Biocomposite Materials Based on Poly(3-hydroxybutyrate) and Chitosan: A Review
Abstract
:1. Introduction
2. Biomaterials Based on Chitosan and PHB
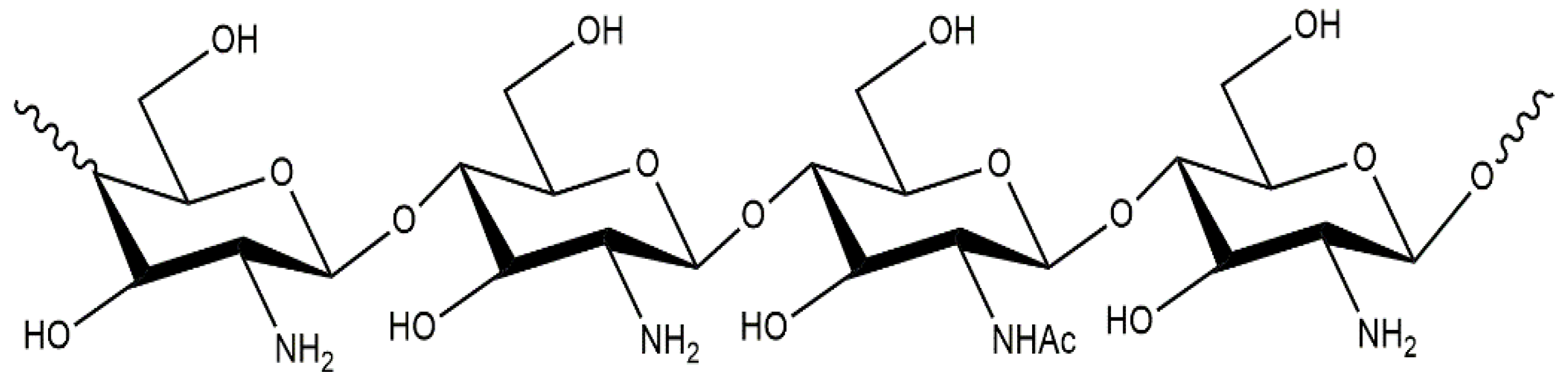
2.1. Chitosan Characteristics and Applications
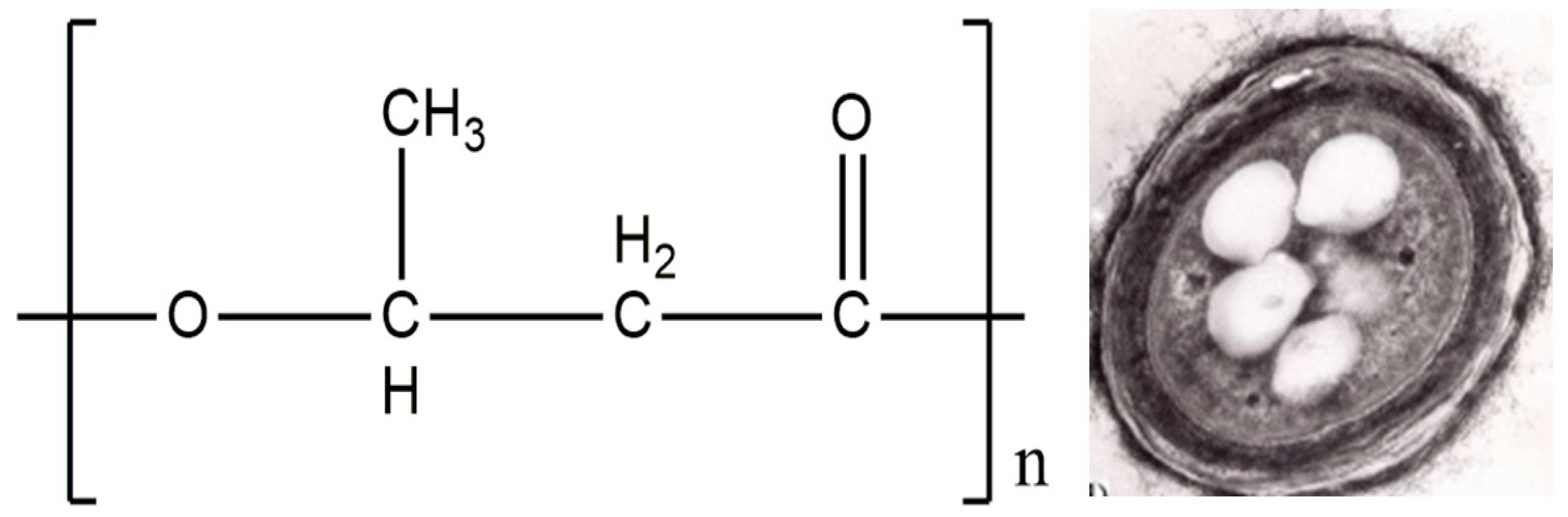
2.2. Poly(3-hydroxybutyrate): Preparation, Structure, Applications
3. Formation of Composites Based on Chitosan and Poly(3-hydroxybutyrate)
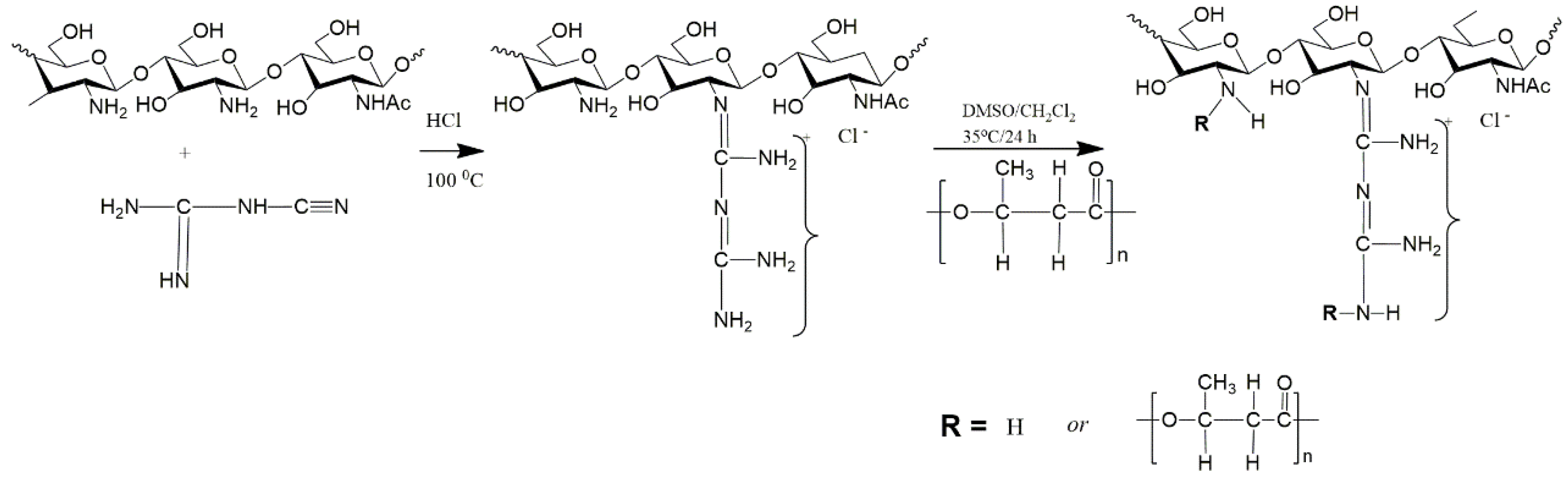
3.1. Copolymerization
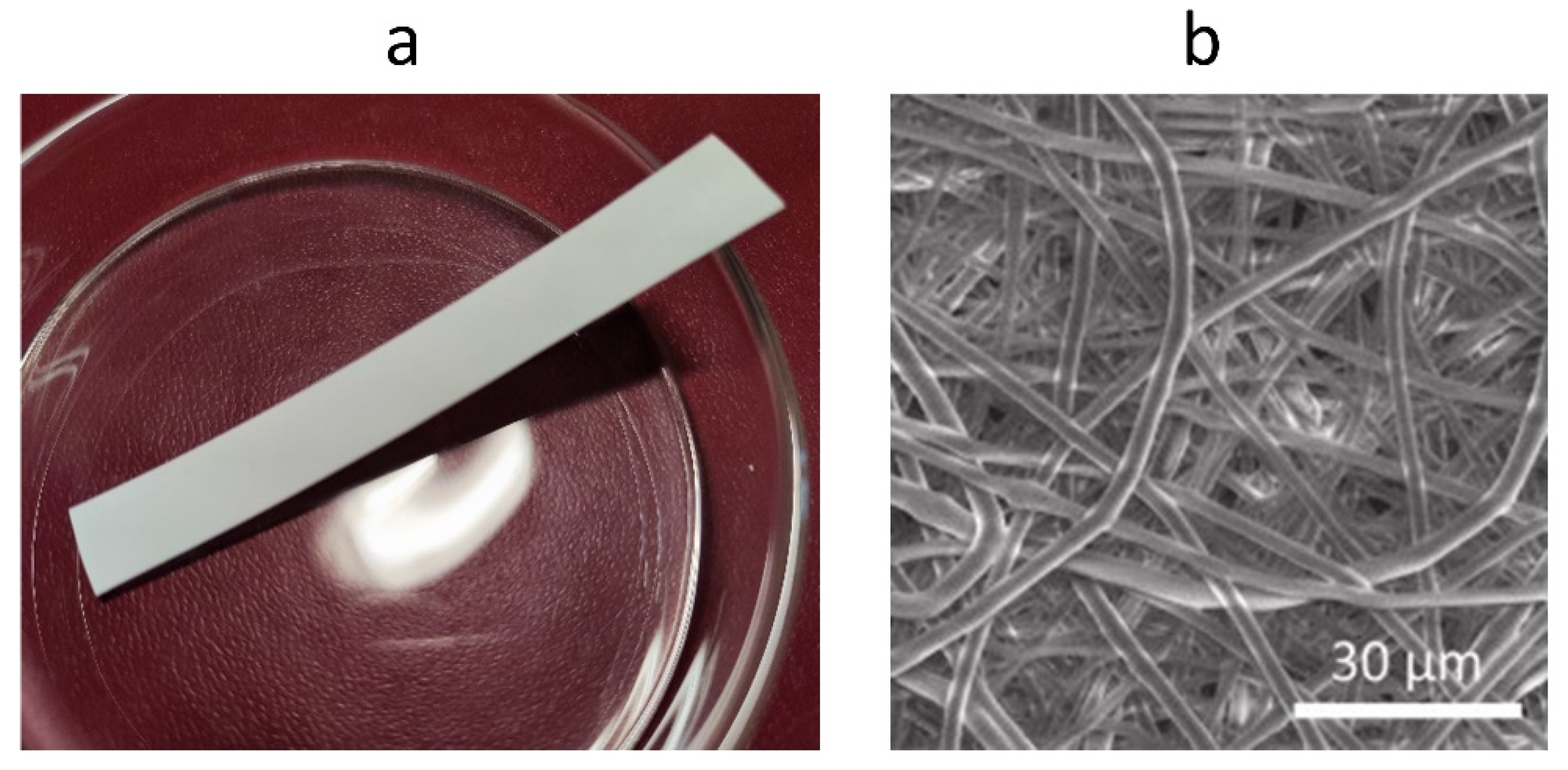
3.2. Electrospinning
3.3. Blending Polymer Solutions in Different Solvents
3.4. Using the Same Solvent for Both Polymers
3.5. Summary of the above Methods
4. Prospects for Applications of PHB/Chitosan Composite Materials
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kabir, E.; Kaur, R.; Lee, J.; Kim, K.-H.; Kwon, E.E. Prospects of biopolymer technology as an alternative option for non-degradable plastics and sustainable management of plastic wastes. J. Clean. Prod. 2020, 258, 120536. [Google Scholar] [CrossRef]
- Sivakanthan, S.; Rajendran, S.; Gamage, A.; Madhujith, T.; Mani, S. Antioxidant and antimicrobial applications of biopolymers: A review. Food Res. Int. 2020, 136, 109327. [Google Scholar] [CrossRef]
- Pradhan, B.; Bharti, D.; Chakravarty, S.; Ray, S.S.; Voinova, V.V.; Bonartsev, A.P.; Pal, K. Internet of Things and Robotics in Transforming Current-Day Healthcare Services. J. Health Eng. 2021, 2021, 1–15. [Google Scholar] [CrossRef]
- Bonartsev, A.P.; Bonartseva, G.A.; Reshetov, I.V.; Kirpichnikov, M.P.; Shaitan, K.V. Application of polyhydroxyalkanoates in medicine and the biological activity of natural poly(3-hydroxybutyrate). Acta Nat. 2019, 11, 4–16. [Google Scholar] [CrossRef]
- Shrivastava, A.; Dondapati, S. Biodegradable composites based on biopolymers and natural bast fibres: A review. Mater. Today Proc. 2021, 46, 1420–1428. [Google Scholar] [CrossRef]
