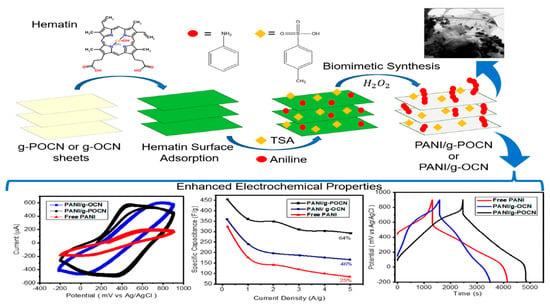
Biomimetic Synthesis of PANI/Graphitic Oxidized Carbon Nitride for Supercapacitor Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
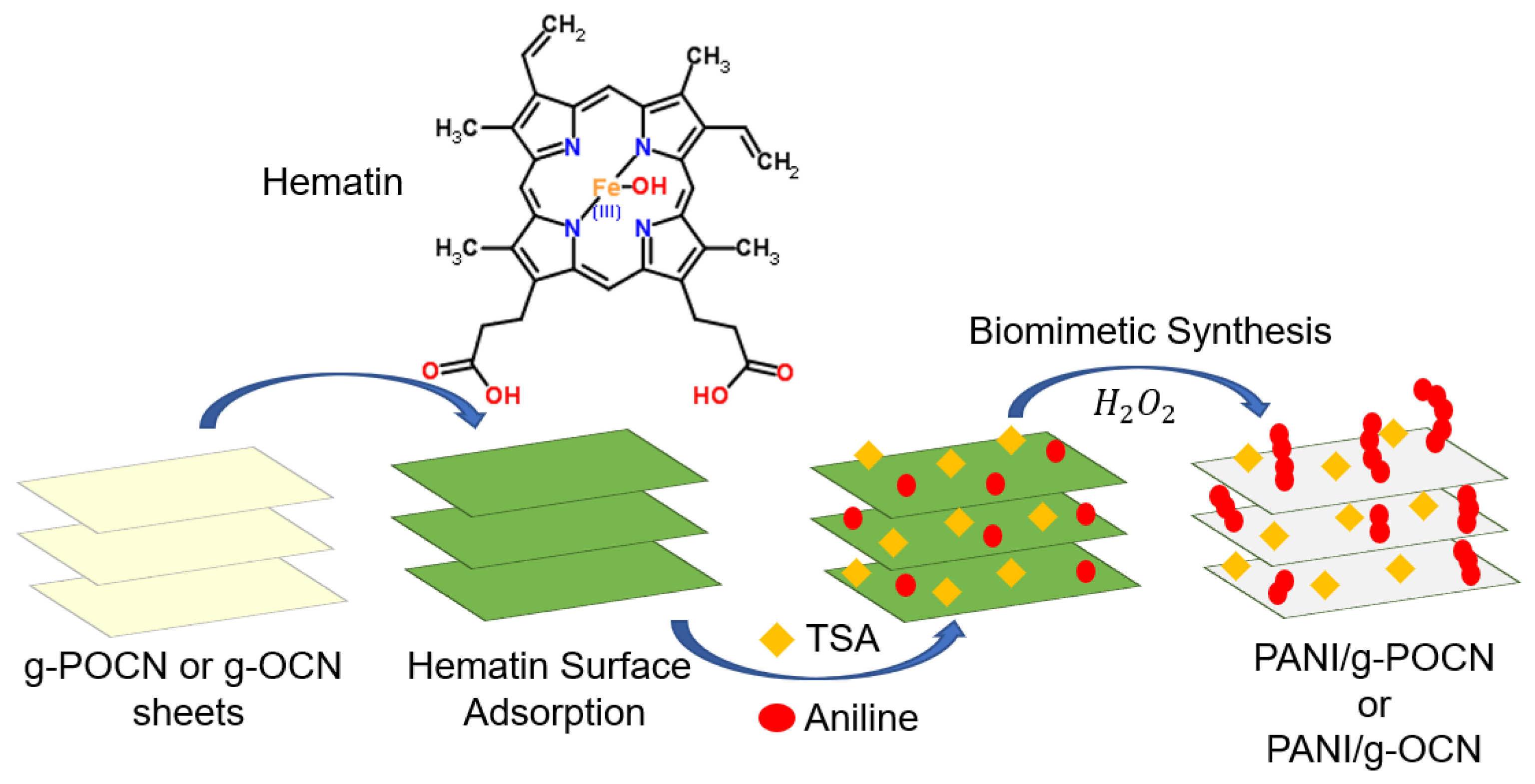
2.2.1. Hematin Deposition on Doped g-OCN
2.2.2. Biomimetic Synthesis Assisted by Hematin Supported in Doped g-OCN
2.2.3. Biomimetic Synthesis Assisted by Free Hematin (Cosolvents)
2.2.4. UV-Vis
2.2.5. Powder X-ray Diffraction (PXRD)
2.2.6. Infrared Spectroscopy (FTIR)
2.2.7. Thermogravimetric Analysis (TGA)
2.2.8. Transmission Electron Microscopy (TEM)
2.2.9. X-ray Emitted Photoelectron Spectroscopy (XPS)
2.2.10. Volumetric Electrical Conductivity Measurement
2.2.11. Preparation of the Working Electrode (We)
2.2.12. Cyclic Voltammetry (CV)
2.2.13. Galvanostatic Charge-Discharge
2.2.14. Electrochemical Impedance Spectroscopy (EIS)
3. Results and Discussion
3.1. Hematin Supported on g-OCN or g-POCN
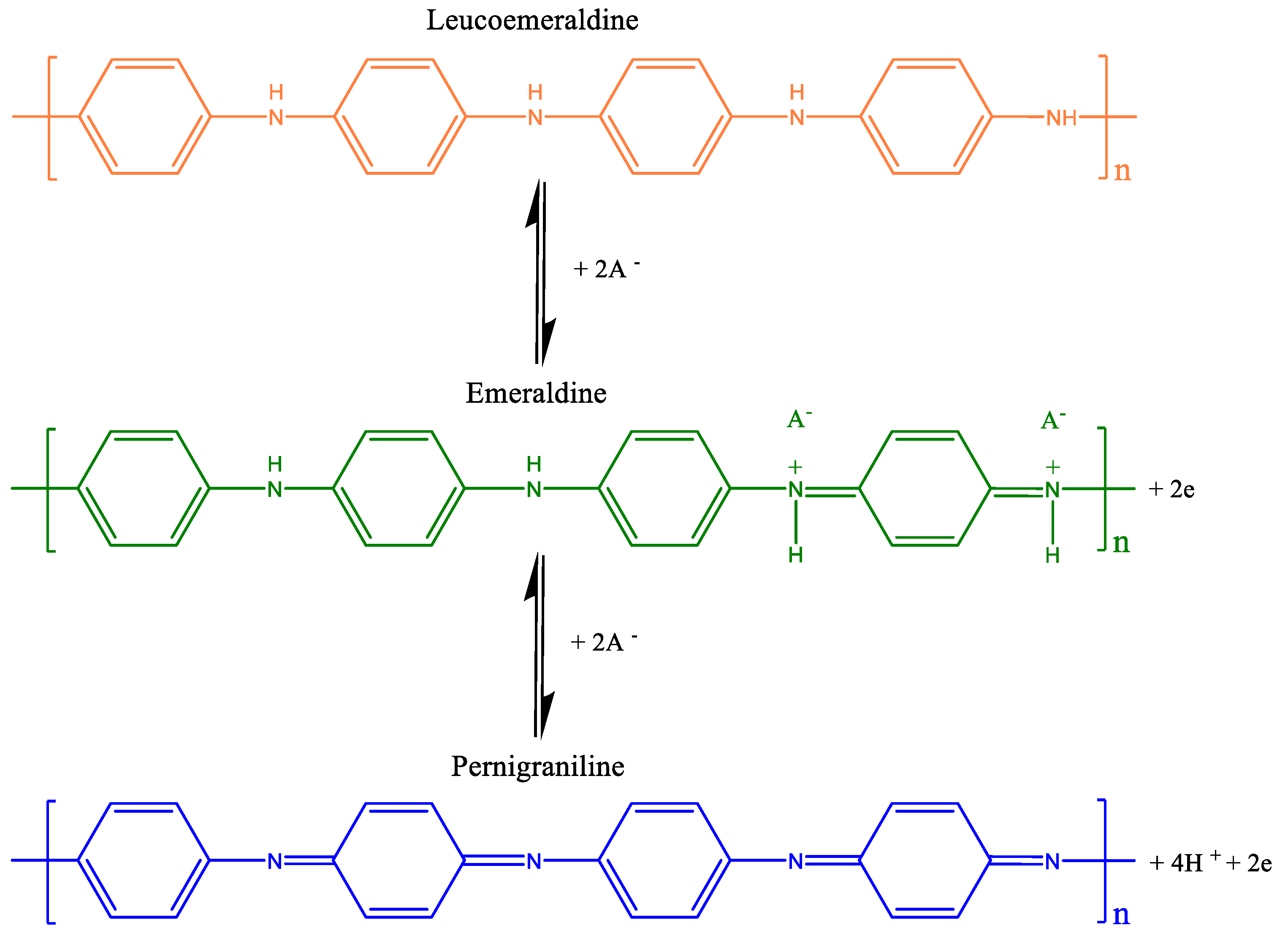
3.2. Free PANI and PANI/g-OCN PANI/g-POCN
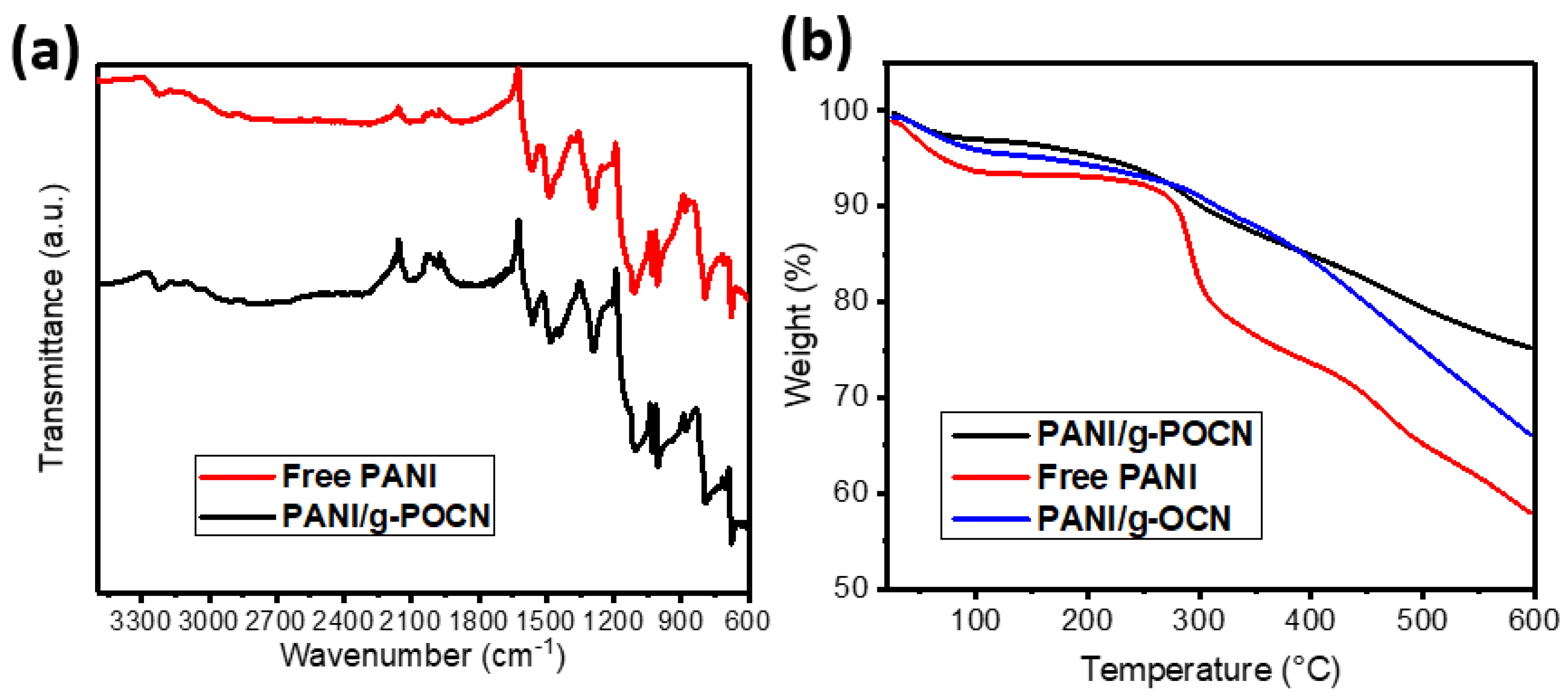
3.2.1. FTIR
3.2.2. Thermal Analysis
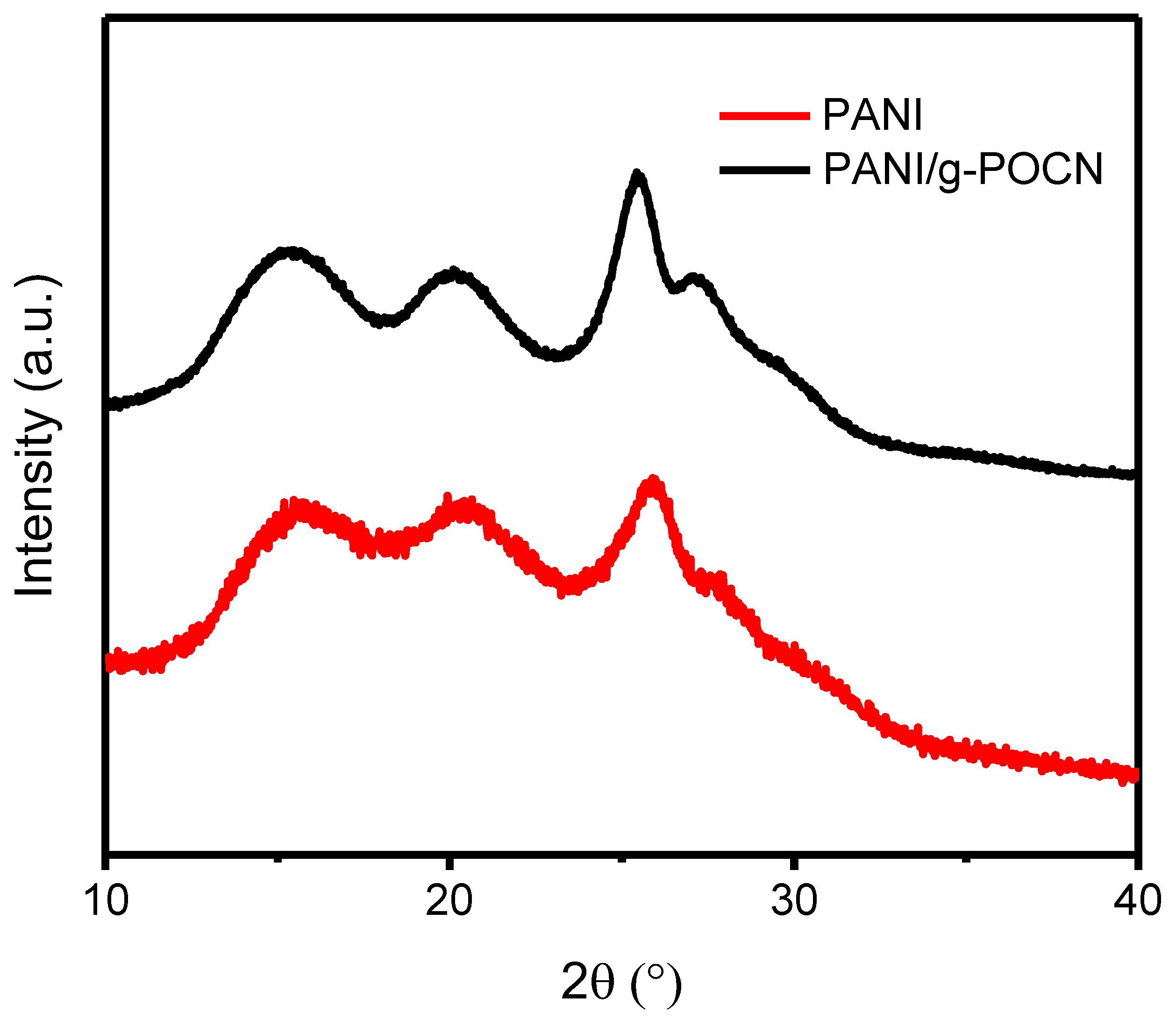
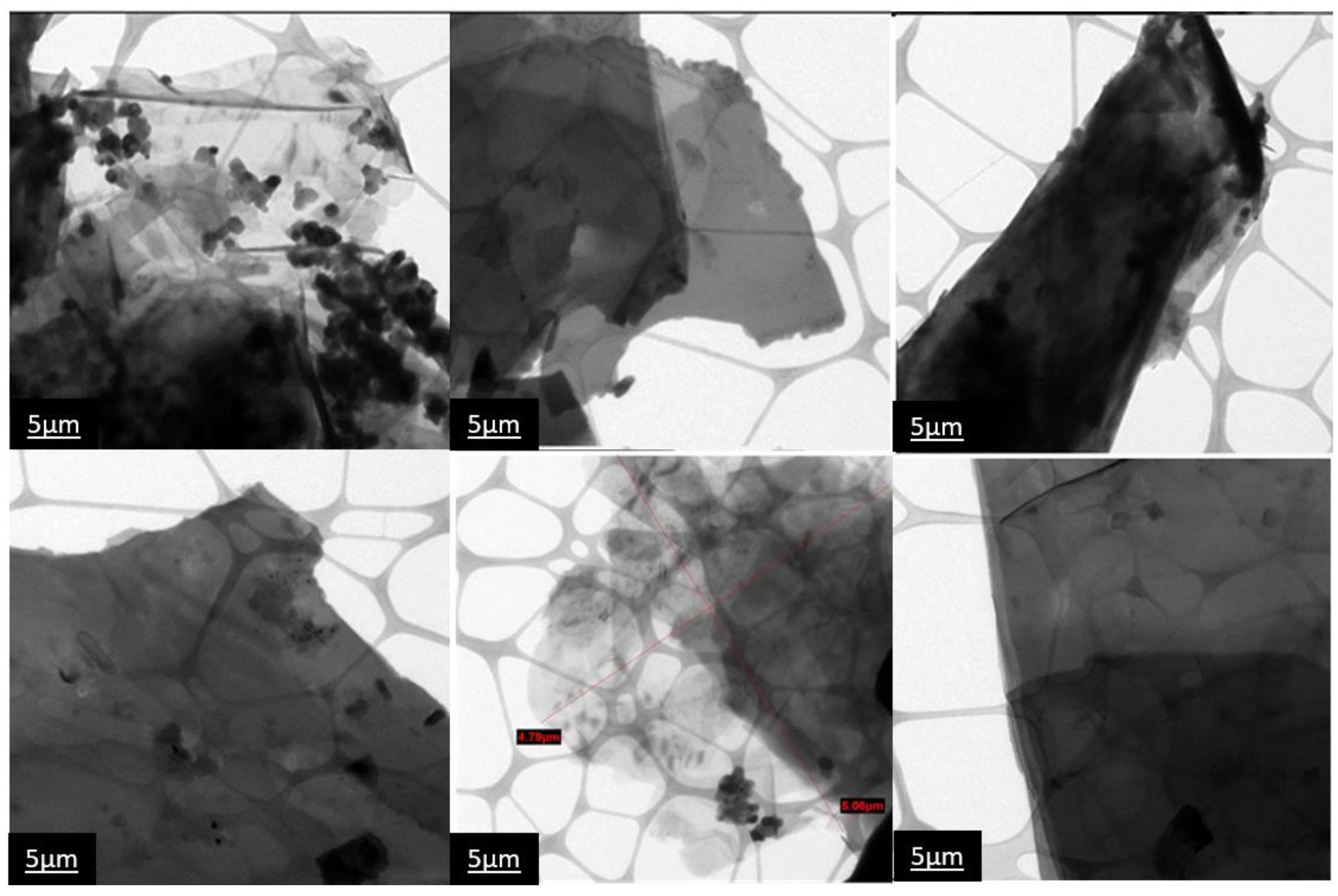
3.2.3. Microstructure Analysis by PXRD, SEM, and TEM
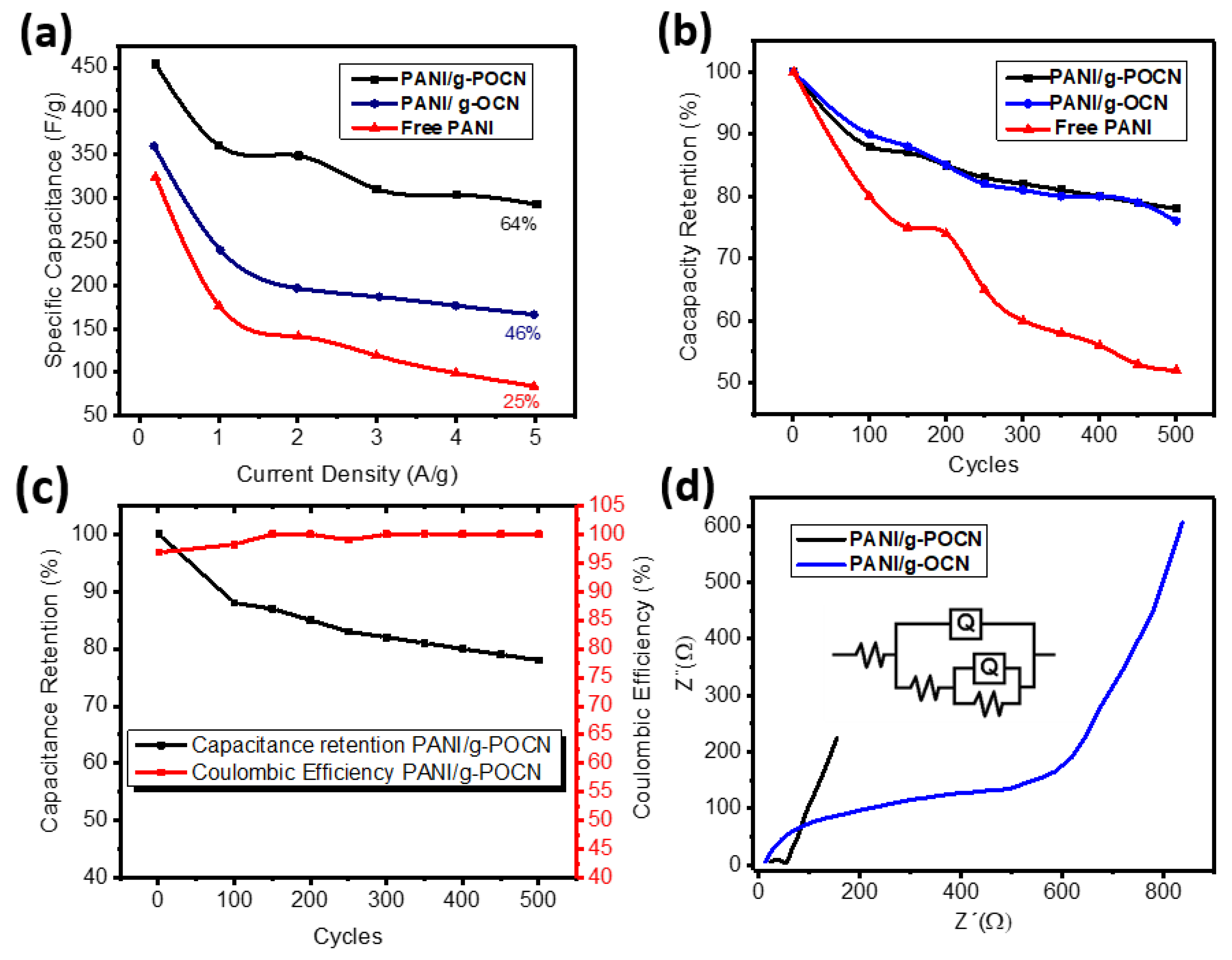
3.3. Electrochemical Characterization
3.3.1. Cyclic Voltammetry
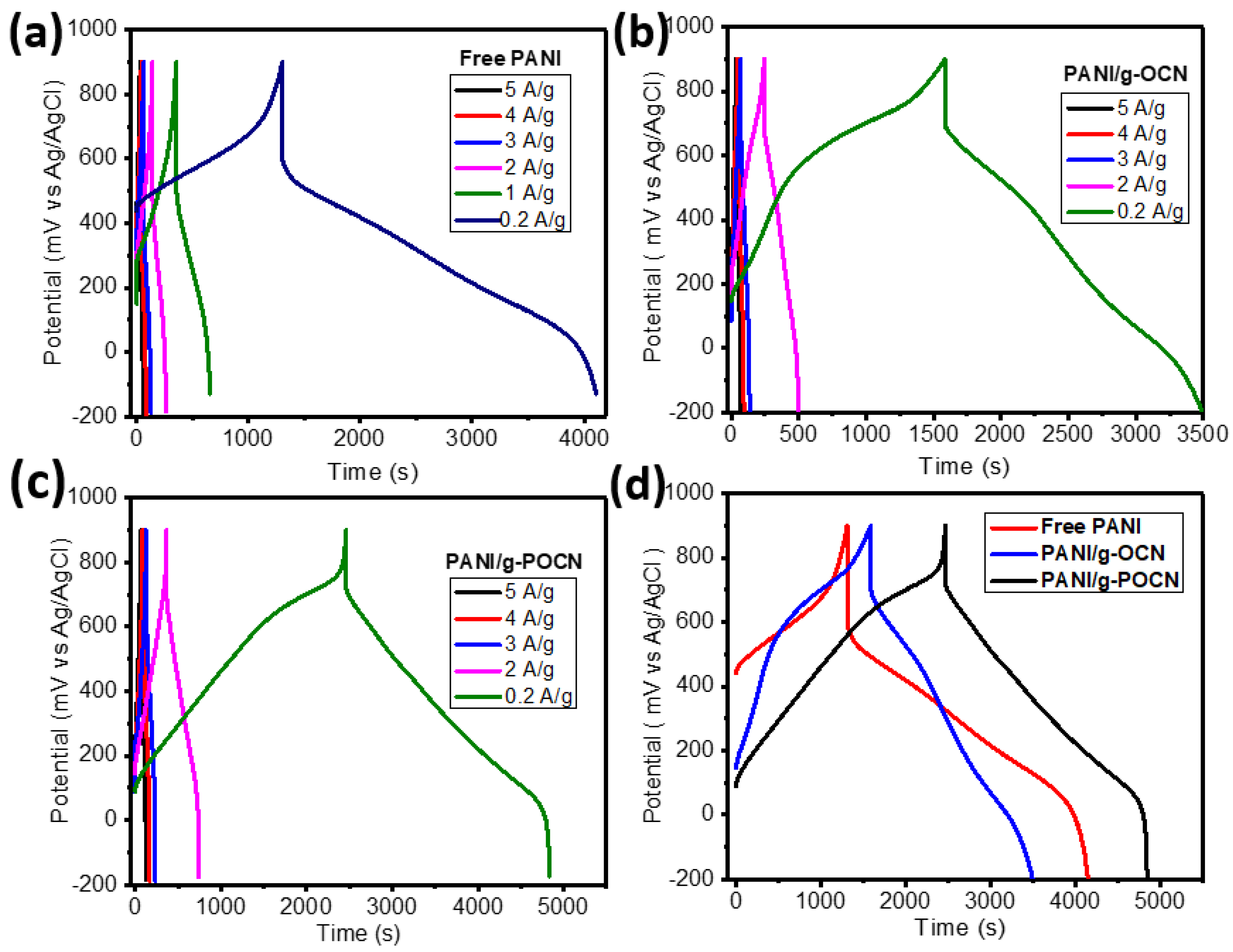
3.3.2. Galvanostatic Charge-Discharge
3.3.3. Electrochemical Impedance Spectroscopy (EIS)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Parravano, G. Chain Reactions Induced by Enzymic Systems. J. Am. Chem. Soc. 1951, 73, 183–184. [Google Scholar] [CrossRef]
- Arias-Marín, E.; Romero, J.; Ledezma-Pérez, A.; Kniajansky, S. Enzymatic mediated polymerization of functional aniline derivatives in nonaqueous media. Polym. Bull. 1996, 37, 581–587. [Google Scholar] [CrossRef]
- Irina Sapurina, J.S. The mechanism of the oxidative polymerization of aniline and the formation of supramolecular polyaniline structures. Polym. Int. 2008, 57, 1295–1325. [Google Scholar] [CrossRef]
- Loos, K. Enzymatic Synthesis of Polyaniline and Other Electrically Conductive Polymers. In Biocatalysis in Polymer Chemistry; Wiley-VCH: Weinheim, Germany, 2010; ISBN 9783527326181. [Google Scholar]
- Cruz-Silva, R.; Romero-García, J.; Angulo-Sánchez, J.L.; Ledezma-Pérez, A.; Arias-Marín, E.; Moggio, I.; Flores-Loyola, E. Template-free enzymatic synthesis of electrically conducting polyaniline using soybean peroxidase. Eur. Polym. J. 2005, 41, 1129–1135. [Google Scholar] [CrossRef]
