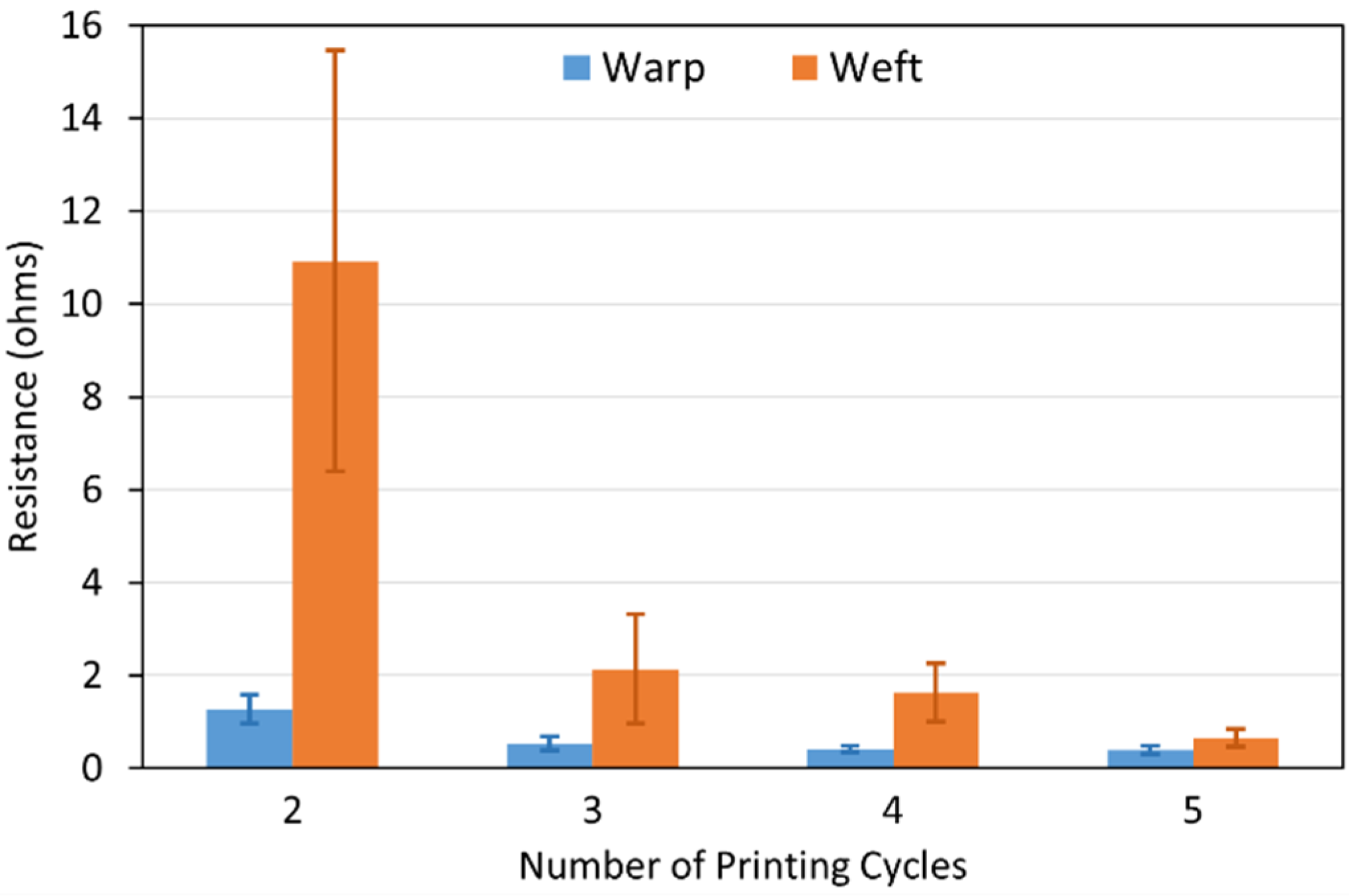
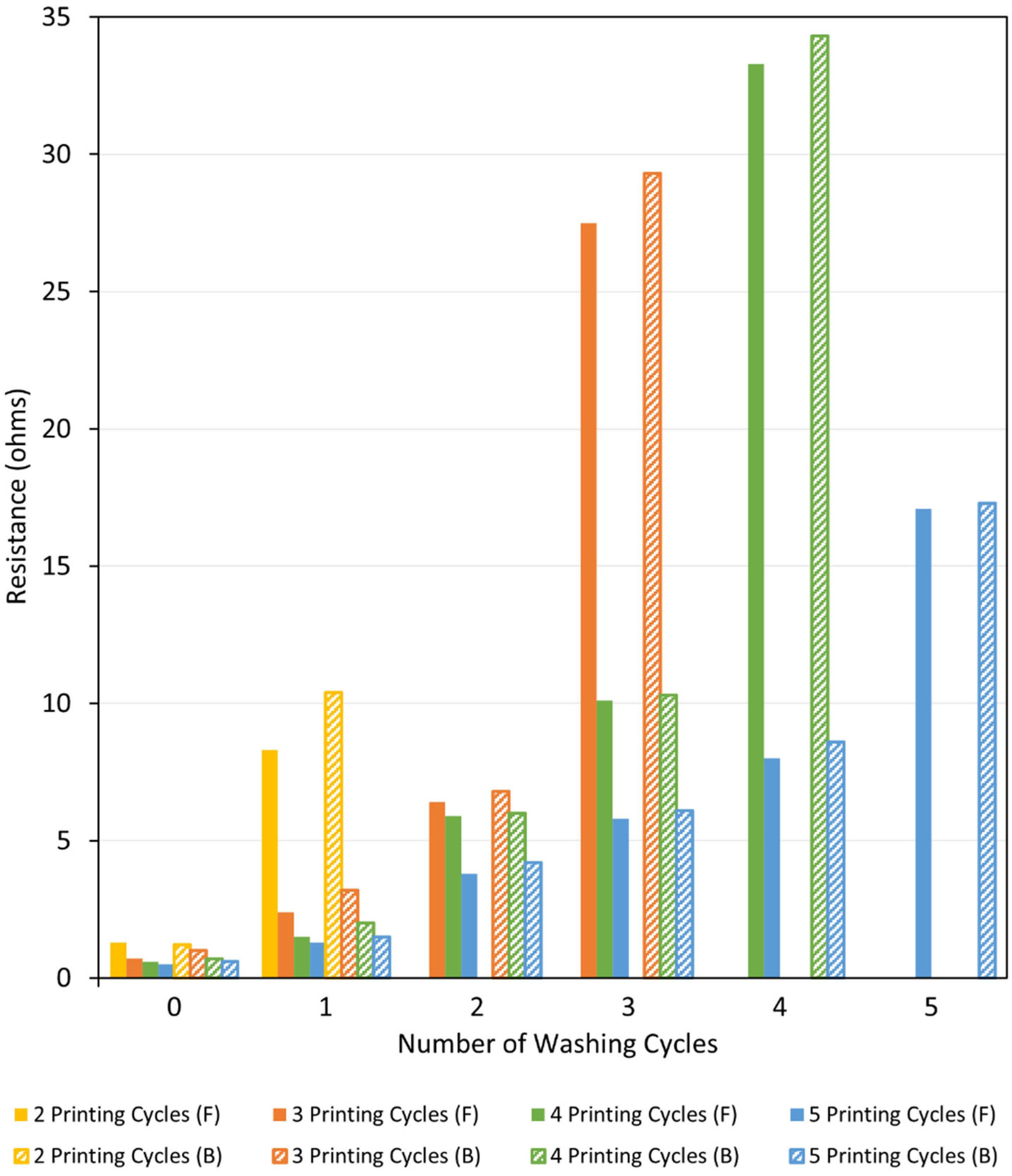
3.2.2. Electrical Resistance Measurements
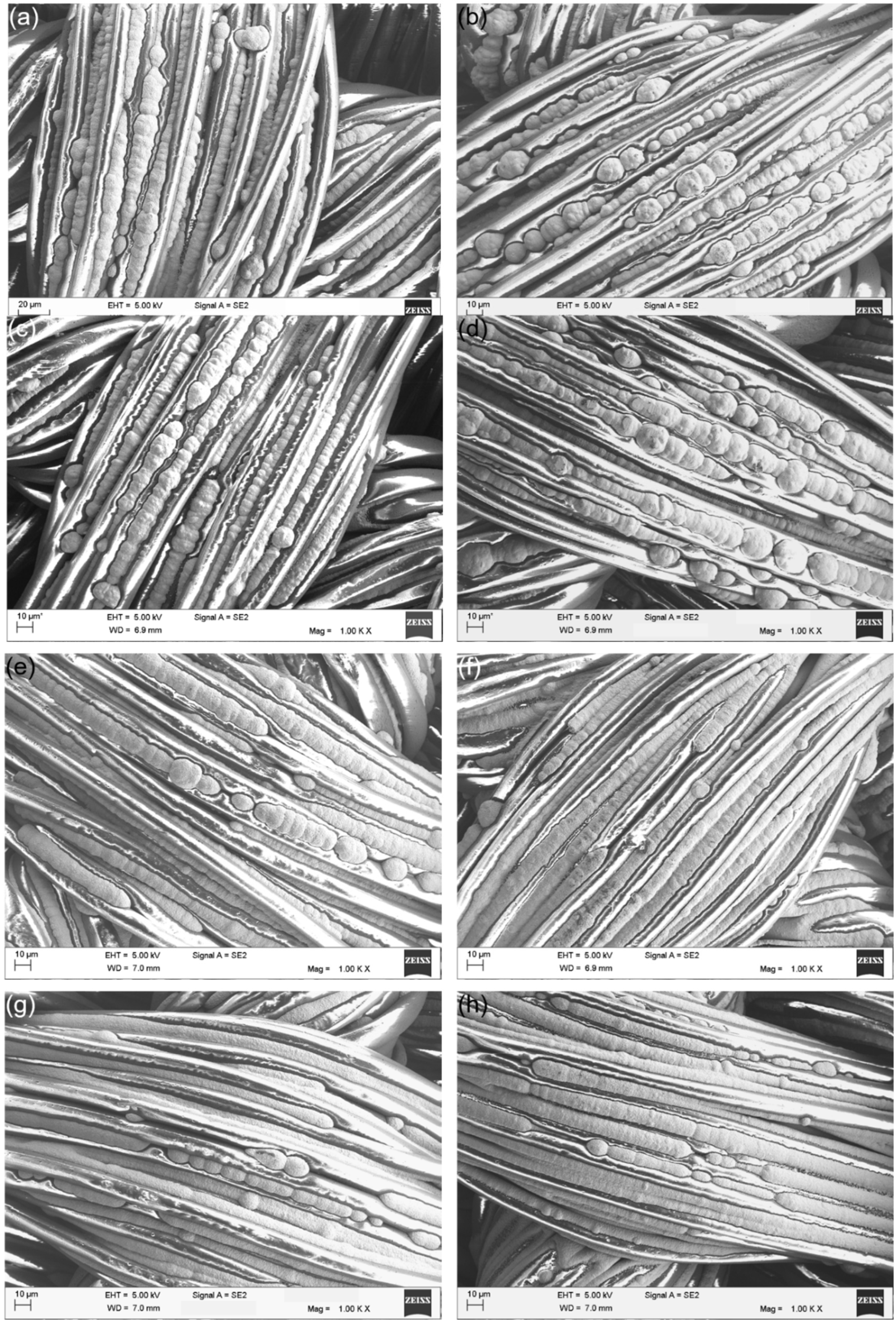
3.2.3. SEM Characterization
3.3. Textile Characterisations
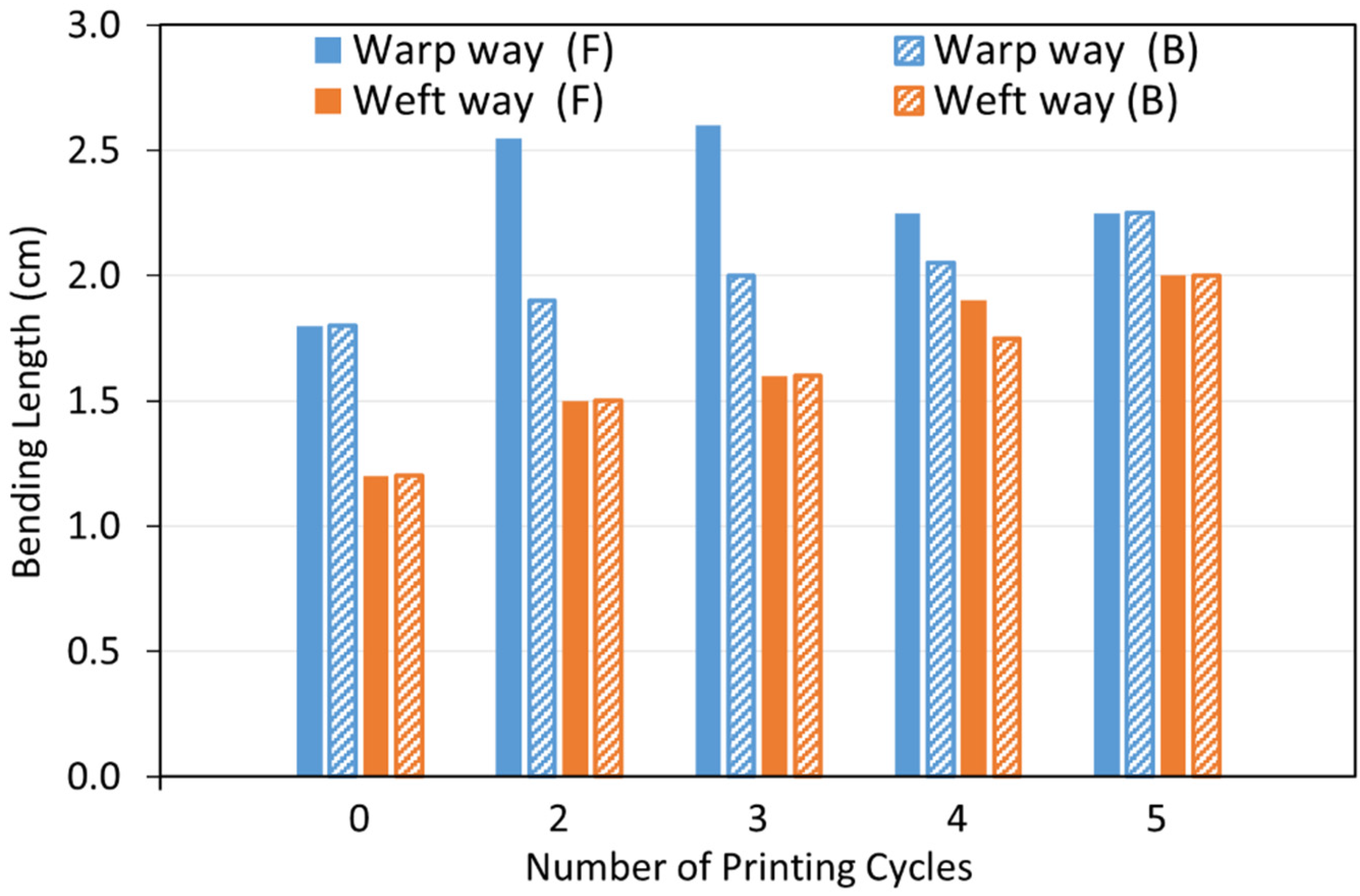
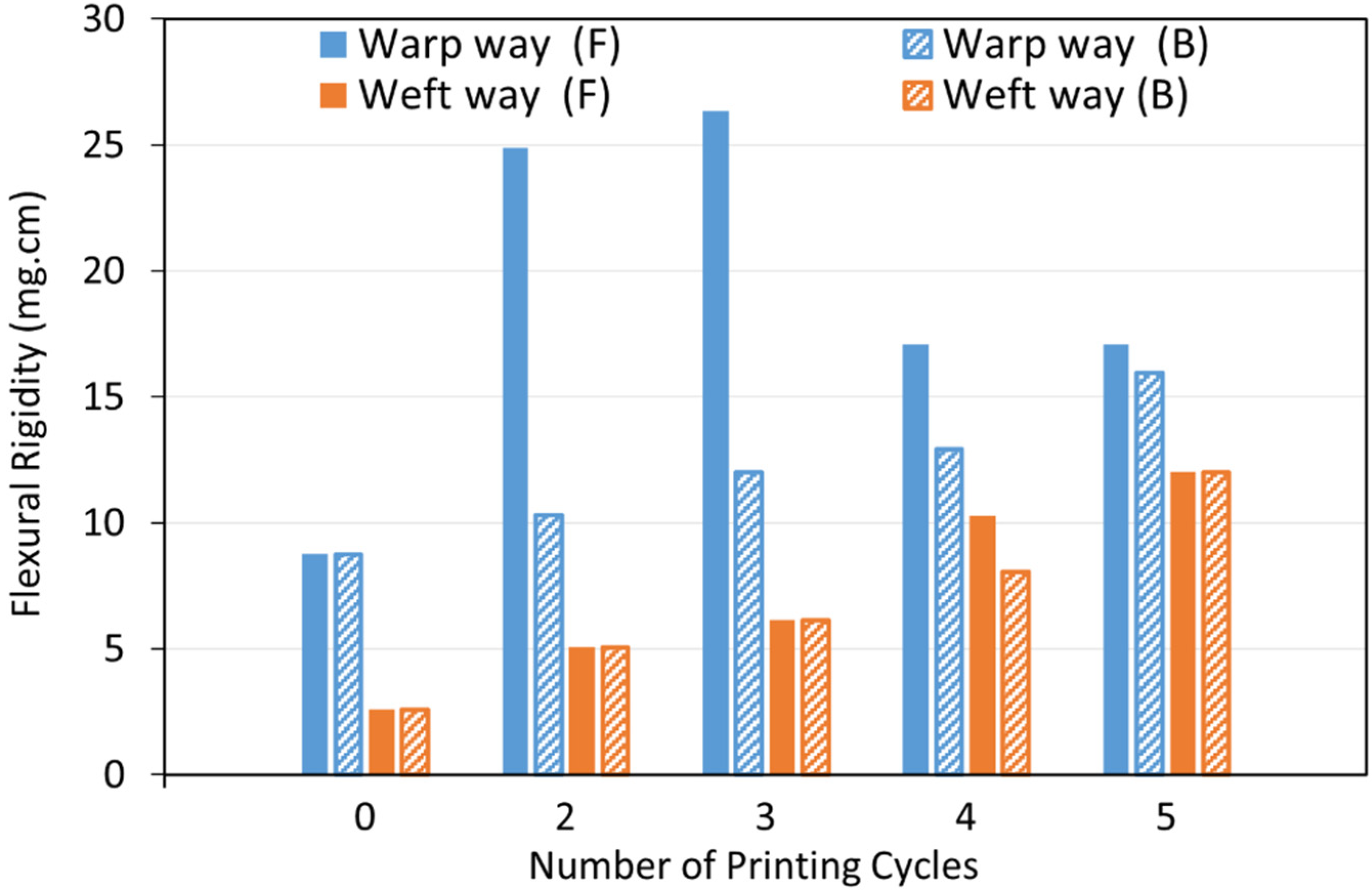
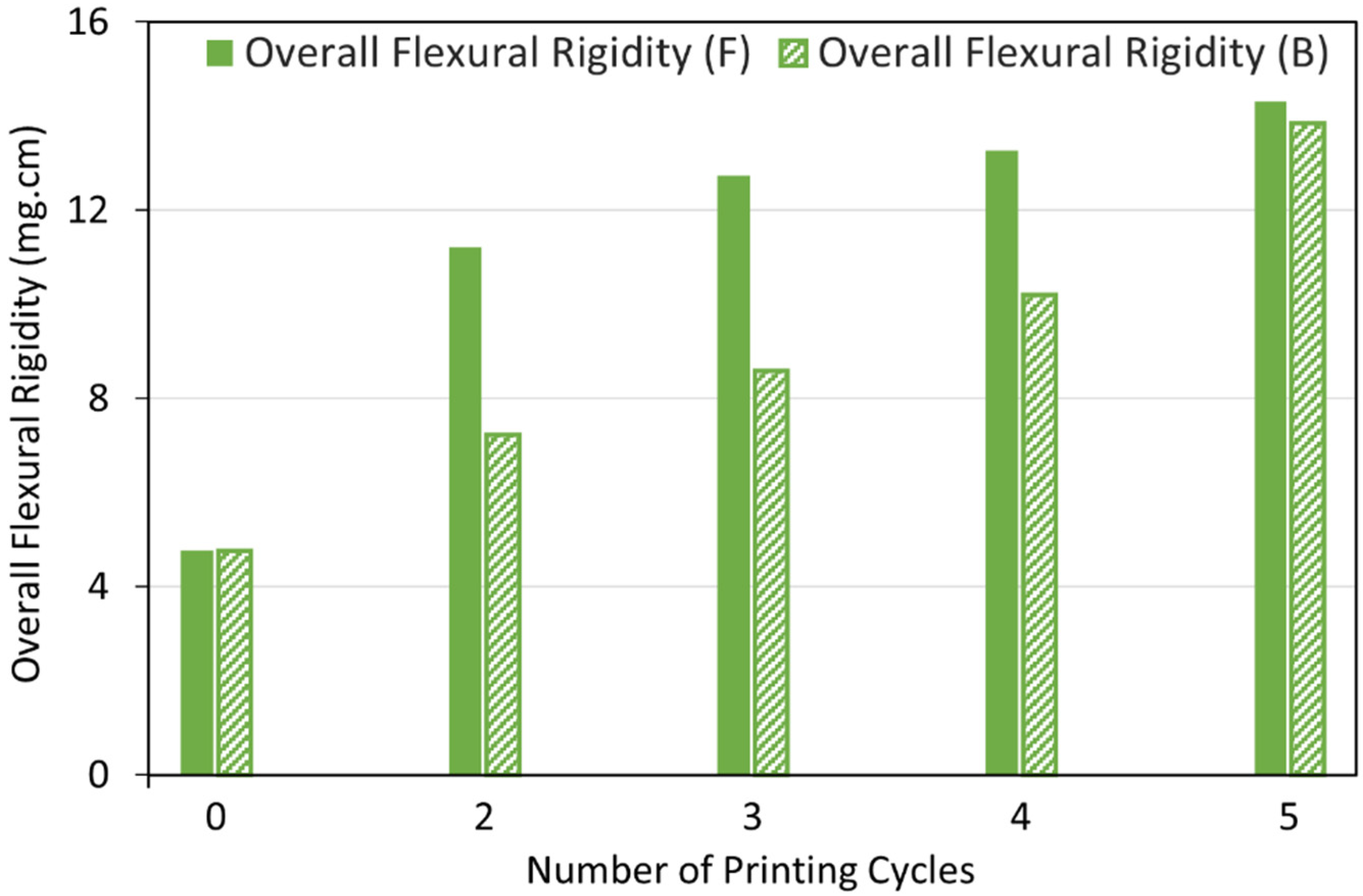
3.3.1. Fabric Stiffness Test
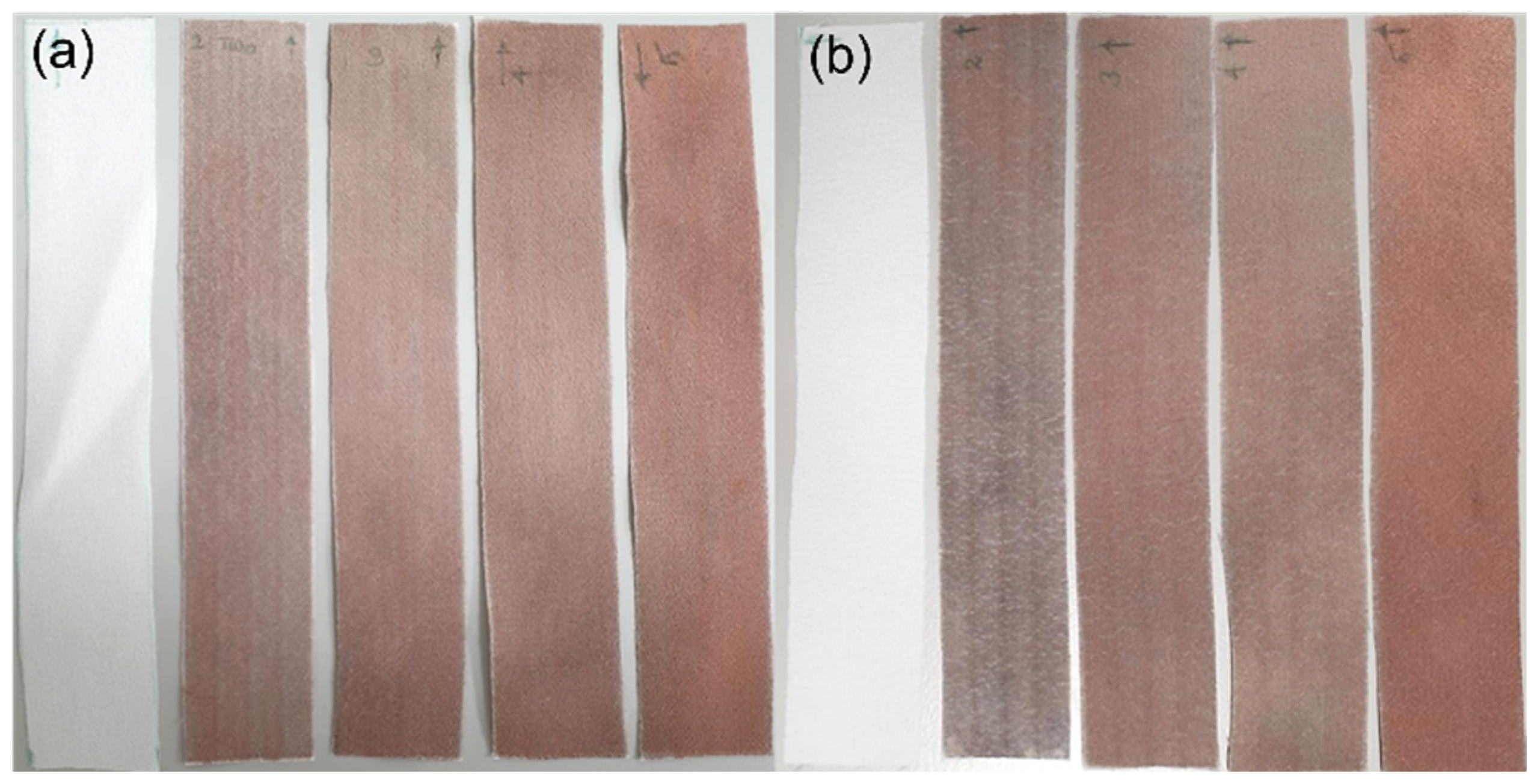
3.3.2. Laundering Durability and Fastness Test (Launder Ometer Test)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Komolafe, A.; Torah, R.; Wei, Y.; Nunes-Matos, H.; Li, M.; Hardy, D.; Dias, T.; Tudor, M.; Beeby, S. Integrating Flexible Filament Circuits for E-Textile Applications. Adv. Mater. Technol. 2019, 4, 1900176. [Google Scholar] [CrossRef]
- Kang, T.K. Highly Stretchable Non-volatile Nylon Thread Memory. Sci. Rep. 2016, 6, 24406. [Google Scholar] [CrossRef] [PubMed]
- Jo, A.; Seo, Y.; Ko, M.; Kim, C.; Kim, H.; Nam, S.; Choi, H.; Hwang, C.S.; Lee, M.J. Textile Resistance Switching Memory for Fabric Electronics. Adv. Funct. Mater. 2017, 27, 1605593. [Google Scholar] [CrossRef]
- Zhang, Z.; Guo, K.; Li, Y.; Li, X.; Guan, G.; Li, H.; Luo, Y.; Zhao, F.; Zhang, Q.; Wei, B.; et al. A colour-tunable, weavable fibre-shaped polymer light-emitting electrochemical cell. Nat. Photonics 2015, 9, 233–238. [Google Scholar] [CrossRef]
- Kim, W.; Kwon, S.; Han, Y.C.; Kim, E.; Choi, K.C.; Kang, S.H.; Park, B.C. Reliable Actual Fabric-Based Organic Light-Emitting Diodes: Toward a Wearable Display. Adv. Electron. Mater. 2016, 2, 1600220. [Google Scholar] [CrossRef]
- Li, R.; Xiang, X.; Tong, X.; Zou, J.; Li, Q. Wearable Double-Twisted Fibrous Perovskite Solar Cell. Adv. Mater. 2015, 27, 3831–3835. [Google Scholar] [CrossRef]
- Yang, Z.; Deng, J.; Sun, X.; Li, H.; Peng, H. Stretchable, wearable dye-sensitized solar cells. Adv. Mater. 2014, 26, 2643–2647. [Google Scholar] [CrossRef]
