The Green Era of Food Packaging: General Considerations and New Trends
Abstract
:1. Introduction
- Substitution of thermal techniques and chemical sanitisation with green alternatives in order to reduce the consumption of resources and the impact on food quality.
- Extraction of added-value compounds from renewable sources (e.g., food by-products) and their application as alternatives to conventional preservatives and additives.
- Development of bio-based active packaging based on renewable biopolymers, aiming to reduce the use of petroleum-derived plastics in the food packaging sector, and to prolong the shelf-life of the products, preventing the generation of food waste.
2. Bio-Based Packaging: General Considerations
2.1. Compostable, Biodegradable, or Renewable?
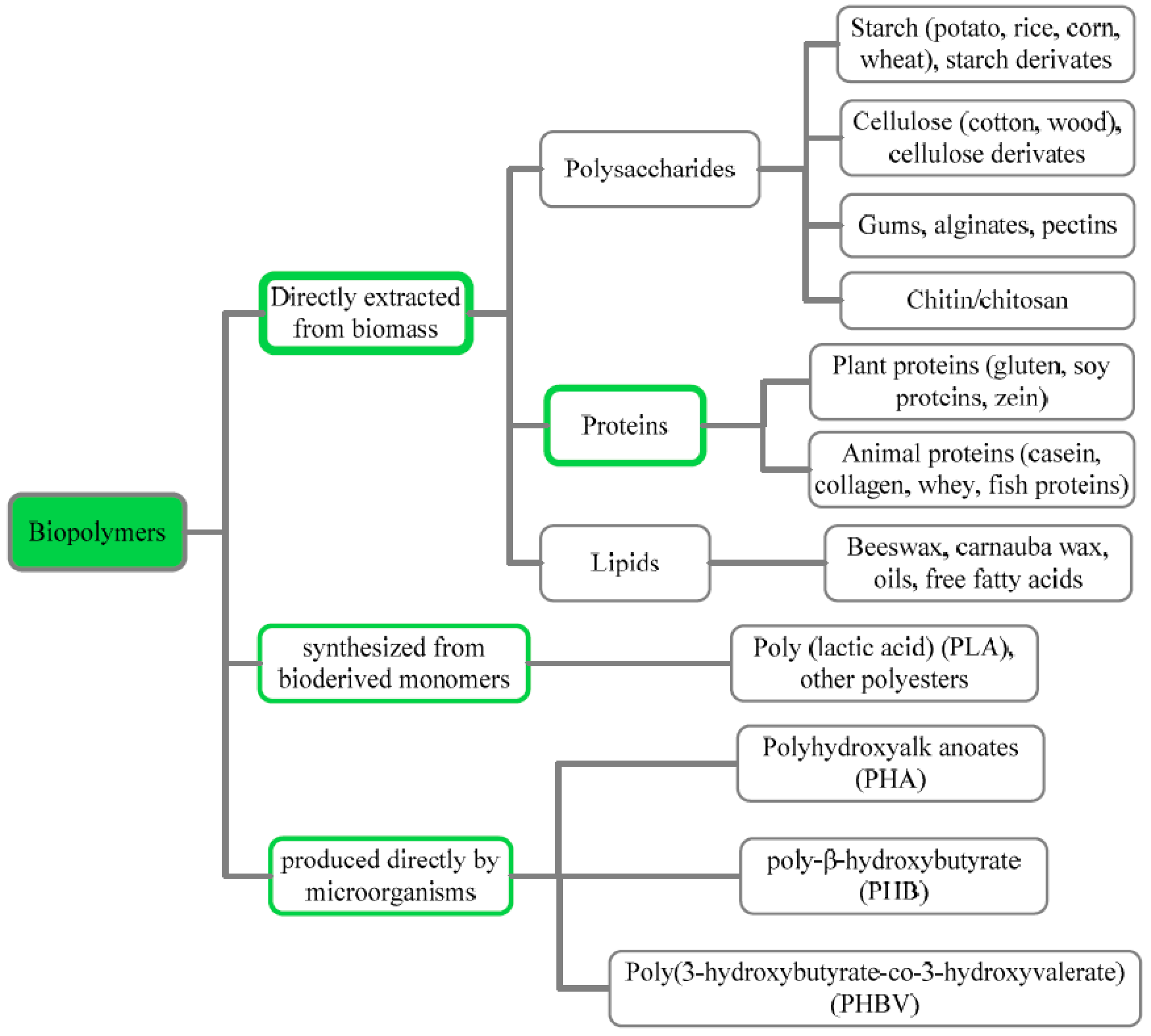
2.2. Biopolymers
2.2.1. Polysaccharides
Chitosan
Cellulose and Derivatives
Starch
Pectin
2.2.2. Proteins
Gelatin
Corn Zein
2.2.3. Polylactic Acid (PLA)
2.2.4. Polycaprolactone (PCL)
2.2.5. Polyhydroxy Butyrate (PHB)
3. Bio-Active Packaging
3.1. Antimicrobial Packaging
3.1.1. Essential Oils (EOs)
3.1.2. Animal-Derived Polypeptides
3.1.3. Antagonistic Microorganisms and Bacteriocins
3.2. Antioxidant Packaging
3.2.1. Natural Antioxidants
3.2.2. Plant Extracts
4. Nanotechnology in Biodegradable Packaging
4.1. Bio-Nanocomposite Materials
4.1.1. Nano-Clays
4.1.2. Metal Nanoparticles
4.1.3. Metal Oxides
4.1.4. Bio-Nanofillers
4.2. Nano-Encapsulation and Nano-Emulsions
5. Biodegradable Packaging from Agri-Food Waste
5.1. Life Cycle Assessment LCA
5.2. Pre-Treatments of By-Products and Application for Packaging Production
5.3. Impact on the Engineering Properties of Packaging
5.4. Impact on Antioxidant and Antimicrobial Capacities of Packaging
6. Future Challenges and Concluding Remarks
- EOs possess a strong biocidal efficacy on a broad range of microorganisms, which virtually makes them suitable alternatives to conventional preservatives. However, each of them also possesses a peculiar aromatic profile, which could negatively affect the flavour of food, and this hinders their broad usage [222];
- Most of the biodegradable packaging films still do not provide a sufficient water barrier for moisture-sensitive foods, and so their feasible applications are mainly restricted to disposable food wrappers for fast foods that do not require improved water barrier properties [174];
- To date, most of the studies focused on packaging with antimicrobial and antioxidant properties are still performed at the in vitro level. For the future, it would be worthy to extend the achieved findings to in vivo experiments in order to provide the food industry with more specific data about the impact of these extracts on food safety, quality, and shelf life.
- Industrial production of biopolymers for the replacement of plastic is still an impossible path to pursue due to the cost of production of these molecules compared with plastics.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaavya, R.; Pandiselvam, R.; Abdullah, S.; Sruthi, N.U.; Jayanath, Y.; Ashokkumar, C.; Chandra Khanashyam, A.; Kothakota, A.; Ramesh, S.V. Emerging Non-Thermal Technologies for Decontamination of Salmonella in Food. Trends Food Sci. Technol. 2021, 112, 400–418. [Google Scholar] [CrossRef]
- María, L.-P.; Díaz-Reinoso, B.; Lorenzo José, M.; Giancarlo, C.; Barba, F.J.; Moure, A.; Domínguez, H.; Daniel, F. Green Technologies for Food Processing: Principal Considerations. In Innovative Thermal and Non-Thermal Processing, Bioaccessibility and Bioavailability of Nutrients and Bioactive Compounds; Elsevier: Amsterdam, The Netherlands, 2019; pp. 55–103. ISBN 978-0-12-814174-8. [Google Scholar]
- Moeini, A.; Germann, N.; Malinconico, M.; Santagata, G. Formulation of Secondary Compounds as Additives of Biopolymer-Based Food Packaging: A Review. Trends Food Sci. Technol. 2021, 114, 342–354. [Google Scholar] [CrossRef]
- Trajkovska Petkoska, A.; Daniloski, D.; D’Cunha, N.M.; Naumovski, N.; Broach, A.T. Edible Packaging: Sustainable Solutions and Novel Trends in Food Packaging. Food Res. Int. 2021, 140, 109981. [Google Scholar] [CrossRef] [PubMed]
- Hoseinnejad, M.; Jafari, S.M.; Katouzian, I. Inorganic and Metal Nanoparticles and Their Antimicrobial Activity in Food Packaging Applications. Crit. Rev. Microbiol. 2018, 44, 161–181. [Google Scholar] [CrossRef]
- Risch, S.J. Food Packaging History and Innovations. J. Agric. Food Chem. 2009, 57, 8089–8092. [Google Scholar] [CrossRef]
- Park, J.H.; Koo, M.S.; Cho, S.H.; Lyu, M.-Y. Comparison of Thermal and Optical Properties and Flowability of Fossil-Based and Bio-Based Polycarbonate. Macromol. Res. 2017, 25, 1135–1144. [Google Scholar] [CrossRef]
- Paletta, A.; Leal Filho, W.; Balogun, A.-L.; Foschi, E.; Bonoli, A. Barriers and Challenges to Plastics Valorisation in the Context of a Circular Economy: Case Studies from Italy. J. Clean. Prod. 2019, 241, 118149. [Google Scholar] [CrossRef]
- Schwarzböck, T.; Van Eygen, E.; Rechberger, H.; Fellner, J. Determining the Amount of Waste Plastics in the Feed of Austrian Waste-to-Energy Facilities. Waste Manag. Res. J. Sustain. Circ. Econ. 2017, 35, 207–216. [Google Scholar] [CrossRef]
- Ghatge, S.; Yang, Y.; Ahn, J.-H.; Hur, H.-G. Biodegradation of Polyethylene: A Brief Review. Appl. Biol. Chem. 2020, 63, 27. [Google Scholar] [CrossRef]
- Fotopoulou, K.N.; Karapanagioti, H.K. Degradation of Various Plastics in the Environment. In Hazardous Chemicals Associated with Plastics in the Marine Environment; The Handbook of Environmental Chemistry; Takada, H., Karapanagioti, H.K., Eds.; Springer International Publishing: Cham, Switzerland, 2017; Volume 78, pp. 71–92. ISBN 978-3-319-95566-7. [Google Scholar]
- Tsironi, T.N.; Chatzidakis, S.M.; Stoforos, N.G. The Future of Polyethylene Terephthalate Bottles: Challenges and Sustainability. Packag. Technol. Sci. 2022, 35, 317–325. [Google Scholar] [CrossRef]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, Use, and Fate of All Plastics Ever Made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [Green Version]
- Hale, R.C.; Seeley, M.E.; La Guardia, M.J.; Mai, L.; Zeng, E.Y. A Global Perspective on Microplastics. J. Geophys. Res. Oceans 2020, 125. [Google Scholar] [CrossRef]
- Amin, U.; Khan, M.U.; Majeed, Y.; Rebezov, M.; Khayrullin, M.; Bobkova, E.; Shariati, M.A.; Chung, I.M.; Thiruvengadam, M. Potentials of Polysaccharides, Lipids and Proteins in Biodegradable Food Packaging Applications. Int. J. Biol. Macromol. 2021, 183, 2184–2198. [Google Scholar] [CrossRef]
- Mir, S.A.; Dar, B.N.; Wani, A.A.; Shah, M.A. Effect of Plant Extracts on the Techno-Functional Properties of Biodegradable Packaging Films. Trends Food Sci. Technol. 2018, 80, 141–154. [Google Scholar] [CrossRef]
- Lambert, S.; Wagner, M. Environmental Performance of Bio-Based and Biodegradable Plastics: The Road Ahead. Chem. Soc. Rev. 2017, 46, 6855–6871. [Google Scholar] [CrossRef]
- Asgher, M.; Qamar, S.A.; Bilal, M.; Iqbal, H.M.N. Bio-Based Active Food Packaging Materials: Sustainable Alternative to Conventional Petrochemical-Based Packaging Materials. Food Res. Int. 2020, 137, 109625. [Google Scholar] [CrossRef]
- Al-Tayyar, N.A.; Youssef, A.M.; Al-hindi, R. Antimicrobial Food Packaging Based on Sustainable Bio-Based Materials for Reducing Foodborne Pathogens: A Review. Food Chem. 2020, 310, 125915. [Google Scholar] [CrossRef]
- Zhong, Y.; Godwin, P.; Jin, Y.; Xiao, H. Biodegradable Polymers and Green-Based Antimicrobial Packaging Materials: A Mini-Review. Adv. Ind. Eng. Polym. Res. 2020, 3, 27–35. [Google Scholar] [CrossRef]
- Al Hosni, A.S.; Pittman, J.K.; Robson, G.D. Microbial Degradation of Four Biodegradable Polymers in Soil and Compost Demonstrating Polycaprolactone as an Ideal Compostable Plastic. Waste Manag. 2019, 97, 105–114. [Google Scholar] [CrossRef]
- Santagata, G.; Valerio, F.; Cimmino, A.; Dal Poggetto, G.; Masi, M.; Di Biase, M.; Malinconico, M.; Lavermicocca, P.; Evidente, A. Chemico-Physical and Antifungal Properties of Poly(Butylene Succinate)/Cavoxin Blend: Study of a Novel Bioactive Polymeric Based System. Eur. Polym. J. 2017, 94, 230–247. [Google Scholar] [CrossRef]
- Rangaraj, V.M.; Rambabu, K.; Banat, F.; Mittal, V. Natural Antioxidants-Based Edible Active Food Packaging: An Overview of Current Advancements. Food Biosci. 2021, 43, 101251. [Google Scholar] [CrossRef]