- Woggon, W.-D. Metalloporphyrines as Active Site AnaloguesLessons from Enzymes and Enzyme Models. Acc. Chem. Res. 2005, 38, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Akkara, J.A.; Wang, J.; Yang, D.P.; Gonsalves, K.E. Hematin-catalyzed polymerization of phenol compounds. Macromolecules 2000, 33, 2377–2382. [Google Scholar] [CrossRef]
- Roy, S.; Fortier, J.M.; Nagarajan, R.; Tripathy, S.; Kumar, J.; Samuelson, L.A.; Bruno, F.F. Biomimetic synthesis of a water soluble conducting molecular complex of polyaniline and lignosulfonate. Biomacromolecules 2002, 3, 937–941. [Google Scholar] [CrossRef]
- Nabid, M.R.; Sedghi, R.; Jamaat, P.R.; Safari, N.; Entezami, A.A. Synthesis of conducting water-soluble polyaniline with iron(III) porphyrin. J. Appl. Polym. Sci. 2006, 102, 2929–2934. [Google Scholar] [CrossRef]
- Nabid, M.R.; Entezami, A.A. Comparative study on the enzymatic polymerization of N-substituted aniline derivatives. Polym. Adv. Technol. 2005, 16, 305–309. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, Y.Y.; Tang, K.; Zou, G.L. Hemoglobin-biocatalysts synthesis of a conducting molecular complex of polyaniline and sulfonated polystyrene. Synth. Met. 2005, 150, 1–7. [Google Scholar] [CrossRef]
- Xue, T.; Jiang, S.; Qu, Y.; Su, Q.; Cheng, R.; Dubin, S.; Chiu, C.Y.; Kaner, R.; Huang, Y.; Duan, X. Graphene-supported hemin as a highly active biomimetic oxidation catalyst. Angew. Chem. Int. Ed. 2012, 51, 3822–3825. [Google Scholar] [CrossRef] [PubMed]
- Cortez, I.E.M.; González, V.A.G.; García, J.R.; Silva, R.C. Hematina como catalizador biomimético en la síntesis de polianilina conductora. Ingenierías 2007, 10, 36–43. [Google Scholar]
- Tierrablanca, E.; Romero-García, J.; Roman, P.; Cruz-Silva, R. Biomimetic polymerization of aniline using hematin supported on halloysite nanotubes. Appl. Catal. A Gen. 2010, 381, 267–273. [Google Scholar] [CrossRef]
- Zhang, Y.; Xia, Z.; Liu, H.; Yang, M.; Lin, L.; Li, Q. Hemin-graphene oxide-pristine carbon nanotubes complexes with intrinsic peroxidase-like activity for the detection of H2O2 and simultaneous determination for Trp, AA, DA, and UA. Sens. Actuators B Chem. 2013, 188, 496–501. [Google Scholar] [CrossRef]
- Javed, M.S.; Shah, S.S.A.; Najam, T.; Siyal, S.H.; Hussain, S.; Saleem, M.; Zhao, Z.; Mai, W. Achieving high-energy density and superior cyclic stability in flexible and lightweight pseudocapacitor through synergic effects of binder-free CoGa2O4 2D-hexagonal nanoplates. Nano Energy 2020, 77, 105276. [Google Scholar] [CrossRef]