- Udayakumar, G.P.; Muthusamy, S.; Selvaganesh, B.; Sivarajasekar, N.; Rambabu, K.; Banat, F.; Sivamani, S.; Sivakumar, N.; Hosseini-Bandegharaei, A.; Show, P.L. Biopolymers and composites: Properties, characterization and their applications in food, medical and pharmaceutical industries. J. Environ. Chem. Eng. 2021, 9, 105322. [Google Scholar] [CrossRef]
- Aaliya, B.; Sunooj, K.V.; Lackner, M. Biopolymer composites: A review. Int. J. Biobased Plast. 2021, 3, 40–84. [Google Scholar] [CrossRef]
- Kartik, A.; Akhil, D.; Lakshmi, D.; Gopinath, K.P.; Arun, J.; Sivaramakrishnan, R.; Pugazhendhi, A. A critical review on production of biopolymers from algae biomass and their applications. Bioresour. Technol. 2021, 329, 124868. [Google Scholar] [CrossRef]
- Han, X.; Zheng, Y.; Munro, C.J.; Ji, Y.; Braunschweig, A.B. Carbohydrate nanotechnology: Hierarchical assembly using nature’s other information carrying biopolymers. Curr. Opin. Biotechnol. 2015, 34, 41–47. [Google Scholar] [CrossRef]
- Rong, S.Y.; Mubarak, N.; Tanjung, F.A. Structure-property relationship of cellulose nanowhiskers reinforced chitosan biocomposite films. J. Environ. Chem. Eng. 2017, 5, 6132–6136. [Google Scholar] [CrossRef]
- Mehrpouya, M.; Vahabi, H.; Janbaz, S.; Darafsheh, A.; Mazur, T.R.; Ramakrishna, S. 4D printing of shape memory polylactic acid (PLA). Polymer 2021, 230, 124080. [Google Scholar] [CrossRef]
- Kumari, S.V.G.; Pakshirajan, K.; Pugazhenthi, G. Recent advances and future prospects of cellulose, starch, chitosan, polylactic acid and polyhydroxyalkanoates for sustainable food packaging applications. Int. J. Biol. Macromol. 2022, 221, 163–182. [Google Scholar] [CrossRef]
- Haghighi, H.; Licciardello, F.; Fava, P.; Siesler, H.W.; Pulvirenti, A. Recent advances on chitosan-based films for sustainable food packaging applications. Food Packag. Shelf Life 2020, 26, 100551. [Google Scholar] [CrossRef]
