The Improvement of Kaolinite Supported Cerium Oxide for Styrene–Butadiene Rubber Composite: Mechanical, Ageing Properties and Mechanism
Abstract
:1. Introduction
2. Experimental
2.1. Material
2.2. Preparation of CeOx/Kaol Material and CeOx/Kaol/SBR Composites
2.3. Characterization
3. Results and Discussion
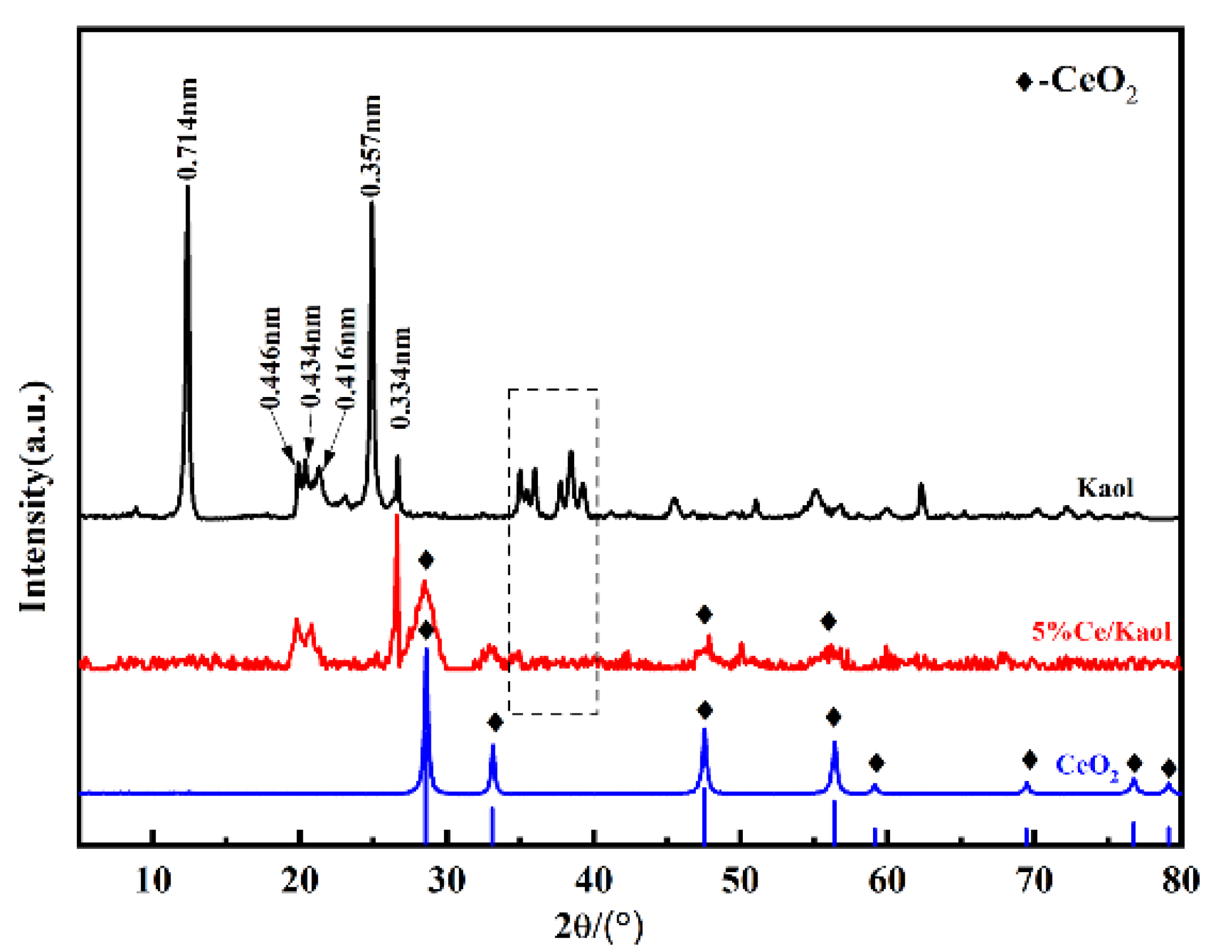
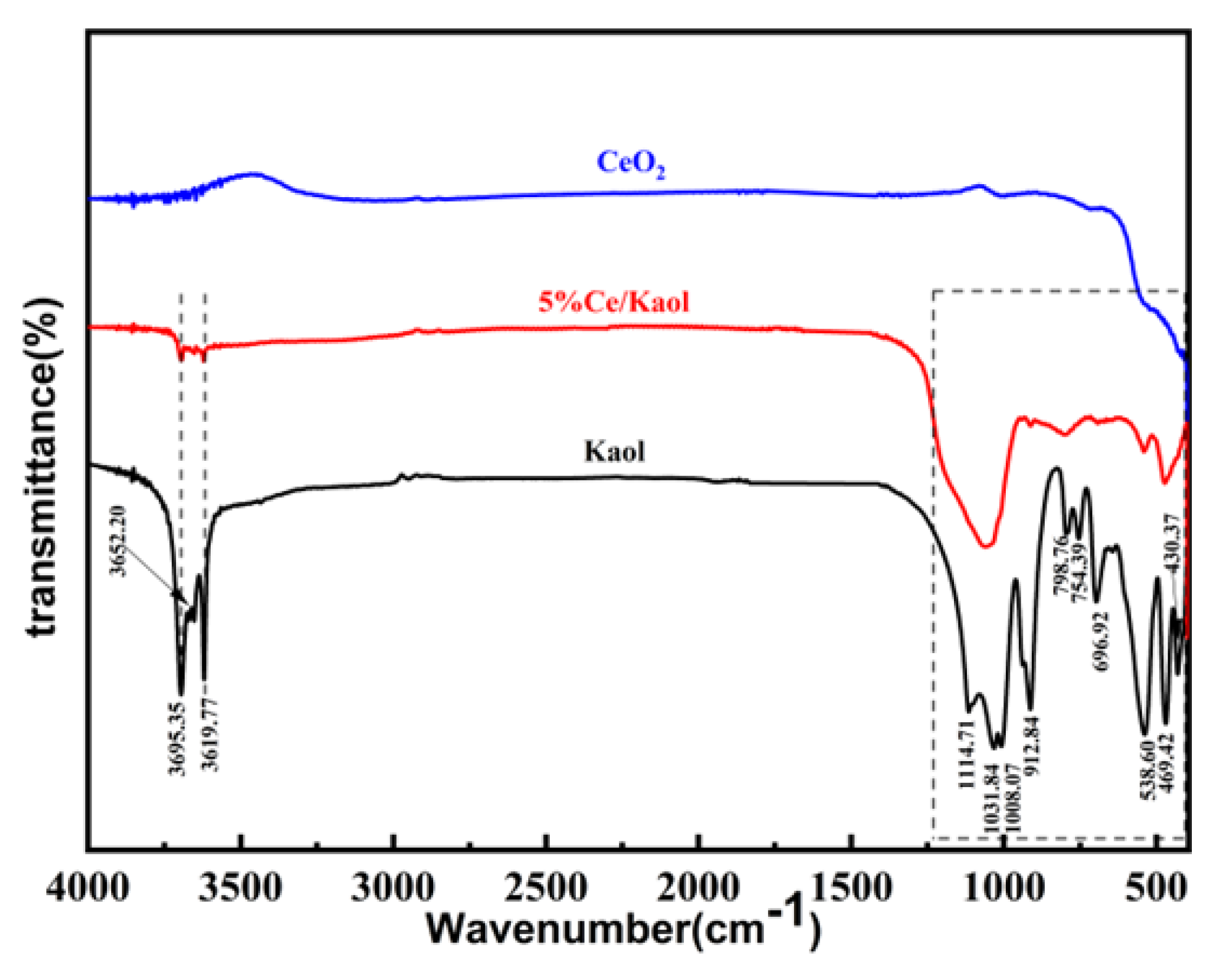
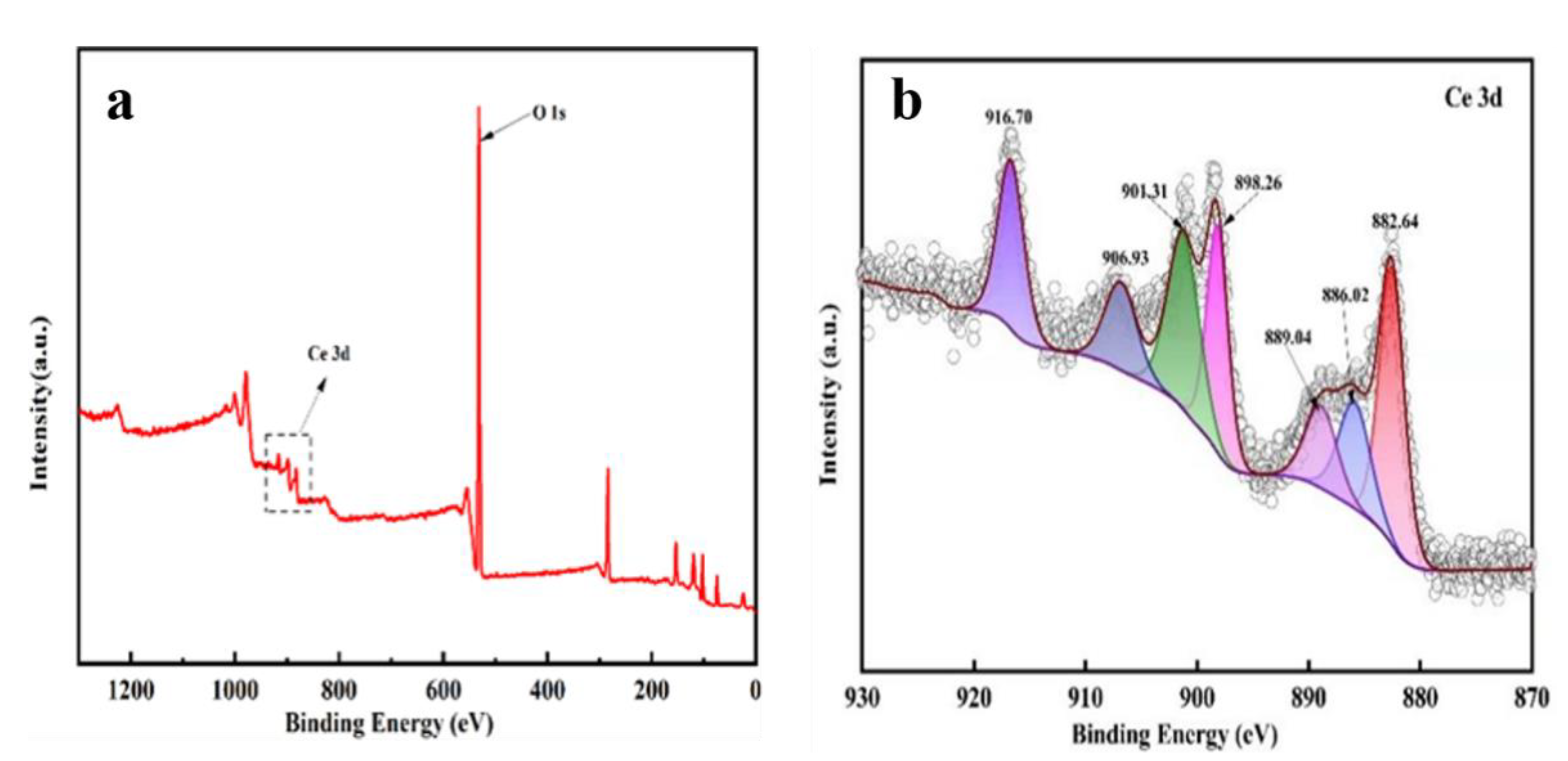
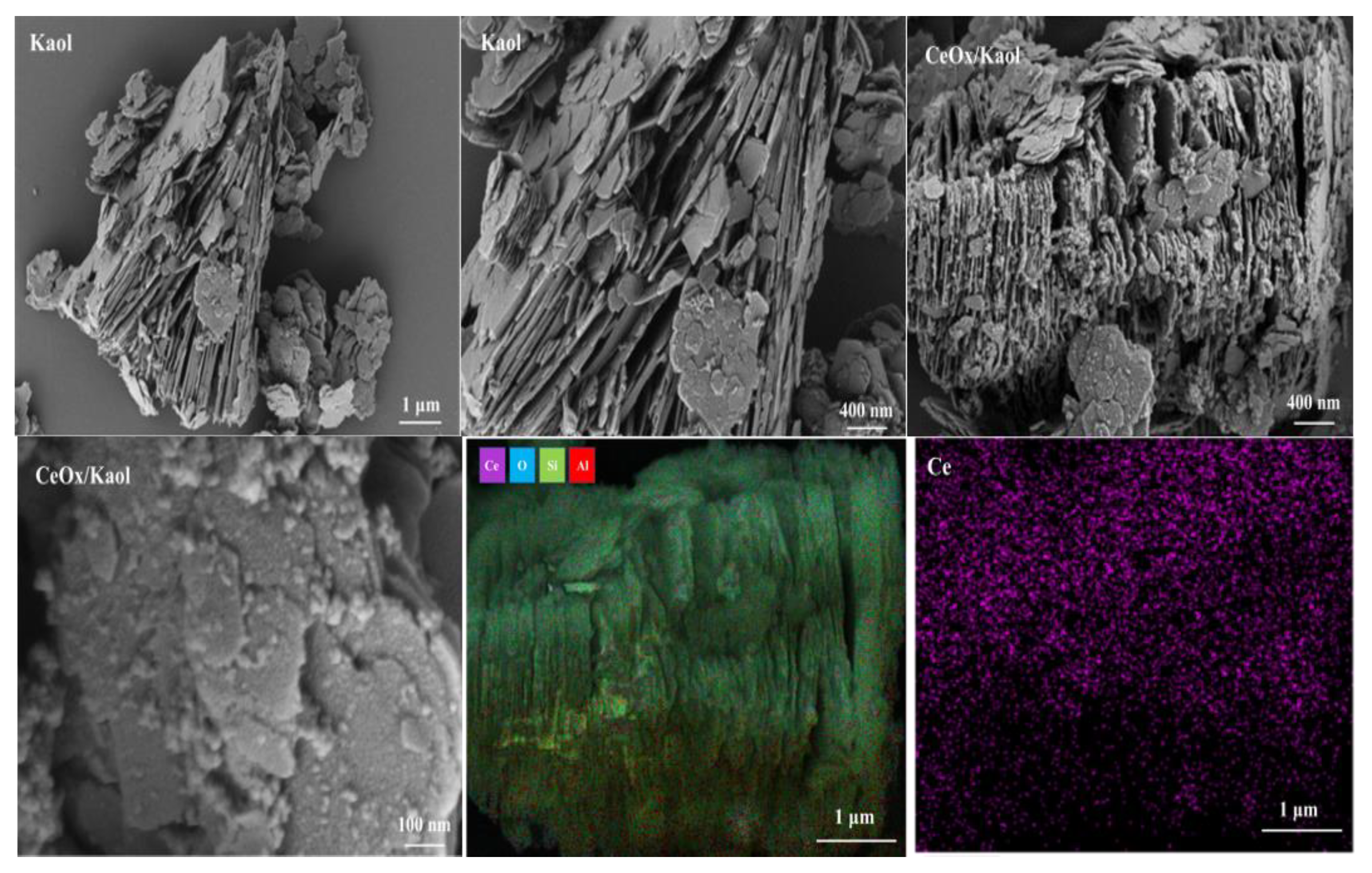
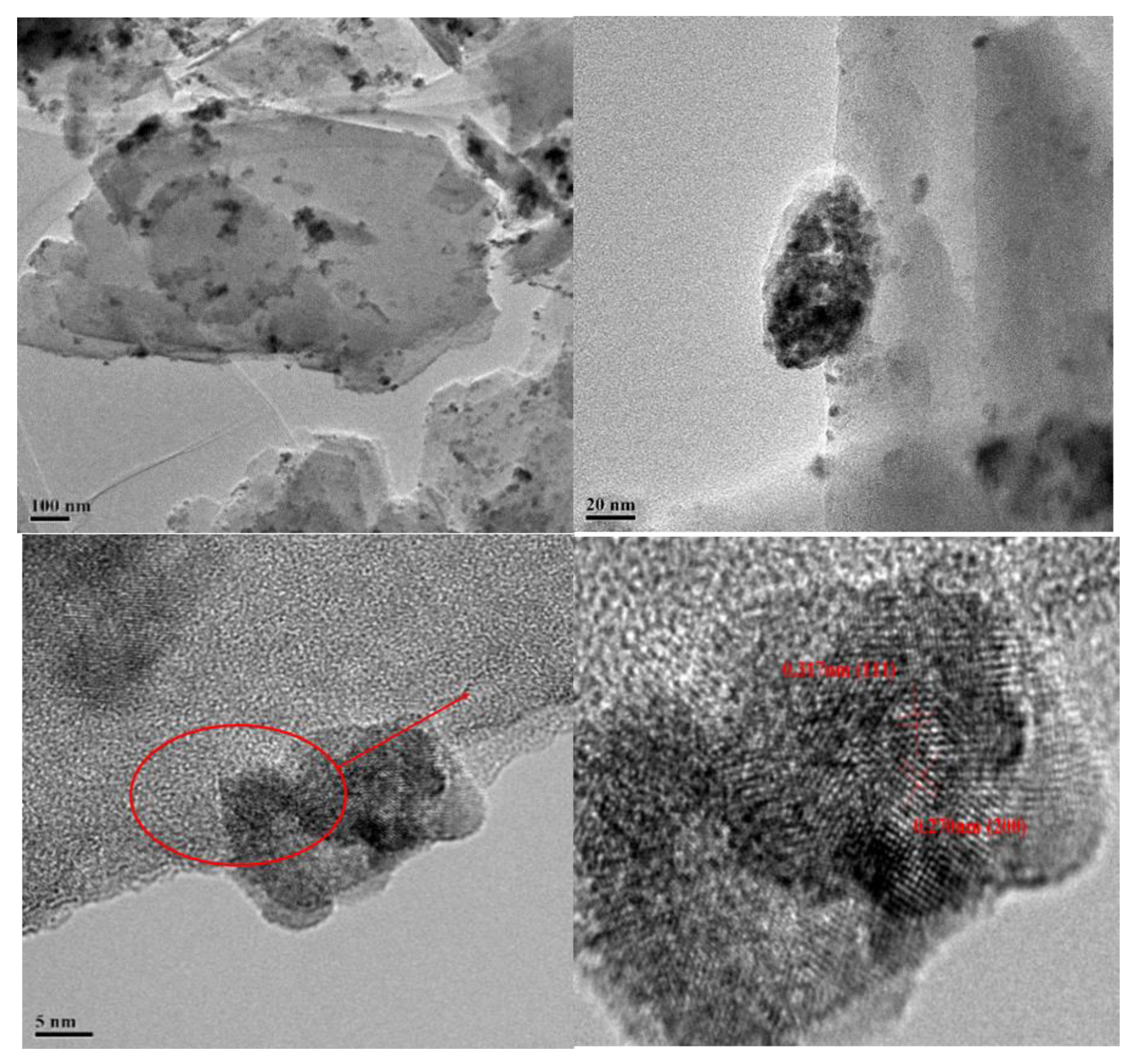
3.1. The Characterization of the Prepared CeOx/Kaol Materials
3.2. Characterization of the Prepared CeOx/Kaol Filled SBR Composites
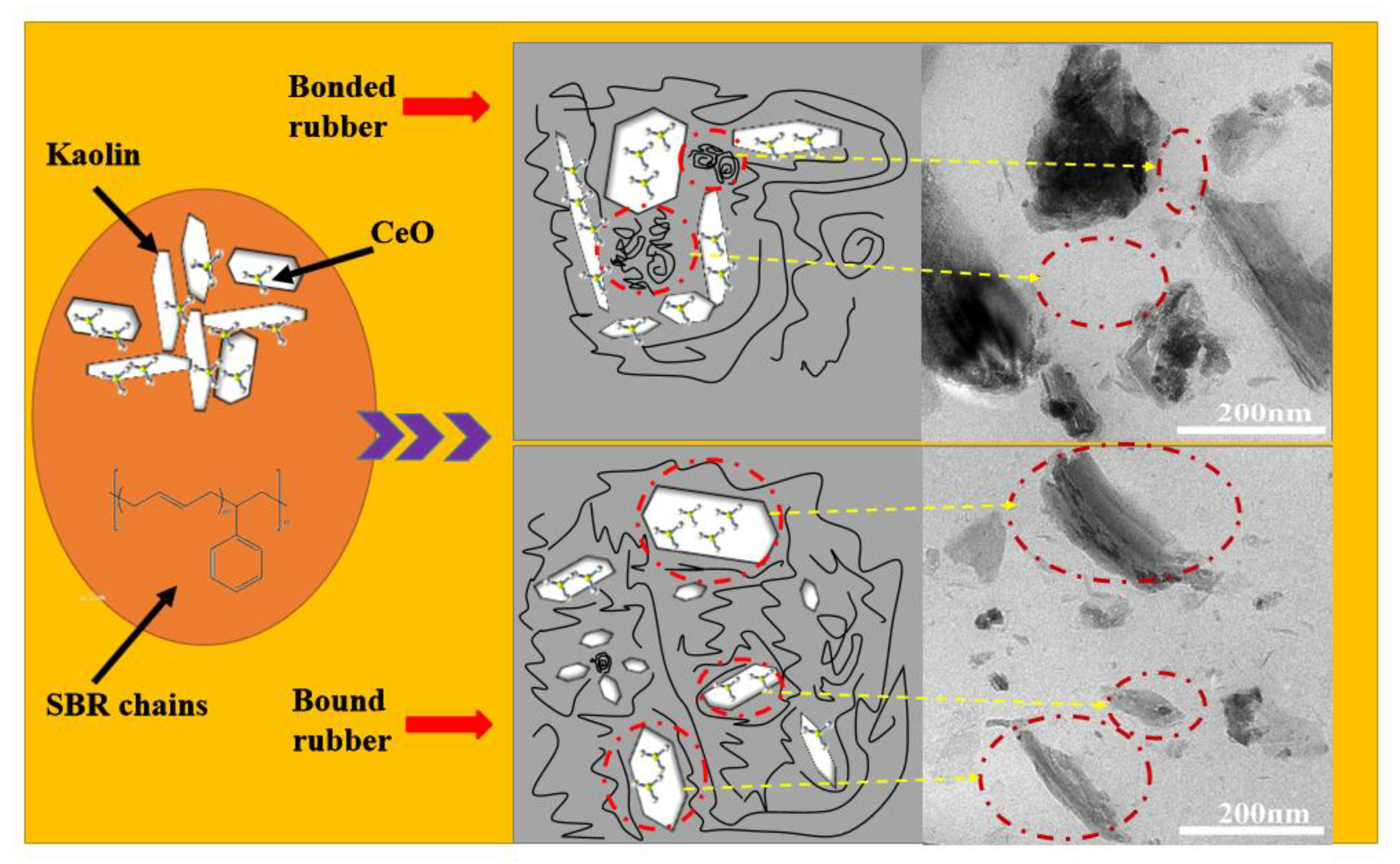
3.2.1. The Microstructure of the Prepared CeOx/Kaol Filled SBR Composites
3.2.2. The Processing Properties of the Prepared CeOx/Kaol Filled SBR Composites
3.2.3. The Mechanical Properties of the Prepared CeOx/Kaol Filled SBR Composites
3.2.4. The Ageing Behaviors of the Resulting SBR Composites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, S. Kaolin/Rubber Nanocomposites Prepared by Co-Coagulating of Rubber Latex and Kaolin Suspensions; School of Geoscience and Surveying Engineering, China University of Mining & Technology: Beijing, China, 2018. [Google Scholar]
- Zhang, A.; Zhang, Y.; Zhang, Y. Characterization of kaolinite/emulsion-polymerization styrene butadiene rubber (ESBR) nanocomposite prepared by latex blending method: Dynamic mechanic properties and mechanism. Polym. Test. 2020, 89, 106600. [Google Scholar] [CrossRef]
- Senetakis, K.; Anastasiadis, A.; Pitilakis, K. Dynamic properties of dry sand/rubber (SRM) and gravel/rubber (GRM) mixtures in a wide range of shearing strain amplitudes. Soil Dyn. Earthq. Eng. 2012, 33, 38–53. [Google Scholar] [CrossRef]
- Pimolsiriphol, V.; Saeoui, P.; Sirisinha, C. Relationship among thermal ageing degradation, dynamic properties, cure systems, and antioxidants in natural rubber vulcanisates. Polym-Plast. Technol. 2007, 46, 113–121. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Q.; Liu, Q.; Cheng, H.; Frost, R.L. Thermal stability of styrene butadiene rubber (SBR) composites filled with kaolinite/silica hybrid filler. J. Therm. Anal. Calorim. 