Functional Properties and Storage Stability of Astaxanthin-Loaded Polysaccharide/Gelatin Blend Films—A Comparative Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Film Preparation
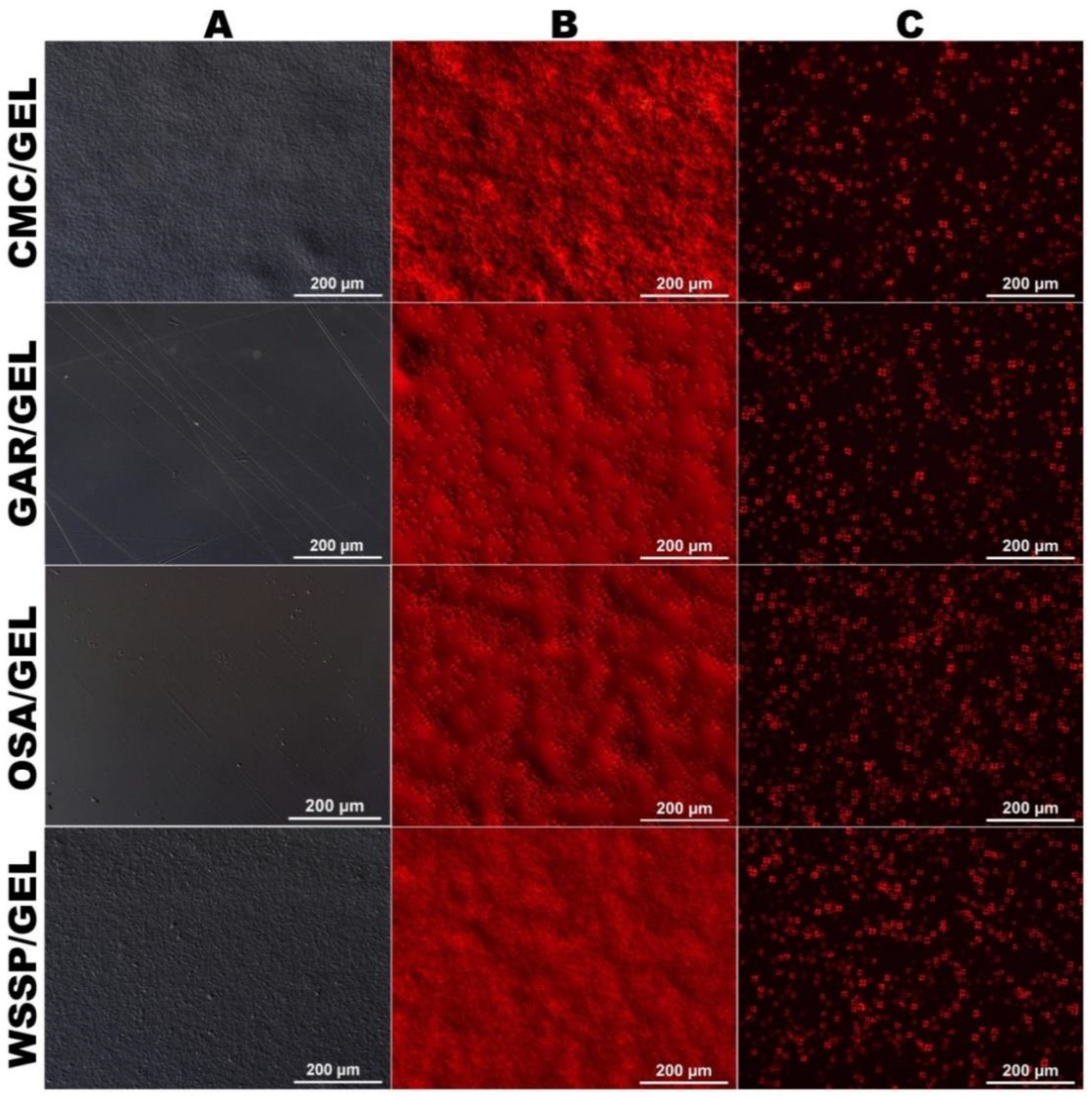
2.3. Microscopic Analysis
2.4. Moisture Content (MC)
2.5. Mechanical Properties
2.6. Light Transmission (T)
2.7. Colour Stability
2.8. Impact of Storage on the Antioxidant Activity of the Films
2.9. Statistical Analysis
3. Results and Discussion
3.1. Microstructure
3.2. MC
3.3. Mechanical Properties
3.4. T
3.5. Colour Stability at Room Temperature
3.6. Accelerated Colour Stability Test
3.7. Impact of Storage on the Antioxidant Activity of the Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lim, G.B.; Lee, S.Y.; Lee, E.K.; Haam, S.J.; Kim, W.S. Separation of astaxanthin from red yeast Phaffia rhodozyma by supercritical carbon dioxide extraction. Biochem. Eng. J. 2002, 11, 181–187. [Google Scholar] [CrossRef]
- McCall, B.; McPartland, C.K.; Moore, R.; Frank-Kamenetskii, A.; Booth, B.W. Effects of astaxanthin on the proliferation and migration of breast cancer cells in vitro. Antioxidants 2018, 7, 135. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kim, H. Inhibitory effect of astaxanthin on oxidative stress-induced mitochondrial dysfunction—A mini-review. Nutrients 2018, 10, 1137. [Google Scholar] [CrossRef] [PubMed]
- Giannaccare, G.; Pellegrini, M.; Senni, C.; Bernabei, F.; Scorcia, V.; Cicero, A.F.G. Clinical applications of astaxanthin in the treatment of ocular diseases: Emerging insights. Mar. Drugs 2020, 18, 239. [Google Scholar] [CrossRef]
- Colín-Chávez, C.; Soto-Valdez, H.; Peralta, E.; Lizardi-Mendoza, J.; Balandrán-Quintana, R. Fabrication and Properties of Antioxidant Polyethylene-based Films Containing Marigold (Tagetes erecta) Extract and Application on Soybean Oil Stability. Packag. Technol. Sci. 2013, 29, 399–412. [Google Scholar] [CrossRef]
- Xu, J.; Wei, R.; Jia, Z.; Song, R. Characteristics and bioactive functions of chitosan/gelatin-based film incorporated with ε-polylysine and astaxanthin extracts derived from by-products of shrimp (Litopenaeus vannamei). Food Hydrocoll. 2020, 100, 105436. [Google Scholar] [CrossRef]
- Inthamat, P.; Boonsiriwit, A.; Lee, Y.S.; Siripatrawan, U. Effects of genipin as natural crosslinker on barrier and mechanical properties of chitosan-astaxanthin film. J. Food Process. Preserv. 2021, 46, e15707. [Google Scholar] [CrossRef]
