Amphiphilic Block Copolymers: Their Structures, and Self-Assembly to Polymeric Micelles and Polymersomes as Drug Delivery Vehicles
Abstract
:1. Introduction
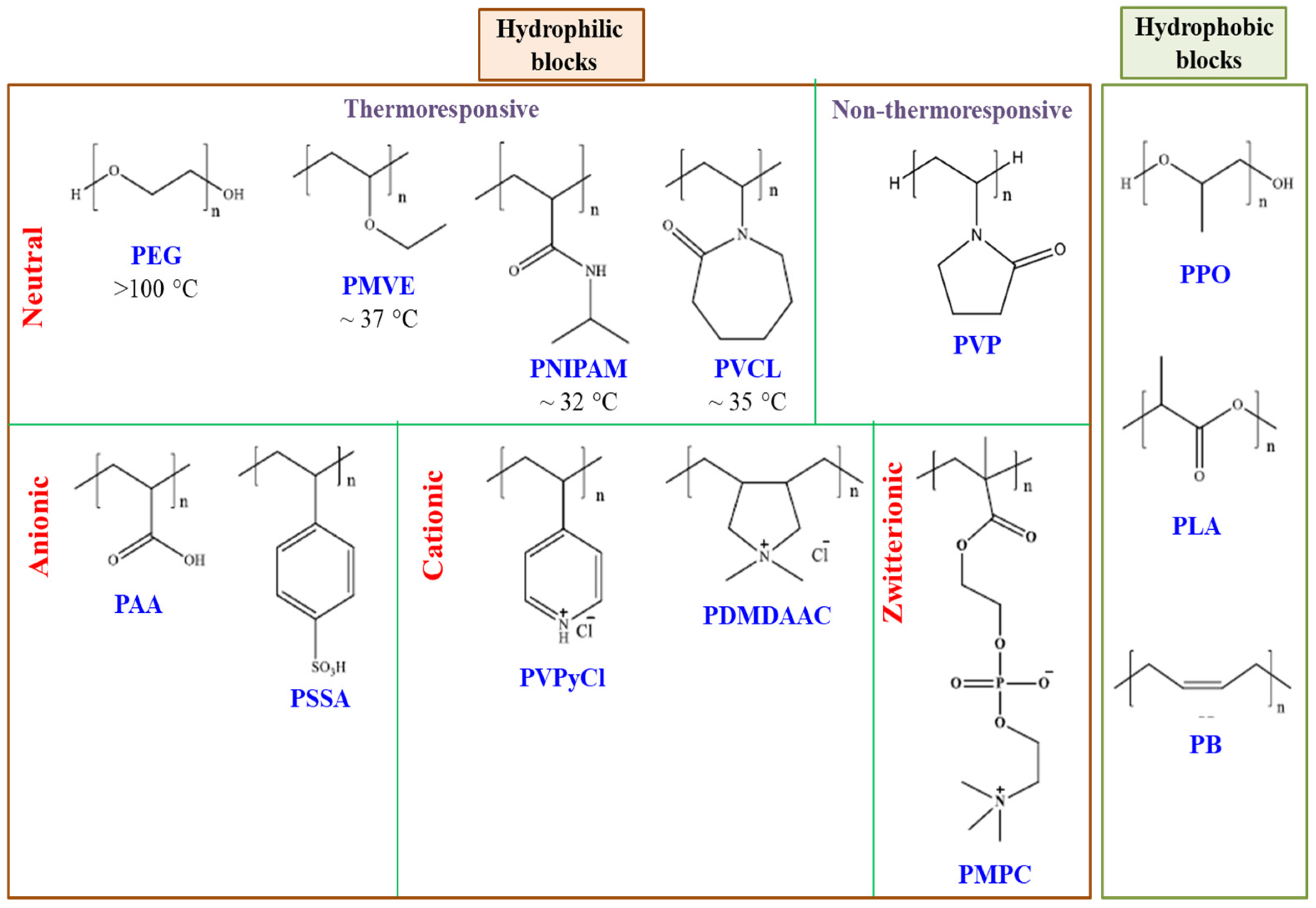
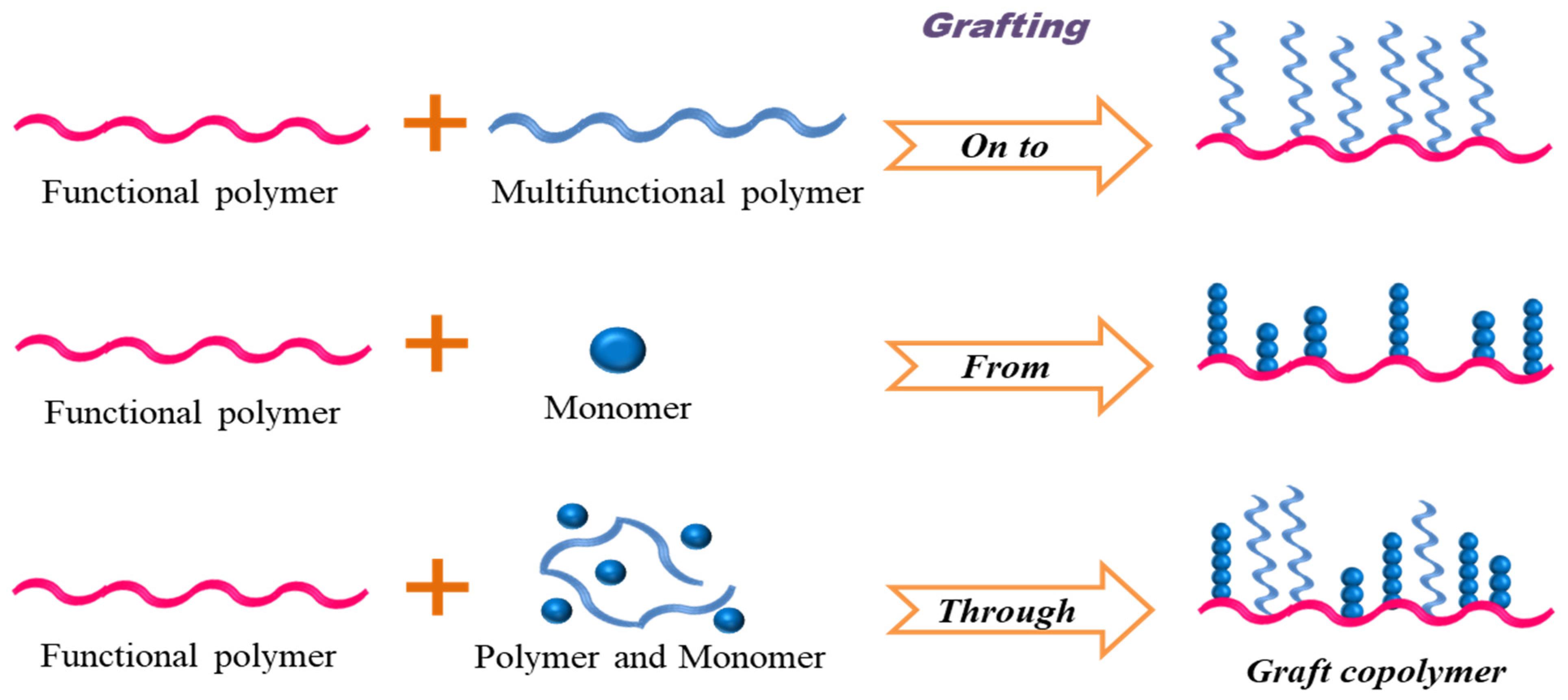
2. Synthesis Strategies
3. Self-Assembly
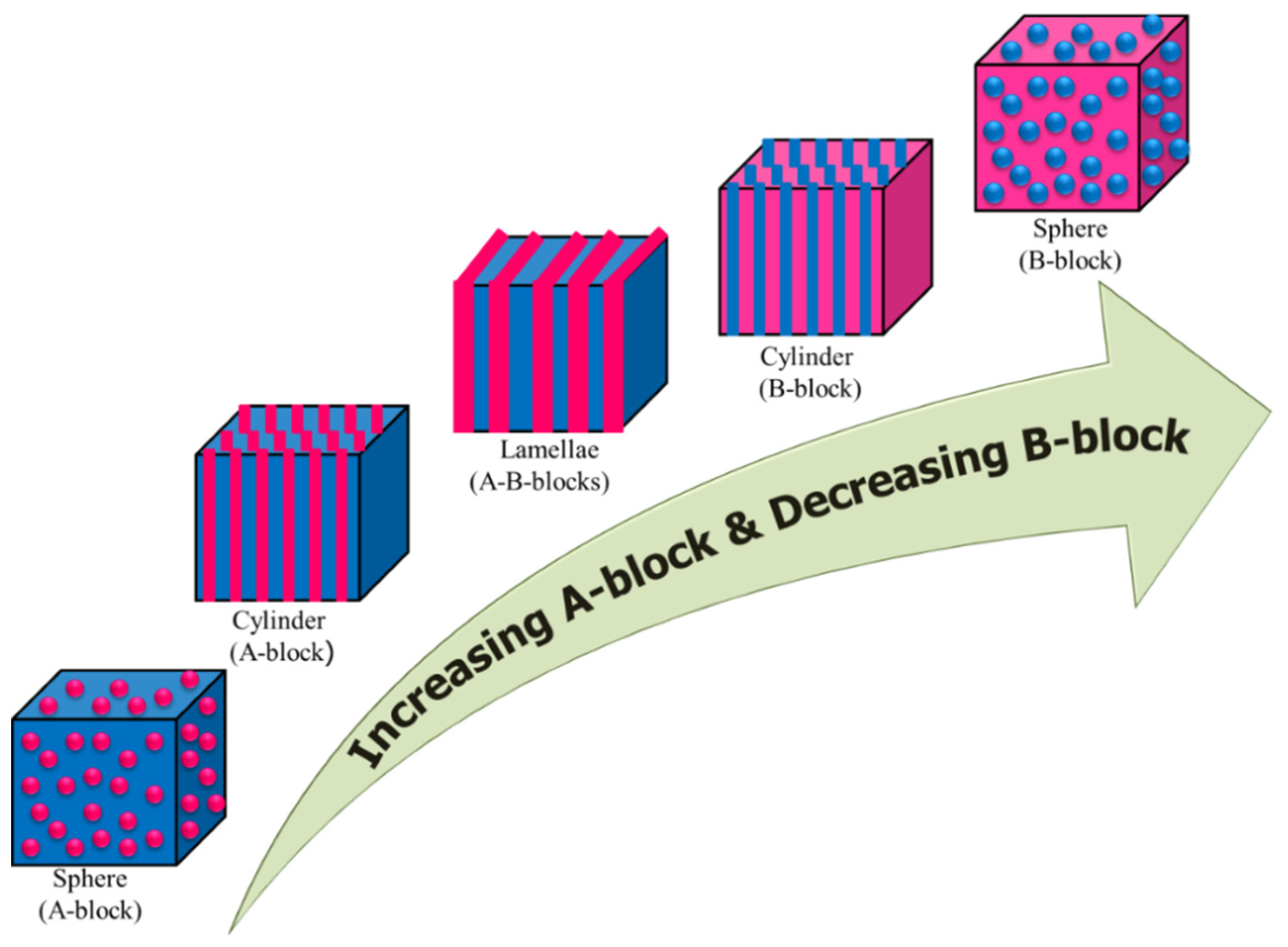
3.1. In Solid State
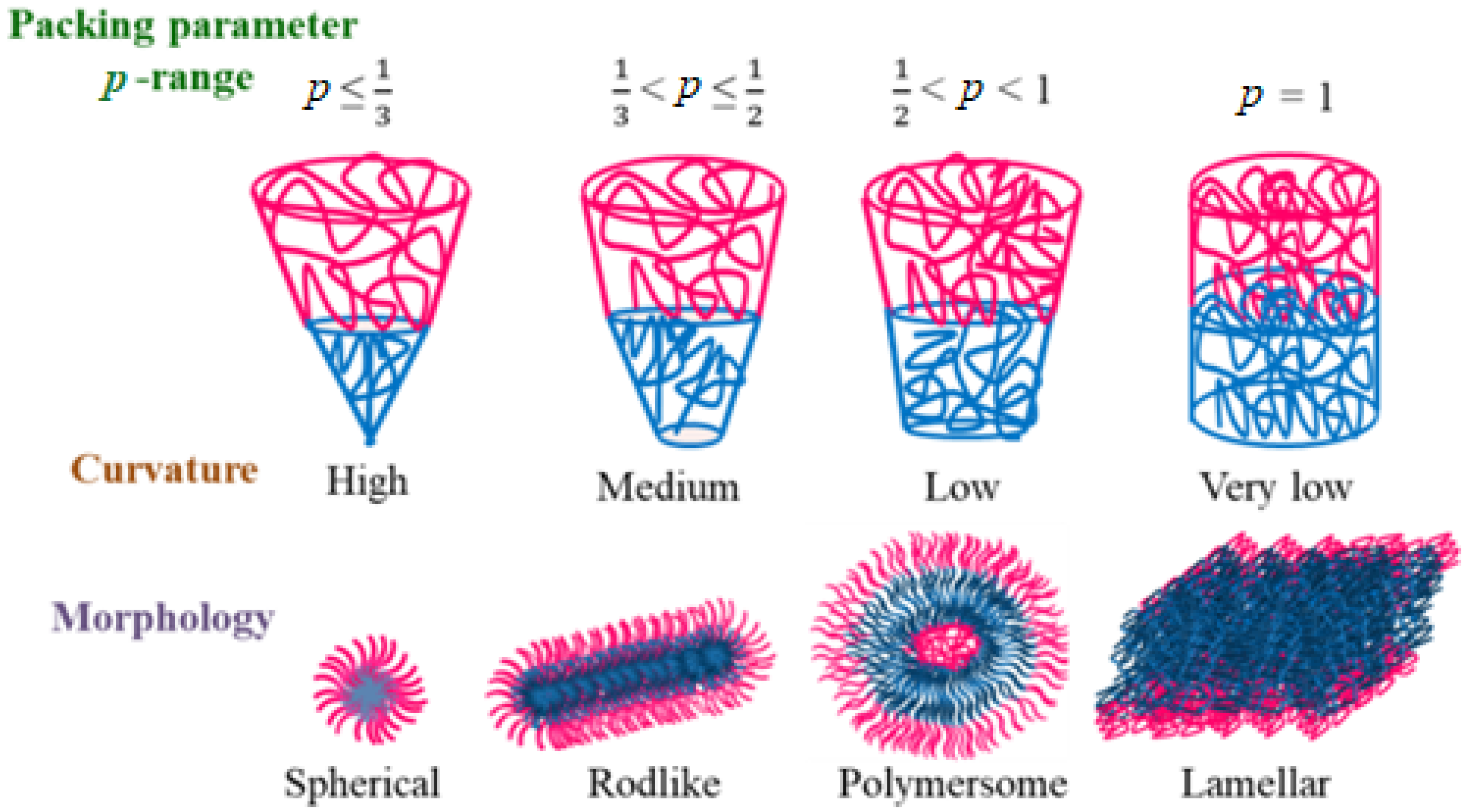
3.2. In Liquid State
4. Types of Micellar Assemblies
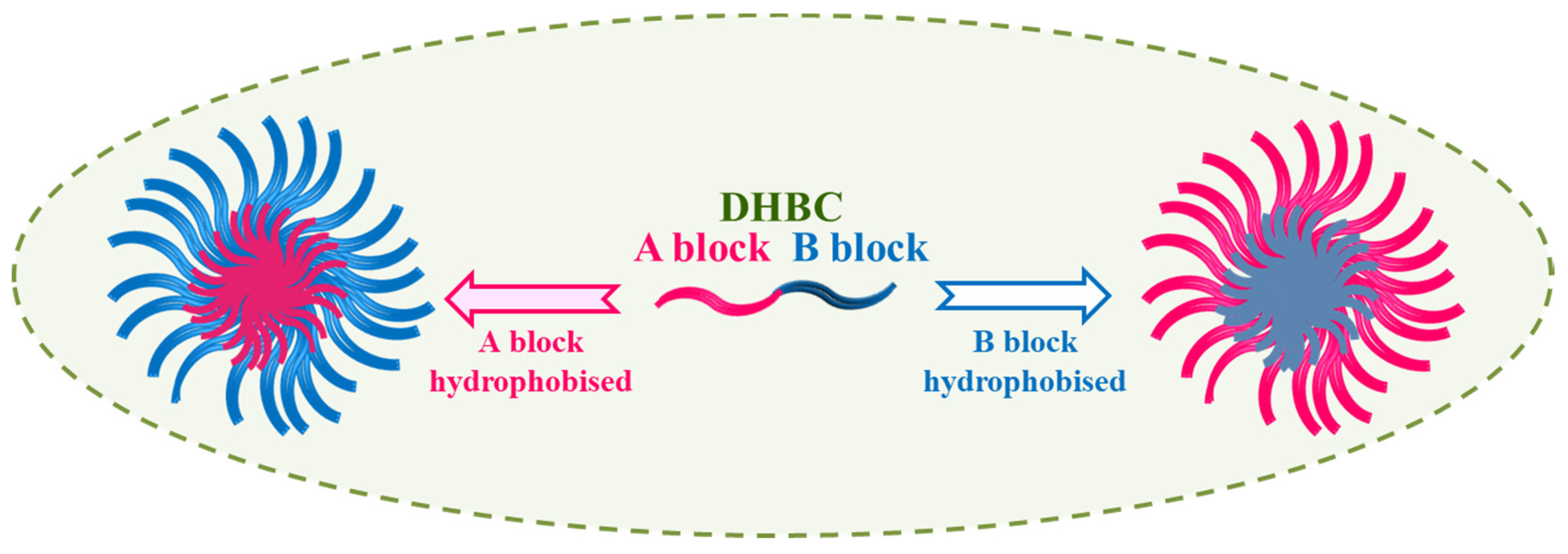
4.1. Schizophrenic Micelles
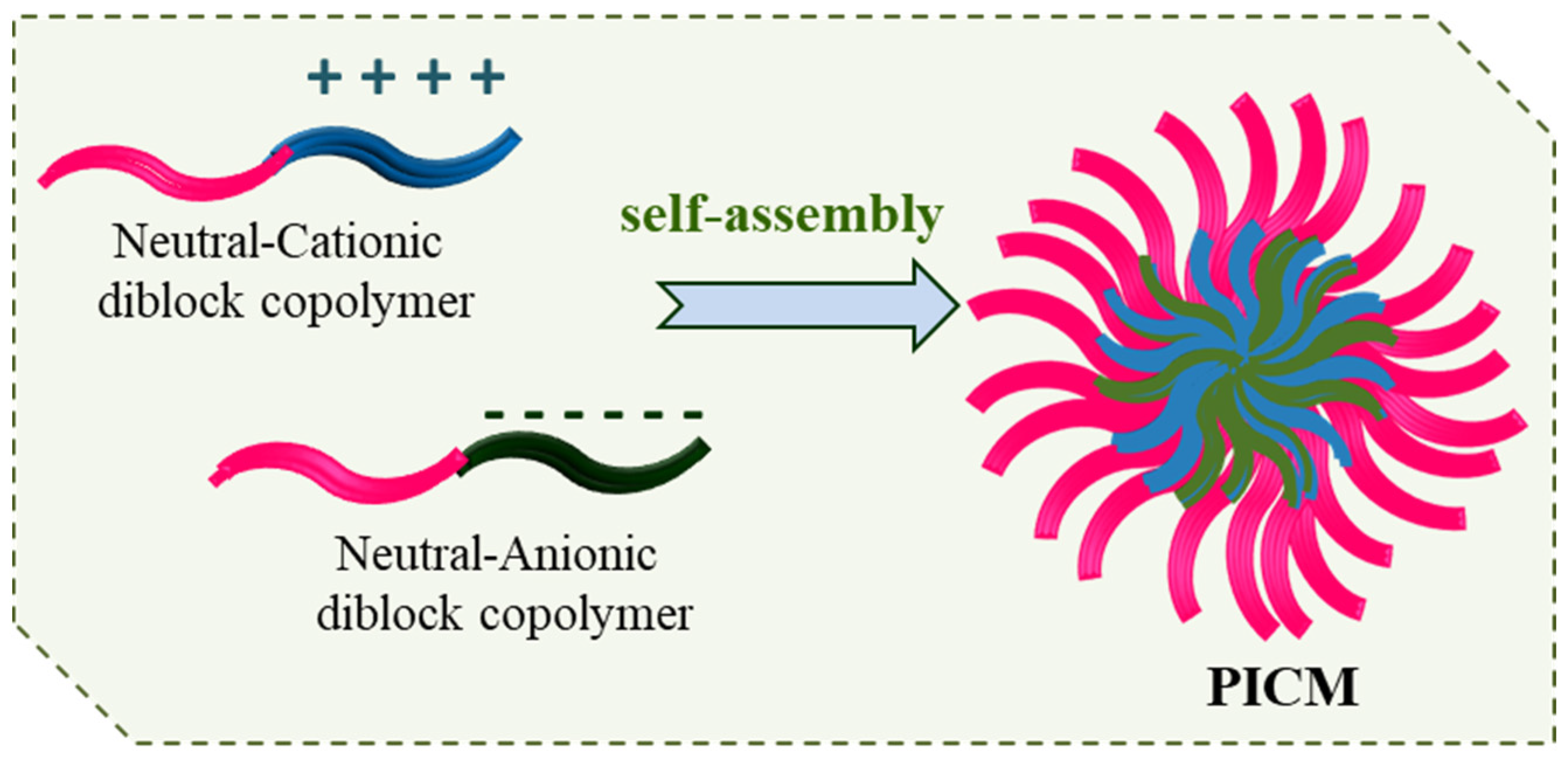
4.2. Polyion Complex Micelles (PICMs)
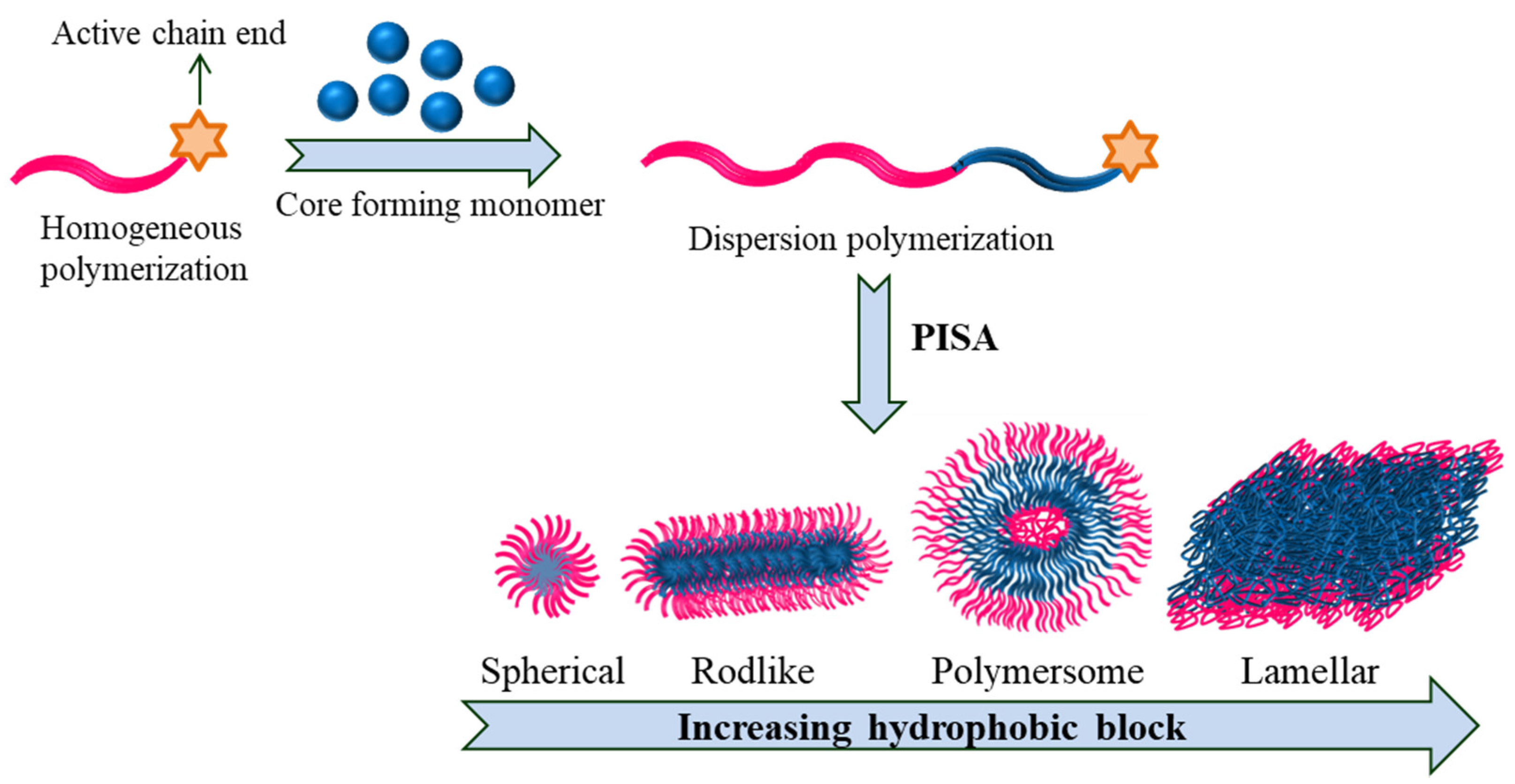
4.3. Polymerization Induced Self-Assembly (PISA)
4.4. Crystallization-Driven Self-Assembly (CDSA)
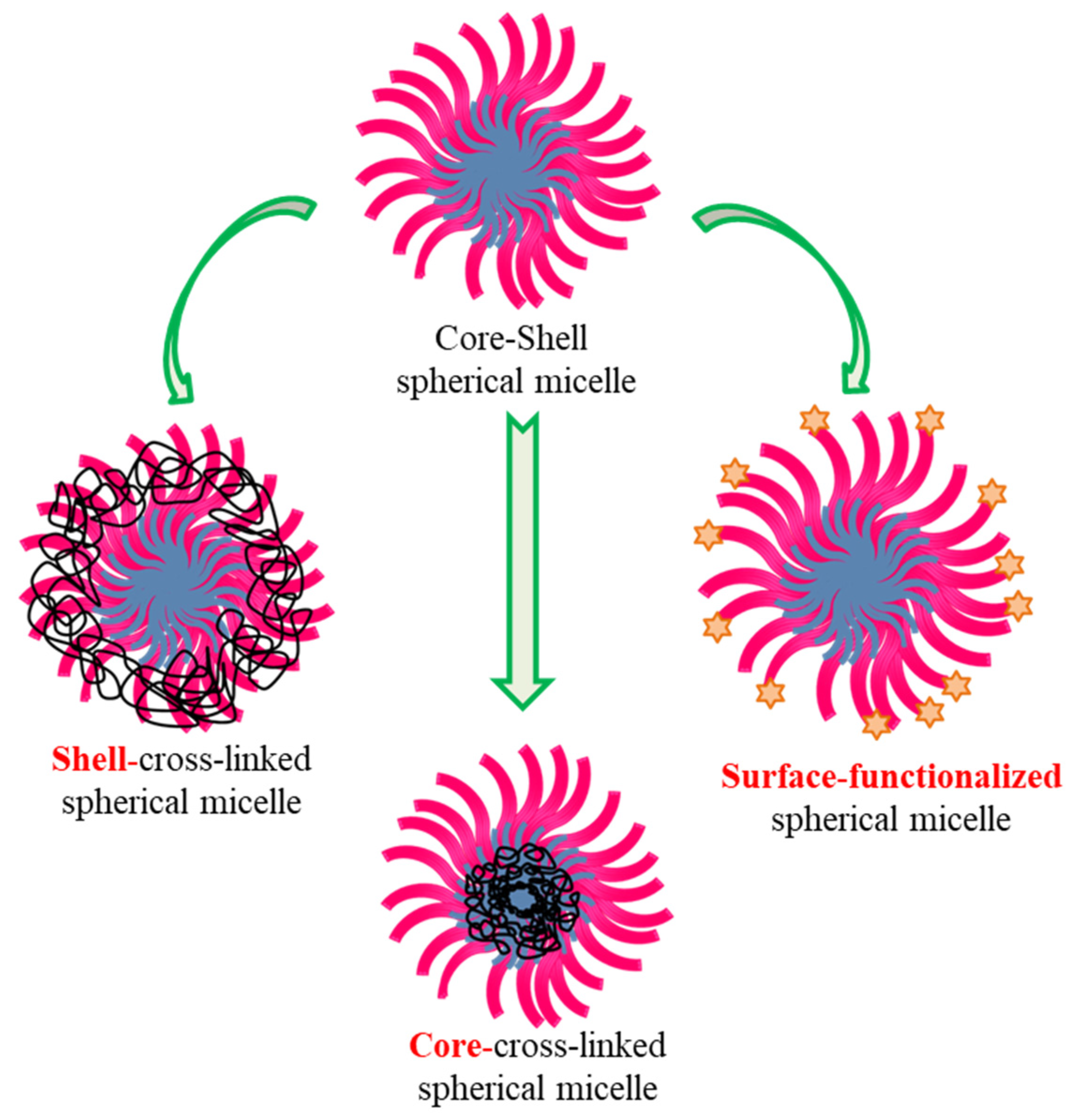
4.5. Cross-Linked, Functionalised and Stimuli-Responsive Micelles
4.6. Mixed Micelles
4.7. Polymer-Drug Conjugates
5. Self-Assembly in Drug Delivery Application
6. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Mai, Y.; Eisenberg, A. Self-assembly of block copolymers. Chem. Rev. 2012, 41, 5969–5985. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chan, C.; Zhao, T.; Parker, R.; Vignolini, S. Recent Advances in Block Copolymer Self-Assembly for the Fabrication of Photonic Films and Pigments. Adv. Opt. Mater. 2021, 9, 2100519. [Google Scholar] [CrossRef]
- Cabral, H.; Miyata, K.; Osada, K.; Kataoka, K. Block Copolymer Micelles in Nanomedicine Applications. Chem. Rev. 2018, 118, 6844–6892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartnikowski, M.; Dargaville, T.R.; Ivanovski, S.; Hutmacher, D.W. Degradation mechanisms of polycaprolactone in the context of chemistry, geometry and environment. Prog. Polym. Sci. 2019, 96, 1–20. [Google Scholar] [CrossRef]
- Espinoza, S.M.; Patil, H.I.; San Martin Martinez, E.; Casanas, R.; Ige, P.P. Poly-ε-caprolactone (PCL), a promising polymer for pharmaceutical and biomedical applications: Focus on nanomedicine in cancer. Int. J. Polym. Mat. Polym. Biomater. 2020, 69, 85–126. [Google Scholar] [CrossRef]
- Raina, N.; Pahwa, R.; Khosla, J.K.; Gupta, P.N.; Gupta, M. Polycaprolactone-based materials in wound healing applications. Polym. Bull. 2021, 79, 7041–7063. [Google Scholar] [CrossRef]
- Atanase, L.I.; Salhi, S.; Cucoveica, O.; Ponjavic, M.; Nikodinovic-Runic, J.; Delaite, C. Biodegradability Assessment of Polyester Copolymers Based on Poly(ethylene adipate) and Poly(ε-caprolactone). Polymers 2022, 14, 3736. [Google Scholar] [CrossRef]
- Atanase, L.I.; Lerch, J.P.; Caprarescu, S.; Iurciuc (Tincu), C.E.; Riess, G. Micellization of pH-sensitive poly(butadiene)-block-poly(2 vinylpyridine)-block-poly(ethylene oxide) triblock copolymers: Complex formation with anionic surfactants. J. Appl. Polym. Sci. 2017, 134, 45313. [Google Scholar] [CrossRef]
- Winninger, J.; Iurea, D.M.; Atanase, L.I.; Salhi, S.; Delaite, C.; Riess, G. Micellization of novel biocompatible thermo-sensitive graft copolymers based on poly(ε-caprolactone), poly(N-vinylcaprolactam) and poly(N-vinylpyrrolidone). Eur. Polym. J. 2019, 119, 74–82. [Google Scholar] [CrossRef]
- Aqeel, R.; Srivastava, N.; Kushwaha, P. Micelles in Cancer Therapy: An Update on Preclinical and Clinical Status. Recent Pat. Nanotechnol. 2022, 16, 283–294. [Google Scholar] [CrossRef]
- Kuperkar, K.; Tiwari, S.; Bahadur, P. Self-assembled block copolymer nanoaggregates for drug delivery applications. In Applications of Polymers in Drug Delivery, 2nd ed.; eBook; Elsevier: Amsterdam, The Netherlands, 2021; pp. 423–447. ISBN 978-0-12-819659-5. [Google Scholar]
- Bláhová, M.; Randárová, E.; Konefał, R.; Nottelet, B.; Etrych, T. Graft copolymers with tunable amphiphilicity tailored for efficient dual drug delivery: Via encapsulation and pH-sensitive drug conjugation. Polym. Chem. 2020, 11, 4438–4453. [Google Scholar] [CrossRef]
- Li, D.; Qin, J.; Sun, M.; Yan, G.; Tang, R. pH-sensitive, dynamic graft polymer micelles via simple synthesis for enhanced chemotherapeutic efficacy. J. Biomater. Appl. 2020, 34, 1059–1070. [Google Scholar] [CrossRef] [PubMed]
- Atanase, L.I.; Winninger, J.; Delaite, C.; Riess, G. Reversible addition–fragmentation chain transfer synthesis and micellar characteristics of biocompatible amphiphilic poly(vinyl acetate)-graft-poly(N-vinyl-2-pyrrolidone) copolymers. Eur. Polym. J. 2014, 53, 109–117. [Google Scholar] [CrossRef]
