One-Part Alkali-Activated Materials: State of the Art and Perspectives
Abstract
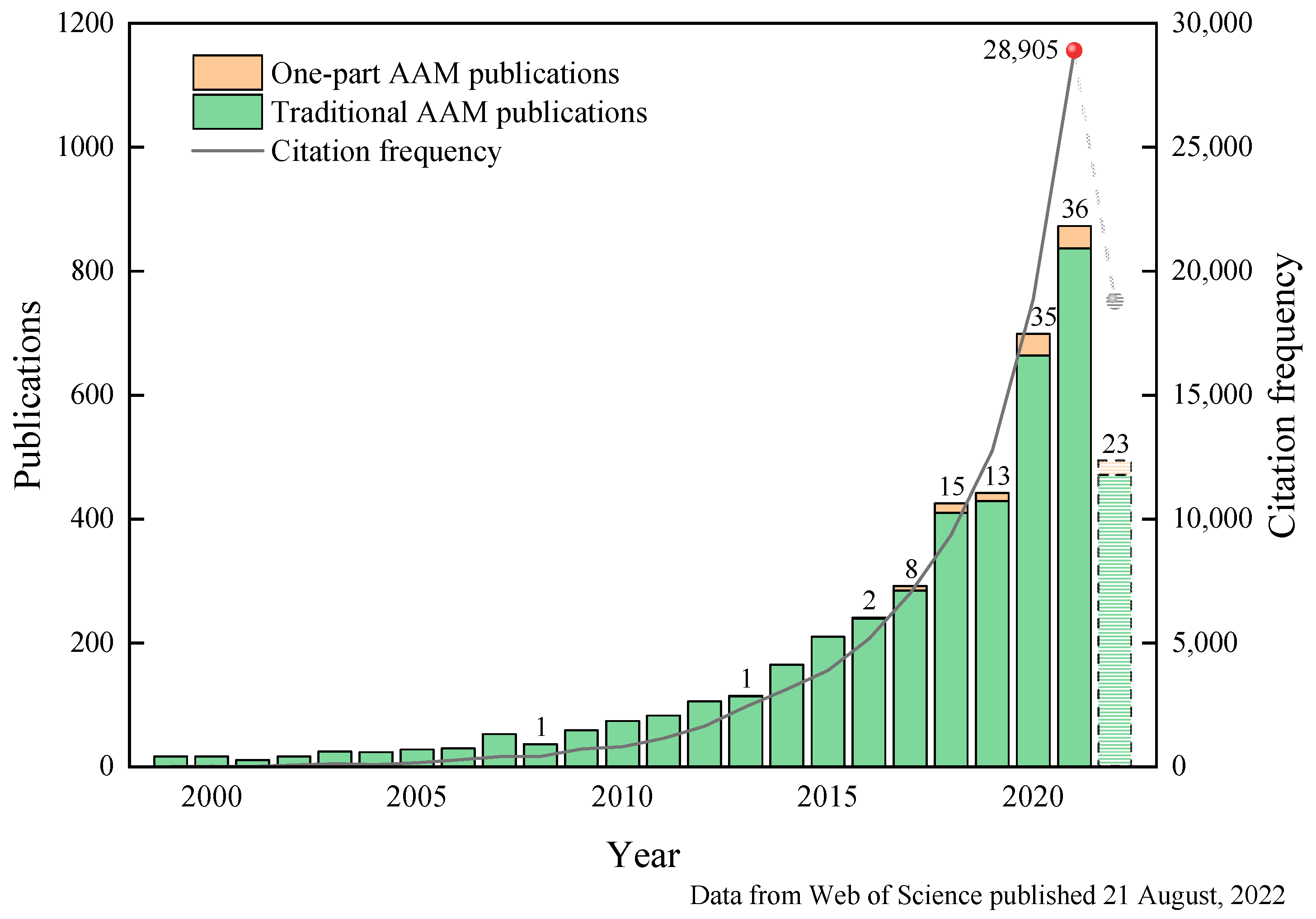
:1. Introduction
2. Raw Materials and Preparation
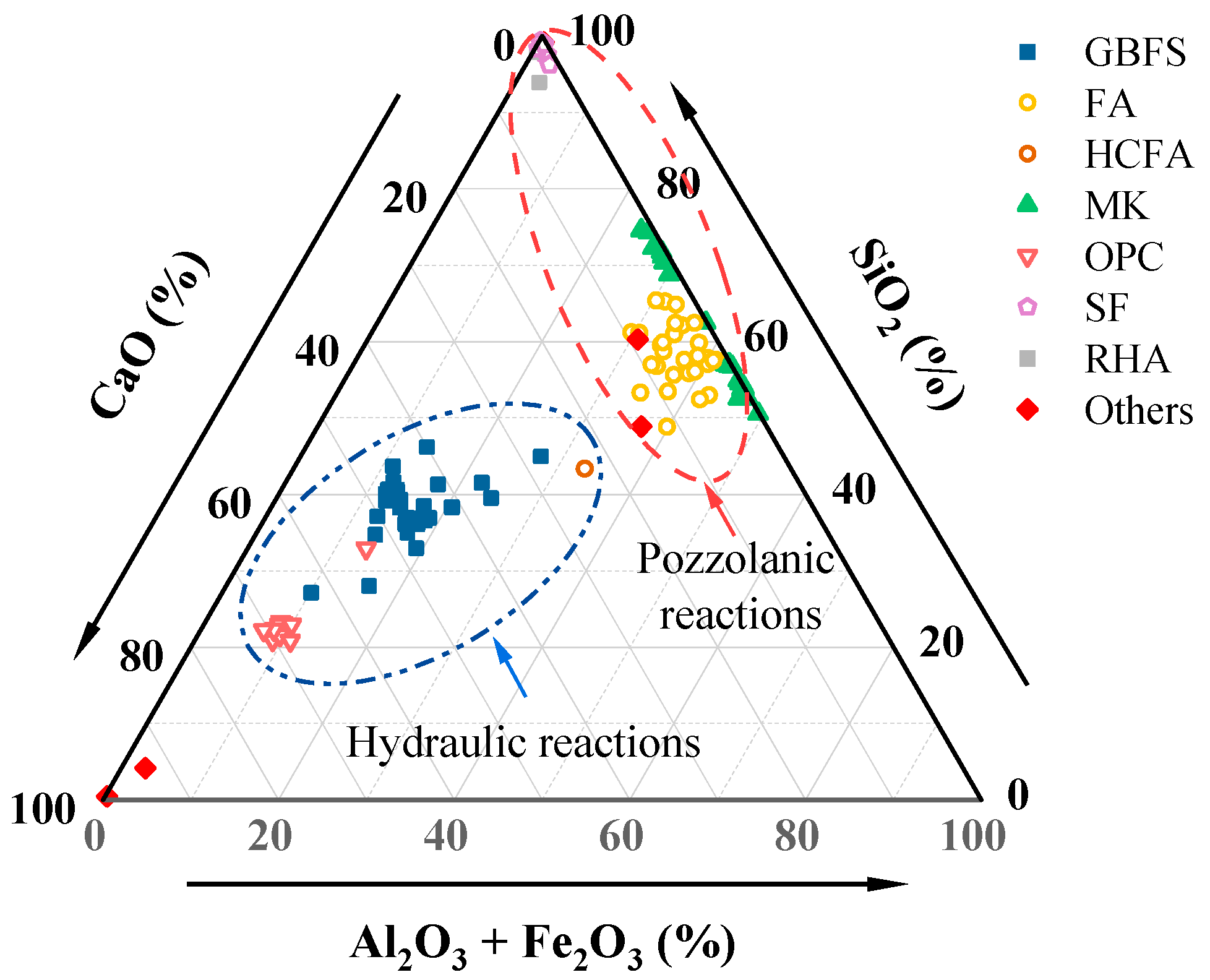
2.1. Aluminosilicate Precursors
2.2. Solid Alkali Sources
2.3. Admixtures
2.4. Preprocessing
2.5. Mix Proportion
2.6. Fiber Reinforcement
2.7. Curing Method
3. Properties of One-Part AAM
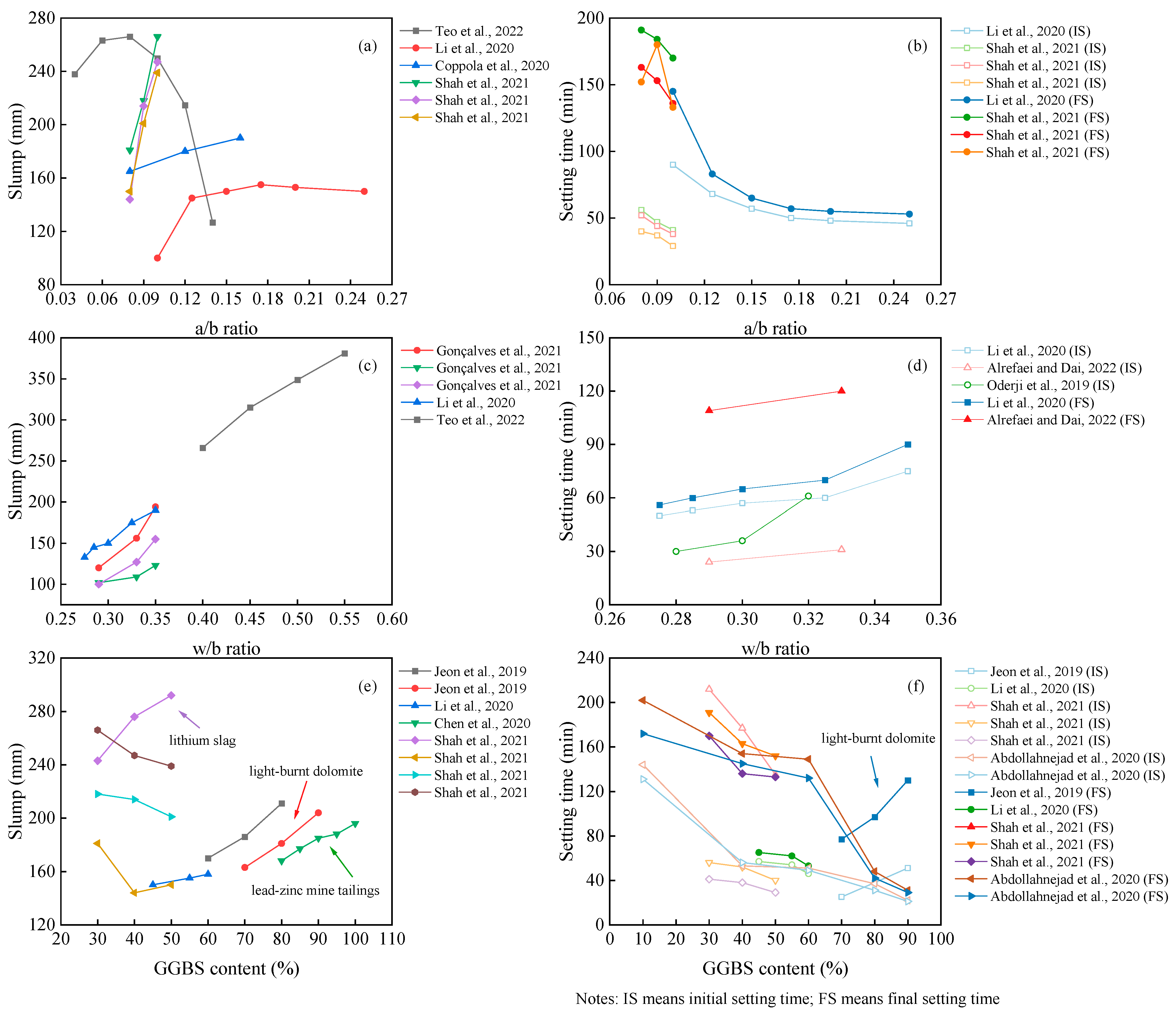
3.1. Workability
3.2. Rheology
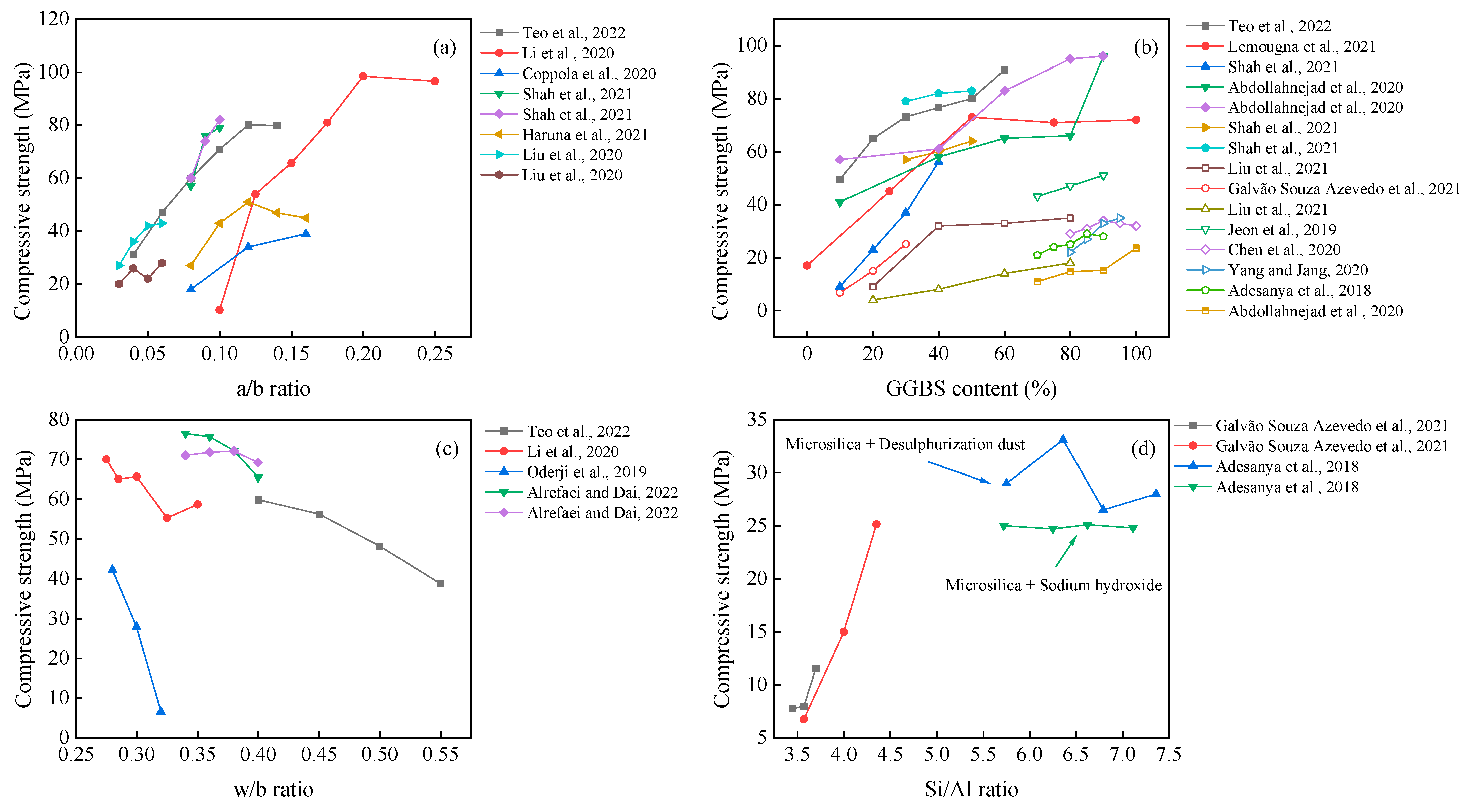
3.3. Compressive Strength
3.4. Durability
4. Conclusions and Future Research Need
- Ground granulated blast-furnace slag (GGBS) and fly ash (FA) are the two most popular raw materials in the precursors. GGBS obtains the highest mechanical strength attributed to its excellent reactivity. However, the high shrinkage and low flowability should be well considered. The excellent mechanical properties of either the GGBS or the FA are still the best choice at present.
- Sodium silicate (SS) and sodium hydroxide (SH) alone or in combination can form a good performance, but SH poses some safety risks. The alkali content of the mixture can be increased by other alkaline earth metal hydroxides (e.g., CaO, Ca(OH)2). Moreover, some treated solid wastes (e.g., paper sludge, oyster shell powder, biomass ash) can be used as the potential alkali source materials.
- Polycarboxylic is an efficient superplasticizer for the AAM compared with its counterparts. Other precursor components, such as lignosulphonate, naphthalene, and borax, can also be beneficial to certain materials.
- Different from the pretreatment of raw materials by calcination and ball milling to improve their reactivity, the effects of the chemical activation or changing water temperature are not always obvious. A new treatment method with the additional delayed components can artificially regulate the reaction process and minimize the competitive adsorption phenomenon.
- Considering the high alkali equivalent of the AAM, these mineral fibers (basalt fibers, glass fibers, etc.) are not highly recommended. However, the stability of the steel fibers in acid and alkaline conditions is relatively constant. Compared with a single fiber, the mixed fibers may overcome some defects of the AAM. Regardless of the fiber type, fiber reinforcing has a better contribution to the tensile and flexural properties of the AAM.
- Normal temperature sealing is an effective curing method for slag-based AAM. For the fly ash-based AAM, a high temperature is recommended if the high performance of the AAM is requested. The other effective method is to add different materials into the binary or multiple precursors. Note that the water bath curing may lead to a significant strength reduction attributed to the alkali leaching.
- For the workability and rheological behavior of the OP-AAM, the solid activator may produce a different behavior from the liquid activator, and the dissolution heat may accelerate the hydration reaction and increase the yield stress. However, it is also noted that the evaporation of water makes the hydration process inadequate.