- Chen, H.; Wang, J.; Cheng, Y.; Wang, C.; Liu, H.; Bian, H.; Pan, Y.; Sun, J.; Han, W. Application of Protein-Based Films and Coatings for Food Packaging: A Review. Polymers 2019, 11, 2039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoseinabadi, A.; Rasooli, I.; Taran, M. Isolation and Identification of Poly β-Hydroxybutyrate Over-Producing Bacteria and Optimization of Production Medium. Jundishapur J. Microbiol. 2015, 8, e16965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kathuria, A.; Abiad, M.G.; Auras, R. PLLA-ZIF-8 Metal Organic Framework Composites for Potential Use in Food Applications: Production, Characterization and Migration Studies. Packag. Technol. Sci. 2021, 34, 393–400. [Google Scholar] [CrossRef]
- Lin, J.; Pan, D.; Sun, Y.; Ou, C.; Wang, Y.; Cao, J. The Modification of Gelatin Films: Based on Various Cross-linking Mechanism of Glutaraldehyde at Acidic and Alkaline Conditions. Food Sci. Nutr. 2019, 7, 4140–4146. [Google Scholar] [CrossRef] [PubMed]
- López de Dicastillo, C.; Bustos, F.; Guarda, A.; Galotto, M.J. Cross-Linked Methyl Cellulose Films with Murta Fruit Extract for Antioxidant and Antimicrobial Active Food Packaging. Food Hydrocoll. 2016, 60, 335–344. [Google Scholar] [CrossRef]
- Łupina, K.; Kowalczyk, D.; Zięba, E.; Kazimierczak, W.; Mężyńska, M.; Basiura-Cembala, M.; Wiącek, A.E. Edible Films Made from Blends of Gelatin and Polysaccharide-Based Emulsifiers—A Comparative Study. Food Hydrocoll. 2019, 96, 555–567. [Google Scholar] [CrossRef]
- Mahmoodi, A.; Ghodrati, S.; Khorasani, M. High-Strength, Low-Permeable, and Light-Protective Nanocomposite Films Based on a Hybrid Nanopigment and Biodegradable PLA for Food Packaging Applications. ACS Omega 2019, 4, 14947–14954. [Google Scholar] [CrossRef] [Green Version]
- Moeini, A.; van Reenen, A.; Van Otterlo, W.; Cimmino, A.; Masi, M.; Lavermicocca, P.; Valerio, F.; Immirzi, B.; Santagata, G.; Malinconico, M.; et al. α-Costic Acid, a Plant Sesquiterpenoid from Dittrichia Viscosa, as Modifier of Poly (Lactic Acid) Properties: A Novel Exploitation of the Autochthone Biomass Metabolite for a Wholly Biodegradable System. Ind. Crops Prod. 2020, 146, 112134. [Google Scholar] [CrossRef]
- Nair, S.S.; Chen, H.; Peng, Y.; Huang, Y.; Yan, N. Polylactic Acid Biocomposites Reinforced with Nanocellulose Fibrils with High Lignin Content for Improved Mechanical, Thermal and Barrier Properties. ACS Sustain. Chem 2018, 6, 10058–10068. [Google Scholar] [CrossRef]
- Qamar, S.A.; Asgher, M.; Bilal, M. Immobilization of Alkaline Protease From Bacillus Brevis Using Ca-Alginate Entrapment Strategy for Improved Catalytic Stability, Silver Recovery, and Dehairing Potentialities. Catal. Lett. 2020, 150, 3572–3583. [Google Scholar] [CrossRef]
- Shahbazi, M.; Ahmadi, S.J.; Seif, A.; Rajabzadeh, G. Carboxymethyl Cellulose Film Modification through Surface Photo-Crosslinking and Chemical Crosslinking for Food Packaging Applications. Food Hydrocoll. 2016, 61, 378–389. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Fernando, A.L.; Pires, J.R.A.; Rodrigues, P.F.; Lopes, A.A.S.; Fernandes, F.M.B. Physical Properties of Chitosan Films Incorporated with Natural Antioxidants. Ind. Crops Prod. 2017, 107, 565–572. [Google Scholar] [CrossRef]
- Talón, E.; Trifkovic, K.T.; Nedovic, V.A.; Bugarski, B.M.; Vargas, M.; Chiralt, A.; González-Martínez, C. Antioxidant Edible Films Based on Chitosan and Starch Containing Polyphenols from Thyme Extracts. Carbohydr. Polym. 2017, 157, 1153–1161. [Google Scholar] [CrossRef]
- Turco, R.; Ortega-Toro, R.; Tesser, R.; Mallardo, S.; Collazo-Bigliardi, S.; Chiralt Boix, A.; Malinconico, M.; Rippa, M.; Di Serio, M.; Santagata, G. Poly (Lactic Acid)/Thermoplastic Starch Films: Effect of Cardoon Seed Epoxidized Oil on Their Chemicophysical, Mechanical, and Barrier Properties. Coatings 2019, 9, 574. [Google Scholar] [CrossRef] [Green Version]
- Xia, C.; Wang, W.; Wang, L.; Liu, H.; Xiao, J. Multilayer Zein/Gelatin Films with Tunable Water Barrier Property and Prolonged Antioxidant Activity. Food Packag. Shelf Life 2019, 19, 76–85. [Google Scholar] [CrossRef]
- Xu, Q.; Chen, C.; Rosswurm, K.; Yao, T.; Janaswamy, S. A Facile Route to Prepare Cellulose-Based Films. Carbohydr. Polym. 2016, 149, 274–281. [Google Scholar] [CrossRef]
- Yu, D.; Feng, Y.; Xu, J.; Kong, B.; Liu, Q.; Wang, H. Fabrication, Characterization, and Antibacterial Properties of Citric Acid Crosslinked PVA Electrospun Microfibre Mats for Active Food Packaging. Packag. Technol. Sci. 2021, 34, 361–370. [Google Scholar] [CrossRef]
- Zhai, X.; Zhang, X.; Ao, H.; Yin, Y.; Li, X.; Ren, D. Preparation and Characterization of Whey Protein Isolate/Chitosan/Microcrystalline Cellulose Composite Films. Packag. Technol. Sci. 2021, 34, 589–599. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Z.; Yang, W.; Xue, C.; Wang, Y.; Dong, J.; Xue, Y. Modification of Gelatine with Galla Chinensis Extract, a Natural Crosslinker. Int. J. Food Prop. 2016, 19, 731–744. [Google Scholar] [CrossRef]
- Ferreira, A.; Alves, V.; Coelhoso, I. Polysaccharide-Based Membranes in Food Packaging Applications. Membranes 2016, 6, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nesic, A.; Moeini, A.; Santagata, G. 4 Marine Biopolymers: Alginate and Chitosan. In 4 Marine Biopolymers: Alginate and Chitosan; De Gruyter: Berlin, Germany, 2020; pp. 73–92. ISBN 978-3-11-059058-6. [Google Scholar]
- Priyadarshi, R.; Rhim, J.-W. Chitosan-Based Biodegradable Functional Films for Food Packaging Applications. Innov. Food Sci. Emerg. Technol. 2020, 62, 102346. [Google Scholar] [CrossRef]
- Nordtveit, R. Degradation of Partially N-Acetylated Chitosans with Hen Egg White and Human Lysozyme. Carbohydr. Polym. 1996, 29, 163–167. [Google Scholar] [CrossRef]
- Cazón, P.; Velazquez, G.; Ramírez, J.A.; Vázquez, M. Polysaccharide-Based Films and Coatings for Food Packaging: A Review. Food Hydrocoll. 2017, 68, 136–148. [Google Scholar] [CrossRef]
- Machado, B.R.; Facchi, S.P.; de Oliveira, A.C.; Nunes, C.S.; Souza, P.R.; Vilsinski, B.H.; Popat, K.C.; Kipper, M.J.; Muniz, E.C.; Martins, A.F. Bactericidal Pectin/Chitosan/Glycerol Films for Food Pack Coatings: A Critical Viewpoint. Int. J. Mol. Sci. 2020, 21, 8663. [Google Scholar] [CrossRef]
- Higueras, L.; López-Carballo, G.; Gavara, R.; Hernández-Muñoz, P. Reversible Covalent Immobilization of Cinnamaldehyde on Chitosan Films via Schiff Base Formation and Their Application in Active Food Packaging. Food Bioprocess Technol. 2015, 8, 526–538. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Mukherjee, A.; Dutta, J. Chitosan Based Nanocomposite Films and Coatings: Emerging Antimicrobial Food Packaging Alternatives. Trends Food Sci. Technol. 2020, 97, 196–209. [Google Scholar] [CrossRef]
- Wang, S.; Lu, A.; Zhang, L. Recent Advances in Regenerated Cellulose Materials. Prog. Polym. Sci. 2016, 53, 169–206. [Google Scholar] [CrossRef]
- Pérez, J.; Muñoz-Dorado, J.; de la Rubia, T.; Martínez, J. Biodegradation and Biological Treatments of Cellulose, Hemicellulose and Lignin: An Overview. Int. Microbiol. 2002, 5, 53–63. [Google Scholar] [CrossRef]
- Béguin, P.; Aubert, J.-P. The Biological Degradation of Cellulose. FEMS Microbiol. Rev. 1994, 13, 25–58. [Google Scholar] [CrossRef]
- Shaghaleh, H.; Xu, X.; Wang, S. Current Progress in Production of Biopolymeric Materials Based on Cellulose, Cellulose Nanofibers, and Cellulose Derivatives. RSC Adv. 2018, 8, 825–842. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.; Yu, M.; Wang, L. Physical and Antimicrobial Properties of Sodium Alginate/Carboxymethyl Cellulose Films Incorporated with Cinnamon Essential Oil. Food Packag. Shelf Life 2018, 15, 35–42. [Google Scholar] [CrossRef]
- Singh, P.; Magalhães, S.; Alves, L.; Antunes, F.; Miguel, M.; Lindman, B.; Medronho, B. Cellulose-Based Edible Films for Probiotic Entrapment. Food Hydrocoll. 2019, 88, 68–74. [Google Scholar] [CrossRef]
- Tyagi, P.; Salem, K.S.; Hubbe, M.A.; Pal, L. Advances in Barrier Coatings and Film Technologies for Achieving Sustainable Packaging of Food Products—A Review. Trends Food Sci. Technol. 2021, 115, 461–485. [Google Scholar] [CrossRef]
- Paunonen, S. Strength and Barrier Enhancements of Cellophane and Cellulose Derivative Films: A Review. BioResources 2013, 8, 3098–3121. [Google Scholar] [CrossRef]
- Puls, J.; Wilson, S.A.; Hölter, D. Degradation of Cellulose Acetate-Based Materials: A Review. J. Polym. Environ. 2011, 19, 152–165. [Google Scholar] [CrossRef] [Green Version]
- Yun, Y.-H.; Wee, Y.-J.; Byun, H.-S.; Yoon, S.-D. Biodegradability of Chemically Modified Starch (RS4)/PVA Blend Films: Part 2. J. Polym. Environ. 2008, 16, 12–18. [Google Scholar] [CrossRef]
