Waste Eggshells as a Natural Filler for the Poly(Vinyl Chloride) Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Eggshell Filler (ES) Preparation
2.3. PVC/ES Composites Processing
2.4. Testing Methods of ES and PVC/ES Composites
2.4.1. SEM Analysis
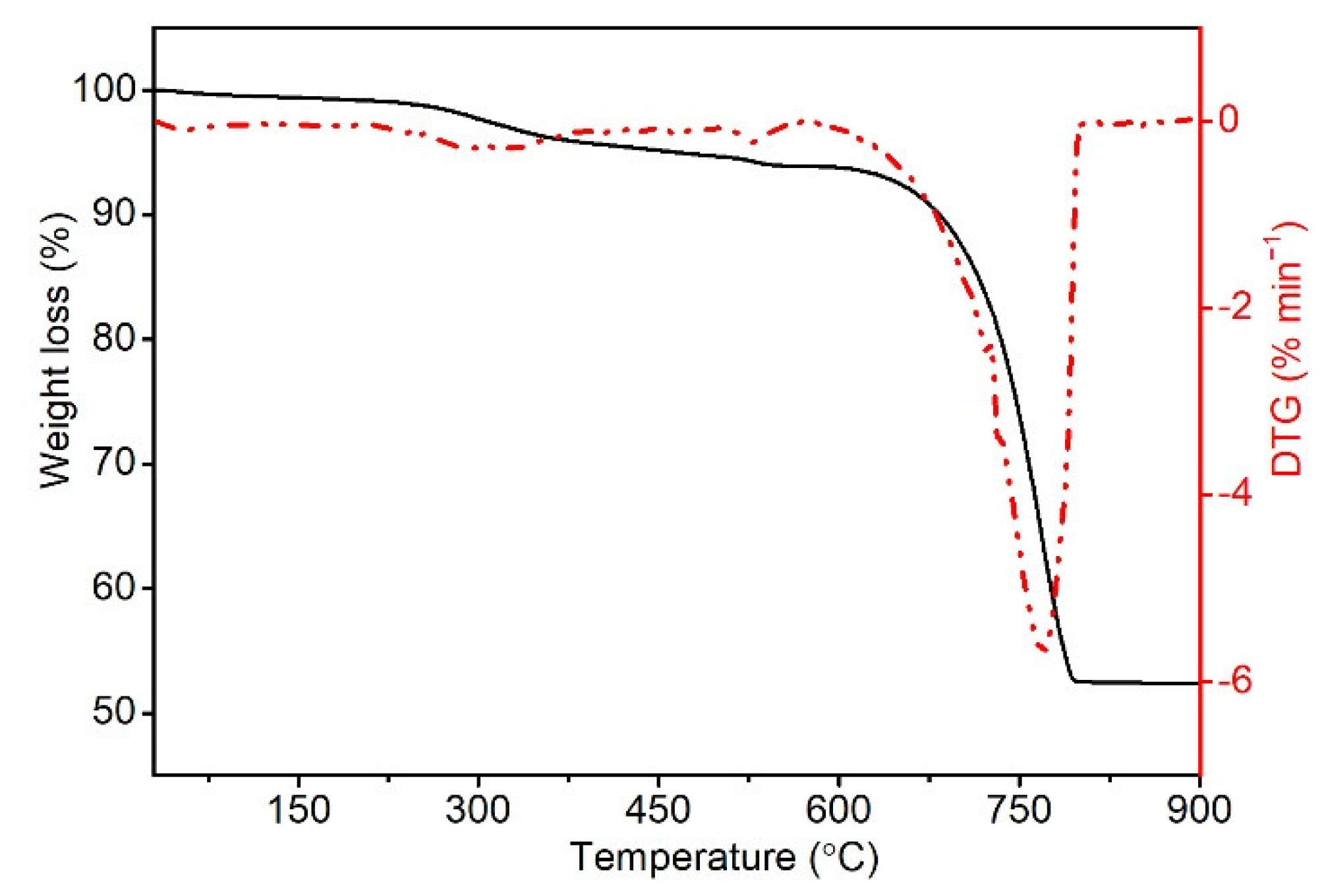
2.4.2. Thermogravimetric Analysis (TG)
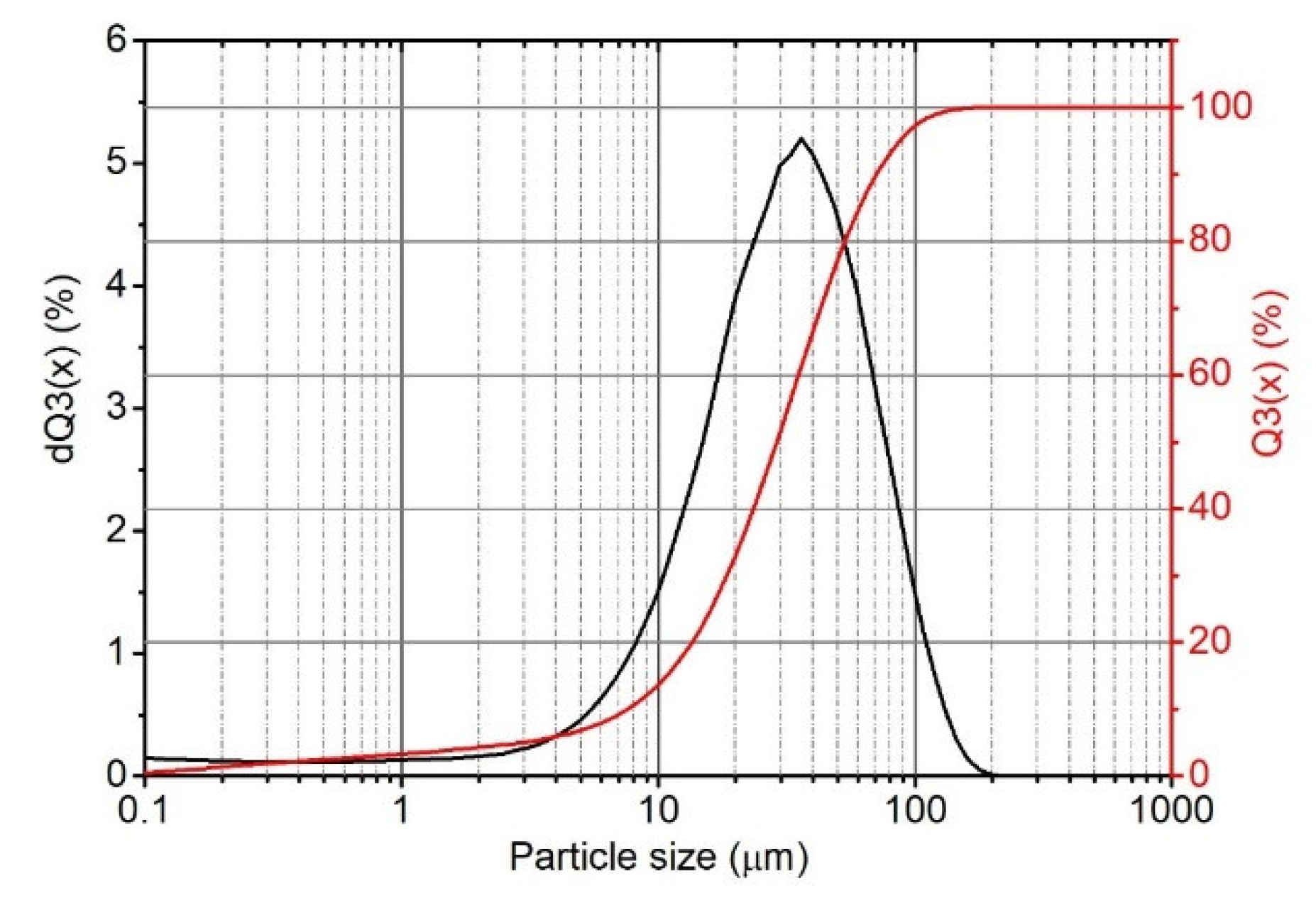
2.4.3. Particle Size Distribution of Filler
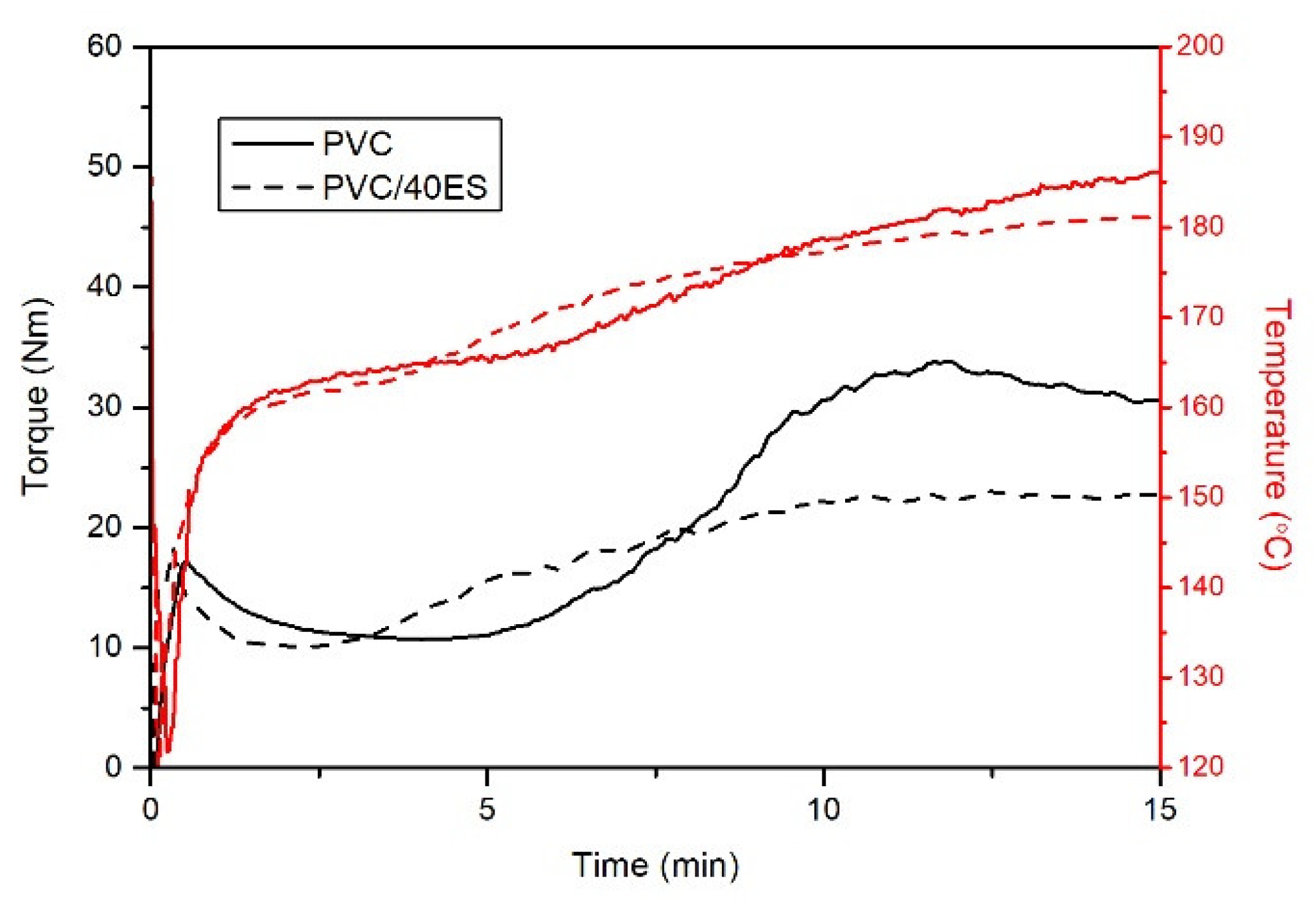
2.4.4. Plastographometric Analysis
2.4.5. Determination of Melt Mass Flow Rate (MFR)