- Qu, G.; Cheng, J.; Li, X.; Yuan, D.; Chen, P.; Chen, X.; Wang, B.; Peng, H. A Fiber Supercapacitor with High Energy Density Based on Hollow Graphene/Conducting Polymer Fiber Electrode. Adv. Mater. 2016, 28, 3646–3652. [Google Scholar] [CrossRef]
- Sun, H.; Xie, S.; Li, Y.; Jiang, Y.; Sun, X.; Wang, B.; Peng, H. Large-Area Supercapacitor Textiles with Novel Hierarchical Conducting Structures. Adv. Mater. 2016, 28, 8431–8438. [Google Scholar] [CrossRef]
- Ojstršek, A.; Plohl, O.; Gorgieva, S.; Kurečič, M.; Jančič, U.; Hribernik, S.; Fakin, D. Metallisation of Textiles and Protection of Conductive Layers: An Overview of Application Techniques. Sensors 2021, 21, 3508. [Google Scholar] [CrossRef]
- Locher, I.; Tröster, G. Enabling Technologies for Electrical Circuits on a Woven Monofilament Hybrid Fabric. Text. Res. J. 2008, 78, 583–594. [Google Scholar] [CrossRef]
- Onggar, T.; Amrhein, G.; Abdkader, A.; Hund, R.D.; Cherif, C. Wet-chemical method for the metallization of a para-aramid filament yarn wound on a cylindrical dyeing package. Text. Res. J. 2017, 87, 1192–1202. [Google Scholar] [CrossRef]
- Takamatsu, S.; Kobayashi, T.; Shibayama, N.; Miyake, K.; Itoh, T. Fabric pressure sensor array fabricated with die-coating and weaving techniques. Sens. Actuators A Phys. 2012, 184, 57–63. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, H.; Yoo, H.J. Electrical characterization of screen-printed circuits on the fabric. IEEE Trans. Adv. Packag. 2010, 33, 196–205. [Google Scholar] [CrossRef]
- Park, B.K.; Kim, D.; Jeong, S.; Moon, J.; Kim, J.S. Direct writing of copper conductive patterns by ink-jet printing. Thin Solid Film 2007, 515, 7706–7711. [Google Scholar] [CrossRef]
- Karim, N.; Afroj, S.; Malandraki, A.; Butterworth, S.; Beach, C.; Rigout, M.; Novoselov, K.S.; Casson, A.J.; Yeates, S.G. All inkjet-printed graphene-based conductive patterns for wearable e-textile applications. J. Mater. Chem. C 2017, 5, 11640–11648. [Google Scholar] [CrossRef]