- Su, C.; Li, D.; Wang, L.; Wang, Y. Biodegradation Behavior and Digestive Properties of Starch-Based Film for Food Packaging—A Review. Crit. Rev. Food Sci. Nutr. 2022, 1–23. [Google Scholar] [CrossRef]
- Abid, M.; Cheikhrouhou, S.; Renard, C.M.G.C.; Bureau, S.; Cuvelier, G.; Attia, H.; Ayadi, M.A. Characterization of Pectins Extracted from Pomegranate Peel and Their Gelling Properties. Food Chem. 2017, 215, 318–325. [Google Scholar] [CrossRef]
- Espitia, P.J.P.; Du, W.-X.; Avena-Bustillos, R.d.J.; Soares, N.d.F.F.; McHugh, T.H. Edible Films from Pectin: Physical-Mechanical and Antimicrobial Properties - A Review. Food Hydrocoll. 2014, 35, 287–296. [Google Scholar] [CrossRef]
- Willats, W.G.T.; Knox, J.P.; Mikkelsen, J.D. Pectin: New Insights into an Old Polymer Are Starting to Gel. Trends Food Sci. Technol. 2006, 17, 97–104. [Google Scholar] [CrossRef]
- De Cindio, B.; Gabriele, D.; Lupi, F.R. Pectin: Properties Determination and Uses. In Encyclopedia of Food and Health; Elsevier: Amsterdam, The Netherlands, 2016; pp. 294–300. ISBN 978-0-12-384953-3. [Google Scholar]
- Mosaad Khattab, A. The Microbial Degradation for Pectin. In Pectins—The New-Old Polysaccharides; Alberto Masuelli, M., Ed.; IntechOpen: London, UK, 2022; ISBN 978-1-83969-596-4. [Google Scholar]
- Satapathy, S.; Rout, J.R.; Kerry, R.G.; Thatoi, H.; Sahoo, S.L. Biochemical Prospects of Various Microbial Pectinase and Pectin: An Approachable Concept in Pharmaceutical Bioprocessing. Front. Nutr. 2020, 7, 117. [Google Scholar] [CrossRef]
- Šešlija, S.; Nešić, A.; Ružić, J.; Kalagasidis Krušić, M.; Veličković, S.; Avolio, R.; Santagata, G.; Malinconico, M. Edible Blend Films of Pectin and Poly(Ethylene Glycol): Preparation and Physico-Chemical Evaluation. Food Hydrocoll. 2018, 77, 494–501. [Google Scholar] [CrossRef]
- Calva-Estrada, S.J.; Jiménez-Fernández, M.; Lugo-Cervantes, E. Protein-Based Films: Advances in the Development of Biomaterials Applicable to Food Packaging. Food Eng. Rev. 2019, 11, 78–92. [Google Scholar] [CrossRef]
- Murrieta-Martínez, C.L.; Soto-Valdez, H.; Pacheco-Aguilar, R.; Torres-Arreola, W.; Rodríguez-Felix, F.; Márquez Ríos, E. Edible Protein Films: Sources and Behavior. Packag. Technol. Sci. 2018, 31, 113–122. [Google Scholar] [CrossRef]
- Umaraw, P.; Verma, A.K. Comprehensive Review on Application of Edible Film on Meat and Meat Products: An Eco-Friendly Approach. Crit. Rev. Food Sci. Nutr. 2017, 57, 1270–1279. [Google Scholar] [CrossRef]
- Ramos, M.; Valdés, A.; Beltrán, A.; Garrigós, M. Gelatin-Based Films and Coatings for Food Packaging Applications. Coatings 2016, 6, 41. [Google Scholar] [CrossRef] [Green Version]
- Sahraee, S.; Milani, J.M.; Regenstein, J.M.; Kafil, H.S. Protection of Foods against Oxidative Deterioration Using Edible Films and Coatings: A Review. Food Biosci. 2019, 32, 100451. [Google Scholar] [CrossRef]
- Corradini, E.; Curti, P.; Meniqueti, A.; Martins, A.; Rubira, A.; Muniz, E. Recent Advances in Food-Packing, Pharmaceutical and Biomedical Applications of Zein and Zein-Based Materials. Int. J. Mol. Sci. 2014, 15, 22438–22470. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Liu, Y.; Yong, H.; Qin, Y.; Liu, J.; Liu, J. Development of Multifunctional Food Packaging Films Based on Chitosan, TiO2 Nanoparticles and Anthocyanin-Rich Black Plum Peel Extract. Food Hydrocoll. 2019, 94, 80–92. [Google Scholar] [CrossRef]
- Qi, X.; Ren, Y.; Wang, X. New Advances in the Biodegradation of Poly(Lactic) Acid. Int. Biodeterior. Biodegrad. 2017, 117, 215–223. [Google Scholar] [CrossRef]
- Marra, A.; Silvestre, C.; Duraccio, D.; Cimmino, S. Polylactic Acid/Zinc Oxide Biocomposite Films for Food Packaging Application. Int. J. Biol. Macromol. 2016, 88, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Siakeng, R.; Jawaid, M.; Ariffin, H.; Sapuan, S.M.; Asim, M.; Saba, N. Natural Fiber Reinforced Polylactic Acid Composites: A Review. Polym. Compos. 2019, 40, 446–463. [Google Scholar] [CrossRef]
- Rasal, R.M.; Janorkar, A.V.; Hirt, D.E. Poly(Lactic Acid) Modifications. Prog. Polym. Sci. 2010, 35, 338–356. [Google Scholar] [CrossRef]
- Thakur, M.; Majid, I.; Hussain, S.; Nanda, V. Poly(ε-caprolactone): A Potential Polymer for Biodegradable Food Packaging Applications. Packag. Technol. Sci. 2021, 34, 449–461. [Google Scholar] [CrossRef]
- Bartnikowski, M.; Dargaville, T.R.; Ivanovski, S.; Hutmacher, D.W. Degradation Mechanisms of Polycaprolactone in the Context of Chemistry, Geometry and Environment. Prog. Polym. Sci. 2019, 96, 1–20. [Google Scholar] [CrossRef]
- Ilyas, R.A.; Zuhri, M.Y.M.; Norrrahim, M.N.F.; Misenan, M.S.M.; Jenol, M.A.; Samsudin, S.A.; Nurazzi, N.M.; Asyraf, M.R.M.; Supian, A.B.M.; Bangar, S.P.; et al. Natural Fiber-Reinforced Polycaprolactone Green and Hybrid Biocomposites for Various Advanced Applications. Polymers 2022, 14, 182. [Google Scholar] [CrossRef]
- Gan, Z.; Yu, D.; Zhong, Z.; Liang, Q.; Jing, X. Enzymatic Degradation of Poly(ε-Caprolactone)/Poly(Dl-Lactide) Blends in Phosphate Buffer Solution. Polymer 1999, 4, 2859–2862. [Google Scholar] [CrossRef]
- Dos Santos, A.J.; Oliveira Dalla Valentina, L.V.; Hidalgo Schulz, A.A.; Tomaz Duarte, M.A. From Obtaining to Degradation of PHB: A Literature Review. Part II. Ing. Cienc. 2018, 14, 207–228. [Google Scholar] [CrossRef] [Green Version]
- Markl, E. PHB-Bio Based and Biodegradable Replacement for PP: A Review. Nov. Tech. Nutr. Food Sci. 2018, 2, 206–209. [Google Scholar] [CrossRef]
- Panaitescu, D.M.; Frone, A.N.; Chiulan, I.; Nicolae, C.A.; Trusca, R.; Ghiurea, M.; Gabor, A.R.; Mihailescu, M.; Casarica, A.; Lupescu, I. Role of Bacterial Cellulose and Poly (3-Hydroxyhexanoate-Co-3-Hydroxyoctanoate) in Poly (3-Hydroxybutyrate) Blends and Composites. Cellulose 2018, 25, 5569–5591. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Fortunati, E.; Dominici, F.; López, J.; Kenny, J.M. Bionanocomposite Films Based on Plasticized PLA–PHB/Cellulose Nanocrystal Blends. Carbohydr. Polym. 2015, 121, 265–275. [Google Scholar] [CrossRef] [Green Version]
- Battisti, R.; Fronza, N.; Vargas Júnior, Á.; da Silveira, S.M.; Damas, M.S.P.; Quadri, M.G.N. Gelatin-Coated Paper with Antimicrobial and Antioxidant Effect for Beef Packaging. Food Packag. Shelf Life 2017, 11, 115–124. [Google Scholar] [CrossRef]
- Valerio, F.; Masi, M.; Cimmino, A.; Moeini, S.A.; Lavermicocca, P.; Evidente, A. Antimould Microbial and Plant Metabolites with Potential Use in Intelligent Food Packaging. Nat. Prod. Res. 2018, 32, 1605–1610. [Google Scholar] [CrossRef]
- Yildirim, S.; Röcker, B.; Pettersen, M.K.; Nilsen-Nygaard, J.; Ayhan, Z.; Rutkaite, R.; Radusin, T.; Suminska, P.; Marcos, B.; Coma, V. Active Packaging Applications for Food: Active Packaging Applications for Food. Compr. Rev. Food Sci. Food Saf. 2018, 17, 165–199. [Google Scholar] [CrossRef] [Green Version]
- Chawla, R.; Sivakumar, S.; Kaur, H. Antimicrobial Edible Films in Food Packaging: Current Scenario and Recent Nanotechnological Advancements- a Review. Carbohydr. Polym. Technol. Appl. 2021, 2, 100024. [Google Scholar] [CrossRef]
- Jideani, V.A.; Vogt, K. Antimicrobial Packaging for Extending the Shelf Life of Bread—A Review. Crit. Rev. Food Sci. Nutr. 2016, 56, 1313–1324. [Google Scholar] [CrossRef]
- Vilela, C.; Kurek, M.; Hayouka, Z.; Röcker, B.; Yildirim, S.; Antunes, M.D.C.; Nilsen-Nygaard, J.; Pettersen, M.K.; Freire, C.S.R. A Concise Guide to Active Agents for Active Food Packaging. Trends Food Sci. Technol. 2018, 80, 212–222. [Google Scholar] [CrossRef]
- Landi, C.; Paciello, L.; de Alteriis, E.; Brambilla, L.; Parascandola, P. High Cell Density Culture with S. Cerevisiae CEN.PK113-5D for IL-1β Production: Optimization, Modeling, and Physiological Aspects. Bioprocess Biosyst. Eng. 2015, 38, 251–261. [Google Scholar] [CrossRef]
- Gonçalves, A.A.; Rocha, M. Safety and Quality of Antimicrobial Packaging Applied to Seafood. MOJ Food Process. Technol. 2017, 4, 00079. [Google Scholar] [CrossRef]
- Sofi, S.A.; Singh, J.; Rafiq, S.; Ashraf, U.; Dar, B.N.; Nayik, G.A. A Comprehensive Review on Antimicrobial Packaging and Its Use in Food Packaging. Curr. Nutr. Food Sci. 2018, 14, 305–312. [Google Scholar] [CrossRef]
- Blanco Massani, M.; Molina, V.; Sanchez, M.; Renaud, V.; Eisenberg, P.; Vignolo, G. Active Polymers Containing Lactobacillus Curvatus CRL705 Bacteriocins: Effectiveness Assessment in Wieners. Int. J. Food Microbiol. 2014, 178, 7–12. [Google Scholar] [CrossRef]