2013, 115, 1013–1020. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Q.; Zhang, S.; Zhang, Y.; Cheng, H. Gas barrier properties and mechanism of kaolin/styrene–butadiene rubber nanocomposites. Appl. Clay Sci. 2015, 111, 37–43. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Q.; Xiang, J.; Frost, R.L. Thermal stability and decomposition kinetics of styrene-butadiene rubber nanocomposites filled with different particle sized kaolinites. Appl. Clay Sci. 2014, 95, 159–166. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Liu, Q.; Zhang, S.; Zhang, Y.; Zhang, Y.; Liang, P. Characterization of kaolinite/styrene butadiene rubber composite: Mechanical properties and thermal stability. Appl. Clay Sci. 2016, 124–125, 167–174. [Google Scholar] [CrossRef]
- Wu, Y.; Wen, S.; Shen, J.; Jiang, J.; Hu, S.; Zhang, L.; Liu, L. Improved dynamic properties of natural rubber filled with irradiation-modified carbon black. Radiat. Phys. Chem. 2015, 111, 91–97. [Google Scholar] [CrossRef]
- Zhong, B.C.; Shi, Q.F.; Jia, Z.X.; Luo, Y.F.; Chen, Y.J.; Jia, D.M. Preparation of silica-supported 2-mercaptobenzimidazole and its antioxidative behavior in styrene-butadiene rubber. Polym. Degrad. Stab. 2014, 110, 260–267. [Google Scholar] [CrossRef]
- Shimizu, T.; Kishi, R.; Kobashi, K.; Morimoto, T.; Okazaki, T.; Yamada, T.; Hata, K. Improved thermal stability of silicone rubber nanocomposites with low filler content, achieved by well-dispersed carbon nanotubes. Compos. Commun. 2020, 22, 100482. [Google Scholar] [CrossRef]
- Sabbagh, F.; Khatir, N.M.; Karim, A.K.; Amineh, O.; Zahra, N.; Reza, J. Mechanical properties and swelling behavior of acrylamide hydrogels using montmorillonite and kaolinite as clays. J. Environ. Treat. Tech. 2019, 7, 211–219. [Google Scholar]
- Zhong, B.C.; Jia, Z.X.; Luo, Y.F.; Guo, B.C.; Jia, D.M. Preparation of halloysite nanotubes supported 2-mercaptobenzimidazole and its application in natural rubber. Compos. Part A-Appl. S 2015, 73, 63–71. [Google Scholar] [CrossRef]
