Abstract
Doxorubicin (DOX) is one of the most commonly used drugs in liver cancer. Unfortunately, the traditional chemotherapy with DOX presents many limitations, such as a systematic release of DOX, affecting both tumor tissue and healthy tissue, leading to the apparition of many side effects, multidrug resistance (MDR), and poor water solubility. Furthermore, drug delivery systems’ responsiveness has been intensively studied according to the influence of different internal and external stimuli on the efficiency of therapeutic drugs. In this review, we discuss both internal stimuli-responsive drug-delivery systems, such as redox, pH and temperature variation, and external stimuli-responsive drug-delivery systems, such as the application of magnetic, photo-thermal, and electrical stimuli, for the controlled release of Doxorubicin in liver cancer therapy, along with the future perspectives of these smart delivery systems in liver cancer therapy.
1. Introduction
Liver cancer is one of the most common types of cancer, ranking in the top five in incidence with up to 1,000,000 cases reported per year worldwide []. From an etiological point of view, the main risk factors that favor liver cancer are viral infection with Hepatitis B and Hepatitis C, liver cirrhosis, obesity, and type II diabetes [,]. There is information linking the increased incidence of developing liver cancer by up to 50% in patients with cirrhosis []. Along with liver cancer, cirrhosis results from a repercussion of different conditions, such as chronic viral hepatitis, alcohol intake, fatty liver disease, and other liver dysfunction [,]. The principal malignancy of the liver accounting for more than 5% of all types of liver cancers that occurs predominantly in patients with underlying chronic liver disease such as cirrhosis is hepatocellular carcinoma (HCC) []. Unfortunately, liver cancer is most often diagnosed in the terminal phase because the early stages have an asymptomatic nature [,,]. The average life expectancy of patients without treatment is 1–3 months and for patients with treatment is 6–20 months [,]. The most used method of treating cancer is to surgically remove the cancerous tissue, but in the case of liver cancer, the survival rate is 47% to 53% []. In addition, chemotherapy and radiotherapy represent other alternatives as cancer treatments []. Chemotherapy is a mandatory part of clinical cancer treatment, through the administration of antitumoral drugs in order to inhibit the growth of the tumoral cells and generate cellular apoptosis. The recently reported studies have shown poor results correlated with chemotherapy []. Unfortunately, the administration of anticancer agents is performed systemically, and the antitumor agent can affect both tumor cells and healthy cells. This can cause both side effects and poor treatment effectiveness due to the off-target phagocytic uptake and nonspecific biodistribution of the drugs []. Another disadvantage of chemotherapy is multidrug resistance (MDR), which is closely related to tumor recurrence and therapeutic failure, and poor water-solubility [,]. The most used antitumoral agent in the chemotherapy treatment for HCC is Doxorubicin (DOX), along with chemotherapeutic drugs, such as 5-fluorouracil and cisplatin [,,].
Doxorubicin (DOX) (Figure 1), isolated from Streptomyces peucetius, is one of the most commonly used drugs in cancer treatment, including breast, bile ducts, prostate, uterus, ovary, esophagus, stomach, and liver cancer [,,,]. DOX was one of the first antitumoral agents used in liver cancer treatment []. It was reported that the antitumor activity of doxorubicin is due to its ability to intercalate into the DNA helix and/or bind covalently to proteins involved in DNA replication and transcription, resulting in an inhibitory effect on DNA, RNA, and proteins’ synthesis, leading to cell death [,]. However, the limitations of DOX are drug resistance and the side effects, mainly because of its toxicity on non-cancerous cells. The main side effects of the usage of DOX are nausea, vomiting, myelosuppression, and arrhythmia in a very short time after administration []. Unfortunately, this cellular damage caused by DOX administration not only occurs in cancer cells but also in healthy cells, such as a DNA alteration caused by the presence of Adriamycin, which leads to a slowing or stopping of the cells’ growth [,]. Furthermore, studies have shown that the use of DOX in conventional chemotherapy can lead to cardiotoxicity through increased oxidative stress, affecting the heart tissue and leading to cardiomyopathy [,]. Nowadays, researchers are developing new forms of drugs able to optimize the pharmacodynamics and pharmacokinetics that specially target the tumor tissue, without affecting the surrounding healthy tissues [,].
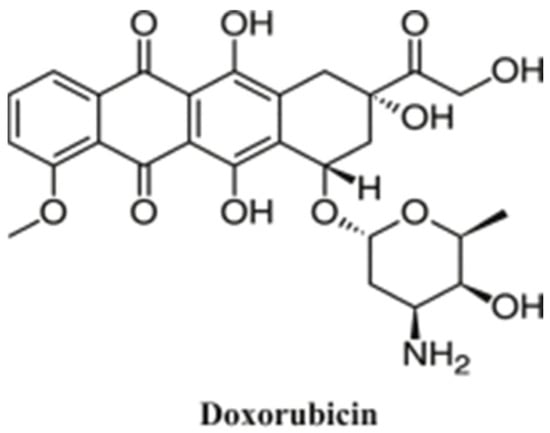
Figure 1.
Chemical structure of DOX (Reprinted with permission from Ref. []. 2019, Gopalakrishna Pillai).
Materials science with its interdisciplinary character has provided a lot of solutions in the field of biomedical sciences for different applications in recent decades, solving a lot of problems that were previously impossible to address. In the twentieth century, membranes for hemodialysis appeared for kidney failure disease [,], a large number of materials were discovered for tissue engineering from soft materials [,,] to composites for bone repair and regeneration [,], or even solutions that minimize the impact of the health system through the environment and everyday life in terms of waste. One of the applications that attracted the interest of researchers in the field of materials for drug delivery.
In the hopes of solving the problem related to the efficiency of cancer treatment, various drug delivery systems (DDSs) have been researched in the past few decades, aiming at targeted delivery and controlled release of antitumor agents [,,,,,,]. The principal interest is to develop new drug delivery systems to guarantee a safe administration of the therapeutic agent, in order to improve the delivery efficiency, and not harm the human body by the presence of toxic side-effects [,]. The development of targeted release systems for cancer treatment may overpower the limitations of standard treatment, such as low stability, fast inactivation or degradation in vivo, non-specific toxicity, poor solubility, unfavorable pharmacokinetics, and low biodistribution []. A multitude of controlled and targeted release systems have been reported in the literature, based on the use of organic [] or inorganic [,] nanoparticles, polymeric micelles [], dendrimers [], nano shells [], and nanotubes []. Smart drug delivery systems are able to respond to a range of diverse stimuli, such as internal stimuli (pH, redox conditions, enzymatic activity, the concentration of specific biomolecules, temperature), or external stimuli (such as applying magnetic fields, electric fields, ultrasound, mechanical pressure, etc.) []. Additionally, many reviews in the domain of liver cancer therapy are continuously published due to the extensive interest in the subject. Some of the latest topics are related to the use of nanoparticles and a multitude of active pharmaceutical compounds [,,,], the nature of materials used for different types of technological solutions for cancer problems related to drug delivery [,,], or medical aspects of liver cancer [,,]. This review generally presents the main recent trends in the obtaining of smart drug-delivery systems field which are internal or external stimuli-responsive for the efficient release of DOX for the treatment of hepatocellular carcinoma. These intelligent release systems are obtained in particular from polymeric materials, which have the role of facilitating the controlled and targeted release of Doxorubicin, reducing systemic toxicity, increasing drug bioavailability, reducing and preventing the many side effects of Doxorubicin, improving selectivity, biocompatibility, dispersibility and the stability of Doxorubicin. A summary of the reviewed studies in tabulated form is presented at the conclusion (Table 1).

Table 1.
Summary of featured stimuli-responsive polymers.
2. Internal Stimuli-Responsive Drug Delivery System
2.1. pH-Responsive Drug Delivery Systems
The variation of pH values in different regions of the human body can provide a suitable physiological stimulus for pH-responsive drug delivery in order to deliver the active substances in the area of interest []. For example, the pH range of the stomach is between 1.5–3.5 pH, 5.5–6.8 pH of the intestine, 6.4–7 pH of the colon, and up to 7.4 pH of the blood []. The pH of cancerous tissue exhibits a decreasing trend due to the Warburg effect, which explained that the hypoxic cells produce lactic acid due to glycolysis []. Further, pH-responsive drug delivery systems have excellent advantages and have attracted much attention in the past decade because the pH values in tumors and inflammatory tissues are significantly lower than those in blood and normal tissues and could increase the therapeutic efficacy of administrated drugs [,,,,]. The main principle of pH-responsive drug delivery systems is to release the active substance when the pH trigger point is achieved, and the intracellular concentration of drugs is equal to the therapeutic dose needed []. This release system time has the role of releasing the active substance preferentially, in order to eliminate the disadvantages of chemotherapy treatment.
One modality to obtain this type of release system is to introduce “ionizable” chemical groups, for example, amines, phosphoric acids, and carboxylic acids among others, with nanomaterials []. These groups, with varying pKa values and chemical structures, have the ability to accept or donate protons and be subjected to pH-dependent alterations in the chemical or physical properties, such as solubility and swelling ratio, culminating in drug release []. Various biomaterials have been reported in the obtaining of pH-responsive drug delivery systems, such as inorganic nanoparticles, core-shell nanoparticles, liposomes, hydrogels, and polymer micelles [,,,]. An example of some widely used materials for the production of pH-sensitive drug delivery systems are pH-sensitive polymers, which are polyelectrolytes with ionizable groups in their backbones, side groups, or end groups. When the pH of an aqueous solution changes, these pH-sensitive polymers are ionized, resulting in a change in their conformation. These “smart” polymers can either accept or donate H+ ions in response to the pH changes in the environment. The protonation or deprotonation of these ionizable groups can lead to changes in the structure of the polymer chain by electrostatic repulsion of the generated charges, which causes the transition of the chains from collapsed to an expanded state []. For example, pH induces protonation/deprotonation in the -NH2 groups of chitosan, making it susceptible to use in obtaining pH-sensitive release systems [,]. At acidic pH (<6), primary amines are protonated and positively charged, making chitosan soluble in an aqueous solution []. In addition to sensitivity to pH, chitosan is low toxicity, biocompatible, has antibacterial properties, mucoadhesive properties, and permeation-enhancing effects [,]. Due to these beneficial characteristics of chitosan, many researchers have used chitosan in various pH-sensitive release systems for liver cancer [,,,,]. Mi et al. [] reported pH-sensitive drug delivery for DOX based on Carboxymethyl-β-Cyclodextrin/Chitosan Nanoparticles. In vitro release showed higher cumulative release rates of DOX from HF-DOX-CD NPs at a low pH, than cumulative release at neutral pH or slightly basic pH due to the tendency of chitosan nanoparticles to swell at low pH, and also, due to the acidic pH, the protons might penetrate the interior and attack the inner secondary bonds of the nanoparticle. These two reasons lead to a release of DOX in an acidic medium. Zhan et al. [] developed pH-sensitive and self-healing properties based on 4armPEGDA and N-carboxyethyl chitosan for liver cancer. The degradation rate was observed to be higher in the case of exposure to an acidic environment indicating that hydrogels have pH-dependent degradation behavior. Cytotoxicity tests were performed on human hepatocytes (L02) and the therapeutic effect of DOX-loaded CEC/4armPEGDA hydrogels on HepG2 cells. The results showed no toxicity for human hepatocyte cells and even at a fairly small load of DOX the obtained system was able to kill tumor cells over time due to the continuous release of DOX in the hydrogel during this process. Qu et al. [] presented the obtaining of a pH-sensitive hydrogel that was able to release DOX into an acidic environment based on N-carboxyethyl chitosan (CEC) and dibenzaldehyde-terminated poly(ethylene glycol) (PEGDA) as a possible treatment for hepatocellular carcinoma. At an acidic pH, the amino groups of chitosan become protonated and positively charged, resulting in weaker -NH2 and -CHO bonds, that also decompose and the release of the DOX takes place.
Furthermore, many polymeric networks have been studied for showing pH-sensitive bioerodible properties such as Poly(Ortho Ester Amides) (POEAd) due to the hydrolysis of ortho esters at low pH []. Yan et al. [] proposed a solution for the promotion of drug accumulation and efficient killing ability of tumoral cells via galactose-grafted on an ultra-pH-sensitive drug carrier (POEAd-g-LA-DOX micelles), which can respond to both intracellular and extracellular pH, to remain stable at pH 7, responding to extracellular tumor pH, conjugating receptors in the cell membrane of liver cancer through surface galactose-ligands of micelles, and being sensitive to intracellular tumor pH following further swelling for rapid drug release. The POEAd-g-LA copolymers were successfully obtained via facile polycondensation followed by the grafting of lactobionic acid. Figure 2 shows the percentage of main chain hydrolysis over time, as well as size and accumulation release over time, at different pH values. In order to assess the influence of pH, Yan carried out an NMR analysis to follow the time-course of the hydrolysis of ortho esters in the main-chain for up to 72 h in deuterated water buffers (pH 7.4, 6.5, and 5.5). The hydrolysis of the ortho ester did not occur at neutral pH, while at pH 5.5 it was observed that the hydrolyzation of ortho esters was accelerated due to the acidic pH, which could lead to a size reduction and drug release. Furthermore, it was highlighted that the ultra-pH-sensitivity of ortho ester can influence the size, observing that at an acidic pH the size of the particles increases, swelling itself, which was probably due to the interaction between the hydrogen bonding interaction of the hydrophilic products with plenty of hydroxyl groups and amide, and gradually increasing hydration. Additionally, the rate of drug release was reported to be higher in the case of an acidic medium, which can thus be a benefit due to the acidic pH of the tumor tissue. The in vivo therapeutic efficacy of the reported formulations was tested in the mice bearing subcutaneous-inoculated H22 tumors, and it was observed that in relation to the mice treated with POEAD-g-LA20-DOX a decrease in the tumor size was reported, reducing the growth, and demonstrating an inhibitory effect on tumoral tissue.
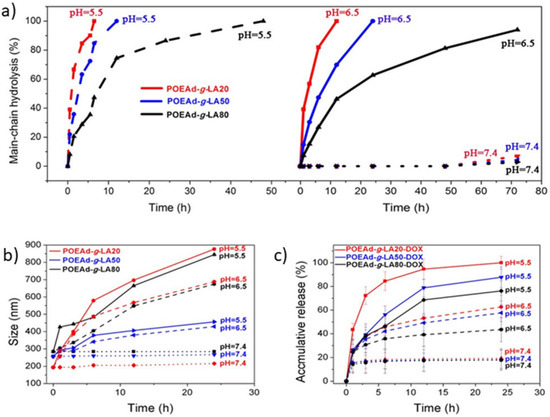
Figure 2.
(a) Hydrolysis kinetics of POEAd-g-LA micelles at pH 7.4, 6.5 and 5.5; (b) Time and pH-dependent changes of average size of POEAd-g-LA micelles in aqueous phosphate buffer measured by DLS; (c) Release kinetics of loaded DOX in POEAd-g-LA micelles at pH 7.4, 6.5, and 5.5 (Reprinted with permission from Ref. []. 2017, Guoqing Yan).
Hyaluronic acid (HA) has been widely used in targeted drug delivery systems due to its good biocompatibility, good water solubility, high selectivity, and affinity to CD44 receptors found in different tumor cells [,]. In order to increase the drug/cargo accumulation specifically in CD44 over-expressing cancer cells, HA-attached pharmaceuticals and nanocarriers have been created. This is because HA has numerous functional groups available for chemical conjugation with anticancer medications or nanocarriers of drugs or genes []. Further, it was reported that DOX could be covalently bounded on the backbone of HA through the hydrazone linkage [,]. Li et al. [] described that hydrazone bonds disintegrated in lysosomal pH and remained stable at neutral pH. Liao et al. [] reported a pH-responsive drug delivery of hyaluronic acid-hydrazone linkage-Doxorubicin (HA-hyd-DOX), which is illustrated in Figure 3. Due to the amphiphilic structure between the hydrophilic glucopyranose ring and hydrophobic DOX segment, a series of HA-hyd-DOX NPs were generated by self-assembling in an aqueous solution. The results showed a burst release in the first 6 h in buffers at pH 5.0 due to a looser structure of particles with the cleavage of hydrazone bonds at a lower pH. The cell cytotoxicity was studied on HeLa and L929 cells for 48 h of incubation and a nontoxicity against HeLa or L929 cells was observed, suggesting that HA is nontoxic, having good biocompatibility. Additionally, an internalization was observed due to the receptor-mediated binding affinity of CD44 for HA with high specificity.
