Abstract
Dispersing graphene nanosheets in polymer-derived ceramics (PDCs) has become a promising route to produce exceptional mechanical and functional properties. To reveal the complex nanodomain structures of graphene–PDC composites, a novel reduced graphene oxide aerogel embedded silicon oxycarbide (RGOA-SiOC) nanocomposite was fabricated bottom-up using a 3D reduced graphene oxide aerogel as a skeleton followed by infiltration of a ceramic precursor and high-temperature pyrolysis. The reduced graphene oxide played a critical role in not only the form of the free carbon phase but also the distribution of SiOxC4−x structural units in SiOC. Long-ordered and continuous graphene layers were then embedded into the amorphous SiOC phase. The oxygen-rich SiOxC4−x units were more prone to forming than carbon-rich SiOxC4−x units in SiOC after the introduction of reduced graphene oxide, which we attributed to the bonding of Si atoms in SiOC with O atoms in reduced graphene oxide during the pyrolysis process.
1. Introduction
Graphene-polymer-derived ceramics (PDCs) nanocomposites are an emerging material system actively researched in advanced structural and functional fields. Proper integration between graphene and PDC is of high practical value, taking advantage of the beneficial properties of both. For instance, adding PDCs to graphene can improve its thermal stability, especially at high temperatures [1,2]. PDCs can be mechanically strengthened by graphene fillers and endowed with multiple functions, such as thermal, electrical, or shielding properties. A series of PDCs (e.g., SiOC [3,4,5,6,7,8,9,10], SiCN [2,11,12,13], SiBCN [1,14], SiOCN [15,16], and Si3N4 [17]) matrix composites containing graphene filler have been obtained so far. For example, graphene nanosheets serve as a reinforcement phase for improving the mechanical properties of SiOC [4,6] or SiC [18]. Graphene–SiOC nanocomposites are being explored as stable anodes for lithium-ion batteries, using the synergistic contribution of graphene for rapid electron transfer and SiOC for robust electrochemical Li+ ion storage [3,7,9,19]. The graphene–SiOC, –SiCN, –SiBCN, or –Si3N4 nanocomposites with good oxidation resistance have been recently applied as high-temperature electromagnetic wave-absorbing material under harsh environments [1,10,11,17]. Additionally, a graphene–SiOCN composite was used for thermal management applications through surface modification of graphene with electrical insulated SiOCN coating [15].
However, there are two main challenges in the field of graphene–PDC composites that have not yet exhibited substantial progress. First, controllable dispersion of the graphene nanosheets, especially with high loadings, in the PDCs is extremely difficult using conventional top-down physical mixing or chemical bonding methods due to the strong van der Waals force between the 2D graphene nanosheets. Thus, graphene tends to agglomerate in the PDC matrix, deteriorating the mechanical, electrical, or shielding properties of the composites. The second challenge is that the nanodomain structures of graphene–PDC material systems, which are critical in clarifying structure–property relationships and ultimately in determining their properties for specific engineering applications, are not yet fundamentally understood [20]. Most work has mainly focused on the increased free carbon phase of graphene–PDC systems, and little attention has been paid to the effect of graphene oxide on the structure of Si-based nanodomains within PDCs. For example, SiOC generally possesses a complex nanostructure in which corner-shared SiOxC4−x (0 ≤ x ≤ 4) tetrahedral structures are surrounded by a fraction of free carbon (Cfree) [21,22,23]. This unique network was extensively documented with various spectroscopic, scattering, and electron microscopic techniques [20], while few reports have focused on revealing the nanodomain structure of the graphene–SiOC hybridized nanocomposites. It is difficult to distinguish between the nanodomains of the graphene–PDC composite prepared by the top-down approaches due to the lack of a fine and homogeneous structure.
In this work, we put forward a universal and delicate bottom-up processing strategy to uniformly disperse graphene into SiOC ceramic by using a 3D reduced graphene oxide aerogel as a skeleton to infiltrate polysiloxane precursors. This strategy has great potential to enable both mechanical reinforcement and multifunctionalization, to which a more controllable design of the geometrical morphology and material constituents is fundamentally attributed. The nanodomain structures, especially Cfree and SiOxC1−x tetrahedral units of reduced graphene oxide embedded SiOC nanocomposites, were investigated using several characterization techniques, including HR-TEM, Raman, XPS, and NMR. The insights into the nanodomain structure of graphene–SiOC composites allow for a greater understanding of structure–property relationships and describe an efficient pathway for designing high-performance graphene–PDCs nanocomposites.
2. Materials and Methods
2.1. Preparation of 3D Porous Reduced Graphene Oxide Aerogel Skeleton
Three-dimensional reduced graphene oxide aerogel (RGOA) was synthesized using a hydrothermal method, followed by freeze-drying and thermal reduction. First, graphene oxide (GO) was prepared according to our previous work [24] and then ultrasonically dispersed in cold deionized water (5 mg GO/mL) for 2 h. An aqueous mixture of pyrrole (Py) monomer and GO suspension with a weight ratio of Py:GO = 5:1 was ultrasonically dispersed for 30 min and then transferred into a Teflon-lined autoclave at 150 °C for 6 h. The resulting product, a black reduced graphene oxide hydrogel (RGOH), was then rinsed with a water/ethanol solution with a volume ratio of 5:1 for 24 h to remove residual Py and polypyrrole (PPy). The reduced graphene oxide polypyrrole (RGO/PPy) aerogel was obtained by freeze-drying the RGOH for 24 h. RGO/PPy aerogel was further thermally reduced at 600 °C and 1000 °C for 1 h in a tube furnace under flowing argon at a heating rate of 3 °C/min to combust the organic content and form reduced graphene oxide aerogel (RGOA). The aerogels obtained after thermolysis at 600 and 1000 °C are denoted hereafter as RGOA-600 and RGOA-1000, respectively.
