Removal of Heavy Metal Ions from Wastewater with Poly-ε-Caprolactone-Reinforced Chitosan Composite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Ch-PCL Thermoplastic Matrix Composite (MCP)
2.3. Polymers and MCP Characterization
2.4. Adsorption Experiments
2.4.1. Adsorption Equilibrium Studies
2.4.2. Adsorption Kinetic Studies
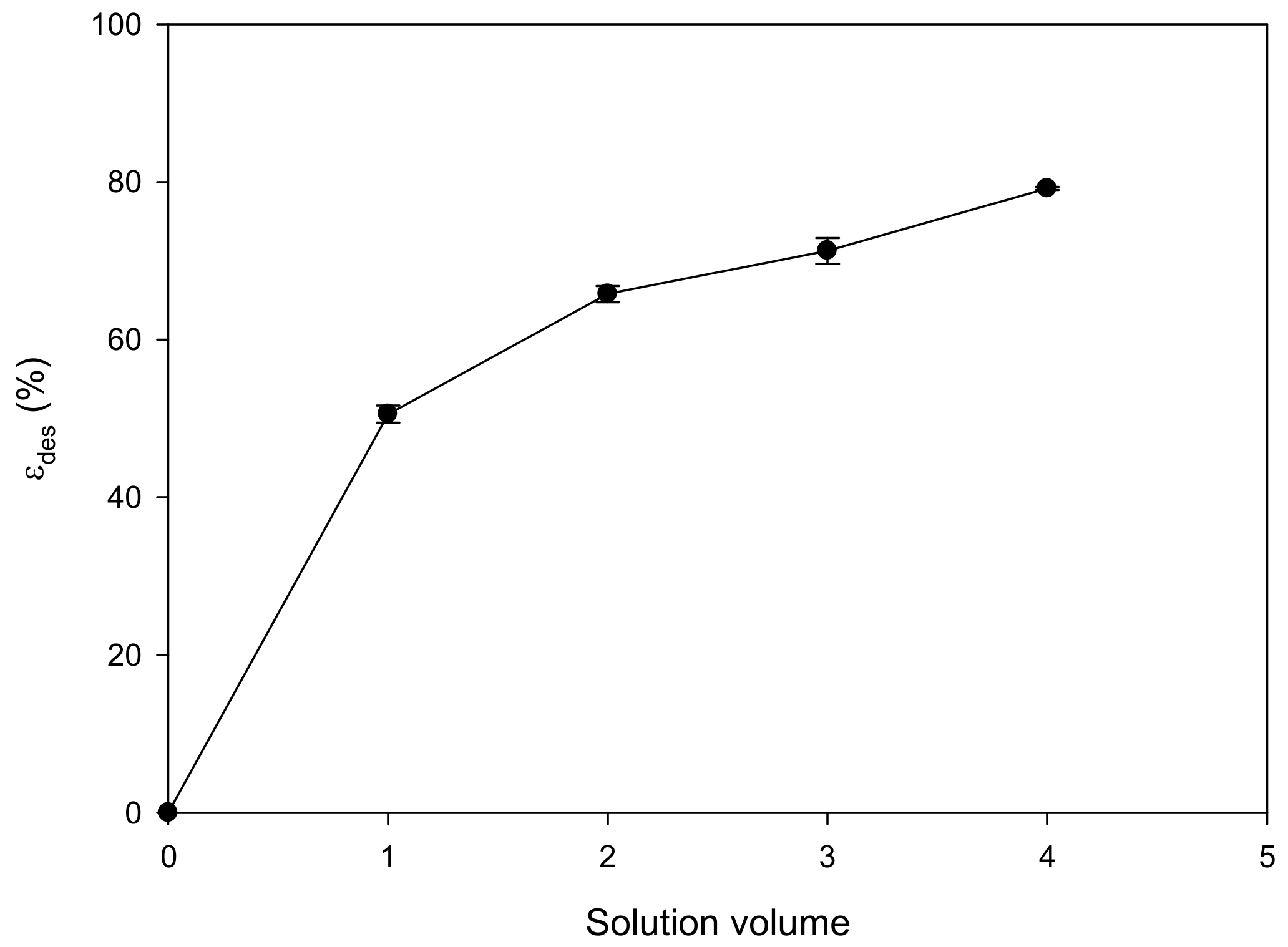
2.5. Desorption and Reusability
3. Results and Discussion
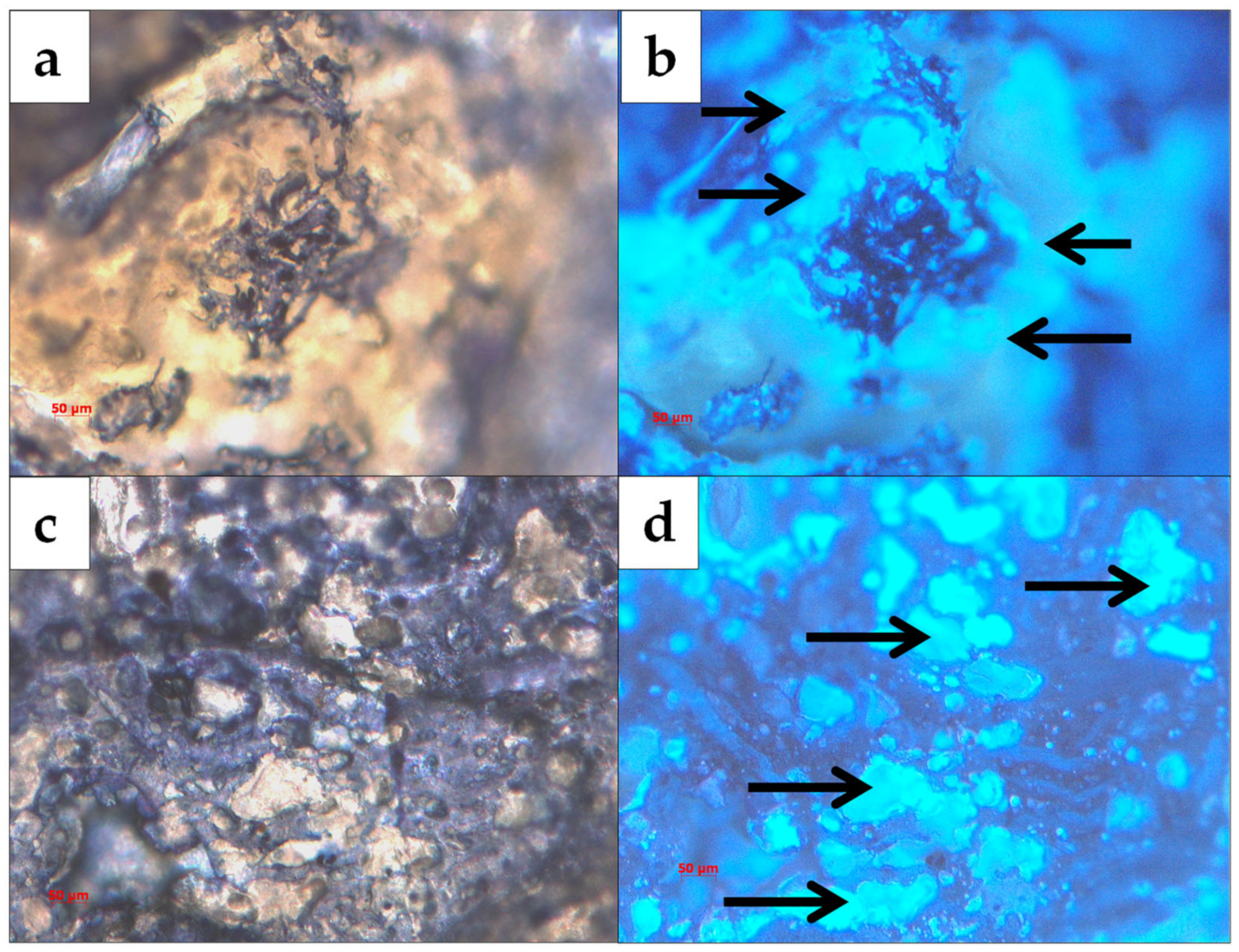
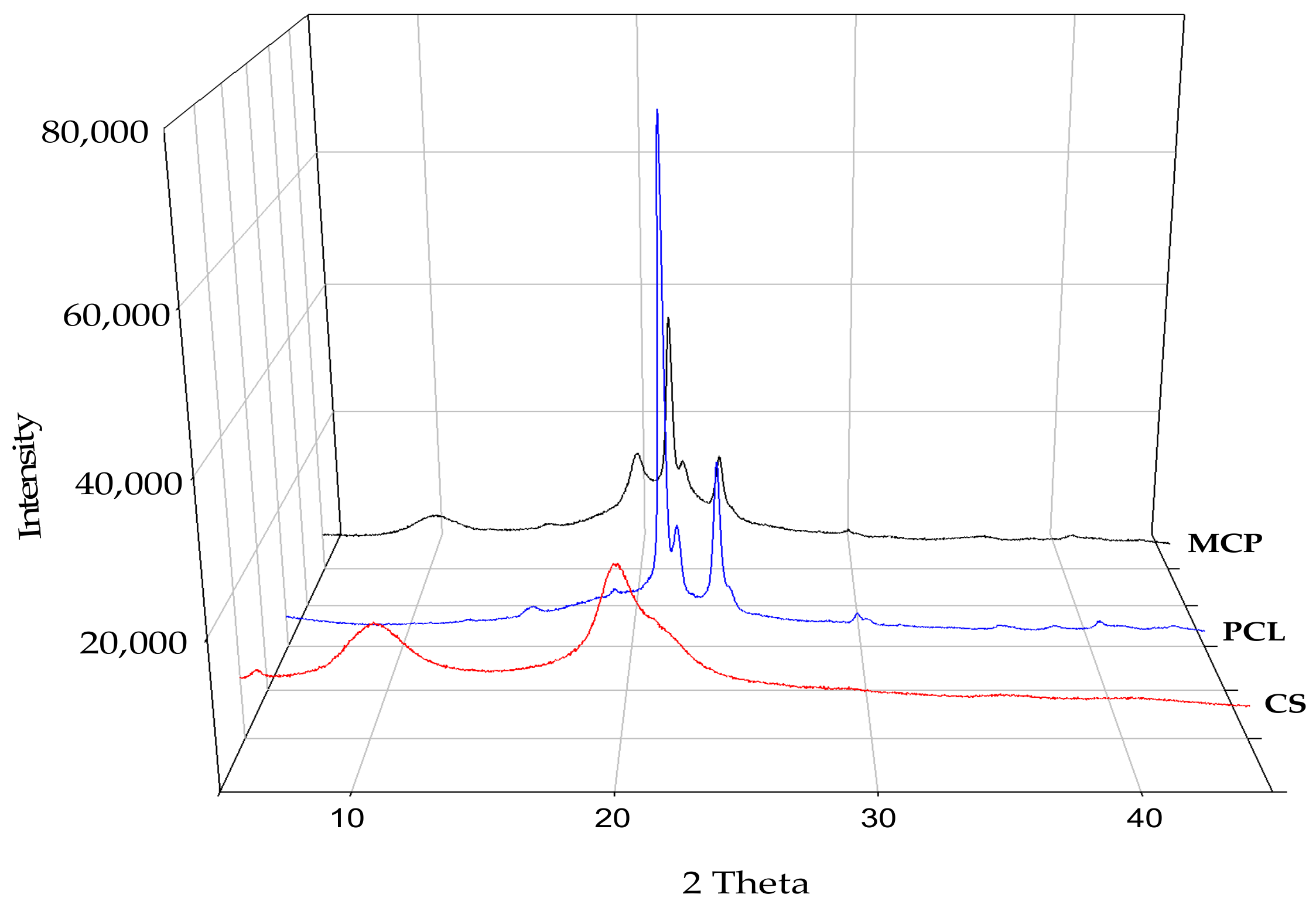
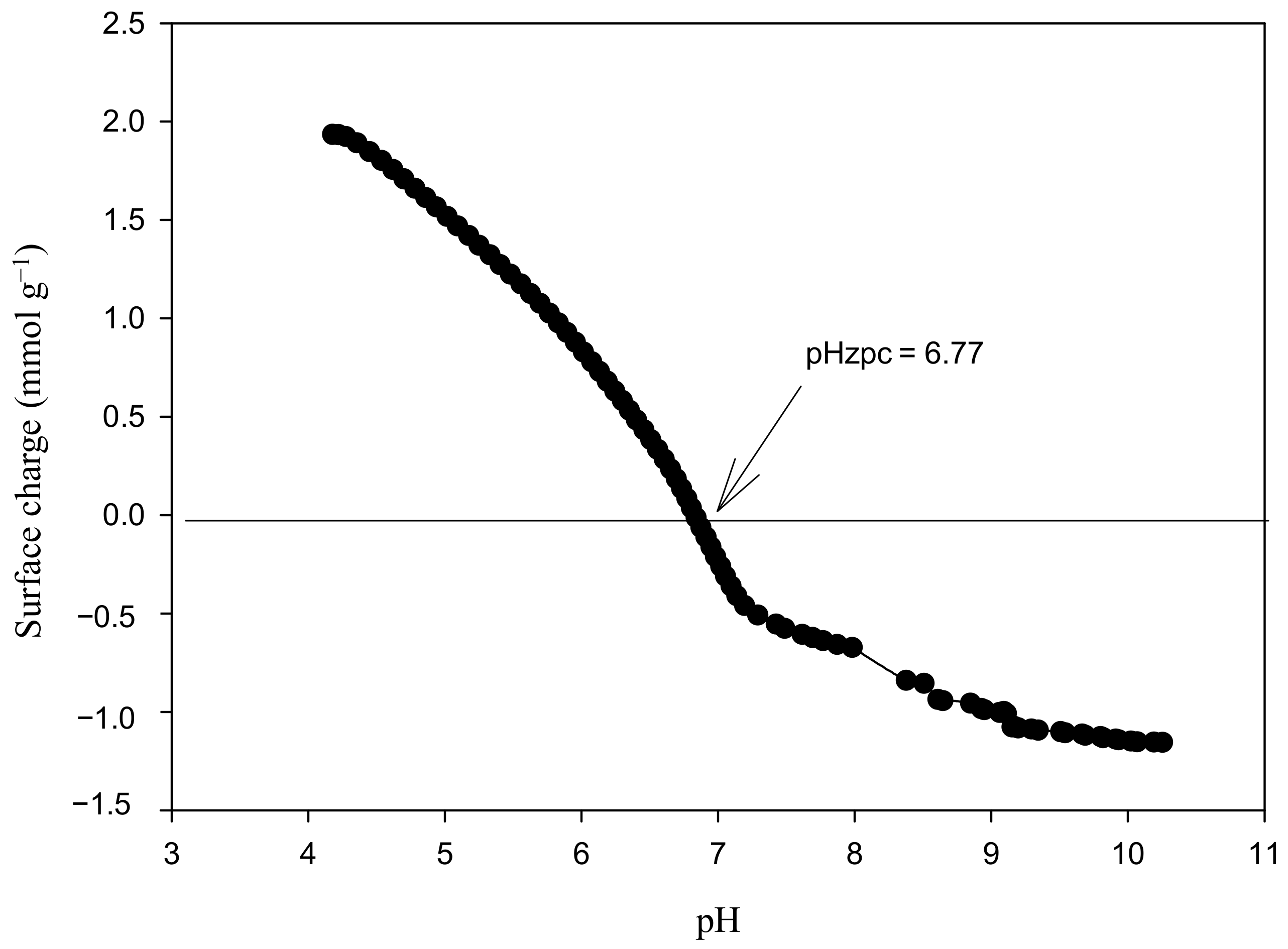
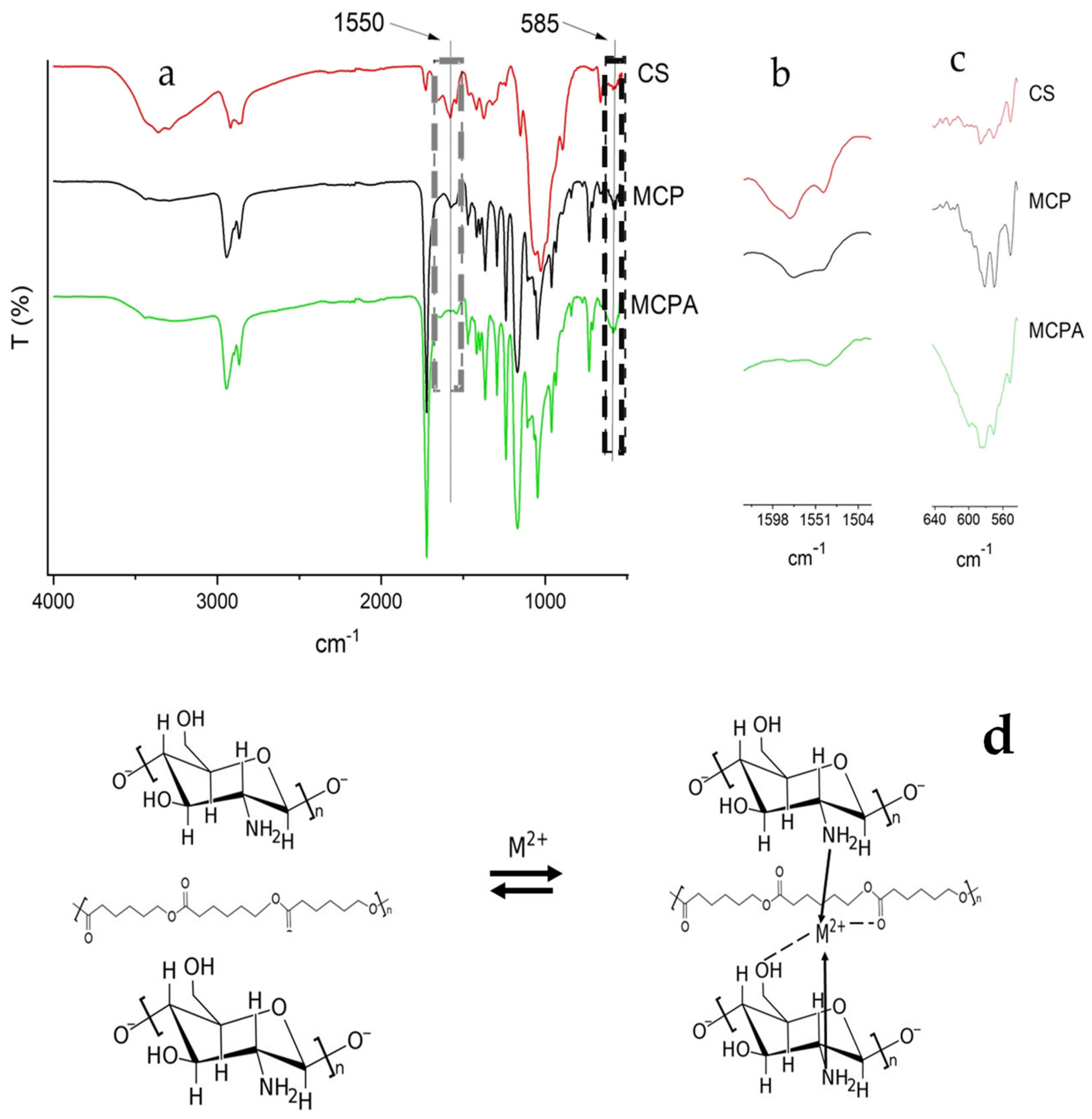