- The AAM has a high performance in carbonation resistance and high-temperature resistance. For acid resistance or sulfate resistance, slag-based AAM is more affected by expansion products, such as gypsum, due to its higher calcium content, and proper blending of fly ash and metakaolin can improve sulfate erosion resistance. However, the freezing resistance of the AAM should be well investigated.
- The main features of the OP-AAM are its cost-effectiveness and safe working compared with the traditional AAM. It is thus feasible to cast the AAM on-site with the one-step preparation process. Nonetheless, the commercialization of this technology has not yet been fully developed. Hopefully, these alkali-activated or geopolymer materials may be more widely used in the construction industry in the future.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Andrew, R. Global CO2 Emissions from Cement Production, 1928–2018. Earth Syst. Sci. Data 2019, 11, 1675–1710. [Google Scholar] [CrossRef] [Green Version]
- de Brito, J.; Kurda, R. The Past and Future of Sustainable Concrete: A Critical Review and New Strategies on Cement-Based Materials. J. Clean. Prod. 2021, 281, 123558. [Google Scholar] [CrossRef]
- Liang, C.; Pan, B.; Ma, Z.; He, Z.; Duan, Z. Utilization of CO2 Curing to Enhance the Properties of Recycled Aggregate and Prepared Concrete: A Review. Cem. Concr. Compos. 2020, 105, 103446. [Google Scholar] [CrossRef]
- Purdon, A.O. The Action of Alkalis on Blast-Furnace Slag. J. Soc. Chem. Ind. 1940, 59, 191–202. [Google Scholar]
- Glukhovsky, V.D. Soil Silicates; Gosstroyizdat Publishing: Kiev, Ukraine, 1959. [Google Scholar]
- Krivenko, P.V. Alkaline Cements. In Proceedings of the 1st International Conference on Alkaline Cements and Concretes, Kiev, Ukraine, 11–14 October 1994; Volume 1, pp. 11–129. [Google Scholar]
- Davidovits, J. Geopolymers. J. Therm. Anal. 1991, 37, 1633–1656. [Google Scholar] [CrossRef]
- Douglas, E.; Bilodeau, A.; Brandstetr, J.; Malhotra, V.M. Alkali Activated Ground Granulated Blast-Furnace Slag Concrete: Preliminary Investigation. Cem. Concr. Res. 1991, 21, 101–108. [Google Scholar] [CrossRef]
- Shi, C. Strength, Pore Structure and Permeability of Alkali-Activated Slag Mortars. Cem. Concr. Res. 1996, 26, 1789–1799. [Google Scholar] [CrossRef]
- Richardson, I.G. Tobermorite/Jennite- and Tobermorite/Calcium Hydroxide-Based Models for the Structure of C-S-H: Applicability to Hardened Pastes of Tricalcium Silicate, β-Dicalcium Silicate, Portland Cement, and Blends of Portland Cement with Blast-Furnace Slag, Metakaolin, or Silica Fume. Cem. Concr. Res. 2004, 34, 1733–1777. [Google Scholar] [CrossRef]
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; van Deventer, J.S.J. Geopolymer Technology: The Current State of the Art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Davidovits, J. The Need to Create a New Technical Language for the Transfer of Basic Scientific Information. In Proceedings of the Transfer and Exploitation of Scientific and Technical Information, Luxembourg, 10–12 June 1981; pp. 316–320. [Google Scholar]
- Sun, B.; Sun, Y.; Ye, G.; De Schutter, G. A Mix Design Methodology of Slag and Fly Ash-Based Alkali-Activated Paste. Cem. Concr. Compos. 2022, 126, 104368. [Google Scholar] [CrossRef]
- Hany, E.; Fouad, N.; Abdel-Wahab, M.; Sadek, E. Compressive Strength of Mortars Incorporating Alkali-Activated Materials as Partial or Full Replacement of Cement. Constr. Build. Mater. 2020, 261, 120518. [Google Scholar] [CrossRef]
- Atiş, C.; Görür, E.; Karahan, O.; Bilim, C.; İlkentapar, S.; Luga, E. Very High Strength (120 MPa) Class F Fly Ash Geopolymer Mortar Activated at Different NaOH Amount, Heat Curing Temperature and Heat Curing Duration. Constr. Build. Mater. 2015, 96, 673–678. [Google Scholar] [CrossRef]
- Mohamed, O.A.; Al Khattab, R. Fresh Properties and Sulfuric Acid Resistance of Sustainable Mortar Using Alkali-Activated GGBS/Fly Ash Binder. Polymers 2022, 14, 591. [Google Scholar] [CrossRef] [PubMed]
- Aiken, T.A.; Kwasny, J.; Sha, W.; Tong, K.T. Mechanical and Durability Properties of Alkali-Activated Fly Ash Concrete with Increasing Slag Content. Constr. Build. Mater. 2021, 301, 124330. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, C.; Zhang, Z.; Hu, X. Reaction Mechanism of Sulfate Attack on Alkali-Activated Slag/Fly Ash Cements. Constr. Build. Mater. 2022, 318, 126052. [Google Scholar] [CrossRef]
- Ali, H.A.; Xuan, D.; Lu, J.-X.; Poon, C.S. Enhancing the Resistance to Microbial Induced Corrosion of Alkali-Activated Glass Powder/GGBS Mortars by Calcium Aluminate Cement. Constr. Build. Mater. 2022, 341, 127912. [Google Scholar] [CrossRef]
- Adesanya, E.; Ohenoja, K.; Di Maria, A.; Kinnunen, P.; Illikainen, M. Alternative Alkali-Activator from Steel-Making Waste for One-Part Alkali-Activated Slag. J. Clean. Prod. 2020, 274, 123020. [Google Scholar] [CrossRef]
- Ababneh, A.; Matalkah, F.; Aqel, R. Synthesis of Kaolin-Based Alkali-Activated Cement: Carbon Footprint, Cost and Energy Assessment. J. Mater. Res. Technol. 2020, 9, 8367–8378. [Google Scholar] [CrossRef]
- Liang, G.; Liu, T.; Li, H.; Dong, B.; Shi, T. A Novel Synthesis of Lightweight and High-Strength Green Geopolymer Foamed Material by Rice Husk Ash and Ground-Granulated Blast-Furnace Slag. Resour. Conserv. Recycl. 2022, 176, 105922. [Google Scholar] [CrossRef]
- Maleki, A.; Hajizadeh, Z.; Sharifi, V.; Emdadi, Z. A Green, Porous and Eco-Friendly Magnetic Geopolymer Adsorbent for Heavy Metals Removal from Aqueous Solutions. J. Clean. Prod. 2019, 215, 1233–1245. [Google Scholar] [CrossRef]
- Bruschi, G.J.; dos Santos, C.P.; Tonini de Araujo, M.; Ferrazzo, S.T.; Marques, S.F.V.; Consoli, N.C. Green Stabilization of Bauxite Tailings: Mechanical Study on Alkali-Activated Materials. J. Mater. Civ. Eng. 2021, 33, 06021007. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, C.; Zhang, Z.; Li, N.; Shi, D. Mechanical and Fracture Properties of Ultra-High Performance Geopolymer Concrete: Effects of Steel Fiber and Silica Fume. Cem. Concr. Compos. 2020, 112, 103665. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Z.; Ghiassi, B.; Yin, S.; Ye, G. Fracture Properties and Microstructure Formation of Hardened Alkali-Activated Slag/Fly Ash Pastes. Cem. Concr. Res. 2021, 144, 106447. [Google Scholar] [CrossRef]
- Xue, L.; Zhang, Z.; Liu, H.; Jiang, Y.; Wang, H. Drying Shrinkage Behavior of Hybrid Alkali Activated Cement (HAAC) Mortars. Constr. Build. Mater. 2022, 316, 126068. [Google Scholar] [CrossRef]
- Liang, G.; Liu, T.; Li, H.; Wu, K. Shrinkage Mitigation, Strength Enhancement and Microstructure Improvement of Alkali-Activated Slag/Fly Ash Binders by Ultrafine Waste Concrete Powder. Compos. Part B Eng. 2022, 231, 109570. [Google Scholar] [CrossRef]
