Behavior of Calcium Phosphate–Chitosan–Collagen Composite Coating on AISI 304 for Orthopedic Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Galvanic Deposition
2.3. Morphology Analysis
2.4. X-ray Diffraction Analysis
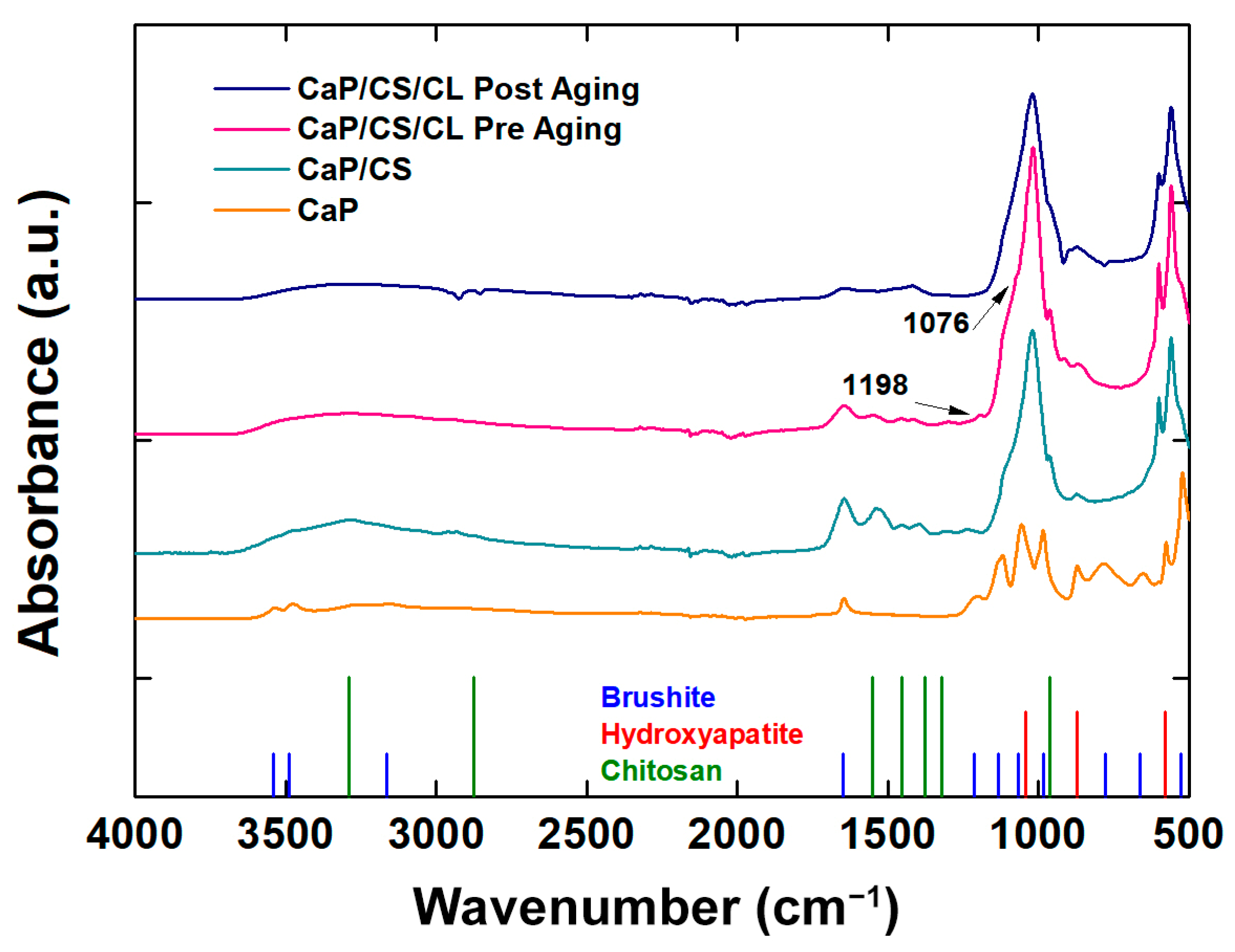
2.5. Raman Spectroscopy and FT-IR/ATR Analysis
2.6. ICP-OES Analysis
2.7. Corrosion Tests
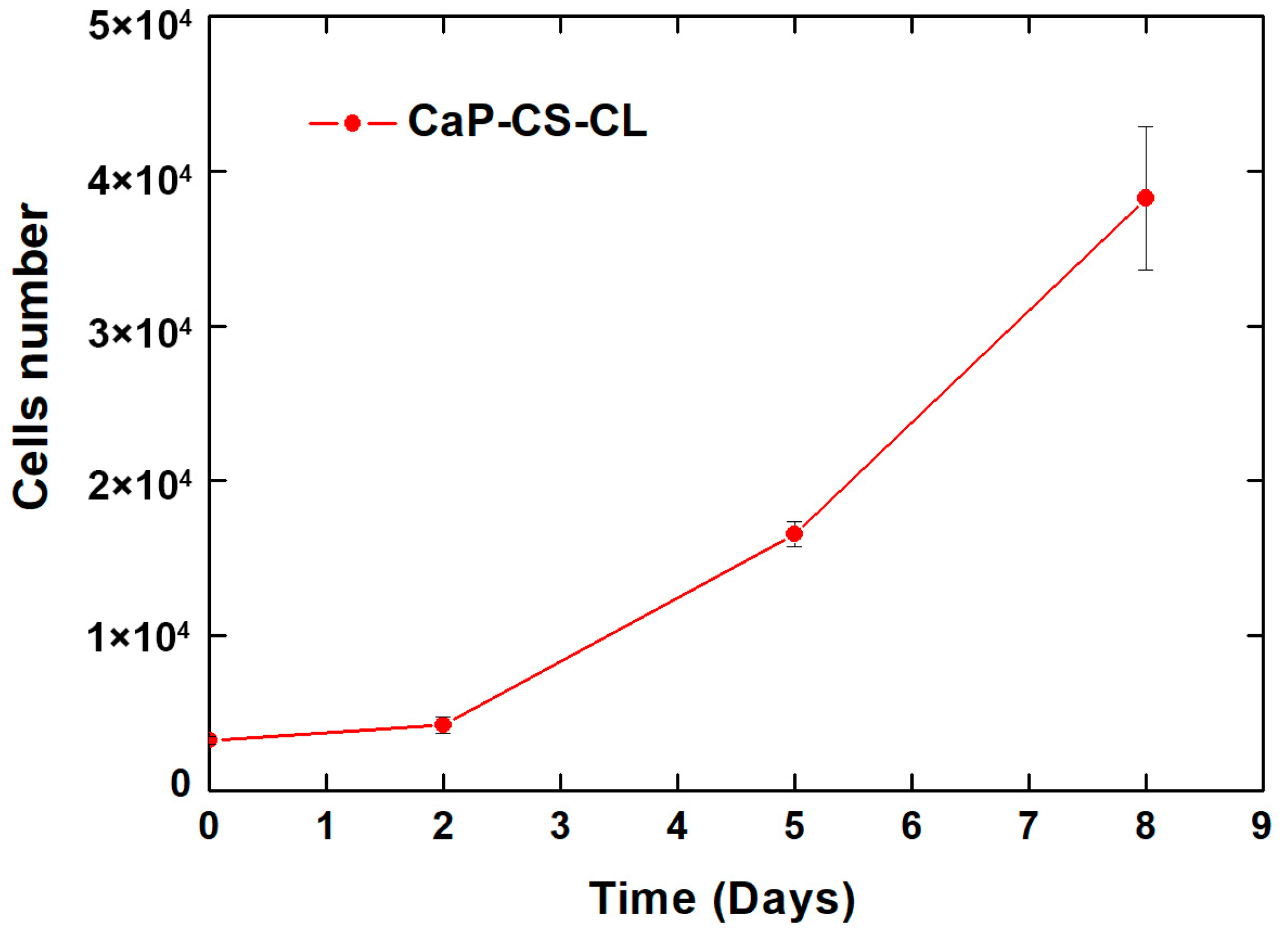
2.8. Cytotoxicity
3. Results
3.1. Galvanic Deposition
3.2. Morphological Analysis
3.3. Raman Spectroscopy and FT-IR/ATR Analysis
3.4. Corrosion Tests
3.5. ICP-OES Analysis
3.6. Cytotoxicity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rony, L.; Lancigu, R.; Hubert, L. Intraosseous metal implants in orthopedics: A review. Morphologie 2018, 102, 231–242. [Google Scholar] [CrossRef]
- Renganathan, G.; Tanneru, N.; Madurai, S.L. Orthopedical and biomedical applications of titanium and zirconium metals. In Fundamental Biomaterials: Metals; Elsevier: Amsterdam, The Netherlands, 2018; pp. 211–241. ISBN 978-0-08-102205-4. [Google Scholar]
- Jin, W.; Chu, P.K. Orthopedic Implants. In Encyclopedia of Biomedical Engineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 425–439. ISBN 978-0-12-805144-3. [Google Scholar]
- Hudecki, A.; Kiryczyński, G.; Łos, M.J. Biomaterials, Definition, Overview. In Stem Cells and Biomaterials for Regenerative Medicine; Elsevier: Amsterdam, The Netherlands, 2019; pp. 85–98. ISBN 978-0-12-812258-7. [Google Scholar]
- Gittens, R.A.; Olivares-Navarrete, R.; Schwartz, Z.; Boyan, B.D. Implant osseointegration and the role of microroughness and nanostructures: Lessons for spine implants. Acta Biomater. 2014, 10, 3363–3371. [Google Scholar] [CrossRef] [Green Version]
- Andrukhov, O.; Huber, R.; Shi, B.; Berner, S.; Rausch-Fan, X.; Moritz, A.; Spencer, N.D.; Schedle, A. Proliferation, behavior, and differentiation of osteoblasts on surfaces of different microroughness. Dent. Mater. 2016, 32, 1374–1384. [Google Scholar] [CrossRef]
- Gao, C.; Peng, S.; Feng, P.; Shuai, C. Bone biomaterials and interactions with stem cells. Bone Res. 2017, 5, 17059. [Google Scholar] [CrossRef] [Green Version]
- Rho, J.-Y.; Kuhn-Spearing, L.; Zioupos, P. Mechanical properties and the hierarchical structure of bone. Med. Eng. Phys. 1998, 20, 92–102. [Google Scholar] [CrossRef]
- Lian, J.B.; McKee, M.D.; Todd, A.M.; Gerstenfeld, L.C. Induction of bone-related proteins, osteocalcin and osteopontin, and their matrix ultrastructural localization with development of chondrocyte hypertrophy in vitro. J. Cell. Biochem. 1993, 52, 206–219. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, D.; Wang, T. Hierarchical Structures of Bone and Bioinspired Bone Tissue Engineering. Small 2016, 12, 4611–4632. [Google Scholar] [CrossRef]
- Monesi, V. Istologia, 7th ed.; Piccin-Nuova Libraria: Padua, Italy, 2018; ISBN 88-299-2813-5. [Google Scholar]
- Huang, J.; Best, S. Ceramic biomaterials for tissue engineering. In Tissue Engineering Using Ceramics and Polymers; Elsevier: Amsterdam, The Netherlands, 2014; pp. 3–34. ISBN 978-0-85709-712-5. [Google Scholar]
- Fiume, E.; Magnaterra, G.; Rahdar, A.; Verné, E.; Baino, F. Hydroxyapatite for Biomedical Applications: A Short Overview. Ceramics 2021, 4, 39. [Google Scholar] [CrossRef]
- Firdaus Hussin, M.S.; Abdullah, H.Z.; Idris, M.I.; Abdul Wahap, M.A. Extraction of natural hydroxyapatite for biomedical applications—A review. Heliyon 2022, 8, e10356. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-W.; Kim, H.-E.; Salih, V.; Knowles, J.C. Sol-gel-modified titanium with hydroxyapatite thin films and effect on osteoblast-like cell responses. J. Biomed. Mater. Res. 2005, 74A, 294–305. [Google Scholar] [CrossRef]
- Walter, N.; Stich, T.; Docheva, D.; Alt, V.; Rupp, M. Evolution of implants and advancements for osseointegration: A narrative review. Injury 2022, 53, S69–S73. [Google Scholar] [CrossRef]
- Bohara, S.; Suthakorn, J. Surface coating of orthopedic implant to enhance the osseointegration and reduction of bacterial colonization: A review. Biomater. Res. 2022, 26, 26. [Google Scholar] [CrossRef]
- Kumar, S.; Nehra, M.; Kedia, D.; Dilbaghi, N.; Tankeshwar, K.; Kim, K.-H. Nanotechnology-based biomaterials for orthopaedic applications: Recent advances and future prospects. Mater. Sci. Eng. C 2020, 106, 110154. [Google Scholar] [CrossRef]
- Eltit, F.; Wang, Q.; Wang, R. Mechanisms of Adverse Local Tissue Reactions to Hip Implants. Front. Bioeng. Biotechnol. 2019, 7, 176. [Google Scholar] [CrossRef]
- Kocak, F.Z.; Yar, M.; Rehman, I.U. Hydroxyapatite-Integrated, Heparin- and Glycerol-Functionalized Chitosan-Based Injectable Hydrogels with Improved Mechanical and Proangiogenic Performance. Int. J. Mol. Sci. 2022, 23, 5370. [Google Scholar] [CrossRef]