3.1. Composite Characterization
3.2. Adsorption Kinetics and Equilibrium
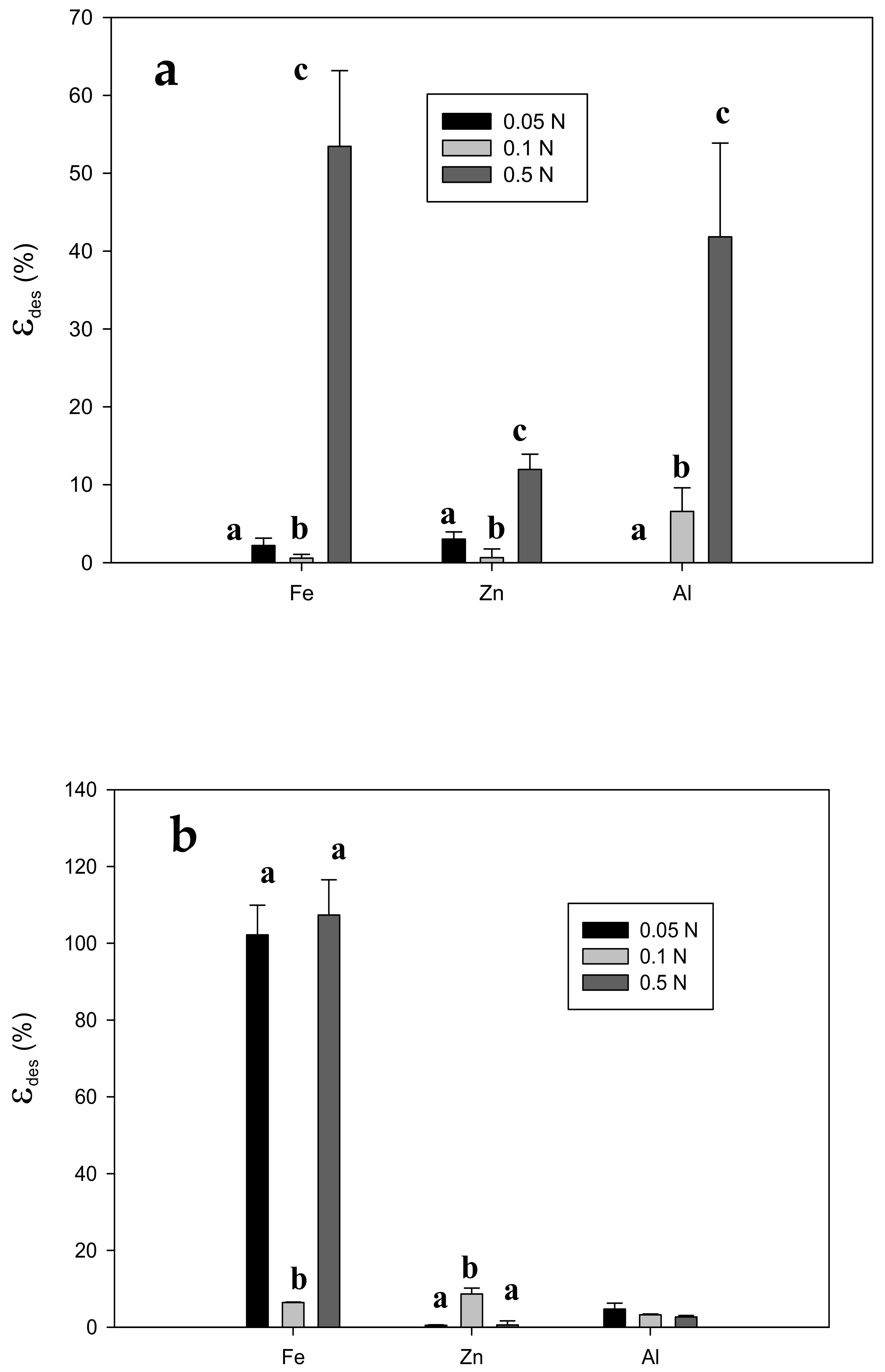
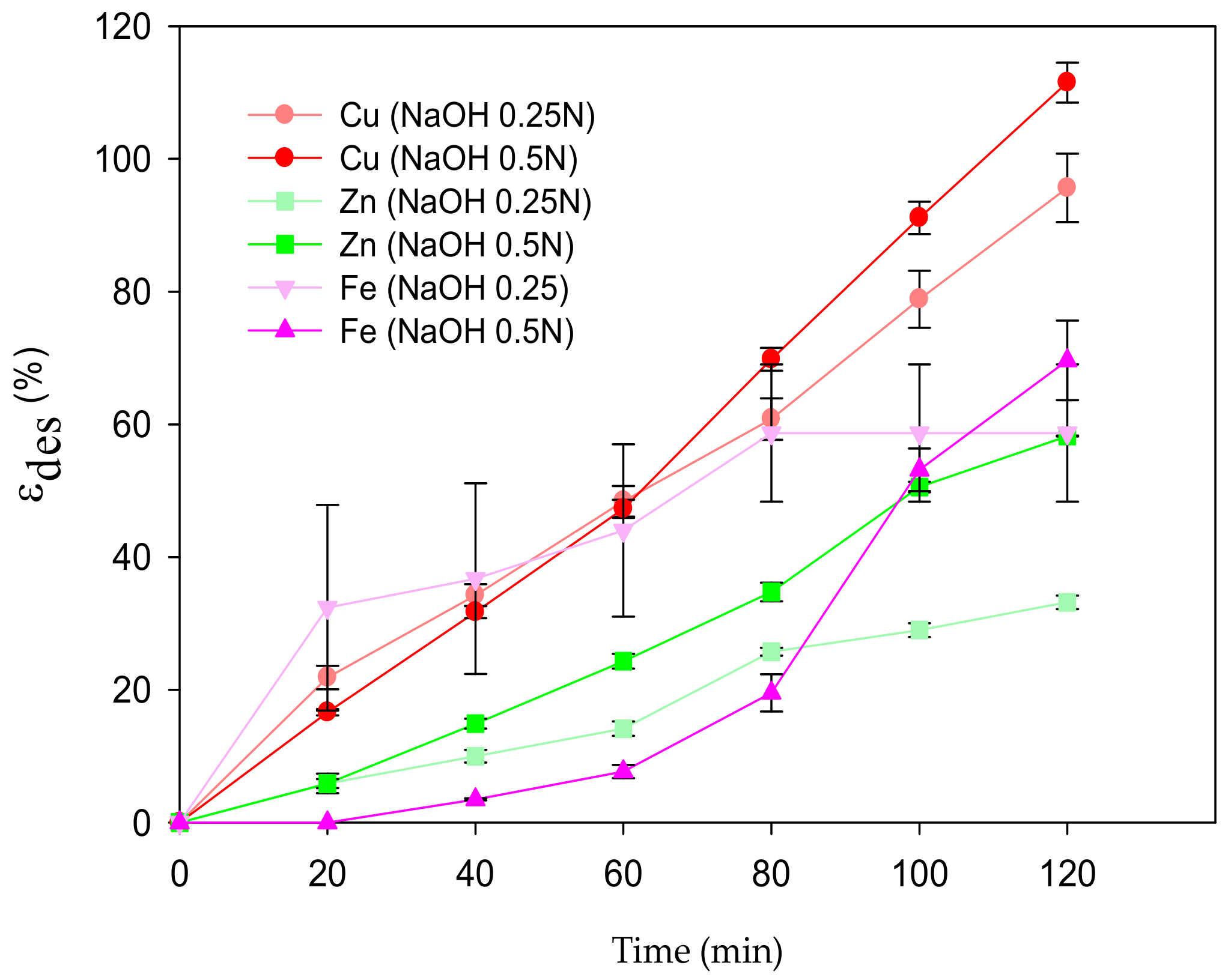
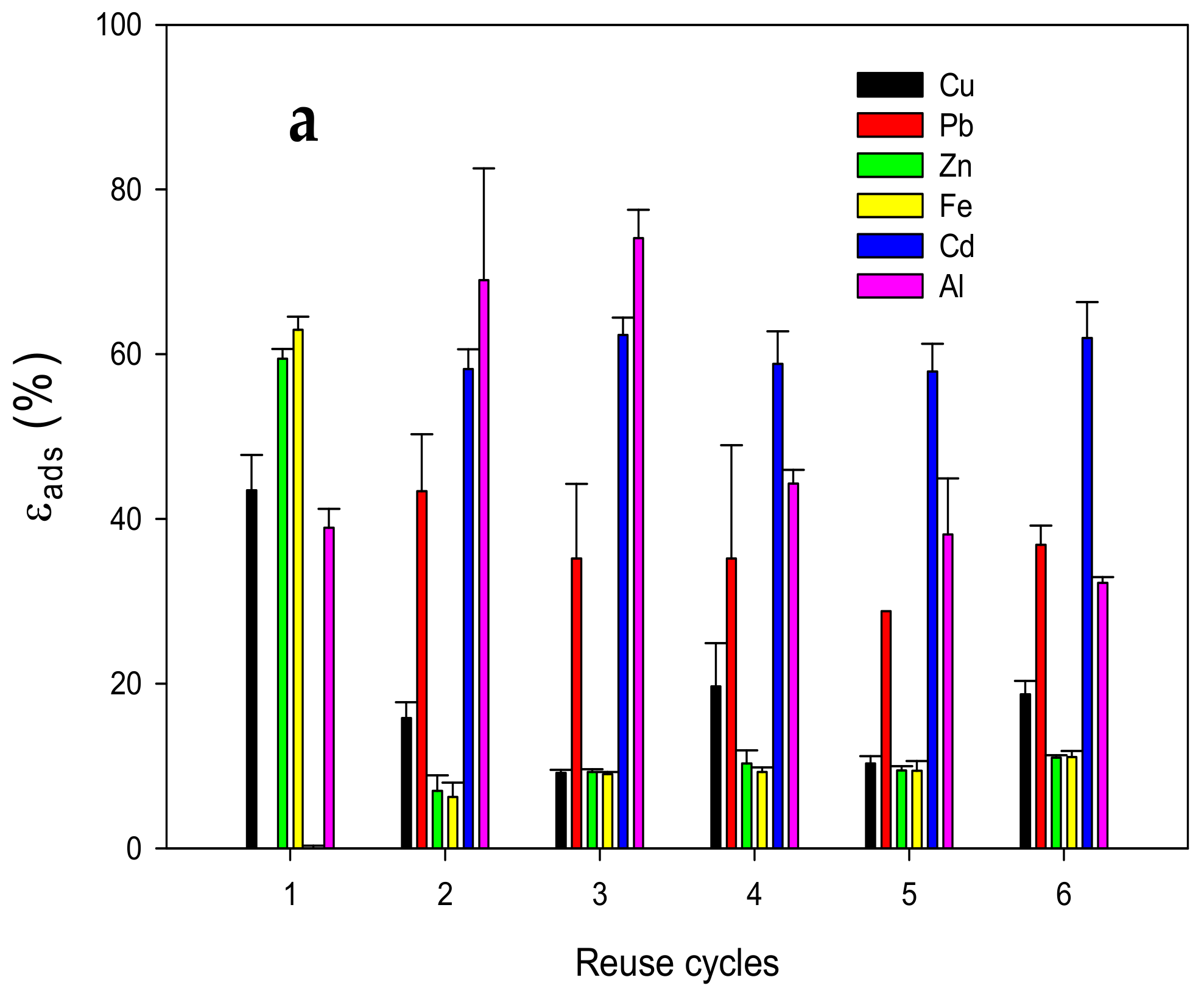
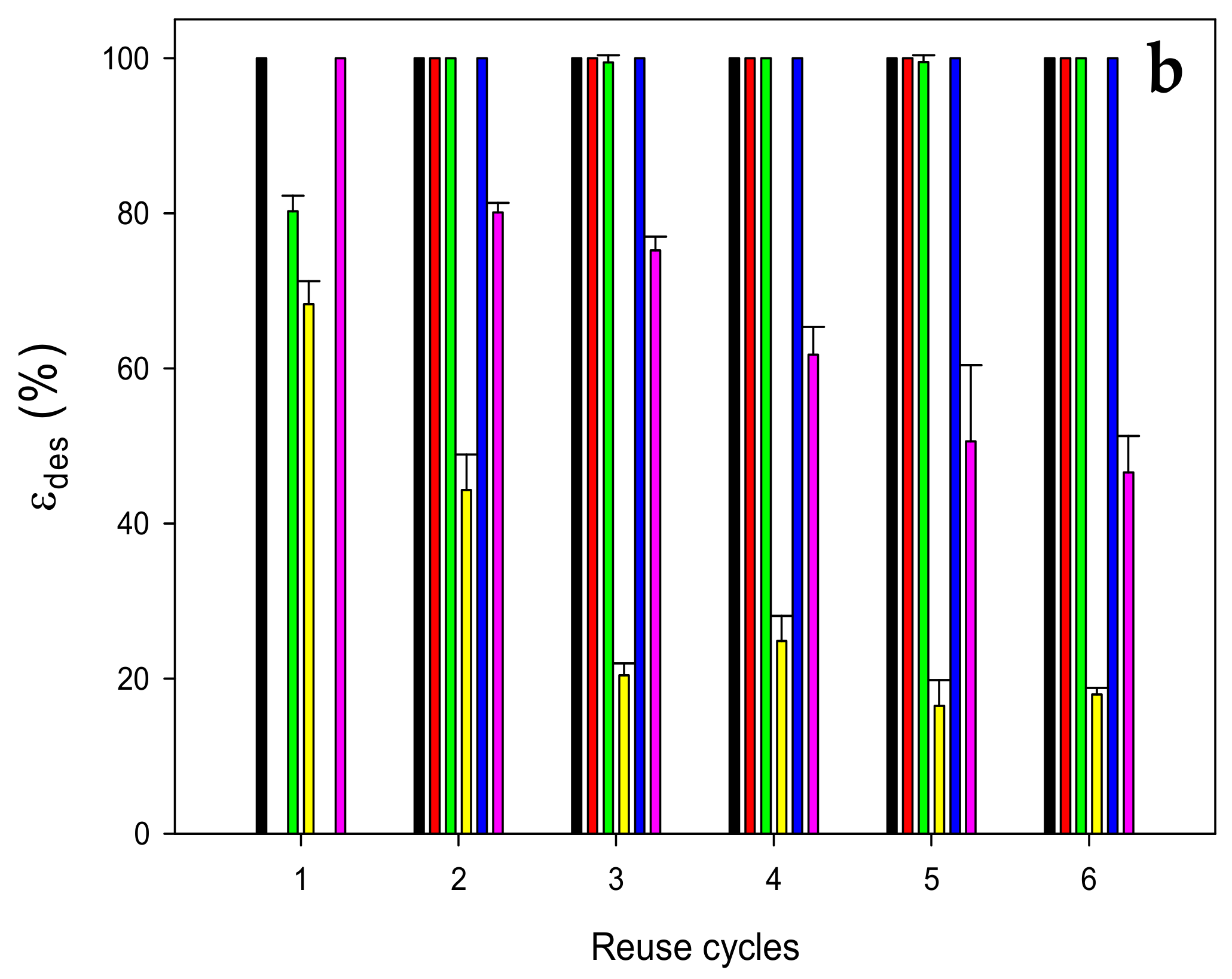
3.3. Desorption and Reusability
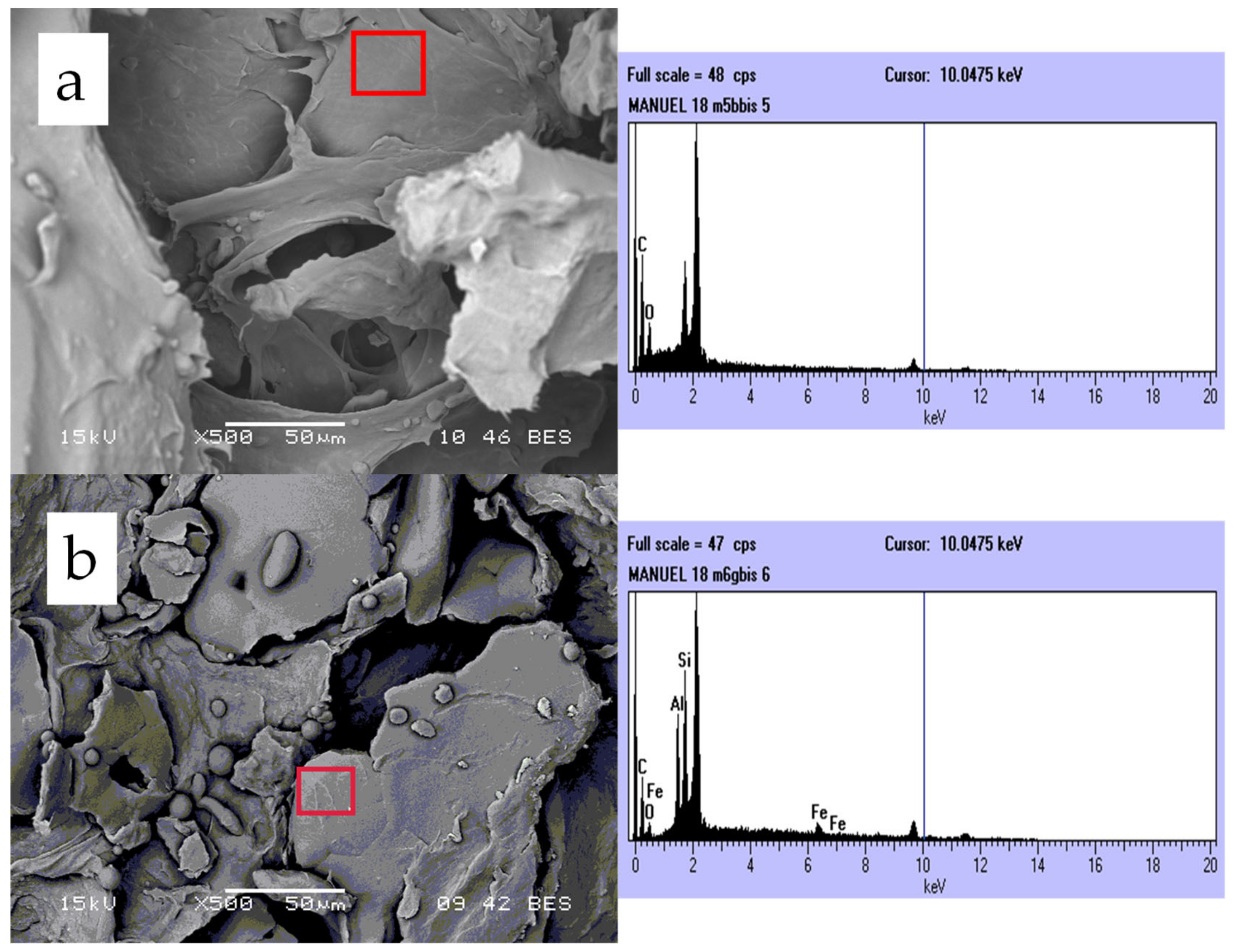
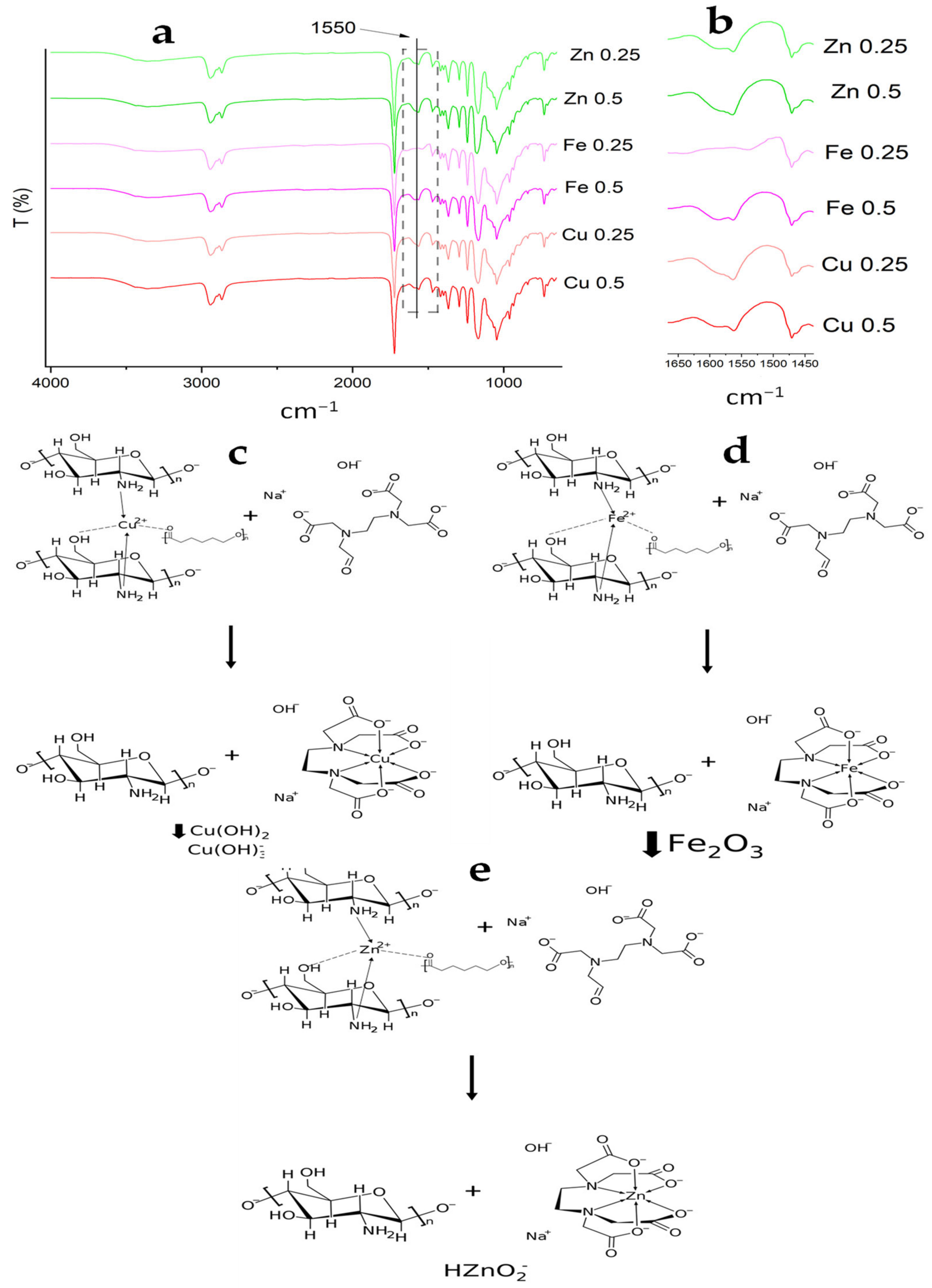
3.4. Elemental Analysis by EDS
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
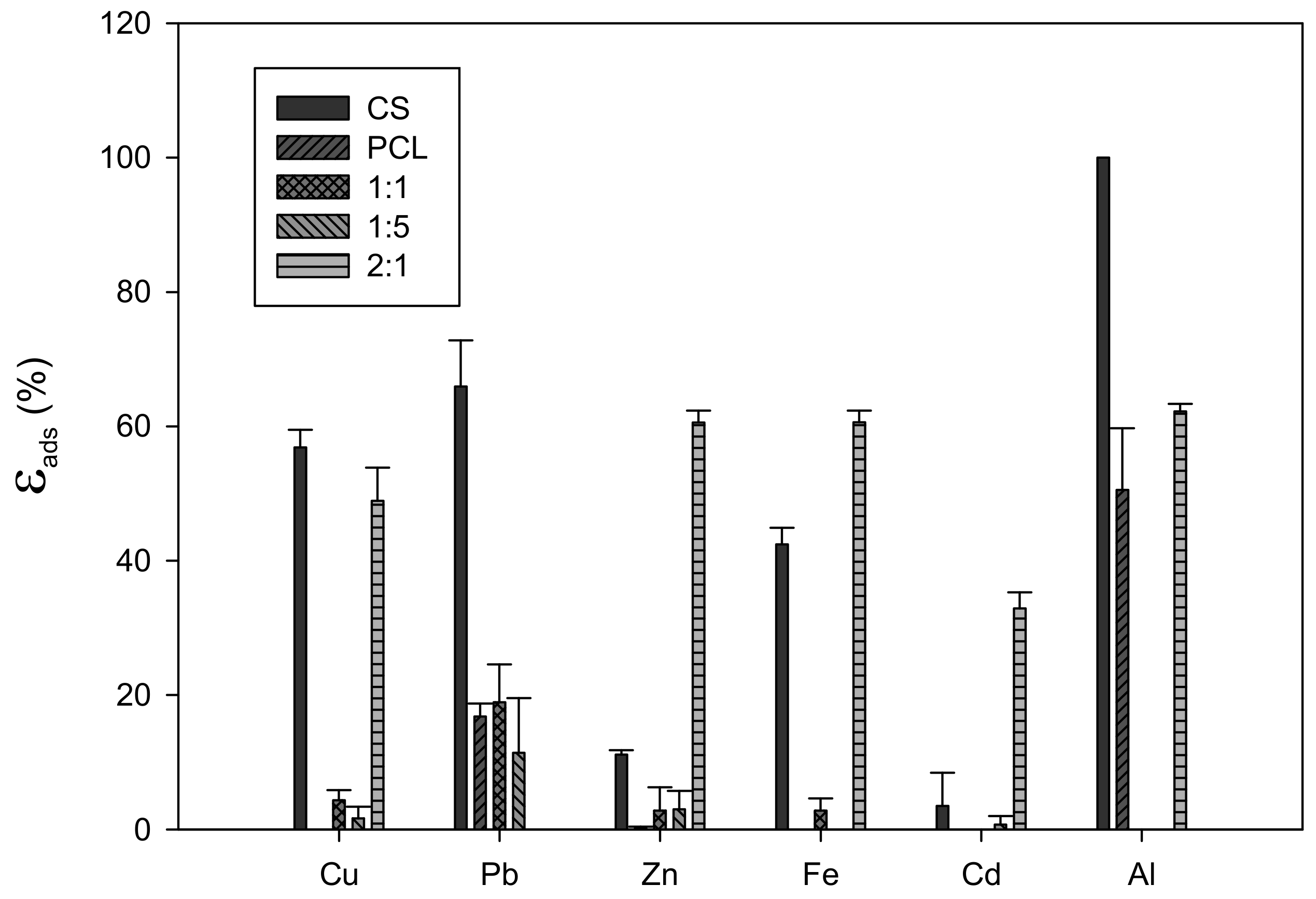
Appendix B

Appendix C
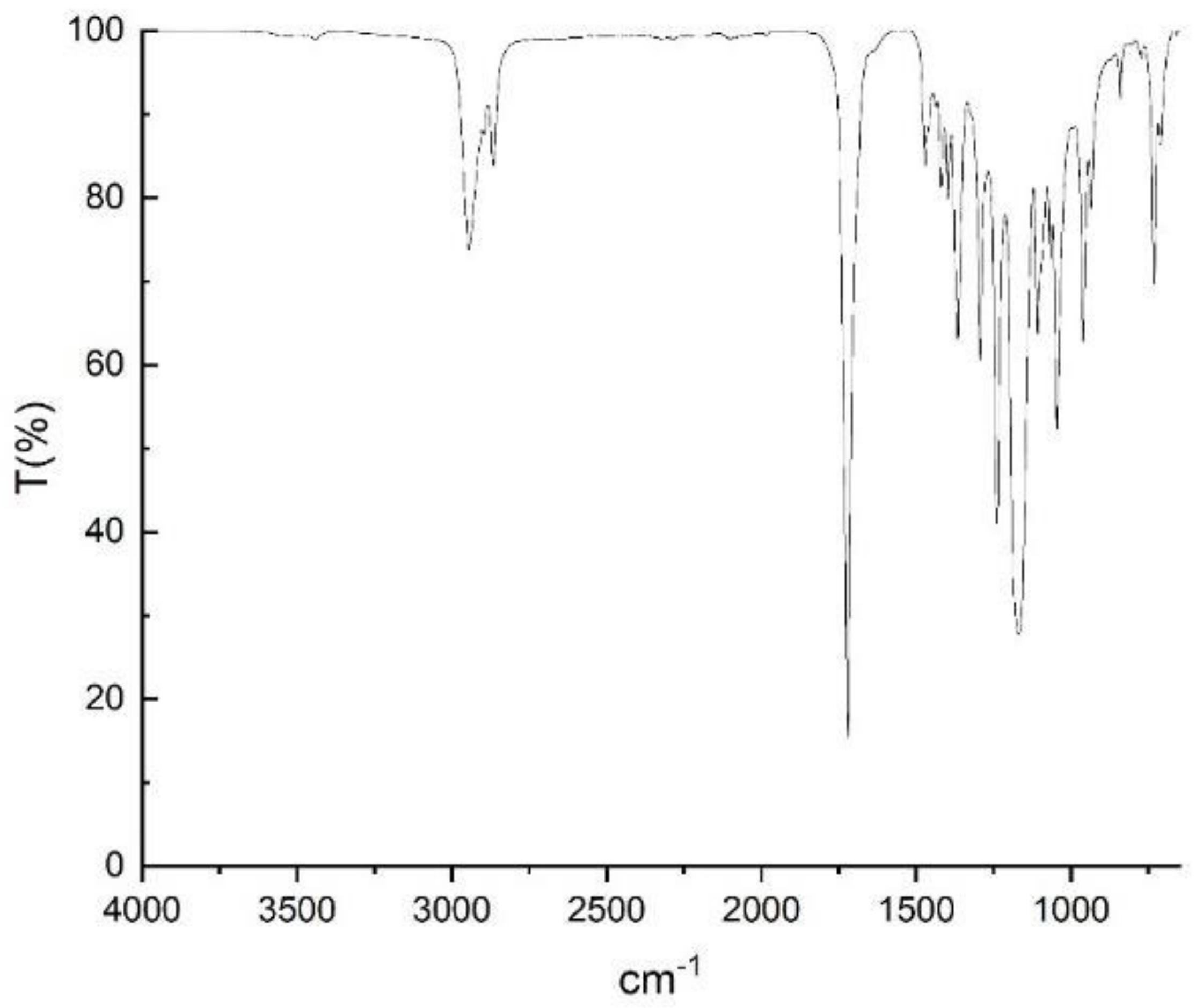
Appendix D
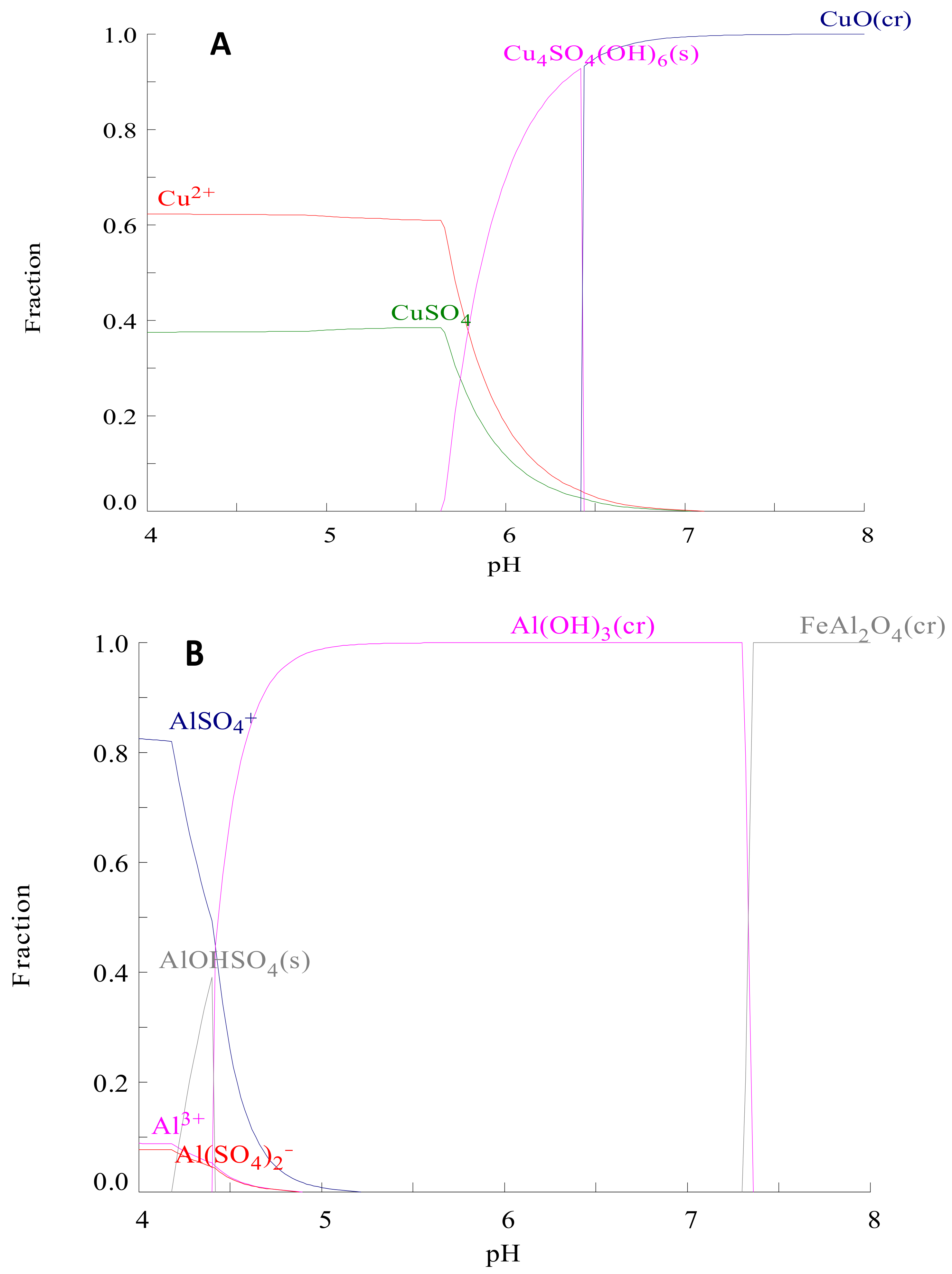