- Javed, M.S.; Shaheen, N.; Hussain, S.; Li, J.; Shah, S.S.A.; Abbas, Y.; Ahmad, M.A.; Raza, R.; Mai, W. An ultra-high energy density flexible asymmetric supercapacitor based on hierarchical fabric decorated with 2D bimetallic oxide nanosheets and MOF-derived porous carbon polyhedra. J. Mater. Chem. A 2019, 7, 946–957. [Google Scholar] [CrossRef]
- Wang, H.; Lin, J.; Shen, Z.X. Polyaniline (PANi) based electrode materials for energy storage and conversion. J. Sci. Adv. Mater. Devices 2016, 1, 225–255. [Google Scholar] [CrossRef]
- Li, Z.; Gong, L. Research Progress on Applications of Polyaniline (PANI) for Electrochemical Energy Storage and Conversion. Materials 2020, 13, 548. [Google Scholar] [CrossRef]
- Bryan, A.M.; Santino, L.M.; Lu, Y.; Acharya, S.; D’Arcy, J.M. Conducting Polymers for Pseudocapacitive Energy Storage. Chem. Mater. 2016, 28, 5989–5998. [Google Scholar] [CrossRef]
- Li, H.; Wang, J.; Chu, Q.; Wang, Z.; Zhang, F.; Wang, S. Theoretical and experimental specific capacitance of polyaniline in sulfuric acid. J. Power Sources 2009, 190, 578–586. [Google Scholar] [CrossRef]
- Li, C.; Bai, H.; Shi, G. Conducting polymer nanomaterials: Electrosynthesis and applications. Chem. Soc. Rev. 2009, 38, 2397. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.K.; Barai, K.; Munichandraiah, N. High capacitance properties of polyaniline by electrochemical deposition on a porous carbon substrate. Electrochim. Acta 2007, 52, 3258–3264. [Google Scholar] [CrossRef]
- Yan, Y.; Cheng, Q.; Zhu, Z.; Pavlinek, V.; Saha, P.; Li, C. Controlled synthesis of hierarchical polyaniline nanowires/ordered bimodal mesoporous carbon nanocomposites with high surface area for supercapacitor electrodes. J. Power Sources 2013, 240, 544–550. [Google Scholar] [CrossRef]
- Galpaya, D.; Wang, M.; Liu, M.; Motta, N.; Waclawik, E.; Yan, C. Recent Advances in Fabrication and Characterization of Graphene-Polymer Nanocomposites. Graphene 2012, 1, 30–49. [Google Scholar] [CrossRef]
- Cao, H.; Zhou, X.; Zhang, Y.; Chen, L.; Liu, Z. Microspherical polyaniline/graphene nanocomposites for high performance supercapacitors. J. Power Sources 2013, 243, 715–720. [Google Scholar] [CrossRef]
- Ye, S.; Wang, R.; Wu, M.-Z.; Yuan, Y.-P. A review on g-C3N4 for photocatalytic water splitting and CO2 reduction. Appl. Surf. Sci. 2015, 358, 15–27. [Google Scholar] [CrossRef]
