Development of an Atmospheric Pressure Plasma Jet Device Using Four-Bore Tubing and Its Applications of In-Liquid Material Decomposition and Solution Plasma Polymerization
Abstract
1. Introduction
2. Materials and Methods
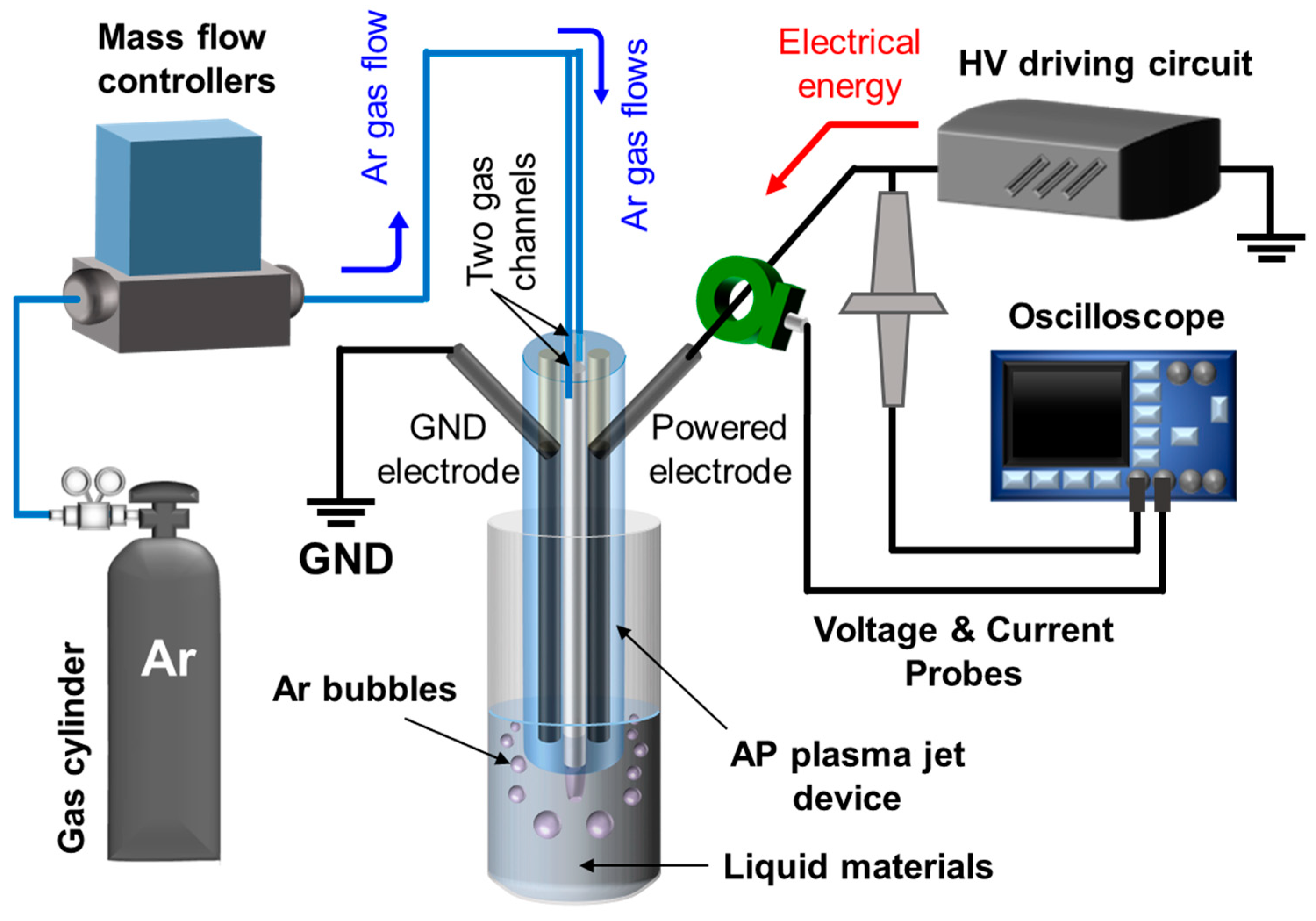
2.1. Atmospheric Pressure Plasma Jet Device and Plasma Operation System
2.2. Preparing a Phosphorus Compound Solution for Assessing Plasma Decomposition
2.3. Ascorbic Acid Reduction Method Using Phosphate Standard Solutions
2.4. Solution Plasma Polymerization for Examination of Plasma Synthesis
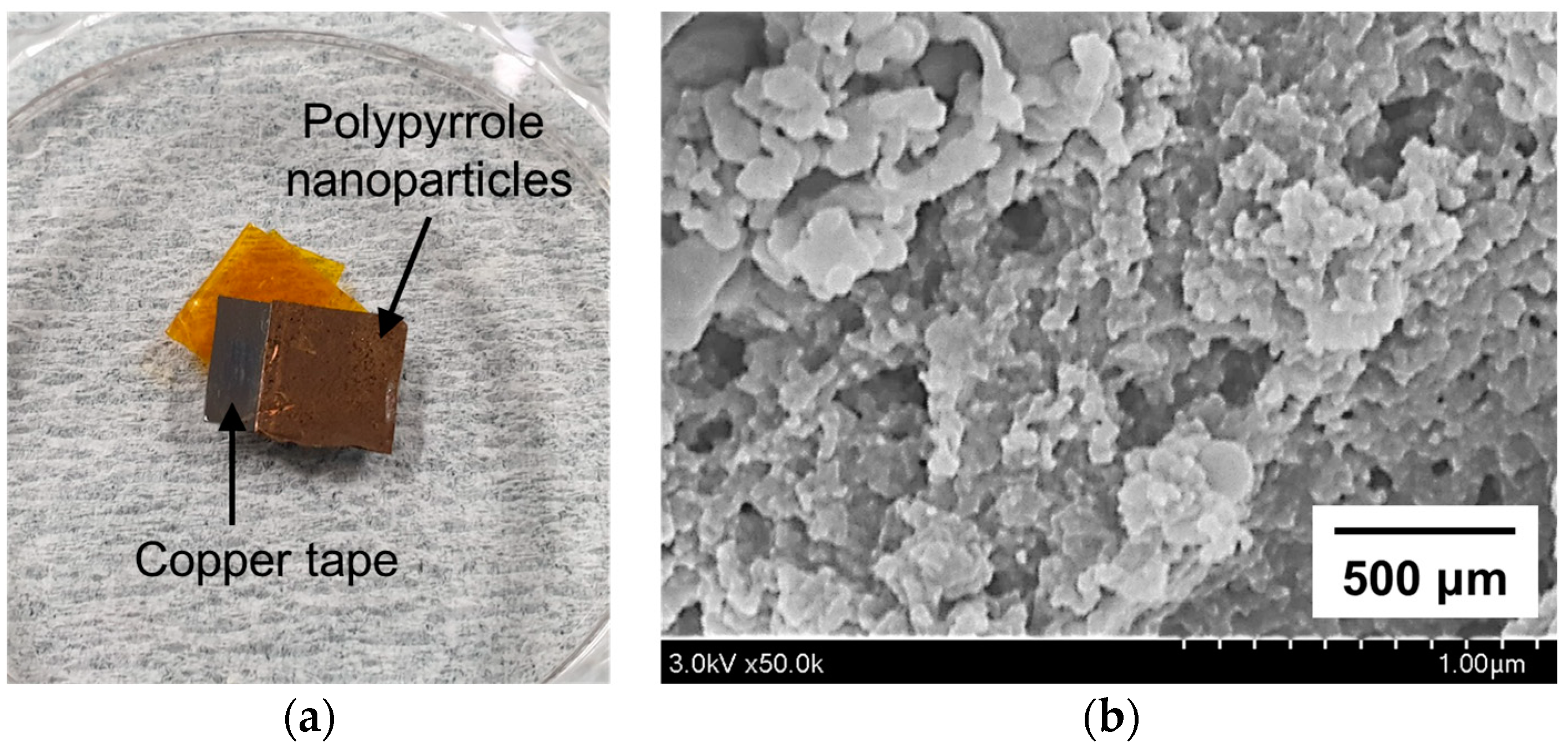
2.5. Preparation of Polypyrrole Nanoparticles
2.6. Analysis and Characterization of Polypyrrole Nanoparticles
2.7. Statistical Analysis
3. Results and Discussion
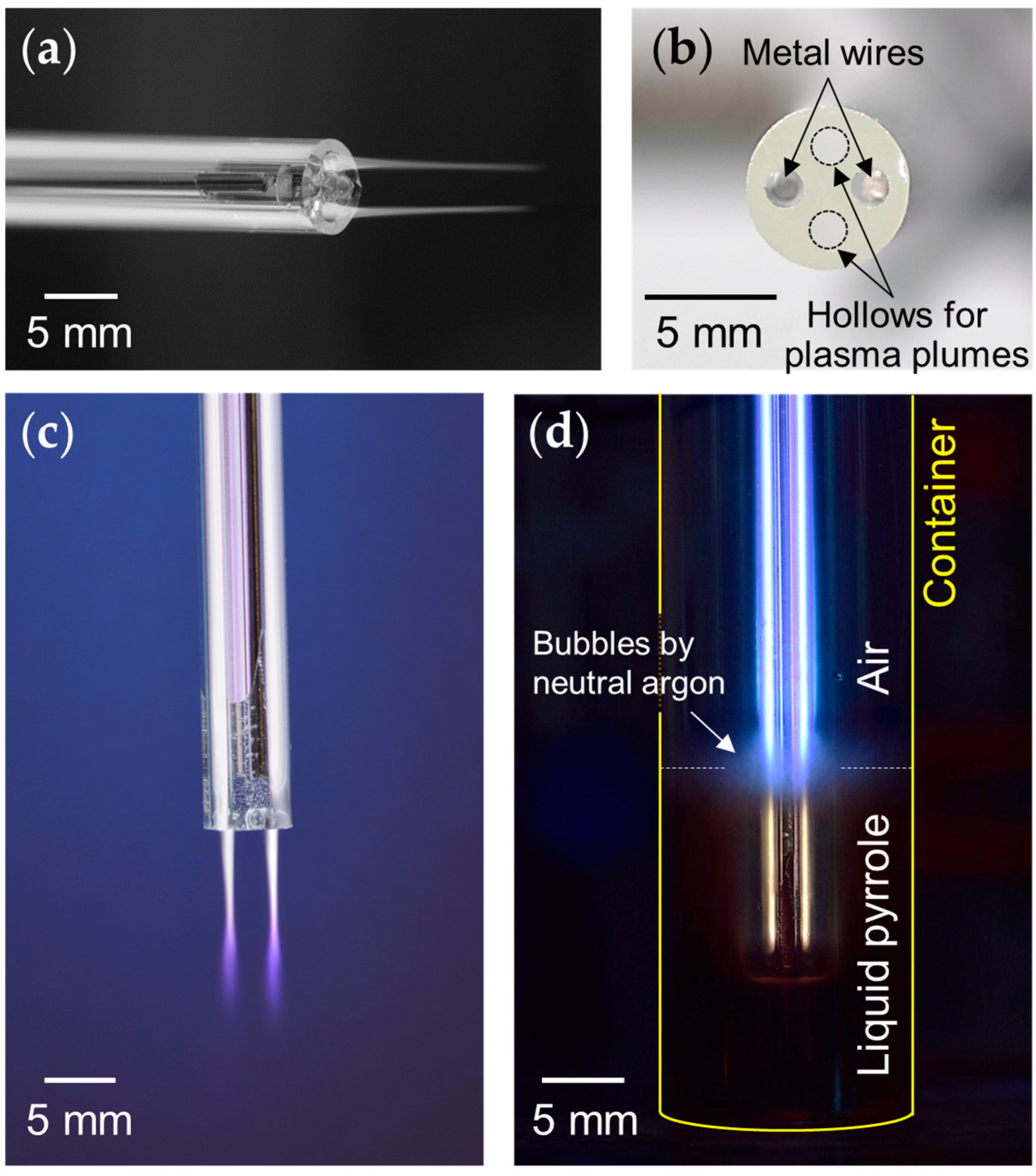
3.1. Electrode-Embedded Atmospheric Pressure Plasma Jet Device
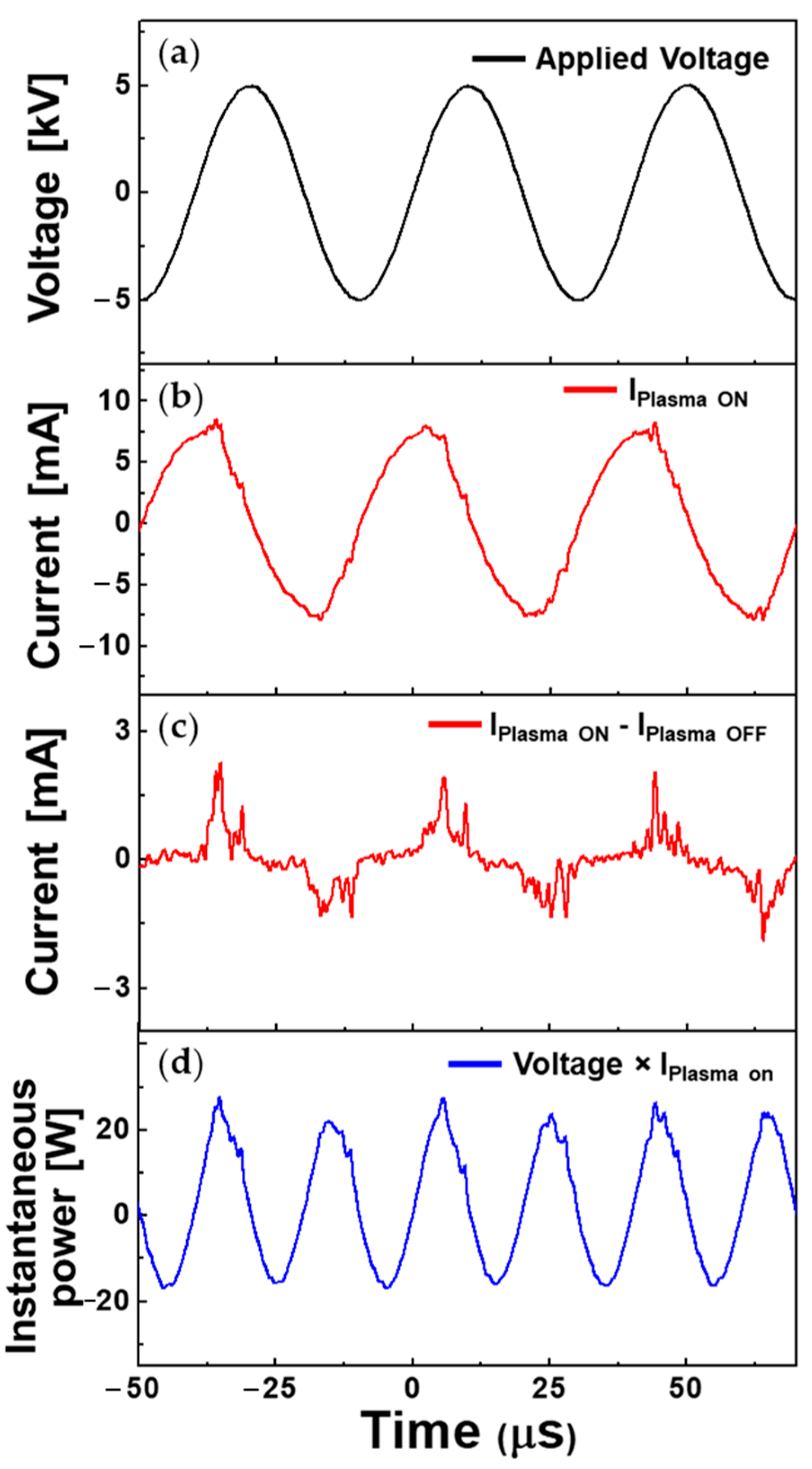
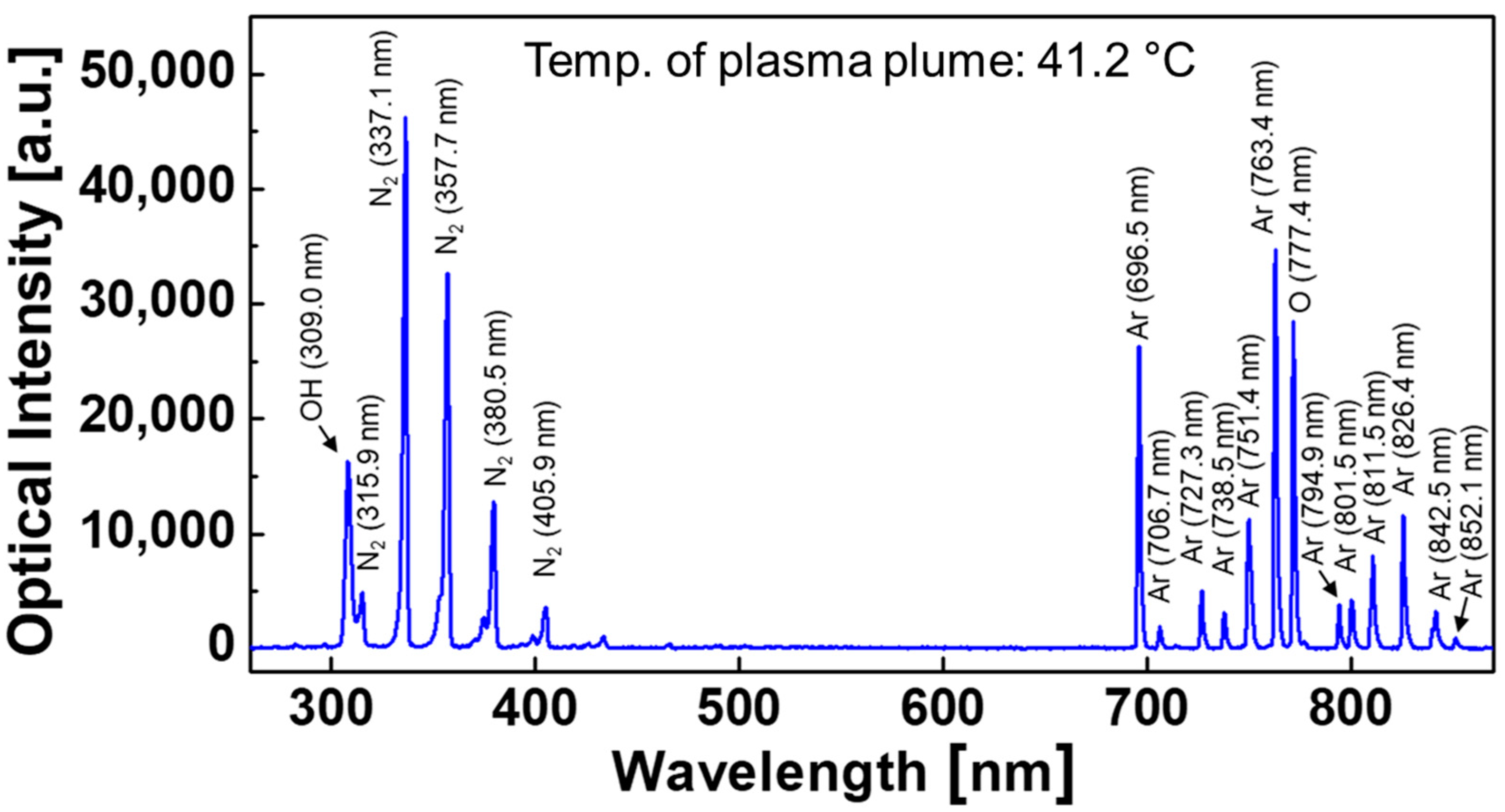
3.2. Optical and Electrical Characteristics of Atmospheric Pressure Plasma Jet
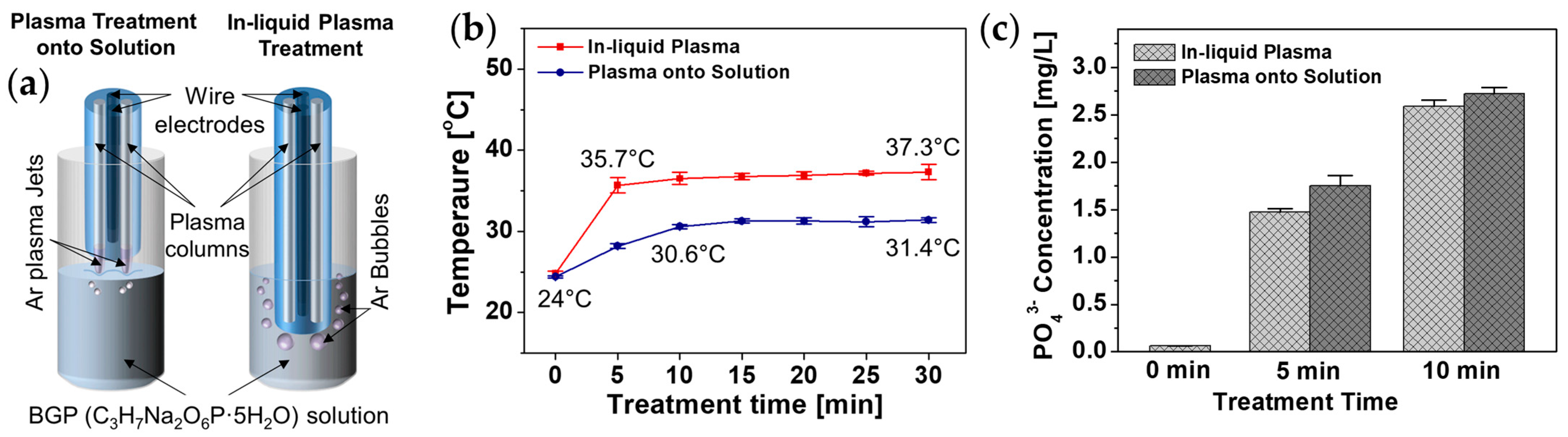
3.3. Decomposition of Aquaeous Phosphorus Compounds by Atmospheric Pressure Plasma Jet
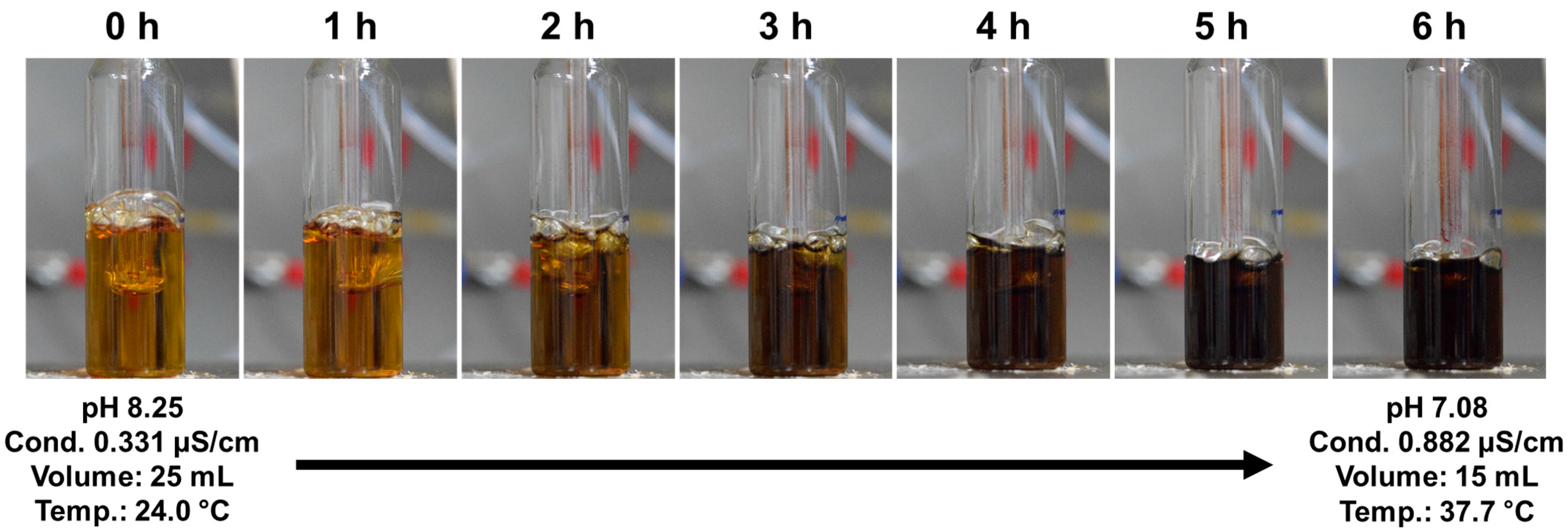
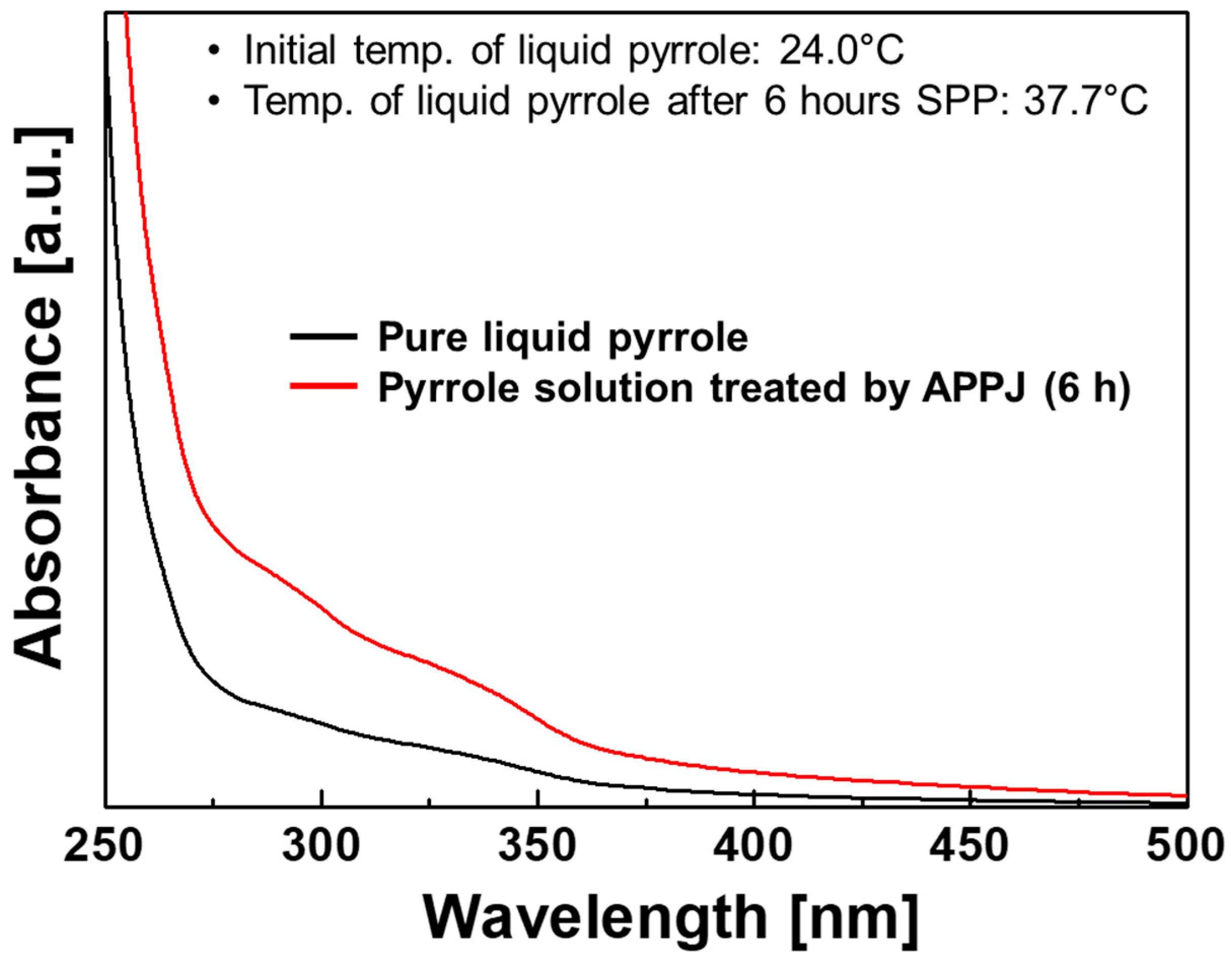
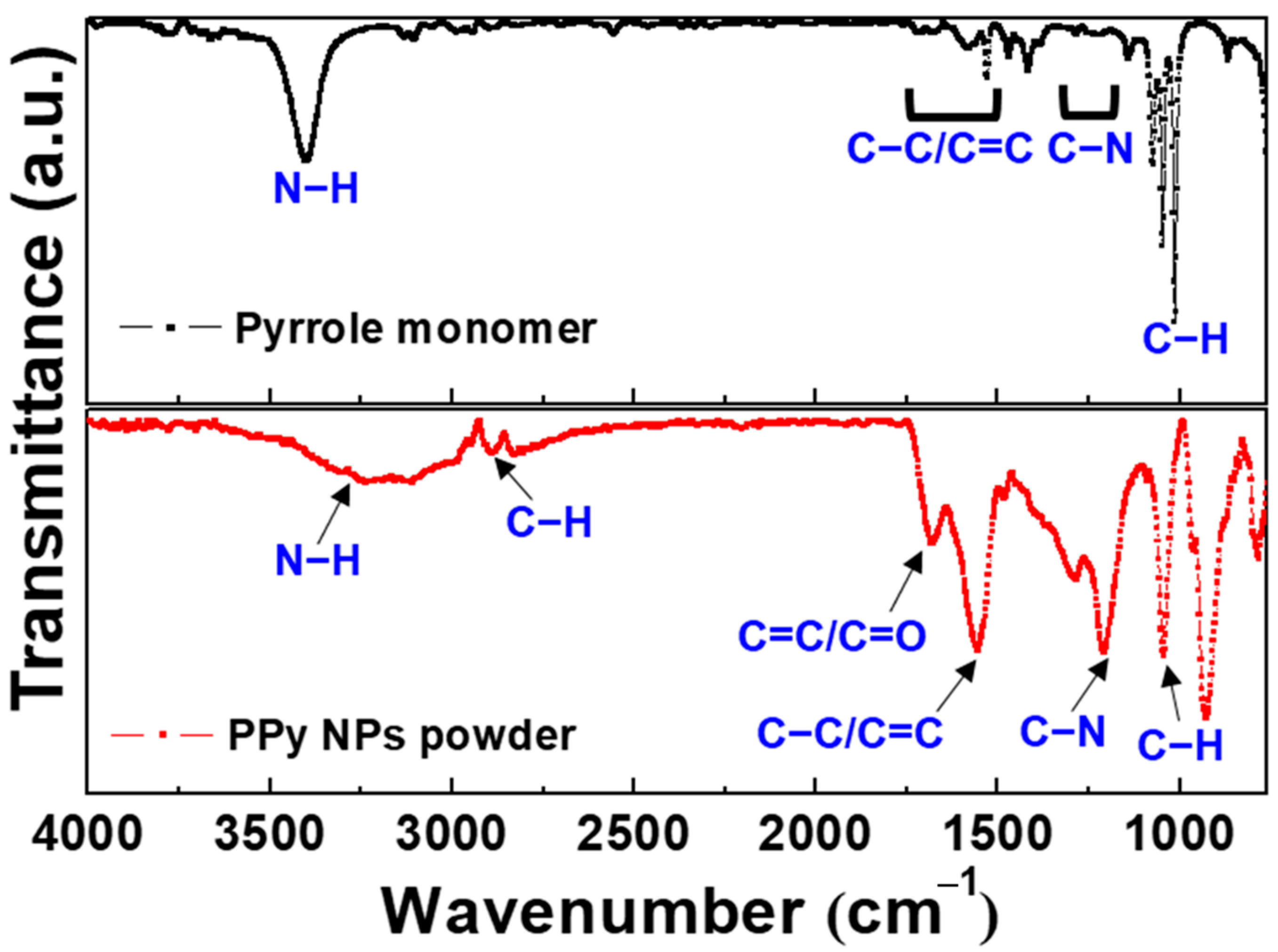
3.4. Plasma Polymerization in Liquid Monomer Using Electrode-Embedded Atmospheric Pressure Plasma Device
