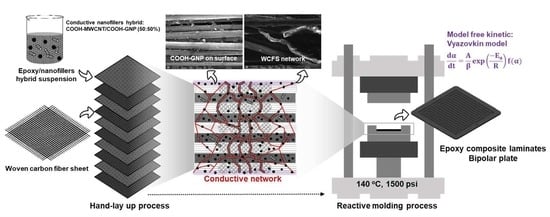
Performance Evaluation for Ultra-Lightweight Epoxy-Based Bipolar Plate Production with Cycle Time Reduction of Reactive Molding Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Bipolar Plate Fabrication
2.3. Characterizations
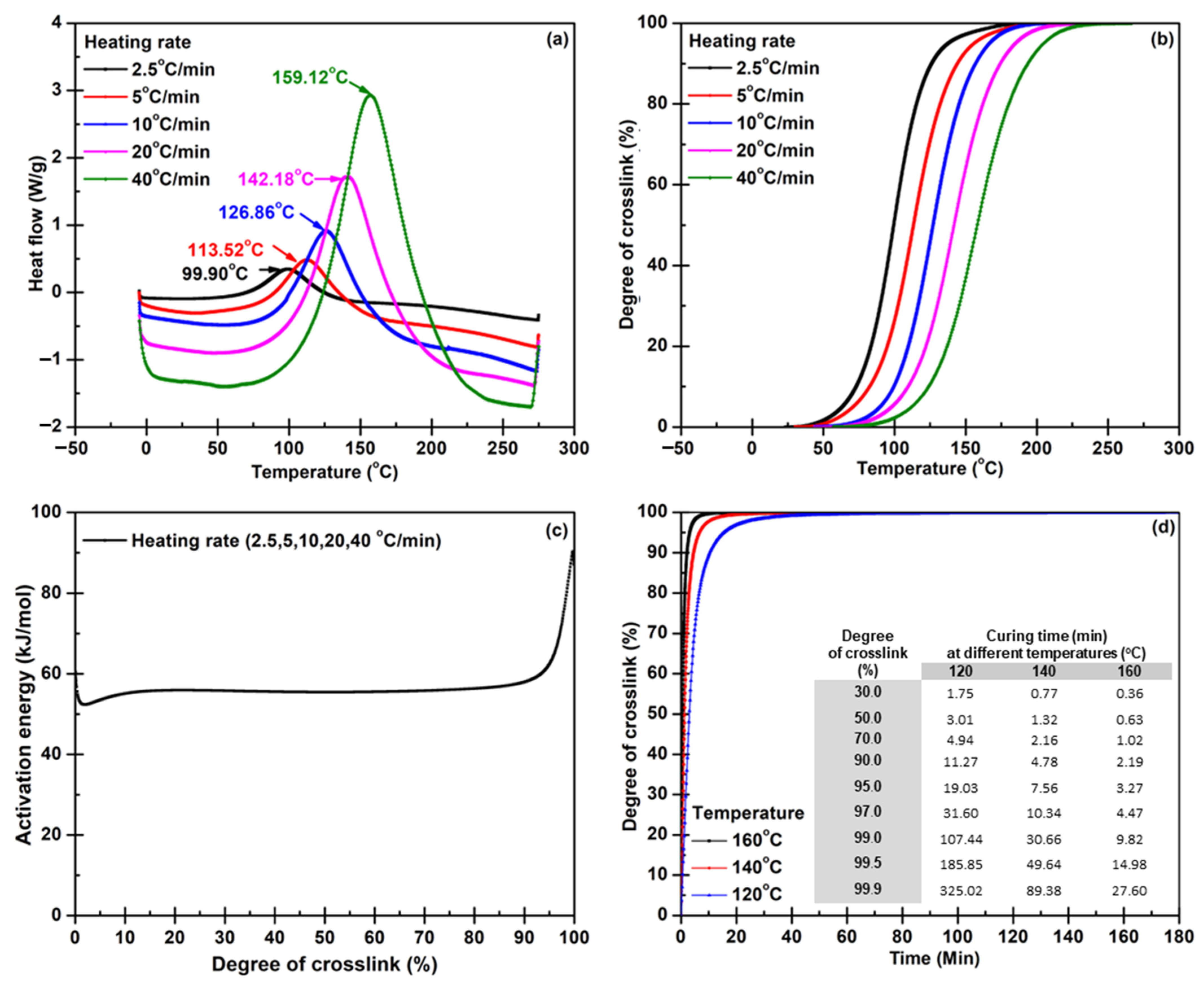
2.3.1. Curing Kinetic Analysis
2.3.2. Electrical Conductivity and Mechanical Property Measurements
2.3.3. Bipolar Plate Performance Diagnosis
3. Results and Discussion
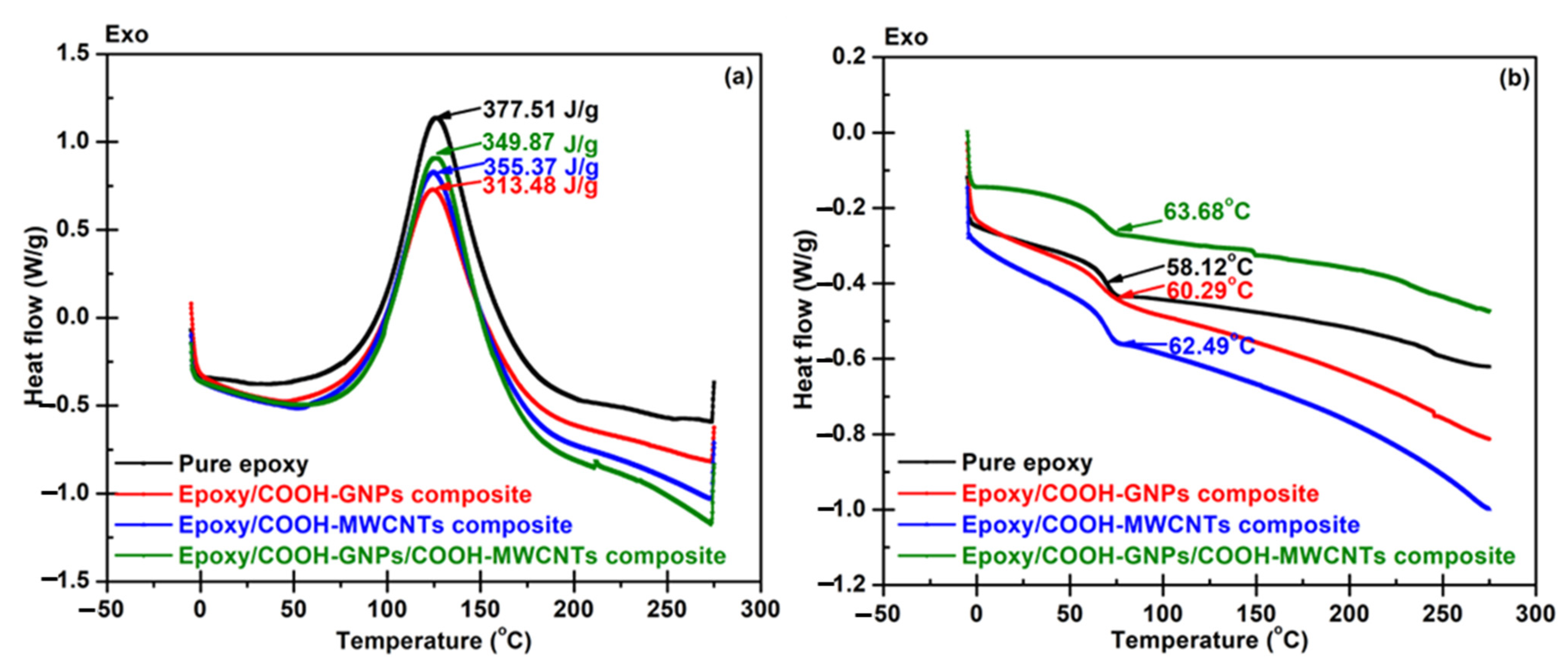
3.1. Predicting Reaction Behavior from DSC to Minimize Cycle Time for BP Molding
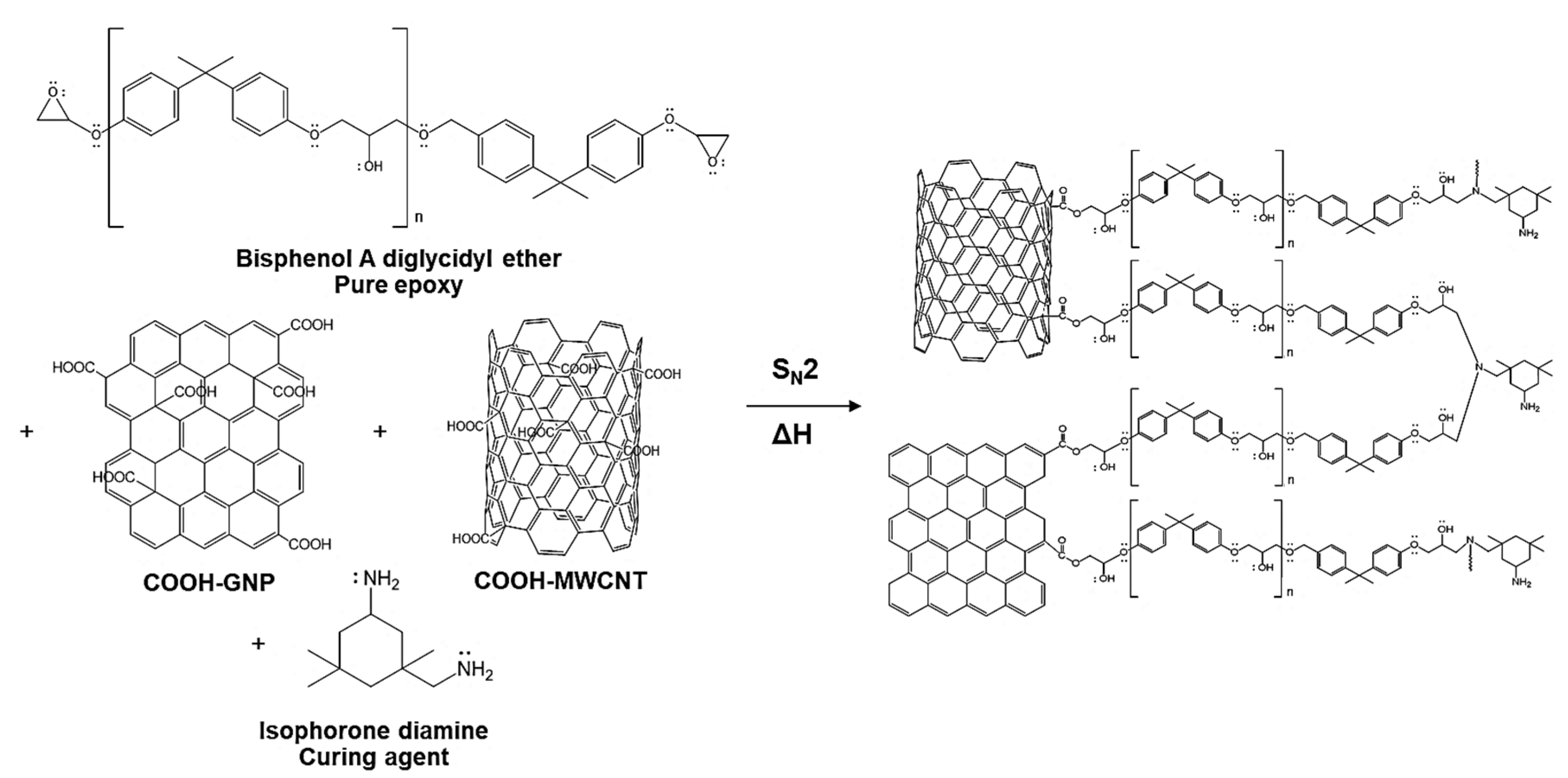
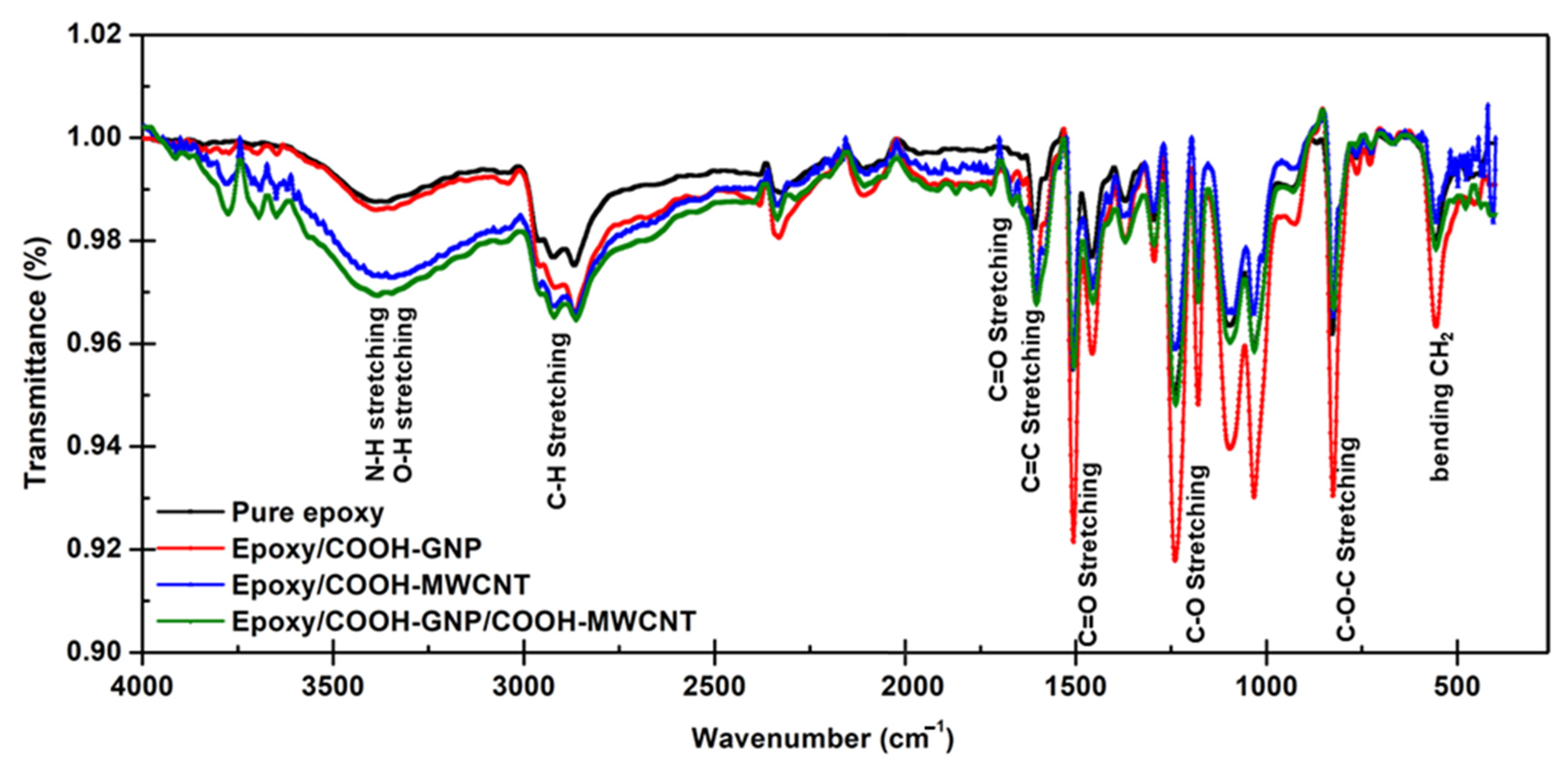
3.2. Reactions Generated in the Laminated Composite during the Curing Process
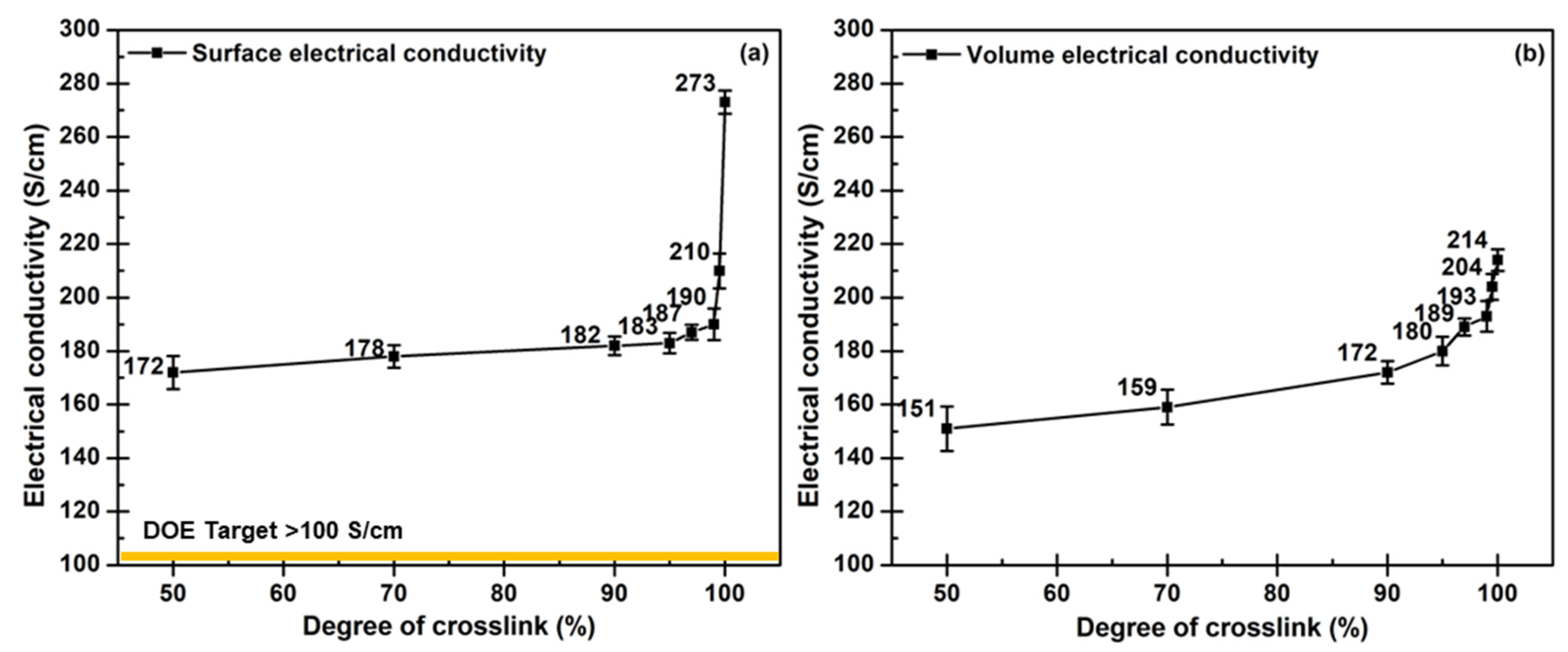
3.3. Relation between Cycle Time Consideration and Mechanical Property Requirement
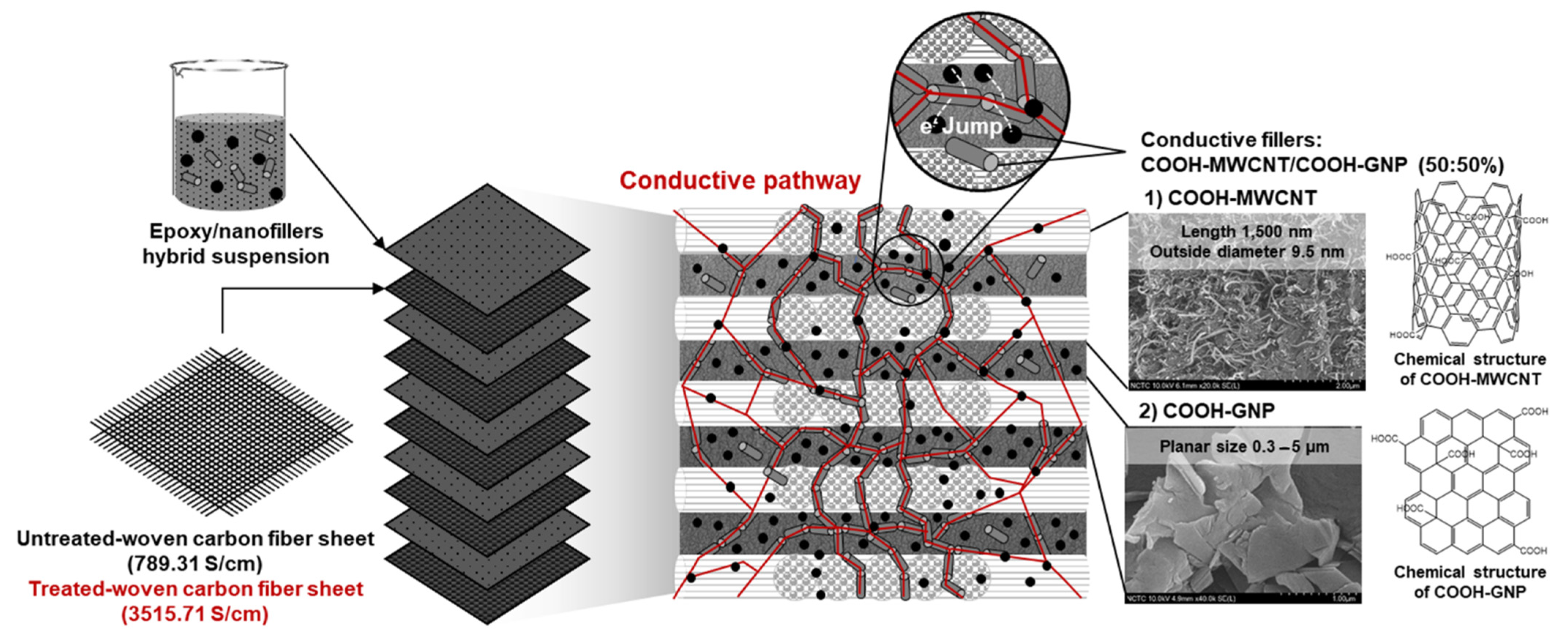
3.4. Composite Structure Design to Create an Electrically Conductive Pathway
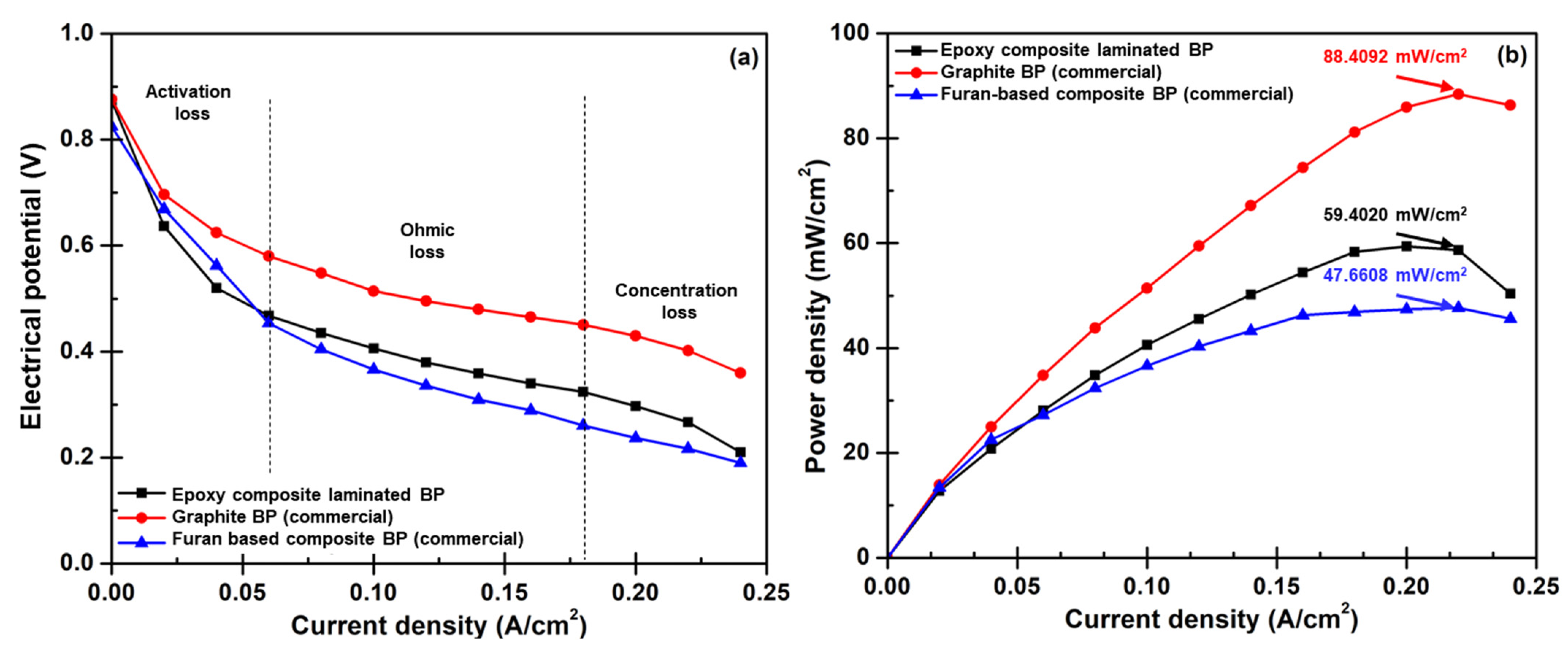
3.5. Bipolar Plate Performance Diagnosis
4. Conclusions
- (1)
- Enhancing connections between WCFS and nanofiller particles is the strategy to form a conductive network within the epoxy matrix, resulting in the enhancement of the surface and volume electrical conductivity. The WCFS layers can increase the mechanical strength of the bipolar plates, and mechanical strength is a key factor for long-term resistance to compressive force in fuel cells.
- (2)
- The information content of thermo-analytical curves and the evaluation of kinetic parameters were found to be the most significant for the reduction of reactive molding time.
- (3)
- Chemical bonding characterization via XPS presented the synergistic effect provided by COOH-GNP and COOH-MWCNT. These nanofillers generated the covalent bonds with epoxy molecules as crosslinking that promotes the conductive network formation and escalates the toughness of the composite BPs.
- (4)
- The cycle time of molding epoxy composite BPs was reduced from one and a half hours to 10 min at 140 °C and under a pressure of 1500 psi.
- (5)
- The weight of the invented composite BP accounts for 1.1% of the overall PEMFC single-cell weight, and this desired weight of BPs can reduce the overall PEMFC weight by approximately 60% in comparison with the single cell used in traditional graphite plates.
- (6)
- The sufficed crosslinking degree for the composite BP is 97 since this degree offers the satisfied BP properties: 596 MPa of flexural strength, 52.4 MPa of compressive strength, 0.05 mm of strain displacement, 202 S/cm of surface electrical conductivity, and 193 S/cm of volume electrical conductivity. These characteristics meet the DOE requirement for PEMFC application.
- (7)
