Preparation and Characterization of Cardanol-Based Flame Retardant for Enhancing the Flame Retardancy of Epoxy Adhesives
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
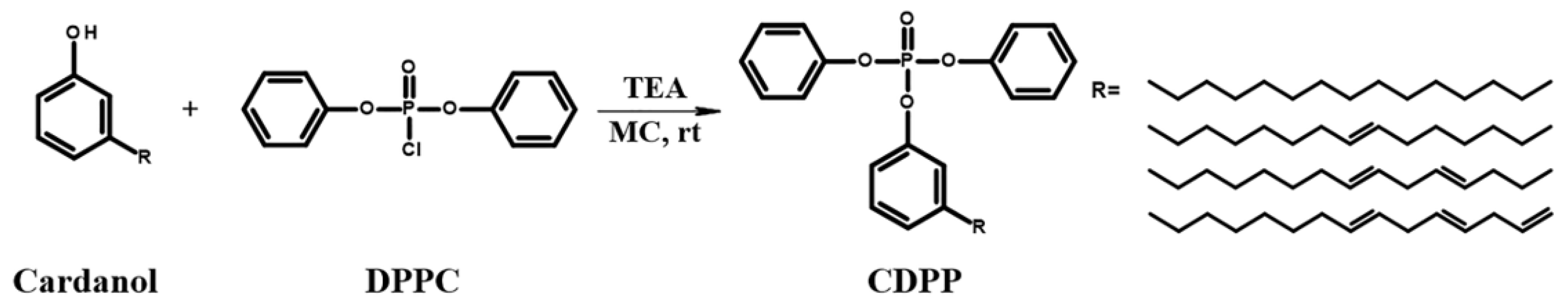
2.2. Synthesis of Cardanyl Diphenylphosphate (CDPP)
2.3. Preparation of Epoxy Adhesive Series with CDPP
2.4. Characterization
3. Results
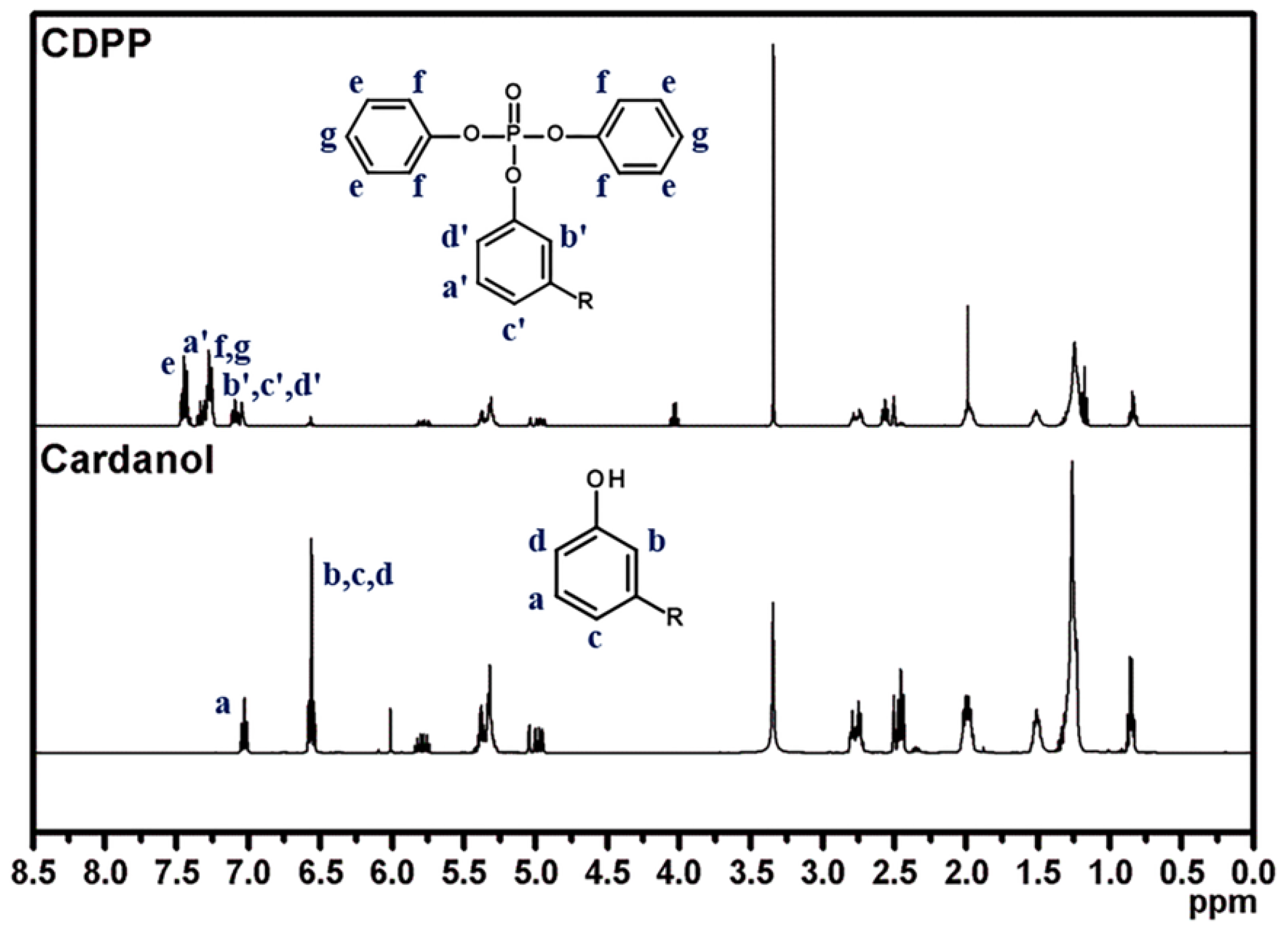
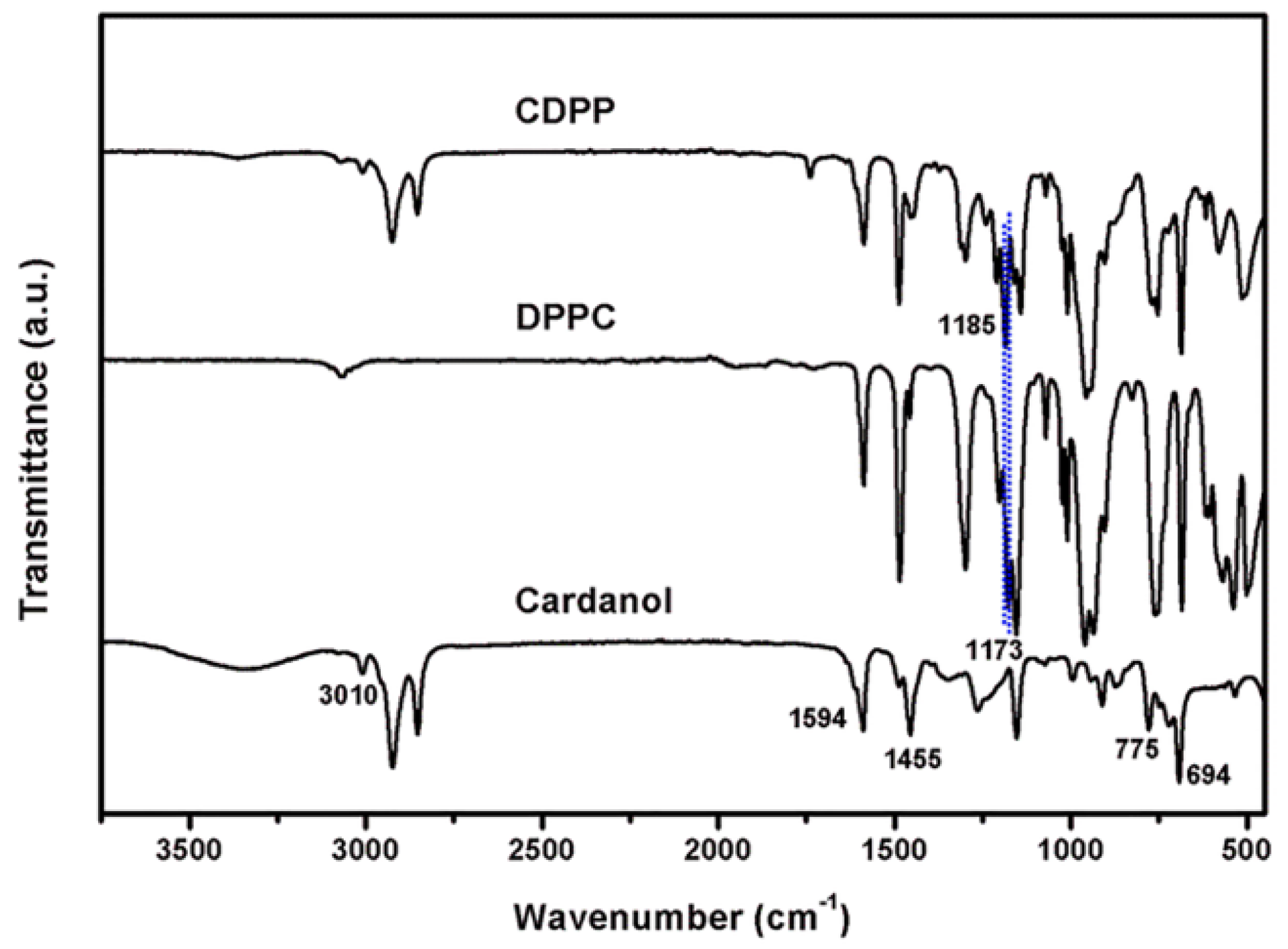
3.1. Synthesis and Characterization of CDPP
3.2. Thermogravimetric Curves of CDPP and EP Series
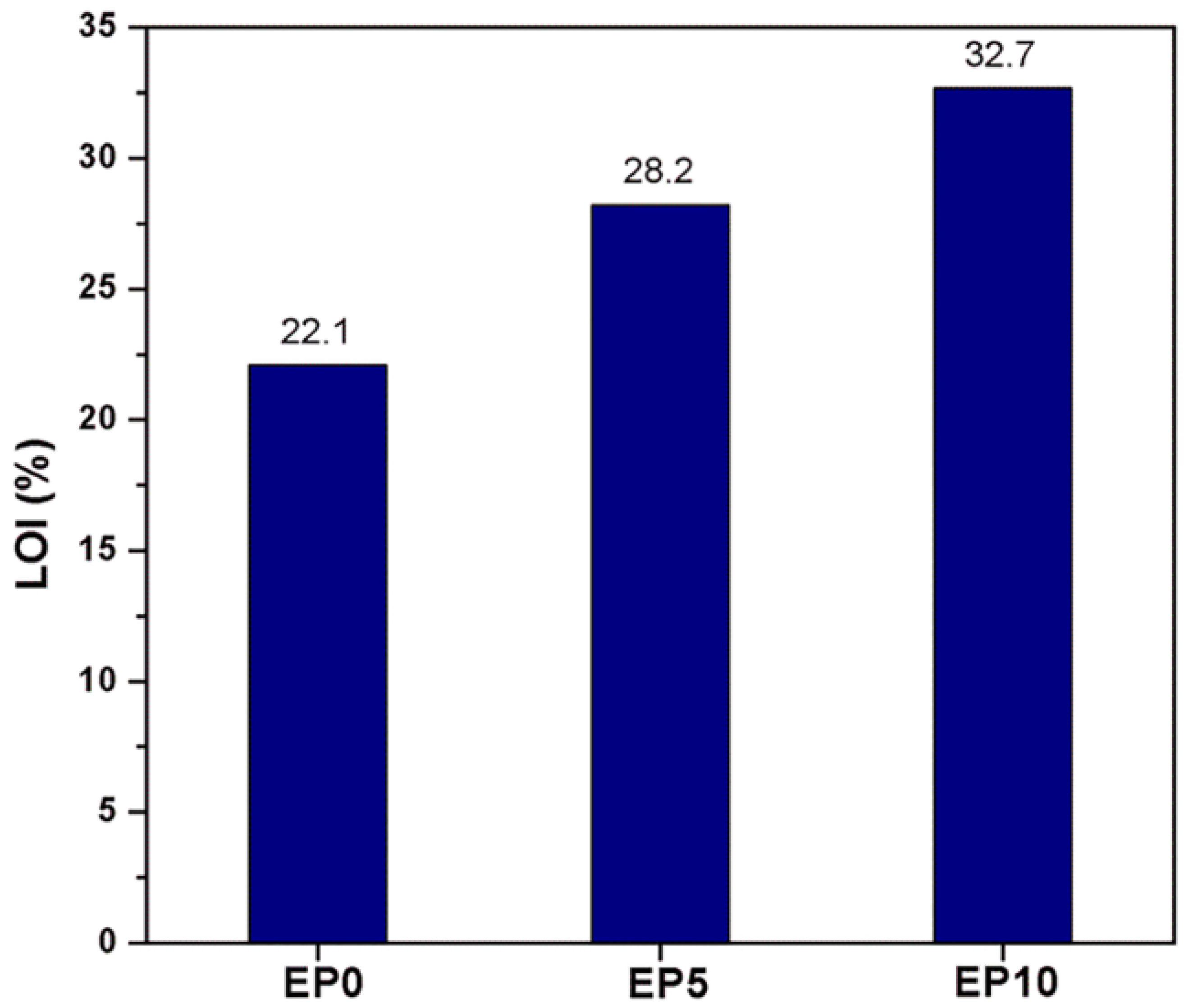
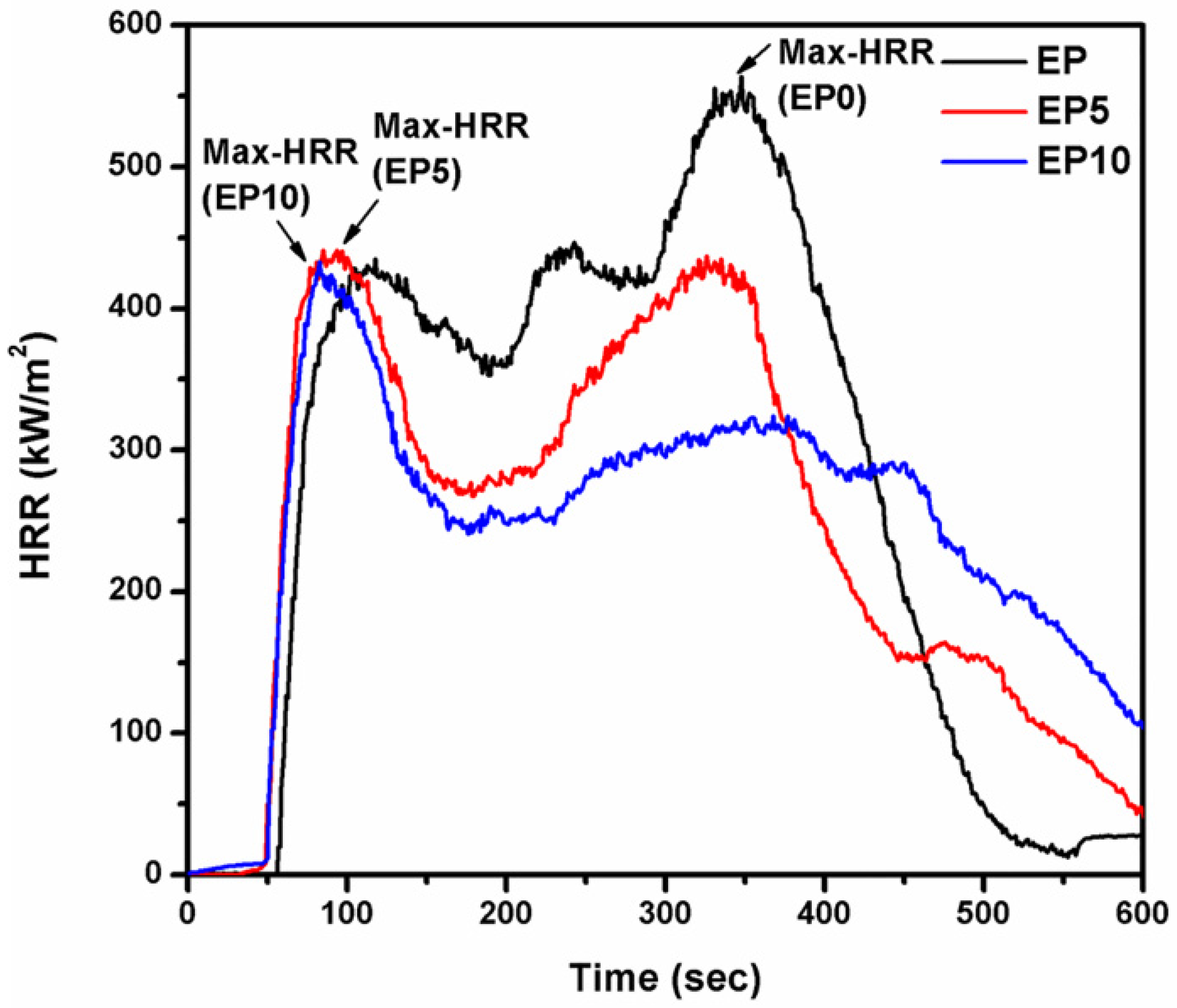
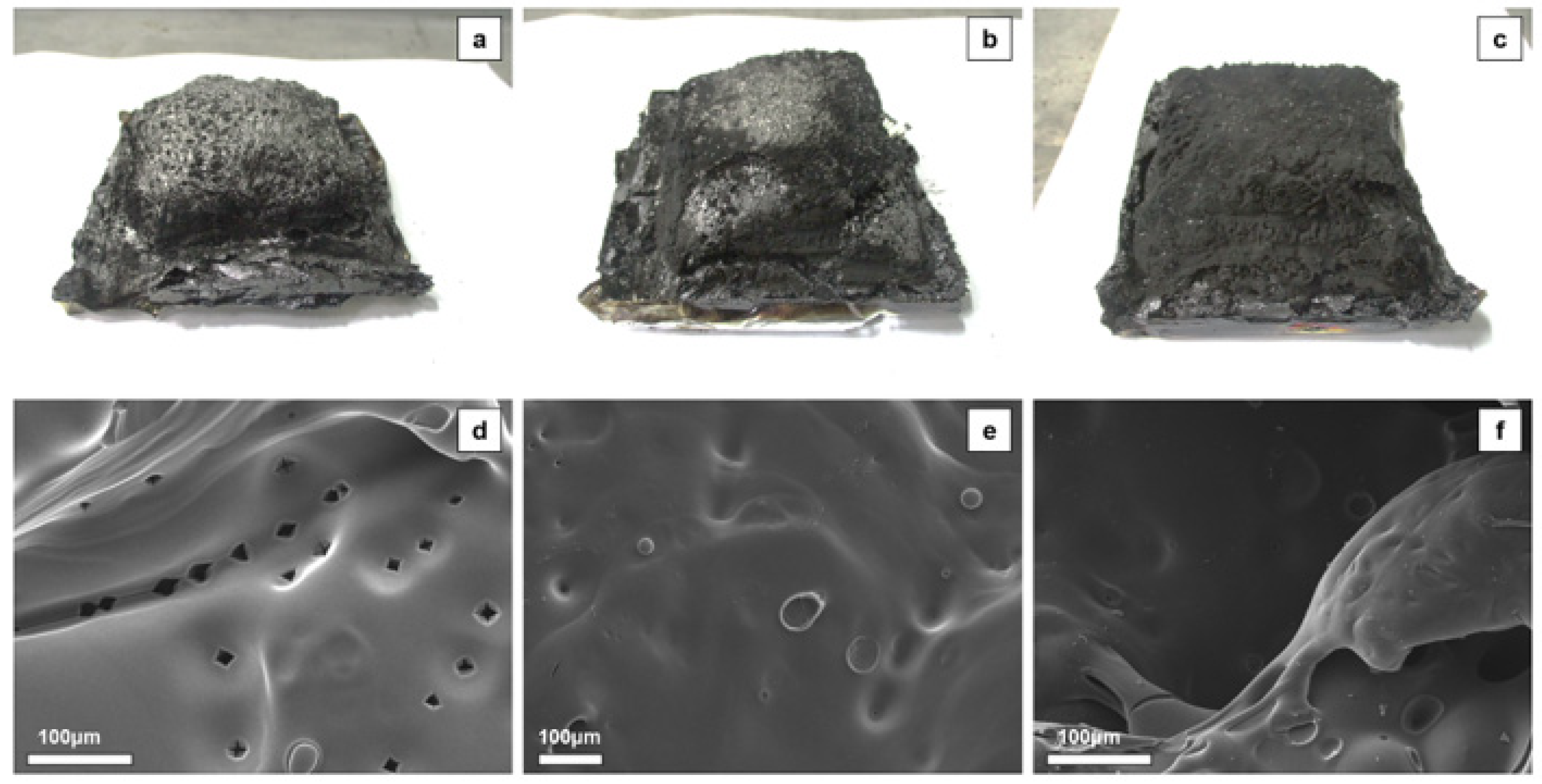
3.3. Flame Retardancy of EP Series with CDPP
3.4. Adhesion Property of EP Series Containing CDPP
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, W.; Wang, X.; Gangireddy, C.S.R.; Wang, J.; Pan, Y.; Xing, W.; Song, L.; Hu, Y. Cardanol derived benzoxazine in combination with boron-doped graphene toward simultaneously improved toughening and flame retardant epoxy composites. Compos. Part A Appl. Sci. Manuf. 2019, 116, 13–23. [Google Scholar] [CrossRef]
- Li, L.; Wang, H.; Hua, F.; Wang, M.; Zhang, Y.; Xi, H.; Yang, J.; Yang, Z.; Lei, Z. Flame retardancy of epoxy resin improved by graphene hybrid containing phosphorous, boron, nitrogen and silicon elements. Macromol. Res. 2021, 29, 625–635. [Google Scholar] [CrossRef]
- Huo, S.; Song, P.; Yu, B.; Ran, S.; Chevali, V.S.; Liu, L.; Fang, Z.; Wang, H. Phosphorus-containing flame retardant epoxy thermosets: Recent advances and future perspectives. Prog. Polym. Sci. 2021, 114, 101366. [Google Scholar] [CrossRef]
- Kim, J.S.; Yoon, K.H.; Lee, Y.S.; Han, J.H. Mechanical Properties and Thermal Conductivity of Epoxy Composites Containing Aluminum-Exfoliated Graphite Nanoplatelets Hybrid Powder. Macromol. Res. 2021, 29, 252–256. [Google Scholar] [CrossRef]
- Wang, L.; Yan, S.; Zhang, L.; Mai, Y.; Li, W.; Pang, H. An Acid-/Base-Degradable Epoxy Resin Cured by 1, 3, 5-Triacroylamino-hexahydro-s-triazine Derivative. Macromol. Res. 2021, 29, 462–469. [Google Scholar] [CrossRef]