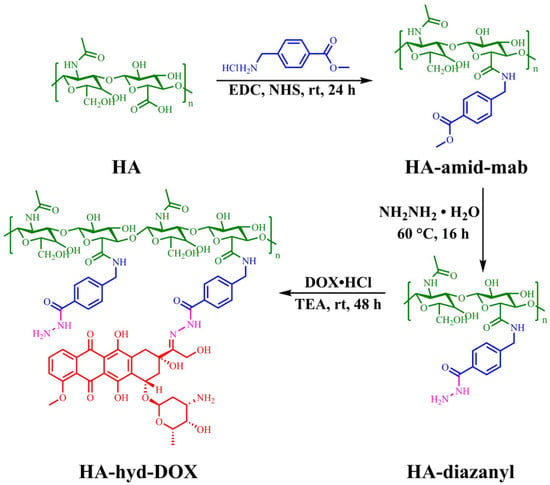
Figure 3.
Synthetic routes of HA-hyd-DOX prodrugs. (Reprinted with permission from Ref. []. 2018, Jianhong Liao).
Wu et al. [] reported on the liver cancer-targeting mixed micelles based on hyaluronic acid–glycyrrhetinic acid conjugate and a hyaluronic acid-L-histidine conjugate S(HA–GA/HA–His) were prepared via ultrasonic dispersion, and the in vitro and in vivo investigation of antitumor effect of Doxorubicin (DOX)-loaded micelles (Figure 4). It was related to the pH sensibility of DOX-loaded HA–GA/HA–His is micelles and the remarkable absorption of HepG2 cells. It was reported that the hydrophobic DOX molecules were efficiently encapsulated into the HA–GA/HA–His micelles in an aqueous solution because of the presence of a hydrophobic core in the micelles. It was shown that the obtained DOX-loaded micelles exhibited a sustained DOX release under the acid pH of hepatocellular carcinoma cells, due to protonation of His, resulting in the swelling of the core, followed by the DOX release.
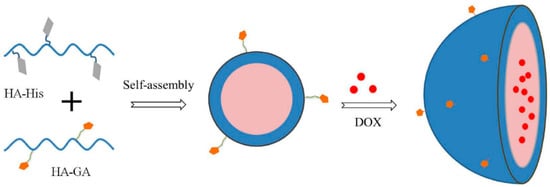
Figure 4.
Preparation of DOX-loaded micelles (Reprinted with permission from Ref. []. 2016, Jing-Liang Wu).
Anirudhan et al. [] delivered DOX and cisplatin (CDDP) via pH-responsive drug delivery, also based on hyaluronic acid (HA) and chitosan (CS-NSA). The release was studied via immersion in two buffer media at pH 7.4 and 5.5 to mimic the intestinal fluid and tumor environment, at 37 °C, for 48 h. A better release of DOX was observed in comparison with cisplatin, for both pH levels. Further, a higher release was observed for both drugs at pH 5.5 (for CS-NSA/A-HA/DOX, the release was 89.0% at pH 5.5 and 42.0% at pH 7.4 and for CS-NSA/A-HA/CDDP, the release was 87.0% at pH 5.5 and 44.6% pH 7.4). Lei et al. [] developed a pH-responsive drug delivery based on HA showed a great release under the endosomal/lysosomal environment for DOX (56.5%) (Figure 5).
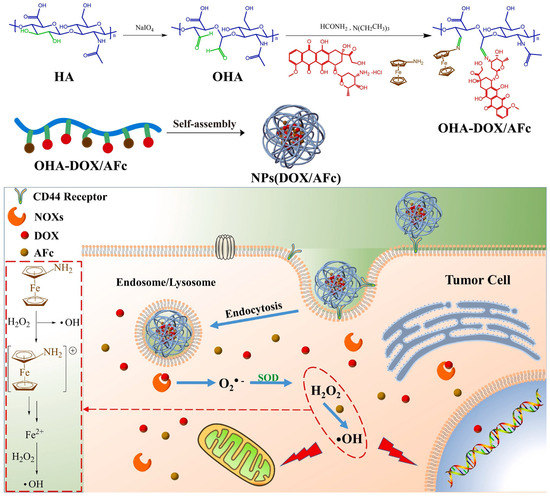
Figure 5.
The schematic illustration of the synthetic routes of OHA-DOX/AFc prodrugs and the schematic diagram of NPs(DOX/AFc) entering tumor cells to play a combined therapeutic effect (Reprinted with permission from Ref. []. 2021, Mengheng Lei).
Folic acid (FA) was reported as an effective targeting prodrug due to the selective binding of FA to the folate receptor (FR), which is overexpressed in cancer cells []. Zeolitic imidazolate framework (ZIF) coordination bonds are sensitive to low pH and this could be used for the delivery of an active substance, such as DOX []. Bi et al. [] reported the delivery of DOX for human hepatocellular carcinoma via folic acid-modified and zeolitic imidazolate framework (ZIF) nanoparticles. It highlighted the pH sensitivity of the obtained nanocarrier; thus, it was observed that drug release of the drug delivery system based on folic acid is increased by the acidic environment. Further, it was reported that the release mechanism of the DOX is influenced by the ZIF degradation in acid environments and the increased solubility of DOX at lower pH as a result of increased protonation of amino groups in DOX molecules. In addition to the previously listed advantages of pH-sensitive systems, the limitations of pH-responsive drug delivery systems are that the pH level is an endogenous stimulus and this makes it difficult to control, a narrow range of pH variation, and steady kinetics for the drug release [,,,]. These limitations can be overcome by the development of multi-responsive drug delivery systems, so that the release is not based only on a single stimulus, such as pH.
2.2. Temperature-Responsive Drug Delivery Systems
One of the most closely studied stimuli of controlled release systems is temperature. Temperature-sensitive polymers are used in order to obtain this responsive DDS, for different biomedical applications, such as temperature-sensitive gels, liposomes, micelles, colloidal particles, mRNA recovery, and gene delivery [,,]. These thermosensitive polymers are able to release the encapsulated active substance even at small temperature variations. Numerous thermosensitive compounds are used for obtaining thermoresponsive hydrogels in DDS applications, such as poly(N-isopro-pylacrylamide (PNIPAAm) derivatives, poly(ethylene oxide)-poly(propylene oxide) (PEO–PPO) pluronic copolymers), core–shell thermoresponsive NPs, polymeric nanotubes, polymeric micelles, layer-by-layer (LBL)-assembled nanocapsules, microbeads (MBs), and elastin-like polypeptides (ELPs) [,,]. However, thermo-responsive release systems can retain the load at systemic circulation temperatures of 37 °C, but release a load rapidly when the temperature exceeds 40 °C, due to the locally heated tumor. These DDS experience a reversible phase transition from a molecularly dissolved hydrated state in an aqueous solution (hydrophilic) to a dehydrated state (hydrophobic) as a reaction to the slight variation in temperature leading to an induced sharp globule-to-coil conversion that generates the release of encapsulated antitumoral agents from these polymeric nanocarriers []. Depending on the critical solution temperature (CST), thermo-responsive polymers are classified into two categories: (i) polymers that have a low critical solution temperature (LCST), which means that these polymers are water-soluble and make homogenous systems below this temperature; and (ii) polymers that have an upper critical solution temperature (UCST), which means that these polymers are water-soluble and make homogenous systems above this temperature [].
Poly(N-isopropyl acrylamide) (PNIPAA)-based materials show thermo–thermo responsiveness behavior which could be very useful in the development of different temperature-sensitive release systems []. Cheng et al. [] developed a PEGylated star-shaped polymer based on the conjugation of the β-CD core with thermosensitive poly(N-isopropyl acrylamide) (PNIPAAm) and biocompatible poly(oligo(ethylene glycol) acrylate) (POEGA) arms in order to obtain temperature-sensitive drug delivery systems for liver cancer. The DOX was used as a model chemotherapeutic in order to study its in vitro cellular uptake. It was shown that only DOX and β-CD-g-(PNIPAAm-b-POEGA)x/DOX at 25 °C have a slow cellular uptake, but when the tempera ture was increased to 37 °C, a faster and higher cellular uptake was reported. Interestingly, at a temperature above LCST, PNIPAAm becomes hydrophobic, having a tendency to aggregate, forming the core of nanoparticles, meanwhile, the POEGA chains help maintain the integrity of the formed nanoparticles. In addition, it was reported at 80% in the first 6 h and 90% release after 24 h of DOX at 37 °C condition; meanwhile, at the 25 °C condition, a slow release of DOX was observed, where after 24 h, the release decreased to 53.9%. In Figure 6, the decrease in the cell viability is reported in the case of β-CD-g-(PNIPAAm-b-POEGA)x/DOX in comparison with neat DOX. The test was performed on HepG2 and H460 cancer cells. The same results were shown in the case of hydrophobic paclitaxel (PTX), which is a hydrophobic anticancer drug. Kunene et al. [] reported pH and temperature-responsive based on magnetic graphene nanosheets (MGNSs), functionalized by poly(N-isopropylacrylamide) (PNIPAM) and polyethylenemine (PEI) nanogel for DOX delivery for liver cancer. The cell viability was reported at above 90% against HEK293 cells and HepG2 cancerous cells. The DOX release was reported higher at pH 5.4 than at pH 7.4 and above, the LCST was rapid, due to the swelling and de-swelling of the PNIPAM/ PEI nanogel at variations of the medium’s temperature.
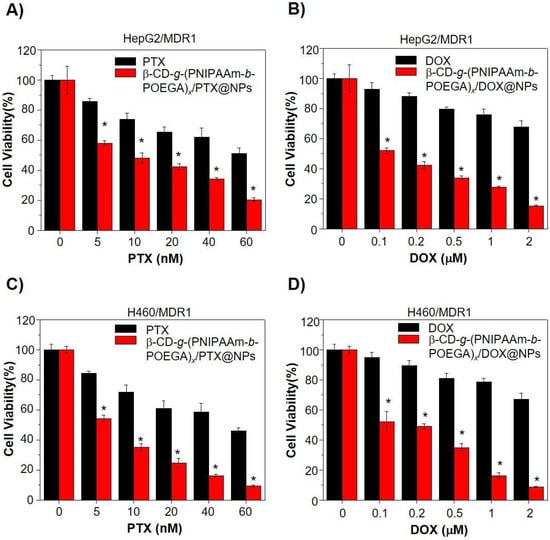
Figure 6.
Cell viability assay to study β-CD-g-(PNIPAAm-b-POEGA)x/drug complex nanoparticles for reversing MDR1-related drug resistance. (A,C) Cell viability of (A) HepG2/MDR1 and (C) H460/MDR1 after 24 h treatment with indicated doses of PTX complex nanoparticles or equivalent PTX only. (B,D) Treatment with DOX complex nanoparticles or equivalent DOX only in (B) HepG2/MDR1 and (D) H460/MDR1 cell lines for 24 h. Data represented in the form of mean ± standard deviation. * p < 0.05, with comparison to growth inhibition of PTX or DOX treatment only, n = 6 (Reprinted with permission from Ref. []. 2018, Hongwei Cheng).
Furthermore, other studies were reported in order to release DOX for liver cancer through temperature-responsive drug delivery. Mdlovu et al. [] described a dual-responsive drug delivery system (pH- and thermo-responsive drug delivery system) based on magnetic iron oxide (MIO) nanoparticles functionalized with Pluronic F127 (PF127) and branched polyethylenimine (bPEI) and loaded with DOX. The DOX releases were dependent on temperature and pH as the highest release rate (54.8%) was in acidic conditions (pH 5.4) and when the temperature was increased from 37 °C to 42 °C an increased rate release (51%) was reported due to the LCST of the PF127 polymer which is 42 °C []. Furthermore, Mdlovu et al. [] described pH- and thermo-sensitive Doxorubicin-conjugated magnetic SBA-15 mesoporous for hepatocellular carcinoma with a release rate of 70% in acidic conditions and 69% at 42 °C (pH = 7.4), which in comparison with previous work, showed an increase in the release values of DOX. Sebeke et al. [] reported thermo-responsive drug delivery based on phosphatidylglycerol (DPPG2) via magnetic resonance-guided high-intensity focused ultrasound (MR-HIFU)-mediated hyperthermia. A rapid release was reported at 42 °C due to the melting temperature of DPPG2-TSL. Classical methods of chemotherapy agents are hemofiltration or plasma filtration [,]. Removal of ~30% of the administered dose was reported and a reduction in the toxicity of the remaining drug in the organism.
2.3. Redox-Responsive Drug Delivery Systems
At this moment, redox-responsive drug delivery systems have been intensely studied, to improve the controlled release of the antitumoral agents, via targeting the tumoral tissue through redox-response in the presence of glutathione (GSH) []. The cancerous tissue presents particular abnormal cellular environments, such as the presence of enzymes and reducing environments []. The main principle of redox-responsive drug delivery systems is employing the distinct differences in redox potentials between tumors and normal tissues. The reducing environment of cancerous tissue is based on the reduction and oxidation state of NADPH/NADP+ and glutathione (GSH, GSH/GSSG) [,]. The most popular redox couple is GSH/glutathione disulfide (GSSG) []. The GSH is a tripeptide of glutamate, cysteine, and glycine found at an increased concentration in ovarian, breast, lung cancer, head and neck cancer, and in lower concentration in brain and liver tumors compared to healthy tissue []. In addition, it was reported that the GSH plays an important role in cell differentiation, proliferation, and apoptosis, and imbalanced values may indicate cancer presence []. The GSH levels can be influenced by oxidative stress, and can act as a biomarker, to indicate the severity of cancer. The highest concentration of GSH values for healthy tissues is in the liver and hepatic GSH plays an important role in interorgan GSH homeostasis by being the main source of plasma GSH. Liver disease can decrease the concentration of GSH values due to multiple factors, such as reduction during oxidative stress, increased utilization and export, and decreased synthesis [].
The main advantages of redox-responsive delivery systems are the stability in contact with the normal tissue, which can decrease the systematic toxicity and side effects, the prompt response to high values of GSH concentration in tumoral cells to release the therapeutic agents, and the release of the therapeutic agent in the cytoplasm, which improves the therapeutic effect []. The main categories of redox-responsive drug delivery systems are disulfide bonds (which are able to break down via reducing glutathione to sulfhydryl group and break the drug delivery system and facilitate the therapeutic agent release) and diselenide bonds (the Se–Se bond and C–Se bond are with lower bond energy than that of the S–S bonds) [,,]. Luo et al. [] reported the pH and redox-responsive drug delivery for DOX via deprotonation/protonation under acidic pH and cleavage of disulfide bonds (Figure 7).
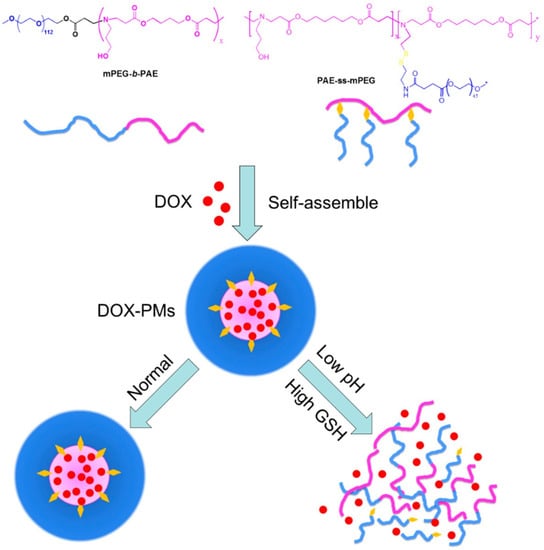
Figure 7.
Co-micellization of pH/redox-responsive diblock copolymers for drug delivery and controlled release triggered by pH and glutathione (GSH). DOX: Doxorubicin; PMs: polymeric micelles. (Reprinted with permission from Ref. []. 2019, Yongle Luo).
These linkages express great stability in the oxidative extracellular medium, the therapeutic agent is released in the increased reductive intracellular compartments by thiol–sulfide exchange reactions []. Chen et al. [] developed a new antibody-targeted and redox-responsive drug delivery system by binding the anti-carbonic anhydrase IX antibody (A-CAIX Ab) on the surface of mesoporous silica nanoparticles (MSNs) via disulfide linkages used as the vehicle to load the chemotherapy drug, Doxorubicin (DOX). In Figure 8, it is shown how the MSNs are used as a vehicle to load the DOX and CAIX grafted on MSNs by disulfide bonds. The in vivo tests performed on mice showed a reduction in tumor weight after only 11 days as well as MSNs loaded with DOX and DOX@MSNs-CAIX.
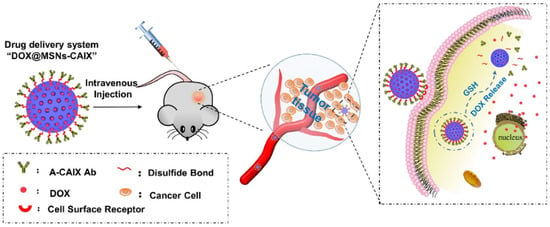
Figure 8.
Illustration of A-CAIX Ab targeted mesoporous silica nanoparticles as a redox-responsive drug delivery system (Reprinted with permission from Ref. []. 2020, Minmin Chen).