- Łupina, K.; Kowalczyk, D.; Lis, M.; Raszkowska-Kaczor, A.; Drozłowska, E. Controlled release of water-soluble astaxanthin from carboxymethyl cellulose/gelatin and octenyl succinic anhydride starch/gelatin blend films. Food Hydrocoll. 2021, 123, 107179. [Google Scholar] [CrossRef]
- Łupina, K.; Kowalczyk, D.; Drozłowska, E. Polysaccharide/gelatin blend films as carriers of ascorbyl palmitate—A comparative study. Food Chem. 2020, 333, 127465. [Google Scholar] [CrossRef]
- Kowalczyk, D.; Pytka, M.; Szymanowska, U.; Skrzypek, T.; Łupina, K.; Biendl, M. Release kinetics and antibacterial activity of potassium salts of iso-α-acids loaded into the films based on gelatin, carboxymethyl cellulose and their blends. Food Hydrocoll. 2020, 109, 106104. [Google Scholar] [CrossRef]
- Łupina, K.; Kowalczyk, D.; Kazimierczak, W. Gum Arabic/gelatin and water-soluble soy polysaccharides/gelatin blend films as carriers of astaxanthin—A comparative study of the kinetics of release and antioxidant properties. Polymers 2021, 13, 1062. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Liu, C.; Huang, J.; Zheng, X.; Liu, S.; Lu, K.; Tang, K.; Liu, J. Heat sealable soluble soybean polysaccharide/gelatin blend edible films for food packaging applications. Food Packag. Shelf Life 2020, 24, 100485. [Google Scholar] [CrossRef]
- Łupina, K.; Kowalczyk, D.; Zięba, E.; Kazimierczak, W.; Mężyńska, M.; Basiura-Cembala, M.; Wiącek, A.E. Edible films made from blends of gelatin and polysaccharide-based emulsifiers—A comparative study. Food Hydrocoll. 2019, 96, 555–567. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, L.; Li, D.; Wang, Y.; Chen, Z.; Zou, C.; Liu, W.; Ma, Y.; Cao, M.J.; Liu, G.M. Re-assembled oleic acid-protein complexes as nano-vehicles for astaxanthin: Multispectral analysis and molecular docking. Food Hydrocoll. 2020, 103, 105689. [Google Scholar] [CrossRef]
- Gökkaya Erdem, B.; Dıblan, S.; Kaya, S. A Comprehensive Study on Sorption, Water Barrier, and Physicochemical Properties of Some Protein- and Carbohydrate-Based Edible Films. Food Bioprocess Technol. 2021, 14, 2161–2179. [Google Scholar] [CrossRef]
- Poletto, M.; Pistor, V.; Zattera, A.J. Structural Characteristics and Thermal Properties of Native Cellulose. In Cellulose—Fundamental Aspects; van de Ven, T., Godbout, L., Eds.; IntechOpen: Rijeka, Croatia, 2013; pp. 45–68. ISBN 978-953-51-1183-2. [Google Scholar]
- Shrivastava, A. Polymerization. In Introduction to Plastics Engineering; William Andrew: Norwich, NY, USA, 2018; pp. 17–48. ISBN 9780323396196. [Google Scholar]