- Sigurdson, G.T.; Giusti, M.M. Bathochromic and hyperchromic effects of aluminum salt complexation by anthocyanins from edible sources for blue color development. J. Agric. Food Chem. 2014, 62, 6955–6965. [Google Scholar] [CrossRef]
- Guo, Y.; Li, J.; Dong, S. Hemin functionalized graphene nanosheets-based dual biosensor platforms for hydrogen peroxide and glucose. Sens. Actuators B Chem. 2011, 160, 295–300. [Google Scholar] [CrossRef]
- Yu, S.; Chen, L.; Yan, Z. Graphene/Hemin Hybrid Material as a Catalyst for Degradation of Alkaline Lignin with Hydrogen Peroxide. BioResources 2017, 12, 2354–2366. [Google Scholar] [CrossRef]
- Kong, F.Y.; Li, W.W.; Wang, J.Y.; Fang, H.L.; Fan, D.H.; Wang, W. Direct electrolytic exfoliation of graphite with hemin and single-walled carbon nanotube: Creating functional hybrid nanomaterial for hydrogen peroxide detection. Anal. Chim. Acta 2015, 884, 37–43. [Google Scholar] [CrossRef]
- Trchová, M.; Stejskal, J. Polyaniline: The infrared spectroscopy of conducting polymer nanotubes (IUPAC Technical Report). Pure Appl. Chem. 2011, 83, 1803–1817. [Google Scholar] [CrossRef]
- Mostafaei, A.; Zolriasatein, A. Synthesis and characterization of conducting polyaniline nanocomposites containing ZnO nanorods. Prog. Nat. Sci. Mater. Int. 2012, 22, 273–280. [Google Scholar] [CrossRef]
- Gautam, V.; Srivastava, A.; Singh, K.P.; Yadav, V.L. Preparation and characterization of polyaniline, multiwall carbon nanotubes, and starch bionanocomposite material for potential bioanalytical applications. Polym. Compos. 2017, 38, 496–506. [Google Scholar] [CrossRef]
- Ahmad, S.; Sultan, A.; Raza, W.; Muneer, M.; Mohammad, F. Boron nitride based polyaniline nanocomposite: Preparation, property, and application. J. Appl. Polym. Sci. 2016, 133, 43989. [Google Scholar] [CrossRef]
- Tran, V.C.; Nguyen, V.H.; Nguyen, T.T.; Lee, J.H.; Huynh, D.C.; Shim, J.-J. Polyaniline and multi-walled carbon nanotube-intercalated graphene aerogel and its electrochemical properties. Synth. Met. 2016, 215, 150–157. [Google Scholar] [CrossRef]
- Shao, D.; Hou, G.; Li, J.; Wen, T.; Ren, X.; Wang, X. PANI/GO as a super adsorbent for the selective adsorption of uranium(VI). Chem. Eng. J. 2014, 255, 604–612. [Google Scholar] [CrossRef]