- Claro, P.I.C.; Neto, A.R.S.; Bibbo, A.C.C.; Mattoso, L.H.C.; Bastos, M.S.R.; Marconcini, J.M. Biodegradable Blends with Potential Use in Packaging: A Comparison of PLA/Chitosan and PLA/Cellulose Acetate Films. J. Polym. Environ. 2016, 24, 363–371. [Google Scholar] [CrossRef]
- Hazrati, K.; Sapuan, S.; Zuhri, M.; Jumaidin, R. Preparation and characterization of starch-based biocomposite films reinforced by Dioscorea hispida fibers. J. Mater. Res. Technol. 2021, 15, 1342–1355. [Google Scholar] [CrossRef]
- Chang, I.; Lee, M.; Tran, A.T.P.; Lee, S.; Kwon, Y.-M.; Im, J.; Cho, G.-C. Review on biopolymer-based soil treatment (BPST) technology in geotechnical engineering practices. Transp. Geotech. 2020, 24, 100385. [Google Scholar] [CrossRef]
- Priyadarshi, R.; Roy, S.; Ghosh, T.; Biswas, D.; Rhim, J.-W. Antimicrobial nanofillers reinforced biopolymer composite films for active food packaging applications—A review. Sustain. Mater. Technol. 2021, 321, e00353. [Google Scholar] [CrossRef]
- Manu, T.; Nazmi, A.R.; Shahri, B.; Emerson, N.; Huber, T. Biocomposites: A review of materials and perception. Mater. Today Commun. 2022, 31, 103308. [Google Scholar] [CrossRef]
- Shanmugam, V.; Mensah, R.A.; Försth, M.; Sas, G.; Restás, Á.; Addy, C.; Xu, Q.; Jiang, L.; Neisiany, R.E.; Singha, S.; et al. Circular economy in biocomposite development: State-of-the-art, challenges and emerging trends. Compos. Part C Open Access 2021, 5, 100138. [Google Scholar] [CrossRef]
- Komal, U.K.; Lila, M.K.; Singh, I. PLA/banana fiber based sustainable biocomposites: A manufacturing perspective. Compos. Part B Eng. 2020, 180, 107535. [Google Scholar] [CrossRef]
- Makvandi, P.; Ghomi, M.; Ashrafizadeh, M.; Tafazoli, A.; Agarwal, T.; Delfi, M.; Akhtari, J.; Zare, E.N.; Padil, V.V.; Zarrabi, A.; et al. A review on advances in graphene-derivative/polysaccharide bionanocomposites: Therapeutics, pharmacogenomics and toxicity. Carbohydr. Polym. 2020, 250, 116952. [Google Scholar] [CrossRef]