- The efficiency of single-cell PEMFC assembled with as-fabricated composite BPs was around 71% together with 59.4020 mW/cm2 of maximum power density. This performance is superior to the performance of the PEMFC assembled with commercial furan-based composite BPs, however; its internal resistance must be reduced for the efficiency to be equivalent to the performance of the cell that utilized commercial graphite BPs.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Di Trolio, P.; Di Giorgio, P.; Genovese, M.; Frasci, E.; Minutillo, M. A Hybrid Power-Unit Based on a Passive Fuel Cell/Battery System for Lightweight Vehicles. Appl. Energy 2020, 279, 115734. [Google Scholar] [CrossRef]
- Ehsani, M.; Gao, Y.; Gay, S.E.; Emadi, A. Modern Electric, Hybris Electric and Fuel Cell Vehicles Fundamentals, Theory, and Design, 2nd ed.; Taylor & Francis Group: Abingdon, UK, 2010; pp. 1–419. [Google Scholar]
- Das, S. Lightweight Opportunities for Fuel Cell Vehicles Reprinted From: Applications of Fuel Cells in Vehicles. In Proceedings of the SAE World Congress, Detroit, MI, USA, 11–14 April 2005. [Google Scholar]
- Kadyk, T.; Winnefeld, C.; Hanke-Rauschenbach, R.; Krewer, U. Analysis and Design of Fuel Cell Systems for Aviation. Energies 2018, 11, 375. [Google Scholar] [CrossRef] [Green Version]
- Kengo, I.; . Toru, E.; Kazuhiko, M.; Tohru, O. Air-Cooled Fuel Cell System in Hybrid Scooter, Auto Tech Rev; Suzuki Motor Corporation: Hamamatsu, Japan, 2014; Volume 3, pp. 24–29. [Google Scholar] [CrossRef]
- Huang, P.H.; Kuo, J.K.; Han, C.Y. Numerical Investigation into Slope-Climbing Capability of Fuel Cell Hybrid Scooter. Appl. Therm. Eng. 2017, 110, 921–930. [Google Scholar] [CrossRef]
- Wang, Y.; Ruiz, D.; Daniela, F.; Chen, K.S.; Wang, Z.; Adroher, X.C. Materials, technological status, and fundamentals of PEM fuel cells – A review. Mater. Today 2019, 32, 178. [Google Scholar] [CrossRef]
- Boukoberine, M.N.; Zia, M.F.; Benbouzid, M.; Zhou, Z.; Donateo, T. Hybrid Fuel Cell Powered Drones Energy Management Strategy Improvement and Hydrogen Saving Using Real Flight Test Data. Energy Convers. Manag. 2021, 236, 113987. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Fernandes, S.; Shihab, M.; Waleed, D. Using Small Capacity Fuel Cells Onboard Drones for Battery Cooling: An Experimental Study. Appl. Sci. 2018, 8, 942. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.; Kang, G.H.; Park, H.W.; Park, Y.B.; Park, Y.H.; Yoon, K.H. Design, Manufacturing, and Characterization of High-Performance Lightweight Bipolar Plates Based on Carbon Nanotube-Exfoliated Graphite Nanoplatelet Hybrid Nanocomposites. J. Nanomater. 2012, 2012, 159737. [Google Scholar] [CrossRef] [Green Version]
- Jayakumar, K.; Pandiyan, S.; Rajalakshmi, N.; Dhathathreyan, K.S. Cost-Benefit Analysis of Commercial Bipolar Plates for PEMFC’s. J. Power Sources 2006, 161, 454–459. [Google Scholar] [CrossRef]
- Energy Efficiency and Renewable Energy (EERE). Hydrogen and Fuel Cell Technologies Office Multi-Year Research, Development, and Demonstration Plan 2011–2020. Office of Energy Efficiency & Renewable Energy: Washington, DC, USA, 2012; pp. 1–378. [Google Scholar] [CrossRef]
