Impact of Graphene Oxide on Properties and Structure of Thin-Film Composite Forward Osmosis Membranes
Abstract
:1. Introduction
2. Experimental Methods
2.1. Material
2.2. Preparation of PSF/GO Support Layer
2.3. Preparation of TFC-FO Membranes
2.3.1. TFC-FOPSF/GO Membrane Preparation
2.3.2. Preparation of TFC-FOPA/GO Membranes
2.3.3. Preparation of TFC-FOPSF-PA/GO Membranes
2.4. Characterization of GO
2.5. Characterization of Membrane
2.6. FO Performance Evaluation
3. Results
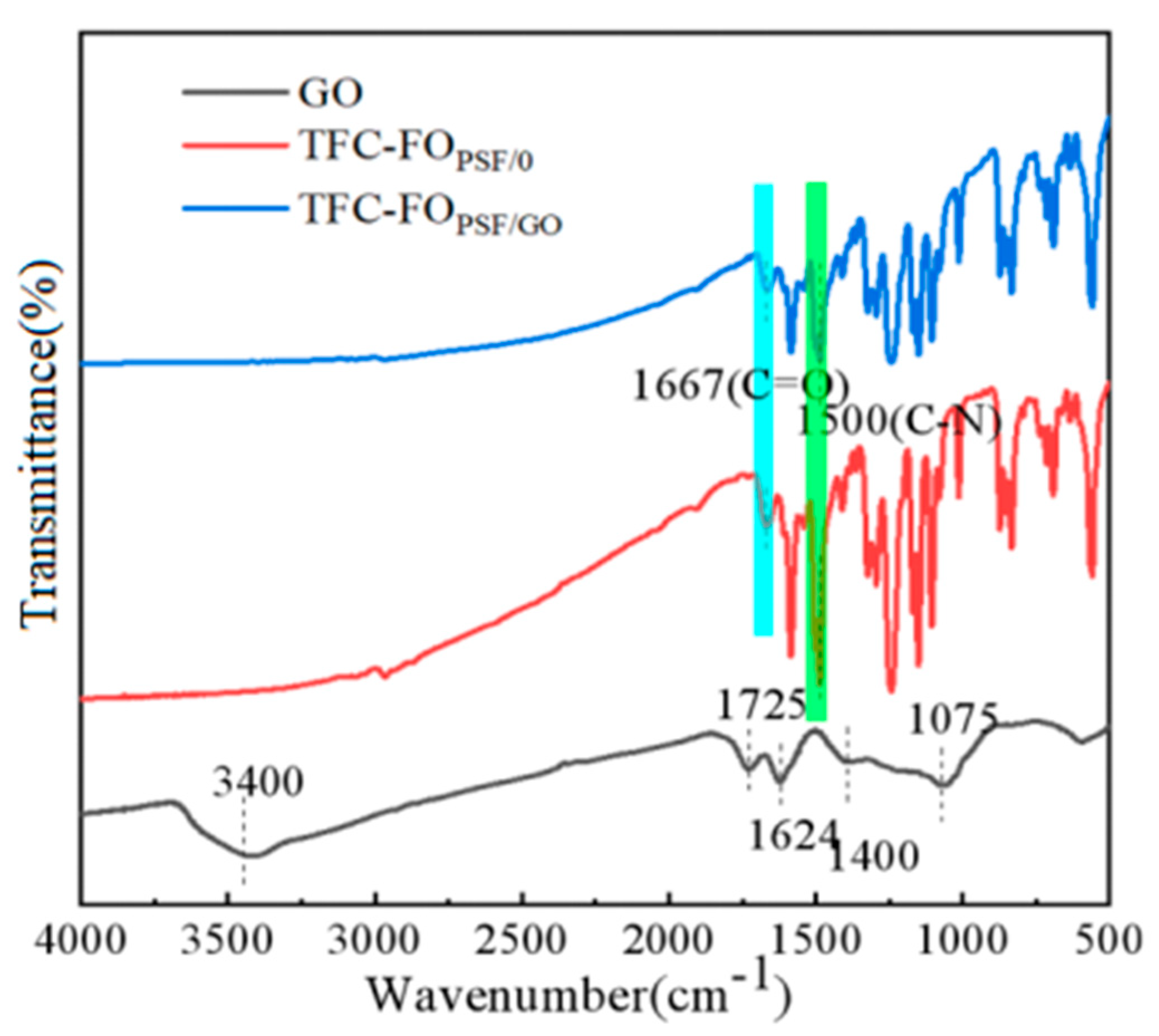
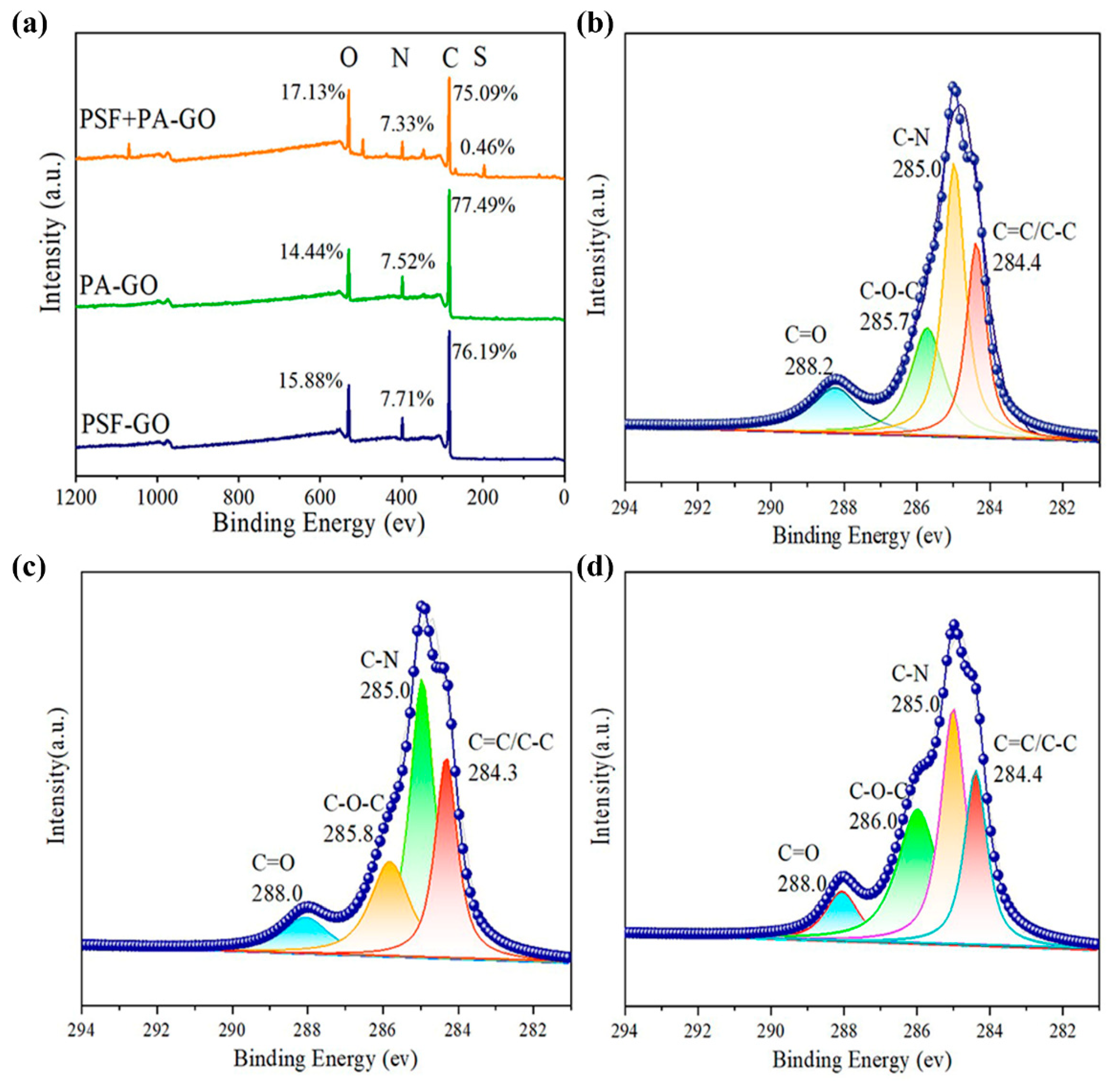
3.1. GO Characterization
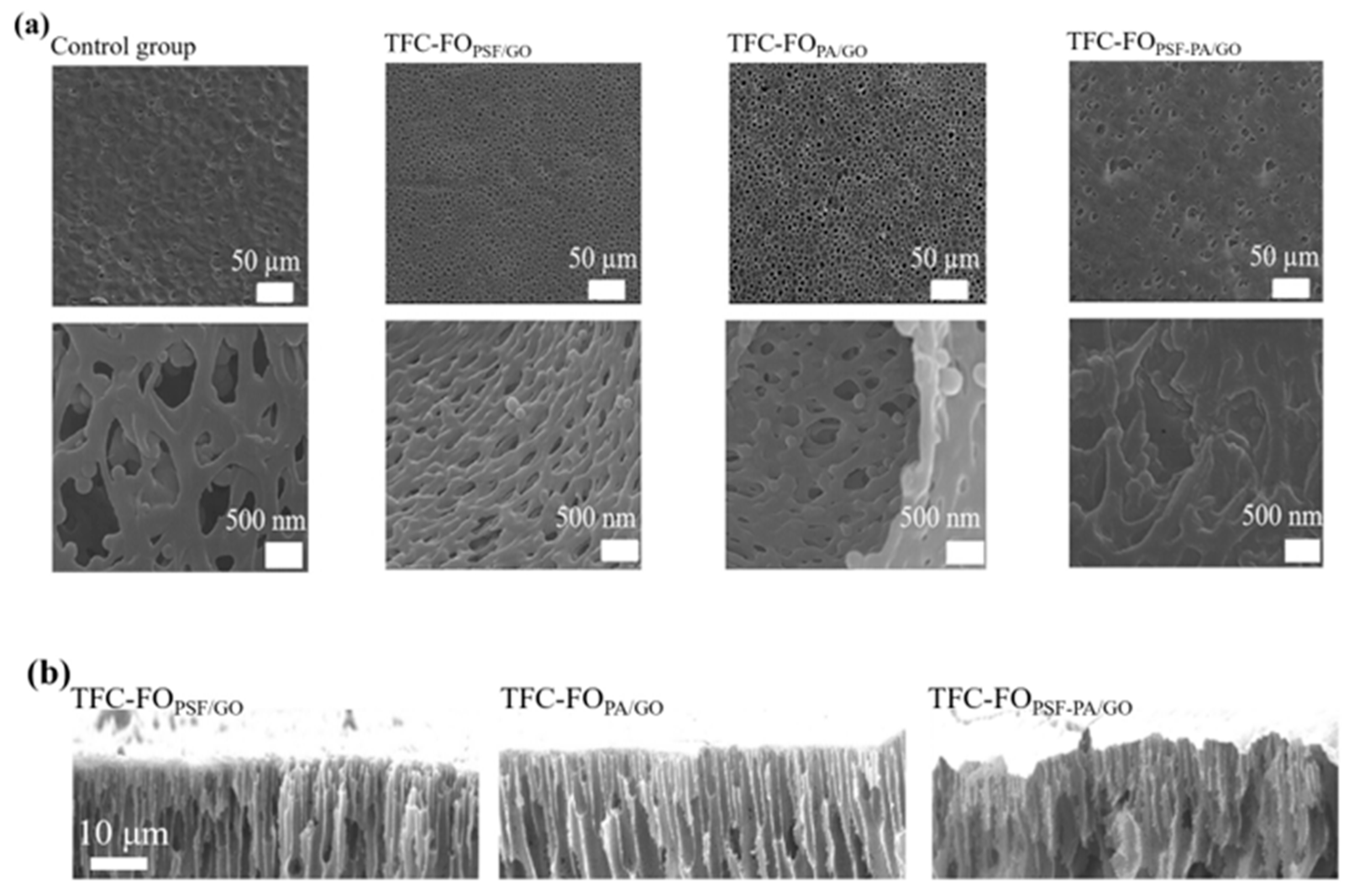
3.2. Surface Morphology and Microstructure of TFC-FO Membranes
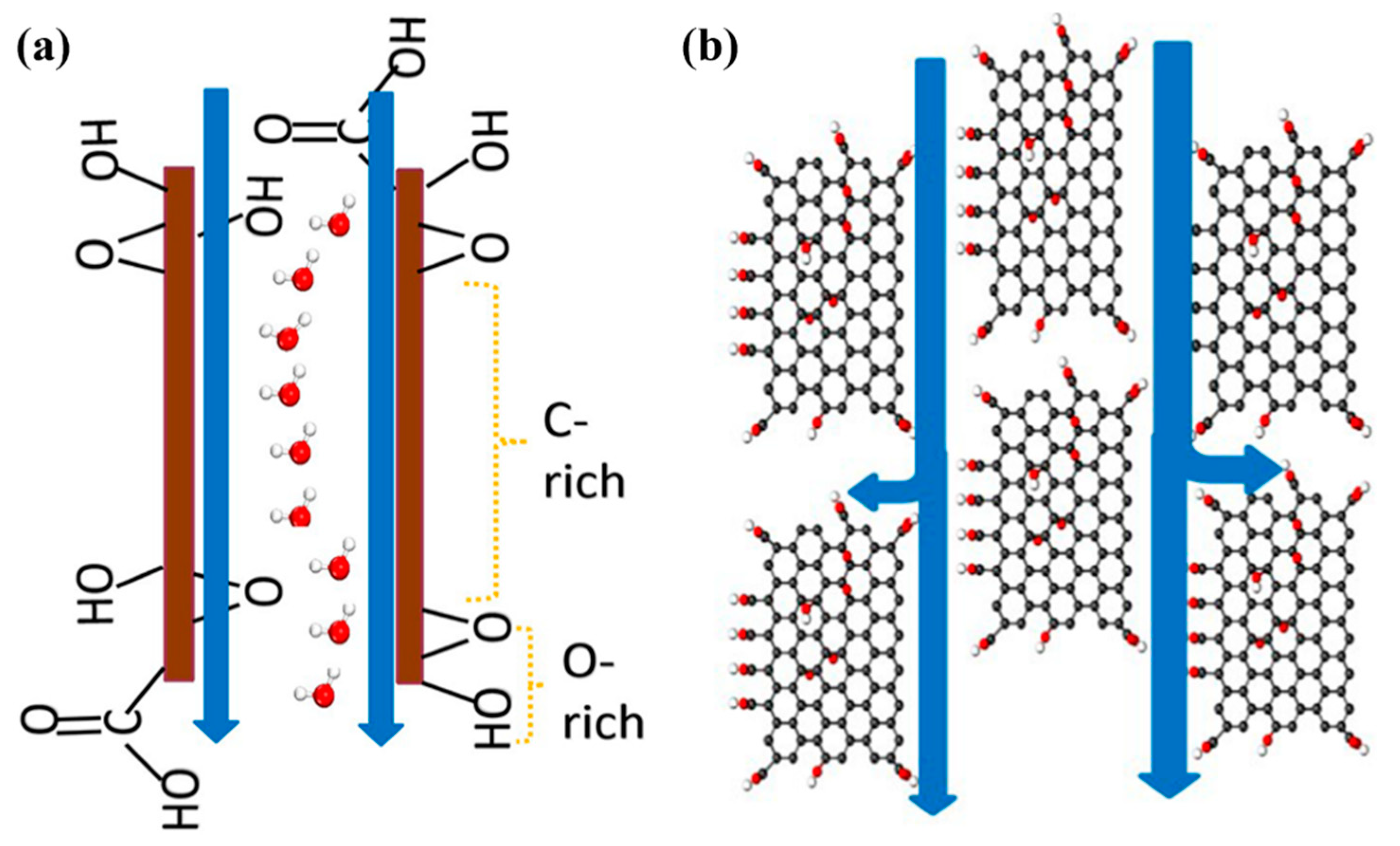
3.3. Formation Mechanism of Water Channels in Membranes
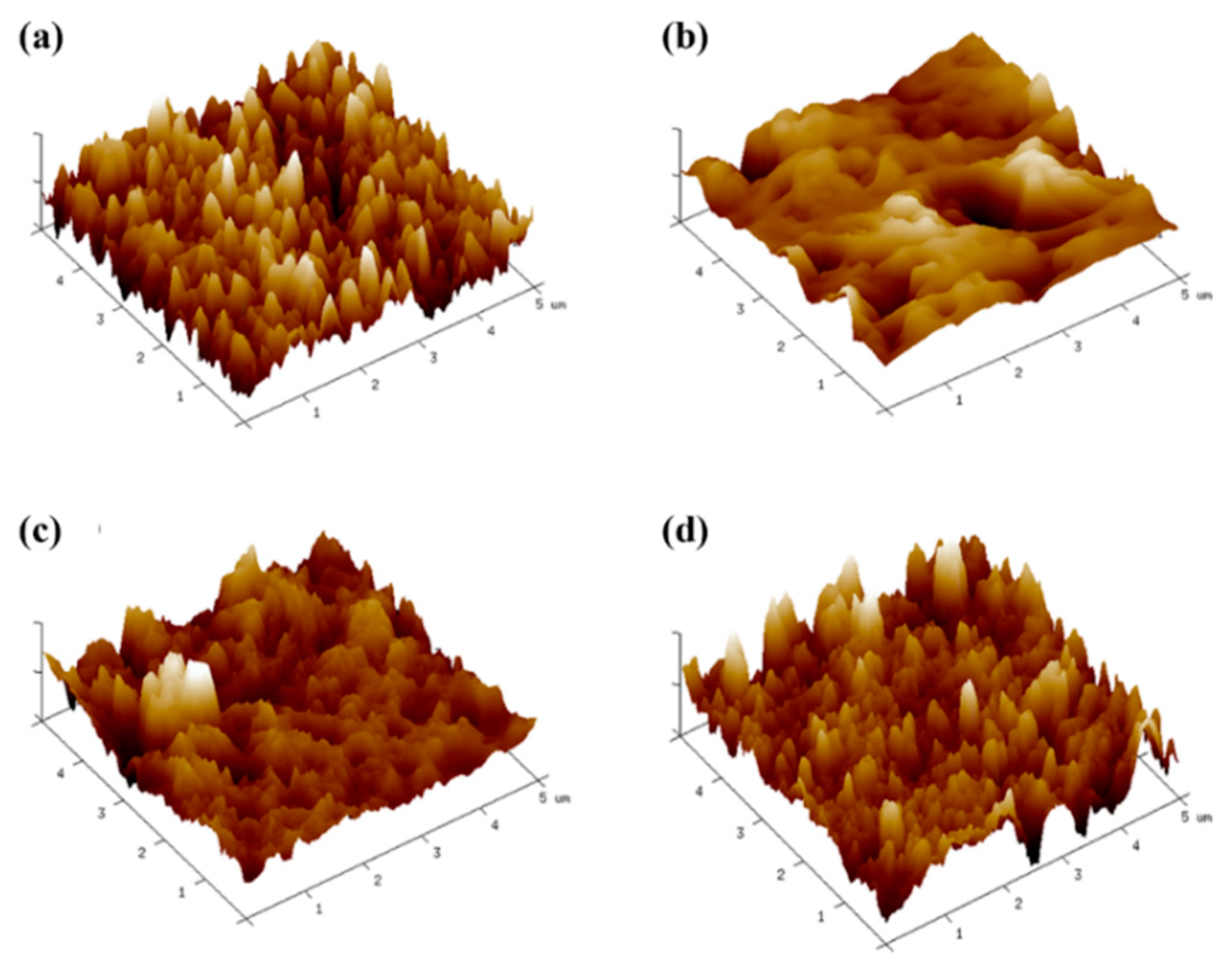
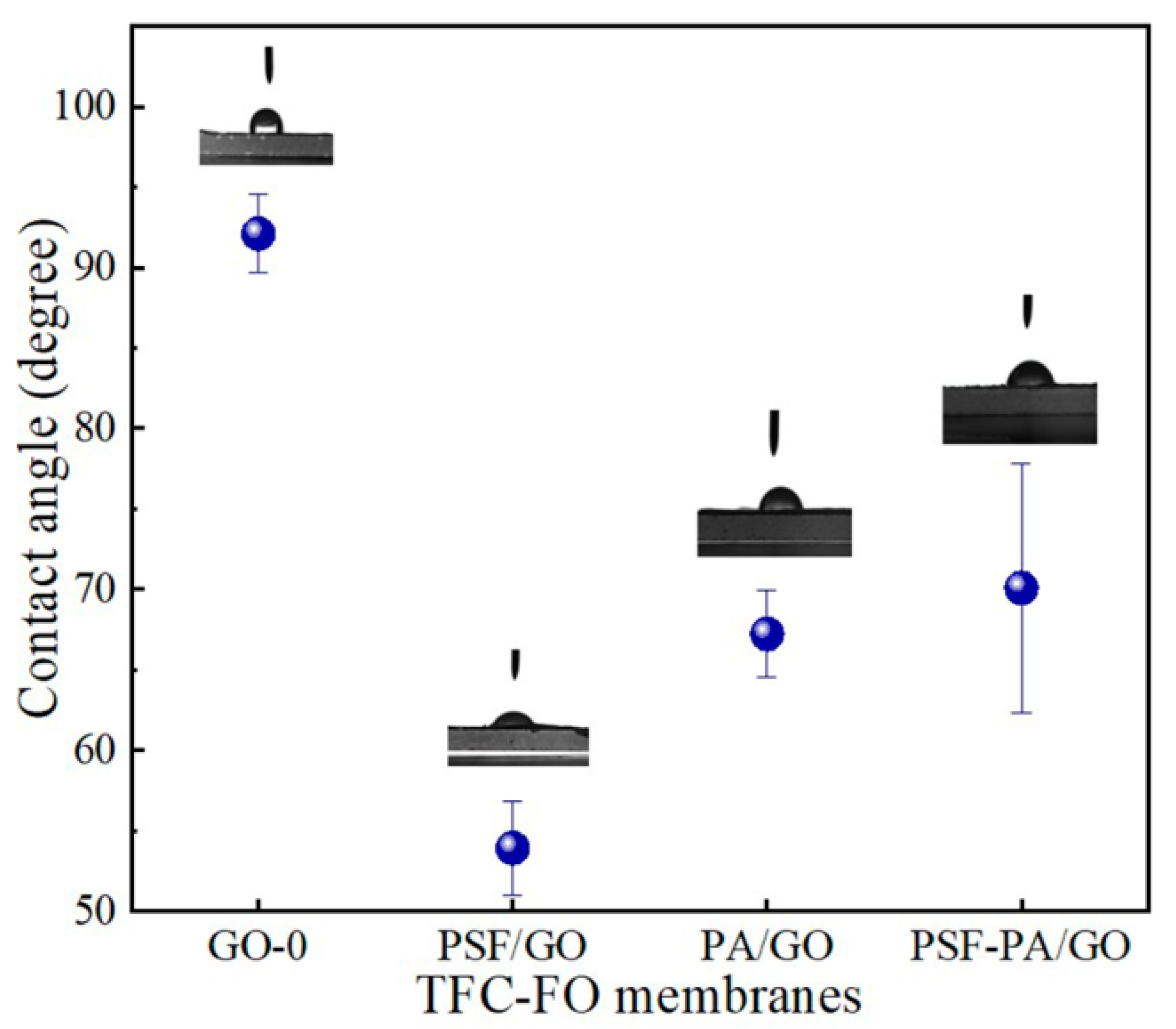
3.4. Effects of GO on Hydrophilicity of Different Membranes
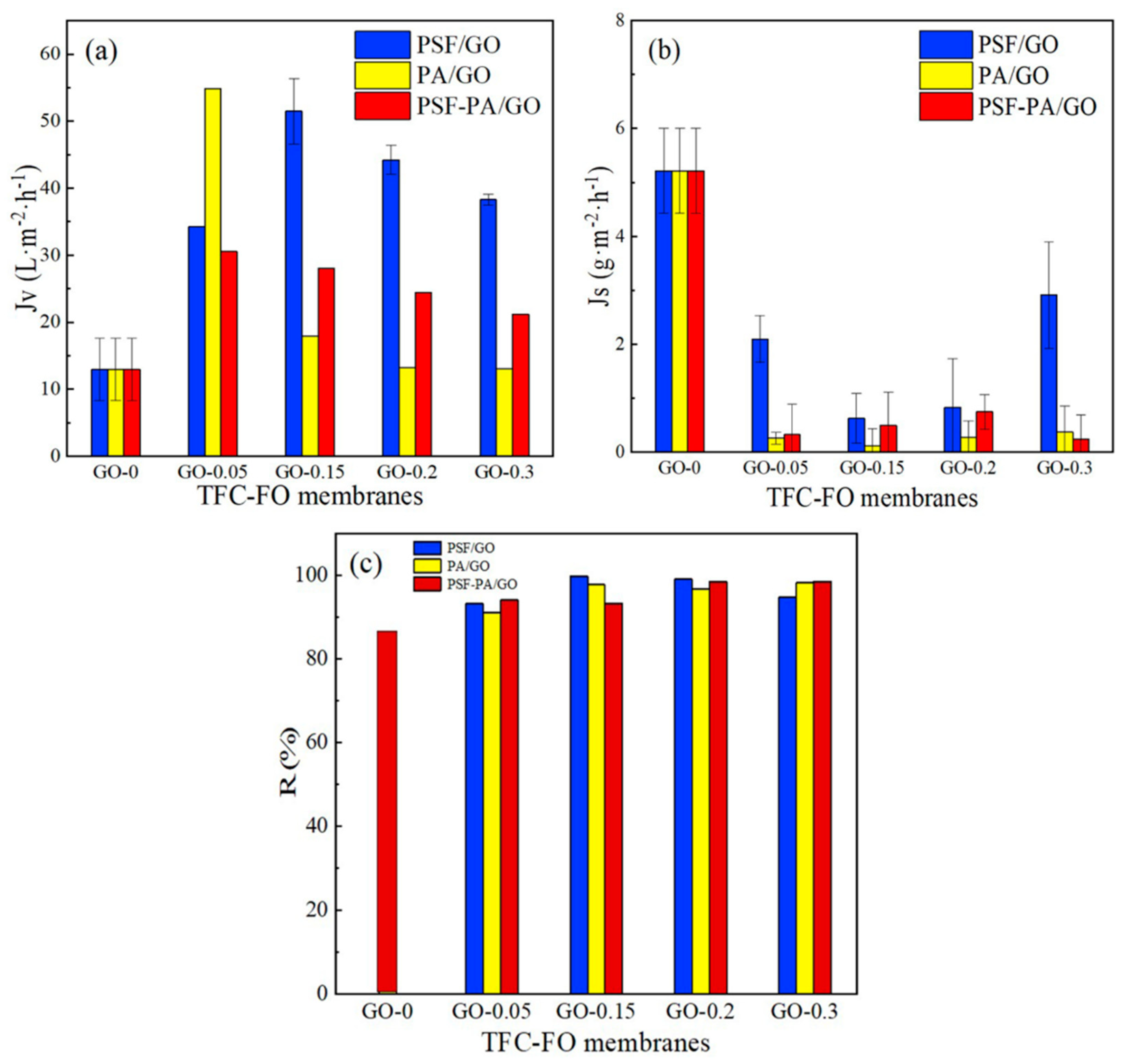
3.5. Analyses of TFC Membrane Permeability
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saleem, H.; Trabzon, L.; Kilic, A. Recent advances in nanofibrous membranes: Production and applications in water treatment and desalination. Desalination 2020, 478, 114178. [Google Scholar] [CrossRef]
- Nguyen, T.; Lee, C.; Field, R.W. Insight into organic fouling behavior in polyamide thin-film composite forward osmosis membrane: Critical flux and its impact on the economics of water reclamation. J. Membr. Sci. 2020, 606, 118118. [Google Scholar] [CrossRef]
- She, Q.; Wang, R.; Fane, A.G. Membrane fouling in osmotically driven membrane processes: A review. J. Membr. Sci. 2016, 499, 201–233. [Google Scholar] [CrossRef]