- Yaashikaa, P.; Kumar, P.S.; Karishma, S. Review on biopolymers and composites—Evolving material as adsorbents in removal of environmental pollutants. Environ. Res. 2022, 212, 113114. [Google Scholar] [CrossRef]
- Kaur, S.; Dhillon, G.S. Recent trends in biological extraction of chitin from marine shell wastes: A review. Crit. Rev. Biotechnol. 2015, 35, 44–61. [Google Scholar] [CrossRef]
- Younes, I.; Rinaudo, M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef] [Green Version]
- Domard, A. A perspective on 30 years research on chitin and chitosan. Carbohydr. Polym. 2011, 84, 696–703. [Google Scholar] [CrossRef]
- Tao, F.; Cheng, Y.; Shi, X.; Zheng, H.; Du, Y.; Xiang, W.; Deng, H. Applications of chitin and chitosan nanofibers in bone regenerative engineering. Carbohydr. Polym. 2020, 230, 115658. [Google Scholar] [CrossRef]
- Bakshi, P.S.; Selvakumar, D.; Kadirvelu, K.; Kumar, N.S. Chitosan as an environment friendly biomaterial—A review on recent modifications and applications. Int. J. Biol. Macromol. 2020, 150, 1072–1083. [Google Scholar] [CrossRef]
- Ghormade, V.; Pathan, E.K.; Deshpande, M.V. Can fungi compete with marine sources for chitosan production? Int. J. Biol. Macromol. 2017, 104, 1415–1421. [Google Scholar] [CrossRef]
- Muzzarelli, R.; Ilari, P.; Tarsi, R.; Dubini, B.; Xia, W. Chitosan from Absidia coerulea. Carbohydr. Polym. 1994, 25, 45–50. [Google Scholar] [CrossRef]
- Di Mario, F.; Rapanà, P.; Tomati, U.; Galli, E. Chitin and chitosan from Basidiomycetes. Int. J. Biol. Macromol. 2008, 43, 8–12. [Google Scholar] [CrossRef]
- Cai, J.; Yang, J.; Du, Y.; Fan, L.; Qiu, Y.; Li, J.; Kennedy, J.F. Enzymatic preparation of chitosan from the waste Aspergillus niger mycelium of citric acid production plant. Carbohydr. Polym. 2006, 64, 151–157. [Google Scholar] [CrossRef]
- Gonil, P.; Sajomsang, W. Applications of magnetic resonance spectroscopy to chitin from insect cuticles. Int. J. Biol. Macromol. 2012, 51, 514–522. [Google Scholar] [CrossRef]