- Nechyporchuk, O.; Yu, J.; Nierstrasz, V.A.; Bordes, R. Cellulose Nanofibril-Based Coatings of Woven Cotton Fabrics for Improved Inkjet Printing with a Potential in E-Textile Manufacturing. ACS Sustain. Chem. Eng. 2017, 5, 4793–4801. [Google Scholar] [CrossRef]
- Liu, X.; Chang, H.; Li, Y.; Huck, W.T.S.; Zheng, Z. Polyelectrolyte-bridged metal/cotton hierarchical structures for highly durable conductive yarns. ACS Appl. Mater. Interfaces 2010, 2, 529–535. [Google Scholar] [CrossRef]
- Hassan, Z.; Kalaoglu, F.; Atalay, O. Development and characterization of conductive textile (cotton) for wearable electronics and soft robotic applications. Text. Res. J. 2020, 90, 1792–1804. [Google Scholar] [CrossRef]
- Taghavi Pourian Azar, G.; Fox, D.; Fedutik, Y.; Krishnan, L.; Cobley, A.J. Functionalised copper nanoparticle catalysts for electroless copper plating on textiles. Surf. Coat. Technol. 2020, 396, 125971. [Google Scholar] [CrossRef]
- Bonaldi, R.R. 12a—Electronics used in high-performance apparel—Part ½. In High-Performance Apparel; McLoughlin, J., Sabir, T., Eds.; Woodhead Publishing: Cambridge, UK, 2018; pp. 245–284. [Google Scholar] [CrossRef]
- Mallory, G.O.; Hadju, J.B. Electroless Plating: Fundamentals and Applications; AESF: Orlando, FL, USA, 1990. [Google Scholar]
- Dubin, V.M.; Shacham-Diamand, Y.; Zhoo, B.; Vasudev, P.K.; Ting, C.H. Selective and blanket electroless copper deposition for ultralarge scale integration. J. Electrochem. Soc. 1997, 144, 898–908. [Google Scholar] [CrossRef]
- Lee, C.L.; Tsai, Y.L.; Chen, C.W. Specific and mass activity of silver nanocube and nanoparticle-based catalysts for electroless copper deposition. Electrochim. Acta. 2013, 104, 185–190. [Google Scholar] [CrossRef]
- Fritz, N.; Koo, H.-C.; Wilson, Z.; Uzunlar, E.; Wen, Z.; Yeow, X.; Bidstrup Allen, S.A.; Kohl, P.A. Electroless Deposition of Copper on Organic and Inorganic Substrates Using a Sn/Ag Catalyst. J. Electrochem. Soc. 2012, 159, D386–D392. [Google Scholar] [CrossRef]