- Yuan, X.Z.; Nayoze-Coynel, C.; Shaigan, N.; Fisher, D.; Zhao, N.; Zamel, N.; Gazdzicki, P.; Ulsh, M.; Friedrich, K.A.; Girard, F.; et al. A Review of Functions, Attributes, Properties and Measurements for the Quality Control of Proton Exchange Membrane Fuel Cell Components. J. Power Sources 2021, 491, 229540. [Google Scholar] [CrossRef]
- Youssef, M.E.S.; Amin, R.S.; El-Khatib, K.M. Development and Performance Analysis of PEMFC Stack Based on Bipolar Plates Fabricated Employing Different Designs. Arab. J. Chem. 2018, 11, 609–614. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.; Lim, J.W.; Lee, D.G. Cathode/Anode Integrated Composite Bipolar Plate for High-Temperature PEMFC. Compos. Struct. 2017, 167, 144–151. [Google Scholar] [CrossRef]
- Kim, M.; Lim, J.W.; Kim, K.H.; Lee, D.G. Bipolar Plates Made of Carbon Fabric/Phenolic Composite Reinforced with Carbon Black for PEMFC. Compos. Struct. 2013, 96, 569–575. [Google Scholar] [CrossRef]
- Saadat, N.; Dhakal, H.N.; Tjong, J.; Jaffer, S.; Yang, W.; Sain, M. Recent advances and future perspectives of carbon materials for fuel cell. Renew. Sust. Energ. Rev. 2021, 138, 110535. [Google Scholar] [CrossRef]
- Lee, D.; Lee, D.G. Carbon Composite Bipolar Plate for High-Temperature Proton Exchange Membrane Fuel Cells (HT-PEMFCs). J. Power Sources 2016, 327, 119–126. [Google Scholar] [CrossRef]
- Yeetsorn, R.; Fowler, M.; Tzoganakis, C. A Review of Thermoplastic Composites for Bipolar Plate Materials in PEM Fuel Cells. In Nanocomposites with Unique Properties and Applications in Medicine and Industry; Cuppoletti, J., Ed.; Intechopen: London, UK, 2011; pp. 1–374. [Google Scholar]
- Bortz, D.R.; Heras, E.G.; Martin-Gullon, I. Impressive Fatigue Life and Fracture Toughness Improvements in Graphene Oxide/Epoxy Composites. Macromolecules 2012, 45, 238–245. [Google Scholar] [CrossRef]
- Heo, S.I.; Yun, J.C.; Oh, K.S.; Han, K.S. Influence of Particle Size and Shape on Electrical and Mechanical Properties of Graphite Reinforced Conductive Polymer Composites for the Bipolar Plate of PEM Fuel Cells. Adv. Compos. Mater. Off. J. Jpn. Soc. Compos. Mater. 2006, 15, 115–126. [Google Scholar] [CrossRef]
- Zeng, W.J.; Li, C.; Feng, Y.; Zeng, S.H.; Fu, B.X.; Zhang, X.L. Carboxylated Multi-Walled Carbon Nanotubes (MWCNTs-COOH)-Intercalated Graphene Oxide Membranes for Highly Efficient Treatment of Organic Wastewater. J. Water Process Eng. 2021, 40, 101901. [Google Scholar] [CrossRef]
- Du, C.; Ming, P.; Hou, M.; Fu, J.; Shen, Q.; Liang, D.; Fu, Y.; Luo, X.; Shao, Z.; Yi, B. Preparation and Properties of Thin Epoxy/Compressed Expanded Graphite Composite Bipolar Plates for Proton Exchange Membrane Fuel Cells. J. Power Sources 2010, 195, 794–800. [Google Scholar] [CrossRef]
- Saba, N.; Jawaid, M.; Alothman, O.Y.; Paridah, M.T.; Hassan, A. Recent Advances in Epoxy Resin, Natural Fiber-Reinforced Epoxy Composites and Their Applications. J. Reinf. Plast. Compos. 2016, 35, 447–470. [Google Scholar] [CrossRef]
- Serban, D.; Opran, C.G. Injection Moulded Composite Bipolar Plates for a Portable Hydrogen Fuel Cell Charger. IOP Conf. Ser. Mater. Sci. Eng. 2020, 916, 012104. [Google Scholar] [CrossRef]