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Becker, K.H.; Schoenbach, K.H.; Eden, J.G. Microplasmas and applications. J. Phys. D Appl. Phys. 2006, 39, R55–R70. [Google Scholar] [CrossRef]
- Fridman, A.; Chirokov, A.; Gutsol, A. Non-thermal atmospheric pressure discharges. J. Phys. D Appl. Phys. 2005, 38, R1–R24. [Google Scholar] [CrossRef]
- Schutze, A.; Jeong, J.Y.; Babayan, S.E.; Park, J.; Selwyn, G.S.; Hicks, R.F. The atmospheric-pressure plasma jet: A review and comparison to other plasma sources. IEEE Trans. Plasma Sci. 1998, 26, 1685–1694. [Google Scholar] [CrossRef]
- Laroussi, M.; Akan, T. Arc-free atmospheric pressure cold plasma jets: A review. Plasma Process. Polym. 2007, 4, 777–788. [Google Scholar] [CrossRef]
- Tendero, C.; Tixier, C.; Tristant, P.; Desmaison, J.; Leprince, P. Atmospheric pressure plasmas: A review. Spectrochim. Acta B 2006, 61, 2–30. [Google Scholar] [CrossRef]
- Bhatt, S.; Pulpytel, J.; Aref-Khonsari, F. Low and atmospheric plasma polymerisation of nanocoatings for bio-applications. Surf. Innov. 2015, 3, 63–83. [Google Scholar] [CrossRef]
- Penkov, O.V.; Khadem, M.; Lim, W.-S.; Kim, D.-E. A review of recent applications of atmospheric pressure plasma jets for materials processing. J. Coat. Technol. Res. 2015, 12, 225–235. [Google Scholar] [CrossRef]
- Jang, H.J.; Jung, E.Y.; Parsons, T.; Tae, H.-S.; Park, C.-S. A review of plasma synthesis methods for polymer films and nanoparticles under atmospheric pressure conditions. Polymers 2021, 13, 2267. [Google Scholar] [CrossRef]