- Lan, J.-L.; Wan, C.-C.; Wang, Y.-Y. Mechanistic Study of Ag/Pd-PVP Nanoparticles and Their Functions as Catalyst for Electroless Copper Deposition. J. Electrochem. Soc. 2008, 155, K77. [Google Scholar] [CrossRef]
- Chen, C.-H.; Yang, H.-L.; Chen, H.-R.; Lee, C.-L. Activity on Electrochemical Surface Area: Silver Nanoplates as New Catalysts for Electroless Copper Deposition. J. Electrochem. Soc. 2012, 159, D507–D511. [Google Scholar] [CrossRef]
- Graves, J.E.; Sugden, M.; Litchfield, R.E.; Hutt, D.A.; Mason, T.J.; Cobley, A.J. Ultrasound assisted dispersal of a copper nanopowder for electroless copper activation. Ultrason. Sonochem. 2016, 29, 428–438. [Google Scholar] [CrossRef]
- Van Den Meerakker, J.E.A.M. On the mechanism of electroless plating. I. Oxidation of formaldehyde at different electrode surfaces. J. Appl. Electrochem. 1981, 11, 387–393. [Google Scholar] [CrossRef]
- Ohno, I.; Wakabayashi, O.; Haruyama, S. Anodic Oxidation of Reductants in Electroless Plating. J. Electrochem. Soc. 1985, 132, 2323. [Google Scholar] [CrossRef]
- Bindra, P.; Roldan, J. Mechanisms of Electroless Metal Plating II. Formaldehyde Oxidation. J. Electrochem. Soc. 1985, 132, 2581–2589. [Google Scholar] [CrossRef]
- Steinhäuser, E. Potential low-cost palladium-alternatives for activating electroless copper deposition. Circuit World 2010, 36, 4–8. [Google Scholar] [CrossRef]
- Duan, D.; Liu, H.; You, X.; Wei, H.; Liu, S. Anodic behavior of carbon supported Cu@Ag core-shell nanocatalysts in direct borohydride fuel cells. J. Power Sources 2015, 293, 292–300. [Google Scholar] [CrossRef]
- Li, C.; Yao, Y. Synthesis of bimetallic core-shell silver-copper nanoparticles decorated on reduced graphene oxide with enhanced electrocatalytic performance. Chem. Phys. Lett. 2020, 761, 137726. [Google Scholar] [CrossRef]