- Zhai, L.; Ling, G.; Wang, Y. Effect of nano-Al2O3 on adhesion strength of epoxy adhesive and steel. Int. J. Adhes. Adhes. 2008, 28, 23–28. [Google Scholar] [CrossRef]
- Zhou, X.; Qiu, S.; Xing, W.; Gangireddy, C.S.R.; Gui, Z.; Hu, Y. Hierarchical polyphosphazene@ molybdenum disulfide hybrid structure for enhancing the flame retardancy and mechanical property of epoxy resins. ACS Appl. Mater. Interfaces 2017, 9, 29147–29156. [Google Scholar] [CrossRef]
- Zhang, J.; Kong, Q.; Wang, D.-Y. Simultaneously improving the fire safety and mechanical properties of epoxy resin with Fe-CNTs via large-scale preparation. J. Mater. Chem. A 2018, 6, 6376–6386. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, S.; Guo, W.-W.; Wang, P.-L.; Xing, W.; Song, L.; Hu, Y. Renewable cardanol-based phosphate as a flame retardant toughening agent for epoxy resins. ACS Sustain. Chem. Eng. 2017, 5, 3409–3416. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, D.; Li, Z.; Li, Z.; Peng, X.; Liu, C.; Zhang, Y.; Zheng, P. Recent developments in the flame-retardant system of epoxy resin. Materials 2020, 13, 2145. [Google Scholar] [CrossRef]
- Zhang, W.; He, X.; Song, T.; Jiao, Q.; Yang, R. The influence of the phosphorus-based flame retardant on the flame retardancy of the epoxy resins. Polym. Degrad. Stab. 2014, 109, 209–217. [Google Scholar] [CrossRef]
- Li, S.; Wang, X.; Chen, T.; Xu, M.; Liu, L.; Gao, S.; Wei, Y.; Li, B. Effects of a composite flame retardant system on the flame retardancy and mechanical performance of epoxy resin adhesive. J. Vinyl Addit. Technol. 2022, 28, 775–787. [Google Scholar] [CrossRef]
- Xie, R.; Yuan, Y.; Sun, P.; Liu, Z.; Ma, J.; Yang, G.; Wang, K.; Li, M.; Shang, L.; Ao, Y. Design of epoxy resin with sustainability, high adhesion and excellent flame retardancy based on bio-based molecules. J. Mater. Sci. 2022, 57, 13078–13096. [Google Scholar] [CrossRef]