Wang et al. [] developed a redox-responsive liposome, capable of probably leading both cancer stem cells and bulk cancer via the incorporation of Salinomycin (Sal), which is a hydrophobic drug, into the lipid layers of the obtained liposomes, and Doxorubicin (DOX), a hydrophilic drug, encapsulated into the aqueous cavity of the liposomes. It was shown that GSH potentially affects the disulfide bonds of obtained liposome lipid layers destabilizing the liposomal nanostructure and the presence of GSH may influence the fast release of DOX and Sel, leading to the synergistic inhibition of tumor growth and reduction in cancer stem cells’ stemness. Mezghani et al. [] described that the introduction of disulfide linkages acts as a burst release constituent and the hydrophobic groups of the glycyrrhetic acid were covalently linked to the hydrophilic backbone of hyaluronic acid over amide bond development, with the mixture of an intermediate disulfide bond as a major component. The resulting swelled nanoparticles from the reduction in the disulfide bonds lead to hydrophobicity modification of the core of nanoparticles conducting to the production of aggregates, which can lead to the deconstruction of the nanoparticle in the deeply reductive environment. In addition, it was observed that the size of the nanoparticles remains unchanged in the absence of GSH, which indicates the stability of the obtained nanoparticles in the non-reductive media. Furthermore, the performed in vivo test showed that the obtained DDS accumulated in hepatic tissue, approving their targeting abilities.
Additionally, Yang et al. [] reported the obtaining of a redox-responsive drug delivery system based on a hyaluronic acid (HA)-grafted polymer loaded with DOX. The HA is conjugated with folic acid (FA) through a reduction-sensitive disulfide linkage in order to design an amphiphilic polymer (HA-ss-FA). Further, cystamine (CYS) was used as a cross-linking agent to link HA and FA. The DOX release was studied in a phosphate buffer solution (PBS), at physiological pH and temperature, in the presence of GSH, in order to simulate the environment of the tumor cells and it was observed that the presence of GSH influenced a faster release of DOX because of the disruption of the disulfide bond in the HA-ss-FA molecules in the reductive environment. Pandey et al. [] presented a dual-stimuli-responsive drug-delivery system based on micelles for cancer therapy using Doxorubicin. The micelles are represented by an amphiphilic biocompatible miktoarm star copolymer comprising two hydrophobic poly(ε-caprolactone) (PCL) blocks, a short poly(propargyl glycine) middle block, and a hydrophilic glycopolypeptide (GP) block containing galactose units for targeting liver cancer cells and were tested for responsiveness via two stimuli, such as redox and enzymes. It was observed that with a higher concentration of GSH, the release of DOX was increased, and in the case of the absence or lower concentration of GSH in the media, the release was radically decreased probably due to the presence of a few lightly cross-linked micelles. Additionally, the TEM images showed that after 24 h, the obtained micelles showed aggregation, and after 48 h the micelles disappeared in the presence of GSH treatment showing that the micellar assembly is only rattled by GSH-mediated cleavage of disulfide bonds. A multifunctional dual-responsive drug delivery system for targeting tumor therapy based on hollow mesoporous nanosilica loaded with Doxorubicin was proposed by Huang et al. []. Cytochrome C (CytC) was used as a sealing agent for mesoporous nanosilica and also as a mediator of apoptosis by recruiting and activating caspase once it is released from the cell mitochondria to the cytoplasm via conjugation with Apoptotic protease activating factor-1 (Apaf-1) in the presence of Deoxyadenosine triphosphate (dATP) which is a nucleotide used for DNA synthesis as a substrate in DNA polymerase [,]. In addition, the usage of lactobionic acid (LA) was utilized as a targeting agent, especially for HepG2 cells due to the particular ligand binding to the asialoglycoprotein receptor (ASGP-R) of HepG2 cells. The purpose of this dual-responsive drug delivery was to create a special delivery of DOX in the presence of glutathione (GSH) and acidic pH in the tumor microenvironment. The drug-release results showed that an increased release was observed in the case of an acidic environment and in the presence of a higher concentration of GSH via the simultaneous breakage of disulfide bonds and disassociation of boronated ester bonds of the system, which can lead to cell apoptosis and tumor growth inhibition []. Saedi et al. [] described a dual-sensitive drug delivery (redox and pH-sensitive) based on a folate-modified star-like amphiphilic copolymer based on castor oil for DOX. The drug release was studied in PBS (pH = 7.4, 2 μM GSH) and ABS (pH = 5.5), with or without 10 mM GSH, and the results showed that at pH 7.4 the release of DOX was slow and inefficient, but at pH 7.4 and 2 μM GSH, the release was approximately 20% and by increasing the concentration of GSH (10 mM GSH), the release was increased up to 39%. The acidic condition also favors the release, such as when at pH 5.5 and 10 mM GSH, the biggest release of DOX was observed. Yan et al. [] described polyethyleneimine (disulfide cross-linked PEI, PSP)/tetrahedral DNA (TDNs)/Doxorubicin (DOX) nanocomplexes (NCs)-based redox-responsive drug delivery system. Figure 9 details the gradually disassembled drug delivery system through the breakage of the disulfide when it interacts with the intracellular high concentration of GSH at the tumor site. Further, the disassembled DDS penetrated the tumoral tissue, improving the therapeutic efficacy. An increase in the release was observed in the presence of an acidic environment and GSH (50%) due to cleavage of the disulfide linkages in the high concentration of GSH.
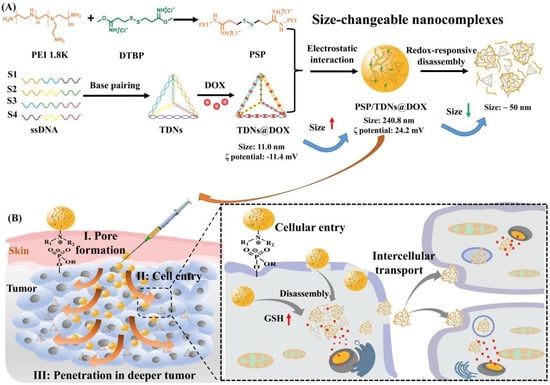
Figure 9.
Schematic of the redox-responsive PEI/TDNs/DOX nanocomplexes with membrane-breaking and size-changeable properties for combating MDR tumors. (A) Synthesis and size-change mechanism of PSP/TDNs@DOX nanocomplexes. (B) Proposed process and underlying mechanism of PSP/TDNs@DOX nanocomplexes overcoming MDR via the formation of pore in plasma membranes, promoted cellular entry and cell-to-cell spread, and enhanced penetration in deeper tumor tissue (Reprinted with permission from Ref. []. 2021, Jianqin Yan).
2.4. Enzyme-Responsive Drug Delivery System
In recent years, many intelligent systems have been studied for the release of Doxorubicin based on enzyme responsiveness [,,,]. Enzymatic-responsive drug delivery systems represent a very promising category, due to the fact that changes in the expression can be found in tumor cells of specific enzymes, such as proteases, phosphatases, and glycosidases, which can be very easily targeted by enzyme-mediated drugs’ release []. The enzyme-responsive drug delivery systems have ester bonds in their composition or the peptide structure that can be degraded by various enzymes specific to inflammation of the tumor location [,]. The main properties of enzyme-responsive drug delivery systems are biorecognition, selectivity, and catalytic efficacy []. Generally, enzymatic-responsive drug delivery systems are obtained from peptide hydrogels, polymers and polymer conjugates, and polymeric nanoparticles, but also from mesoporous silica nanoparticles, metal nanoparticles, and semiconducting nanoparticles [,,]. Enzyme-responsive polymers are used to incorporate the therapeutic agent, and in the presence of the enzyme found in the body, to release the therapeutic agent in a targeted way []. The most widely used enzymes for drug delivery systems are the hydrolases, including proteases, lipases, and glycosidases, due to the simple design requiring the attachment of bioactive moieties to the carrier through enzyme cleavable unit []. Proteases are enzymes that break down the peptides at the level of amino acids, being involved in many physiological processes such as tissue remodeling, wound healing, and tumor invasion []. An overexpression of proteases has been associated with cancer, so proteases can be used to allow for the selective activation of smart drug delivery platforms []. Yildiz et al. [] reported core–shell nanoparticles based on amphiphilic copolymers poly(lactic-co-glycolic acid)-b-poly-l-lysine and poly(lactic acid)-b-poly(ethylene glycol) for Doxorubicin-loaded protease-activated drug delivery systems. The cytocompatibility was evaluated on MDA-MB-231 breast cancer cells and a significantly reduced cell viability was observed at drug concentrations of 0.10 µM.
Lipase, such as phospholipase, are enzymes that hydrolyze fats. However, in this case, it has also been observed that it can play the role of a pathological indicator for various conditions, such as many kinds of cancers and other conditions such as thrombosis, congestive heart failure, inflammation, neurodegeneration, and infectious pathogens [].
Another enzyme intensively studied in the enzyme-responsive drug delivery field is azoreductase, which is an enzyme produced by micro-organism species generally present in the colon [].
Another studied enzyme is azoreductase, which is a reductase enzyme extensively studied in the case of liver cancer, so that Medina et al. [] reported the development of enzyme-activated nanoconjugates for the treatment of liver cancer through the release of DOX by using L1-L4 azo-linkers to conjugate a generation of 5 of poly(amidoamine) dendrimer and designed to be able to bind to hepatic azoreductase enzymes. The obtained enzymatic-responsive system was tested on Hep G2 and Hep 3B cells. The results showed a non-toxicity for cardiomyocytes, comparing with the silenced toxicity after the classical administration of DOX at the same concentration and are readily cleavable by intracellular azoreductase enzymes proven to be effective in killing liver cancer cells, having IC50 value similar to free DOX. Sun et al. [] reported an NTR-responsive 4-nitrobenzyl group, hydrophobic AIE, tetraphenyl ethylene (TPE), and polyethylene glycol hydrophilic moieties for DOX drug delivery trough nitro reductase (NTR)–catalyzed. It was reported that 4 HeLa and HEK 293T cells were used in order to evaluate the cytotoxicity. The TNP-based drug delivery system showed that the DOX release was possible in the presence of NADH due to high sensitivity and selectivity to NTR, leading to the breakdown of the micelles and DOX release.
The main disadvantage of enzyme-responsive drug delivery systems is the release of the therapeutic agent before reaching the target area due to possible exposure to an enzyme trigger, or a closely related enzyme could release the load prematurely. This limitation can be overcome by obtaining a release system sensitive to dual stimuli, with a component sensitive to the pH variation, which favors a much more targeted release []. Gao et al. [] reported a dual-responsive drug delivery based on hydrophobic-modified sodium alginate. In vitro cellular uptake and cytotoxicity were tested on HepG2 or Hela cells, and the results showed had good growth inhibition effects on HepG2 cells or Hela cells and also a slow release effect was shown. In Figure 10, the schematic synthesis and stimuli-responsive release is shown. This dual-stimulus drug delivery system is capable of releasing DOX in a much more targeted manner, due to lysosomal enzyme which is present in all mammalian cells.
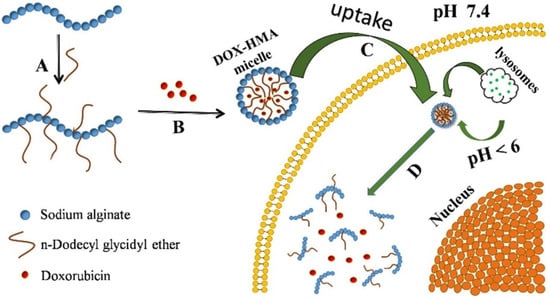
Figure 10.
Schematic of synthesis and stimuli-responsive release of DOX-HMA micelles: (A) dodecyl glycidyl ether-modified alginate; (B) DOX encapsulated into HMA micelles; (C) DOX-loaded HMA micelles accumulated into tumor cells; (D) intracellular stimuli-responsive degradation and drug release. (Reprinted with permission from Ref. []. 2020, Xue Gao).
3. External Stimuli-Responsive Drug Delivery Systems
3.1. Magnetic-Responsive Drug Delivery Systems
The extensively studied external stimuli-responsive drug delivery systems for cancer are the magnetic-responsive drug delivery systems. These kinds of smart drug-delivery systems can be achieved only if the nanocarrier possesses a strong magnetic property and can be employed by an applied magnetic field []. The first time was reported in 1980 by Kenneth et al. where the authors proposed the in vitro release of active Adriamycin via the utilization of magnetic-responsive albumin microspheres []. The magnetic-responsive DDS is able to be a selective target of antitumoral agents without disturbing the reticuloendothelial system (RES) in which an external magnetic field is applied to increase the drug concentration at the tumor site after administration of magnetic particles []. The mechanism of the magnetic drug-delivery system is that the therapeutic agent is linked to/or encapsulates magnetic nano/microparticles. Once attached, the magnetic-responsive DDS is injected into the bloodstream as a biocompatible ferrofluid and applied to a magnetic field in order to direct the DDS to the targeted tissue []. The magnetic susceptibility allows nuclear magnetic resonance (NMR) observation, tracking, and quantification of the antitumoral agent to the targeted tumoral tissue []. The most utilized magnetic-responsive nanocarriers are based on iron, cobalt, nickel, metallic oxides, mesoporous silica, calcium silicates, liposomes, and polymers []. Magnetic-responsive drug delivery systems consist of magnetic core-shell and polymer coatings []. In addition to the properties of the targeted release of the active substance, magnetic-responsive DDS have properties in diagnosis, being considered as theragnostic delivery systems combining imaging agents and effective therapeutic drugs [].
In the last few years, many research papers based on developing a magnetic-responsive carrier have been published [,,,]. Jeon et al. reported the obtaining of smart porous nanoclusters used as potential drug delivery for DOX in liver cancer therapy via transcatheter intra-arterial infusion, which is a widely used medical technique based on image-guidance using X-ray angiography performed for inoperable liver tumors []. Superparamagnetic iron oxide nanoparticles (SPIONs) are frequently used in the production of magnetic-responsive drug delivery systems, that in comparison with ferromagnetic-based materials which are permanently magnetized, these types of materials are magnetized only in an applied field []. SPIONs are constituted through iron oxide cores (magnetite (Fe3O4) and/or maghemite (gFe2O3) nanocrystals) which could be targeted via application of an external magnetic field [,]. Li et al. presented the development of core-shell drug delivery based on SPIONs and DOX for dual purposes (tumor targeting and MRI diagnosis) []. In addition, superparamagnetic iron oxide (SPIO) is commonly used as an MRI contrast agent in order to evaluate or diagnose liver cancer [,]. Maeng et al. [] proposed a magnetic-responsive drug delivery system based on poly(ethylene oxide)-trimellitic anhydride chloride-folate (PEO-TMA-FA), Doxorubicin (DOX), superparamagnetic iron oxide (Fe3O4) and folate, for liver cancer. The efficacy of the delivery system was demonstrated by in vivo tests on rats and rabbits with liver cancer, compared with the administration of DOX and a commercial liposome-based drug, DOXIL. In Figure 11, the authors graphically represent the model of the release system obtained for a better understanding of its structure. It can be seen that the core consists of iron oxides, and the core is coated in PEO-TMA-FA, and DOX is attached to the polymer surface. Folic acid (FA) was used to enter the receptor-expressing cancer cells through folate receptor-mediated endocytosis [,] and a special delivery of the targeting agent due to increased concentration of folate receptor (FR) in tumoral tissues. First, it was shown that there is a high level of FR in the case of liver cancer, compared to healthy tissue, and later, the effectiveness of DOX release was demonstrated using the DDS obtained. The in vitro test that investigates the anticancer effect shows that the obtained DDS was able to inhibit the proliferation of liver cancer cells more than DOXIL. Additionally, the in vivo test shows a significantly decreased tumor volume and did not produce visible side-effects [].
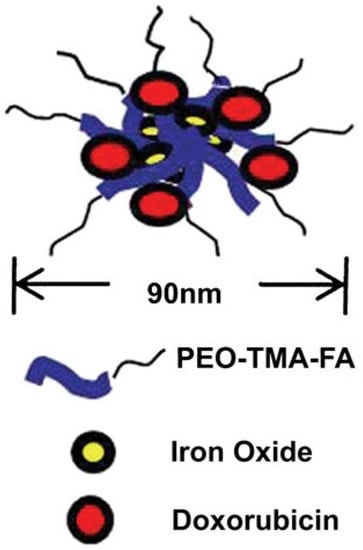
Figure 11.
Structure of DDS based on PEO-TMA-FA, Iron Oxide and DOX (Reprinted with permission from Ref. []. 2010, Jin Hee).
Further, for an improved release, an attempt is made to combine two or more multiple stimuli, as in the case of Tang et al. [], who developed a release system so sensitive to the magnetic stimulus as to direct the path of the therapeutic substance to the target tissue, as well as being sensitive to the reducing environment, so that at the moment of interaction with the tumor tissue the release is performed through the obtaining of star-shaped magnetic micelles based on self-assembly poly(ethylene glycol) (PEG)-poly(e-caprolactone) (PCL) copolymers with magnetic iron oxide nanoparticles (Fe3O4) and DOX encapsulated into the hydrophobic core of the micelles (Figure 12) [].