Appendix E
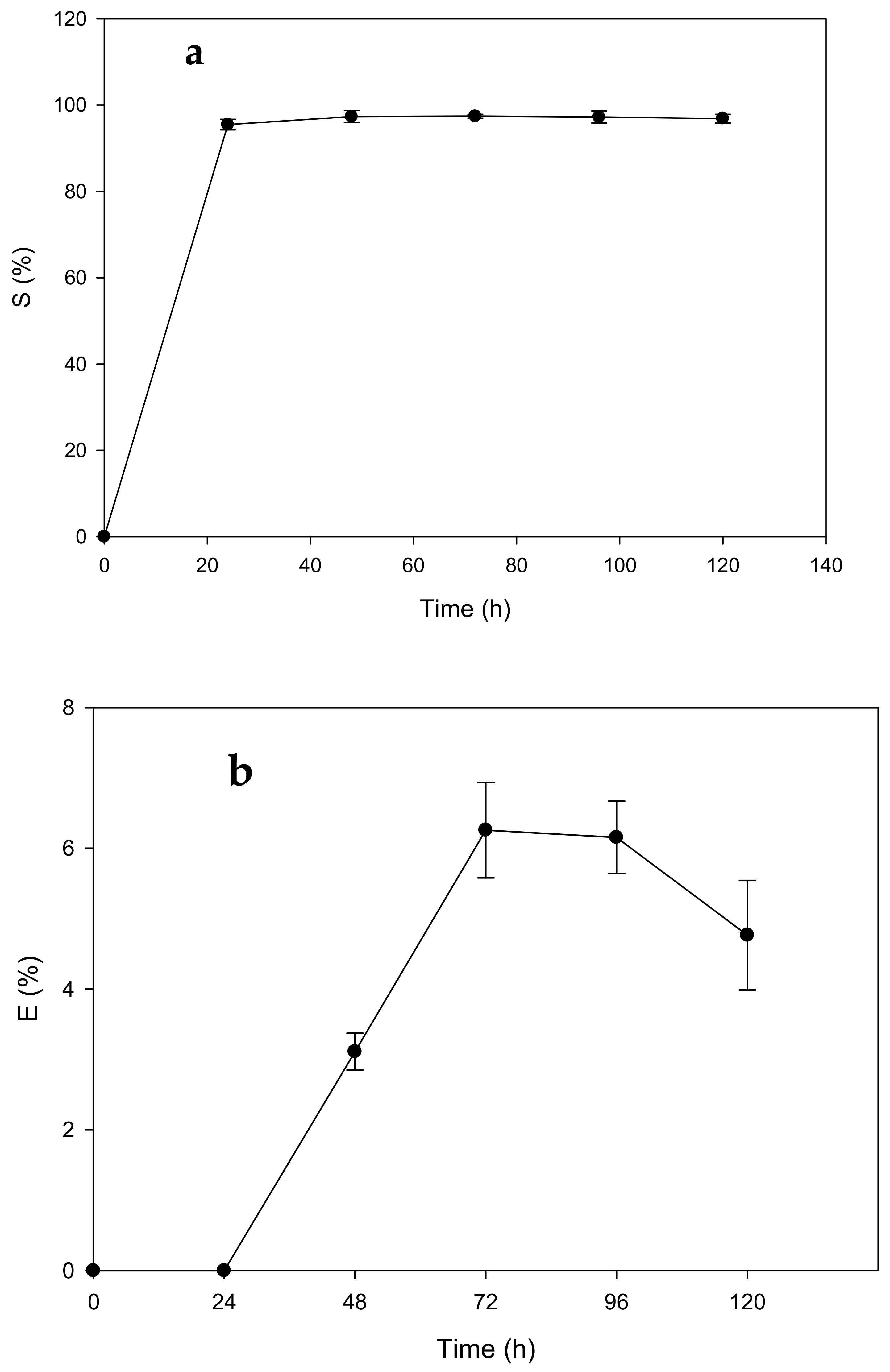
Appendix F

Appendix G

Appendix H
Appendix I
References
- Rangel-Mendez, J.R.; Monroy-Zepeda, R.; Leyva-Ramos, E.; Diaz-Flores, P.E.; Shirai, K. Chitosan Selectivity for Removing Cadmium (II), Copper (II), and Lead (II) from Aqueous Phase: PH and Organic Matter Effect. J. Hazard. Mater. 2009, 162, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Pastor, J.; Hernández, A.J. Heavy Metals, Salts and Organic Residues in Old Solid Urban Waste Landfills and Surface Waters in Their Discharge Areas: Determinants for Restoring Their Impact. J. Environ. Manag. 