- Jia, M.; Fang, M.; Jin, Y. In situ one-pot synthesis of graphene–polyaniline nanofiber composite for high-performance electrochemical capacitors. Appl. Surf. Sci. 2014, 308, 333–340. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, P.; Joshi, C.; Manchanda, M.; Boukherroub, R.; Jain, S.L. Nickel decorated on phosphorous-doped carbon nitride as an efficient photocatalyst for reduction of nitrobenzenes. Nanomaterials 2016, 6, 59. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, L.L.; Zhao, X.S.; Wu, J. Graphene/polyaniline nanofiber composites as supercapacitor electrodes. Chem. Mater. 2010, 22, 1392–1401. [Google Scholar] [CrossRef]
- Shishov, M.A.; Moshnikov, V.A.; Sapurina, I.Y. Self-organization of polyaniline during oxidative polymerization: Formation of granular structure. Chem. Pap. 2013, 67, 909–918. [Google Scholar] [CrossRef]
- Yilmaz, F. Polyaniline: Synthesis, Characterization, Solution Properties and Composites. Ph.D. Thesis, Middle East Technical University, Ankara, Turkey, 2007. [Google Scholar]
- Boeva, Z.A.; Sergeyev, V.G. Polyaniline: Synthesis, properties, and application. Polym. Sci. Ser. C 2014, 56, 144–153. [Google Scholar] [CrossRef]
- Yang, Q.-L. Synthesis and Characterization of Conducting Polyaniline/γ–Fe2O3 magnetic nanocomposite. Synth. Met. 2003, 135–136, 819–820. [Google Scholar] [CrossRef]
- Cruz-Silva, R.; Roman, P.; Romero, J. Enzymatic synthesis of polyaniline and other electrically conductive polymers. In Biocatalysis in Polymer Chemistry; Wiley: Hoboken, NJ, USA, 2010; pp. 187–210. [Google Scholar] [CrossRef]
- Halper, M.; Ellenbogen, J. Supercapacitors: A Brief Overview; Report No. MP 05W0000272; MITRE: McLean, WV, USA, 2006; pp. 1–29. [Google Scholar]
- Beéguin, F.; Frąckowiak, E. Supercapacitors: Materials, Systems, and Applications; Wiley-VCH: Weinheim, Germany, 2013; ISBN 3527328831. [Google Scholar]
- Ansari, S.A.; Ansari, M.O.; Cho, M.H. Facile and Scale Up Synthesis of Red Phosphorus-Graphitic Carbon Nitride Heterostructures for Energy and Environment Applications. Sci. Rep. 2016, 6, 27713. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, X.; Guan, J.; Jiang, Y.; Hou, T.; Mu, X. Facile synthesis of phosphorus doped graphitic carbon nitride polymers with enhanced visible-light photocatalytic activity. Mater. Res. Bull. 2013, 48, 3485–3491. [Google Scholar] [CrossRef]
- Pruneanu, S.; Veress, E.; Marian, I.; Oniciu, L. Characterization of polyaniline by cyclic voltammetry and UV-Vis absorption spectroscopy. J. Mater. Sci. 1999, 34, 2733–2739. [Google Scholar] [CrossRef]