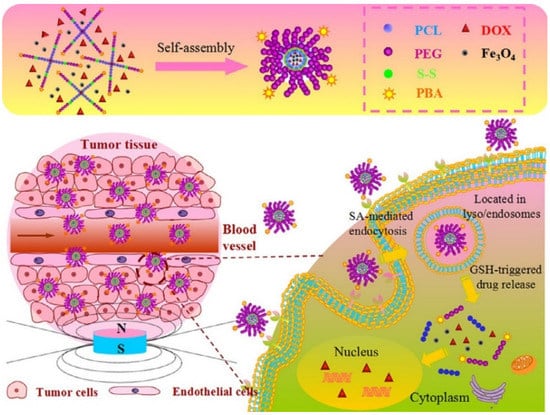
Figure 12.
Schematic illustration of the fabrication of DOX-loaded magnetic star-shaped polymer micelles and the procedure to selectively target the SA-positive HepG2 cells by the dual-targeting, and the fast response by GSH on drug release in the cytoplasm (Reprinted with permission from Ref. []. 2016, Zhaomin Tang).
Shao et al. [] developed magnetic nanoparticles as delivery carriers based on magnetic Fe3O4 and a body of mesoporous SiO2 containing Doxorubicin (DOX) as “nano-bullets” (Figure 13). The loading efficiency was reported as being 60% and the drug-loading content was 20%. It was observed that the drug retention is increased by the magnetic field, and the acidic pH increased the release rate of DOX (50% of DOX is released at pH 5.5 in 24 h in comparison with less than 5% at pH 7.4 in 24 h). Cheraghi et al. [] proposed cobalt ferrite magnetic nanoparticles for antitumoral drug release. The treatment with lemon juice with different volumes was used as a chelating agent. It was observed that an increase in the lemon juice contents can partially replace the Fe3+ ions with Fe2+ ions, reducing the super-exchange interaction between the magnetic atoms at octahedral and tetrahedral sites (Fe3+-O-Fe3+), leading to a drop at the net magnetic moment. Additionally, the obtained drug delivery was dual-responsive, such that the pH values influenced the drug release along with the magnetic response, leading to the best release of DOX by more than 42% at pH 5.4 in 75 h. Li et al. [] employed polyethylene glycol (PEG)-functionalized γ-Fe2O3 particles (γ-Fe2O3/PEG) as a dual drug delivery of DOX. After applying an AMF (45 kA/m and 186 kHz), the cumulative releases were increased to 32, 45, 55, and 63% under the pH values of 7.2, 6.5, 6.0, and 5.5. Further, a higher release of DOX was observed in acidic pH due to higher DOX solubility in the acid environment. Ren et al. [] presented magnetite nanoparticles and graphene oxide (GO/Fe3O4) drug delivery loaded with Doxorubicin hydrochloride via a chemical precipitation method. The release was increased by the addition of a magnetic field by inducing a temperature increase through magnetic hyperthermia. The challenges for using magnetic-responsive drug delivery systems are: the potential toxicity of metallic nanoparticles, so that in many studies the accumulation of various magnetic nanoparticles in various vital organs has been reported [,]; a fairly large magnetic field is needed; and it is very difficult to focus the alternating magnetic field [,,].
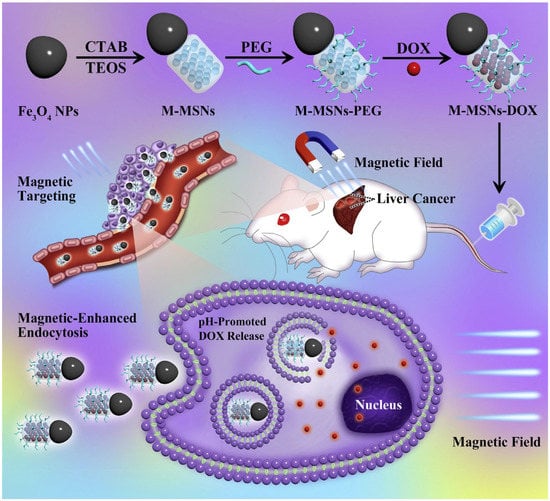
Figure 13.
Schematic illustration of the synthetic procedure for the Janus M-MSNs-DOX nano-bullet and application for pH-promoted drug release after magnetic-enhanced endocytosis in cancer cells, as well as magnet targeting liver cancer chemotherapy in vivo (Reprinted with permission from Ref. []. 2016, Dan Shao).
3.2. Photo-Responsive and Photothermal-Responsive Drug Delivery Systems
Photo-responsive drug delivery systems have been an attractive option for the controlled release of drugs using light sources, such as ultraviolet (UV), visible, and near-infrared (NIR) light []. The photo-responsive drug delivery systems have numerous advantages, such as being safe, minimally invasive, and a tissue-selective treatment for cancer therapy [,,,]. Most commonly used light-responsive agents are reversible such as azobenzene, spiropyran, dithienylethene, and diazonaphthoquinone, and irreversible, such as o-nitrobenzy, pyrenylmethyl, and coumarin []. These light-responsive agents are capable, when irradiated with light radiation, to disturb the bilayer membrane of the corresponding polymersomes (artificial vesicles enclosing an aqueous cavity) resulting in the self-assembly of the amphiphilic copolymer, which reorganizes into smaller polymers leading to the drug release [,]. Boruah et al. [] reported photo-responsive drug delivery systems based on liposome-azobenzene nanocomposite for DOX. The results showed a drug release of 77.33% in comparison to pure vesicles’ release of ~3.3%. Zhou et al. [] developed azo-bearing diblock copolymers as a photo-responsive and pH-responsive drug delivery system for DOX. The results showed excellent cytocompatibility on human breast-cancer cell (MCF-7) and a burst release was observed in the first 2 h and after 10 h 90% was released. Additionally, when spiropyran (SP) is under the action of irradiation with a specific wavelength of light (200–400 nm), a ring-opening occurs through a cis-trans isomerization, to zwitterionic merocyanine (MC) [,]. Chen et al. [] reported a multi-sensitive drug delivery system, including photo-responsive stimuli based on poly(acrylic acid-co-spiropyran methacrylate) crosslinked by disulfide-containing N,N-bis(acryloyl)cystamine for DOX release. After irradiation, the addition of spiropyran lead to the hydrophobic SP in nanogels isomerized to the hydrophilic MC and then the nanogel swelled, resulting in a disruption of nanogel due to the oxidative scission of the disulphide crosslinkers, facilitating the drug release. The limitations of UV-responsive drug delivery systems are poor tissue penetration and the damaging effects on healthy tissues []. As a solution for these limitations, different near-infrared (NIR) or visible light-responsive materials have been developed [,,].
Photothermal-responsive drug delivery systems are based on external light, having many advantages, such as a noninvasive nature, simplicity of operation, good controllability over both wavelength and intensity, and high spatiotemporal resolution [,,,]. Photothermal therapy uses a photothermal agent that is stimulated by both specific band light and vibrational energy/heat release to selectively target tumoral tissue. Photo-responsive DDS are characterized by three main categories: (i) photothermal responsive DDS; (ii) photodynamic responsive DDS; and (iii) photoconversion-responsive DDS []. Drug delivery systems based on the photothermal response have a dual role, in cancer diagnosis and treatment []. The photothermal-responsive DDS has mandatory main components, such as chromophore which is able to absorb the applied energy and the corresponding wavelength and achieves an excited state, followed by the conversion of the absorbed light energy into thermal, and the second main component is the thermally responsive material that has an increased sensibility at temperature variation [,]. The most used materials for this kind of DDS are gold nanoparticles [,,] and NIPAAm []. Ji et al. [] reported a new drug-release system based on gold nanocages (AuNCs) as the photothermal core, Hyaluronan (HA) as the targeting ligand, P(NIPAM-co-Am) (PM) as the thermally responsive copolymers, and Doxorubicin as the therapeutic agent for liver cancer therapy. It was shown that in vitro results demonstrate that the obtained DOX/AuNCs-PM-HA has increased anticancer activity and in vivo photoacoustic tomography imaging shows effective tumor targeting. Modification by conjugation of HA to the outer surface of the delivery vector improved this delivery system targeting ability, leading to a higher drug concentration at the tumor site, reduced toxicity and side effects of DOX, and an improved curative effect. Huang et al. [] proposed mesoporous core–shell structured nanocomposites (MCSN) of Cu2-xSe@SiO (Figure 14). It was observed that the composites displayed a concentration-dependent temperature change under laser irradiation. The release rate was reported as 42% at pH = 4.6 in 6 h, without irradiation and 55.4% at pH = 4.6 after adding NIR laser irradiation, and became slow without laser irradiation. Chen et al. [] proposed a photothermal drug delivery based on gelatin. After irradiation, an increase was reported in local temperature, up to 55.9 °C. Li et al. [] described an iron-oxide-based transdermal drug delivery with high photothermal conversion efficiency and stability, efficient cellular uptake, synergistic cancer cell-killing effect, and enhanced percutaneous permeability in vitro.
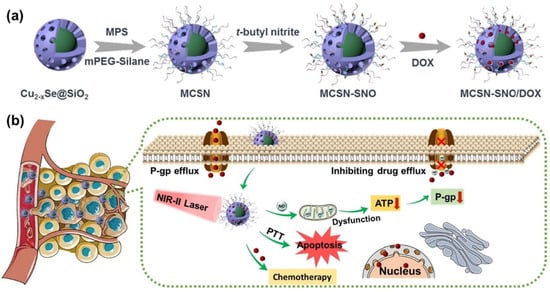
Figure 14.
Schematic illustration of (a) the synthetic procedure of MCSN-SNO/DOX and (b) the process of photothermal-responsive NO gas release for overcoming multidrug resistant tumor by combination therapy (Reprinted with permission from Ref. []. 2021, Youyou Huang).
3.3. Electric-Responsive Drug Delivery Systems
Another studied type of external stimuli drug-delivery system is based on electrical stimulation. Electrical stimuli were used in order to generate the release of the therapeutic agent by conductive polymeric bulk materials and implantable electronic delivery devices []. Conductive polymers are used in many biomedical applications, such as biosensors, in nerve tissue regeneration, and drug delivery systems [,,,], due to their unique electrical and optical properties, very similar to metals and inorganic semiconductors []. The main advantages of conductive polymers are that their chemical, electrical and physical properties can be tailored to the specific needs, and that they have metal conductivity properties, but also have the flexibility of polymers [,,]. The most-used conductive polymers to obtain electric-responsive drug delivery systems are Polyaniline (PANI) [], polypyrrole (PPy) [], and poly(3,4-ethylenedioxythiophene) (PEDOT) []. The conductive polymers are used to customize the drug delivery systems, so that when an electric field is applied, it can facilitate the release of the antitumor agent in the desired area. The accomplishment of this kind of conductive polymer can be influenced by the nature of the chosen dopant and the molecular weight of the antitumoral agent []. The main principle of the utilization of electrically responsive drug delivery systems is the application of a weak electric field over targeted tumoral tissue after the administration of electro-responsive drug carriers for controlled on-site drug release []. The major limitation of electric-responsive drug delivery systems is the fact that they are limited to topical or subdermal implants, due to the need to place electrodes in the polymer matrix [].
3.4. Ultrasonic-Responsive Drug Delivery
Ultrasonic-responsive drug delivery is a less utilized system that is sensitive to external stimuli, but it can represent a promising trigger for stimuli-responsive drug delivery due to its amazing characteristics, such as the ability to non-invasively penetrate deeply into the tissue without damaging it [,]. The ultrasound waves are able to permeate the materials far deeper than light and has the advantage that there is no need for further functionalization of the biomaterials in order to introduce additional functional groups to increase the reactivity of the biomaterial, as the polymers heat up in seconds under exposure to ultrasound []. Ultrasounds are defined as mechanical waves with high frequencies (≥20 kHz) which are able to travel through a medium []. Generally, ultrasound waves have applications in different fields, such as in vivo imaging, physiotherapy, cosmetics and food industry []. In general, release systems based on ultrasound waves are obtained from polymers, in different forms, such as polymer-coated bubbles/emulsions, and polymer hydrogels [,,,,]. It was reported that ultrasonic-responsive drug delivery systems are able to increase drug delivery, but also increase the therapeutic efficacy, and temporal release of drugs []. The use of ultrasound waves can induce thermal effects, mechanical effects, or radiation force, which can lead to the release of the active substance in a non-invasive, remote, and spatiotemporally controlled manner []. Kim et al. [] developed Doxorubicin-loaded albumin nanoparticle-conjugated microbubble complex in an iodized oil emulsion as an ultrasonic-responsive drug delivery system for liver cancer. The delivery of DOX was possible due to the sonoporation effect induced by the cavitation of microbubbles. Wang et al. [] reported poly(lactobionamidoethyl methacrylate)-based amphiphiles as ultrasonic-responsive drug delivery systems (Figure 15). The results showed that the accelerated release was attributed to the ultrasound-induced cleavage of -oa- linkage, leading to intracellular drug release from delivery system increasing the cytotoxic potency at the targeted cells. Zhou et al. [] reported chitosan nanobubbles for ultrasound-mediated targeted delivery of Doxorubicin. The results showed an near-instantaneous cellular entry of DOX due to sonoporation. In addition, Wu et al. [] reported that sonoporation inhibited tumor growth and the decrease in tumor weight was approximately 6.5-fold in comparison, without exposure to ultrasound irradiation. Gao et al. [] developed ultrasound- and pH-responsive delivery of DOX based on alginate/chitosan stabilized perfluoro hexane nanodroplets. The in vitro and in vivo tests showed that DOX-loaded nanodroplets have a good accumulation and tumor-targeting in HepG2 tumors, resulting a growth inhibition of the tumoral tissue under the effect of ultrasound. A very important limitation of ultrasound-responsive drug delivery systems are represented by the possible tissue damage caused by heating and by irreversible pore formation in cell membranes [,,].
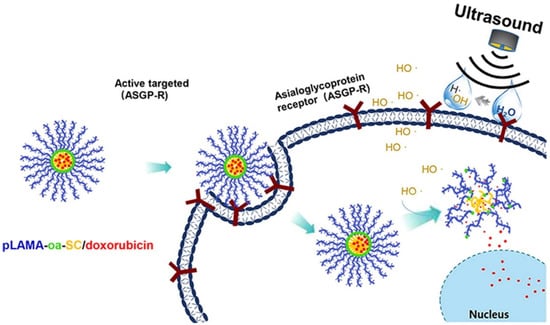
Figure 15.
Schematic illustration of ultrasound-labile motif and hepatocellular carcinoma-targeted drug delivery system (pLAMA-oa-SC/Doxorubicin self-assembled nanostructures) for ultrasound-specified augmented cytotoxic potency to hepatocellular carcinoma (Reprinted with permission from Ref. []. 2019, Jingyun Wang).
4. Conclusions and Future Perspectives
Chemotherapy is the most widely used technique for treating liver cancer. Disadvantages of this method of treatment are numerous side effects, lack of therapeutic agent targeting, and the solubility and stability of anticancer drugs. Furthermore, the most commonly used antitumor agent in the treatment of liver cancer is Doxorubicin. A possible solution for the limitation of the conventional chemotherapy is by using intelligent controlled-release systems, capable of releasing the antitumor agent in a controlled manner in the presence of stimuli. In this review, we present and summarize both internal stimuli-responsive drug delivery systems, such as redox, pH and temperature variation, and external stimuli-responsive drug delivery systems, such as the application of magnetic, photothermal, and electrical stimuli, for the controlled release of Doxorubicin in liver cancer therapy. Furthermore, drug delivery systems sensitive to multiple stimuli are an attractive subject to researchers due to their increased targeted efficiency. The studies of stimuli-responsive drug delivery systems of Doxorubicin are still at an incipient stage as they require multiple preclinical evaluations of their biocompatibility and toxicity. Future trends can be divided into two main directions. The first one is related to ever increasingly precise materials and technologies that will allow precise amounts of DOX to be discarded at the tumor site with minimum impact for the rest of the organism. This would be possible due to higher accessibility at an industrial scale to targeted drug-delivery systems based using as target molecule the concentrations of tumor marker in the blood (especially alpha-fetoprotein) and also by the possibility to concentrate the drug delivery system at the tumor site. Supramolecular architectures that incorporate monoclonal antibodies for tumor markers are an elegant solution to this problem, but their cost remains prohibitive at the moment and the actual synthesis routes make this plan utopian. Another future direction would be represented by coupling the DOX-targeted drug delivery with another medical procedure—hemodialysis. A large number of patients with chronic kidney disease will develop a form of liver cancer due to the accumulation of creatinine, urea, and uric acid. Hemodialysis membranes that incorporate targeted drug delivery systems for liver cancer would increase the life quality of patients that suffer from both medical conditions.