- Choi, H.; Seo, D.J.; Choi, W.Y.; Choi, S.W.; Lee, M.H.; Park, Y.J.; Kim, T.Y.; Yoon, Y.G.; Yi, S.C.; Jung, C.Y. An Ultralight-Weight Polymer Electrolyte Fuel Cell Based on Woven Carbon Fiber-Resin Reinforced Bipolar Plate. J. Power Sources 2021, 484, 229291. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, C.; Ling, C.Y.; Han, M.; Yong, R.Y.; Sun, D.; Chen, J. Review on Current Research of Materials, Fabrication and Application for Bipolar Plate in Proton Exchange Membrane Fuel Cell. Int. J. Hydrogen Energy 2020, 45, 29832–29847. [Google Scholar] [CrossRef]
- Aussawasathien, D.; Hrimchum, K. Carboxylic-Plasma-Treated Nanofiller Hybrids in Carbon Fiber Reinforced Epoxy Composites: Dispersion and Synergetic Effects. Express Polym. Lett. 2021, 15, 262–273. [Google Scholar] [CrossRef]
- Adeli, M.; Seyedein, S.H.; Aboutalebi, M.R.; Kobashi, M.; Kanetake, N. Implementation of DSC Analysis in Reaction Kinetics during Heating of Ti–50 at.% Al Powder Mixture. J. Therm. Anal. Calorim. 2017, 128, 867–874. [Google Scholar] [CrossRef]
- Poosala, A.; Hrimchum, K.; Aussawasathien, D.; Pentrakoon, D. The Effect of Oxygen-Plasma Treated Graphene Nanoplatelets upon the Properties of Multiwalled Carbon Nanotube and Polycarbonate Hybrid Nanocomposites Used for Electrostatic Dissipative Applications. J. Nanomater. 2015, 2015, 470297. [Google Scholar] [CrossRef] [Green Version]
- Barros, J.J.P.; Silva, I.D.D.S.; Jaques, N.G.; Wellen, R.M.R. Approaches on the Non-Isothermal Curing Kinetics of Epoxy/PCL Blends. J. Mater. Res. Technol. 2020, 9, 13539–13554. [Google Scholar] [CrossRef]
- Dong, J.; Pomarède, P.; Chehami, L.; Locquet, A.; Meraghni, F.; Declercq, N.F.; Citrin, D.S. Visualization of Subsurface Damage in Woven Carbon Fiber-Reinforced Composites Using Polarization-Sensitive Terahertz Imaging. NDT E Int. 2018, 99, 72–79. [Google Scholar] [CrossRef] [Green Version]
- Wu, F.; Zhou, X.; Yu, X. Reaction Mechanism, Cure Behavior and Properties of a Multifunctional Epoxy Resin, TGDDM, with Latent Curing Agent Dicyandiamide. RSC Adv. 2018, 8, 8248–8258. [Google Scholar] [CrossRef]
- Lu, L.; Xia, L.; Zengheng, H.; Xingyue, S.; Yi, Z.; Pan, L. Investigation on Cure Kinetics of Epoxy Resin Containing Carbon Nanotubes Modified with Hyper-Branched Polyester. RSC Adv. 2018, 8, 29830–29839. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Álvarez, M.; Velasco, F.; Bautista, A.; Abenojar, J. Effect of Silica Nanoparticles on the Curing Kinetics and Erosion Wear of an Epoxy Powder Coating. J. Mater. Res. Technol. 2020, 9, 455–464. [Google Scholar] [CrossRef]
- Vyazovkin, S. Kissinger Method in Kinetics of Materials: Things to Beware and Be Aware of. Molecules 2020, 25, 2813. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Bu, Z.Y.; Xu, C.J.; Li, B.G.; Fan, H. Learning about Novel Amine-Adduct Curing Agents for Epoxy Resins: Butyl-Glycidylether-Modified Poly(Propyleneimine) Dendrimers. Thermochim. Acta. 2011, 519, 72–82. [Google Scholar] [CrossRef]