- Verma, A.; Gupta, R.K.; Shukla, M.; Malviya, M.; Sinha, I. Ag-Cu Bimetallic Nanoparticles as Efficient Oxygen Reduction Reaction Electrocatalysts in Alkaline Media. J. Nanosci. Nanotechnol. 2019, 20, 1765–1772. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Kaushik, R.; Kumar, R.; Jose, D.A.; Sharma, P.K.; Sharma, A. Facile synthesis of CucAgs based nanoparticles and nanocomposites as highly selective and sensitive colorimetric cyanide sensor. Mater. Chem. Phys. 2021, 260, 124132. [Google Scholar] [CrossRef]
- Mallick, S.; Sanpui, P.; Ghosh, S.S.; Chattopadhyay, A.; Paul, A. Synthesis, characterization and enhanced bactericidal action of a chitosan supported core-shell copper-silver nanoparticle composite. RSC Adv. 2015, 5, 12268–12276. [Google Scholar] [CrossRef]
- Grouchko, M.; Kamyshny, A.; Magdassi, S. Formation of air-stable copper-silver core-shell nanoparticles for inkjet printing. J. Mater. Chem. 2009, 19, 3057–3062. [Google Scholar] [CrossRef]
- Tseng, C.C.; Lin, Y.H.; Shu, Y.Y.; Chen, C.J.; Der Ger, M. Synthesis of vinyl acetate/Pd nanocomposites as activator ink for ink-jet printing technology and electroless copper plating. J. Taiwan Inst. Chem. Eng. 2011, 42, 989–995. [Google Scholar] [CrossRef]
- Kao, C.-Y.; Chou, K.-S. Electroless Copper Plating onto Printed Lines of Nanosized Silver Seeds. Electrochem. Solid-State Lett. 2007, 10, D32. [Google Scholar] [CrossRef]
- Choi, E.K.; Park, J.; Kim, B.S.; Lee, D. Fabrication of electrodes and near-field communication tags based on screen printing of silver seed patterns and copper electroless plating. Int. J. Precis. Eng. Manuf. 2015, 16, 2199–2204. [Google Scholar] [CrossRef]
- Mao, Y.; Zhu, M.; Wang, W.; Yu, D. Well-defined silver conductive pattern fabricated on polyester fabric by screen printing a dopamine surface modifier followed by electroless plating. Soft Matter. 2018, 14, 1260–1269. [Google Scholar] [CrossRef]
- Hidber, P.C.; Helbig, W.; Kim, E.; Whitesides, G.M. Microcontact Printing of Palladium Colloids: Micron-Scale Patterning by Electroless Deposition of Copper. Langmuir 1996, 12, 1375–1380. [Google Scholar] [CrossRef]