Author Contributions
Conceptualization, S.I.V. and E.R.R.; resources, S.I.V.; data curation, S.I.V., A.S. and E.R.R.; writing—original draft preparation, S.I.V. and E.R.R.; writing—review and editing, S.I.V. and E.R.R.; supervision, S.I.V.; project administration, A.S. and S.I.V.; funding acquisition, S.I.V. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a grant of the Ministry of Research, Innovation and Digitization, CNCS/CCCDI—UEFISCDI, project number PN-III-P4-ID-PCE-2020-1154, Hemodialysis combined with stimuli-responsive drug delivery—A new generation of polymeric membranes for advanced biomedical applications within PNCDI III.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Arciero, C.A.; Sigurdson, E.R. Liver-directed therapies for patients with primary liver cancer and hepatic metastases. Curr. Treat. Options Oncol. 2006, 7, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Hashem, B.E.S. Epidemiology of Hepatocellular Carcinoma. Digestive Disease Week; Sesion Handout Book: Bethesda, MA, USA, 2006. [Google Scholar]
- Thomas, M.B.; Zhu, A.X. Hepatocellular carcinoma: The need for progress. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2005, 23, 2892–2899. [Google Scholar] [CrossRef] [PubMed]
- Odze, R.D.; Goldblum, J.R. (Eds.) Chapter 42—Cirrhosis. In Surgical Pathology of the GI Tract, Liver, Biliary Tract, and Pancreas, 2nd ed.; W.B. Saunders: Philadelphia, PA, USA, 2009; pp. 1115–1145. [Google Scholar]
- Le Grazie, M.; Biagini, M.R.; Tarocchi, M.; Polvani, S.; Galli, A. Chemotherapy for hepatocellular carcinoma: The present and the future. World J. Hepatol. 2017, 9, 907–920. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xuan, Z.; Zhu, X.; Sun, H.; Li, J.; Xie, Z. Near-infrared photoresponsive drug delivery nanosystems for cancer photo-chemotherapy. J. Nanobiotechnol. 2020, 18, 108. [Google Scholar] [CrossRef] [PubMed]
- Tam, K. The Roles of Doxorubicin in Hepatocellular Carcinoma. ADMET DMPK 2013, 1, 29–44. [Google Scholar] [CrossRef]
- Liu, C.-Y.; Chen, K.-F.; Chen, P.-J. Treatment of Liver Cancer. Cold Spring Harb. Perspect. Med. 2015, 5, a02153. [Google Scholar] [CrossRef]
- Daher, S.; Massarwa, M.; Benson, A.A.; Khoury, T. Current and Future Treatment of Hepatocellular Carcinoma: An Updated Comprehensive Review. J. Clin. Transl. Hepatol. 2018, 6, 69–78. [Google Scholar] [CrossRef]
- Chi, X.; Liu, K.; Luo, X.; Yin, Z.; Lin, H.; Gao, J. Recent advances of nanomedicines for liver cancer therapy. J. Mater. Chem. B 2020, 8, 3747–3771. [Google Scholar] [CrossRef]
- Wan, L.; Chen, Z.; Deng, Y.; Liao, T.; Kuang, Y.; Liu, J.; Duan, J.; Xu, Z.; Jiang, B.; Li, C. A novel intratumoral pH/redox-dual-responsive nanoplatform for cancer MR imaging and therapy. J. Colloid Interface Sci. 2020, 573, 263–277. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, J.; Wang, L.; Wang, Y.; Chen, M. Tumor pH(e)-triggered charge-reversal and redox-responsive nanoparticles for docetaxel delivery in hepatocellular carcinoma treatment. Nanoscale 2015, 7, 15763–15779. [Google Scholar] [CrossRef]
- Mini, E.; Trave, F.; Rustum, Y.M.; Bertino, J.R. Enhancement of the antitumor effects of 5-fluorouracil by folinic acid. Pharmacol. Ther. 1990, 47, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, M. Chapter 38—Renal Toxicity. In Handbook of Toxicology of Chemical Warfare Agents; Gupta, R.C., Ed.; Academic Press: San Diego, CA, USA, 2009; pp. 561–574. [Google Scholar]
- Cortés-Funes, H.; Coronado, C. Role of anthracyclines in the era of targeted therapy. Cardiovasc. Toxicol. 2007, 7, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Weiss, R.B. The anthracyclines: Will we ever find a better doxorubicin? Semin. Oncol. 1992, 19, 670–686. [Google Scholar] [PubMed]
- Thorn, C.F.; Oshiro, C.; Marsh, S.; Hernandez-Boussard, T.; McLeod, H.; Klein, T.E.; Altman, R.B. Doxorubicin pathways: Pharmacodynamics and adverse effects. Pharm. Genom. 2011, 21, 440–446. [Google Scholar] [CrossRef]
- Pillai, G. Chapter 9—Nanotechnology Toward Treating Cancer: A Comprehensive Review. In Applications of Targeted Nano Drugs and Delivery Systems; Mohapatra, S.S., Ranjan, S., Dasgupta, N., Mishra, R.K., Thomas, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 221–256. [Google Scholar]
- Carvalho, C.; Santos, R.; Cardoso, S.; Correia, S.; Oliveira, P.; Santos, M.; Moreira, P. Doxorubicin: The Good, the Bad and the Ugly Effect. Curr. Med. Chem. 2009, 16, 3267–3285. [Google Scholar] [CrossRef]
- Cagel, M.; Grotz, E.; Bernabeu, E.; Moretton, M.A.; Chiappetta, D.A. Doxorubicin: Nanotechnological overviews from bench to bedside. Drug Discov. Today 2017, 22, 270–281. [Google Scholar] [CrossRef]
- Varela-López, A.; Battino, M.; Navarro-Hortal, M.D.; Giampieri, F.; Forbes-Hernández, T.Y.; Romero-Márquez, J.M.; Collado, R.; Quiles, J.L. An update on the mechanisms related to cell death and toxicity of doxorubicin and the protective role of nutrients. Food Chem. Toxicol. 2019, 134, 110834. [Google Scholar] [CrossRef]
- Mohammadi, M.; Arabi, L.; Alibolandi, M. Doxorubicin-loaded composite nanogels for cancer treatment. J. Control. Release 2020, 328, 171–191. [Google Scholar] [CrossRef]
- Chatterjee, K.; Zhang, J.; Honbo, N.; Karliner, J.S. Doxorubicin Cardiomyopathy. Cardiology 2010, 115, 155–162. [Google Scholar] [CrossRef]
- Jose, P.; Consolacion, M.; Raul, O.; Celia, V.; Pablo, J.A.; Jose, L.A.; Maria, A.R.; Visitacion, G.; Antonia, A. Doxorubicin-Loaded Nanoparticles: New Advances in Breast Cancer Therapy. Anti-Cancer Agents Med. Chem. 2012, 12, 1058–1070. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, Y.; Lan, J.; Kang, Y.; Zhang, T.; Ding, Y.; Zhang, X.; Lu, L. Reduction-sensitive CD44 receptor-targeted hyaluronic acid derivative micelles for doxorubicin delivery. Int. J. Nanomed. 2018, 13, 4361–4378. [Google Scholar] [CrossRef] [PubMed]
- Pandele, A.M.; Oprea, M.; Dutu, A.A.; Miculescu, F.; Voicu, S.I. A Novel Generation of Polysulfone/Crown Ether-Functionalized Reduced Graphene Oxide Membranes with Potential Applications in Hemodialysis. Polymers 2022, 14, 148. [Google Scholar] [CrossRef] [PubMed]
- Radu, E.R.; Voicu, S.I. Functionalized Hemodialysis Polysulfone Membranes with Improved Hemocompatibility. Polymers 2022, 14, 1130. [Google Scholar] [CrossRef] [PubMed]
- Oprea, M.; Voicu, S.I. Recent advances in composites based on cellulose derivatives for biomedical applications. Carbohydr. Polym. 2020, 247, 116683. [Google Scholar] [CrossRef]
- Voicu, S.I.; Thakur, V.K. Aminopropyltriethoxysilane as a linker for cellulose-based functional materials: New horizons and future challenges. Curr. Opin. Green Sustain. Chem. 2021, 30, 100480. [Google Scholar] [CrossRef]
- Oprea, M.; Voicu, S.I. Cellulose Composites with Graphene for Tissue Engineering Applications. Materials 2020, 13, 5347. [Google Scholar] [CrossRef]
- Pandele, A.M.; Constantinescu, A.; Radu, I.C.; Miculescu, F.; Ioan Voicu, S.; Ciocan, L.T. Synthesis and Characterization of PLA-Micro-structured Hydroxyapatite Composite Films. Materials 2020, 13, 274. [Google Scholar] [CrossRef]
- Oprea, M.; Voicu, S.I. Recent Advances in Applications of Cellulose Derivatives-Based Composite Membranes with Hydroxyapatite. Materials 2020, 13, 2481. [Google Scholar] [CrossRef]
- Lee, E.S.; Na, K.; Bae, Y.H. Doxorubicin loaded pH-sensitive polymeric micelles for reversal of resistant MCF-7 tumor. J. Control. Release 2005, 103, 405–418. [Google Scholar] [CrossRef]
- Liu, J.; Huang, Y.; Kumar, A.; Tan, A.; Jin, S.; Mozhi, A.; Liang, X.-J. pH-Sensitive nano-systems for drug delivery in cancer therapy. Biotechnol. Adv. 2013, 32, 693–710. [Google Scholar] [CrossRef]
- Ahmadi, S.; Rabiee, N.; Bagherzadeh, M.; Elmi, F.; Fatahi, Y.; Farjadian, F.; Baheiraei, N.; Nasseri, B.; Rabiee, M.; Dastjerd, N.; et al. Stimulus-Responsive Sequential Release Systems for Drug and Gene Delivery. Nano Today 2020, 34, 100914. [Google Scholar] [CrossRef] [PubMed]
- Palmerston Mendes, L.; Pan, J.; Torchilin, V.P. Dendrimers as Nanocarriers for Nucleic Acid and Drug Delivery in Cancer Therapy. Molecules 2017, 22, 1401. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; He, S.; Yin, Y.; Liu, H.; Hu, J.; Cheng, J.; Wang, W. Combination of Nanomaterials in Cell-Based Drug Delivery Systems for Cancer Treatment. Pharmaceutics 2021, 13, 1888. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Sharipov, M.; Turaev, A.; Azizov, S.; Azizov, I.; Makhado, E.; Rahdar, A.; Kumar, D.; Pandey, S. Polymer-Based Hybrid Nanoarchitectures for Cancer Therapy Applications. Polymers 2022, 14, 3027. [Google Scholar] [CrossRef] [PubMed]
- Salkho, N.M.; Awad, N.S.; Pitt, W.G.; Husseini, G.A. Photo-Induced Drug Release from Polymeric Micelles and Liposomes: Phototriggering Mechanisms in Drug Delivery Systems. Polymers 2022, 14, 1286. [Google Scholar] [CrossRef]
- Bolla, P.K.; Rodriguez, V.A.; Kalhapure, R.S.; Kolli, C.S.; Andrews, S.; Renukuntla, J. A review on pH and temperature responsive gels and other less explored drug delivery systems. J. Drug Deliv. Sci. Technol. 2018, 46, 416–435. [Google Scholar] [CrossRef]
- Luo, Y.-L.; Zhang, X.-Y.; Fu, J.-Y.; Xu, F.; Chen, Y.-S. Novel temperature and pH dual-sensitive PNIPAM/CMCS/MWCNT semi-IPN nanohybrid hydrogels: Synthesis, characterization, and DOX drug release. Int. J. Polym. Mater. Polym. Biomater. 2016, 66, 398–409. [Google Scholar] [CrossRef]
- Torchilin, V.P. Chapter 1 Fundamentals of Stimuli-responsive Drug and Gene Delivery Systems. In Stimuli-Responsive Drug Delivery Systems; The Royal Society of Chemistry: London, UK, 2018; pp. 1–32. [Google Scholar]
- Wang, Z.; Duan, X.; Lv, Y.; Zhao, Y. Low density lipoprotein receptor (LDLR)-targeted lipid nanoparticles for the delivery of sorafenib and Dihydroartemisinin in liver cancers. Life Sci. 2019, 239, 117013. [Google Scholar] [CrossRef]
- Kundu, M.; Chatterjee, S.; Ghosh, N.; Manna, P.; Das, J.; Sil, P.C. Tumor targeted delivery of umbelliferone via a smart mesoporous silica nanoparticles controlled-release drug delivery system for increased anticancer efficiency. Mater. Sci. Eng. C 2020, 116, 111239. [Google Scholar] [CrossRef]
- Ge, X.; Fu, M.; Niu, X.; Kong, X. Atomic layer deposition of γ-Fe2O3 nanoparticles on multi-wall carbon nanotubes for magnetic drug delivery and liver cancer treatment. Ceram. Int. 2020, 46, 26557–26563. [Google Scholar] [CrossRef]
- Yao, Q.; Liu, Y.; Kou, L.; Tu, Y.; Tang, X.; Zhu, L. Tumor-targeted drug delivery and sensitization by MMP2-responsive polymeric micelles. Nanomed. Nanotechnol. Biol. Med. 2019, 19, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Pooja, D.; Srinivasa Reddy, T.; Kulhari, H.; Kadari, A.; Adams, D.J.; Bansal, V.; Sistla, R. N-acetyl-d-glucosamine-conjugated PAMAM dendrimers as dual receptor-targeting nanocarriers for anticancer drug delivery. Eur. J. Pharm. Biopharm. 2020, 154, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, Y.; Luo, L.; Li, L.; Xing, S.; Yin, T.; Bian, K.; Zhu, R.; Gao, D. A chemo-photothermal synergetic antitumor drug delivery system: Gold nanoshell coated wedelolactone liposome. Mater. Sci. Eng. C 2019, 101, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.; Lin, G.; Lu, Q.; Meng, L.; Shen, X.; Dong, L.; Fu, C.; Zhang, X. Targeted therapy of SMMC-7721 liver cancer in vitro and in vivo with carbon nanotubes based drug delivery system. J. Colloid Interface Sci. 2012, 365, 143–149. [Google Scholar] [CrossRef]
- Karimi, M.; Sahandi Zangabad, P.; Ghasemi, A.; Amiri, M.; Bahrami, M.; Malekzad, H.; Ghahramanzadeh Asl, H.; Mahdieh, Z.; Bozorgomid, M.; Ghasemi, A.; et al. Temperature-Responsive Smart Nanocarriers for Delivery Of Therapeutic Agents: Applications and Recent Advances. ACS Appl. Mater. Interfaces 2016, 8, 21107–21133. [Google Scholar] [CrossRef]
- Saadat, M.; Mostafaei, F.; Mahdinloo, S.; Abdi, M.; Zahednezhad, F.; Zakeri-Milani, P.; Valizadeh, H. Drug delivery of pH-Sensitive nanoparticles into the liver cancer cells. J. Drug Deliv. Sci. Technol. 2021, 63, 102557. [Google Scholar] [CrossRef]
- Mateti, T.; Aswath, S.; Vatti, A.K.; Kamath, A.; Laha, A. A review on allopathic and herbal nanofibrous drug delivery vehicles for cancer treatments. Biotechnol. Rep. 2021, 31, e00663. [Google Scholar] [CrossRef]
- Yadav, P.; Jain, J.; Sherje, A.P. Recent advances in nanocarriers-based drug delivery for cancer therapeutics: A review. React. Funct. Polym. 2021, 165, 104970. [Google Scholar] [CrossRef]
- Mintz, K.J.; Leblanc, R.M. The use of nanotechnology to combat liver cancer: Progress and perspectives. Biochim. Biophys. Acta BBA-Rev. Cancer 2021, 1876, 188621. [Google Scholar] [CrossRef]
- Limeres, M.J.; Moretton, M.A.; Bernabeu, E.; Chiappetta, D.A.; Cuestas, M.L. Thinking small, doing big: Current success and future trends in drug delivery systems for improving cancer therapy with special focus on liver cancer. Mater. Sci. Eng. C 2019, 95, 328–341. [Google Scholar] [CrossRef]
- Mazidi, Z.; Javanmardi, S.; Naghib, S.M.; Mohammadpour, Z. Smart stimuli-responsive implantable drug delivery systems for programmed and on-demand cancer treatment: An overview on the emerging materials. Chem. Eng. J. 2022, 433, 134569. [Google Scholar] [CrossRef]