- Wan, J.; Li, C.; Bu, Z.Y.; Xu, C.J.; Li, B.G.; Fan, H. A Comparative Study of Epoxy Resin Cured with a Linear Diamine and a Branched Polyamine. Chem. Eng. J. 2012, 188, 160–172. [Google Scholar] [CrossRef]
- Al-Bayaty, S.A.; Al-Uqaily, R.A.H.; Jubier, N.J. Using the Coats-Redfern Method during Thermogravimetric Analysis and Differential Scanning Calorimetry Analysis of the Thermal Stability of Epoxy and Epoxy/Silica Nanoparticle Nanocomposites. J. Southwest Jiaotong Univ. 2020, 55, 1–12. [Google Scholar] [CrossRef]
- Witold, B.; Sidney H., G.; Joshua, W. Handbook of Thermoset Plastics. Elsevier Inc.: Amsterdam, Netherlands, 2014; pp. 1–768. [Google Scholar]
- Yeetsorn, R. Development of Electrically Conductive Thermoplastic Composites for Bipolar Plate Application in Polymer Electrolyte Membrane Fuel Cell. Doctoral Thesis, University of Waterloo, Waterloo, ON, Canada, 2010. [Google Scholar]
- Yeetsorn, R.; Maiket, Y.; Kaewmanee, W. The Observation of Supercapacitor Effects on PEMFC-Supercapacitor Hybridization Performance through Voltage Degradation and Electrochemical Processes. RSC Adv. 2020, 10, 13100–13111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Childers, C.H.; Hassan, M.K.; Mauritz, K.A.; Wiggins, J.S. Molecular Scale Cure Rate Dependence of Thermoset Matrix Polymers. Arab. J. Chem. 2016, 9, 206–218. [Google Scholar] [CrossRef] [Green Version]
- Zhou, T.; Cheng, P.; Wang, T.; Xiong, D.S. Improving the Thermal Conductivity of Epoxy Resin by the Addition of a Mixture of Graphite Nanoplatelets and Silicon Carbide Microparticles. Express Polym. Lett. 2013, 7, 585–594. [Google Scholar] [CrossRef]
- Acocella, M.R.; Corcione, C.E.; Giuri, A.; Maggio, M.; Maffezzoli, A.; Guerra, G. Graphene Oxide as a Catalyst for Ring Opening Reactions in Amine Crosslinking of Epoxy Resins. RSC Adv. 2016, 6, 23858–23865. [Google Scholar] [CrossRef]
- Boonlert-Uthai, T.; Taki, K.; Somwangthanaroj, A. Curing Behavior, Rheological, and Thermal Properties of Dgeba Modified with Synthesized Bpa/Peg Hyperbranched Epoxy after Their Photo-Initiated Cationic Polymerization. Polymers 2020, 12, 2240. [Google Scholar] [CrossRef]
- Mirmohseni, A.; Pourtaghi-Zahed, H. Polyamidoamines Based on Castor Oil-Styrene Co-Oligomer/Triethylenetetramine as Curing Agents in High-Performance Epoxy Coatings. J. Appl. Polym. Sci. 2020, 137, 1–18. [Google Scholar] [CrossRef]
- Roy, S.; Das, T.; Zhang, L.; Li, Y.; Ming, Y.; Ting, S.; Hu, X.; Yue, C.Y. Triggering Compatibility and Dispersion by Selective Plasma Functionalized Carbon Nanotubes to Fabricate Tough and Enhanced Nylon 12 Composites. Polymer 2015, 58, 153–161. [Google Scholar] [CrossRef]
- González, M.G.; Cabanelas, J.C.; Baselga, J. Applications of FTIR on Epoxy Resins—Identification, Monitoring the Curing Process, Phase Separation and Water Uptake. In Infrared Spectroscopy—Materials Science, Engineering and Technology; Theophanides, T., Ed.; Intechopen: London, UK, 2012; pp. 1–26. [Google Scholar]