- Wang, L.; Li, T.; Chu, H.Q. Natural organic matter separation by forward osmosis: Performance and mechanisms. Water Res. 2021, 191, 116829. [Google Scholar] [CrossRef]
- Wang, L.; Chu, H.Q.; Dong, B.Z. Effects on the purification of tannic acid and natural dissolved organic matter by forward osmosis membrane. J. Membr. Sci. 2014, 455, 31–43. [Google Scholar] [CrossRef]
- Zhao, P.; Gao, B.Y.; Yue, Q.Y. Fatty acid fouling of forward osmosis membrane: Effects of pH, calcium, membrane orientation, initial permeate flux and foulant composition. J. Environ. Sci. 2016, 46, 55–62. [Google Scholar] [CrossRef]
- McGinnis, R.L.; Elimelech, M. Energy requirements of ammonia-carbon dioxide forward osmosis desalination. Desalination 2007, 207, 370–382. [Google Scholar] [CrossRef]
- Shen, Y.; Saboe, P.O.; Sines, I.T. Biomimetic membranes: A review. J. Membr. Sci. 2014, 454, 359–381. [Google Scholar] [CrossRef]
- Kumar, M.; Grzelakowski, M.; Zilles, J. Highly permeable polymeric membranes based on the incorporation of the functional water channel protein Aquaporin, Z. Proc. Natl. Acad. Sci. USA 2007, 104, 20719–20724. [Google Scholar] [CrossRef]
- Liao, G.; Zhou, X.; Chen, L.; Zeng, X.; Xie, X.; Mai, Y. Electrospun aligned PLLA/PCL/functionalised multiwalled carbon nanotube composite fibrous membranes and their bio/mechanical properties. Compos. Sci. Technol. 2012, 72, 248–255. [Google Scholar] [CrossRef]
- Amini, M.; Jahanshahi, M.; Rahimpour, A. Synthesis of novel thin film nanocomposite (TFN) forward osmosis membranes using functionalized multi-walled carbon nanotubes. J. Membr. Sci. 2013, 435, 233–241. [Google Scholar] [CrossRef]
- Ahmed, F.E.; Hashaikeh, R.; Hilal, N. Fouling control in reverse osmosis membranes through modification with conductive carbon nanostructures. Desalination 2019, 470, 114118. [Google Scholar] [CrossRef]