- Choi, I.; Chang, Y.; Shin, S.-H.; Joo, E.; Song, H.; Eom, H.; Han, J. Development of Biopolymer Composite Films Using a Microfluidization Technique for Carboxymethylcellulose and Apple Skin Particles. Int. J. Mol. Sci. 2017, 18, 1278. [Google Scholar] [CrossRef] [Green Version]
- De Moura, M.R.; Mattoso, L.H.C.; Zucolotto, V. Development of Cellulose-Based Bactericidal Nanocomposites Containing Silver Nanoparticles and Their Use as Active Food Packaging. J. Food Eng. 2012, 109, 520–524. [Google Scholar] [CrossRef]
- Hage, M.; Chihib, N.-E.; Abdallah, M.; Khelissa, S.; Crocco, B.; Akoum, H.; Bentiss, F.; Jama, C. Nisin-Based Coatings for the Prevention of Biofilm Formation: Surface Characterization and Antimicrobial Assessments. Surf. Interfaces 2021, 27, 101564. [Google Scholar] [CrossRef]
- Siripatrawan, U.; Kaewklin, P. Fabrication and Characterization of Chitosan-Titanium Dioxide Nanocomposite Film as Ethylene Scavenging and Antimicrobial Active Food Packaging. Food Hydrocoll. 2018, 84, 125–134. [Google Scholar] [CrossRef]
- Sothornvit, R.; Hong, S.-I.; An, D.J.; Rhim, J.-W. Effect of Clay Content on the Physical and Antimicrobial Properties of Whey Protein Isolate/Organo-Clay Composite Films. LWT—Food Sci. Technol. 2010, 43, 279–284. [Google Scholar] [CrossRef]
- Sun, H.; Shao, X.; Zhang, M.; Wang, Z.; Dong, J.; Yu, D. Mechanical, Barrier and Antimicrobial Properties of Corn Distarch Phosphate/Nanocrystalline Cellulose Films Incorporated with Nisin and ε-Polylysine. Int. J. Biol. Macromol. 2019, 136, 839–846. [Google Scholar] [CrossRef]
- Xiong, Y.; Chen, M.; Warner, R.D.; Fang, Z. Incorporating Nisin and Grape Seed Extract in Chitosan-Gelatine Edible Coating and Its Effect on Cold Storage of Fresh Pork. Food Control 2020, 110, 107018. [Google Scholar] [CrossRef]
- Yang, W.; Fortunati, E.; Dominici, F.; Giovanale, G.; Mazzaglia, A.; Balestra, G.M.; Kenny, J.M.; Puglia, D. Synergic Effect of Cellulose and Lignin Nanostructures in PLA Based Systems for Food Antibacterial Packaging. Eur. Polym. J. 2016, 79, 1–12. [Google Scholar] [CrossRef]
- Yu, Z.; Dhital, R.; Wang, W.; Sun, L.; Zeng, W.; Mustapha, A.; Lin, M. Development of Multifunctional Nanocomposites Containing Cellulose Nanofibrils and Soy Proteins as Food Packaging Materials. Food Packag. Shelf Life 2019, 21, 100366. [Google Scholar] [CrossRef]
- Zhao, Y.; An, J.; Su, H.; Li, B.; Liang, D.; Huang, C. Antimicrobial Food Packaging Integrating Polysaccharide-Based Substrates with Green Antimicrobial Agents: A Sustainable Path. Food Res. Int. 2022, 155, 111096. [Google Scholar] [CrossRef]
- Mendonca, A.; Jackson-Davis, A.; Moutiq, R.; Thomas-Popo, E. Use of Natural Antimicrobials of Plant Origin to Improve the Microbiological Safety of Foods. In Food and Feed Safety Systems and Analysis; Elsevier: Amsterdam, The Netherlands, 2018; pp. 249–272. ISBN 978-0-12-811835-1. [Google Scholar]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of Essential Oils on Pathogenic Bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef]
- El-Saber Batiha, G.; Hussein, D.E.; Algammal, A.M.; George, T.T.; Jeandet, P.; Al-Snafi, A.E.; Tiwari, A.; Pagnossa, J.P.; Lima, C.M.; Thorat, N.D.; et al. Application of Natural Antimicrobials in Food Preservation: Recent Views. Food Control 2021, 126, 108066. [Google Scholar] [CrossRef]
- Haghighi, H.; Biard, S.; Bigi, F.; De Leo, R.; Bedin, E.; Pfeifer, F.; Siesler, H.W.; Licciardello, F.; Pulvirenti, A. Comprehensive Characterization of Active Chitosan-Gelatin Blend Films Enriched with Different Essential Oils. Food Hydrocoll. 2019, 95, 33–42. [Google Scholar] [CrossRef]
- Wang, H.; Yang, Z.; Ying, G.; Yang, M.; Nian, Y.; Wei, F.; Kong, W. Antifungal Evaluation of Plant Essential Oils and Their Major Components against Toxigenic Fungi. Ind. Crops Prod. 2018, 120, 180–186. [Google Scholar] [CrossRef]
- Abdollahi, M.; Rezaei, M.; Farzi, G. Improvement of Active Chitosan Film Properties with Rosemary Essential Oil for Food Packaging: Chitosan Film Properties with Rosemary Essential Oil. Int. J. Food Sci. Technol. 2012, 47, 847–853. [Google Scholar] [CrossRef]
- Bonilla, J.; Sobral, P.J.A. Investigation of the Physicochemical, Antimicrobial and Antioxidant Properties of Gelatin-Chitosan Edible Film Mixed with Plant Ethanolic Extracts. Food Biosci. 2016, 16, 17–25. [Google Scholar] [CrossRef]
- Bonilla, J.; Poloni, T.; Lourenço, R.V.; Sobral, P.J.A. Antioxidant Potential of Eugenol and Ginger Essential Oils with Gelatin/Chitosan Films. Food Biosci. 2018, 23, 107–114. [Google Scholar] [CrossRef]
- De Carvalho, S.Y.B.; Almeida, R.R.; Pinto, N.A.R.; de Mayrinck, C.; Vieira, S.S.; Haddad, J.F.; Leitão, A.A.; Guimarães, L.G. de L. Encapsulation of Essential Oils Using Cinnamic Acid Grafted Chitosan Nanogel: Preparation, Characterization and Antifungal Activity. Int. J. Biol. Macromol. 2021, 166, 902–912. [Google Scholar] [CrossRef]
- Donsì, F.; Annunziata, M.; Sessa, M.; Ferrari, G. Nanoencapsulation of Essential Oils to Enhance Their Antimicrobial Activity in Foods. LWT—Food Sci. Technol. 2011, 44, 1908–1914. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Rezaei, M.; Zandi, M.; Farahmandghavi, F. Development of Bioactive Fish Gelatin/Chitosan Nanoparticles Composite Films with Antimicrobial Properties. Food Chem. 2016, 194, 1266–1274. [Google Scholar] [CrossRef] [PubMed]
- Jouki, M.; Yazdi, F.T.; Mortazavi, S.A.; Koocheki, A. Quince Seed Mucilage Films Incorporated with Oregano Essential Oil: Physical, Thermal, Barrier, Antioxidant and Antibacterial Properties. Food Hydrocoll. 2014, 36, 9–19. [Google Scholar] [CrossRef]
- Laroque, D.A.; de Aragão, G.M.F.; de Araújo, P.H.H.; Carciofi, B.A.M. Active Cellulose Acetate-carvacrol Films Antibacterial Physical and Thermal Properties. Packag. Technol. Sci. 2021, 34, 463–474. [Google Scholar] [CrossRef]
- Lee, J.Y.; Garcia, C.V.; Shin, G.H.; Kim, J.T. Antibacterial and Antioxidant Properties of Hydroxypropyl Methylcellulose-Based Active Composite Films Incorporating Oregano Essential Oil Nanoemulsions. LWT 2019, 106, 164–171. [Google Scholar] [CrossRef]
- Li, Y.; Tang, C.; He, Q. Effect of Orange (Citrus Sinensis L.) Peel Essential Oil on Characteristics of Blend Films Based on Chitosan and Fish Skin Gelatin. Food Biosci. 2021, 41, 100927. [Google Scholar] [CrossRef]
- Mohammadi, M.; Mirabzadeh, S.; Shahvalizadeh, R.; Hamishehkar, H. Development of Novel Active Packaging Films Based on Whey Protein Isolate Incorporated with Chitosan Nanofiber and Nano-Formulated Cinnamon Oil. Int. J. Biol. Macromol. 2020, 149, 11–20. [Google Scholar] [CrossRef]
- Nisar, T.; Wang, Z.-C.; Yang, X.; Tian, Y.; Iqbal, M.; Guo, Y. Characterization of Citrus Pectin Films Integrated with Clove Bud Essential Oil: Physical, Thermal, Barrier, Antioxidant and Antibacterial Properties. Int. J. Biol. Macromol. 2018, 106, 670–680. [Google Scholar] [CrossRef]
- Pabast, M.; Shariatifar, N.; Beikzadeh, S.; Jahed, G. Effects of Chitosan Coatings Incorporating with Free or Nano-Encapsulated Satureja Plant Essential Oil on Quality Characteristics of Lamb Meat. Food Control 2018, 91, 185–192. [Google Scholar] [CrossRef]
- Ruiz-Navajas, Y.; Viuda-Martos, M.; Sendra, E.; Perez-Alvarez, J.A.; Fernández-López, J. In Vitro Antibacterial and Antioxidant Properties of Chitosan Edible Films Incorporated with Thymus Moroderi or Thymus Piperella Essential Oils. Food Control 2013, 30, 386–392. [Google Scholar] [CrossRef]
- Sharma, P.; Ahuja, A.; Dilsad Izrayeel, A.M.; Samyn, P.; Rastogi, V.K. Physicochemical and Thermal Characterization of Poly (3-Hydroxybutyrate-Co-4-Hydroxybutyrate) Films Incorporating Thyme Essential Oil for Active Packaging of White Bread. Food Control 2022, 133, 108688. [Google Scholar] [CrossRef]
- Choulitoudi, E.; Ganiari, S.; Tsironi, T.; Ntzimani, A.; Tsimogiannis, D.; Taoukis, P.; Oreopoulou, V. Edible Coating Enriched with Rosemary Extracts to Enhance Oxidative and Microbial Stability of Smoked Eel Fillets. Food Packag. Shelf Life 2017, 12, 107–113. [Google Scholar] [CrossRef]
- Tongnuanchan, P.; Benjakul, S. Essential Oils: Extraction, Bioactivities, and Their Uses for Food Preservation: Bioactivities and Applications of Essential Oils. J. Food Sci. 2014, 79, R1231–R1249. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A.; Georgescu, C.; Turcuş, V.; Olah, N.K.; Mathe, E. An Overview of Natural Antimicrobials Role in Food. Eur. J. Med. Chem. 2018, 143, 922–935. [Google Scholar] [CrossRef]
- Direct Food Substances Affirmed as Generally Recognized as Safe; Egg White Lysozyme; The Food and Drug Administration (FDA): Silver Spring, MD, USA, 1998.