- Tudorachi, N.; Mustata, F. Curing and Thermal Degradation of Diglycidyl Ether of Bisphenol A Epoxy Resin Crosslinked with Natural Hydroxy Acids as Environmentally Friendly Hardeners. Arab. J. Chem. 2020, 13, 671–682. [Google Scholar] [CrossRef]
- Francisco, W.; Ferreira, F.V.; Ferreira, E.V.; de Cividanes, L.S.; dos Coutinho, A.R.; Thim, G.P. Functionalization of Multi-Walled Carbon Nanotube and Mechanical Property of Epoxy-Based Nanocomposite. J. Aerosp. Technol. Manag. 2015, 7, 289–293. [Google Scholar] [CrossRef]
- Sadri, R.; Hosseini, M.; Kazi, S.N.; Bagheri, S.; Zubir, N.; Solangi, K.H.; Zaharinie, T.; Badarudin, A. A Bio-Based, Facile Approach for the Preparation of Covalently Functionalized Carbon Nanotubes Aqueous Suspensions and Their Potential as Heat Transfer Fluids. J. Colloid Interface Sci. 2017, 504, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Rastian, Z.; Khodadadi, A.A.; Vahabzadeh, F.; Bortolini, C.; Dong, M.; Mortazavi, Y.; Mogharei, A.; Naseh, M.V.; Guo, Z. Facile Surface Functionalization of Multiwalled Carbon Nanotubes by Soft Dielectric Barrier Discharge Plasma: Generate Compatible Interface for Lipase Immobilization. Biochem. Eng. J. 2014, 90, 16–26. [Google Scholar] [CrossRef]
- Moraes, M.B.; Cividanes, L.; Thim, G. Synthesis of Graphene Oxide and Functionalized CNT Nanocomposites Based on Epoxy Resin. J. Aerosp. Technol. Manag. 2018, 10, 1–10. [Google Scholar] [CrossRef]
- Alatawna, A.; Birenboim, M.; Nadiv, R.; Buzaglo, M.; Peretz-Damari, S.; Peled, A.; Regev, O.; Sripada, R. The Effect of Compatibility and Dimensionality of Carbon Nanofillers on Cement Composites. Constr. Build. Mater. 2020, 232, 117141. [Google Scholar] [CrossRef]
- Alam, A.; Wan, C.; McNally, T. Surface Amination of Carbon Nanoparticles for Modification of Epoxy Resins: Plasma-Treatment vs. Wet-Chemistry Approach. Eur. Polym. J. 2017, 87, 422–448. [Google Scholar] [CrossRef]
- Khosravi, H.; Eslami-Farsani, R. On the Mechanical Characterizations of Unidirectional Basalt Fiber/Epoxy Laminated Composites with 3-Glycidoxypropyltrimethoxysilane Functionalized Multi-Walled Carbon Nanotubes-Enhanced Matrix. J. Reinf. Plast. Compos. 2016, 35, 421–434. [Google Scholar] [CrossRef]
- Maleki, A.; Hamesadeghi, U.; Daraei, H.; Hayati, B.; Najafi, F.; McKay, G.; Rezaee, R. Amine Functionalized Multi-Walled Carbon Nanotubes: Single and Binary Systems for High Capacity Dye Removal. Chem. Eng. J. 2017, 313, 826–835. [Google Scholar] [CrossRef]
- Rahimian-Koloor, S.M.; Hashemianzadeh, S.M.; Shokrieh, M.M. Effect of CNT Structural Defects on the Mechanical Properties of CNT/Epoxy Nanocomposite. Phys. B Condens. Matter 2018, 540, 16–25. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, S.; Li, Q.; Wang, S.; Huang, K.; Xu, X.; Wang, B.; Zhu, J. Dynamic Transfer Auto-Catalysis of Epoxy Vitrimers Enabled by the Carboxylic Acid/Epoxy Ratio Based on Facilely Synthesized Trifunctional Monoesterified Cyclic Anhydrides. Eur. Polym. J. 2020, 135, 109881. [Google Scholar] [CrossRef]
- Sichina, W.J. Characterization of Epoxy Resins Using DSC; PerkinElmer Instruments: Waltham, MA, USA, 2000; pp. 2–5. [Google Scholar]