- Back, J.-H.; Baek, D.; Kim, T.; Seo, B.; Lee, W.; Kim, H.-J. Synthesis of phosphorus-containing polyol and its effects on impact resistance and flame retardancy of structural epoxy adhesives. Int. J. Adhes. Adhes. 2020, 100, 102601. [Google Scholar] [CrossRef]
- Kim, H.-C. Study on the Compatibility of Brominated Epoxy Resin with Nylon 6 and the Characterization of the Blends. Text. Color. Finish. 2010, 22, 155–162. [Google Scholar] [CrossRef]
- Luda, M.; Balabanovich, A.; Camino, G. Thermal decomposition of fire retardant brominated epoxy resins. J. Anal. Appl. Pyrolysis 2002, 65, 25–40. [Google Scholar] [CrossRef]
- Balabanovich, A.; Hornung, A.; Merz, D.; Seifert, H. The effect of a curing agent on the thermal degradation of fire retardant brominated epoxy resins. Polym. Degrad. Stab. 2004, 85, 713–723. [Google Scholar] [CrossRef]
- Luda, M.; Balabanovich, A.; Zanetti, M.; Guaratto, D. Thermal decomposition of fire retardant brominated epoxy resins cured with different nitrogen containing hardeners. Polym. Degrad. Stab. 2007, 92, 1088–1100. [Google Scholar] [CrossRef]
- Wang, C.; Huo, S.; Ye, G.; Song, P.; Wang, H.; Liu, Z. AP/Si-containing polyethylenimine curing agent towards transparent, durable fire-safe, mechanically-robust and tough epoxy resins. Chem. Eng. J. 2023, 451, 138768. [Google Scholar] [CrossRef]
- Wang, J.; Liu, W.; Liu, H.; Wang, X.; Wu, D.; Zhang, S.; Shi, S.; Liu, W.; Wu, Z. Cyclotriphosphazene-based epoxy resins with excellent mechanical and flame retardant properties. Polymer 2022, 261, 125399. [Google Scholar] [CrossRef]
- Wang, J.; Guo, Y.; Zhao, S.; Zhu, Z.; Huang, Y.; Tian, T.; Zhou, Y.; Bi, L. Low flammability epoxy resin enabled by a phosphaphenanthrene-based oligomer. React. Funct. Polym. 2022, 178, 105335. [Google Scholar] [CrossRef]
- Huo, S.; Sai, T.; Ran, S.; Guo, Z.; Fang, Z.; Song, P.; Wang, H. A hyperbranched P/N/B-containing oligomer as multifunctional flame retardant for epoxy resins. Compos. Part B Eng. 2022, 234, 109701. [Google Scholar] [CrossRef]
- Liu, R.; Wang, X. Synthesis, characterization, thermal properties and flame retardancy of a novel nonflammable phosphazene-based epoxy resin. Polym. Degrad. Stab. 2009, 94, 617–624. [Google Scholar] [CrossRef]