- Wang, G.; Li, R.; Parseh, B.; Du, G. Prospects and challenges of anticancer agents’ delivery via chitosan-based drug carriers to combat breast cancer: A review. Carbohydr. Polym. 2021, 268, 118192. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Ruan, J.; Lv, G.; Shan, Q.; Fan, Z.; Wang, H.; Du, Y.; Ling, L. Cell membrane-based biomimetic nanosystems for advanced drug delivery in cancer therapy: A comprehensive review. Colloids Surf. B Biointerfaces 2022, 215, 112503. [Google Scholar] [CrossRef] [PubMed]
- Khodaei, T.; Inamdar, S.; Suresh, A.P.; Acharya, A.P. Drug delivery for metabolism targeted cancer immunotherapy. Adv. Drug Deliv. Rev. 2022, 184, 114242. [Google Scholar] [CrossRef]
- Kumar, V.; Xin, X.; Ma, J.; Tan, C.; Osna, N.; Mahato, R.I. Therapeutic targets, novel drugs, and delivery systems for diabetes associated NAFLD and liver fibrosis. Adv. Drug Deliv. Rev. 2021, 176, 113888. [Google Scholar] [CrossRef]
- Yan, G.; Wang, J.; Hu, L.; Wang, X.; Yang, G.; Fu, S.; Cheng, X.; Zhang, P.; Tang, R. Stepwise targeted drug delivery to liver cancer cells for enhanced therapeutic efficacy by galactose-grafted, ultra-pH-sensitive micelles. Acta Biomater. 2017, 51, 363–373. [Google Scholar] [CrossRef]
- Liao, J.; Zheng, H.; Fei, Z.; Lu, B.; Zheng, H.; Li, D.; Xiong, X.; Yi, Y. Tumor-targeting and pH-responsive nanoparticles from hyaluronic acid for the enhanced delivery of doxorubicin. Int. J. Biol. Macromol. 2018, 113, 737–747. [Google Scholar] [CrossRef]
- Wu, J.-L.; Tian, G.-X.; Yu, W.-J.; Jia, G.-T.; Sun, T.-Y.; Gao, Z.-Q. pH-Responsive Hyaluronic Acid-Based Mixed Micelles for the Hepatoma-Targeting Delivery of Doxorubicin. Int. J. Mol. Sci. 2016, 17, 364. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Mohan, M.; Rajeev, M.R. Modified chitosan-hyaluronic acid based hydrogel for the pH-responsive Co-delivery of cisplatin and doxorubicin. Int. J. Biol. Macromol. 2022, 201, 378–388. [Google Scholar] [CrossRef]
- Qu, J.; Zhao, X.; Ma, P.X.; Guo, B. pH-responsive self-healing injectable hydrogel based on N-carboxyethyl chitosan for hepatocellular carcinoma therapy. Acta Biomater. 2017, 58, 168–180. [Google Scholar] [CrossRef]
- Bi, J.; Lu, Y.; Dong, Y.; Gao, P. Synthesis of Folic Acid-Modified DOX@ZIF-8 Nanoparticles for Targeted Therapy of Liver Cancer. J. Nanomater. 2018, 2018, 1357812. [Google Scholar] [CrossRef]
- Cheng, H.; Fan, X.; Wang, X.; Ye, E.; Loh, X.J.; Li, Z.; Wu, Y.-L. Hierarchically Self-Assembled Supramolecular Host–Guest Delivery System for Drug Resistant Cancer Therapy. Biomacromolecules 2018, 19, 1926–1938. [Google Scholar] [CrossRef] [PubMed]
- Kunene, S.C.; Lin, K.-S.; Weng, M.-T.; Carrera Espinoza, M.J.; Wu, C.-M. In vitro study of doxorubicin-loaded thermo- and pH-tunable carriers for targeted drug delivery to liver cancer cells. J. Ind. Eng. Chem. 2021, 104, 93–105. [Google Scholar] [CrossRef]
- Mdlovu, N.V.; Lin, K.-S.; Weng, M.-T.; Lin, Y.-S. Design of doxorubicin encapsulated pH-/thermo-responsive and cationic shell-crosslinked magnetic drug delivery system. Colloids Surf. B Biointerfaces 2022, 209, 112168. [Google Scholar] [CrossRef] [PubMed]
- Sebeke, L.C.; Castillo Gómez, J.D.; Heijman, E.; Rademann, P.; Simon, A.C.; Ekdawi, S.; Vlachakis, S.; Toker, D.; Mink, B.L.; Schubert-Quecke, C.; et al. Hyperthermia-induced doxorubicin delivery from thermosensitive liposomes via MR-HIFU in a pig model. J. Control. Release 2022, 343, 798–812. [Google Scholar] [CrossRef]
- Chen, M.; Hu, J.; Wang, L.; Li, Y.; Zhu, C.; Chen, C.; Shi, M.; Ju, Z.; Cao, X.; Zhang, Z. Targeted and redox-responsive drug delivery systems based on carbonic anhydrase IX-decorated mesoporous silica nanoparticles for cancer therapy. Sci. Rep. 2020, 10, 14447. [Google Scholar] [CrossRef]
- Mezghrani, O.; Tang, Y.; Ke, X.; Chen, Y.; Hu, D.; Tu, J.; Zhao, L.; Bourkaib, N. Hepatocellular carcinoma dually-targeted nanoparticles for reduction triggered intracellular delivery of doxorubicin. Int. J. Pharm. 2015, 478, 553–568. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, Q.; Dai, L.; Shen, X.; Chen, W.; Cai, K. Phenylboronic acid-modified hollow silica nanoparticles for dual-responsive delivery of doxorubicin for targeted tumor therapy. Regen. Biomater. 2017, 4, 111–124. [Google Scholar] [CrossRef]
- Yildiz, T.; Gu, R.; Zauscher, S.; Betancourt, T. Doxorubicin-loaded protease-activated near-infrared fluorescent polymeric nanoparticles for imaging and therapy of cancer. Int. J. Nanomed. 2018, 13, 6961–6986. [Google Scholar] [CrossRef]
- Medina, S.H.; Chevliakov, M.V.; Tiruchinapally, G.; Durmaz, Y.Y.; Kuruvilla, S.P.; ElSayed, M.E.H. Enzyme-activated nanoconjugates for tunable release of doxorubicin in hepatic cancer cells. Biomaterials 2013, 34, 4655–4666. [Google Scholar] [CrossRef]
- Maeng, J.H.; Lee, D.-H.; Jung, K.H.; Bae, Y.-H.; Park, I.-S.; Jeong, S.; Jeon, Y.-S.; Shim, C.-K.; Kim, W.; Kim, J.; et al. Multifunctional doxorubicin loaded superparamagnetic iron oxide nanoparticles for chemotherapy and magnetic resonance imaging in liver cancer. Biomaterials 2010, 31, 4995–5006. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Zhang, L.; Wang, Y.; Li, D.; Zhong, Z.; Zhou, S. Redox-responsive star-shaped magnetic micelles with active-targeted and magnetic-guided functions for cancer therapy. Acta Biomater. 2016, 42, 232–246. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Bian, Q.; Wang, P.; Zheng, X.; Lv, L.; Dang, Z.; Wang, G. Photo, pH and redox multi-responsive nanogels for drug delivery and fluorescence cell imaging. Polym. Chem. 2017, 8, 6150–6157. [Google Scholar] [CrossRef]
- Kim, D.; Lee, J.H.; Moon, H.; Seo, M.; Han, H.; Yoo, H.; Seo, H.; Lee, J.; Hong, S.; Kim, P.; et al. Development and evaluation of an ultrasound-triggered microbubble combined transarterial chemoembolization (TACE) formulation on rabbit VX2 liver cancer model. Theranostics 2021, 11, 79–92. [Google Scholar] [CrossRef]
- Wang, J.; Xia, Y.; Liu, H.; Xia, J.; Qian, M.; Zhang, L.; Chen, L.; Chen, Q. Poly(lactobionamidoethyl methacrylate)-based amphiphiles with ultrasound-labile components in manufacture of drug delivery nanoparticulates for augmented cytotoxic efficacy to hepatocellular carcinoma. J. Colloid Interface Sci. 2019, 551, 1–9. [Google Scholar] [CrossRef]
- Gao, Y.; Ma, Q.; Cao, J.; Shi, Y.; Wang, J.; Ma, H.; Sun, Y.; Song, Y. Bifunctional alginate/chitosan stabilized perfluorohexane nanodroplets as smart vehicles for ultrasound and pH responsive delivery of anticancer agents. Int. J. Biol. Macromol. 2021, 191, 1068–1078. [Google Scholar] [CrossRef]
- Zhu, Y.J.; Chen, F. pH-responsive drug-delivery systems. Chem. Asian J. 2015, 10, 284–305. [Google Scholar] [CrossRef]
- Bazban-Shotorbani, S.; Hasani-Sadrabadi, M.M.; Karkhaneh, A.; Serpooshan, V.; Jacob, K.I.; Moshaverinia, A.; Mahmoudi, M. Revisiting structure-property relationship of pH-responsive polymers for drug delivery applications. J. Control. Release 2017, 253, 46–63. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, H.; Li, J.; Wang, Y.; Wang, X. Fabrication of pH-responsive PLGA(UCNPs/DOX) nanocapsules with upconversion luminescence for drug delivery. Sci. Rep. 2017, 7, 18014. [Google Scholar] [CrossRef]
- Ren, H.; Zhang, L.; An, J.; Wang, T.; Li, L.; Si, X.; He, L.; Wu, X.; Wang, C.; Su, Z. Polyacrylic acid@zeolitic imidazolate framework-8 nanoparticles with ultrahigh drug loading capability for pH-sensitive drug release. Chem. Commun. 2014, 50, 1000–1002. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, Z.; Wang, X. Fabrication of pH-responsive PAA-NaMnF3@DOX hybrid nanostructures for magnetic resonance imaging and drug delivery. J. Alloys Compd. 2020, 820, 153142. [Google Scholar] [CrossRef]
- Mu, Y.; Gong, L.; Peng, T.; Yao, J.; Lin, Z. Advances in pH-responsive drug delivery systems. OpenNano 2021, 5, 100031. [Google Scholar] [CrossRef]
- Gierszewska, M.; Ostrowska-Czubenko, J.; Chrzanowska, E. pH-responsive chitosan/alginate polyelectrolyte complex membranes reinforced by tripolyphosphate. Eur. Polym. J. 2018, 101, 282–290. [Google Scholar] [CrossRef]
- Li, X.; Li, H.; Yi, W.; Chen, J.; Liang, B. Acid-triggered core cross-linked nanomicelles for targeted drug delivery and magnetic resonance imaging in liver cancer cells. Int. J. Nanomed. 2013, 8, 3019–3031. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, T.; Ma, X.; Wang, Y.; Lu, Y.; Jia, D.; Huang, X.; Chen, J.; Xu, Z.; Wen, F. The design and synthesis of dextran-doxorubicin prodrug-based pH-sensitive drug delivery system for improving chemotherapy efficacy. Asian J. Pharm. Sci. 2020, 15, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Gerami, S.E.; Pourmadadi, M.; Fatoorehchi, H.; Yazdian, F.; Rashedi, H.; Nigjeh, M.N. Preparation of pH-sensitive chitosan/polyvinylpyrrolidone/α-Fe2O3 nanocomposite for drug delivery application: Emphasis on ameliorating restrictions. Int. J. Biol. Macromol. 2021, 173, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Narayan, R.; Gadag, S.; Cheruku, S.P.; Raichur, A.M.; Day, C.M.; Garg, S.; Manandhar, S.; Pai, K.S.R.; Suresh, A.; Mehta, C.H.; et al. Chitosan-glucuronic acid conjugate coated mesoporous silica nanoparticles: A smart pH-responsive and receptor-targeted system for colorectal cancer therapy. Carbohydr. Polym. 2021, 261, 117893. [Google Scholar] [CrossRef]
- Park, J.J.; Luo, X.; Yi, H.; Valentine, T.M.; Payne, G.F.; Bentley, W.E.; Ghodssi, R.; Rubloff, G.W. Chitosan-mediated in situ biomolecule assembly in completely packaged microfluidic devices. Lab A Chip 2006, 6, 1315–1321. [Google Scholar] [CrossRef]
- Ways, T.M.M.; Lau, W.M.; Khutoryanskiy, V.V. Chitosan and Its Derivatives for Application in Mucoadhesive Drug Delivery Systems. Polymers 2018, 10, 267. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, F.; Li, C.; An, H.; Wan, T.; Zhang, P. Application of Chitosan and Its Derivative Polymers in Clinical Medicine and Agriculture. Polymers 2022, 14, 958. [Google Scholar] [CrossRef]
- Mi, Y.; Zhang, J.; Tan, W.; Miao, Q.; Li, Q.; Guo, Z. Preparation of Doxorubicin-Loaded Carboxymethyl-β-Cyclodextrin/Chitosan Nanoparticles with Antioxidant, Antitumor Activities and pH-Sensitive Release. Mar. Drugs 2022, 20, 278. [Google Scholar] [CrossRef] [PubMed]
- Jhaveri, J.; Raichura, Z.; Khan, T.; Momin, M.; Omri, A. Chitosan Nanoparticles-Insight into Properties, Functionalization and Applications in Drug Delivery and Theranostics. Molecules 2021, 26, 272. [Google Scholar] [CrossRef] [PubMed]
- Azzam, M.; El Safy, S.; Abdelgelil, S.A.; Weiskirchen, R.; Asimakopoulou, A.; de Lorenzi, F.; Lammers, T.; Mansour, S.; Tammam, S. Targeting Activated Hepatic Stellate Cells Using Collagen-Binding Chitosan Nanoparticles for siRNA Delivery to Fibrotic Livers. Pharmaceutics 2020, 12, 590. [Google Scholar] [CrossRef]
- Zhan, J.; Wu, Y.; Wang, H.; Liu, J.; Ma, Q.; Xiao, K.; Li, Z.; Li, J.; Luo, F.; Tan, H. An injectable hydrogel with pH-sensitive and self-healing properties based on 4armPEGDA and N-carboxyethyl chitosan for local treatment of hepatocellular carcinoma. Int. J. Biol. Macromol. 2020, 163, 1208–1222. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.; Wang, J.; Qin, J.; Hu, L.; Zhang, P.; Wang, X.; Tang, R. Well-Defined Poly(Ortho Ester Amides) for Potential Drug Carriers: Probing the Effect of Extra- and Intracellular Drug Release on Chemotherapeutic Efficacy. Macromol. Biosci. 2017, 17, 1600503. [Google Scholar] [CrossRef]
- Zeng, X.; Wang, H.; Zhang, Y.; Xu, X.; Yuan, X.; Li, J. pH-Responsive Hyaluronic Acid Nanoparticles for Enhanced Triple Negative Breast Cancer Therapy. Int. J. Nanomed. 2022, 17, 1437–1457. [Google Scholar] [CrossRef] [PubMed]
- Puluhulawa, L.E.; Joni, I.M.; Elamin, K.M.; Mohammed, A.F.; Muchtaridi, M.; Wathoni, N. Chitosan–Hyaluronic Acid Nanoparticles for Active Targeting in Cancer Therapy. Polymers 2022, 14, 3410. [Google Scholar] [CrossRef]
- Yu, M.; Jambhrunkar, S.; Thorn, P.; Chen, J.; Gu, W.; Yu, C. Hyaluronic acid modified mesoporous silica nanoparticles for targeted drug delivery to CD44-overexpressing cancer cells. Nanoscale 2013, 5, 178–183. [Google Scholar] [CrossRef]
- Das, A.; Chowdhury, G.; Basu, S. Targeted cellular delivery and improved cytotoxicity of pH sensitive hyaluronic acid-doxorubicin conjugates. Mol. Cancer Ther. 2007, 6, C120. [Google Scholar]
- Li, Z.-P.; Tian, G.-X.; Jiang, H.; Pan, R.-Y.; Lian, B.; Wang, M.; Gao, Z.-Q.; Zhang, B.; Wu, J.-L. Liver-Targeting and pH-Sensitive Sulfated Hyaluronic Acid Mixed Micelles for Hepatoma Therapy. Int. J. Nanomed. 2019, 14, 9437–9452. [Google Scholar] [CrossRef]
- Lei, M.; Chen, G.; Zhang, M.; Lei, J.; Li, T.; Li, D.; Zheng, H. A pH-sensitive drug delivery system based on hyaluronic acid co-deliver doxorubicin and aminoferrocene for the combined application of chemotherapy and chemodynamic therapy. Colloids Surf. B Biointerfaces 2021, 203, 111750. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Fan, Z.; Zhang, X.; Li, H.; Sun, Y.; Cao, M.; Wei, G.; Wang, J. pH-Responsive Self-Assemblies from the Designed Folic Acid-Modified Peptide Drug for Dual-Targeting Delivery. Langmuir 2021, 37, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wang, J.; Mao, W.; Zhang, D.; Guo, Y.; Song, Y.; Wang, J.-P.; Qi, T.; Li, G.L. pH-Responsive zeolitic imidazole framework nanoparticles with high active inhibitor content for self-healing anticorrosion coatings. Colloids Surf. A Physicochem. Eng. Asp. 2018, 555, 18–26. [Google Scholar] [CrossRef]