- Huang, Z.; Chen, Y.; Feng, Q.-L.; Zhao, W.; Yu, B.; Tian, J.; Li, S.-J.; Lin, B.-M. In vivo bone regeneration with injectable chitosan/hydroxyapatite/collagen composites and mesenchymal stem cells. Front. Mater. Sci. 2011, 5, 301–310. [Google Scholar] [CrossRef]
- Sun, F.; Pang, X.; Zhitomirsky, I. Electrophoretic deposition of composite hydroxyapatite–chitosan–heparin coatings. J. Mater. Process. Technol. 2009, 209, 1597–1606. [Google Scholar] [CrossRef]
- Kocak, F.Z.; Talari, A.C.S.; Yar, M.; Rehman, I.U. In-Situ Forming pH and Thermosensitive Injectable Hydrogels to Stimulate Angiogenesis: Potential Candidates for Fast Bone Regeneration Applications. Int. J. Mol. Sci. 2020, 21, 1633. [Google Scholar] [CrossRef] [Green Version]
- Xiong Chang, X.; Mujawar Mubarak, N.; Ali Mazari, S.; Sattar Jatoi, A.; Ahmad, A.; Khalid, M.; Walvekar, R.; Abdullah, E.C.; Karri, R.R.; Siddiqui, M.T.H.; et al. A review on the properties and applications of chitosan, cellulose and deep eutectic solvent in green chemistry. J. Ind. Eng. Chem. 2021, 104, 362–380. [Google Scholar] [CrossRef]
- Qin, Z.; Zhao, L. The History of Chito/Chitin Oligosaccharides and Its Monomer. In Oligosaccharides of Chitin and Chitosan; Zhao, L., Ed.; Springer: Singapore, 2019; pp. 3–14. ISBN 9789811394010. [Google Scholar]
- Rkhaila, A.; Chtouki, T.; Erguig, H.; El Haloui, N.; Ounine, K. Chemical Proprieties of Biopolymers (Chitin/Chitosan) and Their Synergic Effects with Endophytic Bacillus Species: Unlimited Applications in Agriculture. Molecules 2021, 26, 1117. [Google Scholar] [CrossRef]
- Pérez-Álvarez, L.; Ruiz-Rubio, L.; Vilas-Vilela, J.L. Determining the Deacetylation Degree of Chitosan: Opportunities To Learn Instrumental Techniques. J. Chem. Educ. 2018, 95, 1022–1028. [Google Scholar] [CrossRef]
- Bhattarai, N.; Gunn, J.; Zhang, M. Chitosan-based hydrogels for controlled, localized drug delivery. Adv. Drug Deliv. Rev. 2010, 62, 83–99. [Google Scholar] [CrossRef]
- Kabanov, V.L.; Novinyuk, L.V. Chitosan Application in Food Technology: A Review of Rescent Advances. Food Syst. 2020, 3, 10–15. [Google Scholar] [CrossRef] [Green Version]
- Morin-Crini, N.; Lichtfouse, E.; Torri, G.; Crini, G. Applications of chitosan in food, pharmaceuticals, medicine, cosmetics, agriculture, textiles, pulp and paper, biotechnology, and environmental chemistry. Environ. Chem. Lett. 2019, 17, 1667–1692. [Google Scholar] [CrossRef] [Green Version]
- de Sousa Victor, R.; Marcelo da Cunha Santos, A.; Viana de Sousa, B.; de Araújo Neves, G.; Navarro de Lima Santana, L.; Rodrigues Menezes, R. A Review on Chitosan’s Uses as Biomaterial: Tissue Engineering, Drug Delivery Systems and Cancer Treatment. Materials 2020, 13, 4995. [Google Scholar] [CrossRef] [PubMed]
- Amirthalingam, S.; Rangasamy, J. Chitosan-Based Biosensor Fabrication and Biosensing Applications. In Chitosan for Biomaterials III; Jayakumar, R., Prabaharan, M., Eds.; Advances in Polymer Science; Springer International Publishing: Cham, Switzerland, 2021; Volume 287, pp. 233–255. ISBN 978-3-030-83806-5. [Google Scholar]
- Liu, Y.; Chen, J.; Li, P.; Ning, N. The Effect of Chitosan in Wound Healing: A Systematic Review. Adv Ski. Wound Care 2021, 34, 262–266. [Google Scholar] [CrossRef]
- Holzwarth, J.M.; Ma, P.X. Biomimetic nanofibrous scaffolds for bone tissue engineering. Biomaterials 2011, 32, 9622–9629. [Google Scholar] [CrossRef] [Green Version]
- Xia, Z.; Yu, X.; Jiang, X.; Brody, H.D.; Rowe, D.W.; Wei, M. Fabrication and characterization of biomimetic collagen–apatite scaffolds with tunable structures for bone tissue engineering. Acta Biomater. 2013, 9, 7308–7319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qian, J.; Zhang, W.; Chen, Y.; Zeng, P.; Wang, J.; Zhou, C.; Zeng, H.; Sang, H.; Huang, N.; Zhang, H.; et al. Osteogenic and angiogenic bioactive collagen entrapped calcium/zinc phosphates coating on biodegradable Zn for orthopedic implant applications. Biomater. Adv. 2022, 136, 212792. [Google Scholar] [CrossRef]
- Guo, Y.; Su, Y.; Gu, R.; Zhang, Z.; Li, G.; Lian, J.; Ren, L. Enhanced corrosion resistance and biocompatibility of biodegradable magnesium alloy modified by calcium phosphate/collagen coating. Surf. Coat. Technol. 2020, 401, 126318. [Google Scholar] [CrossRef]
- Sun, T.; Lee, W.-C.; Wang, M. A comparative study of apatite coating and apatite/collagen composite coating fabricated on NiTi shape memory alloy through electrochemical deposition. Mater. Lett. 2011, 65, 2575–2577. [Google Scholar] [CrossRef]