- Atanase, L.I.; Desbrieres, J.; Riess, R. Micellization of synthetic and polysaccharides-based graft copolymers in aqueous media. Prog. Polym. Sci. 2017, 73, 32–60. [Google Scholar] [CrossRef]
- Atanase, L.I. Micellar Drug Delivery Systems Based on Natural Biopolymers. Polymers 2021, 13, 477. [Google Scholar] [CrossRef]
- Ozturk, M.-R.; Popa, M.; Rata, D.M.; Cadinoiu, A.N.; Parfait, F.; Delaite, C.; Atanase, L.I.; Solcan, C.; Daraba, O.M. Drug-Loaded Polymeric Micelles Based on Smart Biocompatible Graft Copolymers with Potential Applications for the Treatment of Glaucoma. Int. J. Mol. Sci. 2022, 23, 9382. [Google Scholar] [CrossRef]
- Atanase, L.I.; Winninger, J.; Delaite, C.; Riess, G. Micellization and demicellization of amphiphilic poly(vinyl acetate)-graft-poly(N-vinyl-pyrrolidone) graft copolymers in the presence of sodium dodecyl sulfate. Colloid Surf. A Physicochem. Eng. Asp. 2014, 461, 287–294. [Google Scholar] [CrossRef]
- Feng, C.; Li, Y.; Yang, D.; Hu, J.; Zhang, X.; Huang, X. Well-defined graft copolymers: From controlled synthesis to multipurpose applications. Chem. Soc. Rev. 2011, 40, 1282–1295. [Google Scholar] [CrossRef]
- Riess, G.; Hurtrej, G.; Bahadur, P. Block copolymers. In Encyclopedia of Polymer Science Engineering; John Wiley & Sons: Hoboken, NJ, USA, 1985; Volume 2, p. 324. [Google Scholar]
- Hadjichristidis, N.; Pispas, S.; Floudas, G. Block Copolymers: Synthetic Strategies, Physical Properties, and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2003; ISBN 978-0-471-39436-5. [Google Scholar]
- Hamley, I. Block Copolymers in Solution: Fundamentals and Applications. J. Am. Chem. Soc. 2006, 128, 1395. [Google Scholar]
- Li, M.; Coenjarts, C.; Ober, C. Patternable block copolymers. Adv. Polym. Sci. 2005, 190, 183–226. [Google Scholar]
- Lazzari, M.; Tornerio, M. A global view on block copolymers. Polymers 2020, 12, 869. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Park, S.; Hinsberg, W. Block copolymer based nanostructures: Materials, processes, and applications to electronics. Chem. Rev. 2010, 110, 146–177. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Yang, S.Y.; Lee, Y.; Kim, Y. Functional nanomaterials based on block copolymer self-assembly. Prog. Polym. Sci. 2010, 35, 1325–1349. [Google Scholar] [CrossRef]
- Valint, P.; Bock, J.; Schulz, D. Synthesis and characterization of hydrophobically associating polymers. Polym. Mater. Sci. Eng. 1987, 57, 482. [Google Scholar]
- Liu, Z.; Jiao, Y.; Wang, Y.; Zhou, C.; Zhang, Z. Polysaccharides-based nanoparticles as drug delivery systems. Adv. Drug Deliv. Rev. 2008, 60, 1650–1662. [Google Scholar] [CrossRef]
- Myrick, J.M.; Vendra, V.K.; Krishnan, S. Self-assembled polysaccharide nanostructures for controlled-release applications. Nanotechnol. Rev. 2014, 3, 319–346. [Google Scholar]
- Sato, T.; Yang, J.; Terao, K. Micellar structure of hydrophobically modified polysaccharides in aqueous solution. Polym. J. 2022, 54, 403–412. [Google Scholar] [CrossRef]
- Larson, R.G.; Van, A.K.; Chatterjee, T.; Ginzburg, V.V. Associative thickeners for waterborne paints: Structure, characterization, rheology, and modeling. Prog. Polym. Sci. 2022, 129, 101546. [Google Scholar] [CrossRef]
- Szwarc, M. ‘Living’ polymers. Nature 1956, 178, 1168–1169. [Google Scholar] [CrossRef]
- Hadjichristidis, N.; Pitsikalis, M.; Iatrou, H. Synthesis of block copolymers. Adv. Polym. Sci. 2005, 189, 1–124. [Google Scholar]
- Hadjichristidis, N.; Iatrou, H.; Pitsikalis, M.; Mays, J. Macromolecular architectures by living and controlled/living polymerizations. Prog. Polym. Sci. 2006, 31, 1068–1132. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Sumerlin, B.; Tsarevsky, N. Progress in Controlled Radical Polymerization: Mechanisms and Techniques; American Chemical Society: Washington, DC, USA, 2012; Volume 1100, pp. 1–331. [Google Scholar]
- Matyjaszewski, K. Advanced Materials by Atom Transfer Radical Polymerization. Adv. Mater. 2018, 30, 1706441–1706473. [Google Scholar] [CrossRef] [PubMed]
- Kolb, H.; Finn, M.; Sharpless, K. Click chemistry: Diverse chemical function from a few good reactions. Angew. Chem. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Braunecker, W.; Matyjaszewski, K. Controlled/living radical polymerization: Features, developments and perspectives. Prog. Polym. Sci. 2007, 32, 93–146. [Google Scholar]