- Schmaljohann, D. Thermo- and pH-responsive polymers in drug delivery. Adv. Drug Deliv. Rev. 2006, 58, 1655–1670. [Google Scholar] [CrossRef]
- Wang, Z.; Deng, X.; Ding, J.; Zhou, W.; Zheng, X.; Tang, G. Mechanisms of drug release in pH-sensitive micelles for tumour targeted drug delivery system: A review. Int. J. Pharm. 2018, 535, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, R.J.N. Trigger-responsive nanoparticles: Control switches for cancer therapy. Nanomedicine 2011, 6, 1657–1660. [Google Scholar] [CrossRef]
- Gu, X.; Wang, J.; Liu, X.; Zhao, D.; Wang, Y.; Gao, H.; Wu, G. Temperature-responsive drug delivery systems based on polyaspartamides with isopropylamine pendant groups. Soft Matter 2013, 9, 7267–7273. [Google Scholar] [CrossRef]
- Wu, J.; Lu, Q.; Wang, H.; Lu, B.; Huang, B. Controllable Construction of Temperature-Sensitive Supramolecular Hydrogel Based on Cellulose and Cyclodextrin. Polymers 2022, 14, 3801. [Google Scholar] [CrossRef]
- Ortega-García, A.; Martínez-Bernal, B.G.; Ceja, I.; Mendizábal, E.; Puig-Arévalo, J.E.; Pérez-Carrillo, L.A. Drug Delivery from Stimuli-Responsive Poly(N-isopropylacrylamide-co-N-isopropylmethacrylamide)/Chitosan Core/Shell Nanohydrogels. Polymers 2022, 14, 522. [Google Scholar] [CrossRef]
- Sponchioni, M.; Capasso Palmiero, U.; Moscatelli, D. Thermo-responsive polymers: Applications of smart materials in drug delivery and tissue engineering. Mater. Sci. Eng. C 2019, 102, 589–605. [Google Scholar] [CrossRef]
- Van Gheluwe, L.; Chourpa, I.; Gaigne, C.; Munnier, E.J.P. Polymer-based smart drug delivery systems for skin application and demonstration of stimuli-responsiveness. Polymers 2021, 13, 1285. [Google Scholar] [CrossRef] [PubMed]
- Dastidar, D.; Chakrabarti, G. Thermoresponsive Drug Delivery Systems, Characterization and Application. In Applications of Targeted Nano Drugs and Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2019; pp. 133–155. [Google Scholar]
- Alveroglu, E.; İlker, N.; Shah, M.T.; Rajar, K.; Gokceoren, A.T.; Koc, K. Effects of gel morphology on the lysozyme adsorption and desorption kinetics of temperature sensitive magnetic gel composites. Colloids Surf. B Biointerfaces 2019, 181, 981–988. [Google Scholar] [CrossRef] [PubMed]
- Barrera, G.; Allia, P.; Tiberto, P. Dipolar interactions among magnetite nanoparticles for magnetic hyperthermia: A rate-equation approach. Nanoscale 2021, 13, 4103–4121. [Google Scholar] [CrossRef] [PubMed]
- Mdlovu, N.V.; Lin, K.-S.; Weng, M.-T.; Hsieh, C.-C.; Lin, Y.-S.; Carrera Espinoza, M.J. In vitro intracellular studies of pH and thermo-triggered doxorubicin conjugated magnetic SBA-15 mesoporous nanocarriers for anticancer activity against hepatocellular carcinoma. J. Ind. Eng. Chem. 2021, 102, 1–16. [Google Scholar] [CrossRef]
- Ibrahim, R.B.; Liu, C.Y.; Cronin, S.M.; Murphy, B.C.; Cha, R.; Swerdlow, P.; Avila, T.; Smith, S.T.; Lewis, R.A.; Edwards, D.J. Influence of plasma exchange on the disposition of the fourth generation cephalosporin cefepime. J. Oncol. Pharm. Pract. Off. Publ. Int. Soc. Oncol. Pharm. Pract. 2009, 15, 217–222. [Google Scholar] [CrossRef]
- Gouyette, A.; Lemoine, R.; Adhemar, J.P.; Kleinknecht, D.; Man, N.K.; Droz, J.P.; Macquet, J.P. Kinetics of cisplatin in an anuric patient undergoing hemofiltration dialysis. Cancer Treat. Rep. 1981, 65, 665–668. [Google Scholar]
- Guo, X.; Cheng, Y.; Zhao, X.; Luo, Y.; Chen, J.; Yuan, W.-E. Advances in redox-responsive drug delivery systems of tumor microenvironment. J. Nanobiotechnol. 2018, 16, 74. [Google Scholar] [CrossRef]
- Wu, G.; Fang, Y.Z.; Yang, S.; Lupton, J.R.; Turner, N.D. Glutathione metabolism and its implications for health. J. Nutr. 2004, 134, 489–492. [Google Scholar] [CrossRef]
- Huo, M.; Yuan, J.; Tao, L.; Wei, Y. Redox-responsive polymers for drug delivery: From molecular design to applications. Polym. Chem. 2014, 5, 1519–1528. [Google Scholar] [CrossRef]
- Gamcsik, M.P.; Kasibhatla, M.S.; Teeter, S.D.; Colvin, O.M. Glutathione levels in human tumors. Biomarkers 2012, 17, 671–691. [Google Scholar] [CrossRef]
- Traverso, N.; Ricciarelli, R.; Nitti, M.; Marengo, B.; Furfaro, A.L.; Pronzato, M.A.; Marinari, U.M.; Domenicotti, C. Role of Glutathione in Cancer Progression and Chemoresistance. Oxidative Med. Cell. Longev. 2013, 2013, 972913. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.C. Dysregulation of glutathione synthesis in liver disease. Liver Res. 2020, 4, 64–73. [Google Scholar] [CrossRef]
- Ji, S.; Cao, W.; Yu, Y.; Xu, H. Dynamic Diselenide Bonds: Exchange Reaction Induced by Visible Light without Catalysis. Angew. Chem. 2014, 53, 6781–6785. [Google Scholar] [CrossRef] [PubMed]
- Abed, H.F.; Abuwatfa, W.H.; Husseini, G.A. Redox-Responsive Drug Delivery Systems: A Chemical Perspective. Nanomaterials 2022, 12, 3183. [Google Scholar] [CrossRef]
- Luo, Y.; Yin, X.; Yin, X.; Chen, A.; Zhao, L.; Zhang, G.; Liao, W.; Huang, X.; Li, J.; Zhang, C.Y. Dual pH/Redox-Responsive Mixed Polymeric Micelles for Anticancer Drug Delivery and Controlled Release. Pharmaceutics 2019, 11, 176. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, M.; Li, W.; Fan, L.; Zhou, Y.; Hu, Z. A Novel CD133- and EpCAM-Targeted Liposome With Redox-Responsive Properties Capable of Synergistically Eliminating Liver Cancer Stem Cells. Front. Chem. 2020, 8, 649. [Google Scholar] [CrossRef]
- Pandey, B.; Patil, N.G.; Bhosle, G.S.; Ambade, A.V.; Gupta, S.S. Amphiphilic Glycopolypeptide Star Copolymer-Based Cross-Linked Nanocarriers for Targeted and Dual-Stimuli-Responsive Drug Delivery. Bioconjug. Chem. 2019, 30, 633–646. [Google Scholar] [CrossRef]
- Ng, D.; Fahrer, J.; Wu, Y.; Eisele, K.; Kuan, S.; Barth, H.; Weil, T. Efficient Delivery of p53 and Cytochrome C by Supramolecular Assembly of a Dendritic Multi-Domain Delivery System. Adv. Healthc. Mater. 2013, 2, 1620–1629. [Google Scholar] [CrossRef]
- Lee, J.Y.; Park, Y.; Pun, S.; Lee, S.S.; Lo, J.F.; Lee, L.P. Real-time investigation of cytochrome c release profiles in living neuronal cells undergoing amyloid beta oligomer-induced apoptosis. Nanoscale 2015, 7, 10340–10343. [Google Scholar] [CrossRef]
- Saeedi, S.; Murjan, S.; Nabid, M.R. Redox and pH dual sensitive folate-modified star-like amphiphilic copolymer based on castor oil for controlled doxorubicin delivery. J. Drug Deliv. Sci. Technol. 2021, 62, 102391. [Google Scholar] [CrossRef]
- Yan, J.; Zhang, N.; Zhang, Z.; Zhu, W.; Li, B.; Li, L.; Pu, Y.; He, B. Redox-responsive polyethyleneimine/tetrahedron DNA/doxorubicin nanocomplexes for deep cell/tissue penetration to overcome multidrug resistance. J. Control. Release 2021, 329, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhao, G.; Su, W.-K.; Shuai, Q. Enzyme-Responsive Nanoparticles for Anti-tumor Drug Delivery. Front. Chem. 2020, 8, 647. [Google Scholar] [CrossRef]
- Wang, C.; Chen, Q.; Wang, Z.; Zhang, X.J.A.C. An enzyme-responsive polymeric superamphiphile. Angew. Chem. 2010, 122, 8794–8797. [Google Scholar] [CrossRef]
- Song, S.J.; Choi, J.S.J.P. Enzyme-Responsive Amphiphilic Peptide Nanoparticles for Biocompatible and Efficient Drug Delivery. Pharmaceutics 2022, 14, 143. [Google Scholar] [CrossRef]
- Liu, F.; Wang, D.; Wang, J.; Ma, L.; Yu, C.; Wei, H. Construction of Enzyme-Responsive Micelles Based on Theranostic Zwitterionic Conjugated Bottlebrush Copolymers with Brush-on-Brush Architecture for Cell Imaging and Anticancer Drug Delivery. Molecules 2022, 27, 3016. [Google Scholar] [CrossRef] [PubMed]
- Pham, S.H.; Choi, Y.; Choi, J. Stimuli-Responsive Nanomaterials for Application in Antitumor Therapy and Drug Delivery. Pharmaceutics 2020, 12, 630. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Cai, H.; Jiang, L.; Hu, J.; Bains, A.; Hu, J.; Gong, Q.; Luo, K.; Gu, Z. Enzyme-Sensitive and Amphiphilic PEGylated Dendrimer-Paclitaxel Prodrug-Based Nanoparticles for Enhanced Stability and Anticancer Efficacy. ACS Appl. Mater. Interfaces 2017, 9, 6865–6877. [Google Scholar] [CrossRef]
- Zelzer, M.; Todd, S.; Hirst, A.; McDonald, T.; Ulijn, R. Enzyme responsive materials: Design strategies and future developments. Biomater. Sci. 2012, 1, 11–39. [Google Scholar] [CrossRef]
- Sobczak, M. Enzyme-Responsive Hydrogels as Potential Drug Delivery Systems—State of Knowledge and Future Prospects. Int. J. Mol. Sci. 2022, 23, 4421. [Google Scholar] [CrossRef]
- de la Rica, R.; Aili, D.; Stevens, M.M. Enzyme-responsive nanoparticles for drug release and diagnostics. Adv. Drug Deliv. Rev. 2012, 64, 967–978. [Google Scholar] [CrossRef]
- Camara, M.C.; Monteiro, R.A.; Carvalho, L.B.; Oliveira, J.L.; Fraceto, L.F. Enzyme Stimuli–Responsive Nanoparticles for Bioinsecticides: An Emerging Approach for Uses in Crop Protection. ACS Sustain. Chem. Eng. 2021, 9, 106–112. [Google Scholar] [CrossRef]
- Hu, Q.; Katti, P.S.; Gu, Z. Enzyme-responsive nanomaterials for controlled drug delivery. Nanoscale 2014, 6, 12273–12286. [Google Scholar] [CrossRef] [PubMed]
- Das, S.S.; Bharadwaj, P.; Bilal, M.; Barani, M.; Rahdar, A.; Taboada, P.; Bungau, S.; Kyzas, G.Z. Stimuli-Responsive Polymeric Nanocarriers for Drug Delivery, Imaging, and Theragnosis. Polymers 2020, 12, 1397. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.-Y.; Liang, Y.-X.; Wu, C.-Y.; Tang, Q.; Liu, R.; Lu, Z.-L.; He, L. Nitroreductase-responsive polymeric micelles based on 4-nitrobenzyl and AIE moieties for intracellular doxorubicin release. Polym. Chem. 2021, 12, 2618–2626. [Google Scholar] [CrossRef]
- Wells, C.M.; Harris, M.; Choi, L.; Murali, V.P.; Guerra, F.D.; Jennings, J.A. Stimuli-Responsive Drug Release from Smart Polymers. J. Funct. Biomater. 2019, 10, 34. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Yu, Z.; Liu, B.; Yang, J.; Yang, X.; Yu, Y. A smart drug delivery system responsive to pH/enzyme stimuli based on hydrophobic modified sodium alginate. Eur. Polym. J. 2020, 133, 109779. [Google Scholar] [CrossRef]
- Price, P.M.; Mahmoud, W.E.; Al-Ghamdi, A.A.; Bronstein, L.M. Magnetic Drug Delivery: Where the Field Is Going. Front. Chem. 2018, 6, 619. [Google Scholar] [CrossRef]
- Widder, K.J.; Senyei, A.E.; Ranney, D.F. In vitro release of biologically active adriamycin by magnetically responsive albumin microspheres. Cancer Res. 1980, 40, 3512–3517. [Google Scholar]
- Chen, W.; Wei, M.; Wang, Y. Advanced ultrafiltration membranes by leveraging microphase separation in macrophase separation of amphiphilic polysulfone block copolymers. J. Membr. Sci. 2017, 525, 342–348. [Google Scholar] [CrossRef]
- Khoshnevisan, K.; Maleki, H.; Samadian, H.; Shahsavari, S.; Sarrafzadeh, M.H.; Larijani, B.; Dorkoosh, F.A.; Haghpanah, V.; Khorramizadeh, M.R. Cellulose acetate electrospun nanofibers for drug delivery systems: Applications and recent advances. Carbohydr. Polym. 2018, 198, 131–141. [Google Scholar] [CrossRef]
- Zhong, D.; Wang, Z.; Zhou, J.; Wang, Y. Additive-free preparation of hemodialysis membranes from block copolymers of polysulfone and polyethylene glycol. J. Membr. Sci. 2021, 618, 118690. [Google Scholar] [CrossRef]
- Wypych, G. fillers-applications. In Functional Fillers; Wypych, G., Ed.; ChemTec Publishing: Scarborough, ON, Canada, 2018; pp. 153–179. [Google Scholar]
- Liu, Y.; Li, M.; Yang, F.; Gu, N. Magnetic drug delivery systems. Sci. China Mater. 2017, 60, 471–486. [Google Scholar] [CrossRef]
- Ren, M.-X.; Wang, Y.-Q.; Lei, B.-Y.; Yang, X.-X.; Hou, Y.-L.; Meng, W.-J.; Zhao, D.-L. Magnetite nanoparticles anchored on graphene oxide loaded with doxorubicin hydrochloride for magnetic hyperthermia therapy. Ceram. Int. 2021, 47, 20686–20692. [Google Scholar] [CrossRef]
- Liu, J.F.; Jang, B.; Issadore, D.; Tsourkas, A. Use of magnetic fields and nanoparticles to trigger drug release and improve tumor targeting. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2019, 11, e1571. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.-M.; Kang, M.-S.; Choi, G.-E.; Kim, Y.-J.; Bae, C.-H.; Yu, Y.-B.; Jeong, Y.-I.L. Stimuli-Responsive Drug Delivery of Doxorubicin Using Magnetic Nanoparticle Conjugated Poly(ethylene glycol)-g-Chitosan Copolymer. Int. J. Mol. Sci. 2021, 22, 13169. [Google Scholar] [CrossRef] [PubMed]
- Namathoti, S.; Ravindra, K.V.M.; Sreekanth, R.P.S. A review on progress in magnetic, microwave, ultrasonic responsive Shape-memory polymer composites. Mater. Today Proc. 2022, 56, 1182–1191. [Google Scholar] [CrossRef]
- Jeon, M.J.; Gordon, A.C.; Larson, A.C.; Chung, J.W.; Kim, Y.I.; Kim, D.H. Transcatheter intra-arterial infusion of doxorubicin loaded porous magnetic nano-clusters with iodinated oil for the treatment of liver cancer. Biomaterials 2016, 88, 25–33. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Sant, S.; Wang, B.; Laurent, S.; Sen, T. Superparamagnetic iron oxide nanoparticles (SPIONs): Development, surface modification and applications in chemotherapy. Adv. Drug Deliv. Rev. 2011, 63, 24–46. [Google Scholar] [CrossRef] [PubMed]
- Crayton, S.H.; Chen, A.K.; Liu, J.F.; Higbee-Dempsey, E.M.; Huang, C.H.; Tsourkas, A.; Cheng, Z. 3.20 Molecular Imaging☆. In Comprehensive Biomaterials II; Ducheyne, P., Ed.; Elsevier: Oxford, UK, 2017; pp. 424–466. [Google Scholar]
- Lee, R.J.; Low, P.S. Delivery of liposomes into cultured KB cells via folate receptor-mediated endocytosis. J. Biol. Chem. 1994, 269, 3198–3204. [Google Scholar] [CrossRef]