2.2. Preparation of Reduced Graphene Oxide Embedded Silicon Oxycarbide Nanocomposites
Commercial preceramic polymer precursor methylphenylvinylhydrogen polysiloxane (SILRES H62C, Wacker Chemie, Munich, Germany) was infiltrated into the as-prepared RGOA frameworks under vacuum to fabricate 3D graphene-polymer-derived ceramic architectures. We prepared 25 mg/mL polymeric solution by dissolving 100 mg polymeric precursor into 4 mL tert-butanol (TBA, (CH3)3OH); RGOA samples (RGOA-600 and RGOA-1000) were then immersed into this solution under vacuum for 2 h to facilitate precursor penetration into the aerogel and eliminate bubbles. Subsequently, the polymeric precursor-loaded RGOA (hereafter denoted as RGOA-600-P and RGOA-1000-P) samples were removed from the solution and freeze-dried for 12 h to remove the TBA. The obtained RGOA-P cylinders were further cross-linked at 250 °C for 2 h and pyrolyzed at 1000 °C for 3 h in a tube furnace under flowing argon at a heating rate of 100 °C/h to obtain the final 3D RGOA-SiOC materials. The obtained ceramic nanocomposite materials after pyrolysis of RGOA-600-P and RGOA-1000-P are denoted hereafter as RGOA-600/SiOC and RGOA-1000/SiOC, respectively. The final weight ratio (initial weight of RGOA/ weight of RGOA-SiOC nanocomposite) of RGOA in RGOA-600/SiOC and RGOA-1000/SiOC was 22.3 and 20.5 wt%, respectively. For comparison, unmodified SiOC material was prepared by the pyrolysis of preceramic polymer H62C at the same heat-treatment conditions.
2.3. Characterization
The morphology and microstructure of RGOA and RGOA-SiOC composites were investigated with scanning electron microscopy (SEM) using a Leo Gemini 1530 and transmission electron microscopy (TEM) using a JEOL 2100F. X-ray photoelectron spectroscopy (XPS) was performed on a Thermo Fisher Scientific ESCALAB 250Xi to investigate the surface chemical states of different elements in SiOC and reduced graphene oxide modified SiOC nanocomposites. Raman spectra of RGOA and RGOA-SiOC composites were measured using a micro-Raman spectrometer (RENISHAW inVia) with an excitation wavelength of 532 nm. Solid-state -29Si DD/MAS NMR spectra were recorded on an Agilent 600 DD2 spectrometer (Agilent, Santa Clara, CA, USA, magnetic field strength 14.1 T) at a resonance frequency of 199.13 MHz for 29 29Si using dipole decoupling magic angle spinning (DD/MAS) and high-power 1H decoupling. The powder samples were placed in a pencil-type zirconia rotor with an outer diameter of 4.0 mm. The spectra were obtained at a spinning speed of 8 kHz (4 μs 90° pulses) and a recycle delay of 10 s. The Si signal of tetramethylsilane (TMS) at 0 ppm was used as the reference 29Si chemical shift. The scanning number was 5000. Solid-state 13C CP/MAS NMR spectra were recorded on an Agilent 600 DD2 spectrometer (Agilent, Santa Clara, CA, USA, magnetic field strength 14.1 T) at a resonance frequency of 150.72 MHz for 13C using cross-polarization (CP), magic-angle spinning (MAS), and high-power 1H decoupling. The powder samples were placed in a pencil-type zirconia rotor with an outer diameter of 4.0 mm. The spectra were obtained at a spinning speed of 10 kHz (4.2 μs 90° pulses), a 2 ms CP pulse, and a recycle delay of 3 s. The C signal of tetramethylsilane (TMS) at 0 ppm was used to reference the [13] C chemical shift.
3. Results and Discussion
Three-dimensional reduced graphene oxide aerogels (RGOAs) were prepared from GO and PPy via a typical hydrothermal process at 150 °C for 6 h, followed by freeze-drying for 24 h and thermal reduction at 600–1000 °C for 1 h, as illustrated in Figure 1. During the hydrothermal process, the PPy preferentially grows on the surface of GO sheets due to electrostatic interactions between positively charged PPy and negatively charged GO surfaces and π–π interactions between PPy rings and conjugated segments in GO and hydrogen-bonding interactions, resulting in the reduction of GO and the formation of RGO-PPy aerogel [19,24]. Taking advantage of in situ crosslinking of the PPy, GO nanosheets can be aligned along the flow direction during the hydrothermal process (Figure 2a) [25]. During further thermal reduction of RGO-PPy aerogels at 600 and 1000 °C in Ar, PPy decomposes, yielding RGOA with different degrees of reduction. As shown in Figure 2b, the aerogel retained the aligned macropore structure after thermal reduction, which provides sufficient space for the formation of the preceramic precursor and promotes its infiltration into the RGOA skeleton. RGOA/SiOC nanocomposites were then fabricated by precursor infiltration, freeze-drying, and high-temperature pyrolysis (Figure 1). During infiltration, the H62C precursor attaches to RGOA surfaces due to its favorable wetting and adhesion ability and π–π interactions between RGOA and phenyl groups in the H62C precursor. Tert-butanol (TBA) was chosen as the solvent in the infiltration step instead of other organic solvents (e.g., ethanol or tetrahydrofuran) to overcome the problem of surface tension at the gas–liquid–pore wall during the subsequent freeze-drying process. As a result, the 3D graphene skeleton maintained its morphology without shrinkage, collapse, or agglomeration during infiltration and drying. As shown in Figure 2c,d, the obtained RGOA/SiOC nanocomposite after further pyrolysis at 1000 °C was composed of layered graphene-embedded SiOC ceramic, which was more suitable for the further investigation of nanodomains within graphene-SiOC composite than SiOC-based particles with randomly distributed graphene.