- Pyun, S.-I.; Shin, H.-C.; Lee, J.-W.; Go, J.-Y. Electrochemistry of Insertion Materials for Hydrogen and Lithium; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar] [CrossRef]
- Gopalakrishnan, K.; Sultan, S.; Govindaraj, A.; Rao, C.N.R. Supercapacitors based on composites of PANI with nanosheets of nitrogen-doped RGO, BC1.5N, MoS2 and WS2. Nano Energy 2015, 12, 52–58. [Google Scholar] [CrossRef]
- Vivekchand, S.R.C.; Sekhar Rout, C.; Subrahmanyam, K.S.; Govindaraj, A.; Rao, R. Graphene-based electrochemical supercapacitors. J. Chem. Sci 2008, 120, 9–13. [Google Scholar] [CrossRef]
- Brownson, D.A.C.; Banks, C.E. The Handbook of Graphene Electrochemistry; Springer: London, UK, 2014; ISBN 9781447164289. [Google Scholar]
- Skotheim, T.A.; Reynolds, J.R. Handbook of Conducting Polymers: Conjugated Polymers Processing and Applications; CRC Press: Boca Raton, FL, USA, 2007; ISBN 9781420043587. [Google Scholar]
- Lai, L.; Yang, H.; Wang, L.; Teh, B.K.; Zhong, J.; Chou, H.; Chen, L.; Chen, W.; Shen, Z.; Ruoff, R.S.; et al. Preparation of Supercapacitor Electrodes through Selection of Graphene Surface Functionalities. ACS Nano 2012, 6, 5941–5951. [Google Scholar] [CrossRef]
- Karthika, P. Functionalized Exfoliated Graphene Oxide as Supercapacitor Electrodes. Soft Nanosci. Lett. 2012, 02, 59–66. [Google Scholar] [CrossRef] [Green Version]
- Moussa, M.; El-Kady, M.F.; Wang, H.; Michimore, A.; Zhou, Q.; Xu, J.; Majeswki, P.; Ma, J. High-performance supercapacitors using graphene/polyaniline composites deposited on kitchen sponge. Nanotechnology 2015, 26, 075702. [Google Scholar] [CrossRef]
- Gao, Y. Graphene and Polymer Composites for Supercapacitor Applications: A Review. Nanoscale Res. Lett. 2017, 12, 387. [Google Scholar] [CrossRef] [PubMed]
- Scully, J.R.; Silverman, D.C.; Kendig, M.W. Electrochemical Impedance: Analysis and Interpretation; ASTM: West Conshohocken, PA, USA, 1993; ISBN 0803118619. [Google Scholar]
- Brett, C.; Brett, A.M. The Interfacial Region. In Electrochemistry; Oxford University Press Inc.: Oxford, UK, 1993; pp. 39–68. [Google Scholar]
- Ebrahim, S.A.; Harb, M.E.; Soliman, M.M.; Tayel, M.B. Preparation and characterization of a pseudocapacitor electrode by spraying a conducting polymer onto a flexible substrate. J. Taibah Univ. Sci. 2016, 10, 281–285. [Google Scholar] [CrossRef]
- Ryan, T.H. Electrochemical Detectors: Fundamental Aspects and Analytical Applications; Springer: New York, NY, USA, 1984; ISBN 9781461593973. [Google Scholar]