- Choudalakis, G.; Gotsis, A.D. Permeability of polymer/clay nanocomposites: A review. Eur. Polym. J. 2009, 45, 967–984. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Q.; Yang, Y.; Wang, D.; He, J.; Sun, L. Preparation, morphology, and structure of kaolinites with various aspect ratios. Appl. Clay Sci. 2017, 147, 117–122. [Google Scholar] [CrossRef]
- Sadri, S.; Johnson, B.B.; Ruyter-Hooley, M.; Angove, M.J. The adsorption of nortriptyline on montmorillonite, kaolinite and gibbsite. Appl. Clay Sci. 2018, 165, 64–70. [Google Scholar] [CrossRef]
- Liu, Q.; Zuo, X.; Zhang, S.; Zhang, S.; Ji, J. Synthesis and Possible Modeling of Kaolinite-Stearic Acid Intercalation Compound. Chin. J. Inorg. Chem. 2015, 31, 7–14. [Google Scholar]
- Cheng, H.; Hou, X.; Liu, Q.; Li, X.; Frost, R.L. New insights into the molecular structure of kaolinite–methanol intercalation complexes. Appl. Clay Sci. 2015, 109–110, 55–63. [Google Scholar] [CrossRef] [Green Version]
- Sabbagh, F.; Kim, B.S. Microneedles for transdermal drug delivery using clay-based composites. Expert Opin. Drug Deliv. 2022, 19, 1099–1113. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.B.; Gadi, R.; Kalra, S. Synthesis and characterization of novel nanocomposite by using kaolinite and carbon nanotubes. Appl. Clay Sci. 2018, 155, 30–36. [Google Scholar] [CrossRef]
- Villaluenga, J.P.G.; Khayet, M.; López-Manchado, M.A.; Valentin, J.L.; Seoane, B.; Mengual, J.I. Gas transport properties of polypropylene/clay composite membranes. Eur. Polym. J. 2007, 43, 1132–1143. [Google Scholar] [CrossRef]
- Lin, Y.L.; Zhang, A.Q.; Wang, L.S. Rare earth compounds modified carbon black filled powdered natural rubber: Preparation, morphology and properties. J. Appl. Polym. Sci. 2008, 108, 1393–1401. [Google Scholar] [CrossRef]
- Zhang, Q.J.; Wei, F.X.; Zhang, W.G.; Huang, M.Y.; Luo, Y.F. Synthesis, characterization and vulcanizing properties of rare earth complexes with 2-mercaptobenthiazole. J. Rare Earths 2002, 20, 395–399. [Google Scholar]
- Chan, X.; Zhixin, J.; Yuanfang, L.; Demin, J. Antioxidant Effect of SM(Ⅲ) Complex with 2-Mercaptobenzimidazole in Natural Rubber Vulcanizates. Acta Polym. Sin. 2011, 3, 320–326. [Google Scholar]