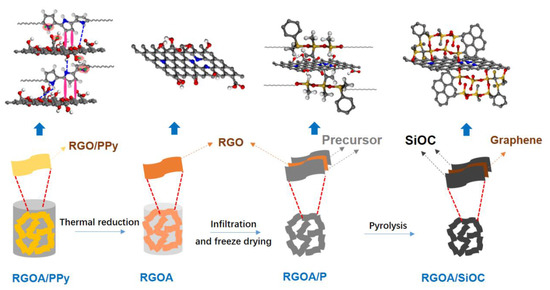
Figure 1.
Schematic of the bottom-up fabrication process of RGOA/SiOC nanocomposites and the evolution of chemical structures and interactions. The hydrothermally synthesized RGOA/PPy aerogels were first reduced at 600–1000 °C in Ar, then infiltrated by a polymeric solution to obtain the RGOA/P precursors, which were subsequently freeze-dried and pyrolyzed at 1000 °C to obtain the RGOA/SiOC nanocomposites.
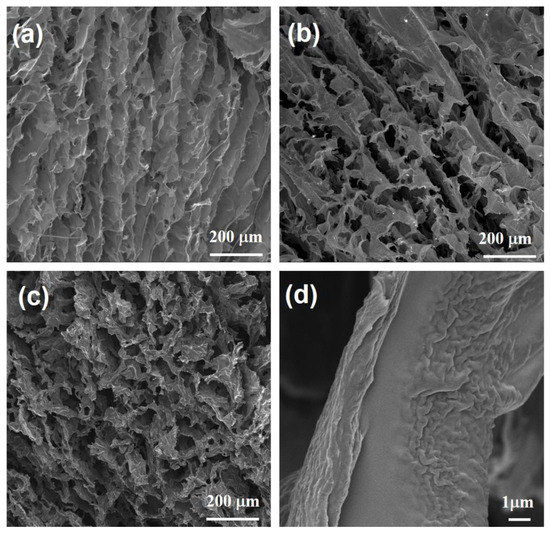
Figure 2.
Low-magnification SEM images of (a) RGOA, (b) RGOA-1000, and (c) RGOA-1000/SiOC samples. (d) High-magnification SEM image of RGOA-1000/SiOC sample.
The TEM images of SiOC pyrolyzed at 1000 °C (Figure 3a,b) show that SiOC ceramic is amorphous and homogenous. The size of the free carbon phase in SiOC pyrolyzed at 1000 °C was rather small, and the so-called basic structural units (BSUs) of the free carbon with few lamellar carbon layers could be detected even at higher magnification (Figure 3b). In contrast, after introducing SiOC into RGO aerogel, a long-ordered carbon phase was embedded in the amorphous SiOC phase (Figure 3d,e), a structure that has not been observed in other similar graphene-modified polymer-derived ceramics [12,13,16].
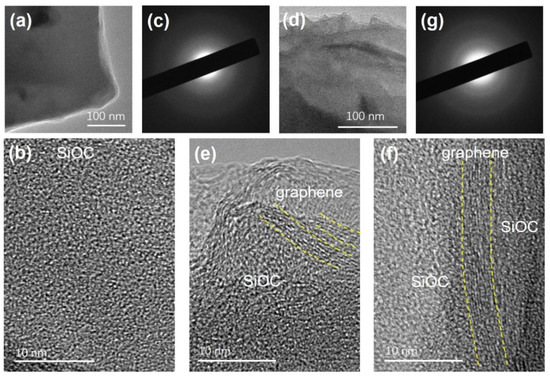
Figure 3.
(a) TEM, (b) HRTEM, and (c) SAED images of SiOC sample. (d) TEM, (e,f) HRTEM, and (g) SAED images of RGOA-1000/SiOC nanocomposite.
Micro-Raman spectroscopy was used to analyze the structure of the carbon phase within reduced graphene oxide aerogels, SiOC, and reduced graphene oxide embedded SiOC samples. Figure 4a shows the Raman spectra of the as-prepared RGOAs with different reduction temperatures. The D and G bands observed at 1345 and 1585 cm−1 could be attributed to disordered sp2-hybridized modes in the carbon rings and in-plane bond stretching of sp2 carbon, respectively. In addition to the typical D and G bands (at 1320 and 1600 cm−1) in the pure SiOC sample, the 2D, D + G, and 2D’ bands were observed in the second-order Raman spectra at around 2610, 2900, and 3195 cm−1, respectively, which could be assigned to the overtones and combinations of different Raman vibration modes in PDC materials [23]. Because the intensity ratio of the D and G modes provides valuable information about the structural arrangement of the free carbon phase present in the SiOC network, the Raman spectra were fitted using the Lorentzian curve fitting for the D1, D4, and G bands and Gaussian curve fitting for the D3 band [26,27,28]. As shown in Figure 4c–g and Table 1, the D4 band was observed as a shoulder of the D1 band at ca. 1190 cm−1 in the spectra of all SiOC, RGOA-600, RGOA-1000, and RGOA-SiOC nanocomposites, which could be attributed to the disordered graphitic lattice (C−C and C=C stretching vibrations and sp2-sp3 bonds) in soot and related carbon materials. The D3 band at 1500–1510 cm−1 could be assigned to the amorphous carbon fractions [20,23].