- Nair, R.R.; Wu, H.A.; Jayaram, P.N.; Grigorieva, I.V.; Geim, A.K. Unimpeded permeation of water through helium-leak-tight graphene-based membranes. Science 2012, 335, 442–444. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wu, B.; Liu, H.; Wang, H.; Su, Y.; Lei, W.; Hu, P.; Liu, Y. Low temperature growth of clean single layer hexagonal boron nitride flakes and film for graphene-based field-effect transistors. Sci. China Mater. 2019, 62, 1218–1225. [Google Scholar] [CrossRef]
- Perreault, F.; Tousley, M.E.; Elimelech, M. Thin-film composite polyamide membranes functionalized with biocidal graphene oxide nanosheets. Environ. Sci. Technol. Lett. 2014, 1, 71–76. [Google Scholar] [CrossRef]
- Bano, S.; Mahmood, A.; Kim, S.; Lee, K. Graphene oxide modified polyamide nanofiltration membrane with improved flux and antifouling properties. J. Mater. Chem. A 2015, 3, 2065–2071. [Google Scholar] [CrossRef]
- Baig, M.I.; Ingole, P.G.; Jeon, J.; Hong, S.U.; Choi, W.K.; Lee, H.K. Water vapor transport properties of interfacially polymerized thin film nanocomposite membranes modified with graphene oxide and GO-TiO2 nanofillers. Chem. Eng. J. 2019, 373, 1190–1202. [Google Scholar] [CrossRef]
- Gao, Y.; Hu, M.; Mi, B. Membrane surface modification with TiO2-graphene oxide for enhanced photocatalytic performance. J. Membr. Sci. 2014, 455, 349–356. [Google Scholar] [CrossRef]
- Emadzadeh, D.; Lau, W.J.; Matsuura, T.; Rahbari-Sisakht, M.; Ismail, A.F. A novel thin film composite forward osmosis membrane prepared from PSF-TiO2 nanocomposite substrate for water desalination. Chem. Eng. J. 2014, 237, 70–80. [Google Scholar] [CrossRef]