- McCormick, C.; Lowe, A. Aqueous RAFT polymerization: Recent developments in synthesis of functional water-soluble copolymers with controlled structures. Acc. Chem. Res. 2004, 37, 312–325. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Park, S.; Hinsberg, W.; Division, I.; Matyjaszewski, K.; Xia, J. Atom transfer radical polymerization. Chem. Rev. 2001, 101, 2921–2990. [Google Scholar]
- Jennings, J.; He, G.; Howdle, S.; Zetterlund, P. Block copolymer synthesis by controlled/living radical polymerisation in heterogeneous systems. Chem. Soc. Rev. 2016, 45, 5055–5084. [Google Scholar] [CrossRef]
- Parkatzidis, K.; Wang, H.; Truong, N.; Anastasaki, A. Recent Developments and Future Challenges in Controlled Radical Polymerization: A 2020 Update. Chem 2020, 6, 1575–1588. [Google Scholar]
- Benoit, D.; Harth, E.; Fox, P.; Waymouth, R.M.; Hawker, C.J. Accurate structural control and block formation in the living polymerization of 1,3-dienes by nitroxide-mediated procedures. Macromolecules 2000, 33, 363–370. [Google Scholar] [CrossRef]
- Gaucher, G.; Dufresne, M.H.; Sant, V.P.; Kang, N.; Maysinger, D.; Leroux, J.C. Block copolymer micelles: Preparation, characterization and application in drug delivery. J. Control. Release 2005, 109, 169–188. [Google Scholar] [CrossRef]
- Kazunori, K.; Atsushi, H.; Yukio, N. Block copolymer micelles for drug delivery: Design, characterization and biological significance. Adv. Drug Deliv. Rev. 2012, 64, 37–48. [Google Scholar]
- Sarkar, B.; Alexandridis, P. Block copolymer–nanoparticle composites: Structure, functional properties, and processing. Prog. Polym. Sci. 2015, 40, 33–62. [Google Scholar] [CrossRef]
- Fesenmeier, D.; Park, S.; Kim, S.; Won, Y. Surface mechanical behavior of water-spread poly(styrene)-poly(ethylene glycol) (PS-PEG) micelles at the air-water interface: Effect of micelle size and polymer end/linking group chemistry. J. Colloid Interface Sci. 2022, 617, 764–777. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, Y.; Xie, Z.; Jing, X.; Bellotti, A.; Gu, Z. Stimuli-Responsive Polymersomes for Biomedical Applications. Biomacromolecules 2017, 18, 649–673. [Google Scholar] [CrossRef] [PubMed]
- Mukerabigwi, J.; Yin, W.; Zha, Z.; Ke, W.; Wang, Y.; Chen, W.; Japir, A.; Wang, Y.; Ge, Z. Polymersome nanoreactors with tumor pH-triggered selective membrane permeability for prodrug delivery, activation, and combined oxidation-chemotherapy. J. Control. Release 2019, 303, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.; Ramezani, M.; Abnous, K.; Alibolandi, M. Biocompatible polymersomes-based cancer theranostics: Towards multifunctional nanomedicine. Int. J. Pharm. 2017, 519, 287–303. [Google Scholar] [CrossRef] [PubMed]
- Robertson, J.; Yealland, G.; Avila-Olias, M.; Chierico, L.; Bandmann, O.; Renshaw, S.; Battaglia, G. pH-sensitive tubular polymersomes: Formation and applications in cellular delivery. ACS Nano 2014, 8, 4650–4661. [Google Scholar] [CrossRef]
- Lei, L.; Gohy, J.; Willet, N.; Zhang, J.; Varshney, S.; Jerome, R. Morphology of core-shell-corona aqueous micelles: II. Addition of core-forming homopolymer. Polymer 2004, 45, 4375–4381. [Google Scholar] [CrossRef]
- Sasidharan, M.; Nakashima, K. Core-Shell-Corona Polymeric Micelles as a Versatile Template for Synthesis of Inorganic Hollow Nanospheres. Acc. Chem. Res. 2014, 47, 157–167. [Google Scholar] [CrossRef]
- Nicolai, T.; Colombani, O.; Chassenieux, C. Dynamic polymeric micelles versus frozen nanoparticles formed by block copolymers. Soft Matter 2010, 6, 3111–3118. [Google Scholar] [CrossRef]
- Xu, R.; Winnik, M.A.; Riess, G.; Chu, B.; Croucher, M.D. Micellization of Polystyrene-Poly(ethylene oxide) Block Copolymers in Water. A Test of the Star and Mean-Field Models. Macromolecules 1992, 25, 644–652. [Google Scholar] [CrossRef]
- Nagarajan, R. “Non-equilibrium” block copolymer micelles with glassy cores: A predictive approach based on theory of equilibrium micelles. J. Colloid Interface Sci. 2015, 449, 416–427. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, G.; Souza, V.; Oliveira, L.; Noronha, M.; Masini, J.; Chaimovich, H.; Salinas, R.; Florenzano, F.; Cuccovia, I. Characterization of PMMA-b-PDMAEMA aggregates in aqueous solutions. Colloid Polym. Sci. 2019, 297, 557–569. [Google Scholar] [CrossRef]