2.4.6. Density Determination and Evaluation of Porosity
2.4.7. Time of Thermal Stability (tts)
2.4.8. Mechanical Properties
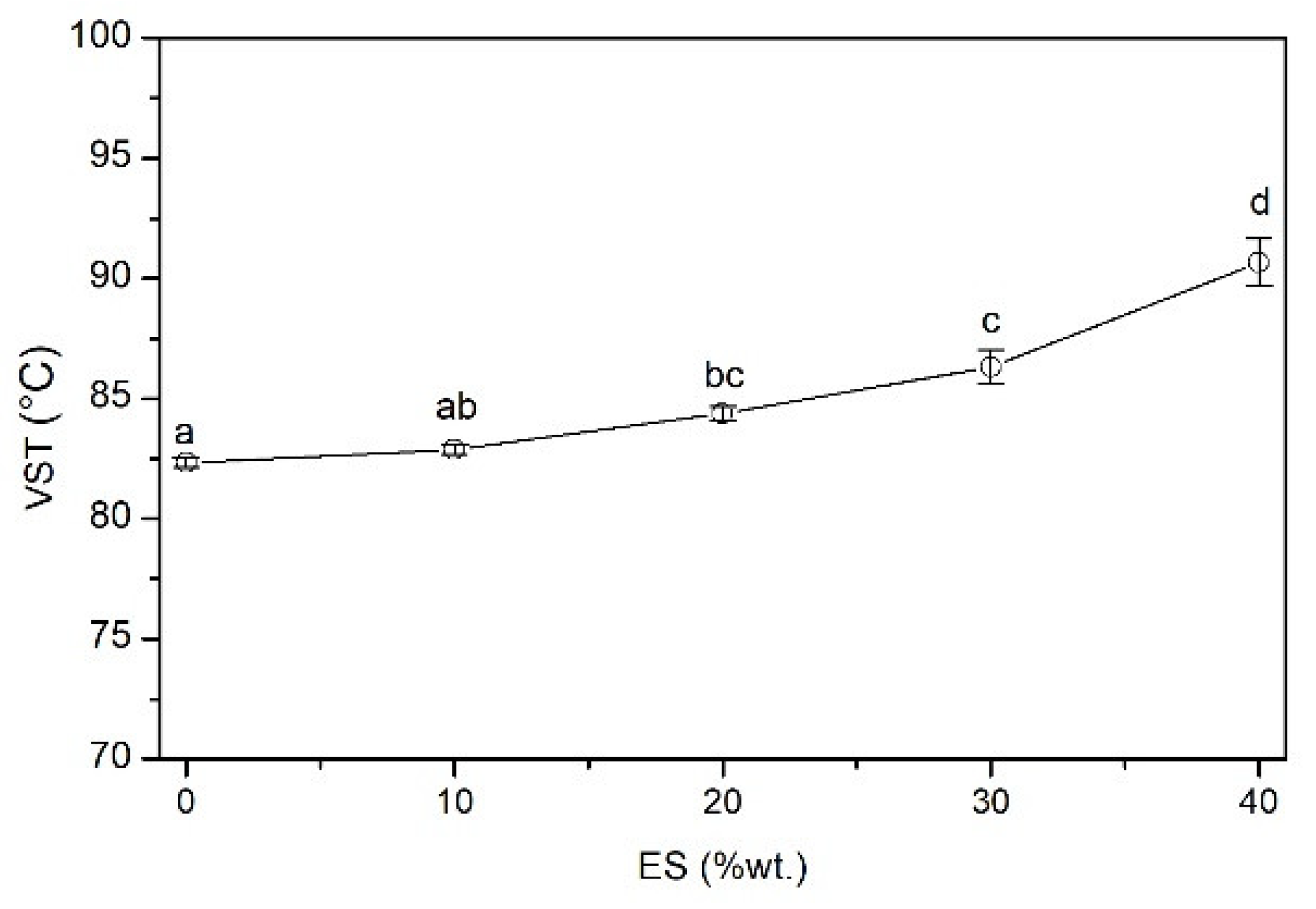
2.4.9. Vicat Softening Temperature (VST)
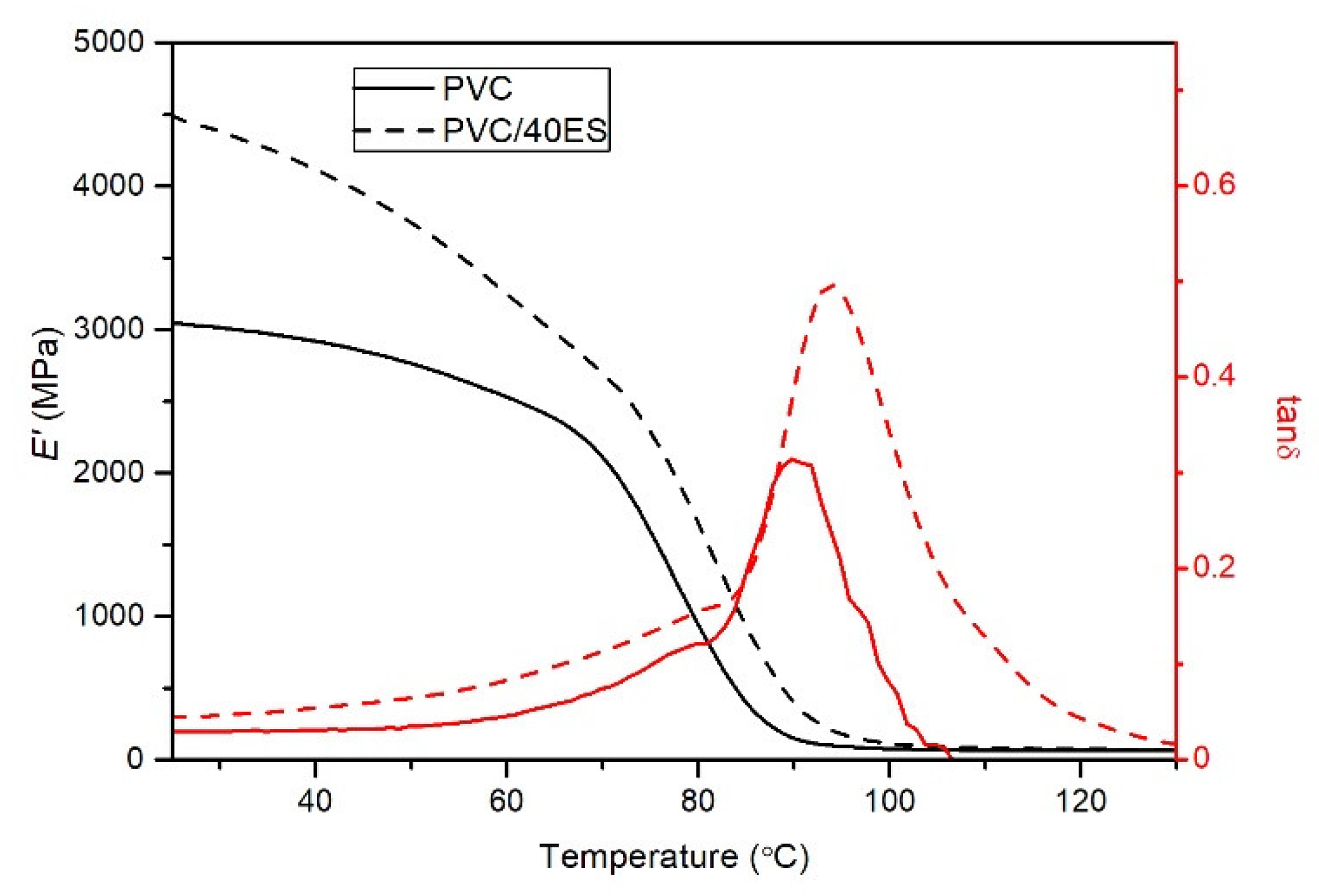
2.4.10. Dynamical Mechanical Thermal Analysis (DMA)
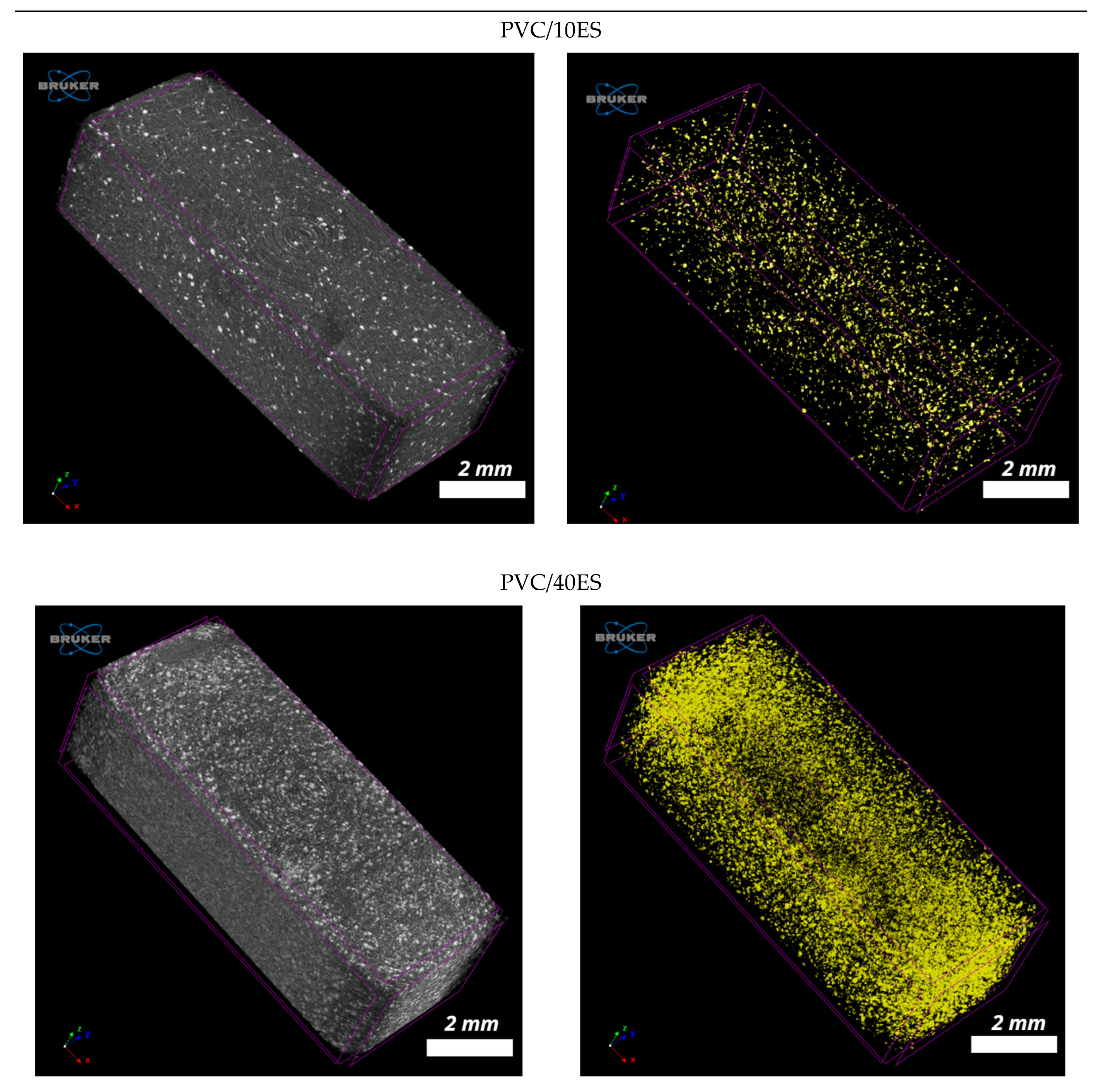
2.4.11. X-ray Microcomputed Tomography (Micro-CT)
2.4.12. Statistical Analysis
3. Results and Discussion
3.1. Analysis of Eggshell Filler (ES)
3.1.1. SEM Observation of ES Filler
3.1.2. Thermogravimetric Analysis (TG)
3.1.3. Particle Size Distribution
3.2. Properties Analysis of PVC/ES Composites
3.2.1. Plastographometric and MFR Analysis
3.2.2. Density and Porosity
3.2.3. Thermogravimetric Analysis (TG)
3.2.4. Time of Thermal Stability (tts)
3.2.5. Mechanical Properties
3.2.6. Vicat Softening Temperature (VST)
3.2.7. Dynamical Mechanical Thermal Analysis (DMA)
3.2.8. SEM Analysis
3.2.9. Micro-CT
4. Summary
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gautron, J.; Dombre, C.; Nau, F.; Feidt, C.; Guillier, L. Review: Production Factors Affecting the Quality of Chicken Table Eggs and Egg Products in Europe. Animal 2022, 16, 100425. [Google Scholar] [CrossRef]
- Available online: https://agridata.ec.europa.eu/extensions/DataPortal/eggs-and-poultry.html (accessed on 29 September 2022).
- Available online: https://agriculture.ec.europa.eu/farming/animal-products/eggs_en (accessed on 29 September 2022).
- Animal Production Expressed in Physical Terms in 2021; Statistics Poland: Warsaw, Poland, 2022.