- Khayrova, A.; Lopatin, S.; Varlamov, V. Black Soldier Fly Hermetia illucens as a Novel Source of Chitin and Chitosan. Int. J. Sci. 2019, 8, 81–86. [Google Scholar] [CrossRef] [Green Version]
- Svirshchevskaya, E. Correlation Analysis of Chitosan Physicochemical Parameters Determined by Different Methods. Org. Med. Chem. Int. J. 2017, 1, 74–82. [Google Scholar] [CrossRef]
- Mourya, V.; Inamdar, N.N. Chitosan-modifications and applications: Opportunities galore. React. Funct. Polym. 2008, 68, 1013–1051. [Google Scholar] [CrossRef]
- Sashiwa, H.; Shigemasa, Y.; Roy, R. Chemical modification of chitosan 8: Preparation of chitosan–dendrimer hybrids via short spacer. Carbohydr. Polym. 2002, 47, 191–199. [Google Scholar] [CrossRef]
- Varlamov, V.P.; Il’Ina, A.V.; Shagdarova, B.T.; Lunkov, A.P.; Mysyakina, I.S. Chitin/Chitosan and Its Derivatives: Fundamental Problems and Practical Approaches. Biochem. Moscow 2020, 85, 154–176. [Google Scholar] [CrossRef]
- Harish Prashanth, K.V.; Tharanathan, R.N. Chitin/chitosan: Modifications and their unlimited application potential—An overview. Trends Food Sci. Technol. 2007, 18, 117–131. [Google Scholar] [CrossRef]
- Fei Liu, X.; Guan, Y.L.; Yang, D.Z.; Li, Z.; Yao, K. De Antibacterial action of chitosan and carboxymethylated chitosan. J. Appl. Polym. Sci. 2000, 79, 1324–1335. [Google Scholar] [CrossRef]
- Vasyukova, N.I.; Zinov’eva, S.V.; Il’inskaya, L.I.; Perekhod, E.A.; Chalenko, G.I.; Gerasimova, N.G.; Il’ina, A.V.; Varlamov, V.P.; Ozeretskovskaya, O.L. Modulation of Plant Resistance to Diseases by Water-Soluble Chitosan. Appl. Biochem. Microbiol. 2001, 371, 103–109. [Google Scholar] [CrossRef]
- Laokuldilok, T.; Potivas, T.; Kanha, N.; Surawang, S.; Seesuriyachan, P.; Wangtueai, S.; Phimolsiripol, Y.; Regenstein, J.M. Physicochemical, antioxidant, and antimicrobial properties of chitooligosaccharides produced using three different enzyme treatments. Food Biosci. 2017, 18, 28–33. [Google Scholar] [CrossRef]