- Xie, C.; Jia, Z.X.; Jia, D.M.; Luo, Y.F.; You, C.J. The Effect of Dy(III) Complex with 2-Mercaptobenzimidazole on the Thermo-Oxidation Aging Behavior of Natural Rubber Vulcanizates. Int. J. Polym. Mater. 2010, 59, 663–679. [Google Scholar] [CrossRef]
- Yaling, L.; Shaoying, H.; Lianshi, W.; Yiyu, Z.; Anqiang, Z. Structure and properties of rare earth oxide and transition metal oxide modified nano calcium carbonate/ natural rubber composites. China Synth. Rubber Ind. 2004, 27, 363–367. [Google Scholar]
- Xu, S.J.; Ren, W.T.; Zhang, Y.; Zhang, Y.X. Study on the preparation of Eu(Pht)3Phen/SBA-15 hybrids and photoluminescence properties of silicone rubber composites with the hybrids. J. Appl. Polym. Sci. 2013, 128, 2375–2384. [Google Scholar] [CrossRef]
- Wen, S.P.; Hu, S.; Zhang, X.P.; Zhang, L.Q.; Liu, L. Physical dispersion state and fluorescent property of Eu-complex in the Eu-complex/silicon rubber composites. J. Rare Earths 2008, 26, 626–632. [Google Scholar] [CrossRef]
- Cheng, H.; Zhang, S.; Liu, Q.; Li, X.; Frost, R.L. The molecular structure of kaolinite–potassium acetate intercalation complexes: A combined experimental and molecular dynamic simulation study. Appl. Clay Sci. 2015, 116–117, 273–280. [Google Scholar] [CrossRef]
- Lin, M.; Liu, Y.-Y.; Lei, S.-M.; Ye, Z.; Pei, Z.-Y.; Li, B. High-efficiency extraction of Al from coal-series kaolinite and its kinetics by calcination and pressure acid leaching. Appl. Clay Sci. 2018, 161, 215–224. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Q.; Wu, Z.; Zheng, Q.; Cheng, H. Thermal behavior analysis of kaolinite–dimethylsulfoxide intercalation complex. J. Therm. Anal. Calorim. 2011, 110, 1167–1172. [Google Scholar] [CrossRef]
- Guo, Y.B.; Wang, L.S.; Zhang, A.Q. Mechanical properties and crosslink density of rare earth-modified high-abrasion furnace-filled powdered natural rubber. J. Appl. Polym. Sci. 2006, 102, 1755–1762. [Google Scholar] [CrossRef]