- de Jonge, L.T.; Leeuwenburgh, S.C.G.; van den Beucken, J.J.J.P.; te Riet, J.; Daamen, W.F.; Wolke, J.G.C.; Scharnweber, D.; Jansen, J.A. The osteogenic effect of electrosprayed nanoscale collagen/calcium phosphate coatings on titanium. Biomaterials 2010, 31, 2461–2469. [Google Scholar] [CrossRef]
- Hu, C.; Yu, L.; Wei, M. Sectioning studies of biomimetic collagen-hydroxyapatite coatings on Ti-6Al-4V substrates using focused ion beam. Appl. Surf. Sci. 2018, 444, 590–597. [Google Scholar] [CrossRef]
- Liu, X.; Liu, W.-C.; Wang, H.-Y.; Li, V.L.; Chen, Y.-C.; Wang, A.-N.; Wu, C.-J.; Li, Y.; Zhao, G.; Lin, C.; et al. Polyelectrolyte multilayer composite coating on 316 L stainless steel for controlled release of dual growth factors accelerating restoration of bone defects. Mater. Sci. Eng. C 2021, 126, 112187. [Google Scholar] [CrossRef] [PubMed]
- Tapsir, Z.; Saidin, S. Synthesis and characterization of collagen–hydroxyapatite immobilized on polydopamine grafted stainless steel. Surf. Coat. Technol. 2016, 285, 11–16. [Google Scholar] [CrossRef]
- Turner, K.R.; Adams, C.; Staelens, S.; Deckmyn, H.; San Antonio, J. Crucial Role for Endothelial Cell α2β1 Integrin Receptor Clustering in Collagen-Induced Angiogenesis. Anat. Rec. 2020, 303, 1604–1618. [Google Scholar] [CrossRef]
- Whelan, M.C.; Senger, D.R. Collagen I Initiates Endothelial Cell Morphogenesis by Inducing Actin Polymerization through Suppression of Cyclic AMP and Protein Kinase A. J. Biol. Chem. 2003, 278, 327–334. [Google Scholar] [CrossRef] [Green Version]
- Niu, X.; Fan, R.; Tian, F.; Guo, X.; Li, P.; Feng, Q.; Fan, Y. Calcium concentration dependent collagen mineralization. Mater. Sci. Eng. C 2017, 73, 137–143. [Google Scholar] [CrossRef]
- Pawłowski, Ł.; Bartmański, M.; Mielewczyk-Gryń, A.; Cieślik, B.M.; Gajowiec, G.; Zieliński, A. Electrophoretically Deposited Chitosan/Eudragit E 100/AgNPs Composite Coatings on Titanium Substrate as a Silver Release System. Materials 2021, 14, 4533. [Google Scholar] [CrossRef]
- Bartmański, M.; Pawłowski, Ł.; Zieliński, A.; Mielewczyk-Gryń, A.; Strugała, G.; Cieślik, B. Electrophoretic Deposition and Characteristics of Chitosan–Nanosilver Composite Coatings on a Nanotubular TiO2 Layer. Coatings 2020, 10, 245. [Google Scholar] [CrossRef]
- Cometa, S.; Bonifacio, M.A.; Baruzzi, F.; de Candia, S.; Giangregorio, M.M.; Giannossa, L.C.; Dicarlo, M.; Mattioli-Belmonte, M.; Sabbatini, L.; De Giglio, E. Silver-loaded chitosan coating as an integrated approach to face titanium implant-associated infections: Analytical characterization and biological activity. Anal. Bioanal. Chem. 2017, 409, 7211–7221. [Google Scholar] [CrossRef]
- Kharitonov, D.S.; Kasach, A.A.; Gibala, A.; Zimowska, M.; Kurilo, I.I.; Wrzesińska, A.; Szyk-Warszyńska, L.; Warszyński, P. Anodic Electrodeposition of Chitosan–AgNP Composites Using In Situ Coordination with Copper Ions. Materials 2021, 14, 2754. [Google Scholar] [CrossRef]
- Li, B.; Xia, X.; Guo, M.; Jiang, Y.; Li, Y.; Zhang, Z.; Liu, S.; Li, H.; Liang, C.; Wang, H. Biological and antibacterial properties of the micro-nanostructured hydroxyapatite/chitosan coating on titanium. Sci. Rep. 2019, 9, 14052. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Q.; Zhang, Z.; Yang, Y.; Jian, Y.; Li, R.; Dai, X.; Wu, W.; Zhong, J.; Chen, C. Synthesis, characterization and biological performance study of Sr-doped hydroxyapatite/chitosan composite coatings. Mater. Chem. Phys. 2021, 270, 124752. [Google Scholar] [CrossRef]
- Schlesinger, M.; Paunovic, M. (Eds.) The electrochemical society series. In Modern Electroplating, 5th ed.; Wiley: Hoboken, NJ, USA, 2010; ISBN 978-0-470-16778-6. [Google Scholar]
- Battaglia, M.; Piazza, S.; Sunseri, C.; Inguanta, R. Amorphous silicon nanotubes via galvanic displacement deposition. Electrochem. Commun. 2013, 34, 134–137. [Google Scholar] [CrossRef]
- Inguanta, R.; Ferrara, G.; Piazza, S.; Sunseri, C. A new route to grow oxide nanostructures based on metal displacement deposition. Lanthanides oxy/hydroxides growth. Electrochim. Acta 2012, 76, 77–87. [Google Scholar] [CrossRef]
- Inguanta, R.; Piazza, S.; Sunseri, C. A Route to Grow Oxide Nanostructures Based on Metal Displacement Deposition: Lanthanides Oxy/Hydroxides Characterization. J. Electrochem. Soc. 2012, 159, D493–D500. [Google Scholar] [CrossRef]
- Patella, B.; Inguanta, R.; Piazza, S.; Sunseri, C. A nanostructured sensor of hydrogen peroxide. Sens. Actuators B Chem. 2017, 245, 44–54. [Google Scholar] [CrossRef]
- Cocchiara, C.; Sunseri, C.; Piazza, S.; Inguanta, R. Pb-PbOHCl Composite Nanowires Synthesized by Galvanic Deposition in Template. J. Nanosci. Nanotechnol. 2019, 19, 4677–4685. [Google Scholar] [CrossRef]