- Dai, X.; Aydin, S.; Yardimci, M.Y.; Lesage, K.; De Schutter, G. Rheology and Microstructure of Alkali-Activated Slag Cements Produced with Silica Fume Activator. Cem. Concr. Compos. 2022, 125, 104303. [Google Scholar] [CrossRef]
- Provis, J.L. Alkali-Activated Materials. Cem. Concr. Res. 2018, 114, 40–48. [Google Scholar] [CrossRef]
- Provis, J.L.; Palomo, A.; Shi, C. Advances in Understanding Alkali-Activated Materials. Cem. Concr. Res. 2015, 78, 110–125. [Google Scholar] [CrossRef]
- Habert, G.; d’Espinose de Lacaillerie, J.B.; Roussel, N. An Environmental Evaluation of Geopolymer Based Concrete Production: Reviewing Current Research Trends. J. Clean. Prod. 2011, 19, 1229–1238. [Google Scholar] [CrossRef]
- Ouellet-Plamondon, C.; Habert, G. Life Cycle Assessment (LCA) of Alkali-Activated Cements and Concretes. In Handbook of Alkali-Activated Cements, Mortars and Concretes; Elsevier: Amsterdam, The Netherlands, 2015; pp. 663–686. ISBN 978-1-78242-276-1. [Google Scholar]
- Yang, T.; Zhang, Z.; Zhang, F.; Gao, Y.; Wu, Q. Chloride and Heavy Metal Binding Capacities of Hydrotalcite-like Phases Formed in Greener One-Part Sodium Carbonate-Activated Slag Cements. J. Clean. Prod. 2020, 253, 120047. [Google Scholar] [CrossRef]
- Samarakoon, M.H.; Ranjith, P.G.; Duan, W.H.; De Silva, V.R.S. Properties of One-Part Fly Ash/Slag-Based Binders Activated by Thermally-Treated Waste Glass/NaOH Blends: A Comparative Study. Cem. Concr. Compos. 2020, 112, 103679. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Puertas, F. Effect of Activator Mix on the Hydration and Strength Behaviour of Alkali-Activated Slag Cements. Adv. Cem. Res. 2003, 15, 129–136. [Google Scholar] [CrossRef]
- Liew, Y.-M.; Heah, C.-Y.; Mohd Mustafa, A.B.; Kamarudin, H. Structure and Properties of Clay-Based Geopolymer Cements: A Review. Prog. Mater. Sci. 2016, 83, 595–629. [Google Scholar] [CrossRef]
- Srividya, T.; Rajkumar, P.R.K.; Sivasakthi, M.; Sujitha, A.; Jeyalakshmi, R. A State-of-the-Art on Development of Geopolymer Concrete and Its Field Applications. Case Stud. Constr. Mater. 2022, 16, e00812. [Google Scholar] [CrossRef]
- Myers, R.J.; Bernal, S.A.; Provis, J.L. A Thermodynamic Model for C-(N-)A-S-H Gel: CNASH_ss. Derivation and Validation. Cem. Concr. Res. 2014, 66, 27–47. [Google Scholar] [CrossRef]
- Garcia-Lodeiro, I.; Palomo, A.; Fernández-Jiménez, A.; Macphee, D.E. Compatibility Studies between N-A-S-H and C-A-S-H Gels. Study in the Ternary Diagram Na2O–CaO–Al2O3–SiO2–H2O. Cem. Concr. Res. 2011, 41, 923–931. [Google Scholar] [CrossRef]
- Ali Shah, S.F.; Chen, B.; Oderji, S.Y.; Haque, M.A.; Ahmad, M.R. Improvement of Early Strength of Fly Ash-Slag Based One-Part Alkali Activated Mortar. Constr. Build. Mater. 2020, 246, 118533. [Google Scholar] [CrossRef]
- Liu, Q.; Li, X.; Cui, M.; Wang, J.; Lyu, X. Preparation of Eco-Friendly One-Part Geopolymers from Gold Mine Tailings by Alkaline Hydrothermal Activation. J. Clean. Prod. 2021, 298, 126806. [Google Scholar] [CrossRef]
- Abdollahnejad, Z.; Mastali, M.; Falah, M.; Shaad, K.M.; Luukkonen, T.; Illikainen, M. Durability of the Reinforced One-Part Alkali-Activated Slag Mortars with Different Fibers. Waste Biomass Valor. 2021, 12, 487–501. [Google Scholar] [CrossRef] [Green Version]
- Teo, W.; Shirai, K.; Lim, J.H.; Jack, L.B.; Nikbakht, E. Experimental Investigation on Ambient-Cured One-Part Alkali-Activated Binders Using Combined High-Calcium Fly Ash (HCFA) and Ground Granulated Blast Furnace Slag (GGBS). Materials 2022, 15, 1612. [Google Scholar] [CrossRef]
- Abudawaba, F.; Gomaa, E.; Gheni, A.; ElGawady, M. Developing Mix Proportions for Class C Fly Ash-Based Alkali-Activated 3D-Printed Concrete Mixtures. Transp. Res. Rec. 2022, 2676, 197–212. [Google Scholar] [CrossRef]
- Jani, P.; Imqam, A. Class C Fly Ash-Based Alkali Activated Cement as a Potential Alternative Cement for CO2 Storage Applications. J. Pet. Sci. Eng. 2021, 201, 108408. [Google Scholar] [CrossRef]
- Zhou, S.; Tan, C.; Gao, Y.; Li, Y.; Guo, S. One-Part Alkali Activated Slag Using Ca(OH)2 and Na2CO3 Instead of NaOH as Activator: More Excellent Compressive Strength and Microstructure. Mater. Res. Express 2021, 8, 085501. [Google Scholar] [CrossRef]
- Perumal, P.; Sreenivasan, H.; Luukkonen, T.; Kantola, A.M.; Telkki, V.-V.; Kinnunen, P.; Illikainen, M. High Strength One-Part Alkali-Activated Slag Blends Designed by Particle Packing Optimization. Constr. Build. Mater. 2021, 299, 124004. [Google Scholar] [CrossRef]
- Nawaz, M.; Heitor, A.; Sivakumar, M. Geopolymers in Construction-Recent Developments. Constr. Build. Mater. 2020, 260, 120472. [Google Scholar] [CrossRef]
- Albidah, A.; Alqarni, A.S.; Abbas, H.; Almusallam, T.; Al-Salloum, Y. Behavior of Metakaolin-Based Geopolymer Concrete at Ambient and Elevated Temperatures. Constr. Build. Mater. 2022, 317, 125910. [Google Scholar] [CrossRef]
- Kuenzel, C.; Vandeperre, L.J.; Donatello, S.; Boccaccini, A.R.; Cheeseman, C. Ambient Temperature Drying Shrinkage and Cracking in Metakaolin-Based Geopolymers. J. Am. Ceram. Soc. 2012, 95, 3270–3277. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.-Y.; Liu, J.-C.; Wu, B. Mechanical Properties and Reaction Mechanism of One-Part Geopolymer Mortars. Constr. Build. Mater. 2021, 273, 121973. [Google Scholar] [CrossRef]
- Wang, X.; Wu, W.; Zhang, L.; Fu, L.; Li, X. Preparation of One-Part Alkali-Activated Nickel Slag Binder Using an Optimal Ball Milling Process. Constr. Build. Mater. 2022, 322, 125902. [Google Scholar] [CrossRef]
- Ali Shah, S.F.; Chen, B.; Ahmad, M.R.; Haque, M.A. Development of Cleaner One-Part Geopolymer from Lithium Slag. J. Clean. Prod. 2021, 291, 125241. [Google Scholar] [CrossRef]
- Alzaza, A.; Ohenoja, K.; Illikainen, M. Enhancing the Mechanical and Durability Properties of Subzero-Cured One-Part Alkali-Activated Blast Furnace Slag Mortar by Using Submicron Metallurgical Residue as an Additive. Cem. Concr. Compos. 2021, 122, 104128. [Google Scholar] [CrossRef]
- Luukkonen, T.; Abdollahnejad, Z.; Yliniemi, J.; Kinnunen, P.; Illikainen, M. Comparison of Alkali and Silica Sources in One-Part Alkali-Activated Blast Furnace Slag Mortar. J. Clean. Prod. 2018, 187, 171–179. [Google Scholar] [CrossRef]
- Li, Z.; Li, S. Carbonation Resistance of Fly Ash and Blast Furnace Slag Based Geopolymer Concrete. Constr. Build. Mater. 2018, 163, 668–680. [Google Scholar] [CrossRef]
- Xie, T.; Visintin, P.; Zhao, X.; Gravina, R. Mix Design and Mechanical Properties of Geopolymer and Alkali Activated Concrete: Review of the State-of-the-Art and the Development of a New Unified Approach. Constr. Build. Mater. 2020, 256, 119380. [Google Scholar] [CrossRef]
- Galvão Souza Azevedo, A.; Strecker, K. Kaolin, Fly-Ash and Ceramic Waste Based Alkali-Activated Materials Production by the “One-Part” Method. Constr. Build. Mater. 2021, 269, 121306. [Google Scholar] [CrossRef]