- Huix-Rotllant, M.; Natarajan, B.; Ipatov, A.; Muhavini Wawire, C.; Deutsch, T.; Casida, M.E. Assessment of Noncollinear Spin-Flip Tamm-Dancoff Approximation Time-Dependent Density-Functional Theory for the Photochemical Ring-Opening of Oxirane. Phys. Chem. Chem. Phys. 2010, 12, 12811–12825. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Zhang, K.; Zhu, S.; Xu, H.; Cao, D.; Zhao, L.; Zhang, R.; Yin, W. Review on the Electrical Resistance/Conductivity of Carbon Fiber Reinforced Polymer. Appl. Sci. 2019, 9, 2390. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.; Xu, L.; Chen, Z.; Wang, Y.; Tusiime, R.; Cheng, C.; Zhou, S.; Liu, Y.; Yu, M.; Zhang, H. Enhancing the Mechanical and Thermal Properties of Epoxy Resin via Blending with Thermoplastic Polysulfone. Polymers 2019, 11, 461. [Google Scholar] [CrossRef] [Green Version]
- Yao, K.; Adams, D.; Hao, A.; Zheng, J.P.; Liang, Z.; Nguyen, N. Highly Conductive and Strong Graphite-Phenolic Resin Composite for Bipolar Plate Applications. Energy Fuels 2017, 31, 14320–14331. [Google Scholar] [CrossRef]
- Pourrahmani, H.; Siavashi, M.; Yavarinasab, A.; Matian, M.; Chitgar, N.; Wang, L.; Van, J. A Review on the Long-Term Performance of Proton Exchange Membrane Fuel Cells: From Degradation Modeling to the Effects of Bipolar Plates, Sealings, and Contaminants. Energies 2022, 15, 5081. [Google Scholar] [CrossRef]
- Arbeiter, D.; Brandt-Wunderlich, C.; Siewert, S.; Kohse, S.; Pfensig, S.; Schmitz, K.P.; Grabow, N. Cyclic Stress-Strain Behavior of Polymeric Nonwoven Structures for the Use as Artificial Leaflet Material for Transcatheter Heart Valve Prostheses. Curr. Dir. Biomed. Eng. 2017, 3, 703–706. [Google Scholar] [CrossRef]
- Onyu, K.; Yeetsorn, R.; Gostick, J. Fabrication of Bipolar Plates from Thermoplastic Elastomer Composites for Vanadium Redox Flow Battery. Polymers 2022, 14, 2143. [Google Scholar] [CrossRef]
- Kim, H.; Choi, J. Subcontinuum Interpretation of Mechanical Behavior for Cross-Linked Epoxy Networks. Macromolecules 2022, 55, 5916–5925. [Google Scholar] [CrossRef]
- Bao, T.; Wang, Z.; Zhao, Y.; Wang, Y.; Yi, X. Improving Tribological Performance of Epoxy Composite by Reinforcing with Polyetheramine-Functionalized Graphene Oxide. J. Mater. Res. Technol. 2021, 12, 1516–1529. [Google Scholar] [CrossRef]
- Bhatt, P.; Goe, A. Carbon Fibres: Production, Properties and Potential Use. Mater. Sci. Res. India 2017, 14, 52–57. [Google Scholar] [CrossRef]
- Hwang, I.U.; Yu, H.N.; Kim, S.S.; Lee, D.G.; Do Suh, J.; Lee, S.H.; Ahn, B.K.; Kim, S.H.; Lim, T.W. Bipolar Plate Made of Carbon Fiber Epoxy Composite for Polymer Electrolyte Membrane Fuel Cells. J. Power Sources 2008, 184, 90–94. [Google Scholar] [CrossRef]
- Ojo, S.O.; Ismail, S.O.; Paggi, M.; Dhakal, H.N. A New Analytical Critical Thrust Force Model for Delamination Analysis of Laminated Composites during Drilling Operation. Compos. Part B Eng. 2017, 124, 207–217. [Google Scholar] [CrossRef] [Green Version]