- Stadelman, W.J. Egg and Egg Products. In Encyclopedia of Food Science and Technology; Francis, F.J., Ed.; John Wiley & Sons: New York, NY, USA, 2000; pp. 593–599. [Google Scholar]
- Laohavisuti, N.; Boonchom, B.; Boonmee, W.; Chaiseeda, K.; Seesanong, S. Simple Recycling of Biowaste Eggshells to Various Calcium Phosphates for Specific Industries. Sci. Rep. 2021, 11, 15143. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, S.; Nie, F.; Feng, L.; Zhu, G.; Jiang, L. Elaborate Architecture of the Hierarchical Hen’s Eggshell. Nano Res. 2011, 4, 171–179. [Google Scholar] [CrossRef]
- Afzal, F. Effect of Eggshell Powder Fortification on the Physicochemical and Organoleptic Characteristics of Muffins. Pure Appl. Biol. 2020, 9, 1488–1496. [Google Scholar] [CrossRef]
- Platon, N.; Arus, V.-A.; Georgescu, A.-M.; Nistor, I.D.; Barsan, N. White Bread Fortified with Calcium from Eggshell Powder. Rev. De Chim. 2020, 71, 299–306. [Google Scholar] [CrossRef]
- El-Shibiny, S.; El-Gawad, M.A.E.-K.M.A.; Assem, F.M.; El-Sayed, S.M. The Use of Nano-Sized Eggshell Powder for Calcium Fortification of Cow?S and Buffalo?S Milk Yogurts. Acta Sci. Pol. Technol. Aliment. 2018, 17, 37–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasheed, S.P.; Shivashankar, M.; Dev, S.; Azeem, A.K. Treatment of Biowaste to Pharmaceutical Excipient. Mater Today Proc. 2019, 15, 316–322. [Google Scholar] [CrossRef]
- Flores-Cano, J.V.; Leyva-Ramos, R.; Mendoza-Barron, J.; Guerrero-Coronado, R.M.; Aragón-Piña, A.; Labrada-Delgado, G.J. Sorption Mechanism of Cd(II) from Water Solution onto Chicken Eggshell. Appl. Surf. Sci. 2013, 276, 682–690. [Google Scholar] [CrossRef]
- Cree, D.; Rutter, A. Sustainable Bio-Inspired Limestone Eggshell Powder for Potential Industrialized Applications. ACS Sustain. Chem. Eng. 2015, 3, 941–949. [Google Scholar] [CrossRef]
- Habte, L.; Shiferaw, N.; Mulatu, D.; Thenepalli, T.; Chilakala, R.; Ahn, J. Synthesis of Nano-Calcium Oxide from Waste Eggshell by Sol-Gel Method. Sustainability 2019, 11, 3196. [Google Scholar] [CrossRef]
- Setiawan, B.D.; Rizqi, O.; Brilianti, N.F.; Wasito, H. Nanoporous of Waste Avian Eggshell to Reduce Heavy Metal and Acidity in Water. Sustain. Chem. Pharm. 2018, 10, 163–167. [Google Scholar] [CrossRef]
- Tan, Y.Y.; Doh, S.I.; Chin, S.C. Eggshell as a Partial Cement Replacement in Concrete Development. Mag. Concr. Res. 2018, 70, 662–670. [Google Scholar] [CrossRef]
- Yang, D.; Zhao, J.; Ahmad, W.; Nasir Amin, M.; Aslam, F.; Khan, K.; Ahmad, A. Potential Use of Waste Eggshells in Cement-Based Materials: A Bibliographic Analysis and Review of the Material Properties. Constr. Build Mater. 2022, 344, 128143. [Google Scholar] [CrossRef]
- Segneanu, A.-E.; Marin, C.N.; Vlase, G.; Cepan, C.; Mihailescu, M.; Muntean, C.; Grozescu, I. Highly Efficient Engineered Waste Eggshell-Fly Ash for Cadmium Removal from Aqueous Solution. Sci. Rep. 2022, 12, 9676. [Google Scholar] [CrossRef]
- Shafiei, S.; Omidi, M.; Nasehi, F.; Golzar, H.; Mohammadrezaei, D.; Rezai Rad, M.; Khojasteh, A. Egg Shell-Derived Calcium Phosphate/Carbon Dot Nanofibrous Scaffolds for Bone Tissue Engineering: Fabrication and Characterization. Mater. Sci. Eng. C 2019, 100, 564–575. [Google Scholar] [CrossRef]
- Oladele, I.O.; Agbabiaka, O.G.; Adediran, A.A.; Akinwekomi, A.D.; Balogun, A.O. Structural Performance of Poultry Eggshell Derived Hydroxyapatite Based High Density Polyethylene Bio-Composites. Heliyon 2019, 5, E02552. [Google Scholar] [CrossRef] [Green Version]
- Pavon, C.; Aldas, M.; Samper, M.D.; Motoc, D.L.; Ferrandiz, S.; López-Martínez, J. Mechanical, Dynamic-Mechanical, Thermal and Decomposition Behavior of 3D-Printed PLA Reinforced with CaCO3 Fillers from Natural Resources. Polymers 2022, 14, 2646. [Google Scholar] [CrossRef]
- Bhagavatheswaran, E.S.; Das, A.; Rastin, H.; Saeidi, H.; Jafari, S.H.; Vahabi, H.; Najafi, F.; Khonakdar, H.A.; Formela, K.; Jouyandeh, M.; et al. The Taste of Waste: The Edge of Eggshell Over Calcium Carbonate in Acrylonitrile Butadiene Rubber. J. Polym. Env. 2019, 27, 2478–2489. [Google Scholar] [CrossRef] [Green Version]
- Kong, J.; Li, Y.; Bai, Y.; Li, Z.; Cao, Z.; Yu, Y.; Han, C.; Dong, L. High-Performance Biodegradable Polylactide Composites Fabricated Using a Novel Plasticizer and Functionalized Eggshell Powder. Int. J. Biol. Macromol. 2018, 112, 46–53. [Google Scholar] [CrossRef]
- Sutapun, W.; Pakdeechote, P.; Suppakarn, N.; Ruksakulpiwat, Y. Application of Calcined Eggshell Powder as Functional Filler for High Density Polyethylene. Polym. Plast. Technol. Eng. 2013, 52, 1025–1033. [Google Scholar] [CrossRef]
- Lin, Z.; Zhang, Z.; Mai, K. Preparation and Properties of Eggshell/β-Polypropylene Bio-Composites. J. Appl. Polym. Sci. 2012, 125, 61–66. [Google Scholar] [CrossRef]