- Huang, S.C.; Tsao, T.C.; Chen, L.J. Selective electroless copper plating on poly(ethylene terephthalate) surfaces by microcontact printing. J. Electrochem. Soc. 2010, 157, 222–227. [Google Scholar] [CrossRef]
- Danilova, S.; Graves, J.E.; Sort, J.; Pellicer, E.; Cave, G.W.V.; Cobley, A. Electroless copper plating obtained by Selective Metallisation using a Magnetic Field (SMMF). Electrochim. Acta. 2021, 389, 138763. [Google Scholar] [CrossRef]
- Cruz, S.M.F.; Rocha, L.A.; Viana, J.C. Printing Technologies on Flexible Substrates for Printed Electronics. In Flexible Electronics; Rackauskas, S., Ed.; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Dai, X.; Xu, W.; Zhang, T.; Shi, H.; Wang, T. Room temperature sintering of Cu-Ag core-shell nanoparticles conductive inks for printed electronics. Chem. Eng. J. 2019, 364, 310–319. [Google Scholar] [CrossRef]
- Lee, C.; Kim, N.R.; Koo, J.; Lee, Y.J.; Lee, H.M. Cu-Ag core-shell nanoparticles with enhanced oxidation stability for printed electronics. Nanotechnology 2015, 26, 455601. [Google Scholar] [CrossRef] [PubMed]
- Pajor-Świerzy, A.; Farraj, Y.; Kamyshny, A.; Magdassi, S. Air stable copper-silver core-shell submicron particles Synthesis and conductive ink formulation. Colloids Surf. A Physicochem. Eng. Asp. 2017, 521, 272–280. [Google Scholar] [CrossRef]
- ASTM Standard D1388-96, 2002; Standard Test Method for Stiffness of Fabrics. ASTM International: West Conshohocken, PA, USA, 2003. [CrossRef]
- Jahan, I. Effect of Fabric Structure on the Mechanical Properties of Woven Fabrics. Adv. Res. Text. Eng. 2017, 2, 1018. [Google Scholar] [CrossRef]
- ISO 105-C06:2010; Textiles—Tests for Colour Fastness—Part C06: Colour Fastness to Domestic and Commercial Laundering. International Organization for Standardization: Geneva, Switzerland, 2010. Available online: https://www.iso.org/standard/51276.html (accessed on 20 January 2022).
- Byeon, J.H.; Kim, Y.-W. Aerosol copper initiated core–shell nanoparticle synthesis and micropatterning. New J. Chem. 2012, 36, 2184–2187. [Google Scholar] [CrossRef]