- Davidson, P.M.; Taylor, T.M.; Schmidt, S.E. Chemical Preservatives and Natural Antimicrobial Compounds. In Food Microbiology; John Wiley & Sons, Ltd: Hoboken, NJ, USA, 2012; pp. 765–801. ISBN 978-1-68367-058-2. [Google Scholar]
- Aloui, H.; Khwaldia, K. Natural Antimicrobial Edible Coatings for Microbial Safety and Food Quality Enhancement: Bioactive Coatings for Food Preservation. Compr. Rev. Food Sci. Food Saf. 2016, 15, 1080–1103. [Google Scholar] [CrossRef]
- Leśnierowski, G.; Yang, T. Lysozyme and Its Modified Forms: A Critical Appraisal of Selected Properties and Potential. Trends Food Sci. Technol. 2021, 107, 333–342. [Google Scholar] [CrossRef]
- Soggiu, A.; Piras, C.; Mortera, S.L.; Alloggio, I.; Urbani, A.; Bonizzi, L.; Roncada, P. Unravelling the Effect of Clostridia Spores and Lysozyme on Microbiota Dynamics in Grana Padano Cheese: A Metaproteomics Approach. J. Proteomics 2016, 147, 21–27. [Google Scholar] [CrossRef]
- Saeed, F.; Afzaal, M.; Tufail, T.; Ahmad, A. Use of Natural Antimicrobial Agents: A Safe Preservation Approach. In Active Antimicrobial Food Packaging; Var, I., Uzunlu, S., Eds.; IntechOpen: London, UK, 2019; ISBN 978-1-78985-003-1. [Google Scholar]
- Buys, E.M.; Seifu, E. Enzymes Indigenous to Milk: Lactoperoxidase. In Encyclopedia of Dairy Sciences, 3rd ed.; McSweeney, P.L.H., McNamara, J.P., Eds.; Academic Press: Oxford, UK, 2022; pp. 670–676. ISBN 978-0-12-818767-8. [Google Scholar]
- Niaz, B.; Saeed, F.; Ahmed, A.; Imran, M.; Maan, A.A.; Khan, M.K.I.; Tufail, T.; Anjum, F.M.; Hussain, S.; Suleria, H.A.R. Lactoferrin (LF): A Natural Antimicrobial Protein. Int. J. Food Prop. 2019, 22, 1626–1641. [Google Scholar] [CrossRef] [Green Version]
- Gyawali, R.; Ibrahim, S.A. Natural Products as Antimicrobial Agents. Food Control 2014, 46, 412–429. [Google Scholar] [CrossRef]
- Quinto, E.J.; Caro, I.; Villalobos-Delgado, L.H.; Mateo, J.; De-Mateo-Silleras, B.; Redondo-Del-Río, M.P. Food Safety through Natural Antimicrobials. Antibiotics 2019, 8, 208. [Google Scholar] [CrossRef]
- Agriopoulou, S.; Stamatelopoulou, E.; Sachadyn-Król, M.; Varzakas, T. Lactic Acid Bacteria as Antibacterial Agents to Extend the Shelf Life of Fresh and Minimally Processed Fruits and Vegetables: Quality and Safety Aspects. Microorganisms 2020, 8, 952. [Google Scholar] [CrossRef]
- Ramos, B.; Brandão, T.R.S.; Teixeira, P.; Silva, C.L.M. Biopreservation Approaches to Reduce Listeria Monocytogenes in Fresh Vegetables. Food Microbiol. 2020, 85, 103282. [Google Scholar] [CrossRef] [PubMed]
- Cheong, E.Y.L.; Sandhu, A.; Jayabalan, J.; Kieu Le, T.T.; Nhiep, N.T.; My Ho, H.T.; Zwielehner, J.; Bansal, N.; Turner, M.S. Isolation of Lactic Acid Bacteria with Antifungal Activity against the Common Cheese Spoilage Mould Penicillium Commune and Their Potential as Biopreservatives in Cheese. Food Control 2014, 46, 91–97. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Li, D.; Liu, X. Effects of Lactobacillus Sakei C2 and Sakacin C2 Individually or in Combination on the Growth of Listeria Monocytogenes, Chemical and Odor Changes of Vacuum-Packed Sliced Cooked Ham. Food Control 2015, 47, 27–31. [Google Scholar] [CrossRef]
- Tiwari, B.K.; Valdramidis, V.P.; O’ Donnell, C.P.; Muthukumarappan, K.; Bourke, P.; Cullen, P.J. Application of Natural Antimicrobials for Food Preservation. J. Agric. Food Chem. 2009, 57, 5987–6000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bauer, R.; Dicks, L.M.T. Mode of Action of Lipid II-Targeting Lantibiotics. Int. J. Food Microbiol. 2005, 101, 201–216. [Google Scholar] [CrossRef] [PubMed]
- Ibarra-Sánchez, L.A.; El-Haddad, N.; Mahmoud, D.; Miller, M.J.; Karam, L. Invited Review: Advances in Nisin Use for Preservation of Dairy Products. J. Dairy Sci. 2020, 103, 2041–2052. [Google Scholar] [CrossRef] [PubMed]
- Gabrielsen, C.; Brede, D.A.; Nes, I.F.; Diep, D.B. Circular Bacteriocins: Biosynthesis and Mode of Action. Appl. Environ. Microbiol. 2014, 80, 6854–6862. [Google Scholar] [CrossRef] [Green Version]
- Verma, S.K.; Sood, S.K.; Saini, R.K.; Saini, N. Pediocin PA-1 Containing Fermented Cheese Whey Reduces Total Viable Count of Raw Buffalo (Bubalis Bubalus) Milk. LWT—Food Sci. Technol. 2017, 83, 193–200. [Google Scholar] [CrossRef]
- Castellano, P.; Pérez Ibarreche, M.; Blanco Massani, M.; Fontana, C.; Vignolo, G. Strategies for Pathogen Biocontrol Using Lactic Acid Bacteria and Their Metabolites: A Focus on Meat Ecosystems and Industrial Environments. Microorganisms 2017, 5, 38. [Google Scholar] [CrossRef]
- Arnon-Rips, H.; Poverenov, E. 10—Biopolymers-Embedded Nanoemulsions and Other Nanotechnological Approaches for Safety, Quality, and Storability Enhancement of Food Products: Active Edible Coatings and Films. In Emulsions; Grumezescu, A.M., Ed.; Nanotechnology in the Agri-Food Industry; Academic Press: Oxford, UK, 2016; pp. 329–363. ISBN 978-0-12-804306-6. [Google Scholar]
- Gómez-Estaca, J.; López-de-Dicastillo, C.; Hernández-Muñoz, P.; Catalá, R.; Gavara, R. Advances in Antioxidant Active Food Packaging. Trends Food Sci. Technol. 2014, 35, 42–51. [Google Scholar] [CrossRef]
- Castro López, M.d.M.; López de Dicastillo, C.; López Vilariño, J.M.; González Rodríguez, M.V. Improving the Capacity of Polypropylene To Be Used in Antioxidant Active Films: Incorporation of Plasticizer and Natural Antioxidants. J. Agric. Food Chem. 2013, 61, 8462–8470. [Google Scholar] [CrossRef] [PubMed]
- Papuc, C.; Goran, G.V.; Predescu, C.N.; Nicorescu, V.; Stefan, G. Plant Polyphenols as Antioxidant and Antibacterial Agents for Shelf-Life Extension of Meat and Meat Products: Classification, Structures, Sources, and Action Mechanisms: Polyphenols Extending Meat Shelf-Life. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1243–1268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, V.T.; Pham, H.N.T.; Bowyer, M.C.; van Altena, I.A.; Scarlett, C.J. Influence of Solvents and Novel Extraction Methods on Bioactive Compounds and Antioxidant Capacity of Phyllanthus Amarus. Chem. Pap. 2016, 0. [Google Scholar] [CrossRef]
- Tuberoso, C.I.G.; Boban, M.; Bifulco, E.; Budimir, D.; Pirisi, F.M. Antioxidant Capacity and Vasodilatory Properties of Mediterranean Food: The Case of Cannonau Wine, Myrtle Berries Liqueur and Strawberry-Tree Honey. Food Chem. 2013, 140, 686–691. [Google Scholar] [CrossRef]
- Oroian, M.; Escriche, I. Antioxidants: Characterization, Natural Sources, Extraction and Analysis. Food Res. Int. Ott. Ont 2015, 74, 10–36. [Google Scholar] [CrossRef]
- Garcia-Vaquero, M.; Ummat, V.; Tiwari, B.; Rajauria, G. Exploring Ultrasound, Microwave and Ultrasound–Microwave Assisted Extraction Technologies to Increase the Extraction of Bioactive Compounds and Antioxidants from Brown Macroalgae. Mar. Drugs 2020, 18, 172. [Google Scholar] [CrossRef] [Green Version]
- Peng, Y.; Wu, Y.; Li, Y. Development of Tea Extracts and Chitosan Composite Films for Active Packaging Materials. Int. J. Biol. Macromol. 2013, 59, 282–289. [Google Scholar] [CrossRef]
- Siripatrawan, U.; Harte, B.R. Physical Properties and Antioxidant Activity of an Active Film from Chitosan Incorporated with Green Tea Extract. Food Hydrocoll. 2010, 24, 770–775. [Google Scholar] [CrossRef]
- Raghav, P.; Saini, M. Development of Mint (Mentha Viridis L.) Herbal Edible Coating for Shelf Life Enhancement of Cucumber (Cucumis Sativus). Int. J. Green Herb. Chem. 2018, 7. [Google Scholar] [CrossRef]
- Oudjedi, K.; Manso, S.; Nerin, C.; Hassissen, N.; Zaidi, F. New Active Antioxidant Multilayer Food Packaging Films Containing Algerian Sage and Bay Leaves Extracts and Their Application for Oxidative Stability of Fried Potatoes. Food Control 2019, 98, 216–226. [Google Scholar] [CrossRef] [Green Version]
- Kumar, N.; Ojha, A.; Singh, R. Preparation and Characterization of Chitosan - Pullulan Blended Edible Films Enrich with Pomegranate Peel Extract. React. Funct. Polym. 2019, 144, 104350. [Google Scholar] [CrossRef]
- Sun, L.; Sun, J.; Chen, L.; Niu, P.; Yang, X.; Guo, Y. Preparation and Characterization of Chitosan Film Incorporated with Thinned Young Apple Polyphenols as an Active Packaging Material. Carbohydr. Polym. 2017, 163, 81–91. [Google Scholar] [CrossRef] [Green Version]
- Farhoodi, M. Nanocomposite Materials for Food Packaging Applications: Characterization and Safety Evaluation. Food Eng. Rev. 2016, 8, 35–51. [Google Scholar] [CrossRef]
- Nile, S.H.; Baskar, V.; Selvaraj, D.; Nile, A.; Xiao, J.; Kai, G. Nanotechnologies in Food Science: Applications, Recent Trends, and Future Perspectives. Nano-Micro Lett. 2020, 12, 45. [Google Scholar] [CrossRef] [Green Version]
- Sharma, R.; Jafari, S.M.; Sharma, S. Antimicrobial Bio-Nanocomposites and Their Potential Applications in Food Packaging. Food Control 2020, 112, 107086. [Google Scholar] [CrossRef]
- Zubair, M.; Ullah, A. Recent Advances in Protein Derived Bionanocomposites for Food Packaging Applications. Crit. Rev. Food Sci. Nutr. 2020, 60, 406–434. [Google Scholar] [CrossRef]
- Honarvar, Z.; Hadian, Z.; Mashayekh, M. Nanocomposites in Food Packaging Applications and Their Risk Assessment for Health. Electron. Physician 2016, 8, 2531–2538. [Google Scholar] [CrossRef] [Green Version]