- Sánchez-Soto, P.; Haro, M.C.J.; Pérez-Maqueda, L.; Varona, I.; Pérez-Rodríguez, J. Effects of dry grinding on the structural changes of kaolinite powders. J. Am. Ceram. Soc. 2000, 83, 1649–1657. [Google Scholar] [CrossRef]
- Franco, F.; Cecila, J.A.; Pérez-Maqueda, L.A.; Pérez-Rodríguez, J.L.; Gomes, C.S.F. Particle-size reduction of dickite by ultrasound treatments: Effect on the structure, shape and particle-size distribution. Appl. Clay Sci. 2007, 35, 119–127. [Google Scholar] [CrossRef]
- Franco, F.; Pérez-Maqueda, L.A.; Pérez-Rodrıguez, J.L. The influence of ultrasound on the thermal behaviour of a well ordered kaolinite. Thermochim. Acta 2003, 404, 71–79. [Google Scholar] [CrossRef]
- Cheng, H.; Liu, Q.; Yang, J.; Zhang, J.; Frost, R.L. Thermal analysis and infrared emission spectroscopic study of halloysite–potassium acetate intercalation compound. Thermochim. Acta 2010, 511, 124–128. [Google Scholar] [CrossRef]
- Cheng, H.; Liu, Q.; Yang, J.; Zhang, J.; Frost, R.L.; Du, X. Infrared spectroscopic study of halloysite-potassium acetate intercalation complex. J. Mol. Struct. 2011, 990, 21–25. [Google Scholar] [CrossRef] [Green Version]
- Frost, R.L.; van der Gaast, S.J.; Zbik, M.; Kloprogge, J.T.; Paroz, G.N. Birdwood kaolinite: A highly ordered kaolinite that is difficult to intercalate—An XRD, SEM and Raman spectroscopic study. Appl. Clay Sci. 2002, 20, 177–187. [Google Scholar] [CrossRef] [Green Version]
- Frost, R.L.; Horváth, E.; Makó, É.; Kristóf, J.; Cseh, T. The effect of mechanochemical activation upon the intercalation of a high-defect kaolinite with formamide. J. Colloid Interface Sci. 2003, 265, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, Q.; Gao, F.; Ma, R.; Wu, Z.; Teppen, B.J. Interfacial Structure and Interaction of Kaolinite Intercalated with N-methylformamide Insight from Molecular Dynamics Modeling. Appl. Clay Sci. 2018, 158, 204–210. [Google Scholar] [CrossRef]
- Zhang, F.; Liao, L.; Wang, Y.; Wang, Y.; Huang, H.; Li, P.; Peng, Z.; Zeng, R. Reinforcement of natural rubber latex with silica modified by cerium oxide: Preparation and properties. J. Rare Earths 2016, 34, 221–226. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Q.; Sun, J.; Zhang, Y.; Zhang, S. Properties and Reinforcement Mechanism of Styrene Butadiene rubber Composites Filled with Modified Kaolin. Polym. Mater. Sci. Eng. 2014, 30, 74–79. [Google Scholar]