- Montenegro, M.; Boiero, L.; Valle, L.; Borsarelli, C. Gum Arabic: More Than an Edible Emulsifier. In Products and Applications of Biopolymers; Verbeek, C.J.R., Ed.; InTech: Rijeka, Croatia, 2012; pp. 3–26. ISBN 978-953-51-0226-7. [Google Scholar]
- Chen, W.; Duizer, L.; Corredig, M.; Goff, H.D. Addition of soluble soybean polysaccharides to dairy products as a source of dietary fiber. J. Food Sci. 2010, 75, 478–484. [Google Scholar] [CrossRef]
- Kowalczyk, D.; Szymanowska, U.; Skrzypek, T.; Basiura-Cembala, M.; Łupina, K.; Biendl, M. Edible films based on gelatin, carboxymethyl cellulose, and their blends as carriers of potassium salts of iso-α-acids: Structural, physicochemical and antioxidant properties. Food Hydrocoll. 2021, 115, 106574. [Google Scholar] [CrossRef]
- Nieto, M.B. Structure and Function of Polysaccharide Gum-Based Edible Films and Coatings. In Edible Films and Coatings for Food Applications; Huber, K.C., Embuscado, M.E., Eds.; Springer: New York, NY, USA, 2009; pp. 57–112. ISBN 9780387928241. [Google Scholar]
- Mathew, A.P.; Dufresne, A. Plasticized waxy maize starch: Effect of polyols and relative humidity on material properties. Biomacromolecules 2002, 3, 1101–1108. [Google Scholar] [CrossRef]
- de Almeida, V.S.; Barretti, B.R.V.; Ito, V.C.; Malucelli, L.; da Silva Carvalho Filho, M.A.; Demiate, I.M.; Pinheiro, L.A.; Lacerda, L.G. Thermal, morphological, and mechanical properties of regular and waxy maize starch films reinforced with cellulose nanofibers (CNF). Mater. Res. 2020, 23, e20190576. [Google Scholar] [CrossRef]
- Matsumura, Y.; Egami, M.; Satake, C.; Maeda, Y.; Takahashi, T.; Nakamura, A.; Mori, T. Inhibitory effects of peptide-bound polysaccharides on lipid oxidation in emulsions. Food Chem. 2003, 83, 107–119. [Google Scholar] [CrossRef]
- Nakamura, A.; Yoshida, R.; Maeda, H.; Furuta, H.; Corredig, M. Study of the role of the carbohydrate and protein moieties of soy soluble polysaccharides in their emulsifying properties. J. Agric. Food Chem. 2004, 52, 5506–5512. [Google Scholar] [CrossRef] [PubMed]
- Campalani, C.; Causin, V.; Selva, M.; Perosa, A. Fish-Waste-Derived Gelatin and Carbon Dots for Biobased UV-Blocking Films. ACS Appl. Mater. Interfaces 2022, 14, 35148–35156. [Google Scholar] [CrossRef]
- Mehta, M.J.; Kumar, A. Ionic Liquid Assisted Gelatin Films: Green, UV Shielding, Antioxidant, and Antibacterial Food Packaging Materials. ACS Sustain. Chem. Eng. 2019, 7, 8631–8636. [Google Scholar] [CrossRef]