- Rhim, J.-W.; Park, H.-M.; Ha, C.-S. Bio-Nanocomposites for Food Packaging Applications. Prog. Polym. Sci. 2013, 38, 1629–1652. [Google Scholar] [CrossRef]
- Vasile, C. Polymeric Nanocomposites and Nanocoatings for Food Packaging: A Review. Materials 2018, 11, 1834. [Google Scholar] [CrossRef]
- Gürler, N.; Torğut, G. Physicomechanical Thermal and Dielectric Properties of Eco-friendly Starch-Microcrystalline Cellulose-Clay Nanocomposite Films for Food Packaging and Electrical Applications. Packag. Technol. Sci. 2022, 35, 473–483. [Google Scholar] [CrossRef]
- Vengatesan, M.R.; Singh, S.; Pillai, V.V.; Mittal, V. Crystallization, Mechanical, and Fracture Behavior of Mullite Fiber-Reinforced Polypropylene Nanocomposites. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Yang, S.; Bai, S.; Wang, Q. Sustainable Packaging Biocomposites from Polylactic Acid and Wheat Straw: Enhanced Physical Performance by Solid State Shear Milling Process. Compos. Sci. Technol. 2018, 158, 34–42. [Google Scholar] [CrossRef]
- De Azeredo, H.M.C. Nanocomposites for Food Packaging Applications. Food Res. Int. 2009, 42, 1240–1253. [Google Scholar] [CrossRef] [Green Version]
- Alboofetileh, M.; Rezaei, M.; Hosseini, H.; Abdollahi, M. Effect of Montmorillonite Clay and Biopolymer Concentration on the Physical and Mechanical Properties of Alginate Nanocomposite Films. J. Food Eng. 2013, 117, 26–33. [Google Scholar] [CrossRef]
- Kirkmeyer, B.P.; Puetter, R.C.; Yahil, A.; Winey, K.I. Deconvolution of Scanning Transmission Electron Microscopy Images of Ionomers. J. Polym. Sci. Part B Polym. Phys. 2003, 41, 319–326. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Kamle, M.; Shukla, S.; Mahato, D.K.; Chandra, P.; Hwang, S.K.; Kumar, P.; Huh, Y.S.; Han, Y.-K. Prospects of Using Nanotechnology for Food Preservation, Safety, and Security. J. Food Drug Anal. 2018, 26, 1201–1214. [Google Scholar] [CrossRef] [PubMed]
- Tamayo, L.; Azócar, M.; Kogan, M.; Riveros, A.; Páez, M. Copper-Polymer Nanocomposites: An Excellent and Cost-Effective Biocide for Use on Antibacterial Surfaces. Mater. Sci. Eng. C 2016, 69, 1391–1409. [Google Scholar] [CrossRef]
- Bhakya, S.; Muthukrishnan, S.; Sukumaran, M.; Muthukumar, M. Biogenic Synthesis of Silver Nanoparticles and Their Antioxidant and Antibacterial Activity. Appl. Nanosci. 2016, 6, 755–766. [Google Scholar] [CrossRef] [Green Version]
- Dos Santos, C.A.; Ingle, A.P.; Rai, M. The Emerging Role of Metallic Nanoparticles in Food. Appl. Microbiol. Biotechnol. 2020, 104, 2373–2383. [Google Scholar] [CrossRef]
- Oun, A.A.; Shankar, S.; Rhim, J.-W. Multifunctional Nanocellulose/Metal and Metal Oxide Nanoparticle Hybrid Nanomaterials. Crit. Rev. Food Sci. Nutr. 2020, 60, 435–460. [Google Scholar] [CrossRef] [PubMed]
- Rajakumar, G.; Thiruvengadam, M.; Mydhili, G.; Gomathi, T.; Chung, I.-M. Green Approach for Synthesis of Zinc Oxide Nanoparticles from Andrographis Paniculata Leaf Extract and Evaluation of Their Antioxidant, Anti-Diabetic, and Anti-Inflammatory Activities. Bioprocess Biosyst. Eng. 2018, 41, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Duan, G.; Zhang, G.; Yang, H.; He, S.; Jiang, S. Electrospun Functional Materials toward Food Packaging Applications: A Review. Nanomaterials 2020, 10, 150. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.N.; Rahman, F. Production and Modification of Nanofibrillated Cellulose Composites and Potential Applications. In Green Composites for Automotive Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 115–141. ISBN 978-0-08-102177-4. [Google Scholar]
- Johansson, C.; Bras, J.; Mondragon, I.; Nechita, P.; Plackett, D.; Simon, P.; Svetec, D.G.; Virtanen, S.; Baschetti, M.G.; Breen, C.; et al. Renewable fibers and bio-based materials for packaging applications—A review of recent developments. BioResources 2012, 7, 2506–2552. [Google Scholar] [CrossRef]
- Vilarinho, F.; Sanches Silva, A.; Vaz, M.F.; Farinha, J.P. Nanocellulose in Green Food Packaging. Crit. Rev. Food Sci. Nutr. 2018, 58, 1526–1537. [Google Scholar] [CrossRef]
- Bharimalla, A.K.; Patil, P.G.; Mukherjee, S.; Yadav, V.; Prasad, V. Nanocellulose-Polymer Composites: Novel Materials for Food Packaging Applications. In Polymers for Agri-Food Applications; Gutiérrez, T.J., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 553–599. ISBN 978-3-030-19415-4. [Google Scholar]
- Yu, S.; Sun, J.; Shi, Y.; Wang, Q.; Wu, J.; Liu, J. Nanocellulose from Various Biomass Wastes: Its Preparation and Potential Usages towards the High Value-Added Products. Environ. Sci. Ecotechnol. 2021, 5, 100077. [Google Scholar] [CrossRef]
- Prakash Menon, M.; Selvakumar, R.; Suresh kumar, P.; Ramakrishna, S. Extraction and Modification of Cellulose Nanofibers Derived from Biomass for Environmental Application. RSC Adv. 2017, 7, 42750–42773. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Han, X.; Zhang, C.; Liu, K.; Duan, G. Source of Nanocellulose and Its Application in Nanocomposite Packaging Material: A Review. Nanomaterials 2022, 12, 3158. [Google Scholar] [CrossRef]
- Moriana, R.; Vilaplana, F.; Ek, M. Cellulose Nanocrystals from Forest Residues as Reinforcing Agents for Composites: A Study from Macro- to Nano-Dimensions. Carbohydr. Polym. 2016, 139, 139–149. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, C.; Chang, P.R.; Cao, X.; Anderson, D.P. Bionanocomposites Based on Pea Starch and Cellulose Nanowhiskers Hydrolyzed from Pea Hull Fibre: Effect of Hydrolysis Time. Carbohydr. Polym. 2009, 76, 607–615. [Google Scholar] [CrossRef]
- Isogai, A.; Zhou, Y. Diverse Nanocelluloses Prepared from TEMPO-Oxidized Wood Cellulose Fibers: Nanonetworks, Nanofibers, and Nanocrystals. Curr. Opin. Solid State Mater. Sci. 2019, 23, 101–106. [Google Scholar] [CrossRef]
- Reshmy, R.; Eapen, P.; Deepa, T.; Aravind, M.; Raveendran, S.; Parameswaran, B.; Sunita, V.; Mukesh, K.A.; Ashok, P. Bacterial Nanocellulose: Engineering, Production, and Applications. Bioengineered 2021, 12, 11463–11483. [Google Scholar] [CrossRef]
- Dufresne, A. Nanocellulose: A New Ageless Bionanomaterial. Mater. Today 2013, 16, 220–227. [Google Scholar] [CrossRef]
- Almasi, H.; Ghanbarzadeh, B.; Dehghannya, J.; Entezami, A.A.; Asl, A.K. Novel Nanocomposites Based on Fatty Acid Modified Cellulose Nanofibers/Poly(Lactic Acid): Morphological and Physical Properties. Food Packag. Shelf Life 2015, 5, 21–31. [Google Scholar] [CrossRef]
- Oyeoka, H.C.; Ewulonu, C.M.; Nwuzor, I.C.; Obele, C.M.; Nwabanne, J.T. Packaging and Degradability Properties of Polyvinyl Alcohol/Gelatin Nanocomposite Films Filled Water Hyacinth Cellulose Nanocrystals. J. Bioresour. Bioprod. 2021, 6, 168–185. [Google Scholar] [CrossRef]
- Donsì, F. Applications of Nanoemulsions in Foods. In Nanoemulsions; Elsevier: Amsterdam, The Netherlands, 2018; pp. 349–377. ISBN 978-0-12-811838-2. [Google Scholar]
- Liu, F.; Avena-Bustillos, R.J.; Chiou, B.-S.; Li, Y.; Ma, Y.; Williams, T.G.; Wood, D.F.; McHugh, T.H.; Zhong, F. Controlled-Release of Tea Polyphenol from Gelatin Films Incorporated with Different Ratios of Free/Nanoencapsulated Tea Polyphenols into Fatty Food Simulants. Food Hydrocoll. 2017, 62, 212–221. [Google Scholar] [CrossRef]
- Cui, H.; Surendhiran, D.; Li, C.; Lin, L. Biodegradable Zein Active Film Containing Chitosan Nanoparticle Encapsulated with Pomegranate Peel Extract for Food Packaging. Food Packag. Shelf Life 2020, 24, 100511. [Google Scholar] [CrossRef]
- Robledo, N.; Vera, P.; López, L.; Yazdani-Pedram, M.; Tapia, C.; Abugoch, L. Thymol Nanoemulsions Incorporated in Quinoa Protein/Chitosan Edible Films; Antifungal Effect in Cherry Tomatoes. Food Chem. 2018, 246, 211–219. [Google Scholar] [CrossRef]
- Hasan, S.M.K.; Ferrentino, G.; Scampicchio, M. Nanoemulsion as Advanced Edible Coatings to Preserve the Quality of Fresh-cut Fruits and Vegetables: A Review. Int. J. Food Sci. Technol. 2020, 55, 1–10. [Google Scholar] [CrossRef]
- Girotto, F.; Alibardi, L.; Cossu, R. Food Waste Generation and Industrial Uses: A Review. Waste Manag. 2015, 45, 32–41. [Google Scholar] [CrossRef]
- Xiong, X.; Yu, I.K.M.; Tsang, D.C.W.; Bolan, N.S.; Sik Ok, Y.; Igalavithana, A.D.; Kirkham, M.B.; Kim, K.-H.; Vikrant, K. Value-Added Chemicals from Food Supply Chain Wastes: State-of-the-Art Review and Future Prospects. Chem. Eng. J. 2019, 375, 121983. [Google Scholar] [CrossRef]
- Dilucia, F.; Lacivita, V.; Conte, A.; Del Nobile, M.A. Sustainable Use of Fruit and Vegetable By-Products to Enhance Food Packaging Performance. Foods 2020, 9, 857. [Google Scholar] [CrossRef] [PubMed]
- Rodsamran, P.; Sothornvit, R. Lime Peel Pectin Integrated with Coconut Water and Lime Peel Extract as a New Bioactive Film Sachet to Retard Soybean Oil Oxidation. Food Hydrocoll. 2019, 97, 105173. [Google Scholar] [CrossRef]
- Plastics Europe Report “The Circular Economy for Plastics—A European Overview”, 2nd ed. 2022, Brussels, Belgium. Available online: https://plasticseurope.org/knowledge-hub/the-circular-economy-for-plastics-a-european-overview-2/ (accessed on 14 September 2022).