- Yang, S.; Wang, J.; Huo, S.; Wang, M.; Cheng, L. Synthesis of a phosphorus/nitrogen-containing additive with multifunctional groups and its flame-retardant effect in epoxy resin. Ind. Eng. Chem. Res. 2015, 54, 7777–7786. [Google Scholar] [CrossRef]
- Battig, A.; Markwart, J.C.; Wurm, F.R.; Schartel, B. Hyperbranched phosphorus flame retardants: Multifunctional additives for epoxy resins. Polym. Chem. 2019, 10, 4346–4358. [Google Scholar] [CrossRef]
- Dogan, M.; Dogan, S.D.; Savas, L.A.; Ozcelik, G.; Tayfun, U. Flame retardant effect of boron compounds in polymeric materials. Compos. Part B Eng. 2021, 222, 109088. [Google Scholar] [CrossRef]
- Sun, Z.; Hou, Y.; Hu, Y.; Hu, W. Effect of additive phosphorus-nitrogen containing flame retardant on char formation and flame retardancy of epoxy resin. Mater. Chem. Phys. 2018, 214, 154–164. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, J.; Ma, L.; Yin, X.; Zhu, Z.; Song, P. Fabrication of anti-dripping and flame-retardant polylactide modified with chitosan derivative/aluminum hypophosphite. Carbohydr. Polym. 2022, 298, 120141. [Google Scholar] [CrossRef]
- Leng, Y.-M.; Zhao, X.; Fu, T.; Wang, X.-L.; Wang, Y.-Z. Bio-Based Flame-Retardant and Smoke-Suppressing Wood Plastic Composites Enabled by Phytic Acid Tyramine Salt. ACS Sustain. Chem. Eng. 2022, 10, 5055–5066. [Google Scholar] [CrossRef]
- Yao, Z.; Qiu, Y.; Qian, L.; Xu, B. A novel high phosphorus-efficiency phosphaphenanthrene curing agent for fabricating flame retardant and toughened epoxy thermoset. Polym. Adv. Technol. 2022, 33, 770–781. [Google Scholar] [CrossRef]
- Liu, L.; Shi, B.; Zhang, A.; Xue, Y.; Zhang, J.; Dai, J.; Hassanpour, M.; Tang, L.-C.; Shi, Y.; Song, P. A polyphosphoramide-grafted lignin enabled thermostable and fire-retardant polylactide with preserved mechanical properties. Compos. Part A Appl. Sci. Manuf. 2022, 160, 107028. [Google Scholar] [CrossRef]