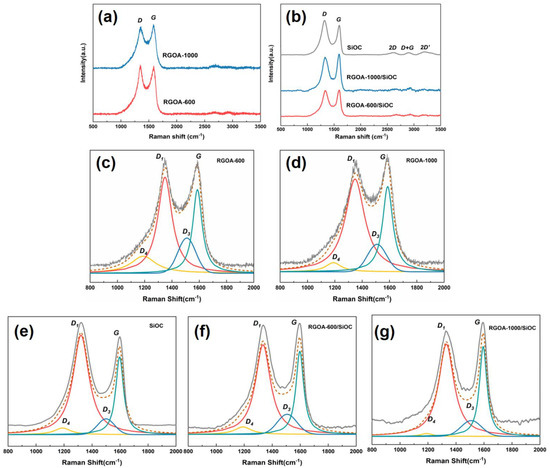
Figure 4.
Raman spectra of (a) RGOA-600 and RGOA-1000 and (b) RGOA modified SiOC nanocomposites and SiOC. (c–g) Deconvoluted spectra of RGOA-600, RGOA-1000, SiOC, RGOA-600/SiOC, and RGOA-1000/SiOC, respectively. The experimental spectra are shown by the solid grey line on top, followed by the simulated spectra (dashed brown lines), and the individual simulation components (solid colored lines).

Table 1.
Band positions, intensity ratios I(D1)/I(G) obtained from curve fitting of the Raman spectra, and lateral crystallite sizes of free carbon of the SiOC, RGOA-600 and -1000, and RGOA-600 and -1000/SiOC samples.
The intensity ratio (ID1/IG) of RGOA, which indicates the reduction degree of graphene oxide, decreased from 1.14 to 1.08 with increasing reduction temperature. This result revealed the removal of defects and recovery of conjugated domains in the reduced graphene oxide, especially at higher thermal reduction temperatures. The extracted ID1/IG intensity ratio can also be used to determine the lateral crystallite size of free carbon (the length of the carbon domain along the sixfold ring plane, donated as La). Until now, two correlations between ID1/IG and La have been proposed, as shown in Equations (1) and (2) [23].
where C(λ) and C’(λ) are wavelength-dependent prefactors, C(λ) = C0 + λC1 (C0 = −12.6 nm and C1 = 0.033), C’(λ) ≈ 0.55 nm−2. λ is the wavelength of the laser; and ID1 and IG are the intensities of the D1 and G band, respectively.
ID1/IG = C(λ)/La1
ID1/IG = C’(λ)La22
Generally, the Tuinstra and Koenig (TK) correlation (Equation (1)) is valid for carbon clusters with La values higher than 2 nm, while the Ferrari–Robertson equation (Equation (2)) is valid for La values lower than 2 nm [23]. In the case of SiOC, the La2 value is about 1.48 nm, which is theoretically acceptable. However, the TK correlation is more suitable for evaluating the La value of graphene–SiOC nanocomposites, which was 4.63 and 4.81 nm for the RGOA-600/SiOC and RGOA-1000/SiOC samples, respectively.
The elemental composition and chemical environment of C and Si on the surface of the samples were examined by XPS to gain insight into not only the reduction progress of graphene oxide [29], but also the evaluation of SiOxC4−x units after the introduction of graphene [30]. As shown in Figure 5a, the intensity of the C 1s peak, located at 284.6 eV, continually increased from GO to RGOA-600 and RGOA-1000, while the change in oxygen content (as indicated by the O 1s peak at ca. 532.4 eV) showed the opposite trend. GO exhibited a C/O atomic ratio of 1.9, which increased to ~9.94 and 25.81 after further thermal reduction at 600 and 1000 °C, respectively, confirming the reduction of graphene oxide sheets. As summarized in Table 2, the highest C/O atomic ratio determined for the RGOA-1000 sample indicated that the reduction eliminated most of the oxygen-containing groups. The surface elemental composition calculated from XPS was found to be 18.6, 34.6, and 46.8 at % for Si, O, and C in SiOC, respectively; the RGOA-600/SiOC and RGOA-1000/SiOC nanocomposites showed lower silicon amounts (i.e., 10.7 and 8.6 at %, respectively), lower oxygen (25.0 and 20.6 at %, respectively), and higher carbon contents (62.5 and 69.0 at %, respectively), with small amounts of nitrogen (1.8 and 1.8 at %, respectively). These results further confirmed that SiOC was successfully integrated into the reduced graphene oxide aerogel.
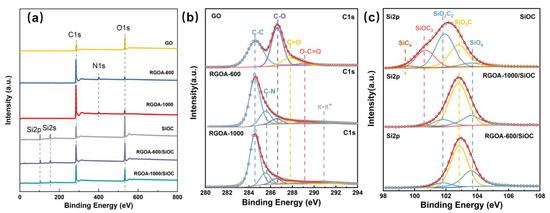
Figure 5.
(a) Survey X-ray photoelectronic spectra of GO, RGOA, and RGOA-SiOC nanocomposites and SiOC. High-resolution (b) C1s spectra of GO, RGOA-600, and RGOA-1000 and (c) Si2p spectra of SiOC, RGOA-1000/SiOC, and RGOA-600/SiOC nanocomposites.

Table 2.
Surface elemental composition from the survey X-ray photoelectronic spectra of GO, RGOA, SiOC, and RGOA-SiOC nanocomposites.