- Casagrande, C.; Fabre, P.; Raphae, E.; Veyssie, M. Janus beads: Realization and behaviour at water/oil interfaces. Europhys. Lett. 1989, 9, 251–255. [Google Scholar] [CrossRef]
- Erhardt, R.; Bo1ker, A.; Zettl, H.; Kaya, H.; Pyckhout-Hintzen, W.; Krausch, G.; Abetz, V.; Muller, A. Janus Micelles. Macromolecules 2001, 34, 1069–1075. [Google Scholar] [CrossRef] [Green Version]
- Walther, A.; Barner-Kowollik, C.; Meuller, A. Mixed, Multicompartment, or Janus Micelles? A Systematic Study of Thermoresponsive Bis-Hydrophilic Block Terpolymers. Langmuir 2010, 26, 12237–12246. [Google Scholar] [CrossRef]
- Gohy, J.; Zhao, Y. Photo-responsive block copolymer micelles: Design and behavior. Chem. Soc. Rev. 2013, 42, 7117–7129. [Google Scholar] [CrossRef] [Green Version]
- Azeri, Ö.; Schönfeld, D.; Noirez, L.; Gradzielski, M. Structural control in micelles of alkyl acrylate-acrylate copolymers via alkyl chain length and block length. Colloid Polym. Sci. 2020, 298, 829–840. [Google Scholar] [CrossRef]
- Gao, Z.; Varshney, S.; Wong, S.; Eisenberg, A. Block Copolymer “Crew-Cut” Micelles in Water. Macromolecules 1994, 27, 7923–7927. [Google Scholar] [CrossRef]
- Zhang, L.; Eisenberg, A. Multiple Morphologies of “Crew-Cut” Aggregates of Polystyrene-b-poly(acrylic acid) Block Copolymers. Science 1995, 268, 1728–1732. [Google Scholar] [CrossRef] [Green Version]
- Talelli, M.; Barz, M.; Rijcken, C.; Kiessling, F.; Hennink, W.; Lammers, T. Core-crosslinked polymeric micelles: Principles, preparation, biomedical applications and clinical translation. Nano Today 2015, 10, 93–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Fliedel, C.; Manoury, E.; Poli, R. Core-crosslinked micelles with a poly-anionic poly (styrene sulfonate)-based outer shell made by RAFT polymerization. Polymer 2022, 243, 124640. [Google Scholar] [CrossRef]
- Procházka, K.; Limpouchová, Z.; Štěpánek, M.; Šindelka, K.; Lísal, M. DPD Modelling of the Self-and Co-Assembly of Polymers and Polyelectrolytes in Aqueous Media: Impact on Polymer Science. Polymers 2022, 14, 404. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Liu, S. Emerging trends in solution self-assembly of block copolymers. Polymer 2020, 207, 122914. [Google Scholar] [CrossRef]
- Ge, Z.; Xie, D.; Chen, D.; Jiang, X.; Zhang, Y.; Liu, H.; Liu, S. Stimuli-Responsive Double Hydrophilic Block Copolymer Micelles with Switchable Catalytic Activity. Macromolecules 2007, 40, 3538–3546. [Google Scholar] [CrossRef]
- Manga, M.; Cayre, O.; Biggs, S.; Hunter, T. Influence of pH-Responsive Monomer Content on the Behavior of Di-Block Copolymers in Solution and as Stabilizers of Pickering Latex Particle Emulsifiers. Front. Chem. 2018, 6, 301–313. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Du, J. Ultrasound and pH Dually Responsive Polymer Vesicles for Anticancer Drug Delivery. Sci. Rep. 2013, 3, 2162–2171. [Google Scholar] [CrossRef] [Green Version]
- Papadakis, C.M.; Müller-Buschbaum, P.; Laschewsky, A. Switch it inside-out: “Schizophrenic” behavior of all thermoresponsive UCST-LCST diblock copolymers. Langmuir 2019, 35, 9660–9676. [Google Scholar] [CrossRef]
- Butun, V.; Armes, S.; Billingham, N.; Tuzar, Z.; Rankin, A.; Eastoe, J.; Heenan, R. Synthesis and aqueous solution properties of a well-defined thermo-responsive schizophrenic diblock copolymer. Macromolecules 2001, 34, 1503. [Google Scholar]
- Kocak, G.; Tuncer, C.; Bütün, V. pH-Responsive polymers. Polym. Chem. 2017, 8, 144–176. [Google Scholar] [CrossRef]
- Checot, F.; Rodrıguez-Hernandez, J.; Gnanou, Y.; Lecommandoux, S. pH-responsive micelles and vesicles nanocapsules based on polypeptide diblock copolymers. Biomol. Eng. 2007, 24, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Atanase, L.I.; Riess, G. Micellization of pH-stimulable poly(2-vinylpyridine)-b-poly(ethylene oxide) copolymers and their complexation with anionic surfactants. J. Colloid Interface Sci. 2013, 395, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Harada, A.; Kataoka, K. Polyion complex micelle formation from double hydrophilic block copolymers composed of charged and non-charged segments in aqueous media. Polym. J. 2018, 50, 95–100. [Google Scholar] [CrossRef]