- Zhan, Y.; Wu, X.; Wang, S.; Yuan, B.; Fang, Q.; Shang, S.; Cao, C.; Chen, G. Synthesis of a bio-based flame retardant via a facile strategy and its synergistic effect with ammonium polyphosphate on the flame retardancy of polylactic acid. Polym. Degrad. Stab. 2021, 191, 109684. [Google Scholar] [CrossRef]
- Zhong, J.; Huang, Y.; Chen, Y.; Li, L.; Guo, C. Synthesis of eugenol-modified epoxy resin and application on wood flame retardant coating. Ind. Crops Prod. 2022, 183, 114979. [Google Scholar] [CrossRef]
- Liu, J.; Dai, J.; Wang, S.; Peng, Y.; Cao, L.; Liu, X. Facile synthesis of bio-based reactive flame retardant from vanillin and guaiacol for epoxy resin. Compos. Part B Eng. 2020, 190, 107926. [Google Scholar] [CrossRef]
- Heinen, M.; Gerbase, A.E.; Petzhold, C.L. Vegetable oil-based rigid polyurethanes and phosphorylated flame-retardants derived from epoxydized soybean oil. Polym. Degrad. Stab. 2014, 108, 76–86. [Google Scholar] [CrossRef]
- Balachandran, V.S.; Jadhav, S.R.; Vemula, P.K.; John, G. Recent advances in cardanol chemistry in a nutshell: From a nut to nanomaterials. Chem. Soc. Rev. 2013, 42, 427–438. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, E.; Tu, Y.; Ye, M.; Chen, N. An Eco-Friendly Wood Adhesive Consisting of Soybean Protein and Cardanol-Based Epoxy for Wood Based Composites. Polymers 2022, 14, 2831. [Google Scholar] [CrossRef]
- Lee, J.-H.; Jeon, J.-S.; Kim, S.-M. Green adhesives using tannin and cashew nut shell liquid for environment-friendly furniture materials. J. Korea Furnit. Soc. 2011, 22, 219–229. [Google Scholar]
- Phalak, G.; Patil, D.; Patil, A.; Mhaske, S. Synthesis of acrylated cardanol diphenyl phosphate for UV curable flame-retardant coating application. Eur. Polym. J. 2019, 121, 109320. [Google Scholar] [CrossRef]
- Bo, C.; Shi, Z.; Hu, L.; Pan, Z.; Hu, Y.; Yang, X.; Jia, P.; Ren, X.; Zhang, M.; Zhou, Y. Cardanol derived P, Si and N based precursors to develop flame retardant phenolic foam. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Guo, W.; Wang, X.; Huang, J.; Mu, X.; Cai, W.; Song, L.; Hu, Y. Phosphorylated cardanol-formaldehyde oligomers as flame-retardant and toughening agents for epoxy thermosets. Chem. Eng. J. 2021, 423, 130192. [Google Scholar] [CrossRef]