- Patella, B.; Inguanta, R.; Piazza, S.; Sunseri, C. Nanowire ordered arrays for electrochemical sensing of H2O2. Chem. Eng. Trans. 2016, 47, 19–24. [Google Scholar] [CrossRef]
- Inguanta, R.; Piazza, S.; Sunseri, C. Synthesis of self-standing Pd nanowires via galvanic displacement deposition. Electrochem. Commun. 2009, 11, 1385–1388. [Google Scholar] [CrossRef]
- Inguanta, R.; Piazza, S.; Sunseri, C. Novel procedure for the template synthesis of metal nanostructures. Electrochem. Commun. 2008, 10, 506–509. [Google Scholar] [CrossRef]
- Inguanta, R.; Ferrara, G.; Piazza, S.; Sunseri, C. Nanostructure fabrication by template deposition into anodic alumina membranes. Chem. Eng. Trans. 2009, 17, 957–962. [Google Scholar] [CrossRef]
- Inguanta, R.; Ferrara, G.; Piazza, S.; Sunseri, C. Fabrication and characterization of metal and metal oxide nanostructures grown by metal displacement deposition into anodic alumina membranes. Chem. Eng. Trans. 2011, 24, 199–204. [Google Scholar] [CrossRef]
- Battaglia, M.; Piazza, S.; Sunseri, C.; Inguanta, R. Amorphous silicon nanotubes. In Silicon Nanomaterials Sourcebook; Sattler, K.D., Ed.; Series: Series in Materials Science and Engineering; CRC Press: Boca Raton, FL, USA, 2017; pp. 565–590. ISBN 978-1-315-15354-4. [Google Scholar]
- Cocchiara, C.; Patella, B.; Ganci, F.; Insinga, M.G.; Piazza, S.; Sunseri, C.; Inguanta, R. Nanostructured Materials Obtained by Electrochemical Methods. In 21st Century Nanoscience—A Handbook; Sattler, K.D., Ed.; CRC Press: Boca Raton, FL, USA, 2020; ISBN 978-0-429-34042-0. [Google Scholar]
- Blanda, G.; Brucato, V.; Pavia, F.C.; Greco, S.; Piazza, S.; Sunseri, C.; Inguanta, R. Galvanic deposition and characterization of brushite/hydroxyapatite coatings on 316L stainless steel. Mater. Sci. Eng. C 2016, 64, 93–101. [Google Scholar] [CrossRef]
- Blanda, G.; Brucato, V.; Pavia, F.C.; Greco, S.; Piazza, S.; Sunseri, C.; Inguanta, R. In Vitro Corrosion and Biocompatibility of Brushite/Hydroxyapatite Coatings Obtained by Galvanic Deposition on 316LSS. J. Electrochem. Soc. 2018, 165, G1–G10. [Google Scholar] [CrossRef]
- Blanda, G.; Brucato, V.; Carfì, F.; Conoscenti, G.; La Carrubba, V.; Piazza, S.; Sunseri, C.; Inguanta, R. Chitosan-Coating Deposition via Galvanic Coupling. ACS Biomater. Sci. Eng. 2019, 5, 1715–1724. [Google Scholar] [CrossRef] [PubMed]
- Mendolia, I.; Zanca, C.; Ganci, F.; Conoscenti, G.; Pavia, F.C.; Brucato, V.; La Carrubba, V.; Lopresti, F.; Piazza, S.; Sunseri, C.; et al. Calcium phosphate/polyvinyl acetate coatings on SS304 via galvanic co-deposition for orthopedic implant applications. Surf. Coat. Technol. 2021, 408, 126771. [Google Scholar] [CrossRef]
- Zanca, C.; Carbone, S.; Patella, B.; Lopresti, F.; Aiello, G.; Brucato, V.; Carfì Pavia, F.; La Carrubba, V.; Inguanta, R. Composite Coatings of Chitosan and Silver Nanoparticles Obtained by Galvanic Deposition for Orthopedic Implants. Polymers 2022, 14, 3915. [Google Scholar] [CrossRef]
- Zanca, C.; Cordaro, G.; Capuana, E.; Brucato, V.; Pavia, F.C.; Carrubba, V.L.; Ghersi, G.; Inguanta, R. Galvanic Deposition Of Hydroxyapatite/Chitosan/Collagen Coatings On 304 Stainless Steel. Chem. Eng. Trans. 2021, 86, 1399–1404. [Google Scholar]
- Zanca, C.; Mendolia, I.; Capuana, E.; Blanda, G.; Carfì Pavia, F.; Brucato, V.; Ghersi, G.; la Carrubba, V.; Piazza, S.; Sunseri, C.; et al. Co-Deposition and Characterization of Hydroxyapatite-Chitosan and Hydroxyapatite-Polyvinylacetate Coatings on 304 SS for Biomedical Devices. KEM 2019, 813, 153–158. [Google Scholar] [CrossRef]
- International Centre for Diffraction Data. Power Diffraction File; International Centre for Diffraction Data: Newtown Square, PA, USA, 2007. [Google Scholar]
- Nobial, M.; Devos, O.; Mattos, O.R.; Tribollet, B. The nitrate reduction process: A way for increasing interfacial pH. J. Electroanal. Chem. 2007, 600, 87–94. [Google Scholar] [CrossRef]
- Dronova, M.; Lair, V.; Vermaut, P.; Ringuedé, A.; An, V. Study of ceria thin films prepared via electrochemical deposition: Role of selected electrochemical parameters on growth kinetics. Thin Solid Film. 2020, 693, 137674. [Google Scholar] [CrossRef]
- Therese, G.H.A.; Kamath, P.V. Electrochemical Synthesis of Metal Oxides and Hydroxides. Chem. Mater. 2000, 12, 1195–1204. [Google Scholar] [CrossRef]
- Indira, L.; Kamath, P.V. Electrogeneration of base by cathodic reduction of anions: Novel one-step route to unary and layered double hydroxides (LDHs). J. Mater. Chem. 1994, 4, 1487. [Google Scholar] [CrossRef]
- Patella, B.; Moukri, N.; Regalbuto, G.; Cipollina, C.; Pace, E.; Di Vincenzo, S.; Aiello, G.; O’Riordan, A.; Inguanta, R. Electrochemical Synthesis of Zinc Oxide Nanostructures on Flexible Substrate and Application as an Electrochemical Immunoglobulin-G Immunosensor. Materials 2022, 15, 713. [Google Scholar] [CrossRef]