- Graves, D.B. The emerging role of reactive oxygen and nitrogen species in redox biology and some implications for plasma applications to medicine and biology. J. Phys. D Appl. Phys. 2012, 45, 263001. [Google Scholar] [CrossRef]
- Jalaber, V.; Del Frari, D.; De Winter, J.; Mehennaoui, K.; Planchon, S.; Choquet, P.; Detrembleur, C.; Moreno-Couranjou, M. Atmospheric aerosol assisted pulsed plasma polymerization: An environmentally friendly technique for tunable catechol-bearing thin films. Front. Chem. 2019, 7, 183. [Google Scholar] [CrossRef] [PubMed]
- Lisco, F.; Shaw, A.; Wright, A.; Walls, J.M.; Iza, F. Atmospheric-pressure plasma surface activation for solution processed photovoltaic devices. Sol. Energy 2017, 146, 287–297. [Google Scholar] [CrossRef]
- Galmiz, O.; Tucekova, Z.K.; Kelar, J.; Zemanek, M.; Stupavska, M.; Kovacik, D.; Cernak, M. Effect of atmospheric pressure plasma on surface modification of paper. AIP Adv. 2019, 9, 105013. [Google Scholar] [CrossRef]
- Jiang, C.; Mohamed, A.-A.H.; Stark, R.H.; Yuan, J.H.; Schoenbach, K.H. Removal of volatile organic compounds in atmospheric pressure air by means of direct current glow discharges. IEEE Trans. Plasma Sci. 2005, 33, 1416–1425. [Google Scholar] [CrossRef]
- Walsh, J.L.; Kong, M.G. Room-temperature atmospheric argon plasma jet sustained with submicrosecond high-voltage pulses. Appl. Phys. Lett. 2007, 91, 221502. [Google Scholar] [CrossRef]