- Ma, H.-X.; Li, J.-J.; Qiu, J.-J.; Liu, Y.; Liu, C.-M. Renewable cardanol-based star-shaped prepolymer containing a phosphazene core as a potential biobased green fire-retardant coating. ACS Sustain. Chem. Eng. 2017, 5, 350–359. [Google Scholar] [CrossRef]
- Chen, L.; Song, L.; Lv, P.; Jie, G.; Tai, Q.; Xing, W.; Hu, Y. A new intumescent flame retardant containing phosphorus and nitrogen: Preparation, thermal properties and application to UV curable coating. Prog. Org. Coat. 2011, 70, 59–66. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Hsiue, G.-H.; Lan, C.-W.; Chiu, Y.-S. Phosphorus-containing epoxy for flame retardance: IV. Kinetics and mechanism of thermal degradation. Polym. Degrad. Stab. 1997, 56, 291–299. [Google Scholar] [CrossRef]
- Wang, X.; Spörer, Y.; Leuteritz, A.; Kuehnert, I.; Wagenknecht, U.; Heinrich, G.; Wang, D.-Y. Comparative study of the synergistic effect of binary and ternary LDH with intumescent flame retardant on the properties of polypropylene composites. RSC Adv. 2015, 5, 78979–78985. [Google Scholar] [CrossRef]
- Guo, W.; Wang, X.; Huang, J.; Cai, W.; Song, L.; Hu, Y. Intrinsically anti-flammable and self-toughened phosphorylated cardanol-derived novolac epoxy thermosets. Ind. Crops Prod. 2021, 166, 113496. [Google Scholar] [CrossRef]
- Chang, M.-K.; Hwang, S.-S.; Liu, S.-P. Flame retardancy and thermal stability of ethylene-vinyl acetate copolymer nanocomposites with alumina trihydrate and montmorillonite. J. Ind. Eng. Chem. 2014, 20, 1596–1601. [Google Scholar] [CrossRef]
- Peng, X.; Li, Z.; Wang, D.; Li, Z.; Liu, C.; Wang, R.; Jiang, L.; Liu, Q.; Zheng, P. A facile crosslinking strategy endows the traditional additive flame retardant with enormous flame retardancy improvement. Chem. Eng. J. 2021, 424, 130404. [Google Scholar] [CrossRef]