- Mustapha, K.; Ayinla, R.; Ottan, A.S.; Owoseni, T.A. Mechanical Properties of Calcium Carbonate/Eggshell Particle Filled Polypropylene Composites. MRS Adv. 2020, 5, 2783–2792. [Google Scholar] [CrossRef]
- Vandeginste, V. Food Waste Eggshell Valorization through Development of New Composites: A Review. Sustain. Mater. Technol. 2021, 29, e00317. [Google Scholar] [CrossRef]
- Xu, Z.; Chu, Z.; Yan, L.; Chen, H.; Jia, H.; Tang, W. Effect of Chicken Eggshell on the Flame-retardant and Smoke Suppression Properties of an Epoxy-based Traditional APP-PER-MEL System. Polym. Compos. 2019, 40, 2712–2723. [Google Scholar] [CrossRef]
- Urtekin, G.; Hazer, S.; Aytac, A. Effect of Eggshell and Intumescent Flame Retardant on the Thermal and Mechanical Properties of Plasticised PLA. Plast. Rubber Compos. 2021, 50, 127–136. [Google Scholar] [CrossRef]
- Sree, G.V.; Nagaraaj, P. Enhancement of PVA Packaging Properties Using Calcined Eggshell Waste as Filler and Nanonutrient. Mater. Chem. Phys. 2022, 291, 126611. [Google Scholar] [CrossRef]
- Dhumal, P.S.; Bhakare, M.A.; Lokhande, K.D.; Bondarde, M.P.; Some, S. Bio-Waste Derived, Phosphorus Decorated Composite for Highly Efficient Flame Retardant for Cotton Fabric. Cellulose 2022, 29, 8879–8888. [Google Scholar] [CrossRef]
- Gomes, H.I.A.S.; Sales, M.G.F. Natural Materials Modified and Applied to the Detection of Drugs In Situ: Modification of Eggshell and Quantification of Oxytetracycline. Sensors 2022, 22, 5746. [Google Scholar] [CrossRef]
- Lewandowski, M.; Piszczek, K.; Pieńkowska, M.; Lewandowski, K. Innovative Oligomeric Poly(Vinyl Chloride) Plasticizers for Specialized Applications. Polimery 2020, 65, 550–556. [Google Scholar] [CrossRef]
- Lewandowski, K.; Skórczewska, K.; Piszczek, K.; Manikowski, M.; Mirowski, J. Modification of Rigid Poly(Vinyl Chloride) for Application in Three-Layer Feed Pipes. Polimery 2020, 65, 304–310. [Google Scholar] [CrossRef]
- Klapiszewski, Ł.; Tomaszewska, J.; Skórczewska, K.; Jesionowski, T. Preparation and Characterization of Eco-Friendly Mg(OH)2/Lignin Hybrid Material and Its Use as a Functional Filler for Poly(Vinyl Chloride). Polymers 2017, 9, 258. [Google Scholar] [CrossRef] [Green Version]
- Tomaszewska, J.; Sterzyński, T.; Walczak, D. Thermal Stability of Nanosilica-Modified Poly(Vinyl Chloride). Polymers 2021, 13, 2057. [Google Scholar] [CrossRef]
- Sharmeeni, M.; Munusamy, Y.; Ismail, H. The Effect of Blending Sequence on the Structure and Properties of Poly(Vinyl Chloride)/Chicken Eggshell Powder Composites. J. Vinyl Addit. Technol. 2017, 23, 298–304. [Google Scholar] [CrossRef]
- Murugan, S.; Munusamy, Y.; Ismail, H. Effects of Chicken Eggshell Filler Size on the Processing, Mechanical and Thermal Properties of PVC Matrix Composite. Plast. Rubber Compos. 2017, 46, 42–51. [Google Scholar] [CrossRef]
- Khaleghi, M. Experimental and Computational Study of Thermal Behavior of PVC Composites Based on Modified Eggshell Biofiller for UPVC Product. J. Polym. Res. 2022, 29, 2. [Google Scholar] [CrossRef]
- Marceneiro, S.; Lobo, I.; Dias, I.; de Pinho, E.; Dias, A.M.A.; de Sousa, H.C. Eco-Friendlier and Sustainable Natural-Based Additives for Poly(Vinyl Chloride)-Based Composites. J. Ind. Eng. Chem. 2022, 110, 248–261. [Google Scholar] [CrossRef]
- Venkatesh, N.; Hanumanthraju, H.G.; Prashanth, K.P.; Venkatesha, B.K.; Yuvaraj, L. Investigation on Dynamic Mechanical Analysis of Bio-Active Coating Materials Coated on Teflon, PVC and Nylon Polymers. Mater. Today Proc. 2022, 54, 421–427. [Google Scholar] [CrossRef]
- Baláž, M.; Boldyreva, E.V.; Rybin, D.; Pavlović, S.; Rodríguez-Padrón, D.; Mudrinić, T.; Luque, R. State-of-the-Art of Eggshell Waste in Materials Science: Recent Advances in Catalysis, Pharmaceutical Applications, and Mechanochemistry. Front. Bioeng. Biotechnol. 2021, 8, 612567. [Google Scholar] [CrossRef]
- Baláž, M.; Baláž, P.; Bujňáková, Z.; Pap, Z.; Kupka, D.; Zorkovská, A. Mechanochemical Dechlorination of PVC by Utilizing Eggshell Waste. Acta Phys. Pol. A 2014, 126, 884–887. [Google Scholar] [CrossRef]
- Lewandowski, K.; Skórczewska, K.; Piszczek, K.; Urbaniak, W. Recycled Glass Fibres from Wind Turbines as a Filler for Poly(Vinyl Chloride). Adv. Polym. Technol. 2019, 2019, 8960503. [Google Scholar] [CrossRef]
- Barczewski, M.; Hejna, A.; Sałasińska, K.; Aniśko, J.; Piasecki, A.; Skórczewska, K.; Andrzejewski, J. Thermomechanical and Fire Properties of Polyethylene-Composite-Filled Ammonium Polyphosphate and Inorganic Fillers: An Evaluation of Their Modification Efficiency. Polymers 2022, 14, 2501. [Google Scholar] [CrossRef]
- Ward, J.M. Mechanical Properties of Solid Polymers; John Wiley & Sons Ltd: New York, NY, USA, 1975. [Google Scholar]