- Banik, M.; Patra, M.; Dutta, D.; Mukherjee, R.; Basu, T. A simple robust method of synthesis of copper–silver core–shell nano-particle: Evaluation of its structural and chemical properties with anticancer potency. Nanotechnology 2018, 29, 325102. [Google Scholar] [CrossRef]
- Sakthisabarimoorthi, A.; Jose, M.; Martin Britto Dhas, S.A.; Jerome Das, S. Fabrication of Cu@Ag core–shell nanoparticles for nonlinear optical applications. J. Mater. Sci. Mater. Electron. 2017, 28, 4545–4552. [Google Scholar] [CrossRef]
- Pellarin, M.; Issa, I.; Langlois, C.; Lebeault, M.A.; Ramade, J.; Lermé, J.; Broyer, M.; Cottancin, E. Plasmon Spectroscopy and Chemical Structure of Small Bimetallic Cu(1−x) Agx Clusters. J. Phys. Chem. C 2015, 119, 5002–5012. [Google Scholar] [CrossRef]
- Paszkiewicz, M.; GoBdbiewska, A.; Rajski, A.; Kowal, E.; Sajdak, A.; Zaleska-Medynska, A. Synthesis and Characterization of Monometallic (Ag, Cu) and Bimetallic Ag-Cu Particles for Antibacterial and Antifungal Applications. J. Nanomater. 2016, 2016, 2187940. [Google Scholar] [CrossRef]
- Singh, A.; Jha, S.; Srivastava, G.; Sarkar, P.; Gogoi, P. Silver Nanoparticles as Fluorescent Probes: New Approach for Bioimaging. Int. J. Sci. Technol. Res. 2013, 2, 153–157. [Google Scholar]
- Hajipour, A.; Nateri, A.S. The effect of weave structure on the quality of inkjet polyester printing. J. Text. Inst. 2019, 110, 799–806. [Google Scholar] [CrossRef]
- Karim, N.; Afroj, S.; Tan, S.; He, P.; Fernando, A.; Carr, C.; Novoselov, K.S. Scalable production of graphene-based wearable e-textiles. ACS Nano 2017, 11, 12266–12275. [Google Scholar] [CrossRef] [PubMed]
- Zaman, S.U.; Tao, X.; Cochrane, C.; Koncar, V. Market readiness of smart textile structures—reliability and washability. IOP Conf. Ser. Mater. Sci. Eng. 2018, 459, 012071. [Google Scholar] [CrossRef]
- Ojstršek, A.; Virant, N.; Fox, D.; Krishnan, L.; Cobley, A. The efficacy of polymer coatings for the protection of electroless copper plated polyester fabric. Polymers 2020, 12, 1277. [Google Scholar] [CrossRef]