- Peng, M.X.; Wang, Z.H.; Xiao, Q.G.; Song, F.; Xie, W.; Yu, L.C.; Huang, H.W.; Yi, S.J. Effects of Alkali on One-Part Alkali-Activated Cement Synthesized by Calcining Bentonite with Dolomite and Na2CO3. Appl. Clay Sci. 2017, 139, 64–71. [Google Scholar] [CrossRef]
- Jeon, I.K.; Ryou, J.S.; Jakhrani, S.H.; Kim, H.G. Effects of Light-Burnt Dolomite Incorporation on the Setting, Strength, and Drying Shrinkage of One-Part Alkali-Activated Slag Cement. Materials 2019, 12, 2874. [Google Scholar] [CrossRef] [Green Version]
- Perumal, P.; Nguyen, H.; Carvelli, V.; Kinnunen, P.; Illikainen, M. High Strength Fiber Reinforced One-Part Alkali Activated Slag Composites from Industrial Side Streams. Constr. Build. Mater. 2022, 319, 126124. [Google Scholar] [CrossRef]
- Choo, H.; Lim, S.; Lee, W.; Lee, C. Compressive Strength of One-Part Alkali Activated Fly Ash Using Red Mud as Alkali Supplier. Constr. Build. Mater. 2016, 125, 21–28. [Google Scholar] [CrossRef]
- Li, L.; Lu, J.-X.; Zhang, B.; Poon, C.-S. Rheology Behavior of One-Part Alkali Activated Slag/Glass Powder (AASG) Pastes. Constr. Build. Mater. 2020, 258, 120381. [Google Scholar] [CrossRef]
- Abdollahnejad, Z.; Miraldo, S.; Pacheco-Torgal, F.; Aguiar, J.B. Cost-Efficient One-Part Alkali-Activated Mortars with Low Global Warming Potential for Floor Heating Systems Applications. Eur. J. Environ. Civ. Eng. 2017, 21, 412–429. [Google Scholar] [CrossRef]
- Provis, J.L. Introduction and Scope. In Alkali Activated Materials: State-of-the-Art Report, RILEM TC 224-AAM; Springer: Berlin/Heidelberg, Germany, 2014; pp. 1–9. [Google Scholar]
- Lemougna, P.N.; Dilissen, N.; Hernandez, G.M.; Kingne, F.; Gu, J.; Rahier, H. Effect of Sodium Disilicate and Metasilicate on the Microstructure and Mechanical Properties of One-Part Alkali-Activated Copper Slag/Ground Granulated Blast Furnace Slag. Materials 2021, 14, 5505. [Google Scholar] [CrossRef]
- Alrefaei, Y.; Dai, J.-G. Effects of Delayed Addition of Polycarboxylate Ether on One-Part Alkali-Activated Fly Ash/Slag Pastes: Adsorption, Reaction Kinetics, and Rheology. Constr. Build. Mater. 2022, 323, 126611. [Google Scholar] [CrossRef]
- Li, C.; Sun, H.; Li, L. A Review: The Comparison between Alkali-Activated Slag (Si+Ca) and Metakaolin (Si+Al) Cements. Cem. Concr. Res. 2010, 40, 1341–1349. [Google Scholar] [CrossRef]
- Abdel Gawwad, H.A.; Hekal, E.E.; El-Didamony, H.; Hashem, F.S.; Mohammed, A.H. A New Method to Create One-Part Non-Portland Cement Powder. J. Anal. Calorim. 2018, 134, 1447–1456. [Google Scholar] [CrossRef]
- Wang, Y.-S.; Alrefaei, Y.; Dai, J.-G. Roles of Hybrid Activators in Improving the Early-Age Properties of One-Part Geopolymer Pastes. Constr. Build. Mater. 2021, 306, 124880. [Google Scholar] [CrossRef]
- Zheng, H.; He, Y.; Zhu, Y.; Liu, L.; Cui, X. Novel Procedure of CO2 Capture of the CaO Sorbent Activator on the Reaction of One-Part Alkali-Activated Slag. RSC Adv. 2021, 11, 12476–12483. [Google Scholar] [CrossRef]
- Wei, T.; Zhao, H.; Ma, C. A Comparison of Water Curing and Standard Curing on One-Part Alkali-Activated Fly Ash Sinking Beads and Slag: Properties, Microstructure and Mechanisms. Constr. Build. Mater. 2021, 273, 121715. [Google Scholar] [CrossRef]
- Samarakoon, M.H.; Ranjith, P.G.; Hui Duan, W.; Haque, A.; Chen, B.K. Extensive Use of Waste Glass in One-Part Alkali-Activated Materials: Towards Sustainable Construction Practices. Waste Manag. 2021, 130, 1–11. [Google Scholar] [CrossRef]
- Ren, J.; Sun, H.; Li, Q.; Li, Z.; Ling, L.; Zhang, X.; Wang, Y.; Xing, F. Experimental Comparisons between One-Part and Normal (Two-Part) Alkali-Activated Slag Binders. Constr. Build. Mater. 2021, 309, 125177. [Google Scholar] [CrossRef]
- Refaat, M.; Mohsen, A.; Nasr, E.-S.A.R.; Kohail, M. Minimizing Energy Consumption to Produce Safe One-Part Alkali-Activated Materials. J. Clean. Prod. 2021, 323, 129137. [Google Scholar] [CrossRef]
- Liu, Q.; Sun, S.; Jia, Z.; Wang, J.; Lyu, X. Effect of CaO on Hydration Properties of One-Part Alkali-Activated Material Prepared from Tailings through Alkaline Hydrothermal Activation. Constr. Build. Mater. 2021, 308, 124931. [Google Scholar] [CrossRef]
- Kadhim, A.; Sadique, M.; Al-Mufti, R.; Hashim, K. Developing One-Part Alkali-Activated Metakaolin/Natural Pozzolan Binders Using Lime Waste. Adv. Cem. Res. 2021, 33, 342–356. [Google Scholar] [CrossRef]
- Haruna, S.; Mohammed, B.S.; Wahab, M.M.A.; Kankia, M.U.; Amran, M.; Gora, A.M. Long-Term Strength Development of Fly Ash-Based One-Part Alkali-Activated Binders. Materials 2021, 14, 4160. [Google Scholar] [CrossRef]
- Gonçalves, M.; Vilarinho, I.S.; Capela, M.; Caetano, A.; Novais, R.M.; Labrincha, J.A.; Seabra, M.P. Waste-Based One-Part Alkali Activated Materials. Materials 2021, 14, 2911. [Google Scholar] [CrossRef]
- Alzaza, A.; Ohenoja, K.; Illikainen, M. One-Part Alkali-Activated Blast Furnace Slag for Sustainable Construction at Subzero Temperatures. Constr. Build. Mater. 2021, 276, 122026. [Google Scholar] [CrossRef]
- Almakhadmeh, M.; Soliman, A.M. Effects of Mixing Water Temperatures on Properties of One-Part Alkali-Activated Slag Paste. Constr. Build. Mater. 2021, 266, 121030. [Google Scholar] [CrossRef]
- Yang, B.; Jang, J.G. Environmentally Benign Production of One-Part Alkali-Activated Slag with Calcined Oyster Shell as an Activator. Constr. Build. Mater. 2020, 257, 119552. [Google Scholar] [CrossRef]
- Mobili, A.; Tittarelli, F.; Rahier, H. One-Part Alkali-Activated Pastes and Mortars Prepared with Metakaolin and Biomass Ash. Appl. Sci. 2020, 10, 5610. [Google Scholar] [CrossRef]
- Lv, W.; Sun, Z.; Su, Z. Study of Seawater Mixed One-Part Alkali Activated GGBFS-Fly Ash. Cem. Concr. Compos. 2020, 106, 103484. [Google Scholar] [CrossRef]
- Luukkonen, T.; Yliniemi, J.; Kinnunen, P.; Illikainen, M. Sustainable Batching Water Options for One-Part Alkali-Activated Slag Mortar: Sea Water and Reverse Osmosis Reject Water. PLoS ONE 2020, 15, e0242462. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yao, X.; Zhang, W. Controlling the Setting Times of One-Part Alkali-Activated Slag by Using Honeycomb Ceramics as Carrier of Sodium Silicate Activator. Constr. Build. Mater. 2020, 235, 117091. [Google Scholar] [CrossRef]
- Kadhim, A.; Sadique, M.; Al-Mufti, R.; Hashim, K. Long-Term Performance of Novel High-Calcium One-Part Alkali-Activated Cement Developed from Thermally Activated Lime Kiln Dust. J. Build. Eng. 2020, 32, 101766. [Google Scholar] [CrossRef]
- Haruna, S.; Mohammed, B.S.; Wahab, M.M.A.; Liew, M.S. Effect of Paste Aggregate Ratio and Curing Methods on the Performance of One-Part Alkali-Activated Concrete. Constr. Build. Mater. 2020, 261, 120024. [Google Scholar] [CrossRef]