- Xiong, Q.; Lu, X.P.; Jiang, Z.H.; Tang, Z.Y.; Hu, J.; Xiong, Z.L.; Pan, Y. An atmospheric pressure nonequilibrium plasma jet device. IEEE Trans. Plasma Sci. 2008, 36, 986–987. [Google Scholar] [CrossRef]
- Park, H.S.; Kim, S.J.; Joh, H.M.; Chung, T.H.; Bae, S.H.; Leem, S.H. Optical and electrical characterization of an atmospheric pressure microplasma jet with a capillary electrode. Phys. Plasmas 2010, 17, 033502. [Google Scholar] [CrossRef]
- Barman, K.; Mudgal, M.; Rane, R.; Bhattacharjee, S. Effect of magnetic field on optical emission from cold atmospheric pressure micro-plasma jet. Phys. Plasmas 2021, 28, 123503. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, S.-O.; Wei, Y.; Li, J. A flexible cold microplasma jet using biocompatible dielectric tubes for cancer therapy. Appl. Phys. Lett. 2010, 96, 203701. [Google Scholar] [CrossRef]
- Sato, T.; Furuya, O.; Ikeda, K.; Nakatani, T. Generation and transportation mechanisms of chemically active species by dielectric barrier discharge in a tube for catheter sterilization. Plasma Process. Polym. 2008, 5, 606–614. [Google Scholar] [CrossRef]
- Schweigert, I.; Zakrevsky, D.; Gugin, P.; Yelak, E.; Golubitskaya, E.; Troitskaya, O.; Koval, O. Interaction of cold atmospheric argon and helium plasma jets with bio-target with grounded substrate beneath. Appl. Sci. 2019, 9, 4528. [Google Scholar] [CrossRef]