- Kim, S.-H.; Kim, S.-B.; Cha, S.-H. Preparation and Characterization of Biopolyurethane Film with a Novel Cross-linkable Biopolyol Based on Cardanol. Polymer (Korea) 2018, 42, 736–746. [Google Scholar] [CrossRef]
- Huggett, C. Estimation of rate of heat release by means of oxygen consumption measurements. Fire Mater. 1980, 4, 61–65. [Google Scholar] [CrossRef]
- Wang, J.; Huo, S.; Wang, J.; Yang, S.; Chen, K.; Li, C.; Fang, D.; Fang, Z.; Song, P.; Wang, H. Green and Facile Synthesis of Bio-Based, Flame-Retardant, Latent Imidazole Curing Agent for Single-Component Epoxy Resin. ACS Appl. Polym. Mater. 2022, 4, 3564–3574. [Google Scholar] [CrossRef]
- Duan, H.; Chen, Y.; Ji, S.; Hu, R.; Ma, H. A novel phosphorus/nitrogen-containing polycarboxylic acid endowing epoxy resin with excellent flame retardance and mechanical properties. Chem. Eng. J. 2019, 375, 121916. [Google Scholar] [CrossRef]
- Sai, T.; Ran, S.; Guo, Z.; Song, P.; Fang, Z. Recent advances in fire-retardant carbon-based polymeric nanocomposites through fighting free radicals. SusMat 2022, 2, 411–434. [Google Scholar] [CrossRef]
- Liu, L.; Zhu, M.; Ma, Z.; Xu, X.; Seraji, S.M.; Yu, B.; Sun, Z.; Wang, H.; Song, P. A reactive copper-organophosphate-MXene heterostructure enabled antibacterial, self-extinguishing and mechanically robust polymer nanocomposites. Chem. Eng. J. 2022, 430, 132712. [Google Scholar] [CrossRef]
- Chu, C.-W.; Zhang, Y.; Obayashi, K.; Kojio, K.; Takahara, A. Single-Lap Joints Bonded with Epoxy Nanocomposite Adhesives: Effect of Organoclay Reinforcement on Adhesion and Fatigue Behaviors. ACS Appl. Polym. Mater. 2021, 3, 3428–3437. [Google Scholar] [CrossRef]
- García del Cid, M.; Prolongo, M.; Salom, C.; Sánchez-Cabezudo, M.; Masegosa, R. Influence of different organoclays on the curing, morphology, and dynamic mechanical properties of an epoxy adhesive. J. Adhes. 2014, 90, 817–834. [Google Scholar] [CrossRef]
- Park, S.-J.; Kim, B.-J. Roles of acidic functional groups of carbon fiber surfaces in enhancing interfacial adhesion behavior. Mater. Sci. Eng. A 2005, 408, 269–273. [Google Scholar] [CrossRef]