- Lai, G.S.; Lau, W.J.; Gray, S.R.; Matsuura, T.; Gohari, R.J.; Subramanian, M.N.; Lai, S.O.; Ong, C.S.; Ismail, A.F.; Emazadah, D.; et al. A practical approach to synthesize polyamide thin film nanocomposite (TFN) membranes with improved separation properties for water/wastewater treatment. J. Mater. Chem. A 2016, 4, 4134–4144. [Google Scholar] [CrossRef]
- Lai, G.S.; Lau, W.J.; Goh, P.S.; Ismail, A.F.; Yusof, N.; Tan, Y.H. Graphene oxide incorporated thin film nanocomposite nanofiltration membrane for enhanced salt removal performance. Desalination 2016, 387, 14–24. [Google Scholar] [CrossRef]
- Ma, N.; Wei, J.; Qi, S.; Zhao, Y.; Gao, Y.; Tang, C.Y. Nanocomposite substrates for controlling internal concentration polarization in forward osmosis membranes. J. Membr. Sci. 2013, 441, 54–62. [Google Scholar] [CrossRef]
- Jin, L.; Wang, Z.; Zheng, S.; Mi, B. Polyamide-crosslinked graphene oxide membrane for forward osmosis. J. Membr. Sci. 2018, 545, 11–18. [Google Scholar] [CrossRef]
- Park, M.J.; Phuntsho, S.; He, T.; Nisola, G.M.; Tijing, L.D.; Li, X.; Chen, G.; Chung, W.; Shon, H.K. Graphene oxide incorporated polysulfone substrate for the fabrication of flat-sheet thin-film composite forward osmosis membranes. J. Membr. Sci. 2015, 493, 496–507. [Google Scholar] [CrossRef]
- Zhang, K.; Dwivedi, V.; Chi, C.; Wu, J. Graphene oxide/ferric hydroxide composites for efficient arsenate removal from drinking water. J. Hazard. Mater. 2010, 182, 162–168. [Google Scholar] [CrossRef]
- Rao, C.N.R.; Sood, A.K.; Subrahmanyam, K.S.; Govindaraj, A. Graphene: The new two-dimensional nanomaterial. Angew. Chem. Int. Ed. 2009, 48, 7752–7777. [Google Scholar] [CrossRef]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef]
- Yang, Z.; Guo, H.; Tang, C.Y. The upper bound of thin-film composite (TFC) polyamide membranes for desalination. J. Membr. Sci. 2019, 590, 117297. [Google Scholar] [CrossRef]