- Elde, A.C.; Pettersen, R.; Bruheim, P.; Järnegren, J.; Johnsen, G. Pigmentation and spectral absorbance signatures in deep-water corals from the Trondheimsfjord, Norway. Mar. Drugs 2012, 10, 1400–1411. [Google Scholar] [CrossRef] [PubMed]
- Mokrzycki, W.; Tatol, M. Color difference Delta E—A survey. Mach. Graph. Vis. 2011, 20, 383–411. [Google Scholar]
- Zha, F.; Dong, S.; Rao, J.; Chen, B. Pea protein isolate-gum Arabic Maillard conjugates improves physical and oxidative stability of oil-in-water emulsions. Food Chem. 2019, 285, 130–138. [Google Scholar] [CrossRef]
- Yang, Y.; Cui, S.W.; Gong, J.; Guo, Q.; Wang, Q.; Hua, Y. A soy protein-polysaccharides Maillard reaction product enhanced the physical stability of oil-in-water emulsions containing citral. Food Hydrocoll. 2015, 48, 155–164. [Google Scholar] [CrossRef]
- Wang, J.; Hu, S.; Nie, S.; Yu, Q.; Xie, M. Reviews on Mechanisms of in Vitro Antioxidant Activity of Polysaccharides. Oxid. Med. Cell. Longev. 2016, 2016, 5692852. [Google Scholar] [CrossRef]
- Abuine, R.; Rathnayake, A.U.; Byun, H.G. Biological activity of peptides purified from fish skin hydrolysates. Fish. Aquat. Sci. 2019, 22, 10. [Google Scholar] [CrossRef]
- Gomez-Guillen, M.C.; Gimenez, B.; Lopez-Caballero, M.E.; Montero, M.P. Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocoll. 2011, 25, 1813–1827. [Google Scholar] [CrossRef] [Green Version]


| Films | AST (%) | MC (%) | TS (MPa) | E (%) | EM (MPa) |
|---|---|---|---|---|---|
| CMC/GEL | 0 | 14.28 ± 0.21 i | 43.04 ± 3.81 h | 22.59 ± 6.53 b | 1242.68 ± 179.48 fg |
| 0.25 | 13.62 ± 0.31 h | 38.36 ± 1.61 g | 20.51 ± 7.06 ab | 1302.23 ± 112.87 g | |
| 0.5 | 12.78 ± 0.24 g | 37.51 ± 2.87 g | 25.20 ± 4.93 bc | 1171.33 ± 135.84 e | |
| 1 | 12.03 ± 0.24 f | 36.88 ± 2.78 g | 12.57 ± 2.94 ab | 1216.89 ± 146.18 ef | |
| GAR/GEL | 0 | 11.58 ± 0.46 ef | 10.31 ± 2.06 bcd | 15.31 ± 4.46 ab | 314.90 ± 96.39 b |
| 0.25 | 11.18 ± 0.43 de | 9.09 ± 2.05 b | 10.21 ± 4.07 a | 231.01 ± 98.31 b | |
| 0.5 | 10.95 ± 0.24 d | 10.28 ± 3.61 bcd | 8.89 ± 4.31 a | 423.41 ± 114.67 c | |
| 1 | 10.14 ± 0.21 bc | 15.62 ± 1.57 f | 14.16 ± 4.13 ab | 673.45 ± 80.14 d | |
| OSA/GEL | 0 | 14.62 ± 0.21 i | 3.26 ± 0.53 a | 139.21 ± 25.21 g | 16.48 ± 5.77 a |
| 0.25 | 12.66 ± 0.64 g | 3.07 ± 0.36 a | 132.08 ± 14.49 fg | 16.16 ± 4.31 a | |
| 0.5 | 11.81 ± 0.66 f | 3.17 ± 0.60 a | 124.53 ± 22.43 f | 19.55 ± 4.87 a | |
| 1 | 10.69 ± 0.48 cd | 2.69 ± 0.26 a | 108.08 ± 14.75 e | 22.98 ± 4.08 a | |
| WSSP/GEL | 0 | 11.01 ± 0.48 de | 13.05 ± 1.47 e | 39.36 ± 8.07 d | 497.80 ± 125.45 c |