- Lei, Y.; Song, B.; van der Weijden, R.D.; Saakes, M.; Buisman, C.J.N. Electrochemical Induced Calcium Phosphate Precipitation: Importance of Local pH. Environ. Sci. Technol. 2017, 51, 11156–11164. [Google Scholar] [CrossRef]
- Ling, T.; Lin, J.; Tu, J.; Liu, S.; Weng, W.; Cheng, K.; Wang, H.; Du, P.; Han, G. Mineralized collagen coatings formed by electrochemical deposition. J. Mater. Sci. Mater. Med. 2013, 24, 2709–2718. [Google Scholar] [CrossRef]
- Toworfe, G.K.; Composto, R.J.; Shapiro, I.M.; Ducheyne, P. Nucleation and growth of calcium phosphate on amine-, carboxyl- and hydroxyl-silane self-assembled monolayers. Biomaterials 2006, 27, 631–642. [Google Scholar] [CrossRef]
- Kikuchi, M.; Itoh, S.; Ichinose, S.; Shinomiya, K.; Tanaka, J. Self-organization mechanism in a bone-like hydroxyapatite/collagen nanocomposite synthesized in vitro and its biological reaction in vivo. Biomaterials 2001, 22, 1705–1711. [Google Scholar] [CrossRef]
- Wang, X.; Sang, L.; Luo, D.; Li, X. From collagen–chitosan blends to three-dimensional scaffolds: The influences of chitosan on collagen nanofibrillar structure and mechanical property. Colloids Surf. B Biointerfaces 2011, 82, 233–240. [Google Scholar] [CrossRef]
- Zhuang, J.; Lin, J.; Li, J.; Wang, H.; Cheng, K.; Weng, W. Electrochemical deposition of mineralized BSA/collagen coating. Mater. Sci. Eng. C 2016, 66, 66–76. [Google Scholar] [CrossRef]
- Nawrotek, K.; Grams, J. Understanding Electrodeposition of Chitosan–Hydroxyapatite Structures for Regeneration of Tubular-Shaped Tissues and Organs. Materials 2021, 14, 1288. [Google Scholar] [CrossRef] [PubMed]
- Witecka, A.; Valet, S.; Basista, M.; Boccaccini, A.R. Electrophoretically deposited high molecular weight chitosan/bioactive glass composite coatings on WE43 magnesium alloy. Surf. Coat. Technol. 2021, 418, 127232. [Google Scholar] [CrossRef]
- Gong, J.; Zhang, W.; Liu, T.; Zhang, L. Facile fabrication of chitosan–calcium carbonate nanowall arrays and their use as a sensitive non-enzymatic organophosphate pesticide sensor. Nanoscale 2011, 3, 3123. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Jia, W.-Z.; Qian, Q.-Y.; Zhou, Y.-G.; Xia, X.-H. Simple Approach for Efficient Encapsulation of Enzyme in Silica Matrix with Retained Bioactivity. Anal. Chem. 2009, 81, 3478–3484. [Google Scholar] [CrossRef]
- Mąkiewicz, M.; Wach, R.A.; Nawrotek, K. Investigation of Parameters Influencing Tubular-Shaped Chitosan-Hydroxyapatite Layer Electrodeposition. Molecules 2020, 26, 104. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aal, E.A.; Dietrich, D.; Steinhaeuser, S.; Wielage, B. Electrocrystallization of nanocrystallite calcium phosphate coatings on titanium substrate at different current densities. Surf. Coat. Technol. 2008, 202, 5895–5900. [Google Scholar] [CrossRef]
- Kumar, M.; Dasarathy, H.; Riley, C. Electrodeposition of brushite coatings and their transformation to hydroxyapatite in aqueous solutions. J. Biomed. Mater. Res. 1999, 45, 302–310. [Google Scholar] [CrossRef]
- Nur, A.; Setyawan, H.; Widjaja, A.; Lenggoro, I.W. Electrochemical Processes for the Formation of Hydroxyapatite Powders. Bull. Chem. React. Eng. Catal. 2014, 9, 168–174. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.; Leng, Y. Theoretical analysis of calcium phosphate precipitation in simulated body fluid. Biomaterials 2005, 26, 1097–1108. [Google Scholar] [CrossRef]
- Penel, G.; Leroy, N.; Van Landuyt, P.; Flautre, B.; Hardouin, P.; Lemaître, J.; Leroy, G. Raman microspectrometry studies of brushite cement: In vivo evolution in a sheep model. Bone 1999, 25, 81S–84S. [Google Scholar] [CrossRef]
- Koutsopoulos, S. Synthesis and characterization of hydroxyapatite crystals: A review study on the analytical methods. J. Biomed. Mater. Res. 2002, 62, 600–612. [Google Scholar] [CrossRef] [PubMed]
- Sauer, G.R.; Zunic, W.B.; Durig, J.R.; Wuthier, R.E. Fourier transform raman spectroscopy of synthetic and biological calcium phosphates. Calcif. Tissue Int. 1994, 54, 414–420. [Google Scholar] [CrossRef]
- Penel, G.; Leroy, G.; Rey, C.; Bres, E. MicroRaman Spectral Study of the PO4 and CO3 Vibrational Modes in Synthetic and Biological Apatites. Calcif. Tissue Int. 1998, 63, 475–481. [Google Scholar] [CrossRef]
- Baradaran, S.; Moghaddam, E.; Basirun, W.J.; Mehrali, M.; Sookhakian, M.; Hamdi, M.; Moghaddam, M.R.N.; Alias, Y. Mechanical properties and biomedical applications of a nanotube hydroxyapatite-reduced graphene oxide composite. Carbon 2014, 69, 32–45. [Google Scholar] [CrossRef]