2012, 95, S42–S49. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.C.; Patel, R.K. Removal of Lead and Zinc Ions from Water by Low Cost Adsorbents. J. Hazard. Mater. 2009, 168, 319–325. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Siafaka, P.I.; Pavlidou, E.G.; Chrissafis, K.J.; Bikiaris, D.N. Synthesis and Adsorption Application of Succinyl-Grafted Chitosan for the Simultaneous Removal of Zinc and Cationic Dye from Binary Hazardous Mixtures. Chem. Eng. J. 2015, 259, 438–448. [Google Scholar] [CrossRef]
- Lu, S.; Gibb, S.W.; Cochrane, E. Effective Removal of Zinc Ions from Aqueous Solutions Using Crab Carapace Biosorbent. J. Hazard. Mater. 2007, 149, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Gautam, N.; Mishra, A.; Gupta, R. Heavy Metals and Living Systems: An Overview. Indian J. Pharmacol. 2011, 43, 246–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKinley, K.; McLellan, I.; Gagné, F.; Quinn, B. The Toxicity of Potentially Toxic Elements (Cu, Fe, Mn, Zn and Ni) to the Cnidarian Hydra Attenuata at Environmentally Relevant Concentrations. Sci. Total Environ. 2019, 665, 848–854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Li, Y.; Ye, Z. Preparation of Macroporous Bead Adsorbents Based on Poly(Vinyl Alcohol)/Chitosan and Their Adsorption Properties for Heavy Metals from Aqueous Solution. Chem. Eng. J. 2011, 178, 60–68. [Google Scholar] [CrossRef]
- Li, X.F.; Wang, P.F.; Feng, C.L.; Liu, D.Q.; Chen, J.K.; Wu, F.C. Acute Toxicity and Hazardous Concentrations of Zinc to Native Freshwater Organisms Under Different PH Values in China. Bull. Environ. Contam. Toxicol. 2019, 103, 120–126. [Google Scholar] [CrossRef] [Green Version]
- US EPA. 1995 Updates: Water Quality Criteria Documents for the Protection of Aquatic Life.