| 0.25 | 10.01 ± 0.50 b | 11.63 ± 2.26 cde | 34.56 ± 11.45 cd | 495.57 ± 148.84 c | |
| 0.5 | 9.30 ± 0.31 a | 13.03 ± 2.03 e | 39.33 ± 9.45 d | 421.01 ± 86.66 c | |
| 1 | 9.33 ± 0.53 a | 12.12 ± 1.63 de | 32.88 ± 8.92 cd | 412.73 ± 43.07 c |
| Films | AST (%) | 25 °C | 60 °C | ||
|---|---|---|---|---|---|
| 30 Days | 60 Days | 30 Days | 60 Days | ||
| CMC/GEL | 0 | 0.88 ± 0.12 abc | 0.98 ± 0.21 abcde | 2.24 ± 0.24 a | 2.73 ± 0.38 a |
| 0.25 | 0.91 ± 0.11 abcd | 1.24 ± 0.20 cdefg | 11.10 ± 0.34 jkl | 13.40 ± 0.56 op | |
| 0.5 | 1.45 ± 0.32 fghi | 1.56 ± 0.23 ghij | 5.26 ± 0.37 bc | 5.38 ± 0.49 bc | |
| 1 | 1.11 ± 0.30 cdefg | 0.94 ± 0.16 abcde | 6.23 ± 0.31 cd | 6.79 ± 1.08 de | |
| GAR/GEL | 0 | 0.60 ± 0.14 a | 1.09 ± 0.18 bcdef | 7.72 ± 0.40 ef | 10.59 ± 0.75 ijk |
| 0.25 | 0.64 ± 0.17 ab | 1.66 ± 0.19 hij | 11.89 ± 0.16 lm | 13.67 ± 0.96 opq | |
| 0.5 | 0.58 ± 0.16 a | 1.37 ± 0.20 bcd | 11.25 ± 0.23 kl | 11.99 ± 0.48 lm | |
| 1 | 1.16 ± 0.23 cda | 1.13 ± 0.18 ab | 7.95 ± 0.71 efg | 8.24 ± 0.59 fg | |
| OSA/GEL | 0 | 1.35 ± 0.07 defgh | 1.88 ± 0.12 ijk | 4.78 ± 0.59 b | 9.06 ± 0.40 gh |
| 0.25 | 1.46 ± 0.13 fghij | 2.31 ± 0.23 kl | 12.76 ± 0.27 mno | 15.50 ± 0.71 r | |
| 0.5 | 1.45 ± 0.47 fghi | 2.54 ± 0.16 l | 11.10 ± 0.31 jkl | 14.31 ± 0.57 pqr | |
| 1 | 1.25 ± 0.21 cdefg | 1.91 ± 0.25 jk | 12.10 ± 0.39 lmn | 14.34 ± 1.11 pqr | |
| WSSP/GEL | 0 | 2.57 ± 0.17 l | 3.70 ± 0.14 m | 7.23 ± 0.63 def | 9.73 ± 1.47 hi |
| 0.25 | 2.70 ± 0.34 l | 1.20 ± 0.20 cdefg | 17.20 ± 0.36 s | 19.52 ± 1.02 t | |
| 0.5 | 3.67 ± 0.31 m | 2.54 ± 0.16 g | 13.30 ± 0.37 nop | 14.65 ± 0.91 qr | |
| 1 | 4.41 ± 0.34 n | 4.02 ± 0.20 mn | 9.74 ± 0.37 hi | 10.03 ± 1.63 hij | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łupina, K.; Kowalczyk, D.; Kazimierczak, W. Functional Properties and Storage Stability of Astaxanthin-Loaded Polysaccharide/Gelatin Blend Films—A Comparative Study. Polymers 2022, 14, 4001. https://doi.org/10.3390/polym14194001
Łupina K, Kowalczyk D, Kazimierczak W. Functional Properties and Storage Stability of Astaxanthin-Loaded Polysaccharide/Gelatin Blend Films—A Comparative Study. Polymers. 2022; 14(19):4001. https://doi.org/10.3390/polym14194001
Chicago/Turabian StyleŁupina, Katarzyna, Dariusz Kowalczyk, and Waldemar Kazimierczak. 2022. "Functional Properties and Storage Stability of Astaxanthin-Loaded Polysaccharide/Gelatin Blend Films—A Comparative Study" Polymers 14, no. 19: 4001. https://doi.org/10.3390/polym14194001