- Wang, F.; Wu, Y.; Huang, Y. Novel application of graphene oxide to improve hydrophilicity and mechanical strength of aramid nanofiber hybrid membrane. Compos. Part A Appl. Sci. Manuf. 2018, 110, 126–132. [Google Scholar] [CrossRef]
- Akther, N.; Phuntsho, S.; Chen, Y.; Ghaffour, N.; Shon, H.K. Recent advances in nanomaterial-modified polyamide thin-film composite membranes for forward osmosis processes. J. Membr. Sci. 2019, 584, 20–45. [Google Scholar] [CrossRef]
- Fan, X.; Liu, Y.; Quan, X. A novel reduced graphene oxide/carbon nanotube hollow fiber membrane with high forward osmosis performance. Desalination 2019, 451, 117–124. [Google Scholar] [CrossRef]
- Hu, M.; Mi, B. Enabling graphene oxide nanosheets as water separation membranes. Environ. Sci. Technol. 2013, 47, 3715–3723. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Shah, A.A.; Nam, S.; Park, Y.; Park, H. Thin-film composite membranes comprising ultrathin hydrophilic polydopamine interlayer with graphene oxide for forward osmosis. Desalination 2019, 449, 41–49. [Google Scholar] [CrossRef]
- Thür, R.; Corvilain, M.; Klaysom, C.; Hartanto, Y.; Vankelecom, I.F.J. Tuning the selectivity of thin film composite forward osmosis membranes: Effect of co-solvent and different interfacial polymerization synthesis routes. Sep. Purif. Technol. 2019, 227, 115671. [Google Scholar] [CrossRef]
- Shen, L.; Xiong, S.; Wang, Y. Graphene oxide incorporated thin-film composite membranes for forward osmosis applications. Chem. Eng. Sci. 2016, 143, 194–205. [Google Scholar] [CrossRef]
- Choi, W.; Choi, J.; Bang, J.; Lee, J. Layer-by-layer assembly of graphene oxide nanosheets on polyamide membranes for durable reverse-osmosis applications. ACS Appl. Mater. Interfaces 2013, 5, 12510–12519. [Google Scholar] [CrossRef]