- Ferry, J.D. Viscoelastic Properties of Polymers; John Wiley & Sons Inc.: New York, NY, USA, 1980. [Google Scholar]
- Nuryantini, A.Y.; Deliana, C.; Sundari, D.; Diah, H.; Nuryadin, B.W. Synthesis and Characterization of Calcium Oxide Nanoparticles from Duck Eggshells Using Ball Milling Methods. J. Kim. Val. 2019, 5, 231–235. [Google Scholar] [CrossRef] [Green Version]
- Kerru, N.; Gummidi, L.; Bhaskaruni, S.V.H.S.; Maddila, S.N.; Jonnalagadda, S.B. One-Pot Green Synthesis of Novel 5,10-Dihydro-1H-Pyrazolo[1,2-b]Phthalazine Derivatives with Eco-Friendly Biodegradable Eggshell Powder as Efficacious Catalyst. Res. Chem. Intermed. 2020, 46, 3067–3083. [Google Scholar] [CrossRef]
- Li, H.-Y.; Tan, Y.-Q.; Zhang, L.; Zhang, Y.-X.; Song, Y.-H.; Ye, Y.; Xia, M.-S. Bio-Filler from Waste Shellfish Shell: Preparation, Characterization, and Its Effect on the Mechanical Properties on Polypropylene Composites. J. Hazard. Mater. 2012, 217–218, 256–262. [Google Scholar] [CrossRef]
- Klapiszewski, Ł.; Pawlak, F.; Tomaszewska, J.; Jesionowski, T. Preparation and Characterization of Novel Pvc/Silica-Lignin Composites. Polymers 2015, 7, 1767–1788. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.H.; Li, H.C.; Teng, C.C.; Yang, C.H. Fusion, Electrical Conductivity, Thermal, and Mechanical Properties of Rigid Poly(Vinyl Chloride) PVO/Carbon Black (CB) Composites. J. Appl. Polym. Sci. 2006, 99, 2167–2173. [Google Scholar] [CrossRef]
- Skórczewska, K.; Tomaszewska, J.; Piszczek, K. Influence of MWCNT on the Processing Properties and Structure of PVC Composites. Macromol. Symp. 2018, 378, 1600177. [Google Scholar] [CrossRef]
- Matuana, L.M.; Kim, J.W. Fusion Characteristics of Rigid PVC/Wood-Flour Composites by Torque Rheometry. J. Vinyl Addit. Technol. 2007, 13, 7–13. [Google Scholar] [CrossRef]
- Ari, G.A.; Aydin, I. A Study on Fusion and Rheological Behaviors of PVC/SiO2 Microcomposites and Nanocomposites: The Effects of SiO2 Particle Size. Polym. Eng. Sci. 2011, 51, 1574–1579. [Google Scholar] [CrossRef]
- Barczewski, M.; Mysiukiewicz, O.; Lewandowski, K.; Nowak, D.; Matykiewicz, D.; Andrzejewski, J.; Skórczewska, K.; Piasecki, A. Effect of Basalt Powder Surface Treatments on Mechanical and Processing Properties of Polylactide-Based Composites. Materials 2020, 13, 5436. [Google Scholar] [CrossRef]
- Xie, X.L.; Liu, Q.X.; Li, R.K.Y.; Zhou, X.P.; Zhang, Q.X.; Yu, Z.Z.; Mai, Y.W. Rheological and Mechanical Properties of PVC/CaCO3 Nanocomposites Prepared by in Situ Polymerization. Polymers 2004, 45, 6665–6673. [Google Scholar] [CrossRef]
- Aniśko, J.; Barczewski, M.; Piasecki, A.; Skórczewska, K.; Szulc, J.; Szostak, M. The Relationship between a Rotational Molding Processing Procedure and the Structure and Properties of Biobased Polyethylene Composites Filled with Expanded Vermiculite. Materials 2022, 15, 5903. [Google Scholar] [CrossRef] [PubMed]
- di Landro, L.; Montalto, A.; Bettini, P.; Guerra, S.; Montagnoli, F.; Rigamonti, M. Detection of Voids in Carbon/Epoxy Laminates and Their Influence on Mechanical Properties. Polym. Polym. Compos. 2017, 25, 371–380. [Google Scholar] [CrossRef]
- Iván, B. The Stability of Poly(Vinyl Chloride). J. Vinyl Addit. Technol. 2003, 9, 1–3. [Google Scholar] [CrossRef]
- Szakács, T.; Iván, B.; Kupai, J. Thermal Stability of Cationically Allylated Poly(Vinyl Chloride) and Poly(Vinyl Chloride-Co-2-Chloropropene) Copolymer. Polym. Degrad. Stab. 2004, 85, 1029–1033. [Google Scholar] [CrossRef]
- Dragan, S.; Ghirisan (Miclaus), A. Kinetic Study of Sulfur Dioxide Absorption into Dolomite-Brucite Suspensions. Studia Univ. Babeș-Bolyai Chem. 2019, 64, 345–355. [Google Scholar] [CrossRef]
- Karayildirim, T.; Yanik, J.; Yuksel, M.; Saglam, M.; Vasile, C.; Bockhorn, H. The Effect of Some Fillers on PVC Degradation. J. Anal. Appl. Pyrolysis 2006, 75, 112–119. [Google Scholar] [CrossRef]
- Schlickmann, K.P.; Howarth, J.L.L.; Silva, D.A.K.; Pezzin, A.P.T. Effect of The Incorporation of Micro and Nanoparticles of Calcium Carbonate in Poly (Vinyl Chloride) Matrix for Industrial Application. Mater. Res. 2019, 22, e20180870. [Google Scholar] [CrossRef]
- Patil, C.B.; Kapadi, U.R.; Hundiwale, D.G.; Mahulikar, P.P. Preparation and Characterization of Poly(Vinyl Chloride) Calcium Carbonate Nanocomposites via Melt Intercalation. J. Mater. Sci. 2009, 44, 3118–3124. [Google Scholar] [CrossRef]
- Baláž, M.; Bujňáková, Z.; Achimovičová, M.; Tešinský, M.; Baláž, P. Simultaneous Valorization of Polyvinyl Chloride and Eggshell Wastes by a Semi-Industrial Mechanochemical Approach. Env. Res 2019, 170, 332–336. [Google Scholar] [CrossRef]