- Kakadellis, S.; Harris, Z.M. Don’t scrap the waste: The need for broader system boundaries in bioplastic food packaging life-cycle assessment—A critical review. J. Clean. Prod. 2020, 274, 122831. [Google Scholar] [CrossRef]
- Horowitz, N.; Frago, J.; Mu, D. Life Cycle Assessment of Bottled Water: A Case Study of Green2O Products. Waste Manag. 2018, 76, 734–743. [Google Scholar] [CrossRef]
- Desole, M.P.; Aversa, C.; Barletta, M.; Gisario, A.; Vosooghnia, A. Life Cycle Assessment (LCA) of PET and PLA Bottles for the Packaging of Fresh Pasteurised Milk: The Role of the Manufacturing Process and the Disposal Scenario. Packag. Technol. Sci. 2022, 35, 135–152. [Google Scholar] [CrossRef]
- Khan, M.M.H.; Laitinen, V.; Havukainen, J.; Horttanainen, M. Carbon Footprint of Different Recovery Options for the Repulping Reject from Liquid Packaging Board Waste Treatment Process. Waste Manag. 2021, 136, 93–103. [Google Scholar] [CrossRef]
- Moghadam, M.; Salami, M.; Mohammadian, M.; Khodadadi, M.; Emam-Djomeh, Z. Development of Antioxidant Edible Films Based on Mung Bean Protein Enriched with Pomegranate Peel. Food Hydrocoll. 2020, 104, 105735. [Google Scholar] [CrossRef]
- Coelho, C.C.d.S.; Silva, R.B.S.; Carvalho, C.W.P.; Rossi, A.L.; Teixeira, J.A.; Freitas-Silva, O.; Cabral, L.M.C. Cellulose Nanocrystals from Grape Pomace and Their Use for the Development of Starch-Based Nanocomposite Films. Int. J. Biol. Macromol. 2020, 159, 1048–1061. [Google Scholar] [CrossRef]
- Azmin, S.N.H.M.; Hayat, N.A.b.M.; Nor, M.S.M. Development and Characterization of Food Packaging Bioplastic Film from Cocoa Pod Husk Cellulose Incorporated with Sugarcane Bagasse Fibre. J. Bioresour. Bioprod. 2020, 5, 248–255. [Google Scholar] [CrossRef]
- De Amorim, J.D.P.; de Souza, K.C.; Duarte, C.R.; da Silva Duarte, I.; de Assis Sales Ribeiro, F.; Silva, G.S.; de Farias, P.M.A.; Stingl, A.; Costa, A.F.S.; Vinhas, G.M.; et al. Plant and Bacterial Nanocellulose: Production, Properties and Applications in Medicine, Food, Cosmetics, Electronics and Engineering. A Review. Environ. Chem. Lett. 2020, 18, 851–869. [Google Scholar] [CrossRef]
- Gowman, A.; Wang, T.; Rodriguez-Uribe, A.; Mohanty, A.K.; Misra, M. Bio-Poly(Butylene Succinate) and Its Composites with Grape Pomace: Mechanical Performance and Thermal Properties. ACS Omega 2018, 3, 15205–15216. [Google Scholar] [CrossRef] [Green Version]
- Melendez-Rodriguez, B.; Castro-Mayorga, J.L.; Reis, M.A.M.; Sammon, C.; Cabedo, L.; Torres-Giner, S.; Lagaron, J.M. Preparation and Characterization of Electrospun Food Biopackaging Films of Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) Derived From Fruit Pulp Biowaste. Front. Sustain. Food Syst. 2018, 2, 38. [Google Scholar] [CrossRef]
- Munir, S.; Hu, Y.; Liu, Y.; Xiong, S. Enhanced Properties of Silver Carp Surimi-Based Edible Films Incorporated with Pomegranate Peel and Grape Seed Extracts under Acidic Condition. Food Packag. Shelf Life 2019, 19, 114–120. [Google Scholar] [CrossRef]
- Quilez-Molina, A.I.; Heredia-Guerrero, J.A.; Armirotti, A.; Paul, U.C.; Athanassiou, A.; Bayer, I.S. Comparison of Physicochemical, Mechanical and Antioxidant Properties of Polyvinyl Alcohol Films Containing Green Tealeaves Waste Extracts and Discarded Balsamic Vinegar. Food Packag. Shelf Life 2020, 23, 100445. [Google Scholar] [CrossRef]
- De Moraes Crizel, T.; de Oliveira Rios, A.; Alves, V.D.; Bandarra, N.; Moldão-Martins, M.; Hickmann Flôres, S. Active Food Packaging Prepared with Chitosan and Olive Pomace. Food Hydrocoll. 2018, 74, 139–150. [Google Scholar] [CrossRef]
- Malhotra, B.; Keshwani, A.; Kharkwal, H. Antimicrobial Food Packaging: Potential and Pitfalls. Front. Microbiol. 2015, 6, 611. [Google Scholar] [CrossRef] [Green Version]
- Ozogul, Y.; Yuvka, İ.; Ucar, Y.; Durmus, M.; Kösker, A.R.; Öz, M.; Ozogul, F. Evaluation of Effects of Nanoemulsion Based on Herb Essential Oils (Rosemary, Laurel, Thyme and Sage) on Sensory, Chemical and Microbiological Quality of Rainbow Trout (Oncorhynchus Mykiss) Fillets during Ice Storage. LWT 2017, 75, 677–684. [Google Scholar] [CrossRef]
- Guo, Q.; Du, G.; Jia, H.; Fan, Q.; Wang, Z.; Gao, Z.; Yue, T.; Yuan, Y. Essential Oils Encapsulated by Biopolymers as Antimicrobials in Fruits and Vegetables: A Review. Food Biosci. 2021, 44, 101367. [Google Scholar] [CrossRef]
- Said, N.S.; Sarbon, N.M. Physical and Mechanical Characteristics of Gelatin-Based Films as a Potential Food Packaging Material: A Review. Membranes 2022, 12, 442. [Google Scholar] [CrossRef]
- Haghighi, H.; Licciardello, F.; Fava, P.; Siesler, H.W.; Pulvirenti, A. Recent Advances on Chitosan-Based Films for Sustainable Food Packaging Applications. Food Packag. Shelf Life 2020, 26, 100551. [Google Scholar] [CrossRef]
- Ahankari, S.S.; Subhedar, A.R.; Bhadauria, S.S.; Dufresne, A. Nanocellulose in Food Packaging: A Review. Carbohydr. Polym. 2021, 255, 117479. [Google Scholar] [CrossRef]
- Olszewska, M.A.; Gędas, A.; Simões, M. Antimicrobial Polyphenol-Rich Extracts: Applications and Limitations in the Food Industry. Food Res. Int. 2020, 134, 109214. [Google Scholar] [CrossRef] [PubMed]










| Polymers | Additives | Treatments | Solvents for the Polymers | The Effects and Advantages | References |
|---|---|---|---|---|---|
| Polybutylene succinate (PBS), Polyhydroxybutyrate (PHB), Polycaprolactone (PCL), Polylactic acid (PLA) | / | Biodegradation test of 10 months at 25, 37, and 50 °C soil and compost | / |
| [21] |
| Poly-β-hydroxybutyrate | / | Fermentation | Oily sludge |
| [25] |
| PLLA-15% ZIF-8 MOF | / | Extrusion | / |
| [26] |
| Gelatin 6% (w/v) | Glutaraldehyde (GTA) 50% (w/v of polymer) | Cross-linking | Distilled water |
| [27] |
| Methyl cellulose (MC) 1% (w/v) | Murta berry extract (MU) 25% (w/w of the polymer)Glutaraldehyde (GA) 10–20% (w/w of polymer) Polyethylene glycol (PEG) (25% w/w of the polymer) | / | Distilled water |
| [28] |
| Binary blend of polymers at a final concentration of 5% of gelatin (GEL) and different polysaccharides: gum arabic (GAR), methylcellulose (MC), octenyl succinic anhydride modified starch (OSA), and water-soluble soy polysaccharides (WSSP) | Glycerol 1% (w/w) | / | Distilled water |
| [29] |
| Polylactic acid (PLA) | Nanocomposite films containing 1−5% (w/w of the polymer) of dye−clay hybrid nano pigments (DCNP) | Cationic exchange reaction between a cationic dye and C20A | Chloroform |
| [30] |
| Polylactic acid (PLA) 1% (w/v) | α-costic acid (α-CA) 7:1 (w/w of the polymer) | / | Chloroform |
| [31] |
| PLA latex | Nanocellulose fibrils with high lignin content (NCFHL) from 5 to 20% (w/w) | Extraction of Thuja plicata bark and fibrillation | Aqueous suspensions of NCFHL |
| [32] |
| Fossil-based and bio-based polycarbonate (PC) | / | Moulding | / |
| [7] |
| Sodium alginate 1–3.5% (w/v) | 200–800 mg/L of protease from Bacillus brevis 1–3.5% CaCl2 | / | Milli-Q water | ∙ Best performance of immobilisation at 2.5–3% of Na-alginate and CaCl2, with 400–600 mg/L of protease | [33] |
| CMC 0.5% (w/v), gelatin (GEL) 0.05–0.25% (w/v) | Sodium benzoate 5–30% (w/v), saturate vapour of glutaraldehyde (GLA) | UV irradiation at (253.7 nm, 30 W) for 30–180 min | Aqueous solution |
| [34] |
| Chitosan 1.5% (w/v) | Glycerol 30% (w/w of the polymer) and tween 80 0.2% (w/v of essential oil) | / | Distilled water |
| [35] |
| Chitosan (CH) 2% (w/w), pea starch (S) 2% (w/w), CH:S 1:4 (w/w) | Lyophilised tannic acid (TA) 1:0.04 (w/w on polymer) or thyme extract (TE) 1:0.15 (w/w on polymer) | / | Water dispersion |
| [36] |
| Corn starch and polylactic acid (PLA) blended at a ratio of 80:20 | Epoxidised cardoon oil (ECO) 3% (w/w of PLA fraction) and glycerol at 30% (w/w of starch fraction) | Melt blending process | / |
| [37] |
| Zein (Z) 15% (w/v), gelatin (G) 10% (w/v), blend ZG at different ratios (2:1, 1:1, 1:2) 15% (w/v) | Tea polyphenol 2.5%–7.5% (w/v), glycerol 0.4–0.8 mL | / | Acetic acid (AA) and water |
| [38] |
| Microcrystalline cellulose 3% (w/w) | 68% ZnCl2 (w/w) | / | Distilled water |
| [39] |
| Polyvinyl alcohol (PVA) 5–12.5% (w/v) | Heat cross-linking with citric acid (CA) 3–12% (w/w of the polymer), Clove oil (CO) 20% (w/w of the polymer) | Electrospinning and cross-linking | Distilled water |
| [40] |
| Chitosan(CS) 4% (w/v) | Whey protein isolate (WPI) 4% (w/v), microcrystalline cellulose (MCC) 4% (w/v) and glycerin 10–50% | / | Distilled water |
| [41] |
| Gelatin 6% (w/v) | Galla chinensis extract powder (GCE) 0.03–0.12 g/100 mL | / | Distilled water |
| [42] |
| Antimicrobial Compounds | Polymers | Solvents for the Antimicrobial Compounds | The Effects and Advantages | Microorganisms | Efficacy | References |
|---|---|---|---|---|---|---|
| Citric acid 0.5–1% (w/w) | Gelatin 2% (w/v) | Distilled water |
| Total bacterial count(TBC) | + | [88] |
| L. curvatus CRL705 bacteriocins | Wheat gluten | Distilled water |