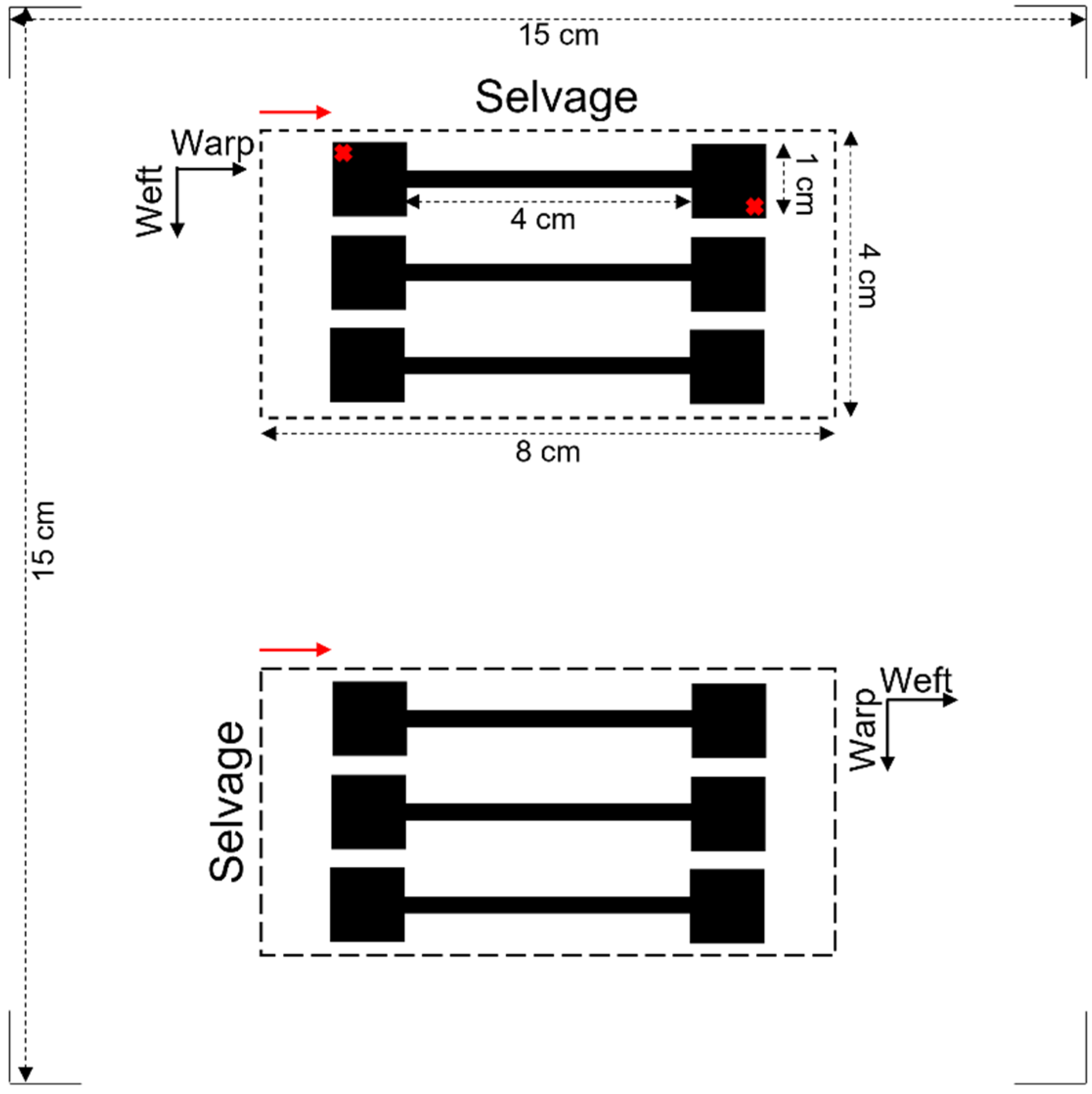

| No. of Printing Cycles/Fastness | Washing Cycles | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Warp Way Sample | Weft Way Sample | |||||||||
| 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 | |
| 2/Colour change | 4/5 | 4 | 3 | 4/5 | 3 | 3 | ||||
| 2/Colour staining | 4/5 | 4/5 | 4/5 | 4/5 | 4/5 | 4/5 | ||||
| 3/Colour change | 4/5 | 4/5 | 4 | 4/5 | 4 | 4 | 3/4 | |||
| 3/Colour staining | 4/5 | 4/5 | 4/5 | 4/5 | 4/5 | 4/5 | 4/5 | 4/5 | ||
| 4/Colour change | 4/5 | 4 | 4 | 3/4 | 4/5 | 4 | 4 | 3/4 | ||
| 4/Colour staining | 4/5 | 4/5 | 4/5 | 4/5 | 4/5 | 4/5 | 4/5 | 4/5 | ||
| 5/Colour change | 4/5 | 4 | 4 | 4 | 4 | 4/5 | 4 | 4 | 3/4 | 3/4 |
| 5/Colour staining | 4/5 | 4/5 | 4/5 | 4/5 | 4/5 | 4/5 | 4/5 | 4/5 | 4/5 | 4/5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azar, G.T.P.; Danilova, S.; Krishnan, L.; Fedutik, Y.; Cobley, A.J. Selective Electroless Copper Plating of Ink-Jet Printed Textiles Using a Copper-Silver Nanoparticle Catalyst. Polymers 2022, 14, 3467. https://doi.org/10.3390/polym14173467
Azar GTP, Danilova S, Krishnan L, Fedutik Y, Cobley AJ. Selective Electroless Copper Plating of Ink-Jet Printed Textiles Using a Copper-Silver Nanoparticle Catalyst. Polymers. 2022; 14(17):3467. https://doi.org/10.3390/polym14173467
Chicago/Turabian StyleAzar, Golnaz Taghavi Pourian, Sofya Danilova, Latha Krishnan, Yirij Fedutik, and Andrew J. Cobley. 2022. "Selective Electroless Copper Plating of Ink-Jet Printed Textiles Using a Copper-Silver Nanoparticle Catalyst" Polymers 14, no. 17: 3467. https://doi.org/10.3390/polym14173467
APA StyleAzar, G. T. P., Danilova, S., Krishnan, L., Fedutik, Y., & Cobley, A. J. (2022). Selective Electroless Copper Plating of Ink-Jet Printed Textiles Using a Copper-Silver Nanoparticle Catalyst. Polymers, 14(17), 3467. https://doi.org/10.3390/polym14173467