- Tongamp, W.; Kano, J.; Zhang, Q.; Saito, F. Simultaneous Treatment of PVC and Oyster-Shell Wastes by Mechanochemical Means. Waste Manag. 2008, 28, 484–488. [Google Scholar] [CrossRef]
- Tuen, B.S.; Hassan, A.; Abu Bakar, A. Thermal Properties and Processability of Talc- and Calcium Carbonate-Filled Poly(Vinyl Chloride) Hybrid Composites. J. Vinyl Addit. Technol. 2012, 18, 87–94. [Google Scholar] [CrossRef]
- Piszczek, K.; Tomaszewska, J. Processing of Precipitated Nongranular PVC in the Brabender Measuring Mixer. J. Vinyl Addit. Technol. 2012, 18, 147–152. [Google Scholar] [CrossRef]
- Piszczek, K.; Tomaszewska, J.; Sterzyński, T. The Universal Temperature Parameter of Rigid PVC Gelation in Brabender Kneader. Polimery 2004, 49, 646–648. [Google Scholar] [CrossRef] [Green Version]
- Szewczykowski, P.; Skarżynski, L. Application of the X-Ray Micro-Computed Tomography to the Analysis of the Structure of Polymeric Materials. Polimery 2019, 64, 12–22. [Google Scholar] [CrossRef]






| Sample | tX (min) | MX (Nm) | TX (°C) | Me (Nm) | Te (°C) | MFR (g/10 min) |
|---|---|---|---|---|---|---|
| PVC | 11.8 ± 0.15 b | 33.9 ± 0.20 a | 181.9 ± 0.72 a | 30.6 ± 0.47 a | 186.2 ± 0.21 a | 9.56 ± 0.25 a |
| PVC/10ES | 10.0 ± 1.02 a | 29.5 ± 0.44 b | 179.9 ± 0.51 a | 25.7 ± 0.31 b | 183.5 ± 0.49 b | 6.39 ± 0.12 b |
| PVC/20ES | 11.4 ± 0.58 a,b | 25.3 ± 0.49 c | 180.8 ± 0.49 a | 24.6 ± 0.25 c | 182.1 ± 0.12 c | 6.28 ± 0.25 b |
| PVC/30ES | 12.1 ± 0.30 b | 24.4 ± 0.20 d | 181.0 ± 0.95 a | 23.7 ± 0.10 d | 181.9 ± 0.15 c | 5.70 ± 0.41 c |
| PVC/40ES | 12.3 ± 0.31 b | 23.0 ± 0.13 e | 180.1 ± 1.0 a | 22.7 ± 0.21 e | 181.0 ± 0.30 d | 5.88 ± 0.21 b,c |
| Sample | Density (g/cm3) | Volume Fraction ES | Porosity (%) |
|---|---|---|---|
| EGS | 2.27 ± 0.002 | - | |
| PVC | 1.35 ± 0.012 | - | |
| PVC/10ES | 1.40 ± 0.001 | 0.063 | 0.661 |
| PVC/20ES | 1.47 ± 0.001 | 0.135 | 0.451 |
| PVC/30ES | 1.54 ± 0.001 | 0.216 | 0.854 |
| PVC/40ES | 1.61 ± 0.002 | 0.308 | 1.804 |
| Sample | T1 (°C) | T5 (°C) | T50 (°C) | TDTG (°C) | RM (%) |
|---|---|---|---|---|---|
| PVC | 240.4 | 265.2 | 318.5 | 291.4 | 11.9 |
| PVC/10ES | 244.9 | 269.8 | 328.1 | 296.4 | 14.8 |
| PVC/20ES | 249.1 | 273.9 | 347.9 | 294.7 | 19.3 |
| PVC/30ES | 253.3 | 277.5 | 427.3 | 295.3 | 23.6 |
| PVC/40ES | 256.7 | 280.8 | 456.2 | 292.7 | 27.6 |
| Sample | Et (MPa) | σM (MPa) | εb (%) | acU (kJ/m2) |
|---|---|---|---|---|
| PVC | 1235 ± 17.6 a | 46.5 ± 1.26 a | 5.9 ± 0.54 c | 17.4 ± 1.17 a |
| PVC/10ES | 1380 ± 18.9 b | 42.6 ± 0.57 b | 20.9 ± 2.03 a | 16.2 ± 2.26 a |
| PVC/20ES | 1532 ± 20.5 c | 35.3 ± 0.85 c | 11.2 ± 1.47 b | 18.2 ± 2.87 a |
| PVC/30ES | 1698 ± 19.4 d | 29.6 ± 0.78 d | 5.8 ± 1.34 c | 15.1 ± 1.88 a |
| PVC/40ES | 1787 ± 45.7 e | 21.2 ± 0.84 e | 4.1 ± 0.58 c | 10.1 ± 1.51 b |
| Sample | Tg (°C) | E′ (MPa) | |||||
|---|---|---|---|---|---|---|---|
| E′ Onset | E′ Inflection | E′ Offset | tanδ Peak | 30 °C | 50 °C | 70 °C | |
| PVC | 73.1 ± 0.86 a | 78.4 ± 0.58 a | 83.8 ± 0.64 a | 90.0 ± 0.92 a | 2946 ± 80.2 a | 2720 ± 74.2 a | 2206 ± 29.7 a |
| PVC/10ES | 73.6 ± 0.27 a,b | 78.7 ± 0.12 a,b | 84.2 ± 0.20 a,b | 90.2 ± 0.20 a | 3070 ± 106.9 a | 2809 ± 107.8 a | 2346 ± 29.0 a |
| PVC/20ES | 74.6 ± 0.39 b,c | 79.6 ± 0.19 b | 84.9 ± 0.16 b | 90.5 ± 0.21 a,b | 3593 ± 83.5 b | 3259 ± 78.6 b | 2565 ± 18.4 b |
| PVC/30ES | 75.9 ± 0.42 c,d | 80.8 ± 0.29 c | 86.3 ± 0.30 c | 91.8 ± 0.50 b | 4003 ± 97.4 c | 3606 ± 75.4 c | 2795 ± 62.2 c |
| PVC/40ES | 77.0 ± 0.42 d | 81.8 ± 0.26 d | 87.5 ± 0.34 d | 93.3 ± 0.65 c | 4356 ± 99.9 d | 3865 ± 97.5 d | 2782 ± 68.9 c |
| Sample | Average Particle Size (µm) |
|---|---|
| PVC/10ES | 37.39 |
| PVC/20ES | 35.00 |
| PVC/30ES | 38.10 |
| PVC/40ES | 34.91 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skórczewska, K.; Lewandowski, K.; Szewczykowski, P.; Wilczewski, S.; Szulc, J.; Stopa, P.; Nowakowska, P. Waste Eggshells as a Natural Filler for the Poly(Vinyl Chloride) Composites. Polymers 2022, 14, 4372. https://doi.org/10.3390/polym14204372
Skórczewska K, Lewandowski K, Szewczykowski P, Wilczewski S, Szulc J, Stopa P, Nowakowska P. Waste Eggshells as a Natural Filler for the Poly(Vinyl Chloride) Composites. Polymers. 2022; 14(20):4372. https://doi.org/10.3390/polym14204372
Chicago/Turabian StyleSkórczewska, Katarzyna, Krzysztof Lewandowski, Piotr Szewczykowski, Sławomir Wilczewski, Joanna Szulc, Paulina Stopa, and Paulina Nowakowska. 2022. "Waste Eggshells as a Natural Filler for the Poly(Vinyl Chloride) Composites" Polymers 14, no. 20: 4372. https://doi.org/10.3390/polym14204372
APA StyleSkórczewska, K., Lewandowski, K., Szewczykowski, P., Wilczewski, S., Szulc, J., Stopa, P., & Nowakowska, P. (2022). Waste Eggshells as a Natural Filler for the Poly(Vinyl Chloride) Composites. Polymers, 14(20), 4372. https://doi.org/10.3390/polym14204372