- Kostov, K.G.; Nishime, T.M.C.; Machida, M.; Borges, A.C.; Prysiazhnyi, V.; Koga-Ito, C.Y. Study of cold atmospheric plasma jet at the end of flexible plastic tube for microbial decontamination. Plasma Process. Polym. 2015, 12, 1383–1391. [Google Scholar] [CrossRef]
- Kim, J.Y.; Ballato, J.; Foy, P.; Hawkins, T.; Wei, Y.; Li, J.; Kim, S.-O. Apoptosis of cultured tumor cells treated with 200 µm-sized flexible microplasma jet. IEEE Trans. Plasma Sci. 2011, 39, 2974–2975. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, J.H.; Tae, H.-S.; Moon, D.W. Electrode-embedded atmospheric pressure plasma jet device for humid environment. IEEE Trans. Plasma Sci. 2014, 42, 2476–2477. [Google Scholar] [CrossRef]
- Bae, G.T.; Kim, J.Y.; Kim, D.Y.; Jung, E.Y.; Jang, H.J.; Park, C.-S.; Jang, H.; Lee, D.H.; Lee, H.-K.; Tae, H.-S. Potential application of pin-to-liquid dielectric barrier discharge structure in decomposing aqueous phosphorus compounds for monitoring water quality. Materials 2021, 14, 7559. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-Z.; Fischer, C.J.; Ortner, P.B. Continuous flow analysis of phosphate in natural waters using hydrazine as a reductant. Intern. J. Environ. Chem. 2001, 80, 61–73. [Google Scholar] [CrossRef]
- Takeuchi, N.; Yasuoka, K. Review of plasma-based water treatment technologies for the decomposition of persistent organic compounds. Jpn. J. Appl. Phys. 2021, 60, SA0801. [Google Scholar] [CrossRef]
- Sano, N.; Kawashima, T.; Fujikawa, J.; Fujimoto, T.; Kitai, T.; Kanki, T.; Toyoda, A. Decomposition of organic compounds in water by direct contact of gas corona discharge: influence of discharge conditions. Ind. Eng. Chem. Res. 2002, 41, 5906–5911. [Google Scholar] [CrossRef]