- Jundi, A.; Buwalda, S.; Bakkourb, Y.; Garric, X.; Nottelet, B. Double hydrophilic block copolymers self-assemblies in biomedical applications. Adv. Colloid Interfac. Sci. 2020, 283, 102213–102225. [Google Scholar] [CrossRef]
- Smith, A.; Xu, X.; Kirkland-York, S.; Savin, D.; McCormick, C. Schizophrenic Self-Assembly of Block Copolymers Synthesized via Aqueous RAFT Polymerization: From Micelles to Vesicles. Macromolecules 2010, 43, 1210–1217. [Google Scholar] [CrossRef]
- Can, A.; Zhang, Q.; Rudolph, T.; Schacher, F.; Gohy, J.; Schubert, U.; Hoogenboom, R. Schizophrenic thermoresponsive block copolymer micelles based on LCST and UCST behavior in ethanol-water mixtures. Eur. Polym. J. 2015, 69, 460–471. [Google Scholar] [CrossRef]
- Colfen, H. Double-Hydrophilic Block Copolymers: Synthesis and Application as Novel Surfactants and Crystal Growth Modifiers. Macromol. Rapid Commun. 2001, 4, 220–252. [Google Scholar]
- Guragain, S.; Bastakoti, B.P.; Malgras, V.; Nakashima, K.; Yamauchi, Y. Multi-Stimuli-Responsive Polymeric Materials. Chem. Eur. J. 2015, 21, 13164–13174. [Google Scholar] [CrossRef]
- Zhuang, J.; Gordon, M.; Ventura, J.; Li, L.; Thayumanavan, S. Multi-stimuli responsive macromolecules and their assemblies. Chem. Soc. Rev. 2013, 42, 7421–7436. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Chen, S.; Pang, M.; Zhang, W. Synthesis and micellization of a multi-stimuli responsive block copolymer based on spiropyran. Polym. Chem. 2016, 7, 6880–6885. [Google Scholar] [CrossRef]
- Appold, M.; Mari, C.; Lederle, C.; Elbert, J.; Schmidt, C.; Ott, I.; Stühn, B.; Gasser, G.; Gallei, M. Multi-stimuli responsive block copolymers as a smart release platform for a polypyridyl ruthenium complex. Polym. Chem. 2016, 8, 890–900. [Google Scholar] [CrossRef]
- Corten, C.; Kretschmer, K.; Kuckling, D. Novel multi-responsive P2VP-block-PNIPAAm block copolymers via nitroxide-mediated radical polymerization. Beilstein J. Org. Chem. 2010, 6, 756–765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, J.; Liu, S. Responsive Polymers for Detection and Sensing Applications: Current Status and Future Developments. Macromolecules 2010, 43, 8315–8330. [Google Scholar] [CrossRef]
- Tan, S.; Zhao, D.; Yuan, D.; Wang, H.; Tu, K.; Wang, L. Influence of indomethacin-loading on the micellization and drug release of thermosensitive dextran-graft-poly(N-isopropylacrylamide). React. Funct. Polym. 2011, 71, 820–827. [Google Scholar] [CrossRef]
- Liu, S.; Billingham, N.C.; Armes, S.P. A Schizophrenic Water-Soluble Diblock Copolymer. Angew. Chem. Int. Ed. 2001, 40, 2328. [Google Scholar] [CrossRef]
- Liu, S.; Armes, S.P. Micelle Formation and Inversion Kinetics of a Schizophrenic Diblock Copolymer. Langmuir 2002, 19, 4432. [Google Scholar] [CrossRef]
- Yusa, S.; Yokoyama, Y.; Morishima, Y. Synthesis of oppositely charged block copolymers of polyethylene glycol via reversible addition-fragmentation chain transfer radical polymerization and characterization of their polyion complex micelles in water. Macromolecules 2009, 42, 376–383. [Google Scholar] [CrossRef]
- Nakai, K.; Nishiuchi, M.; Inoue, M.; Ishihara, K.; Sanada, Y.; Sakurai, K.; Yusa, S. Preparation and characterization of polyion complex micelles with phosphobetaine shells. Langmuir 2013, 29, 9651–9661. [Google Scholar] [CrossRef]
- De Santis, S.; Ladogana, R.; Diociaiuti, M.; Masci, G. PEGylated and thermosensitive polyion complex micelles by self-assembly of two oppositely and permanently charged diblock copolymers. Macromolecules 2010, 43, 1992–2001. [Google Scholar] [CrossRef]
- Amaral, S.; Tawara, M.; Fernandez-Villamarin, M.; Borrajo, E.; Martinez-Costas, J.; Vidal, A.; Riguera, R.; Fernandez-Megia, E. Tuning the Size of Nanoassembles: A Hierarchical Transfer of Information from Dendrimers to Polyion Complexes. Angew. Chem. 2018, 57, 5273–5277. [Google Scholar] [CrossRef]
- Fuoss, R.; Sadek, H. Mutual interaction of polyelectrolytes. Science 1949, 110, 552–554. [Google Scholar] [CrossRef] [PubMed]