- EU Dangerous Substances Discharged into the Aquatic Environment. Off. J. Eur. Union 1976, 23–29.
- Crini, G.; Lichtfouse, E.; Wilson, L.D.; Morin-Crini, N. Conventional and Non-Conventional Adsorbents for Wastewater Treatment. Environ. Chem. Lett. 2019, 17, 195–213. [Google Scholar] [CrossRef]
- Crini, G. Recent Developments in Polysaccharide-Based Materials Used as Adsorbents in Wastewater Treatment. Prog. Polym. Sci. 2005, 30, 38–70. [Google Scholar] [CrossRef]
- Pal, P.; Pal, A. Treatment of Real Wastewater: Kinetic and Thermodynamic Aspects of Cadmium Adsorption onto Surfactant-Modified Chitosan Beads. Int. J. Biol. Macromol. 2019, 131, 1092–1100. [Google Scholar] [CrossRef] [PubMed]
- Hall, G.M.; Barrera, C.H.; Shirai, K. Alternative Methods for Chitin and Chitosan Preparation, Characterization, and Application. In Handbook of Chitin and Chitosan; Elsevier: Amsterdam, The Netherlands, 2020; Volume 1, pp. 225–246. ISBN 9780128179703. [Google Scholar]
- Desbrières, J.; Guibal, E. Chitosan for Wastewater Treatment. Polym. Int. 2018, 67, 7–14. [Google Scholar] [CrossRef]
- Guibal, E.; Van Vooren, M.; Dempsey, B.A.; Roussy, J. A Review of the Use of Chitosan for the Removal of Particulate and Dissolved Contaminants. Sep. Sci. Technol. 2006, 41, 2487–2514. [Google Scholar] [CrossRef]
- Jiang, M.; Han, T.; Wang, J.; Shao, L.; Qi, C.; Zhang, X.M.; Liu, C.; Liu, X. Removal of Heavy Metal Chromium Using Cross-Linked Chitosan Composite Nanofiber Mats. Int. J. Biol. Macromol. 2018, 120, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Salih, S.S.; Ghosh, T.K. Adsorption of Zn(II) Ions by Chitosan Coated Diatomaceous Earth. Int. J. Biol. Macromol. 2018, 106, 602–610. [Google Scholar] [CrossRef]
- Velasco-Garduño, O.; Martínez, M.E.; Gimeno, M.; Tecante, A.; Beristain-Cardoso, R.; Shirai, K. Copper Removal from Wastewater by a Chitosan-Based Biodegradable Composite. Environ. Sci. Pollut. Res. 2020, 27, 28527–28535. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wu, W.; Liu, Y.; Lin, Z.; Sun, X. Chitosan Modified Filter Paper Separators with Specific Ion Adsorption to Inhibit Side Reactions and Induce Uniform Zn Deposition for Aqueous Zn Batteries. Chem. Eng. J. 2022, 450, 137902. [Google Scholar] [CrossRef]
- Wu, C.S. A Comparison of the Structure, Thermal Properties, and Biodegradability of Polycaprolactone/Chitosan and Acrylic Acid Grafted Polycaprolactone/Chitosan. Polymer 2005, 46, 147–155. [Google Scholar] [CrossRef]
- Aranday-García, R.; Román Guerrero, A.; Ifuku, S.; Shirai, K. Successive Inoculation of Lactobacillus Brevis and Rhizopus Oligosporus on Shrimp Wastes for Recovery of Chitin and Added-Value Products. Process Biochem. 2017, 58, 17–24. [Google Scholar] [CrossRef]
- García-Arrazola, R.; Gimeno, M.; Bárzana, E. Use of Liquid 1,1,1,2-Tetrafluoroethane as Solvent Media for Enzyme-Catalyzed Ring-Opening Polymerization of Lactones. Macromolecules 2007, 40, 4119–4120. [Google Scholar] [CrossRef]
- AlMarzooqi, F.A.; Bilad, M.R.; Mansoor, B.; Arafat, H.A. A Comparative Study of Image Analysis and Porometry Techniques for Characterization of Porous Membranes. J. Mater. Sci. 2016, 51, 2017–2032. [Google Scholar] [CrossRef]
- Steele, W.A. Adsorption Surface Area and Porosity. J. Colloid Interface Sci. 1983, 94, 597–598. [Google Scholar] [CrossRef]
- Wang, B.; Zhu, Y.; Bai, Z.; Luque, R.; Xuan, J. Functionalized Chitosan Biosorbents with Ultra-High Performance, Mechanical Strength and Tunable Selectivity for Heavy Metals in Wastewater Treatment. Chem. Eng. J. 2017, 325, 350–359. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption Isotherm Models: Classification, Physical Meaning, Application and Solving Method. Chemosphere 2020, 258, 127279. [Google Scholar] [CrossRef] [PubMed]
- SEMARNAT. Norma Oficial Mexicana NOM-001-SEMARNAT-2021, Que Establece Los Límites Permisibles de Contaminantes En Las Descargas de Aguas Residuales En Cuerpos Receptores Propiedades de La Nación; Secretariat of Environment and Natural Resources: Mexico City, Mexico, 2022; Volume 5, pp. 1–17.