- Barjasteh, A.; Dehghani, Z.; Lamichhane, P.; Kaushik, N.; Choi, E.H.; Kaushik, N.K. Recent progress in applications of non-thermal plasma for water purification, bio-sterilization, and decontamination. Appl. Sci. 2021, 11, 3372. [Google Scholar] [CrossRef]
- Zhou, M.; Struve, D.M. The effects of post-persulfate-digestion procedures on total phosphorus analysis in water. Water Res. 2004, 38, 3893–3898. [Google Scholar] [CrossRef] [PubMed]
- Gross, A.; Boyd, C.E. A digestion procedure for the simultaneous determination of total nitrogen and total phosphorus in pond water. J. World Aquac. Soc. 1998, 29, 300–303. [Google Scholar] [CrossRef]
- To, Y.S.; Randall, C.W. Evaluation of ascorbic acid method for determination of orthophosphates. J. Water Pollut. Control Fed. 1977, 49, 689–692. [Google Scholar]
- Ma, J.; Yuan, Y.; Zhou, T.; Yuan, D. Determination of total phosphorus in natural waters with a simple neutral digestion method using sodium persulfate. Limnol. Oceanogr. Methods 2017, 15, 372–380. [Google Scholar] [CrossRef]
- Morishita, T.; Ueno, T.; Panomsuwan, G.; Hieda, J.; Yoshida, A.; Bratescu, M.A.; Saito, N. Fast formation routes of nanocarbons in solution plasma processes. Sci. Rep. 2016, 6, 36880. [Google Scholar] [CrossRef] [PubMed]