- Zając, A.; Hanuza, J.; Wandas, M.; Dymińska, L. Determination of N-acetylation degree in chitosan using Raman spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 134, 114–120. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts, 3rd ed.; repr. as paperback; Wiley: Chichester, UK, 2010; ISBN 978-0-470-09307-8. [Google Scholar]
- Lin-Vien, D. (Ed.) The Handbook of Infrared and Raman Characteristic Frequencies of Organic Molecules; Academic Press: Boston, MA, USA, 1991; ISBN 978-0-12-451160-6. [Google Scholar]
- Gullekson, C.; Lucas, L.; Hewitt, K.; Kreplak, L. Surface-Sensitive Raman Spectroscopy of Collagen I Fibrils. Biophys. J. 2011, 100, 1837–1845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cárcamo, J.J.; Aliaga, A.E.; Clavijo, R.E.; Brañes, M.R.; Campos-Vallette, M.M. Raman study of the shockwave effect on collagens. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 86, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Purcell, F.J.; Bello, J.M. Fluorescence-Free Raman Spectra of Polymers; Adar, F., Griffiths, J.E., Eds.; SPIE: Bellingham, WA, USA, 1990; pp. 135–143. [Google Scholar]
- Cui, F.-Z.; Li, Y.; Ge, J. Self-assembly of mineralized collagen composites. Mater. Sci. Eng. R Rep. 2007, 57, 1–27. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, D.; Liu, S.; Fu, Y.; Kou, X.; Wang, X.; Sha, Y.; Gan, Y.; Zhou, Y. Effect of Nanostructure of Mineralized Collagen Scaffolds on Their Physical Properties and Osteogenic Potential. J. Biomed. Nanotechnol. 2014, 10, 1049–1060. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Cui, F.Z.; Zhang, W.; Feng, Q.L.; Zhu, X.D.; de Groot, K. Formation of calcium phosphate/collagen composites through mineralization of collagen matrix. J. Biomed. Mater. Res. 2000, 50, 518–527. [Google Scholar] [CrossRef]
- Vigneshwari, S.; Gokula, V. Extraction and FTIR characterization of chitosan from Portunus pelagicus shell wastes. Int. J. Adv. Sci. Res. Manag. 2018, 3, 91–95. [Google Scholar] [CrossRef]
- Belbachir, K.; Noreen, R.; Gouspillou, G.; Petibois, C. Collagen types analysis and differentiation by FTIR spectroscopy. Anal. Bioanal. Chem. 2009, 395, 829–837. [Google Scholar] [CrossRef]
- Gheisari, H.; Karamian, E.; Abdellahi, M. A novel hydroxyapatite –Hardystonite nanocomposite ceramic. Ceram. Int. 2015, 41, 5967–5975. [Google Scholar] [CrossRef]
- Binitha, M.P.; Pradyumnan, P.P. Dielectric Property Studies of Biologically Compatible Brushite Single Crystals Used as Bone Graft Substitute. J. Biomater. Nanobiotechnology 2013, 4, 119–122. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Singh, V.; Aggarwal, S.; Mandal, U. Synthesis of brushite nanoparticles at different temperatures. Chem. Pap. 2010, 64, 491–498. [Google Scholar] [CrossRef]
- Lee, D.; Kumta, P.N. Chemical synthesis and stabilization of magnesium substituted brushite. Mater. Sci. Eng. C 2010, 30, 934–943. [Google Scholar] [CrossRef]
- Ślósarczyk, A.; Paszkiewicz, Z.; Paluszkiewicz, C. FTIR and XRD evaluation of carbonated hydroxyapatite powders synthesized by wet methods. J. Mol. Struct. 2005, 744–747, 657–661. [Google Scholar] [CrossRef]
- Paul, S.; Mandal, C. Biocompatibly Coated 304 Stainless Steel as Superior Corrosion-Resistant Implant Material to 316L Steel. J. Mater. Eng. Perform. 2013, 22, 3147–3154. [Google Scholar] [CrossRef]
- Shibata, H.; Yokoi, T.; Goto, T.; Kim, I.Y.; Kawashita, M.; Kikuta, K.; Ohtsuki, C. Behavior of hydroxyapatite crystals in a simulated body fluid: Effects of crystal face. J. Ceram. Soc. Jpn. 2013, 121, 807–812. [Google Scholar] [CrossRef]
- Thanh, D.T.M.; Nam, P.T.; Phuong, N.T.; Que, L.X.; Anh, N.V.; Hoang, T.; Lam, T.D. Controlling the electrodeposition, morphology and structure of hydroxyapatite coating on 316L stainless steel. Mater. Sci. Eng. C 2013, 33, 2037–2045. [Google Scholar] [CrossRef]
- Orazem, M.E.; Tribollet, B. Electrochemical Impedance Spectroscopy; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017; ISBN 978-1-119-36368-2. [Google Scholar]
- Jüttner, K. Electrochemical impedance spectroscopy (EIS) of corrosion processes on inhomogeneous surfaces. Electrochim. Acta 1990, 35, 1501–1508. [Google Scholar] [CrossRef]
- Bockelmann, M.; Becker, M.; Reining, L.; Kunz, U.; Turek, T. Passivation of Zinc Anodes in Alkaline Electrolyte: Part, I. Determination of the Starting Point of Passive Film Formation. J. Electrochem. Soc. 2018, 165, A3048–A3055. [Google Scholar] [CrossRef]
- Leikin, J.B.; Paloucek, F.P. (Eds.) Poisoning and Toxicology Handbook, 4th ed.; CRC Press/Taylor & Francis Group: Boca Raton, FL, USA, 2008; ISBN 978-1-4200-4479-9. [Google Scholar]
- ISO 10993-5; Biological Evaluation of Medical Devices–Part 5: Tests for In Vitro Cytotoxicity. ISO: Geneva, Switzerland, 2009.