- Kinloch, A. The science of adhesion. J. Mater. Sci. 1980, 15, 2141–2166. [Google Scholar] [CrossRef]
- Mansourian-Tabaei, M.; Jafari, S.H.; Khonakdar, H.A. Lap shear strength and thermal stability of diglycidyl ether of bisphenol a/epoxy novolac adhesives with nanoreinforcing fillers. J. Appl. Polym. Sci. 2014, 131, 40017. [Google Scholar] [CrossRef]


| Samples | BADGE (wt%) | DDM (wt%) | CDPP (wt%) |
|---|---|---|---|
| EP0 | 77.64 | 22.36 | 0 |
| EP5 | 73.75 | 21.24 | 5.01 |
| EP10 | 69.83 | 20.11 | 10.06 |
| Samples | Td5% (°C) | Td30% (°C) | Residue % at 700 °C | |
|---|---|---|---|---|
| Measured | Calculated | |||
| CDPP | 284 | 344 | 2 | - |
| EP0 | 359 | 394 | 18 | - |
| EP5 | 295 | 383 | 21 | 17 |
| EP10 | 285 | 379 | 24 | 16 |
| Samples | Max-HRR (kW/m2) at Time (s) | THR (MJ/m2) |
|---|---|---|
| EP0 | 564.07 (348) | 168.1 |
| EP5 | 441.34 (85) | 148.1 |
| EP10 | 433.03 (83) | 147.9 |
| Samples | Lap Shear Strength (MPa) |
|---|---|
| EP0 | 6.27 ± 0.18 |
| EP5 | 7.18 ± 0.35 |
| EP10 | 5.55 ± 0.41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, W.-J.; Cha, S.-H.; Kim, D.-H. Preparation and Characterization of Cardanol-Based Flame Retardant for Enhancing the Flame Retardancy of Epoxy Adhesives. Polymers 2022, 14, 5205. https://doi.org/10.3390/polym14235205
Lee W-J, Cha S-H, Kim D-H. Preparation and Characterization of Cardanol-Based Flame Retardant for Enhancing the Flame Retardancy of Epoxy Adhesives. Polymers. 2022; 14(23):5205. https://doi.org/10.3390/polym14235205
Chicago/Turabian StyleLee, Won-Ji, Sang-Ho Cha, and Do-Hyun Kim. 2022. "Preparation and Characterization of Cardanol-Based Flame Retardant for Enhancing the Flame Retardancy of Epoxy Adhesives" Polymers 14, no. 23: 5205. https://doi.org/10.3390/polym14235205
APA StyleLee, W.-J., Cha, S.-H., & Kim, D.-H. (2022). Preparation and Characterization of Cardanol-Based Flame Retardant for Enhancing the Flame Retardancy of Epoxy Adhesives. Polymers, 14(23), 5205. https://doi.org/10.3390/polym14235205