- Naveed, M.; Phil, L.; Sohail, M.; Hasnat, M.; Baig, M.M.F.A.; Ihsan, A.U.; Shumzaid, M.; Kakar, M.U.; Khan, T.M.; Akabar, M.D.; et al. Chitosan oligosaccharide (COS): An overview. Int. J. Biol. Macromol. 2019, 129, 827–843. [Google Scholar] [CrossRef]
- Yuan, X.; Zheng, J.; Jiao, S.; Cheng, G.; Feng, C.; Du, Y.; Liu, H. A review on the preparation of chitosan oligosaccharides and application to human health, animal husbandry and agricultural production. Carbohydr. Polym. 2019, 220, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-K.; Rajapakse, N. Enzymatic production and biological activities of chitosan oligosaccharides (COS): A review. Carbohydr. Polym. 2005, 62, 357–368. [Google Scholar] [CrossRef]
- Lee, S.-H.; Park, J.-S.; Kim, S.-K.; Ahn, C.-B.; Je, J.-Y. Chitooligosaccharides suppress the level of protein expression and acetylcholinesterase activity induced by Aβ25–35 in PC12 cells. Bioorg. Med. Chem. Lett. 2009, 19, 860–862. [Google Scholar] [CrossRef] [PubMed]
- Rahman, H.; Hjeljord, L.G.; Aam, B.B.; Sørlie, M.; Tronsmo, A. Antifungal effect of chito-oligosaccharides with different degrees of polymerization. Eur. J. Plant Pathol. 2015, 141, 147–158. [Google Scholar] [CrossRef]
- Azuma, K.; Osaki, T.; Minami, S.; Okamoto, Y. Anticancer and Anti-Inflammatory Properties of Chitin and Chitosan Oligosaccharides. J. Funct. Biomater. 2015, 6, 33–49. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhang, X.; Wang, X.; Wang, C.; Tang, B. Microenvironment of alginate-based microcapsules for cell culture and tissue engineering. J. Biosci. Bioeng. 2012, 114, 640–647. [Google Scholar] [CrossRef]
- Frank, L.A.; Onzi, G.R.; Morawski, A.S.; Pohlmann, A.R.; Guterres, S.S.; Contri, R.V. Chitosan as a coating material for nanoparticles intended for biomedical applications. React. Funct. Polym. 2020, 147, 104459. [Google Scholar] [CrossRef]