Figure 5b shows the high-resolution C 1s spectrum of GO, which could be deconvoluted into four peaks assigned to C−C (284.6 eV), C−O (286.6 eV), C=O (287.8 eV), and O-C=O (289.1 eV) [29] bonds and groups. The relative intensity of C−O, C=O, and O-C=O peaks in RGOA decreased, and two peaks corresponding to C−N bond (285.6 eV) and π−π stacking (290.8 eV) appeared after the thermal removal of oxygenated functional groups. The Si 2p peaks in SiOC and RGOA-SiOC nanocomposites (Figure 5c) could be deconvoluted into five peaks assigned to SiO4 (103.6 eV), SiO3C (102.8 eV), SiO2C2 (101.8 eV), SiOC3 (100.8 eV), and SiC4 (99.5eV) groups [30]. It is worth noting that the relative amount of SiC4, SiOC3, and SiO2C2 units decreased and the content of oxygen-rich SiOxC4−x units (SiO3C and SiO4) increased after the incorporation of RGOA. Especially in the case of lower reduction degrees of RGOA-600, SiC4 and SiOC3 units almost disappeared, and the contribution of SiO2C2 decreased, whereas the proportion of SiO3C and SiO4 increased. These results suggested that RGOA can tailor the structure of SiOxC4-x units in SiOC ceramic by converting the carbon-rich SiOxC4-x units into oxygen-rich SiOxC4−x units (Figure 5c).
29Si MAS-NMR spectroscopy was further used to probe the structure of SiOxC4−x tetrahedra present in the amorphous network of SiOC and RGOA-SiOC nanocomposites. Four different environments were evident in the SiOC sample obtained by pyrolysis at 1000 °C (Figure 6a), which could be simulated by Gaussian curve fitting and assigned to SiO4 (−110 ppm), SiO3C (−74 ppm), SiO2C2 (−37 ppm), and SiC4 (−12 ppm) structural units [31,32], as summarized in Table 3. These results are consistent with those of previous NMR studies on PDCs [33]. However, to the best of our knowledge, no NMR studies of graphene-modified SiOC have been conducted. Intriguingly, the RGOA-1000/SiOC obtained by pyrolysis at the same temperature (i.e., 1000 °C) exhibited the characteristic signals of SiOC3 (7 ppm) along with SiO4 (−107 ppm), SiO3C (−75 ppm), SiO2C2 (−42 ppm), and SiC4 (−13 ppm) units, as shown in Figure 6b. The fraction of the SiC4 unit decreased, while those of the of SiO2C2 and SiO3C units increased (Table 3), indicating the partial conversion of the SiC4 unit into oxygen-containing SiOxC4−x (SiOC3, SiO2C2, and SiO3C) units due to the interaction between RGOA and SiOC. These interactions caused Si atoms to bond with O atoms from remaining oxygen groups in reduced graphene oxide during the pyrolysis process. The 29Si MAS-NMR spectrum of RGOA-600/SiOC nanocomposite (Figure 6c) obtained at lower pyrolysis temperature (i.e., 600 °C) showed the characteristic signals of SiO4 (−110 ppm), SiO3C (−74 ppm), SiO2C2 (−41 ppm), and SiC4 (−10 ppm) units. The relative concentration of SiC4 unit further decreased, and that of SiO4 unit increased. These results further confirmed the interaction between Si atoms of SiOC and O atoms of RGOA and that graphene oxide plays a critical role in the distribution of SiOxC4−x structural units in SiOC. The oxygen-rich SiOxC4−x units are more prone to forming than carbon-rich SiOxC4−x units in SiOC via the modification of graphene oxide, and the lower reduction degree of graphene oxide results in a higher concentration of oxygen-rich SiCxO4−x units.
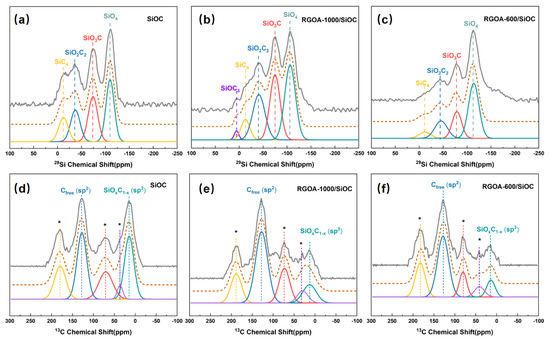
Figure 6.
The 29Si and 13C MAS NMR spectra of (a,d) SiOC, (b,e) RGOA-1000/SiOC, and (c,f) RGOA-600/SiOC. The experimental spectra are shown by the solid grey line on top, followed by the simulated spectra (dashed brown lines), and the individual simulation components (solid colored lines).

Table 3.
Si-containing structural units in SiOC and RGOA-modified SiOC nanocomposites according to the simulation of 29Si MAS NMR spectra.
Figure 6d–f show the experimental and simulated 13C MAS NMR spectra of SiOC and RGOA-SiOC nanocomposites. In the case of SiOC sample (Figure 6d), two apparent peaks centered at 14 and 127 ppm could be assigned to sp3-hybridized carbon within SiOxC4-x units and sp2-hybridized carbon (Cfree), respectively [34,35]. These results suggested the presence of two types of carbon in SiOC ceramic: carbon bonded to Si in SiOxC4-x units and carbon bonded to other carbon atoms forming nanodomains of turbostratic carbon, consistent with the findings in a previous work [31]. The other three peaks located at 36, 70, and 179 ppm were spinning sidebands [31]. After the integration of SiOC into RGOA, the peak corresponded to Cfree, which was present as both turbostratic carbon and graphene sheets and was dominant in RGOA-1000/SiOC and RGOA-600/SiOC nanocomposites (Figure 6e,f), further confirming that sp2-hybridized reduced graphene oxide as Cfree phase was embedded in SiOC.
Based on the above characterizations, a schematic representation of the possible nanodomain structures of the as-prepared graphene-embedded SiOC nanocomposites is shown in Figure 7. The lower thermal treatment temperature resulted in a lower reduction degree of reduced graphene oxide aerogel, which maintained a higher concentration of oxygen groups (Figure 7a,b), promoting the formation of oxygen-rich SiOxC4−x units and improved sp2-hybridized Cfree phase with graphene-SiOC nanocomposite (Figure 7c–e).