- Zheng, S.; Tu, Q.; Urban, J.J.; Li, S.; Mi, B. Swelling of graphene oxide membranes in aqueous solution: Characterization of interlayer spacing and insight into water transport mechanisms. ACS Nano 2017, 11, 6440–6450. [Google Scholar] [CrossRef]
- Kim, S.; Ou, R.; Hu, Y.; Li, X.; Zhang, H.; Simon, G.P.; Wang, H. Non-swelling graphene oxide-polymer nanocomposite membrane for reverse osmosis desalination. J. Membr. Sci. 2018, 562, 47–55. [Google Scholar] [CrossRef]
- Dimiev, A.M.; Tour, J.M. Mechanism of graphene oxide formation. ACS Nano 2014, 8, 3060–3068. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Choi, W.; Jeon, S.; Kwon, S.J.; Park, H.; Park, Y.; Nam, S.; Lee, P.S.; Lee, J.S.; Choi, J.; Hong, S. Thin film composite reverse osmosis membranes prepared via layered interfacial polymerization. J. Membr. Sci. 2017, 527, 121–128. [Google Scholar] [CrossRef]
- Huang, L.W.; Nhungoc, B.; Mark, M.; Hamlin, T.J.; McCutcheon, J.R. Novel hydrophilic nylon 6,6 microfiltration membrane supported thin film composite membranes for engineered osmosis. J. Membr. Sci. 2013, 437, 141–149. [Google Scholar] [CrossRef]
- Yu, Y.B.; Seo, S.K.; Kim, I.C.; Lee, S. Nanoporous polyethersulfone (PES) membrane with enhances flux applied in forward osmosis process. J. Membr. Sci. 2011, 375, 63–68. [Google Scholar] [CrossRef]
- Huang, Y.B.; Jin, H.Y.; Li, H.; Yu, P.; Luo, Y. Synthesis and characterization of a polyamide thin film composite membrane based on a polydopamine coated support layer for forward osmosis. RSC Adv. 2015, 5, 106113–106121. [Google Scholar] [CrossRef]