- Mathias, C.J.; Wang, S.; Lee, R.J.; Waters, D.J.; Low, P.S.; Green, M.A. Tumor-selective radiopharmaceutical targeting via receptor-mediated endocytosis of gallium-67-deferoxamine-folate. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 1996, 37, 1003–1008. [Google Scholar]
- Shao, D.; Li, J.; Zheng, X.; Pan, Y.; Wang, Z.; Zhang, M.; Chen, Q.-X.; Dong, W.-F.; Chen, L. Janus “nano-bullets” for magnetic targeting liver cancer chemotherapy. Biomaterials 2016, 100, 118–133. [Google Scholar] [CrossRef] [PubMed]
- Cheraghi, A.; Davar, F.; Homayoonfal, M.; Hojjati-Najafabadi, A. Effect of lemon juice on microstructure, phase changes, and magnetic performance of CoFe2O4 nanoparticles and their use on release of anti-cancer drugs. Ceram. Int. 2021, 47, 20210–20219. [Google Scholar] [CrossRef]
- Li, S.; Zhang, R.; Wang, D.; Feng, L.; Cui, K. Synthesis of hollow maghemite (γ-Fe2O3) particles for magnetic field and pH-responsive drug delivery and lung cancer treatment. Ceram. Int. 2021, 47, 7457–7464. [Google Scholar] [CrossRef]
- Prijic, S.; Sersa, G. Magnetic nanoparticles as targeted delivery systems in oncology. Radiol. Oncol. 2011, 45, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Soares, G.A.; Faria, J.V.C.; Pinto, L.A.; Prospero, A.G.; Pereira, G.M.; Stoppa, E.G.; Buranello, L.P.; Bakuzis, A.F.; Baffa, O.; Miranda, J.R.A. Long-Term Clearance and Biodistribution of Magnetic Nanoparticles Assessed by AC Biosusceptometry. Materials 2022, 15, 2121. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.; Husni, P.; Kang, K.; Lee, D.; Lee, S.; Lee, E.; Youn, Y.; Oh, K. Recent Advances in pH- or/and Photo-Responsive Nanovehicles. Pharmaceutics 2021, 13, 725. [Google Scholar] [CrossRef] [PubMed]
- Park, W.; Park, S.-j.; Na, K. The controlled photoactivity of nanoparticles derived from ionic interactions between a water soluble polymeric photosensitizer and polysaccharide quencher. Biomaterials 2011, 32, 8261–8270. [Google Scholar] [CrossRef]
- Castano, A.P.; Mroz, P.; Hamblin, M.R. Photodynamic therapy and anti-tumour immunity. Nat. Rev. Cancer 2006, 6, 535–545. [Google Scholar] [CrossRef]
- Cheng, W.; Gu, L.; Ren, W.; Liu, Y. Stimuli-responsive polymers for anti-cancer drug delivery. Mater. Sci. Eng. C 2014, 45, 600–608. [Google Scholar] [CrossRef]
- Kim, D.H.; Hwang, H.S.; Na, K. Photoresponsive Micelle-Incorporated Doxorubicin for Chemo-Photodynamic Therapy to Achieve Synergistic Antitumor Effects. Biomacromolecules 2018, 19, 3301–3310. [Google Scholar] [CrossRef]
- Hou, W.; Liu, R.; Bi, S.; He, Q.; Wang, H.; Gu, J. Photo-Responsive Polymersomes as Drug Delivery System for Potential Medical Applications. Molecules 2020, 25, 5147. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-Y.; Zhang, P.-Y. Polymersomes in Nanomedicine—A Review. Curr. Med. Chem. 2017, 13, 124–129. [Google Scholar] [CrossRef]
- Boruah, J.S.; Chowdhury, D. Liposome-azobenzene nanocomposite as photo-responsive drug delivery vehicle. Appl. Nanosci. 2022, 1–13. [Google Scholar] [CrossRef]
- Zhou, J.; Xu, H.; Tong, Z.; Yang, Y.; Jiang, G. Photo/pH-controlled host–guest interaction between an azobenzene-containing block copolymer and water-soluble pillar [6]arene as a strategy to construct the “compound vesicles” for controlled drug delivery. Mater. Sci. Eng. C 2018, 89, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Fagan, A.; Bartkowski, M.; Giordani, S. Spiropyran-Based Drug Delivery Systems. Front. Chem. 2021, 9, 720087. [Google Scholar] [CrossRef]
- Poelma, S.O.; Oh, S.S.; Helmy, S.; Knight, A.S.; Burnett, G.L.; Soh, H.T.; Hawker, C.J.; Read de Alaniz, J. Controlled drug release to cancer cells from modular one-photon visible light-responsive micellar system. Chem. Commun. 2016, 52, 10525–10528. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Xie, S.; Zhou, C.; Chen, Y.; Li, H.; Liu, P.; Jiang, R.; Hang, L.; Jiang, G. All-In-One Second Near-Infrared Light-Responsive Drug Delivery System for Synergistic Chemo-Photothermal Therapy. ACS Appl. Bio Mater. 2022, 5, 3841–3849. [Google Scholar] [CrossRef]
- Lu, G.; Gao, X.; Zhang, H.; Zhang, Y.; Yu, Y.; Sun, Z.; Li, W.; Wu, W.; Lu, Y.; Zou, H. Near-infrared light (NIR)-responsive nanoliposomes combining photodynamic therapy and chemotherapy for breast tumor control. Chin. Chem. Lett. 2022, 33, 1923–1926. [Google Scholar] [CrossRef]
- Jingchao, L.; Pu, K. Semiconducting Polymer Nanomaterials as Near-Infrared Photoactivatable Protherapeutics for Cancer. Acc. Chem. Res. 2020, 53, 752–762. [Google Scholar] [CrossRef]
- Xiong, Q.; Lim, Y.; Li, D.; Pu, K.; Liang, L.; Duan, H. Photoactive Nanocarriers for Controlled Delivery. Adv. Funct. Mater. 2020, 30, 1903896. [Google Scholar] [CrossRef]
- Shaker, M.A.; Younes, H.M. Photo-irradiation paradigm: Mapping a remarkable facile technique used for advanced drug, gene and cell delivery. J. Control. Release 2015, 217, 10–26. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Sun, Z.; Ren, Y.; Chen, X.; Zhang, W.; Zhu, X.; Mao, Z.; Shen, J.; Nie, S. Advances in nanomaterials for use in photothermal and photodynamic therapeutics (Review). Mol. Med. Rep. 2019, 20, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.R.; Adams, R.L.; Heaton, S.; Dickey, D.T.; Bartels, K.E.; Nordquist, R.E. Chromophore-enhanced laser-tumor tissue photothermal interaction using an 808-nm diode laser. Cancer Lett. 1995, 88, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Linsley, C.S.; Wu, B.M. Recent advances in light-responsive on-demand drug-delivery systems. Ther. Deliv. 2017, 8, 89–107. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Pandit, S.; Mokkapati, V.R.S.S.; Garg, A.; Ravikumar, V.; Mijakovic, I. Gold Nanoparticles in Diagnostics and Therapeutics for Human Cancer. Int. J. Mol. Sci. 2018, 19, 1979. [Google Scholar] [CrossRef]
- Guerrero, A.; Hassan, N.; Escobar, C.; Albericio, F.; Kogan, M.; Araya, E. Gold nanoparticles for photothermally controlled drug release. Nanomedicine 2014, 9, 2023–2039. [Google Scholar] [CrossRef]
- You, J.; Zhang, G.; Li, C. Exceptionally high payload of doxorubicin in hollow gold nanospheres for near-infrared light-triggered drug release. ACS Nano 2010, 4, 1033–1041. [Google Scholar] [CrossRef]
- Xin, Y.; Yin, M.; Zhao, L.; Meng, F.; Luo, L. Recent progress on nanoparticle-based drug delivery systems for cancer therapy. Cancer Biol. Med. 2017, 14, 228–241. [Google Scholar] [CrossRef]
- Ji, M.; Qiu, X.; Hou, L.; Huang, S.; Li, Y.; Liu, Y.; Duan, S.; Hu, Y. Construction and application of a liver cancer-targeting drug delivery system based on core-shell gold nanocages. Int. J. Nanomed. 2018, 13, 1773–1789. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, J.; Zhang, Y.; Shi, L.; Qin, X.; Lu, B.; Ding, Y.; Wang, Y.; Chen, T.; Yao, Y. P-glycoprotein suppression by photothermal-responsive nitric oxide releasing nanoplatform for triple-combination therapy of multidrug resistant cancer. Mater. Des. 2021, 211, 110160. [Google Scholar] [CrossRef]
- Chen, X.; Zou, J.; Zhang, K.; Zhu, J.; Zhang, Y.; Zhu, Z.; Zheng, H.; Li, F.; Piao, J.-G. Photothermal/matrix metalloproteinase-2 dual-responsive gelatin nanoparticles for breast cancer treatment. Acta Pharm. Sin. B 2021, 11, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, F.; Yang, Z.; Xu, J.; Liu, H.; Zhang, L.; Xu, W. Emulsifying performance of near-infrared light responsive polydopamine-based silica particles to control drug release. Powder Technol. 2020, 359, 17–26. [Google Scholar] [CrossRef]
- Senapati, S.; Mahanta, A.; Kumar, S.; Maiti, P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct. Target. Ther. 2018, 3, 7. [Google Scholar] [CrossRef] [PubMed]
- Svirskis, D.; Travas-Sejdic, J.; Rodgers, A.; Garg, S. Electrochemically controlled drug delivery based on intrinsically conducting polymers. J. Control. Release 2010, 146, 6–15. [Google Scholar] [CrossRef]
- García-Fernández, A.; Lozano-Torres, B.; Blandez, J.F.; Monreal-Trigo, J.; Soto, J.; Collazos-Castro, J.E.; Alcañiz, M.; Marcos, M.D.; Sancenón, F.; Martínez-Máñez, R. Electro-responsive films containing voltage responsive gated mesoporous silica nanoparticles grafted onto PEDOT-based conducting polymer. J. Control. Release 2020, 323, 421–430. [Google Scholar] [CrossRef]
- Ferrigno, B.; Bordett, R.; Duraisamy, N.; Moskow, J.; Arul, M.R.; Rudraiah, S.; Nukavarapu, S.P.; Vella, A.T.; Kumbar, S.G. Bioactive polymeric materials and electrical stimulation strategies for musculoskeletal tissue repair and regeneration. Bioact. Mater. 2020, 5, 468–485. [Google Scholar] [CrossRef]
- Wang, H.; Lü, H.; Yang, L.; Song, Z.; Hui, N. Glycyrrhiza polysaccharide doped the conducting polymer PEDOT hybrid-modified biosensors for the ultrasensitive detection of microRNA. Anal. Chim. Acta 2020, 1139, 155–163. [Google Scholar] [CrossRef]
- Nezakati, T.; Seifalian, A.; Tan, A.; Seifalian, A.M. Conductive Polymers: Opportunities and Challenges in Biomedical Applications. Chem. Rev. 2018, 118, 6766–6843. [Google Scholar] [CrossRef]
- Balint, R.; Cassidy, N.J.; Cartmell, S.H. Conductive polymers: Towards a smart biomaterial for tissue engineering. Acta Biomater. 2014, 10, 2341–2353. [Google Scholar] [CrossRef]
- Puiggalí-Jou, A.; del Valle, L.J.; Alemán, C. Drug delivery systems based on intrinsically conducting polymers. J. Control. Release 2019, 309, 244–264. [Google Scholar] [CrossRef]
- Behera, D.; Mishra, K.; Nayak, P. Formulation and Evaluation of Chitosan-Polyaniline Nanocomposites for Controlled Release of Anticancer Drug Doxorubicin. Int. J. Drug Deliv. Technol. 2018, 8, 95–102. [Google Scholar] [CrossRef]
- Chen, J.; Li, X.; Li, J.; Li, J.; Huang, L.; Ren, T.; Yang, X.; Zhong, S. Assembling of stimuli-responsive tumor targeting polypyrrole nanotubes drug carrier system for controlled release. Mater. Sci. Eng. C 2018, 89, 316–327. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Cheng, L.; Xiang, J.; Xu, H.; Feng, L.; Shi, X.; Liu, Z. Near-Infrared Absorbing Polymeric Nanoparticles as a Versatile Drug Carrier for Cancer Combination Therapy. Adv. Funct. Mater. 2013, 23, 6059–6067. [Google Scholar] [CrossRef]
- Hossen, S.; Hossain, M.K.; Basher, M.K.; Mia, M.N.H.; Rahman, M.T.; Uddin, M.J. Smart nanocarrier-based drug delivery systems for cancer therapy and toxicity studies: A review. J. Adv. Res. 2018, 15, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Rasheed, T.; Nabeel, F.; Hayat, U.; Bilal, M.; Iqbal, H.M.N. Endogenous and Exogenous Stimuli-Responsive Drug Delivery Systems for Programmed Site-Specific Release. Molecules 2019, 24, 1117. [Google Scholar] [CrossRef]
- Entzian, K.; Aigner, A. Drug Delivery by Ultrasound-Responsive Nanocarriers for Cancer Treatment. Pharmaceutics 2021, 13, 1135. [Google Scholar] [CrossRef]
- Osei, E.; Al-Asady, A. A review of ultrasound-mediated microbubbles technology for cancer therapy: A vehicle for chemotherapeutic drug delivery. J. Radiother. Pract. 2020, 19, 291–298. [Google Scholar] [CrossRef]
- Wei, P.; Cornel, E.J.; Du, J. Ultrasound-responsive polymer-based drug delivery systems. Drug Deliv. Transl. Res. 2021, 11, 1323–1339. [Google Scholar] [CrossRef]
- Husseini, G.A.; Pitt, W.G. Micelles and nanoparticles for ultrasonic drug and gene delivery. Adv. Drug Deliv. Rev. 2008, 60, 1137–1152. [Google Scholar] [CrossRef]
- Al Sawaftah, N.M.; Husseini, G.A. Ultrasound-Mediated Drug Delivery in Cancer Therapy: A Review. J. Nanosci. Nanotechnol. 2020, 20, 7211–7230. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, L.G.; Fan, X.M.; Pang, J.L. Ultrasound Responsive Smart Implantable Hydrogels for Targeted Delivery of Drugs: Reviewing Current Practices. Int. J. Nanomed. 2022, 17, 5001–5026. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.; Jazani, A.M.; Oh, J.K.; Noh, S.M. Perfluorocarbon Nanodroplets for Dual Delivery with Ultrasound/GSH-Responsive Release of Model Drug and Passive Release of Nitric Oxide. Polymers 2022, 14, 2240. [Google Scholar] [CrossRef] [PubMed]
- Delaney, L.J.; Isguven, S.; Eisenbrey, J.R.; Hickok, N.J.; Forsberg, F. Making waves: How ultrasound-targeted drug delivery is changing pharmaceutical approaches. Mater. Adv. 2022, 3, 3023–3040. [Google Scholar] [CrossRef]
- Luo, Z.; Jin, K.; Pang, Q.; Shen, S.; Yan, Z.; Jiang, T.; Zhu, X.; Yu, L.; Pang, Z.; Jiang, X. On-Demand Drug Release from Dual-Targeting Small Nanoparticles Triggered by High-Intensity Focused Ultrasound Enhanced Glioblastoma-Targeting Therapy. ACS Appl. Mater. Interfaces 2017, 9, 31612–31625. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Guo, L.; Shi, D.; Duan, S.; Li, J. Biocompatible Chitosan Nanobubbles for Ultrasound-Mediated Targeted Delivery of Doxorubicin. Nanoscale Res. Lett. 2019, 14, 24. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Jia, Y.; Qu, F.; Sun, Y.; Wang, P.; Zhang, K.; Xu, C.; Liu, Q.; Wang, X. Ultrasound-Responsive Polymeric Micelles for Sonoporation-Assisted Site-Specific Therapeutic Action. ACS Appl. Mater. Interfaces 2017, 9, 25706–25716. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Jiang, Y.; Lin, M.; Zhang, J.; Guo, H.; Yang, F.; Leung, W.; Xu, C. Ultrasound-responsive materials for drug/gene delivery. Front. Pharmacol. 2020, 10, 1650. [Google Scholar] [CrossRef]
- Mehier-Humbert, S.; Bettinger, T.; Yan, F.; Guy, R.H. Plasma membrane poration induced by ultrasound exposure: Implication for drug delivery. J. Control. Release 2005, 104, 213–222. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).