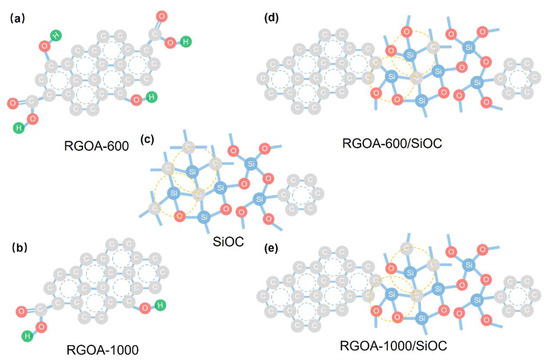
Figure 7.
Schematic illustration of nanodomain structures of SiOC and reduced graphene oxide embedded SiOC samples. (a) RGOA-600, (b) 1000/SiOC, (c) SiOC, (d) RGOA-600/SiOC and (e) RGOA-1000/SiOC.
This work revealed the nanodomain structure of graphene-SiOC composites, allowing for an understanding of the structure–property relationships and providing valuable insights for the creation of high-performance graphene–PDCs nanocomposites in the areas of batteries [36,37], supercapacitors [38], catalysis [39,40], and microwave absorption and shielding [41].
4. Conclusions
In summary, reduced graphene oxide aerogels were prepared by pyrrole-mediated hydrothermal synthesis, followed by freeze-drying and thermal reduction. A higher thermal treatment temperature (1000 °C) resulted in a higher reduction degree of graphene oxide aerogel with fewer remaining oxygen groups. A novel reduced graphene oxide aerogel containing SiOC ceramic was prepared by the infiltration of a preceramic polymer and a subsequent high-temperature pyrolysis process. The as-prepared RGOA-SiOC presented a highly porous structure with a uniform distribution of graphene into SiOC. After the introduction of RGOA, the carbon-rich SiOxC4−x units within SiOC were prone to transforming into oxygen-rich SiOxC4-x units, both confirmed by Si2p XPS and 29Si NMR spectra, which occurred due to the interaction between the Si atoms in SiOC and the O atoms in RGOA during the pyrolysis process. The RGOA-600/SiOC nanocomposites contained a higher concentration of oxygen-rich SiOxC4−x units due to the lower reduction degree of RGOA-600 with a higher concentration of oxygen groups. In addition to the tailorable SiOxC4−x tetrahedral units, the free carbon phase was regulated. Long-ordered, sp2-hybridized graphene sheets embedded into the amorphous SiOC phase have been documented by HR-TEM, Raman, and 13C NMR techniques. Revealing the nanodomain structures of graphene–SiOC nanocomposites may offer a path to investigate structure-property relationships and tailor material properties for practical applications.
Author Contributions
Data curation, D.H. and G.S.; methodology and investigation, G.S., J.W. and D.H; resources, A.G.; conceptualization, M.F.B., G.S. and A.G.; writing—original draft, D.H. and G.S.; writing—review and editing, M.F.B., A.G. and J.W.; supervision, M.F.B. and A.G.; project administration, M.F.B. All authors have read and agreed to the published version of the manuscript.
Funding
The authors are grateful to the National Natural Science Foundation of China (52102361), Natural Science Foundation of Jiangsu Province (BK20200827), and the Startup Foundation for Introducing Talent of NUIST (2020r025).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank Shiyanjia Lab (www.shiyanjia.com, accessed on 8 April 2021) for the NMR and TEM measurements. We acknowledge support from the German Research Foundation and the Open Access Publication Fund of TU Berlin.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Luo, C.; Jiao, T.; Gu, J.; Tang, Y.; Kong, J. Graphene Shield by SiBCN Ceramic: A Promising High-Temperature Electromagnetic Wave-Absorbing Material with Oxidation Resistance. ACS Appl. Mater. Interfaces 2018, 10, 39307–39318. [Google Scholar] [CrossRef] [PubMed]
- Román-Manso, B.; Moyano, J.J.; Pérez-Coll, D.; Belmonte, M.; Miranzo, P.; Osendi, M.I. Polymer-derived ceramic/graphene oxide architected composite with high electrical conductivity and enhanced thermal resistance. J. Eur. Ceram. Soc. 2018, 38, 2265–2271. [Google Scholar] [CrossRef]
- David, L.; Bhandavat, R.; Barrera, U.; Singh, G. Silicon oxycarbide glass-graphene composite paper electrode for long-cycle lithium-ion batteries. Nat. Commun. 2016, 7, 10998. [Google Scholar] [CrossRef]
- Picot, O.T.; Rocha, V.G.; Ferraro, C.; Ni, N.; D’Elia, E.; Meille, S.; Chevalier, J.; Saunders, T.; Peijs, T.; Reece, M.J.; et al. Using graphene networks to build bioinspired self-monitoring ceramics. Nat. Commun. 2017, 8, 14425. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Barrios, E.; Zhai, L. Bulk Polymer-Derived Ceramic Composites of Graphene Oxide. ACS Omega 2018, 3, 4006–4016. [Google Scholar] [CrossRef]