- Saito, N.; Bratescu, M.A.; Hashimi, K. Solution plasma: A new reaction field for nanomaterials synthesis. Jpn. J. Appl. Phys. 2018, 57, 0102A4. [Google Scholar] [CrossRef]
- Kim, H.-J.; Shin, J.-G.; Park, C.-S.; Kum, D.S.; Shin, B.J.; Kim, J.Y.; Park, H.-D.; Choi, M.; Tae, H.-S. In-liquid plasma process for size- and shape-controlled synthesis of silver nanoparticles by controlling gas bubbles in water. Materials 2018, 11, 891. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.-G.; Shin, B.J.; Jung, E.Y.; Park, C.-S.; Kim, J.Y.; Tae, H.-S. Effects of a dielectric barrier discharge (DBD) on characteristics of polyaniline nanoparticles synthesized by a solution plasma process with an Ar gas bubble channel. Polymers 2020, 12, 1939. [Google Scholar] [CrossRef] [PubMed]
- Qian, R.; Pei, Q.; Huang, Z. The role of H+ ions in the electrochemical polymerization of pyrrole. Makromol. Chem. 1991, 192, 1263–1273. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Pozharskii, A.F. Reactivity of heterocycles. In Handbook of Heterocyclic Chemistry, 2nd ed.; Academic Press: Cambridge, MA, USA, 2000; pp. 377–379. [Google Scholar]
- Zotti, G.; Martina, S.; Wegner, G.; Schlüter, A.-D. Well-Defined Pyrrole Oligomers: Electrochemical and UV/vis Studies. Adv. Mater. 1992, 4, 798–801. [Google Scholar] [CrossRef]