- Liu, X.; Ng, H.Y. Fabrication of layered silica-polysulfone mixed matrix substrate membrane for enhancing performance of thin-film composite forward osmosis membrane. J. Membr. Sci. 2015, 481, 148–163. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Qu, R.W.; Ge, Q.Q.; Wang, H.; Xu, T. Preparation of polyethersulfone/carbon nanotube substrate for high-performance forward osmosis membrane. Desalination 2013, 330, 70–78. [Google Scholar] [CrossRef]
- Hegab, H.M.; ElMekawy, A.; Barclay, T.G. Single-step assembly of multifunctional poly(tannic acid)-graphene oxide coating to reduce biofouling of forward osmosis membranes. ACS Appl. Mater. Interfaces 2016, 8, 17519–17528. [Google Scholar] [CrossRef]
- Hegab, H.M.; ElMekawy, A.; Barclay, T.G. Fine-tuning the surface of forward osmosis membranes via grafting graphene oxide: Performance patterns and biofouling propensity. ACS Appl. Mater. Interfaces 2015, 7, 18004–18016. [Google Scholar] [CrossRef]
- Vatanpour, V.; Sanadgol, A. Surface modification of reverse osmosis membranes by grafting of polyamidoamine dendrimer containing graphene oxide nanosheets for desalination improvement. Desalination 2020, 491, 114442. [Google Scholar] [CrossRef]
- Shao, F.F.; Su, X.; Shen, X. Highly improved chlorine resistance of polyamide reverse membrane by grafting layers of graphene oxide. Sep. Purif. Technol. 2021, 254, 117586. [Google Scholar] [CrossRef]
- Huang, X.; Marsh, K.L.; Mcverry, B.T. Low-fouling antibacterial reverse osmosis membranes via surface grafting of graphene oxide. ACS Appl. Mater. Interfaces 2016, 8, 14334–14338. [Google Scholar] [CrossRef] [PubMed]