- Moon, C.; Seo, D.-J.; Song, Y.-S.; Jung, W.-J. Antibacterial activity of various chitosan forms against Xanthomonas axonopodis pv. glycines. Int. J. Biol. Macromol. 2020, 156, 1600–1605. [Google Scholar] [CrossRef]
- Chudinova, Y.V.; Shagdarova, B.T.; Il’ina, A.V.; Varlamov, V.P. Antibacterial effect of peptide conjugates with a quaternized chitosan derivative and its estimation by the method of atomic force microscopy. Appl. Biochem. Microbiol. 2016, 52, 496–501. [Google Scholar] [CrossRef]
- Verlee, A.; Mincke, S.; Stevens, C.V. Recent developments in antibacterial and antifungal chitosan and its derivatives. Carbohydr. Polym. 2017, 164, 268–283. [Google Scholar] [CrossRef]
- Qin, Y.; Li, P.; Guo, Z. Cationic chitosan derivatives as potential antifungals: A review of structural optimization and applications. Carbohydr. Polym. 2020, 236, 116002. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, A.; Talukdar, D.; Pal, A.; Ray, M. Immunomodulation of macrophages by methylglyoxal conjugated with chitosan nanoparticles against Sarcoma-180 tumor in mice. Cell. Immunol. 2014, 287, 27–35. [Google Scholar] [CrossRef]
- Cesari, A.; Fabiano, A.; Piras, A.M.; Zambito, Y.; Uccello-Barretta, G.; Balzano, F. Binding and mucoadhesion of sulfurated derivatives of quaternary ammonium-chitosans and their nanoaggregates: An NMR investigation. J. Pharm. Biomed. Anal. 2020, 177, 112852. [Google Scholar] [CrossRef]
- Badhe, R.V.; Nanda, R.K.; Chejara, D.R.; Choonara, Y.E.; Kumar, P.; du Toit, L.C.; Pillay, V. Microwave-assisted facile synthesis of a new tri-block chitosan conjugate with improved mucoadhesion. Carbohydr. Polym. 2015, 130, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Augustine, R.; Rehman, S.R.U.; Ahmed, R.; Zahid, A.A.; Sharifi, M.; Falahati, M.; Hasan, A. Electrospun chitosan membranes containing bioactive and therapeutic agents for enhanced wound healing. Int. J. Biol. Macromol. 2020, 156, 153–170. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Shitiz, K.; Singh, A. Chitin and chitosan: Biopolymers for wound management. Int. Wound J. 2017, 14, 1276–1289. [Google Scholar] [CrossRef]