| Lactobacillus plantarum | - | [97] |
| Listeria innocua | + | |||||
| Microfluidiser apple skin extract (ASP) 1:1 (v/v of the polymer) and tartaric acid (TA) 0.5–1% | 0.75% CMC | Distilled water |
| Listeria monocytogenes | - | [98] |
| Staphylococcus aureus | - | |||||
| Salmonella enterica | + | |||||
| Shigella flexneri | + | |||||
| AgNPs of 41 and 100 nm | HPMC 3% (w/w) in a PVA-coated silver nanoparticles solution | Distilled water |
| Escherichiacoli | + | [99] |
| Staphylococcus aureus | + | |||||
| Nisin (N), glutaraldehyde (G) and succinic acid (A) | Stainless steel (S)/polydopamine (D) | / |
| Listeria monocytogenes | + | [100] |
| Murta berry extract (MU) 25% (w/w of the polymer), glutaraldehyde (GA) 10–20% (w/w of polymer) | Methyl cellulose (MC) 1% (w/v) | Ethanol solution 70% |
| Listeria innocua | + | [28] |
| TiO2 nanopowders 0–2% (w/w) | Chitosan 2% (w/v) | Distilled water |
| S. aureus | + | [101] |
| E. coli | + | |||||
| P. aeruginosa | + | |||||
| S. Typhimurium | + | |||||
| Aspergillus spp. | + | |||||
| Pennicillium spp. | + | |||||
| Whey Protein Isolate (WPI) | Clay composite 5–20% | Distilled water |
| Listeria monocytogenes | + | [102] |
| Escherichia coli | - | |||||
| Nisin (N) 0.25–0.5% (w/w) and ε-polylysine (PL) 0.2% (w/w) | Corn distarch phosphate 3% (w/w),nanocellulose 0.5% (w/w), CMC 0.8% (w/w) | Distilled water |
| S. aureus | + | [103] |
| E. coli | + | |||||
| Nisin 105 IU/mL in 0.02 M HCl, Grape seed extract 0.5% (w/v) | Chitosan 1% (v/v), gelatin 3% (v/v) | Distilled water |
| Total Viable Count (TVC) | + | [104] |
| Cellulose nanocrystal (CNC) 1% and lignin nanoparticle (LNP) 3% | PLA grafted with GMA at 15% (w/w of the polymer) | / |
| Pseudomonas syringae pv. tomato | + | [105] |
| Clove oil (CO) 20% (w/w of the polymer) | Polyvinyl alcohol (PVA) 5–12.5% (w/v) cross-linked with citric acid (CA) 3–12% (w/w of the polymer) | Distilled water |
| S. aureus | + | [40] |
| E. coli | + | |||||
| Cedrus deodara pine needle extract (PNE) 15% (w/w of SPI) and cellulose nanofibril (CNF) 15% (w/w of SPI) | Soy protein isolate (SPI) 6% (w/v) | Distilled water |
| Escherichia coli | + | [106] |
| SalmonellaTyphimurium | + | |||||
| Staphylococcusaureus | + | |||||
| Listeriamonocytogenes | + | |||||
| Nanosized TiO2 1% (w/v) and black plum peel extract (BPPE) 1% (w/v) | Chitosan 2% (w/v) | Distilled water |
| Escherichia coli | + | [75] |
| Staphylococcus aureus | + | |||||
| Salmonella spp. | + | |||||
| Listeria monocytogenes | + |
| EOs and Plants Extracts | Polymers | Solvents for EOs | The Effect and Advantages | References |
|---|---|---|---|---|
| Rosemary EO at 0.5, 1.0, and 1.5% (v/v) | Chitosan 2% (w/v) | Distilled water |
| [113] |
| Extracts of cinnamon, guarana, rosemary and boldo-do-chile | Blend of gelatin 4% and chitosan 1% (w/v) | Absolute ethanol |
| [114] |
| Eugenol (E) and ginger (G) EOs (0.5 g/g biopolymer) | Blend of gelatin 4% and chitosan 1% (w/v) | Distilled water |
| [115] |
| EOs of Cinnamomum ssp. and Syzygium aromaticum | Chitosan | Ethanol |
| [116] |
| D-Limonene and terpenes from Melaleuca alternifolia (25 g/L to 0.1 g/L) | / | Sunflower oil and palm oil |
| [117] |
| Cinnamon, citronella, pink clove, nutmeg and thyme EOs at 1% (v/v) | Chitosan 2%, gelatin 2% (w/v) | Distilled water |
| [111] |
| Cinnamon essential oil (EO) 5–15 g/L | Sodium alginate 0.75%, CMC 0.25% | Distilled water |
| [55] |
| Cinnamaldehyde 5.33% | Chitosan 1.5% | Ethanol 96% |
| [49] |
| Origanum vulgare L. EO 0.4–1.2%, (w/v) | Chitosan nanoparticles (CSNPs) and fish gelatin 4% | Distilled water |
| [118] |
| Oregano EO 0–2% (v/v) | Mucilage from quince seeds 1% | Distilled water |
| [119] |
| Carvacrol 0–10% (w/v of the polymer) | Cellulose acetate (CA) 5% (w/v) | Acetone |
| [120] |
| Oregano essential-oil nanoemulsion (ORNE) 0–7.5% (v/v) | Hydroxypropyl methylcellulose (HPMC) 2.5% | Distilled water |
| [121] |
| Oregano essential-oil nanoemulsion (ORNE) 0–7.5% (v/v) | Fish gelatin 3%, Chitosan 2% | Distilled water |
| [122] |
| Cinnamon EO | Chitosan nanofibre (CSNF) emulsified in Nanostructured lipid carriers (NLC) | Molten cocoa butter |
| [123] |
| Clove bud EO 0%–1.5% | Pectin 3% (w/v) | Distilled water |
| [124] |
| Satureja khuzestanica Jamzad EO 1% | Lecithin:cholesterol (60:0, 50:10, 40:20, and 30:30) dissolved in dichloromethane/methanol (1:1), added to chitosan 2% (w/v) | Methanol |
| [125] |
| T. moroderi (TM) and T. piperella (TP) extracted EOs 0.5–2% (v/v) | Chitosan 2% (w/v) | Distilled water |
| [126] |
| Clove bud, tagetes, thyme, eucalyptus, neem, cinnamon leaf, himalayan pine needle, tea tree EOs 0–40% (v/w) | poly(3-hydroxybutyrate-co-4-hydroxybutyrate) 4% | Chloroform |
| [127] |
| Plant EOs extracted from Cinnamomum cassia Presl, Litsea cubeba, Cymbopogon martini, Thymus mongolicus Ronn, Syringa Linn., Lavendula angustifolia Mill., Foeniculum uulgare Mill, Citrus reticulata Banco, Mentha haplocalyx Briq., Allium sativum and Artemisia argyi | / | / |
| [112] |
| Antioxidant Compounds | Polymers | Solvents of Antioxidant Compounds | The Effects and Advantages | References |
|---|---|---|---|---|
| Catechin (2% or 5%) or green tea extract (2% or 5%) | Polypropylene | / |
| [153] |
| Microfluidiser apple skin extract (ASP) 1:1 (v/v of the polymer) and tartaric acid (TA) 0.5–1% | 0.75% CMC | Distilled water |
| [98] |
| Murta berry extract (MU) 25% (w/w of the polymer), glutaraldehyde (GA) 10–20% (w/w of polymer) | Methyl cellulose (MC) 1% (w/v) | Solution in ethanol 70% (v/v) |
| [28] |
| Thyme extract (TE) with a ratio of 0.04:1 on the polymer | Chitosan 2% (w/w) and pea starch 2% (w/w) solutions blended together in a ratio of 1:4 w/w | Ethanol 50% |
| [36] |
| Tea polyphenol 2.5%–7.5% w/w | Zein (Z) 15% (w/v), gelatin (G) 10% (w/v), blend ZG at different ratios (2:1, 1:1, 1:2) 15% (w/v) | Acetic acid (AA) and water |
| [38] |
| Cedrus deodara pine needle extract (PNE) 15% (w/w of SPI) and cellulose nanofibril (CNF) 15% (w/w of SPI) | Soy protein isolate (SPI) 6% (w/v) | Distilled water |
| [106] |
| Anthocyanins from black plum peel extract (BPPE) 1% (w/v) | Chitosan 2% (w/v)Nanosized TiO2 1% (w/v) | Distilled water |
| [75] |
| Nanoencapsulated or Nanofiller Molecules | Polymers | Solvent | The Effects and Advantages | References |
|---|---|---|---|---|
| Silver nanoparticles (AgNPs) of 79 mM silver nitrate incapsulated in 45 mM of Poly(vinyl alcohol) (PVA) | Hydroxypropyl methylcellulose (HPMC) 3% (w/w) | Distilled water |
| [99] |
| Montmorillonite clay (MMT) 1–10% | Potato starch (PS) and Microcrystalline cellulose (MCC) | Distilled water |
| [172] |
| TiO2 nanopowders 0–2% (w/w) | Chitosan 2% (w/v) | Distilled water |
| [101] |
| Nisin (N) 0.25–0.5% (w/w) and ε-polylysine (PL) 0.2% (w/w) | Corn distarch phosphate 3% (w/w),nanocellulose 0.5% (w/w), CMC 0.8% (w/w) | Distilled water |
| [103] |
| Amine functionalised mullite fibres(AMUF) from 0.5 to 10 %wt | Polypropylene-grafted-maleic anhydride (PP-g-MA) | o-xylene |
| [173] |
| Nanofibril of cellulose 10–40 % w/w from wheat straw | Polylatic acid (PLA) | / |
| [174] |
| Cellulose nanofibril (CNF) 15% (w/w of SPI) | Soy protein isolate (SPI) 6% (w/v) | Distilled water |
| [106] |
| Microcrystalline cellulose 3% (w/w) | Cellulose 3% in 68% ZnCl2 (w/w) | Distilled water |
| [39] |
| Microcrystalline cellulose (MCC) 4% (w/v) | Chitosan (CS) 4% (w/v), whey protein isolate (WPI) 4% (w/v) and glycerin 10–50% | Distilled water |
| [41] |
| Nanosized TiO2 1% (w/v) and black plum peel extract (BPPE) 1% (w/v) | Chitosan 2% (w/v) | Distilled water |
| [75] |
| Topics | Advantages | Disadvantages | References |
|---|---|---|---|
| Essential oils |
|
| [109,129,224] |
| LABs |
|
| [141,150] |
| Biopolymers |
|
| [3,15,17,18,19,24,45,46,47,51,52,53,54,57,60,63,65,66,67,69,71,72,73,74,76,79,80,81,82,83,84,91,168,199,225,226] |
| Nanotechnology |
|
| [90,167,170,178,179,182,187,188,196,203,227] |
| NaturalAntimicrobials |
|
| [1,5,19,20,50,90,91,92,96,107,108,110,133,139,145,147,148,154,179,203,222,228] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maurizzi, E.; Bigi, F.; Quartieri, A.; De Leo, R.; Volpelli, L.A.; Pulvirenti, A. The Green Era of Food Packaging: General Considerations and New Trends. Polymers 2022, 14, 4257. https://doi.org/10.3390/polym14204257
Maurizzi E, Bigi F, Quartieri A, De Leo R, Volpelli LA, Pulvirenti A. The Green Era of Food Packaging: General Considerations and New Trends. Polymers. 2022; 14(20):4257. https://doi.org/10.3390/polym14204257
Chicago/Turabian StyleMaurizzi, Enrico, Francesco Bigi, Andrea Quartieri, Riccardo De Leo, Luisa Antonella Volpelli, and Andrea Pulvirenti. 2022. "The Green Era of Food Packaging: General Considerations and New Trends" Polymers 14, no. 20: 4257. https://doi.org/10.3390/polym14204257