- Nakahata, R.; Yusa, S. Preparation of water-soluble polyion complex (PIC) micelles covered with amphoteric random copolymer shells with pendant sulfonate and quaternary amino groups. Polymers 2018, 10, 205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohno, S.; Ishihara, K.; Yusa, S. Formation of Polyion Complex (PIC) Micelles and Vesicles with Anionic pH-Responsive Unimer Micelles and Cationic Diblock Copolymers in Water. Langmuir 2016, 32, 3945–3953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, R.; Sato, T.; Terao, K.; Yusa, S. Reversible Vesicle-Spherical Micelle Transition in a Polyion Complex Micellar System Induced by Changing the Mixing Ratio of Copolymer Components. Macromolecules 2016, 49, 3091–3099. [Google Scholar] [CrossRef]
- Kim, H.; Zheng, M.; Miyata, K.; Kataoka, K. Preparation of polyion complex micelles using block copolymers for siRNA delivery. Methods Mol. Biol. 2016, 1364, 89–103. [Google Scholar] [PubMed]
- Penfold, N.; Yeow, J.; Boyer, C.; Armes, S. Emerging Trends in Polymerization-Induced Self-Assembly. ACS Macro Lett. 2019, 8, 1029–1054. [Google Scholar] [CrossRef] [Green Version]
- Phan, H.; Taresco, V.; Penelle, J.; Couturaud, B. Polymerisation-Induced Self-Assembly (PISA) as a straightforward formulation strategy for Stimuli-Responsive Drug Delivery Systems and Biomaterials: Recent Advances. Biomater. Sci. 2020, 9, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Kadirkhanov, J.; Wang, C.; Ding, S.; Hong, C.; Wang, F.; You, Y. Polymerization-induced self-assembly for the fabrication of polymeric nano-objects with enhanced structural stability by cross-linking. Polym. Chem. 2020, 11, 3654–3672. [Google Scholar] [CrossRef]
- D’Agosto, F.; Rieger, J.; Lansalot, M. RAFT-Mediated Polymerization-Induced Self-Assembly. Angew. Chem. 2020, 59, 8368–8392. [Google Scholar] [CrossRef]
- Liu, C.; Hong, C.; Pan, C. Polymerization techniques in polymerization-induced self-assembly (PISA). Polym. Chem. 2020, 11, 3673–3689. [Google Scholar] [CrossRef]
- Cao, J.; Tan, Y.; Chen, Y.; Zhang, L.; Tan, J. Expanding the Scope of Polymerization-Induced Self-Assembly: Recent Advances and New Horizons. Macromol. Rapid Commun. 2021, 42, 2100498–2100510. [Google Scholar] [CrossRef] [PubMed]
- An, N.; Chen, X.; Yuan, J. Non-thermally initiated RAFT polymerization-induced self-assembly. Polym. Chem. 2021, 12, 3220–3232. [Google Scholar] [CrossRef]
- Damsongsang, P.; Hoven, Y. Core-functionalized nanoaggregates: Preparation via polymerization-induced self-assembly and their applications. New J. Chem. 2021, 45, 12776–12791. [Google Scholar] [CrossRef]
- Ganda, S.; Stenzel, M. Concepts, fabrication methods and applications of living crystallization-driven self-assembly of block copolymers. Prog. Polym. Sci. 2020, 101, 101195–101205. [Google Scholar] [CrossRef]
- Noack, S.; Schanzenbach, D.; Koetz, J.; Schlaad, H. Polylactide-Based Amphiphilic Block Copolymers: Crystallization-Induced Self-Assembly and Stereocomplexation. Macromol. Rapid Commun. 2018, 40, 1800639–1800650. [Google Scholar] [CrossRef]
- MacFarlane, L.; Shaikh, H.; Garcia-Hernandez, J.; Vespa, M.; Fukui, T.; Manners, I. Functional nanoparticles through π-conjugated polymer self-assembly. Nat. Rev. Mater. 2021, 6, 7–26. [Google Scholar] [CrossRef]
- MacFarlane, L.; Zhao, C.; Cai, J.; Qiu, H.; Manners, I. Emerging applications for living crystallization-driven self-assembly. Chem. Sci. 2021, 12, 4661–4682. [Google Scholar] [CrossRef]
- Finnegan, J.; Pilkington, E.; Alt, K.; Rahim, A.; Kent, S.; Davis, T.; Kempe, K. Stealth Nanorods via the Aqueous Living Crystallisation-Driven Self-Assembly of Poly(2-oxazoline)s. Chem. Sci. 2021, 12, 7350–7360. [Google Scholar] [CrossRef]
- Ringsdorf, H. Structure and properties of pharmacologically active polymers. J. Polym. Sci. Symp. 1975, 51, 135–153. [Google Scholar] [CrossRef]
- Shi, A.C. Self-Consistent Field Theory of Block Copolymers, Developments in Block Copolymer Science and Technology; John Wiley & Sons: Hoboken, NJ, USA, 2004; pp. 265–294. [Google Scholar]
- Chantawansri, T.L.; Bosse, A.W.; Hexemer, A.; Ceniceros, H.D.; Garcia-Cervera, C.J.; Kramer, E.J.; Fredrickson, G.H. Self-consistent Field Theory Simulations of Block Copolymer Assembly on a Sphere. Phys. Rev. E 2007, 75, 031802. [Google Scholar] [CrossRef] [Green Version]
- Lyubimov, I.; Daniel, J.; Beltran, V.; Jayaraman, A. PRISM Theory Study of Amphiphilic Block Copolymer Solutions with Varying Copolymer Sequence and Composition. Macromolecules 2017, 50, 7419–7431. [Google Scholar] [CrossRef]