- Morales-Torres, S.; Esteves, C.M.P.; Figueireddo, J. Thin-film composite forward osmosis membranes based on polysulfone supports blended with nanostructured carbon materials. J. Membr. Sci. 2016, 520, 326–336. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, Y.; He, M. Antifouling membranes for sustainable water purification: Strategies and mechanisms. Chem. Soc. Rev. 2016, 45, 5888–5924. [Google Scholar] [CrossRef] [PubMed]
- Rana, D.; Matsuura, T. Surface Modifications for Antifouling Membranes. Chem. Rev. 2010, 110, 2448–2471. [Google Scholar] [CrossRef]
- Chen, G.; Liu, R.; Shon, H.K. Open porous hydrophilic supported thin-film composite forward osmosis membrane via co-casting for treatment of high-salinity wastewater. Desalination 2017, 405, 76–84. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, C.; Zhao, D.; Wang, Y.; Zhao, R.; Wang, H.; Wu, X.; Liu, S.; Zhu, H.; Fu, J.; Zhang, M.; et al. Impact of Graphene Oxide on Properties and Structure of Thin-Film Composite Forward Osmosis Membranes. Polymers 2022, 14, 3874. https://doi.org/10.3390/polym14183874
Dai C, Zhao D, Wang Y, Zhao R, Wang H, Wu X, Liu S, Zhu H, Fu J, Zhang M, et al. Impact of Graphene Oxide on Properties and Structure of Thin-Film Composite Forward Osmosis Membranes. Polymers. 2022; 14(18):3874. https://doi.org/10.3390/polym14183874
Chicago/Turabian StyleDai, Chenglong, Dan Zhao, Yongqiang Wang, Rui Zhao, Han Wang, Xiangci Wu, Shejiang Liu, Huizhen Zhu, Jianfeng Fu, Mengling Zhang, and et al. 2022. "Impact of Graphene Oxide on Properties and Structure of Thin-Film Composite Forward Osmosis Membranes" Polymers 14, no. 18: 3874. https://doi.org/10.3390/polym14183874
APA StyleDai, C., Zhao, D., Wang, Y., Zhao, R., Wang, H., Wu, X., Liu, S., Zhu, H., Fu, J., Zhang, M., & Ding, H. (2022). Impact of Graphene Oxide on Properties and Structure of Thin-Film Composite Forward Osmosis Membranes. Polymers, 14(18), 3874. https://doi.org/10.3390/polym14183874