- Rezig, N.; Bellahcene, T.; Aberkane, M.; Abdelaziz, M.N. Thermo-oxidative ageing of a SBR rubber: Effects on mechanical and chemical properties. J. Polym. Res. 2020, 27, 1–13. [Google Scholar] [CrossRef]
- Ooi, Z.X.; Ismail, H.; Bakar, A.A. Thermal properties and aging characteristics of chemically modified oil palm ash-filled natural rubber composites. Iran Polym. J. 2014, 23, 723–730. [Google Scholar] [CrossRef]
- Gu, H.S.; Itoh, Y. Ageing Behaviour of Natural Rubber and High Damping Rubber Materials Used in Bridge Rubber Bearings. Adv. Struct. Eng. 2010, 13, 1105–1113. [Google Scholar] [CrossRef]
- Chen, M.; Ao, N.-J.; Liao, Y.-Y.; Chen, Y.; Zhou, H.-L. Thermooxidative degradation of natural rubber/clay composite. J. Appl. Polym. Sci. 2006, 100, 3809–3815. [Google Scholar] [CrossRef]
- Salinas, D.; Escalona, N.; Pecchi, G.; Fierro, J.L.G. Lanthanum oxide behavior in La2O3-Al2O3 and La2O3-ZrO2 catalysts with application in FAME production. Fuel 2019, 253, 400–408. [Google Scholar] [CrossRef]



| Ingredient | E-SBR | Ce/Kaol | ZnO | SA | NS | Sulfur |
|---|---|---|---|---|---|---|
| Content/phr | 100.00 | Variable | 3.00 | 1.00 | 1.00 | 1.75 |
| CeOx (%) | MH /(dN·m) | ML /(dN·m) | ΔM /(dN·m) | t10 /(min) | t90 /(min) |
|---|---|---|---|---|---|
| Pure SBR | 3.30 | 0.40 | 2.90 | 3.4 ± 0.6 | 14.5 ± 0.7 |
| 0 | 6.70 | 0.90 | 5.80 | 3.5 ± 0.4 | 17.3 ± 0.9 |
| 1 | 6.40 | 1.00 | 5.40 | 5.0 ± 0.7 | 19.2 ± 0.6 |
| 2 | 6.70 | 1.10 | 5.60 | 5.4 ± 0.6 | 21.4 ± 0.9 |
| 3 | 6.40 | 1.10 | 5.30 | 5.3 ± 0.9 | 21.3 ± 0.6 |
| 4 | 7.10 | 1.20 | 5.90 | 5.4 ± 0.9 | 19.0 ± 0.7 |
| 5 | 5.90 | 1.00 | 4.90 | 6.0 ± 0.5 | 19.4 ± 0.3 |
| 6 | 6.00 | 1.30 | 4.70 | 6.0 ± 0.7 | 19.1 ± 0.8 |
| 7 | 5.90 | 1.20 | 4.70 | 6.4 ± 0.6 | 19.1 ± 0.9 |
| 8 | 6.00 | 1.40 | 4.60 | 6.3 ± 0.7 | 20.3 ± 0.3 |
| CeOx /Kaol (phr) | MH /(dN·m) | ML /(dN·m) | ΔM /(dN·m) | t10 /(min) | t90 /(min) |
|---|---|---|---|---|---|