- Fan, X.; Yang, Z.; Liu, Z. One-Step Synthesis of Graphene/Polyaniline Nanotube Composite for Supercapacitor Electrode. Chinese J. Chem. 2016, 34, 107–113. [Google Scholar] [CrossRef]
- Yan, J.; Wei, T.; Fan, Z.; Qian, W.; Zhang, M.; Shen, X.; Wei, F. Preparation of graphene nanosheet/carbon nanotube/polyaniline composite as electrode material for supercapacitors. J. Power Sources 2010, 195, 3041–3045. [Google Scholar] [CrossRef]
- Mahmood, A.; Zhao, B.; Javed, M.S.; He, D.; Cheong, W.; Han, D.; Niu, L. Unprecedented Dual Role of Polyaniline for Enhanced Pseudocapacitance of Cobalt–Iron Layered Double Hydroxide. Macromol. Rapid Commun. 2022, 43, 2100905. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Deng, J.; Li, X.; Yang, Z.; He, S.; Chen, X.; Guan, G.; Ren, J.; Peng, H. Superelastic Supercapacitors with High Performances during Stretching. Adv. Mater. 2015, 27, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.; Dong, L.; Wang, J.; Ma, X.; Xu, C.; Kang, F. Facile Preparation of High-Performance Stretchable Fiber-Like Electrodes and Supercapacitors. ChemistrySelect 2018, 3, 4179–4184. [Google Scholar] [CrossRef]
- Thomas, L.; Pete, S.; Chaitra, K.; Venkatesh, K.; Gopalkrishna, B.; Nagaraju, K. Facile synthesis of PANI-MWCNT-Ni(OH)2 ternary composites and study of their performance as electrode material for supercapacitors. Diam. Relat. Mater. 2020, 106, 107853. [Google Scholar] [CrossRef]
- Deyab, M.A.; Mele, G. PANI@Co-Porphyrins composite for the construction of supercapacitors. J. Energy Storage 2019, 26, 101013. [Google Scholar] [CrossRef]
- Dasdevan, N.; Abdah, M.A.A.M.; Sulaiman, Y. Facile Electrodeposition of Poly(3,4-ethylenedioxythiophene) on Poly(vinyl alcohol) Nanofibers as the Positive Electrode for High-Performance Asymmetric Supercapacitor. Energies 2019, 12, 3382. [Google Scholar] [CrossRef]
- Khoh, W.H.; Wee, B.H.; Hong, J.D. High performance flexible solid-state asymmetric supercapacitor composed of a polyaniline/PEDOT/polyaniline/ultralarge reduced graphene oxide tetralayer film and a PEDOT/MoS2 composite film. Colloids Surfaces A Physicochem. Eng. Asp. 2019, 581, 123815. [Google Scholar] [CrossRef]
- Li, K.; Huang, Y.; Liu, J.; Sarfraz, M.; Agboola, P.O.; Shakir, I.; Xu, Y. A three-dimensional graphene framework-enabled high-performance stretchable asymmetric supercapacitor. J. Mater. Chem. A 2018, 6, 1802–1808. [Google Scholar] [CrossRef]