- Zhang, Q.; Lin, J.; Wang, L.; Xu, Z. Theoretical modeling and simulations of self-assembly of copolymers in solution. Prog. Polym. Sci. 2017, 75, 1–30. [Google Scholar] [CrossRef]
- Duncan, R. Polymer conjugates as anticancer nanomedicines. Nat. Rev. Cancer 2006, 6, 688–701. [Google Scholar] [CrossRef]
- Greco, F.; Vicent, M.J. Combination therapy: Opportunities and challenges for polymer-drug conjugates as anticancer nanomedicines. Adv. Drug. Deliv. Rev. 2009, 61, 1203–1213. [Google Scholar] [CrossRef]
- Kopeček, J. Polymer-drug conjugates: Origins, progress to date and future directions. Adv. Drug. Deliv. Rev. 2013, 65, 49–59. [Google Scholar] [CrossRef] [Green Version]
- Feng, Q.; Tong, R. Anticancer nanoparticulate polymer-drug conjugate. Bioeng. Trans. Med. 2016, 1, 277–296. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.; Baker, S.L.; Colina, C.M.; Figg, C.A.; Kaar, J.L.; Matyjaszewski, K.; Simakova, A.; Summerlin, B.S. Next Generation Protein-Polymer Conjugates. AIChE J. 2018, 64, 3230–3245. [Google Scholar] [CrossRef]
- Murthy, K.S.; Ma, Q.; Clark, C.G., Jr.; Remsen, E.E.; Wooley, K.L. Fundamental design aspects of amphiphilic shell-cross-linked nanoparticles for controlled release applications. Chem. Commun. 2001, 8, 773–774. [Google Scholar] [CrossRef]
- Xi, S.; Wang, L.; Liu, J.; Chapman, W. Thermodynamics, microstructures, and solubilization of block copolymer micelles by density functional theory. Langmuir 2019, 35, 5081–5092. [Google Scholar] [CrossRef]
- Biswas, S.; Kumari, P.; Lakhani, P.M.; Ghosh, B. Recent advances in polymeric micelles for anti-cancer drug delivery. Eur. J. Pharm. Sci. 2016, 83, 184–202. [Google Scholar] [CrossRef]
- Lee, J.S.; Feijen, J. Polymersomes for drug delivery: Design, formation and characterization. J. Cont. Release 2012, 161, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V. Micellar nanocarriers: Pharmaceutical perspectives. Pharm. Res. 2007, 24, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Guiraud, S.; Alimi-Guez, D.; Wittenberghe, L.; Scherman, D.; Kichler, A. The Reverse Block Copolymer Pluronic 25R2 Promotes DNA Transfection of Skeletal Muscle. Macromol. Biosci. 2011, 11, 590–594. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Du, J.; Duan, Y.; Zang, Y.; Zhang, H.; Yang, C.; Cao, F.; Zhai, G. Curcumin loaded mixed micelles composed of Pluronic P123 and F68: Preparation, optimization and in-vitro characterization. Colloids Surf. B Biointerfaces 2012, 97, 101–108. [Google Scholar] [CrossRef]
- Hassanzadeh, S.; Feng, Z.; Pettersson, T.; Hakkarainen, M. A proof-of-concept for folate-conjugated and quercetin-anchored Pluronic mixed micelles as molecularly modulated polymeric carriers for doxorubicin. Polymer 2015, 74, 193–204. [Google Scholar] [CrossRef]
- Patel, D.; Rathod, S.; Tiwari, S.; Ray, D.; Kuperkar, K.; Aswal, V.; Bahadur, P. Self-Association in EO-BO-EO Triblock Copolymers as a Nanocarrier Template for Sustainable Release of Anticancer Drugs. J. Phys. Chem. B 2020, 124, 11750–11761. [Google Scholar] [CrossRef]
- Patel, D.; Kuperkar, K.; Bahadur, P. Temperature stimulated self-association and micellar transition for star shaped normal and reverse EO-PO block copolymers and their mixed systems as potential use for anticancer drug solubilization. Soft Matter 2022, 18, 4543–4553. [Google Scholar] [CrossRef]
- Basak, R.; Bandyopadhyay, R. Encapsulation of Hydrophobic Drugs in Pluronic F127 Micelles: Effects of Drug Hydrophobicity, Solution Temperature, and pH. Langmuir 2013, 29, 4350–4356. [Google Scholar] [CrossRef] [Green Version]
- Yao, M.; Hu, Y.; Sheng, X.; Lin, J.; Ling, D.; Gao, J. Toxicity analysis of various Pluronic F-68-coated carbon nanotubes on mesenchymal stem cells. Chem.-Biol. Interact. 2016, 250, 47–58. [Google Scholar] [CrossRef] [Green Version]
- Letchford, K.; Burt, H. A review of the formation and classification of amphiphilic block copolymer nanoparticulate structures: Micelles, nanospheres, nanocapsules and polymersomes. Eur. J. Pharm. Biopharm. 2007, 65, 259–269. [Google Scholar] [CrossRef]
- Bajpai, A.; Shukla, S.; Bhanu, S.; Kankane, S. Responsive polymers in controlled drug delivery. Prog. Polym. Sci. 2008, 33, 1088–1118. [Google Scholar] [CrossRef]
- Popovici, C.; Popa, M.; Sunel, V.; Atanase, L.I.; Ichim, D.L. Drug Delivery Systems Based on Pluronic Micelles with Antimicrobial Activity. Polymers 2022, 14, 3007. [Google Scholar] [CrossRef] [PubMed]