| Pure | 3.30 | 0.40 | 2.90 | 3.4 ± 0.6 | 14.5 ± 0.2 |
| 10 | 4.30 | 0.60 | 3.70 | 2.5 ± 0.8 | 15.0 ± 0.9 |
| 20 | 5.90 | 0.90 | 5.00 | 2.5 ± 0.4 | 15.3 ± 06 |
| 30 | 8.20 | 1.30 | 6.90 | 5.2 ± 0.8 | 17.5 ± 0.8 |
| 40 | 13.30 | 2.30 | 11.10 | 5.1 ± 0.6 | 17.5 ± 0.6 |
| 50 | 8.10 | 1.20 | 6.90 | 5.3 ± 0.9 | 18.0 ± 0.7 |
| 60 | 9.00 | 1.50 | 7.50 | 5.3 ± 0.7 | 18.2 ± 0.2 |
| 70 | 8.80 | 2.40 | 6.40 | 5.4 ± 0.4 | 20.0 ± 0.7 |
| 80 | 9.30 | 2.60 | 8.70 | 6.0 ± 0.6 | 20.1 ± 0.3 |
| CeOx (%) | Hardness | Tensile Strength/MPa | Tensile Modulus/MPa | Tear Strength/MPa | ||
|---|---|---|---|---|---|---|
| 100% | 300% | 500% | ||||
| Pure | 37 ± 1 | 2.08 ± 0.07 | 0.99 ± 0.03 | 1.69 ± 0.02 | - | 10.02 ± 0.33 |
| 0 | 56 ± 1 | 9.45 ± 0.33 | 1.73 ± 0.08 | 4.76 ± 0.24 | 6.64 ± 0.22 | 36.99 ± 1.59 |
| 1 | 51 ± 1 | 10.06 ± 0.96 | 1.03 ± 0.22 | 2.15 ± 0.67 | 3.99 ± 0.81 | 42.51 ± 1.36 |
| 2 | 52 ± 1 | 9.83 ± 0.99 | 1.11 ± 0.05 | 2.48 ± 0.21 | 4.56 ± 0.31 | 41.43 ± 3.58 |
| 3 | 52 ± 1 | 9.50 ± 0.13 | 1.15 ± 0.05 | 2.78 ± 0.23 | 5.18 ± 0.28 | 42.92 ± 1.19 |
| 4 | 57 ± 1 | 12.85 ± 0.12 | 1.33 ± 0.10 | 3.41 ± 0.31 | 6.06 ± 0.38 | 51.16 ± 2.42 |
| 5 | 54 ± 1 | 11.61 ± 0.14 | 1.32 ± 0.04 | 3.26 ± 0.16 | 5.59 ± 0.25 | 48.82 ± 1.32 |
| 6 | 53 ± 1 | 10.49 ± 0.65 | 1.14 ± 0.06 | 2.35 ± 0.13 | 4.05 ± 0.18 | 41.67 ± 0.62 |
| 7 | 54 ± 1 | 9.49 ± 0.70 | 1.10 ± 0.05 | 2.10 ± 0.16 | 3.70 ± 0.28 | 35.63 ± 2.63 |
| 8 | 52 ± 1 | 9.64 ± 0.25 | 1.21 ± 0.04 | 2.34 ± 0.08 | 4.10 ± 0.16 | 36.07 ± 2.21 |
| CeOx/Kaol (phr) | Hardness/Shore A | Tensile Strength/MPa | Tensile Modulus/MPa | Tear Strength/MPa | ||
|---|---|---|---|---|---|---|
| 100% | 300% | 500% | ||||
| Pure | 37 ± 1 | 2.08 ± 0.07 | 0.99 ± 0.03 | 1.69 ± 0.02 | - | 10.02 ± 0.33 |
| 10 | 43 ± 1 | 3.27 ± 0.03 | 0.73 ± 0.03 | 1.28 ± 0.10 | 2.25 ± 0.21 | 13.74 ± 0.82 |
| 20 | 48 ± 1 | 5.18 ± 0.50 | 0.91 ± 0.02 | 1.87 ± 0.05 | 3.26 ± 0.09 | 20.09 ± 1.61 |
| 30 | 54 ± 1 | 7.47 ± 0.65 | 1.14 ± 0.01 | 2.24 ± 0.05 | 3.84 ± 0.08 | 23.85 ± 1.83 |
| 40 | 59 ± 1 | 13.79 ± 0.86 | 1.88 ± 0.09 | 4.86 ± 0.28 | 7.81 ± 0.43 | 37.45 ± 2.42 |
| 50 | 57 ± 1 | 12.85 ± 0.12 | 1.33 ± 0.10 | 3.41 ± 0.31 | 6.06 ± 0.38 | 51.16 ± 1.32 |