- Srinivasan, R.; Elaiyappillai, E.; Anandaraj, S.; Duvaragan, B.K.; Johnson, P.M. Study on the electrochemical behavior of BiVO4/PANI composite as a high performance supercapacitor material with excellent cyclic stability. J. Electroanal. Chem. 2020, 861, 113972. [Google Scholar] [CrossRef]
- Srinivasan, R.; Elaiyappillai, E.; Nixon, E.J.; Sharmila Lydia, I.; Johnson, P.M. Enhanced electrochemical behaviour of Co-MOF/PANI composite electrode for supercapacitors. Inorg. Chim. Acta 2020, 502, 119393. [Google Scholar] [CrossRef]
- Nallappan, M.; Gopalan, M. Fabrication of CeO2/PANI composites for high energy density supercapacitors. Mater. Res. Bull. 2018, 106, 357–364. [Google Scholar] [CrossRef]



| Material | Conductivity (S/cm) | Cs (F/g) 0.2 A/g | Cs (F/g) 5 A/g |
|---|---|---|---|
| Free PANI | 0.0045 | 322 | 82 |
| PANI/g-OCN | 0.0171 | 361 | 165 |
| PANI/g-POCN | 0.0375 | 450.5 | 294 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Cartagena, M.E.; Bernal-Martínez, J.; Banda-Villanueva, A.; Enríquez-Medrano, J.; Lechuga-Islas, V.D.; Magaña, I.; Córdova, T.; Morales-Acosta, D.; Olivares-Romero, J.L.; Díaz-de-León, R. Biomimetic Synthesis of PANI/Graphitic Oxidized Carbon Nitride for Supercapacitor Applications. Polymers 2022, 14, 3913. https://doi.org/10.3390/polym14183913
Martínez-Cartagena ME, Bernal-Martínez J, Banda-Villanueva A, Enríquez-Medrano J, Lechuga-Islas VD, Magaña I, Córdova T, Morales-Acosta D, Olivares-Romero JL, Díaz-de-León R. Biomimetic Synthesis of PANI/Graphitic Oxidized Carbon Nitride for Supercapacitor Applications. Polymers. 2022; 14(18):3913. https://doi.org/10.3390/polym14183913
Chicago/Turabian StyleMartínez-Cartagena, Manuel Eduardo, Juan Bernal-Martínez, Arnulfo Banda-Villanueva, Javier Enríquez-Medrano, Víctor D. Lechuga-Islas, Ilse Magaña, Teresa Córdova, Diana Morales-Acosta, José Luis Olivares-Romero, and Ramón Díaz-de-León. 2022. "Biomimetic Synthesis of PANI/Graphitic Oxidized Carbon Nitride for Supercapacitor Applications" Polymers 14, no. 18: 3913. https://doi.org/10.3390/polym14183913
APA StyleMartínez-Cartagena, M. E., Bernal-Martínez, J., Banda-Villanueva, A., Enríquez-Medrano, J., Lechuga-Islas, V. D., Magaña, I., Córdova, T., Morales-Acosta, D., Olivares-Romero, J. L., & Díaz-de-León, R. (2022). Biomimetic Synthesis of PANI/Graphitic Oxidized Carbon Nitride for Supercapacitor Applications. Polymers, 14(18), 3913. https://doi.org/10.3390/polym14183913