- Vernitskaya, T.V.; Efimov, O.N. Polypyrrole: A conducting polymer; its synthesis, properties and applications. Russ. Chem. Rev. 1997, 66, 443–457. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of infrared spectra, a practical approach. Encycl. Anal. Chem. 2006, 20, 10815–10837. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bae, G.T.; Jang, H.J.; Jung, E.Y.; Lee, Y.R.; Park, C.-S.; Kim, J.Y.; Tae, H.-S. Development of an Atmospheric Pressure Plasma Jet Device Using Four-Bore Tubing and Its Applications of In-Liquid Material Decomposition and Solution Plasma Polymerization. Polymers 2022, 14, 4917. https://doi.org/10.3390/polym14224917
Bae GT, Jang HJ, Jung EY, Lee YR, Park C-S, Kim JY, Tae H-S. Development of an Atmospheric Pressure Plasma Jet Device Using Four-Bore Tubing and Its Applications of In-Liquid Material Decomposition and Solution Plasma Polymerization. Polymers. 2022; 14(22):4917. https://doi.org/10.3390/polym14224917
Chicago/Turabian StyleBae, Gyu Tae, Hyo Jun Jang, Eun Young Jung, Ye Rin Lee, Choon-Sang Park, Jae Young Kim, and Heung-Sik Tae. 2022. "Development of an Atmospheric Pressure Plasma Jet Device Using Four-Bore Tubing and Its Applications of In-Liquid Material Decomposition and Solution Plasma Polymerization" Polymers 14, no. 22: 4917. https://doi.org/10.3390/polym14224917
APA StyleBae, G. T., Jang, H. J., Jung, E. Y., Lee, Y. R., Park, C.-S., Kim, J. Y., & Tae, H.-S. (2022). Development of an Atmospheric Pressure Plasma Jet Device Using Four-Bore Tubing and Its Applications of In-Liquid Material Decomposition and Solution Plasma Polymerization. Polymers, 14(22), 4917. https://doi.org/10.3390/polym14224917