- Coppola, L.; Coffetti, D.; Crotti, E.; Gazzaniga, G.; Pastore, T. The Durability of One-Part Alkali-Activated Slag-Based Mortars in Different Environments. Sustainability 2020, 12, 3561. [Google Scholar] [CrossRef]
- Chen, W.; Peng, R.; Straub, C.; Yuan, B. Promoting the Performance of One-Part Alkali-Activated Slag Using Fine Lead-Zinc Mine Tailings. Constr. Build. Mater. 2020, 236, 117745. [Google Scholar] [CrossRef]
- Ahmad, M.R.; Chen, B.; Shah, S.F.A. Influence of Different Admixtures on the Mechanical and Durability Properties of One-Part Alkali-Activated Mortars. Constr. Build. Mater. 2020, 265, 120320. [Google Scholar] [CrossRef]
- Oderji, S.Y.; Chen, B.; Shakya, C.; Ahmad, M.R.; Shah, S.F.A. Influence of Superplasticizers and Retarders on the Workability and Strength of One-Part Alkali-Activated Fly Ash/Slag Binders Cured at Room Temperature. Constr. Build. Mater. 2019, 229, 116891. [Google Scholar] [CrossRef]
- Sturm, P.; Gluth, G.J.G.; Jäger, C.; Brouwers, H.J.H.; Kühne, H.-C. Sulfuric Acid Resistance of One-Part Alkali-Activated Mortars. Cem. Concr. Res. 2018, 109, 54–63. [Google Scholar] [CrossRef]
- Adesanya, E.; Ohenoja, K.; Luukkonen, T.; Kinnunen, P.; Illikainen, M. One-Part Geopolymer Cement from Slag and Pretreated Paper Sludge. J. Clean. Prod. 2018, 185, 168–175. [Google Scholar] [CrossRef]
- Almalkawi, A.T.; Hamadna, S.; Soroushian, P. One-Part Alkali Activated Cement Based Volcanic Pumice. Constr. Build. Mater. 2017, 152, 367–374. [Google Scholar] [CrossRef]
- Ye, N.; Chen, Y.; Yang, J.; Liang, S.; Hu, Y.; Xiao, B.; Huang, Q.; Shi, Y.; Hu, J.; Wu, X. Co-Disposal of MSWI Fly Ash and Bayer Red Mud Using an One-Part Geopolymeric System. J. Hazard. Mater. 2016, 318, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Alrefaei, Y.; Wang, Y.-S.; Dai, J.-G.; Xu, Q.-F. Effect of Superplasticizers on Properties of One-Part Ca(OH)2/Na2SO4 Activated Geopolymer Pastes. Constr. Build. Mater. 2020, 241, 117990. [Google Scholar] [CrossRef]
- Luukkonen, T.; Abdollahnejad, Z.; Yliniemi, J.; Kinnunen, P.; Illikainen, M. One-Part Alkali-Activated Materials: A Review. Cem. Concr. Res. 2018, 103, 21–34. [Google Scholar] [CrossRef]
- Peng, M.X.; Wang, Z.H.; Shen, S.H.; Xiao, Q.G.; Li, L.J.; Tang, Y.C.; Hu, L.L. Alkali Fusion of Bentonite to Synthesize One-Part Geopolymeric Cements Cured at Elevated Temperature by Comparison with Two-Part Ones. Constr. Build. Mater. 2017, 130, 103–112. [Google Scholar] [CrossRef]
- Abdel-Gawwad, H.A. Thermo-Alkali Activation of Talc for the Production of a Novel White One-Part Alkali-Activated Magnesia-Based Cement. Constr. Build. Mater. 2021, 306, 124909. [Google Scholar] [CrossRef]
- Lima, F.S.; Gomes, T.C.F.; Moraes, J.C.B.d. Novel One-Part Alkali-Activated Binder Produced with Coffee Husk Ash. Mater. Lett. 2022, 313, 131733. [Google Scholar] [CrossRef]
- Ali, H.A.; Lu, J.X.; Sun, K.; Poon, C.S. Valorization of Spent Fluorescent Lamp Waste Glass Powder as an Activator for Eco-Efficient Binder Materials. Constr. Build. Mater. 2022, 352, 129020. [Google Scholar] [CrossRef]
- Ouyang, G.; Wang, J.; Wang, R.; Chen, L.; Bu, B. Rheokinetics and Fluidity Modification of Alkali Activated Ultrafine Metakaolin Based Geopolymers. Constr. Build. Mater. 2021, 269, 121268. [Google Scholar] [CrossRef]
- Luukkonen, T.; Abdollahnejad, Z.; Ohenoja, K.; Kinnunen, P.; Illikainen, M. Suitability of Commercial Superplasticizers for One-Part Alkali-Activated Blast-Furnace Slag Mortar. J. Sustain. Cem.-Based Mater. 2019, 8, 244–257. [Google Scholar] [CrossRef]
- Alrefaei, Y.; Wang, Y.-S.; Dai, J.-G. The Effectiveness of Different Superplasticizers in Ambient Cured One-Part Alkali Activated Pastes. Cem. Concr. Compos. 2019, 97, 166–174. [Google Scholar] [CrossRef]
- Nedunuri, A.S.S.S.; Muhammad, S. Fundamental Understanding of the Setting Behaviour of the Alkali Activated Binders Based on Ground Granulated Blast Furnace Slag and Fly Ash. Constr. Build. Mater. 2021, 291, 123243. [Google Scholar] [CrossRef]
- Abdollahnejad, Z.; Mastali, M.; Woof, B.; Illikainen, M. High Strength Fiber Reinforced One-Part Alkali Activated Slag/Fly Ash Binders with Ceramic Aggregates: Microscopic Analysis, Mechanical Properties, Drying Shrinkage, and Freeze-Thaw Resistance. Constr. Build. Mater. 2020, 241, 118129. [Google Scholar] [CrossRef]
- Wang, K.; Du, L.; Lv, X.; He, Y.; Cui, X. Preparation of Drying Powder Inorganic Polymer Cement Based on Alkali-Activated Slag Technology. Powder Technol. 2017, 312, 204–209. [Google Scholar] [CrossRef]
- Matalkah, F.; Xu, L.; Wu, W.; Soroushian, P. Mechanochemical Synthesis of One-Part Alkali Aluminosilicate Hydraulic Cement. Mater. Struct. 2017, 50, 97. [Google Scholar] [CrossRef]
- Hajimohammadi, A.; van Deventer, J.S.J. Characterisation of One-Part Geopolymer Binders Made from Fly Ash. Waste Biomass Valor. 2017, 8, 225–233. [Google Scholar] [CrossRef]
- Suwan, T.; Fan, M. Effect of Manufacturing Process on the Mechanisms and Mechanical Properties of Fly Ash-Based Geopolymer in Ambient Curing Temperature. Mater. Manuf. Process. 2017, 32, 461–467. [Google Scholar] [CrossRef]
- Abdollahnejad, Z.; Mastali, M.; Luukkonen, T.; Kinnunen, P.; Illikainen, M. Fiber-Reinforced One-Part Alkali-Activated Slag/Ceramic Binders. Ceram. Int. 2018, 44, 8963–8976. [Google Scholar] [CrossRef]
- Ali Shah, S.F.; Chen, B.; Oderji, S.Y.; Haque, M.A.; Ahmad, M.R. Comparative Study on the Effect of Fiber Type and Content on the Performance of One-Part Alkali-Activated Mortar. Constr. Build. Mater. 2020, 243, 118221. [Google Scholar] [CrossRef]
- Alrefaei, Y.; Dai, J. Deflection Hardening Behavior and Elastic Modulus of One-Part Hybrid Fiber-Reinforced Geopolymer Composites. J. Asian Concr. Fed. 2019, 5, 37–51. [Google Scholar] [CrossRef]
- Lee, S.; van Riessen, A.; Chon, C. Benefits of Sealed-Curing on Compressive Strength of Fly Ash-Based Geopolymers. Materials 2016, 9, 598. [Google Scholar] [CrossRef] [PubMed]
- Nodehi, M.; Ozbakkaloglu, T.; Gholampour, A.; Mohammed, T.; Shi, X. The Effect of Curing Regimes on Physico-Mechanical, Microstructural and Durability Properties of Alkali-Activated Materials: A Review. Constr. Build. Mater. 2022, 321, 126335. [Google Scholar] [CrossRef]
- Abdollahnejad, Z.; Luukkonen, T.; Mastali, M.; Kinnunen, P.; Illikainen, M. Development of One-Part Alkali-Activated Ceramic/Slag Binders Containing Recycled Ceramic Aggregates. J. Mater. Civ. Eng. 2019, 31, 04018386. [Google Scholar] [CrossRef] [Green Version]
- Romagnoli, M.; Leonelli, C.; Kamse, E.; Lassinantti Gualtieri, M. Rheology of Geopolymer by DOE Approach. Constr. Build. Mater. 2012, 36, 251–258. [Google Scholar] [CrossRef]
- Boca Santa, R.A.A.; Kessler, J.C.; Soares, C.; Riella, H.G. Microstructural Evaluation of Initial Dissolution of Aluminosilicate Particles and Formation of Geopolymer Material. Particuology 2018, 41, 101–111. [Google Scholar] [CrossRef]