- Yu, M.; Picot, O.T.; Saunders, T.G.; Dlouhý, I.; Feng, J.; Titirici, M.-M.; Mahajan, A.; Reece, M.J. Graphene-reinforced silicon oxycarbide composites prepared by phase transfer. Carbon 2018, 139, 813–823. [Google Scholar] [CrossRef]
- Sang, Z.; Yan, X.; Wen, L.; Su, D.; Zhao, Z.; Liu, Y.; Ji, H.; Liang, J.; Dou, S.X. A graphene-modified flexible SiOC ceramic cloth for high-performance lithium storage. Energy Storage Mater. 2020, 25, 876–884. [Google Scholar] [CrossRef]
- Sujith, R.; Chauhan, P.K.; Gangadhar, J.; Maheshwari, A. Graphene nanoplatelets as nanofillers in mesoporous silicon oxycarbide polymer derived ceramics. Sci. Rep. 2018, 8, 17633. [Google Scholar] [CrossRef] [PubMed]
- Sang, Z.; Zhao, Z.; Su, D.; Miao, P.; Zhang, F.; Ji, H.; Yan, X. SiOC nanolayer wrapped 3D interconnected graphene sponge as a high-performance anode for lithium ion batteries. J. Mater. Chem. A 2018, 6, 9064–9073. [Google Scholar] [CrossRef]
- Han, M.; Yin, X.; Duan, W.; Ren, S.; Zhang, L.; Cheng, L. Hierarchical graphene/SiC nanowire networks in polymer-derived ceramics with enhanced electromagnetic wave absorbing capability. J. Eur. Ceram. Soc. 2016, 36, 2695–2703. [Google Scholar] [CrossRef]
- Liu, X.; Yu, Z.; Ishikawa, R.; Chen, L.; Liu, X.; Yin, X.; Ikuhara, Y.; Riedel, R. Single-source-precursor derived RGO/CNTs-SiCN ceramic nanocomposite with ultra-high electromagnetic shielding effectiveness. Acta Mater. 2017, 130, 83–93. [Google Scholar] [CrossRef]
- Shen, C.; Calderon, J.E.; Barrios, E.; Soliman, M.; Khater, A.; Jeyaranjan, A.; Tetard, L.; Gordon, A.; Seal, S.; Zhai, L. Anisotropic electrical conductivity in polymer derived ceramics induced by graphene aerogels. J. Mater. Chem. C 2017, 5, 11708–11716. [Google Scholar] [CrossRef]
- Yu, Y.; Xia, F.; Huang, Q.; Fang, J.; An, L. Electrical conductivity of silicon carbonitride-reduced graphene oxide composites. J. Am. Ceram. Soc. 2017, 100, 5113–5119. [Google Scholar] [CrossRef]
- Wei, Y.; Yongsheng, L.; Mingxi, Z.; Fang, Y.; Laifei, C. Effect of heat treatment temperature on microstructure and electromagnetic shielding properties of graphene/SiBCN composites. J. Mater. Sci. Technol. 2019, 35, 2897–2905. [Google Scholar] [CrossRef]
- Oh, H.; Kim, Y.; Wie, J.; Kim, K.; Kim, J. Tailoring of Si–C–N–O ceramic-coated reduced graphene oxide by oil/water-solution process for high thermal conductive epoxy composite with electrical insulation. Compos. Sci. Technol. 2020, 197, 108257. [Google Scholar] [CrossRef]
- Yu, Y.; Huang, Q.; Rhodes, S.; Fang, J.; An, L. SiCNO–GO composites with the negative temperature coefficient of resistance for high-temperature sensor applications. J. Am. Ceram. Soc. 2017, 100, 592–601. [Google Scholar] [CrossRef]
- Wang, X.; Mera, G.; Morita, K.; Ionescu, E. Synthesis of polymer-derived graphene/silicon nitride-based nanocomposites with tunable dielectric properties. J. Ceram. Soc. Jpn. 2016, 124, 981–988. [Google Scholar] [CrossRef]
- Cheng, Y.; Hu, P.; Zhou, S.; Zhang, X.; Han, W. Using macroporous graphene networks to toughen ZrC–SiC ceramic. J. Eur. Ceram. Soc. 2018, 38, 3752–3758. [Google Scholar] [CrossRef]
- Shao, G.; Hanaor, D.A.H.; Wang, J.; Kober, D.; Li, S.; Wang, X.; Shen, X.; Bekheet, M.F.; Gurlo, A. Polymer-Derived SiOC Integrated with a Graphene Aerogel As a Highly Stable Li-Ion Battery Anode. ACS Appl. Mater. Interfaces 2020, 12, 46045–46056. [Google Scholar] [CrossRef]
- Mera, G.; Navrotsky, A.; Sen, S.; Kleebe, H.-J.; Riedel, R. Polymer-derived SiCN and SiOC ceramics—Structure and energetics at the nanoscale. J. Mater. Chem. A 2013, 1, 3826–3836. [Google Scholar] [CrossRef]
- Colombo, P.; Mera, G.; Riedel, R.; Sorarù, G.D. Polymer-Derived Ceramics: 40 Years of Research and Innovation in Advanced Ceramics. J. Am. Ceram. Soc. 2010, 93, 1805–1837. [Google Scholar] [CrossRef]
- Stabler, C.; Ionescu, E.; Graczyk-Zajac, M.; Gonzalo-Juan, I.; Riedel, R. Silicon oxycarbide glasses and glass-ceramics: “All-Rounder” materials for advanced structural and functional applications. J. Am. Ceram. Soc. 2018, 101, 4817–4856. [Google Scholar] [CrossRef]
- Wen, Q.; Yu, Z.; Riedel, R. The fate and role of in situ formed carbon in polymer-derived ceramics. Prog. Mater. Sci. 2020, 109, 100623. [Google Scholar] [CrossRef]