- Senthil, R.; Vedakumari, S.W.; Hemalatha, T.; Das, B.N.; Sastry, T.P. Leather Fibres as Reinforcement for Epoxy Composites: A Novel Perspective. Fibers Polym. 2015, 16, 181–187. [Google Scholar] [CrossRef]
- Vryonis, O.; Andritsch, T.; Vaughan, A.S.; Lewin, P.L. Structural and Chemical Comparison between Moderately Oxygenated and Edge Oxygenated Graphene: Mechanical, Electrical and Thermal Performance of the Epoxy Nanocomposites. SN Appl. Sci. 2019, 1, 1275. [Google Scholar] [CrossRef] [Green Version]
- Epstein, W.M. Book Review: Quixote’s Ghost: The Right, the Liberati, and the Future of Social Policy. Res. Soc. Work Pract. 2006, 16, 238–240. [Google Scholar] [CrossRef]

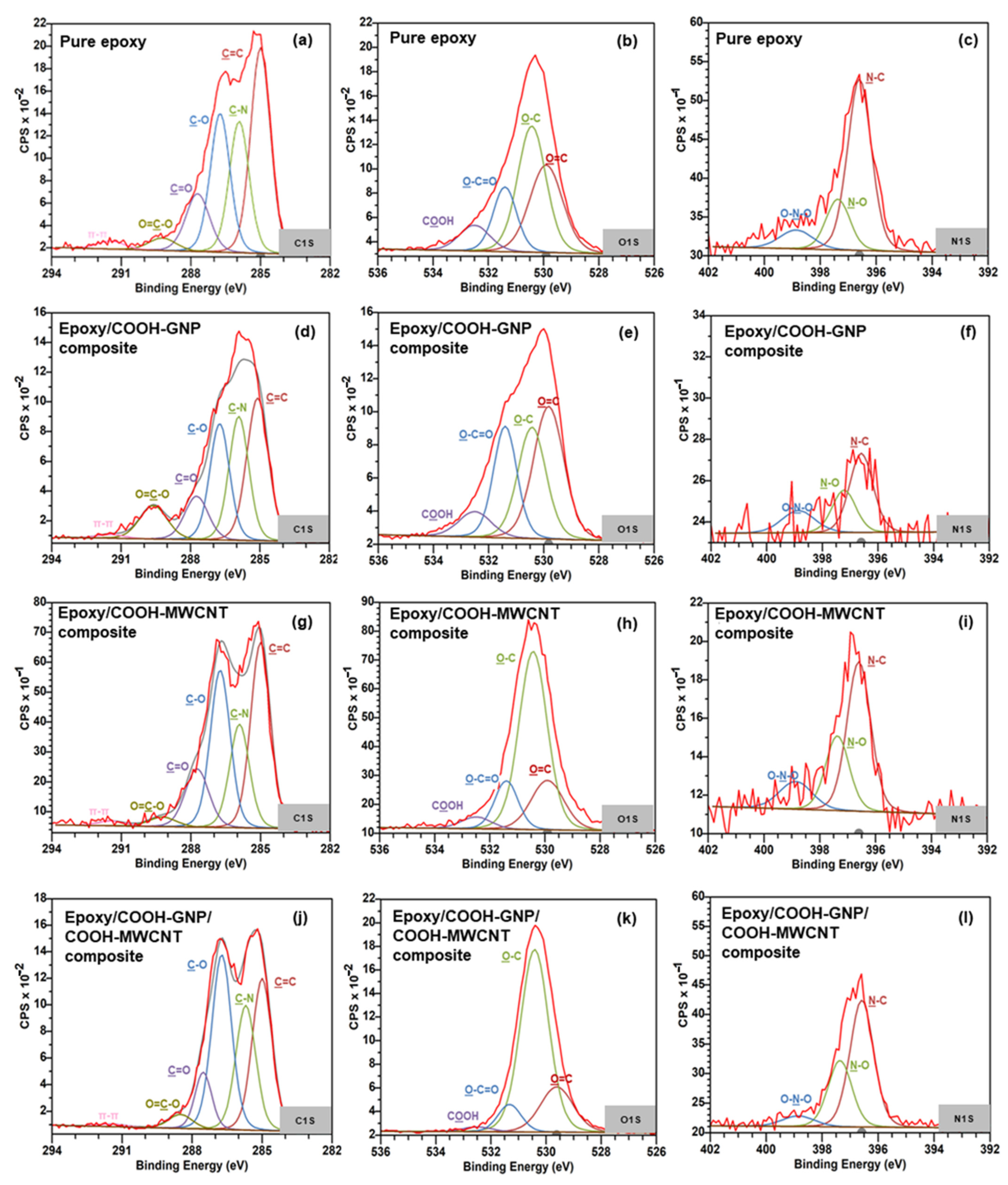



| Peak | Position (eV) | Pure Epoxy | Epoxy with COOH-MWCNT | Epoxy with COOH-GNP | Epoxy with COOH-MWCNT and COOH-GNP | |
|---|---|---|---|---|---|---|
| C1s | C=C/C-C | 285.00 | 35.81 | 34.57 | 31.27 | 29.22 |
| C-N | 285.92 | 22.87 | 19.30 | 24.70 | 23.97 | |
| C-O | 286.74 | 24.18 | 29.33 | 23.21 | 33.66 | |
| C=O | 287.71 | 12.13 | 13.04 | 10.41 | 9.23 | |
| O-C=O | 289.23 | 3.08 | 2.51 | 9.35 | 3.23 | |
| π-π* | 291.44 | 1.94 | 1.26 | 1.07 | 0.69 | |
| O1s | O=C | 529.91 | 32.92 | 19.15 | 38.01 | 20.02 |
| O-C | 530.43 | 49.68 | 61.60 | 29.47 | 69.28 | |
| O-C=O | 531.40 | 17.37 | 13.96 | 24.63 | 8.68 | |
| COOH | 532.50 | 9.03 | 4.48 | 7.89 | 2.01 | |
| N1s | N-C | 396.60 | 68.92 | 56.79 | 51.71 | 60.96 |
| N-O | 397.37 | 20.44 | 28.41 | 27.87 | 31.94 | |
| O-N-O | 398.86 | 10.64 | 14.80 | 20.42 | 7.10 | |
| Type of Bipolar Plate | OCV (V) | Efficiency at OCV (%) | Maximum Power Density (mW/cm2) | Weight of BP (g/Plate) | Weight of PEMFC (g/Cell) | Weight of BP Per Weight of PEMFC (%) |
|---|---|---|---|---|---|---|
| Epoxy composite laminated BP | 0.8732 | 70.9 | 59.4020 | 4.832 | 846.504 | 1.1 |
| Graphite BP (commercial) | 0.8759 | 71.2 | 88.4092 | 12.213 | 861.266 | 2.9 |
| Furan-based composite BP (commercial) | 0.8248 | 67.1 | 47.6608 | 6.228 | 849.256 | 1.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karoonsit, B.; Yeetsorn, R.; Aussawasathien, D.; Prissanaroon-Ouajai, W.; Yogesh, G.K.; Maiket, Y. Performance Evaluation for Ultra-Lightweight Epoxy-Based Bipolar Plate Production with Cycle Time Reduction of Reactive Molding Process. Polymers 2022, 14, 5226. https://doi.org/10.3390/polym14235226
Karoonsit B, Yeetsorn R, Aussawasathien D, Prissanaroon-Ouajai W, Yogesh GK, Maiket Y. Performance Evaluation for Ultra-Lightweight Epoxy-Based Bipolar Plate Production with Cycle Time Reduction of Reactive Molding Process. Polymers. 2022; 14(23):5226. https://doi.org/10.3390/polym14235226
Chicago/Turabian StyleKaroonsit, Budsaba, Rungsima Yeetsorn, Darunee Aussawasathien, Walaiporn Prissanaroon-Ouajai, Gaurav Kumar Yogesh, and Yaowaret Maiket. 2022. "Performance Evaluation for Ultra-Lightweight Epoxy-Based Bipolar Plate Production with Cycle Time Reduction of Reactive Molding Process" Polymers 14, no. 23: 5226. https://doi.org/10.3390/polym14235226