- Fan, J.; Zhu, H.; Shi, J.; Li, Z.; Yang, S. Influence of Slag Content on the Bond Strength, Chloride Penetration Resistance, and Interface Phase Evolution of Concrete Repaired with Alkali Activated Slag/Fly Ash. Constr. Build. Mater. 2020, 263, 120639. [Google Scholar] [CrossRef]
- Yang, W.-H.; Ryu, D.-W.; Park, D.-C.; Kim, W.-J.; Seo, C.-H. A Study of the Effect of Light-Burnt Dolomite on the Hydration of Alkali-Activated Portland Blast-Furnace Slag Cement. Constr. Build. Mater. 2014, 57, 24–29. [Google Scholar] [CrossRef]
- Lu, J.; Yu, Z.; Zhu, Y.; Huang, S.; Luo, Q.; Zhang, S. Effect of Lithium-Slag in the Performance of Slag Cement Mortar Based on Least-Squares Support Vector Machine Prediction. Materials 2019, 12, 1652. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Kumar, V.; Prasad, B. Strength Development and Flexural Behavior of Reinforced Concrete Beam Using One-Part Alkali-Activated Binder. Constr. Build. Mater. 2021, 281, 122619. [Google Scholar] [CrossRef]
- Lu, C.; Zhang, Z.; Shi, C.; Li, N.; Jiao, D.; Yuan, Q. Rheology of Alkali-Activated Materials: A Review. Cem. Concr. Compos. 2021, 121, 104061. [Google Scholar] [CrossRef]
- Alrefaei, Y.; Wang, Y.-S.; Qian, Y.; Dai, J.-G. Effects of Solid Activator and Fly Ash on Rheology and Thixotropy of One-Part Alkali-Activated Pastes. J. Adv. Concr. Technol. 2022, 20, 139–151. [Google Scholar] [CrossRef]
- Panda, B.; Ruan, S.; Unluer, C.; Tan, M.J. Investigation of the Properties of Alkali-Activated Slag Mixes Involving the Use of Nanoclay and Nucleation Seeds for 3D Printing. Compos. Part B Eng. 2020, 186, 107826. [Google Scholar] [CrossRef]
- Ismail, I.; Bernal, S.A.; Provis, J.L.; San Nicolas, R.; Hamdan, S.; van Deventer, J.S.J. Modification of Phase Evolution in Alkali-Activated Blast Furnace Slag by the Incorporation of Fly Ash. Cem. Concr. Compos. 2014, 45, 125–135. [Google Scholar] [CrossRef]
- Qian, L.-P.; Wang, Y.-S.; Alrefaei, Y.; Dai, J.-G. Experimental Study on Full-Volume Fly Ash Geopolymer Mortars: Sintered Fly Ash versus Sand as Fine Aggregates. J. Clean. Prod. 2020, 263, 121445. [Google Scholar] [CrossRef]
- Panda, B.; Unluer, C.; Tan, M.J. Investigation of the Rheology and Strength of Geopolymer Mixtures for Extrusion-Based 3D Printing. Cem. Concr. Compos. 2018, 94, 307–314. [Google Scholar] [CrossRef]
- Ishwarya, G.A.; Singh, B.; Deshwal, S.; Bhattacharyya, S.K. Effect of Sodium Carbonate/Sodium Silicate Activator on the Rheology, Geopolymerization and Strength of Fly Ash/Slag Geopolymer Pastes. Cem. Concr. Compos. 2019, 97, 226–238. [Google Scholar] [CrossRef]
- Aboulayt, A.; Jaafri, R.; Samouh, H.; Cherki El Idrissi, A.; Roziere, E.; Moussa, R.; Loukili, A. Stability of a New Geopolymer Grout: Rheological and Mechanical Performances of Metakaolin-Fly Ash Binary Mixtures. Constr. Build. Mater. 2018, 181, 420–436. [Google Scholar] [CrossRef]
- Xiang, J.; Liu, L.; Cui, X.; He, Y.; Zheng, G.; Shi, C. Effect of Fuller-Fine Sand on Rheological, Drying Shrinkage, and Microstructural Properties of Metakaolin-Based Geopolymer Grouting Materials. Cem. Concr. Compos. 2019, 104, 103381. [Google Scholar] [CrossRef]
- Coppola, L.; Coffetti, D.; Crotti, E.; Candamano, S.; Crea, F.; Gazzaniga, G.; Pastore, T. The Combined Use of Admixtures for Shrinkage Reduction in One-Part Alkali Activated Slag-Based Mortars and Pastes. Constr. Build. Mater. 2020, 248, 118682. [Google Scholar] [CrossRef]
- Nematollahi, B.; Sanjayan, J.; Shaikh, F.U.A. Synthesis of Heat and Ambient Cured One-Part Geopolymer Mixes with Different Grades of Sodium Silicate. Ceram. Int. 2015, 41, 5696–5704. [Google Scholar] [CrossRef]
- Duxson, P.; Provis, J.L.; Lukey, G.C.; Mallicoat, S.W.; Kriven, W.M.; Van, D.J. Understanding the Relationship between Geopolymer Composition, Microstructure and Mechanical Properties. Colloids Surf. A Physicochem. Eng. Asp. 2005, 269, 47–58. [Google Scholar] [CrossRef]
- Simon, S.; Gluth, G.J.G.; Peys, A.; Onisei, S.; Banerjee, D.; Pontikes, Y. The Fate of Iron during the Alkali-Activation of Synthetic (CaO-)FeOx-SiO2 Slags: An Fe K-Edge XANES Study. J. Am. Ceram. Soc. 2018, 101, 2107–2118. [Google Scholar] [CrossRef]
- Adesanya, E.; Sreenivasan, H.; Kantola, A.M.; Telkki, V.-V.; Ohenoja, K.; Kinnunen, P.; Illikainen, M. Ladle Slag Cement–Characterization of Hydration and Conversion. Constr. Build. Mater. 2018, 193, 128–134. [Google Scholar] [CrossRef]
- Winnefeld, F.; Gluth, G.J.G.; Bernal, S.A.; Bignozzi, M.C.; Carabba, L.; Chithiraputhiran, S.; Dehghan, A.; Dolenec, S.; Dombrowski-Daube, K.; Dubey, A.; et al. RILEM TC 247-DTA Round Robin Test: Sulfate Resistance, Alkali-Silica Reaction and Freeze–Thaw Resistance of Alkali-Activated Concretes. Mater. Struct. 2020, 53, 140. [Google Scholar] [CrossRef]



| Author | Precursor | Activator | w/b Ratio | Slump (mm) | 28 d UCS/MPa |
|---|---|---|---|---|---|
| Wang et al. [53] | NS | Na2SiO3 | 0.35 | - | 21–53 |
| Teo et al. [44] | GGBS + FA(C) | Na2SiO3 | 0.4 | 196–214 | 49–91 |
| Alrefaei and Dai [68] | GGBS + FA | Na2SiO3 | 0.42 | - | 49–63 |
| Zhou et al. [47] | GGBS | Na2CO3 + Ca(OH)2 | 0.46 | - | 31–36 |
| Zheng et al. [72] | GGBS | Na2CO3 + CaO | 0.4 | - | 45–48 |
| Wei et al. [73] | GGBS + FASB | Na2SiO3, Na2CO3 | 0.35 | - | 42–76 |
| Wang et al. [71] | GGBS + FA | Na2SiO3, Na2CO3, NaAlO2 | 0.36 | 205–249 | 68–95 |
| Samarakoon et al. [74] | GGBS + FA + WS | NaOH | 0.5 | - | 21–28 |
| Ren et al. [75] | GGBS | Na2SiO3 | 0.4 | 235–239 | 58–67 |
| Refaat et al. [76] | GGBS | NaOH | 0.27–0.31 | 60–150 | 9–53 |
| Perumal et al. [48] | GGBS + SF + PD | Na2SiO3 | 0.25–0.35 | - | 46–145 |
| Liu et al. [77] | GGBS + GMT | NaOH + CaO | 0.5 | - | 14–45 |
| Liu et al. [42] | GGBS + GMT | NaOH | 0.4 | - | 4–36 |
| Lemougna et al. [67] | GGBS + CS | Na2SiO3 | 0.32–0.4 | - | 28–76 |
| Kadhim et al. [78] | MK + NP | LKD | 0.45 | - | 6–20 |
| Haruna et al. [79] | FA | Na2SiO3 | 0.25 | - | 46–68 |
| Gonçalves et al. [80] | GGBS | Na2SiO3 | 0.29–0.35 | 102–196 | 41–56 |
| Galvão Souza Azevedo et al. [59] | FA + WS/MK | NaOH + Na2SiO3 | 0.6, 0.9 | - | 5–12 |
| Alzaza et al. [81] | GGBS | Na2SiO3 | 0.35 | - | 15–101 |
| Alzaza et al. [55] | GGBS + SMP | Na2SiO3 | 0.35 | - | 3–43 |
| Almakhadmeh and Soliman [82] | GGBS | Na2SiO3 | 0.4 | 190 | 77–89 |
| Ali Shah et al. [54] | GGBS + LS | Na2SiO3 | 0.3 | 101–285 | 9–56 |
| Yang et al. [34] | GGBS + CD | Na2CO3 | 0.6 | - | 27–45 |
| Yang and Jang [83] | GGBS | COS | 0.4 | - | 22–35 |
| Samarakoon et al. [35] | GGBS + FA | SLGP + NaOH | 0.4 | - | 28–45 |
| Mobili et al. [84] | MK | BA | 0.49–0.65 | - | 1.6–3.7 |
| Lv et al. [85] | GGBS + FA | NaOH + Na2SiO3 + Na2CO3 | 0.45 | - | 41–44 |
| Luukkonen et al. [86] | GGBS | Na2SiO3 | 0.35 | - | 70–90 |
| Liu et al. [87] | GGBS | Na2SiO3 + HCC | 0.5 | - | 21–44 |
| Li et al. [64] | GGBS + WS + CAC | Na2SiO3 | 0.275–0.35 | 70–175 | 46–70 |