- Shao, G.; Ovsianytskyi, O.; Bekheet, M.F.; Gurlo, A. On-chip assembly of 3D graphene-based aerogels for chemiresistive gas sensing. Chem. Commun. 2020, 56, 450–453. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.-Y.; Yu, Z.-L.; Xu, Z.-L.; Bu, L.-F.; Liu, H.-R.; Zhu, Y.-B.; Qin, B.; Ma, T.; Zhan, H.-J.; Xu, L.; et al. Origin of Batch Hydrothermal Fluid Behavior and Its Influence on Nanomaterial Synthesis. Matter 2020, 2, 1270–1282. [Google Scholar] [CrossRef]
- Sadezky, A.; Muckenhuber, H.; Grothe, H.; Niessner, R.; Pöschl, U. Raman microspectroscopy of soot and related carbonaceous materials: Spectral analysis and structural information. Carbon 2005, 43, 1731–1742. [Google Scholar] [CrossRef]
- Wilamowska-Zawlocka, M.; Puczkarski, P.; Grabowska, Z.; Kaspar, J.; Graczyk-Zajac, M.; Riedel, R.; Sorarù, G.D. Silicon oxycarbide ceramics as anodes for lithium ion batteries: Influence of carbon content on lithium storage capacity. RSC Adv. 2016, 6, 104597–104607. [Google Scholar] [CrossRef]
- Chandra, C.; Cahyadi, H.S.; Alvin, S.; Devina, W.; Park, J.-H.; Chang, W.; Chung, K.Y.; Kwak, S.K.; Kim, J. Revealing the Sodium Storage Mechanism in High-Temperature-Synthesized Silicon Oxycarbides. Chem. Mater. 2020, 32, 410–423. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Roualdes, S.; Berjoan, R.; Durand, J. 29Si NMR and Si2p XPS correlation in polysiloxane membranes prepared by plasma enhanced chemical vapor deposition. Sep. Purif. Technol. 2001, 25, 391–397. [Google Scholar] [CrossRef]
- Widgeon, S.J.; Sen, S.; Mera, G.; Ionescu, E.; Riedel, R.; Navrotsky, A. 29Si and 13C Solid-State NMR Spectroscopic Study of Nanometer-Scale Structure and Mass Fractal Characteristics of Amorphous Polymer Derived Silicon Oxycarbide Ceramics. Chem. Mater. 2010, 22, 6221–6228. [Google Scholar] [CrossRef]
- Nimmo, J.P.; Kroll, P. First-Principles Calculations and Analysis of 29Si Nuclear Magnetic Resonance Chemical Shifts in Silicon Oxycarbide Ceramics. J. Phys. Chem. C 2014, 118, 29952–29961. [Google Scholar] [CrossRef]
- Tavakoli, A.H.; Armentrout, M.M.; Narisawa, M.; Sen, S.; Navrotsky, A. White Si–O–C Ceramic: Structure and Thermodynamic Stability. J. Am. Ceram. Soc. 2015, 98, 242–246. [Google Scholar] [CrossRef]
- Dubey, R.J.C.; Sasikumar, P.V.W.; Cerboni, N.; Aebli, M.; Krumeich, F.; Blugan, G.; Kravchyk, K.V.; Graule, T.; Kovalenko, M.V. Silicon oxycarbide-antimony nanocomposites for high-performance Li-ion battery anodes. Nanoscale 2020, 12, 13540–13547. [Google Scholar] [CrossRef]
- Sen, S.; Widgeon, S.J.; Navrotsky, A.; Mera, G.; Tavakoli, A.; Ionescu, E.; Riedel, R. Carbon substitution for oxygen in silicates in planetary interiors. Proc. Natl. Acad. Sci. USA 2013, 110, 15904–15907. [Google Scholar] [CrossRef]
- Wang, M.; Xia, Y.; Wang, X.; Xiao, Y.; Liu, R.; Wu, Q.; Qiu, B.; Metwalli, E.; Xia, S.; Yao, Y.; et al. Silicon Oxycarbide/Carbon Nanohybrids with Tiny Silicon Oxycarbide Particles Embedded in Free Carbon Matrix Based on Photoactive Dental Methacrylates. ACS Appl. Mater. Interfaces 2016, 8, 13982–13992. [Google Scholar] [CrossRef]
- Wang, J.; Kober, D.; Shao, G.; Epping, J.D.; Görke, O.; Li, S.; Gurlo, A.; Bekheet, M.F. Stable anodes for lithium-ion batteries based on tin-containing silicon oxycarbonitride ceramic nanocomposites. Mater. Today Energy 2022, 26, 100989. [Google Scholar] [CrossRef]
- Wen, Q.; Qu, F.; Yu, Z.; Graczyk-Zajac, M.; Xiong, X.; Riedel, R. Si-based polymer-derived ceramics for energy conversion and storage. J. Adv. Ceram. 2022, 11, 197–246. [Google Scholar] [CrossRef]
- Wang, J.; Gili, A.; Grünbacher, M.; Praetz, S.; Epping, J.D.; Görke, O.; Schuck, G.; Penner, S.; Schlesiger, C.; Schomäcker, R.; et al. Silicon oxycarbonitride ceramic containing nickel nanoparticles: From design to catalytic application. Mater. Adv. 2021, 2, 1715–1730. [Google Scholar] [CrossRef]
- Wang, J.; Grünbacher, M.; Penner, S.; Bekheet, M.F.; Gurlo, A. Porous silicon oxycarbonitride ceramics with palladium nanoparticles for dry reforming of methane. Polymers 2022, 14, 3470. [Google Scholar] [CrossRef]
- Shao, G.; Shen, X.; Huang, X. Multilevel Structural Design and Heterointerface Engineering of a Host–Guest Binary Aerogel toward Multifunctional Broadband Microwave Absorption. ACS Mater. Lett. 2022, 4, 1787–1797. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).