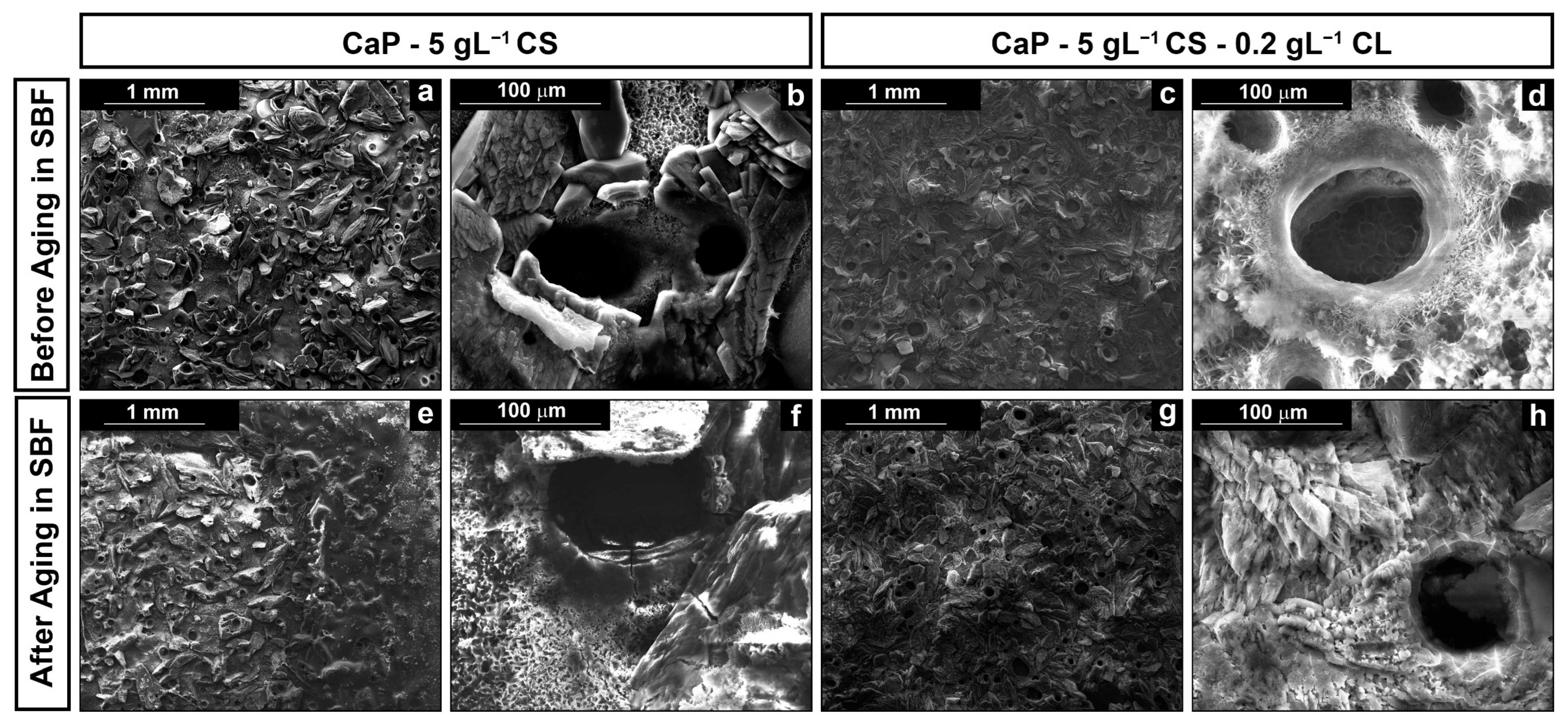
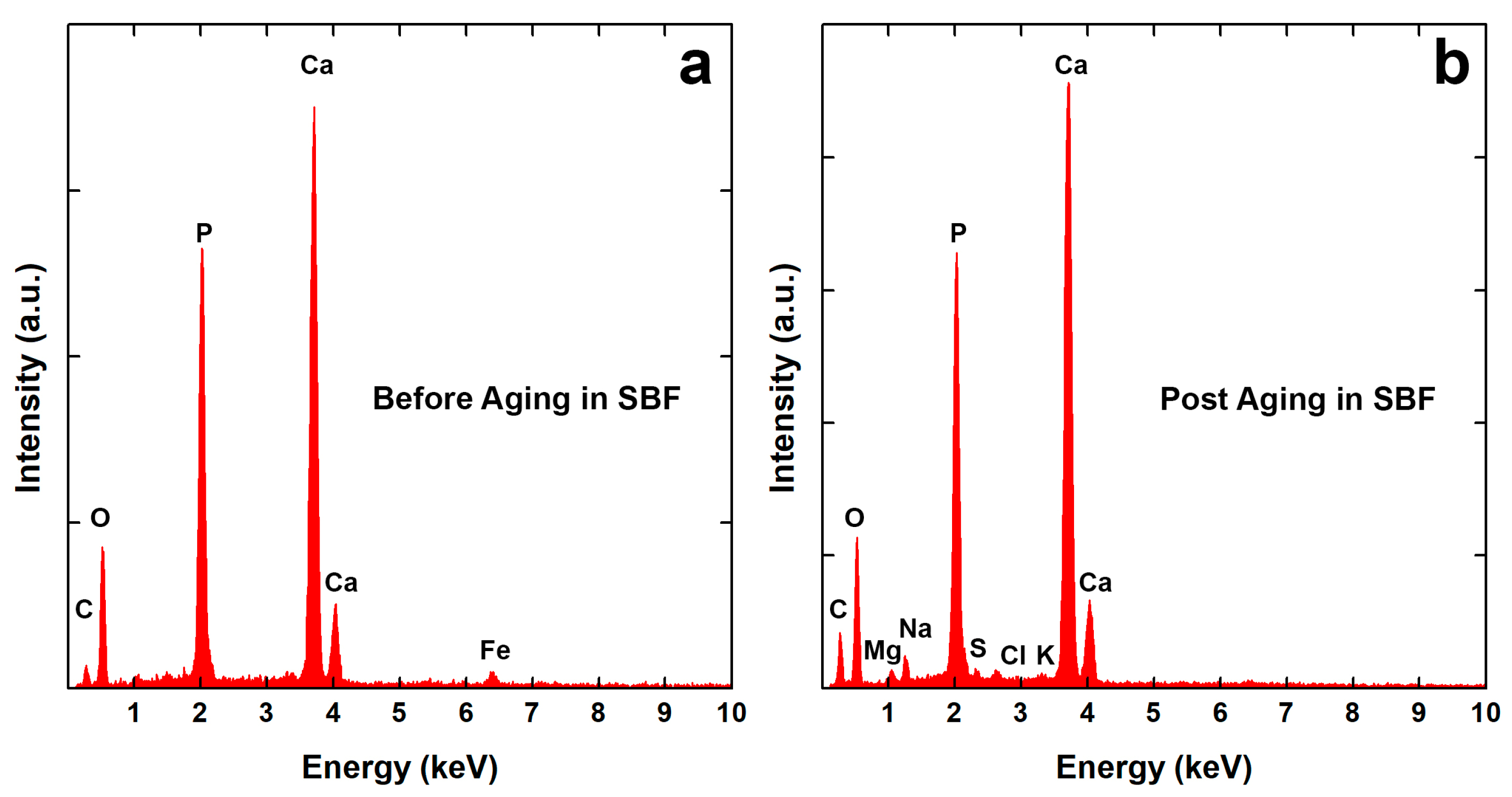
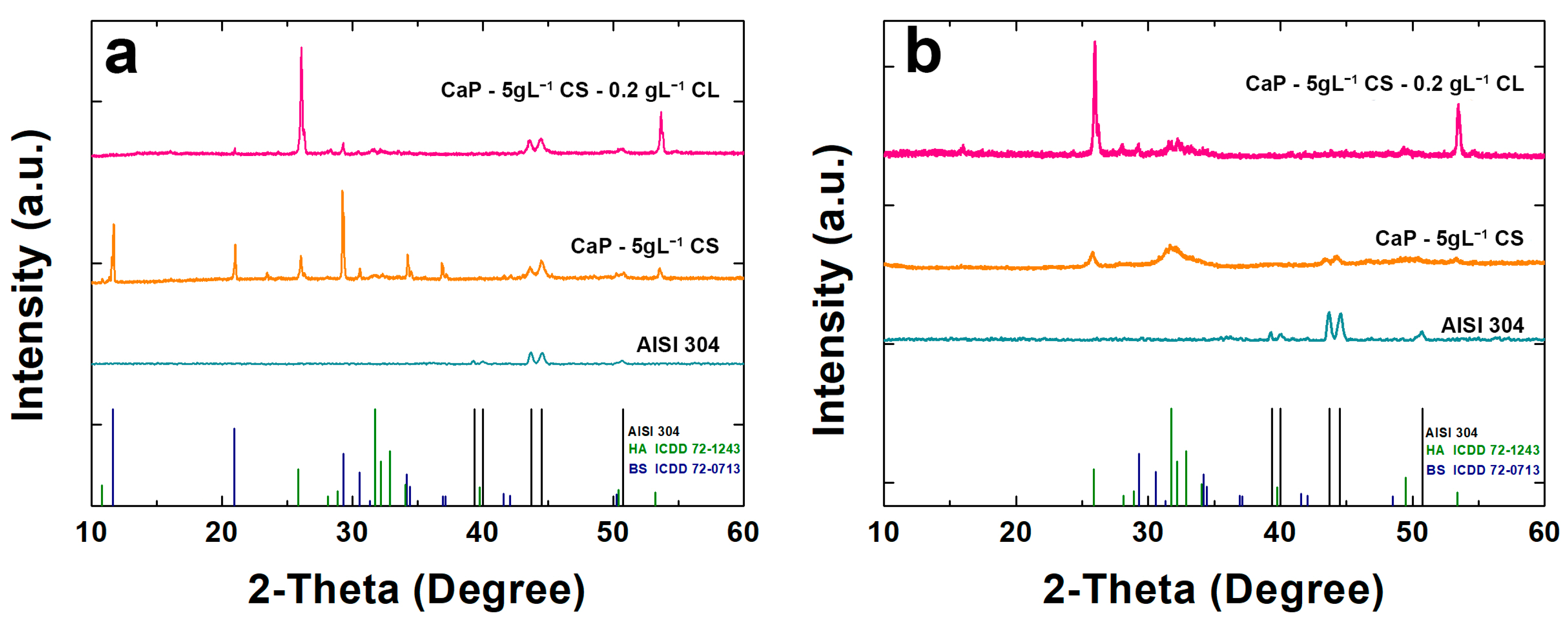
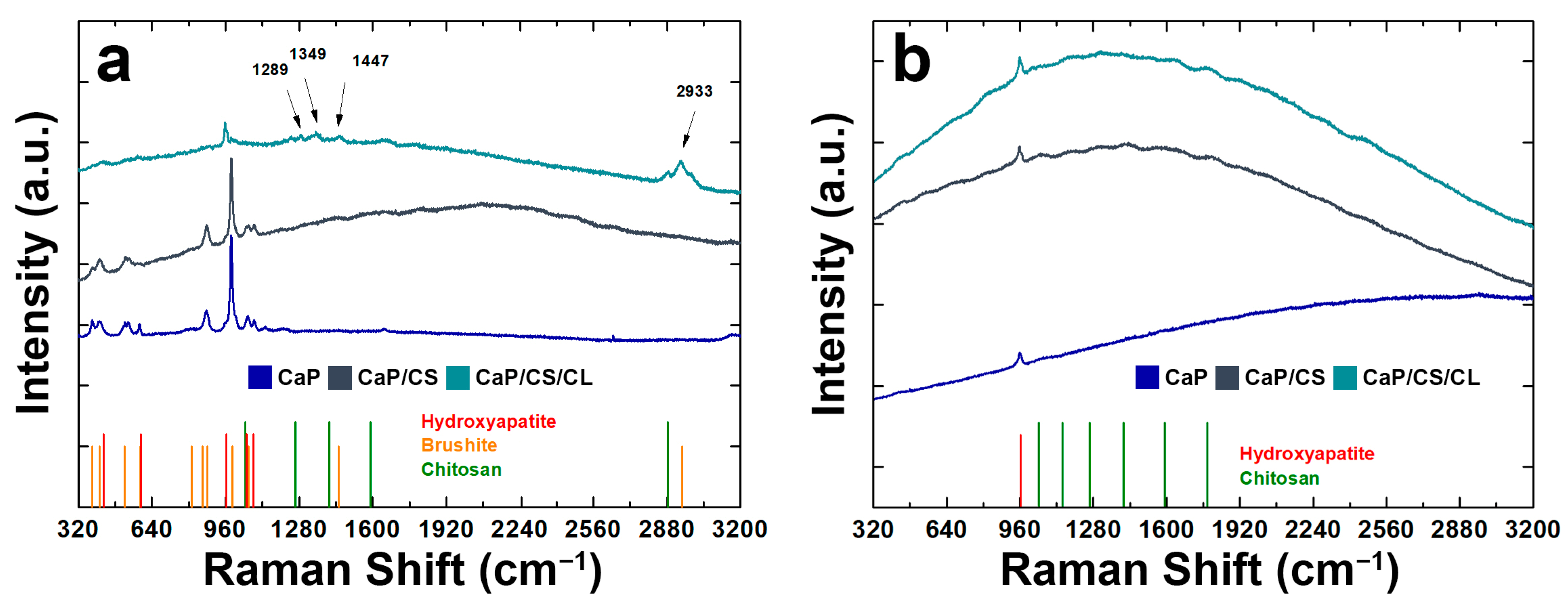


| Before Aging | Post Aging | |||
|---|---|---|---|---|
| Ca/P | Ca/Fe | Ca/P | Ca/Fe | |
| CaP—5 gL−1 CS | 1.25 | 11.7 | 1.83 | no Fe |
| CaP—5 gL−1 CS—0.2 gL−1 CL | 1.29 | 35.97 | 1.81 | no Fe |
| Time (Day) | ||||||
|---|---|---|---|---|---|---|
| 0 | 1 | 7 | 14 | 21 | AISI 304 | |
| CaP—5gL−1 CS—0.2 gL−1 CL | ||||||
| Ecorr (mV) | 24 | 43 | 85 | 92 | 71 | −183 |
| icorr (Acm−2) | 2.75 × 10−7 | 2.12 × 10−7 | 2.45 × 10−7 | 2.15 × 10−7 | 2.01 × 10−7 | 8.92 × 10−7 |
| CaP—5gL−1 CS | ||||||
| Ecorr (mV) | −147 | −67 | −24 | −38 | 68 | −183 |
| icorr (Acm−2) | 4.86 × 10−7 | 2.35 × 10−7 | 2.13 × 10−7 | 2.09 × 10−7 | 2.12 × 10−7 | 8.92 × 10−7 |
| Concentration (ppm) | |||||
|---|---|---|---|---|---|
| Fe | Cr | Ni | Ca | P | |
| SBF (measured) | 0 | 0 | 0 | 103.75 | 31.74 |
| SBF (calculated) | 0 | 0 | 0 | 105 | 31 |
| AISI 304 | 0.037 | 0 | 0 | 82.22 | 25.44 |