- Zhang, L.; Zeng, Y.; Cheng, Z. Removal of Heavy Metal Ions Using Chitosan and Modified Chitosan: A Review. J. Mol. Liq. 2016, 214, 175–191. [Google Scholar] [CrossRef]
- Guibal, E. Interactions of Metal Ions with Chitosan-Based Sorbents: A Review. Sep. Purif. Technol. 2004, 38, 43–74. [Google Scholar] [CrossRef]
- Abdolali, A.; Ngo, H.H.; Guo, W.; Zhou, J.L.; Zhang, J.; Liang, S.; Chang, S.W.; Nguyen, D.D.; Liu, Y. Application of a Breakthrough Biosorbent for Removing Heavy Metals from Synthetic and Real Wastewaters in a Lab-Scale Continuous Fixed-Bed Column. Bioresour. Technol. 2017, 229, 78–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Futalan, C.M.; Yang, J.H.; Phatai, P.; Chen, I.P.; Wan, M.W. Fixed-Bed Adsorption of Copper from Aqueous Media Using Chitosan-Coated Bentonite, Chitosan-Coated Sand, and Chitosan-Coated Kaolinite. Environ. Sci. Pollut. Res. 2020, 27, 24659–24670. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, U.; Sreedhar, I.; Singh, S.A.; Patel, C.M.; Anitha, K.L. Recent Advances in Heavy Metal Removal by Chitosan Based Adsorbents. Carbohydr. Polym. 2021, 251, 117000. [Google Scholar] [CrossRef] [PubMed]
- Salih, S.S.; Ghosh, T.K. Highly Efficient Competitive Removal of Pb(II) and Ni(II) by Chitosan/Diatomaceous Earth Composite. J. Environ. Chem. Eng. 2018, 6, 435–443. [Google Scholar] [CrossRef]
- Correlo, V.M.; Boesel, L.F.; Bhattacharya, M.; Mano, J.F.; Neves, N.M.; Reis, R.L. Properties of Melt Processed Chitosan and Aliphatic Polyester Blends. Mater. Sci. Eng. A 2005, 403, 57–68. [Google Scholar] [CrossRef] [Green Version]
- Rouquerol, J.; Avnir, D.; Fairbridge, C.W.; Everett, D.H.; Haynes, J.H.; Pernicone, N.; Ramsay, J.D.F.; Sing, K.S.W.; Unger, K.K. Recommendations for the Characterization of Porous Solids (Technical Report). Pure Appl. Chem. 1994, 66, 1739–1758. [Google Scholar] [CrossRef]
- Elzein, T.; Nasser-Eddine, M.; Delaite, C.; Bistac, S.; Dumas, P. FTIR Study of Polycaprolactone Chain Organization at Interfaces. J. Colloid Interface Sci. 2004, 273, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Pawar, V.; Srivastava, R. Chitosan-Polycaprolactone Blend Sponges for Management of Chronic Osteomyelitis: A Preliminary Characterization and in Vitro Evaluation. Int. J. Pharm. 2019, 568, 118553. [Google Scholar] [CrossRef] [PubMed]
- Pillai, C.K.S.; Paul, W.; Sharma, C.P. Chitin and Chitosan Polymers: Chemistry, Solubility and Fiber Formation. Prog. Polym. Sci. 2009, 34, 641–678. [Google Scholar]
- Pourbaix, M.; Zhang, H.; Pourbaix, A. Presentation of an Atlas of Chemical and Electrochemical Equilibria in the Presence of a Gaseous Phase. Mater. Sci. Forum 1997, 251–254, 143–148. [Google Scholar] [CrossRef]
- Mekahlia, S.; Bouzid, B. Chitosan-Copper (II) Complex as Antibacterial Agent: Synthesis, Characterization and Coordinating Bond- Activity Correlation Study. Phys. Procedia 2009, 2, 1045–1053. [Google Scholar] [CrossRef] [Green Version]
- Rex, G.C.; Schlick, S. Binding of Cu2+ in Polyacrylamide Gels. An Electron Spin Resonance Study. J. Phys. Chem. 1985, 89, 3598–3601. [Google Scholar] [CrossRef]
- Rhazi, M.; Desbrie Áres, J.; Tolaimate, A.; Rinaudo, M.; Vottero, P.; Alagui, A. Contribution to the Study of the Complexation of Copper by Chitosan and Oligomers. Polymer 2001, 43, 1267–1276. [Google Scholar] [CrossRef]
- Bhatia, S.C.; Ravi, N. A Magnetic Study of an Fe-Chitosan Complex and Its Relevance to Other Biomolecules. Biomacromolecules 2000, 1, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Sownthari, K.; Suthanthiraraj, S.A. Synthesis and Characterization of an Electrolyte System Based on a Biodegradable Polymer. Express Polym. Lett. 2013, 7, 495–504. [Google Scholar] [CrossRef]
- Cuppett, J.D.; Duncan, S.E.; Dietrich, A.M. Evaluation of Copper Speciation and Water Quality Factors That Affect Aqueous Copper Tasting Response. Chem. Senses 2006, 31, 689–697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krężel, A.; Maret, W. The Biological Inorganic Chemistry of Zinc Ions. Arch. Biochem. Biophys. 2016, 611, 3–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rabajczyk, A.; Namieśnik, J. Speciation of Iron in the Aquatic Environment. Water Environ. Res. 2014, 86, 741–758. [Google Scholar] [CrossRef] [PubMed]
- Bikiaris, D.; Panayiotou, C. LDPE/Starch Blends Compatibilized with PE-g-MA Copolymers. J. Appl. Polym. Sci. 1998, 70, 1503–1521. [Google Scholar] [CrossRef]
- Sutirman, Z.A.; Sanagi, M.M.; Abd Karim, K.J.; Wan Ibrahim, W.A.; Jume, B.H. Equilibrium, Kinetic and Mechanism Studies of Cu(II) and Cd(II) Ions Adsorption by Modified Chitosan Beads. Int. J. Biol. Macromol. 2018, 116, 255–263. [Google Scholar] [CrossRef]
- Ayawei, N.; Ebelegi, A.N.; Wankasi, D. Modelling and Interpretation of Adsorption Isotherms. J. Chem. 2017, 2017, 3039817. [Google Scholar] [CrossRef] [Green Version]
- Igberase, E.; Osifo, P. Equilibrium, Kinetic, Thermodynamic and Desorption Studies of Cadmium and Lead by Polyaniline Grafted Cross-Linked Chitosan Beads from Aqueous Solution. J. Ind. Eng. Chem. 2015, 26, 340–347. [Google Scholar] [CrossRef]
- Chen, C.; Tang, Y.; Liu, Y.; Liang, Y.; Zhang, K.; Wang, S.; Liang, Y. Effect of Competitive Adsorption on Zinc Removal from Aqueous Solution and Zinc Smelting Effluent by Eucalyptus Leaf-Based Magnetic Biosorbent. J. Environ. Sci. Health—Part A Toxic/Hazard. Subst. Environ. Eng. 2017, 52, 873–889. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Luo, C.; Lv, Z.; Lu, F.; Qiu, H. Preparation of Magnetic Modified Chitosan and Adsorption of Zn2+ from Aqueous Solutions. Colloids Surf. B Biointerfaces 2011, 88, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Ding, P.; Huang, K.L.; Li, G.Y.; Liu, Y.F.; Zeng, W.W. Kinetics of Adsorption of Zn(II) Ion on Chitosan Derivatives. Int. J. Biol. Macromol. 2006, 39, 222–227. [Google Scholar] [CrossRef]
- Mubarak, M.F.; Ragab, A.H.; Hosny, R.; Ahmed, I.A.; Ahmed, H.A.; El-Bahy, S.M.; El Shahawy, A. Enhanced Performance of Chitosan via a Novel Quaternary Magnetic Nanocomposite Chitosan/Grafted Halloysitenanotubes@znγfe3o4 for Uptake of Cr (III), Fe (III), and Mn (II) from Wastewater. Polymers 2021, 13, 2714. [Google Scholar] [CrossRef] [PubMed]
- Liu, C. Removal of Cobalt (II) Ions from Aqueous Solution on Zinc(II) Ions Doping Chitosan/Hydroxyapatite Composite. Adv. Compos. Lett. 2013, 22, 143–150. [Google Scholar] [CrossRef]
- Shariful, M.I.; Sharif, S.B.; Lee, J.J.L.; Habiba, U.; Ang, B.C.; Amalina, M.A. Adsorption of Divalent Heavy Metal Ion by Mesoporous-High Surface Area Chitosan/Poly (Ethylene Oxide) Nanofibrous Membrane. Carbohydr. Polym. 2017, 157, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Amuda, O.S.; Giwa, A.A.; Bello, I.A. Removal of Heavy Metal from Industrial Wastewater Using Modified Activated Coconut Shell Carbon. Biochem. Eng. J. 2007, 36, 174–181. [Google Scholar] [CrossRef]
- Classification of EDTA Complexes. In Applied Complexometry; Elsevier: Amsterdam, The Netherlands, 1982; pp. 91–222.
- Ralph, G. Pearson Hard and Soft Acids and Bases. J. Am. Chem. Soc. 1963, 85, 3533–3539. [Google Scholar]
- Shinde, R.N.; Pandey, A.K.; Acharya, R.; Guin, R.; Das, S.K.; Rajurkar, N.S.; Pujari, P.K. Chitosan-Transition Metal Ions Complexes for Selective Arsenic(V) Preconcentration. Water Res. 2013, 47, 3497–3506. [Google Scholar] [CrossRef] [PubMed]



| CS Based Composite | qe (mg g−1)/Model | qmax (mg g−1)/Model |
|---|---|---|
| CS-PCL composite (this work) | Synthetic solution 165.59 ± 3.4 Pseudo-second order | Zn solution 495.75 Dubinin-Radushkevich |
| CS and CS grafted materials [4] | Zn Solution 151.7 Pseudo-second order | Binary solution of Zn and cationic dye 168.8 with neat CS 290 with succinyl-grafted Tóth |
| PVA/CS beads [8] | Quaternary solution of Zn, Cu, Pb and Cd 35.71 Pseudo-second order | Zn Solution 83.33 Langmuir |
| CS coated diatomaceous earth [19] | Zn Solution 130.7 Pseudo-second order | Zn Solution 140.84 Langmuir |
| CS/poly(ethylene oxide) membrane [59] | Zn Solution 84.43 Pseudo-second order | Zn Solution 117 Langmuir |
| Activated coconut shell carbon modified with CS [60] | ND | Zn Solution 60.41 Langmuir |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez, M.E.; Rangel-Méndez, J.R.; Gimeno, M.; Tecante, A.; Lapidus, G.T.; Shirai, K. Removal of Heavy Metal Ions from Wastewater with Poly-ε-Caprolactone-Reinforced Chitosan Composite. Polymers 2022, 14, 5196. https://doi.org/10.3390/polym14235196
Martínez ME, Rangel-Méndez JR, Gimeno M, Tecante A, Lapidus GT, Shirai K. Removal of Heavy Metal Ions from Wastewater with Poly-ε-Caprolactone-Reinforced Chitosan Composite. Polymers. 2022; 14(23):5196. https://doi.org/10.3390/polym14235196
Chicago/Turabian StyleMartínez, Manuel E., José René Rangel-Méndez, Miquel Gimeno, Alberto Tecante, Gretchen T. Lapidus, and Keiko Shirai. 2022. "Removal of Heavy Metal Ions from Wastewater with Poly-ε-Caprolactone-Reinforced Chitosan Composite" Polymers 14, no. 23: 5196. https://doi.org/10.3390/polym14235196