| Kadhim et al. [88] | MK + NP | LKD | 0.55 | - | 9–27 |
| Haruna et al. [89] | FA(C) | Na2SiO3 | 0.25 | 34–165 | 41–70 |
| Coppola et al. [90] | GGBS | Na2SiO3 + KOH + Na2CO3 | 0.55 | 150–220 | 14–48 |
| Chen et al. [91] | GGBS + LZMT | Na2SiO3 | 0.45 | 168–191 | 29–34 |
| Ahmad et al. [92] | GGBS + FA + MK/SF/MgO/OPC | Na2SiO3 | 0.16 | 223–305 | 28–41 |
| Oderji et al. [93] | GGBS + FA | Na2SiO3 | 0.3 | 231–278 | 8–43 |
| Ababneh et al. [21] | MK | Na2SiO3 + CaO + Na2CO3 | 0.4–0.53 | - | 7–20 |
| Jeon et al. [61] | GGBS + CD | Na2SiO3 | 0.4 | 165–218 | 28–52 |
| Sturm et al. [94] | GGBS + SF/SiO2/RHA | NaAlO2 | 0.38–0.5 | - | 30–58 |
| Luukkonen et al. [56] | GGBS + SF/RHA | Na2SiO3 | 0.35 | - | 30–107 |
| Adesanya et al. [95] | GGBS | NaOH + PS | 0.31 | - | 33–42 |
| Abdel Gawwad et al. [70] | GGBS | NaOH + MgCO3 | 0.3 | - | 56–83 |
| Peng et al. [60] | CB + CD | Na2CO3 | 0.35 | - | 18–38 |
| Almalkawi et al. [96] | VP | CaO + Na2SO4 + Na2CO3 | 0.5 | - | 5–22 |
| Ye et al. [97] | RM + FA | NaOH | 0.5 | - | 1.1–1.8 |
| Alrefaei et al. [98] | GGBS + FA | Ca(OH)2 + Na2SO4 | 0.27–0.4 | - | 21–74 |
| Type | Treatment | Parameter | Major Process | Evaluation | References |
|---|---|---|---|---|---|
| Mechanical | Ball milling, crushing | Ball loading rate; ball material ratio | Increase the contact area and improve reactivity by mechanical treatment to accelerate the destabilization process of aluminosilicate structure | Highest reactivity and easy to handle | [53,101,110] |
| High temperature | Calcination | Temperature; duration | High temperature changes the mineralogical phase, loss of its long-range ordered structure and transformed into an amorphous form, improving the crystallinity and reactivity | High reactivity but increases carbon emissions | [59,83,88,96,101] |
| Chemical | Immersion, thermochemical, adsorption | - | The crystal original structure has been destroyed, which is conducive for the dissolution of silicon and aluminum | Medium reactivity, need to control chemical treatment level and not easy to use | [42,72,76,87] |
| Delayed addition | Delayed adding PCE | Delay time | Minimized the competitive adsorption phenomenon | No change in reactivity, slight increase in strength | [68] |
| Mixing process | Water temperature | Hot water, cold water | High temperature could speed up the initial reaction or reduce setting time; cold water can offset the early thermal shrinkage | Low reactivity, but provides controlled field mixing conditions | [82] |
| References | Fiber Type | Dosage of Fiber (Vol.) | Compressive Strength | Flexural Strength | Effect Evaluation |
|---|---|---|---|---|---|
| Abdollahnejad et al. [113] | ST | 0.5% and 1.0% | ST: 53–64 MPa | n.r. | ST can get the best mechanical properties and has the highest chemical stability, while PP/PVA may have chemical adhesion. |
| PP | PP: 45–60 MPa | ||||
| PVA | PVA: 47–61 MPa | ||||
| Abdollahnejad et al. [108] | PP | 1.5% | PP: 50 MPa PVA: 56 MPa BA: 52 MPa Hybrid: 42–61 MPa | PP: 7.8 MPa PVA: 10.7 MPa BA: 11.3 MPa Hybrid: 7.8–10.9 MPa | All groups showed a decreased compressive strength from the original state; PVA has a better mechanical performance. |
| PVA | |||||
| BA | |||||
| Shah et al. [114] | ST | 0.5%, 1.0%, 1.5% and 2.0% | ST: 28–33 MPa | ST: 2.8–3.1 MPa | The strength decreased when the dosage of the fiber was beyond 1.5%; BA has bad adhesion with the OP-AAM. |
| PVA | PVA: 25–30 MPa | PVA: 2.8–3.2 MPa | |||
| BA | BA: 29–31 MPa | BA: 2.3–2.7 MPa | |||
| Abdollahnejad et al. [43] | ST | 1.0% | ST: 82 MPa PVA: 76 MPa BA: 33 MPa CEL: 53 MPa Hybrid: 54–72 MPa | ST: 10.7 MPa PVA: 9.6 MPa BA: 6.1 MPa CEL: 5.2 MPa Hybrid: 6–11 MPa | Except for ST, all single and hybrid fibers increased porosity and water absorption; ST can improve both fire resistance and freezing resistance. |
| PVA | |||||
| BA | |||||
| CEL | |||||
| Perumal et al. [62] | ST | 1.0% | ST: 150–233 MPa | n.r. | ST outperformed other fibers in the AAM, and longer the fiber, the better was the load-carrying capacity. Mineral fibers were unstable in high alkaline environment. |
| GLA | GLA: 143–177 MPa | ||||
| BA | BA: 135–168 MPa | ||||
| Alrefaei and Dai [115] | ST | 0.5%, 1.0%, 1.5% and 2.0% | ST: 60–81 MPa | ST: 6.6–8.2 MPa | PE showed a comparative modulus of rupture relative to that of ST, and ST showed minor effects on flexural cracking and ultimate strengths. |
| PE | PE: 45–63 MPa | PE: 7.3–8.0 MPa |
| References | Curing Method | Mix Parameter | Major Results | Evaluation |
|---|---|---|---|---|
| Abdollahnejad et al. [113] | Sealing/water curing | GGBS/PCW + Na2SiO3 | Water curing strength +3%–+13% | Water curing has better properties for compressive strength and flexural strength |
| Abdollahnejad et al. [118] | Thermal curing | GGBS/PCW (porcelain and raw) + Na2SiO3 | Compressive strength −20%–+25% | Thermal curing led to the maximum improvement of strength |
| Ahmad et al. [92] | Ambient/water/thermal curing | FA/GGBS + Na2SiO3 | Water curing strength −92–−60%; thermal curing strength +5%–+135% | Water curing had an adverse effect |
| Haruna et al. [89] | Ambient/solar/water curing | FA(C) + Na2SiO3 | Water curing strength –3%–−7%; Solar curing strength +6%–+23% | Solar curing had the best compressive strength; ambient curing had nice flexural strength |
| Alzaza et al. [81] | Subzero curing (−5/−10/−20 °C) | GGBS + Na2SiO3 | Subzero curing strength –45%–−86% | The reactivity of AAS at low temperature is better than that of OPC; the strengths of AAS and OPC are 68 and 9.7 MPa, respectively |
| Alzaza et al. [55] | Subzero curing (−5/−10/−20 °C) | GGBS/SMP + Na2SiO3 | Compressive strength under −5 °C is much higher then −10 and −20 °C | Low temperature hinders the reaction process, and adding SMP can obtain a better performance under subzero curing |
| Wei et al. [73] | Ambient/water curing | FASB + Na2SiO3/Na2CO3 | Water curing strength –14%–−20% | Water curing decreases the quantity of hydration products and makes the microstructure much coarser |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, Y.; Qu, C.; Ma, C.; Zhou, L. One-Part Alkali-Activated Materials: State of the Art and Perspectives. Polymers 2022, 14, 5046. https://doi.org/10.3390/polym14225046
Qin Y, Qu C, Ma C, Zhou L. One-Part Alkali-Activated Materials: State of the Art and Perspectives. Polymers. 2022; 14(22):5046. https://doi.org/10.3390/polym14225046
Chicago/Turabian StyleQin, Yongjun, Changwei Qu, Cailong Ma, and Lina Zhou. 2022. "One-Part Alkali-Activated Materials: State of the Art and Perspectives" Polymers 14, no. 22: 5046. https://doi.org/10.3390/polym14225046
APA StyleQin, Y., Qu, C., Ma, C., & Zhou, L. (2022). One-Part Alkali-Activated Materials: State of the Art and Perspectives. Polymers, 14(22), 5046. https://doi.org/10.3390/polym14225046