| CaP—5 gL−1 CS | 0.001 | 0 | 0 | 2 | 110 |
| CaP—5 gL−1 CS—0.2 gL−1 CL | 0.001 | 0 | 0 | 4 | 117 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zanca, C.; Patella, B.; Capuana, E.; Lopresti, F.; Brucato, V.; Carfì Pavia, F.; La Carrubba, V.; Inguanta, R. Behavior of Calcium Phosphate–Chitosan–Collagen Composite Coating on AISI 304 for Orthopedic Applications. Polymers 2022, 14, 5108. https://doi.org/10.3390/polym14235108
Zanca C, Patella B, Capuana E, Lopresti F, Brucato V, Carfì Pavia F, La Carrubba V, Inguanta R. Behavior of Calcium Phosphate–Chitosan–Collagen Composite Coating on AISI 304 for Orthopedic Applications. Polymers. 2022; 14(23):5108. https://doi.org/10.3390/polym14235108
Chicago/Turabian StyleZanca, Claudio, Bernardo Patella, Elisa Capuana, Francesco Lopresti, Valerio Brucato, Francesco Carfì Pavia, Vincenzo La Carrubba, and Rosalinda Inguanta. 2022. "Behavior of Calcium Phosphate–Chitosan–Collagen Composite Coating on AISI 304 for Orthopedic Applications" Polymers 14, no. 23: 5108. https://doi.org/10.3390/polym14235108