- Iurciuc-Tincu, C.-E.; Cretan, M.S.; Purcar, V.; Popa, M.; Daraba, O.M.; Atanase, L.I.; Ochiuz, L. Drug Delivery System Based on pH-Sensitive Biocompatible Poly(2-vinyl pyridine)-b-poly(ethylene oxide) Nanomicelles Loaded with Curcumin and 5-Fluorouracil. Polymers 2020, 12, 1450. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Patil, R.; Bahadur, P. Polysaccharide based scaffolds for soft tissue engineering applications. Polymers 2019, 11, 1. [Google Scholar] [CrossRef] [Green Version]
- Pethe, A.; Yadav, K. Polymers, responsiveness and cancer therapy. Artif. Cells Nanomed. Biotechnol. 2019, 47, 395–405. [Google Scholar] [CrossRef]
- Sun, C.; Lu, C.; Zhao, Y.; Guo, P.; Tian, J.; Zhang, L.; Li, X.; Lv, H.; Dai, D.; Li, X. Characterization of the Doxorubicin-Pluronic F68 Conjugate Micelles and Their Effect on Doxorubicin Resistant Human Erythroleukemic Cancer Cells. J. Nanomed. Nanotechnol. 2011, 2, 1000114–1000119. [Google Scholar]
- Zhao, L.; Zhang, W. Recent progress in drug delivery of pluronic P123: Pharmaceutical perspectives. J. Drug Target. 2017, 25, 471–484. [Google Scholar] [CrossRef]
- Tang, Y.; Varyambath, A.; Ding, Y.; Chen, B.; Huang, X.; Zhang, Y.; Yu, D.; Kim, I.; Song, W. Porous organic polymers for drug delivery: Hierarchical pore structures, variable morphologies, and biological properties. Biomater. Sci. 2022, 10, 5369–5390. [Google Scholar] [CrossRef]
- Zhang, Y.; Kim, I.; Lu, Y.; Xu, Y.; Yu, D.; Song, W. Intelligent poly(l-histidine)-based nanovehicles for controlled drug delivery. J. Control. Release 2022, 349, 963–982. [Google Scholar] [CrossRef]
- Sharma, A.; Prasher, P.; Aljabali, A.; Mishra, V.; Gandhi, H.; Kumar, S.; Mutalik, S.; Chellappan, D.; Tambuwala, M.; Dua, K.; et al. Emerging era of “somes”: Polymersomes as versatile drug delivery carrier for cancer diagnostics and therapy. Drug Deliv. Transl. Res. 2020, 10, 1171–1190. [Google Scholar] [CrossRef]
- Mu, X.; Gan, S.; Wang, Y.; Li, H.; Zhou, G. Stimulus-responsive vesicular polymer nano-integrators for drug and gene delivery. Int. J. Nanomed. 2019, 14, 5415–5434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tawfik, S.M.; Elmasry, M.R.; Lee, Y.I. Recent advances on amphiphilic polymer-based fluorescence spectroscopic techniques for sensing and imaging. Appl. Spectrosc. Rev. 2019, 54, 204–236. [Google Scholar] [CrossRef]
- Zou, Y.; Zhou, X.; Ma, J.; Yang, X.; Deng, Y. Recent advances in amphiphilic block copolymer templated mesoporous metal-based materials: Assembly engineering and applications. Chem. Soc. Rev. 2020, 49, 1173–1208. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Patil, R.; Dubey, S.; Bahadur, P. Derivatization approaches and applications of pullulan. Adv. Colloid Interface Sci. 2019, 269, 296–308. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Bahadur, P. Modified hyaluronic acid-based materials for biomedical applications. Int. J. Biol. Macromol. 2019, 121, 556–571. [Google Scholar] [CrossRef]
- Agrahari, A.; Agrahari, V. Advances and applications of block copolymer based nanoformulations. Drug Discov. Today 2018, 5, 1139–1151. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuperkar, K.; Patel, D.; Atanase, L.I.; Bahadur, P. Amphiphilic Block Copolymers: Their Structures, and Self-Assembly to Polymeric Micelles and Polymersomes as Drug Delivery Vehicles. Polymers 2022, 14, 4702. https://doi.org/10.3390/polym14214702
Kuperkar K, Patel D, Atanase LI, Bahadur P. Amphiphilic Block Copolymers: Their Structures, and Self-Assembly to Polymeric Micelles and Polymersomes as Drug Delivery Vehicles. Polymers. 2022; 14(21):4702. https://doi.org/10.3390/polym14214702
Chicago/Turabian StyleKuperkar, Ketan, Dhruvi Patel, Leonard Ionut Atanase, and Pratap Bahadur. 2022. "Amphiphilic Block Copolymers: Their Structures, and Self-Assembly to Polymeric Micelles and Polymersomes as Drug Delivery Vehicles" Polymers 14, no. 21: 4702. https://doi.org/10.3390/polym14214702
APA StyleKuperkar, K., Patel, D., Atanase, L. I., & Bahadur, P. (2022). Amphiphilic Block Copolymers: Their Structures, and Self-Assembly to Polymeric Micelles and Polymersomes as Drug Delivery Vehicles. Polymers, 14(21), 4702. https://doi.org/10.3390/polym14214702