| 60 | 58 ± 1 | 12.16 ± 0.71 | 1.31 ± 0.03 | 3.16 ± 0.03 | 5.69 ± 0.17 | 50.95 ± 3.36 |
| 70 | 61 ± 1 | 12.14 ± 0.51 | 1.58 ± 0.02 | 4.07 ± 0.09 | 6.64 ± 0.09 | 54.20 ± 2.38 |
| 80 | 68 ± 1 | 12.19 ± 0.55 | 2.29 ± 0.09 | 6.85 ± 0.22 | 10.26 ± 0.21 | 40.59 ± 2.72 |
| Samples | Time/h | Tensile Strength/Mpa (Rate of Change/%) | Shore (A) Hardness (Rate of Change/%) |
|---|---|---|---|
| Pure SBR | 0 | 2.08 ± 0.07 | 37 ± 1 |
| 24 | 1.60 ± 0.03 (−18.75%) | 41 ± 1 (+ 10.81%) | |
| 48 | 1.51 ± 0.09 (−27.40%) | 45 ± 1 (+ 21.62%) | |
| 72 | 1.45 ± 0.03 (−30.28%) | 47 ± 1 (+27.02%) | |
| Kaol/SBR | 0 | 9.45 ± 0.33 | 56 ± 1 |
| 24 | 6.29 ± 0.09 (−33.43%) | 62 ± 1 (+5.08%) | |
| 48 | 3.96 ± 0.19 (−58.09%) | 68 ± 1 (+15.25%) | |
| 72 | 3.79 ± 0.17 (−59.89%) | 68 ± 1 (+15.25%) | |
| CeOx/Kaol/SBR | 0 | 13.79 ± 0.86 | 59 ± 1 |
| 24 | 7.28 ± 0.23 (−24.48%) | 58 ± 1 (+11.53%) | |
| 48 | 6.08 ± 0.39 (−36.92%) | 61 ± 1 (+17.30%) | |
| 72 | 5.32 ± 0.21 (−44.81%) | 66 ± 1 (+26.92%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Xiao, K.; Zhang, Y.; Gong, Y.; Zhang, Y. The Improvement of Kaolinite Supported Cerium Oxide for Styrene–Butadiene Rubber Composite: Mechanical, Ageing Properties and Mechanism. Polymers 2022, 14, 5187. https://doi.org/10.3390/polym14235187
Liu H, Xiao K, Zhang Y, Gong Y, Zhang Y. The Improvement of Kaolinite Supported Cerium Oxide for Styrene–Butadiene Rubber Composite: Mechanical, Ageing Properties and Mechanism. Polymers. 2022; 14(23):5187. https://doi.org/10.3390/polym14235187
Chicago/Turabian StyleLiu, Honglei, Kaiyuan Xiao, Yinmin Zhang, Yanbing Gong, and Yongfeng Zhang. 2022. "The Improvement of Kaolinite Supported Cerium Oxide for Styrene–Butadiene Rubber Composite: Mechanical, Ageing Properties and Mechanism" Polymers 14, no. 23: 5187. https://doi.org/10.3390/polym14235187
APA StyleLiu, H., Xiao, K., Zhang, Y., Gong, Y., & Zhang, Y. (2022). The Improvement of Kaolinite Supported Cerium Oxide for Styrene–Butadiene Rubber Composite: Mechanical, Ageing Properties and Mechanism. Polymers, 14(23), 5187. https://doi.org/10.3390/polym14235187