- Wang, L.; Hao, F.; Tian, S.; Dong, H.; Nie, J.; Ma, G. Targeting polysaccharides such as chitosan, cellulose, alginate and starch for designing hemostatic dressings. Carbohydr. Polym. 2022, 291, 119574. [Google Scholar] [CrossRef]
- Rodríguez-Vázquez, M.; Vega-Ruiz, B.; Ramos-Zúñiga, R.; Saldaña-Koppel, D.A.; Quiñones-Olvera, L.F. Chitosan and Its Potential Use as a Scaffold for Tissue Engineering in Regenerative Medicine. Biomed. Res. Int. 2015, 2015, 821279. [Google Scholar] [CrossRef]
- Chen, M.-C.; Liu, C.-T.; Tsai, H.-W.; Lai, W.-Y.; Chang, Y.; Sung, H.-W. Mechanical properties, drug eluting characteristics and in vivo performance of a genipin-crosslinked chitosan polymeric stent. Biomaterials 2009, 30, 5560–5571. [Google Scholar] [CrossRef] [PubMed]
- Perrin, N.; Mohammadkhani, G.; Moghadam, F.H.; Delattre, C.; Zamani, A. Biocompatible fibers from fungal and shrimp chitosans for suture application. Curr. Res. Biotechnol. 2022, 4, 530–536. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, Y.; Wei, P.; Zhong, Y.; Chen, C.; Cai, J. Extremely strong and tough chitosan films mediated by unique hydrated chitosan crystal structures. Mater. Today 2021, 51, 27–38. [Google Scholar] [CrossRef]
- Luna, R.; Touhami, F.; Uddin, M.J.; Touhami, A. Effect of temperature and pH on nanostructural and nanomechanical properties of chitosan films. Surf. Interfaces 2022, 29, 101706. [Google Scholar] [CrossRef]
- Lewandowska, K.; Sionkowska, A.; Kaczmarek, B.; Furtos, G. Mechanical and Morphological Studies of Chitosan/Clay Composites. Mol. Cryst. Liq. Cryst. 2014, 590, 193–198. [Google Scholar] [CrossRef]
- Leceta, I.; Peñalba, M.; Arana, P.; Guerrero, P.; De La Caba, K. Ageing of chitosan films: Effect of storage time on structure and optical, barrier and mechanical properties. Eur. Polym. J. 2015, 66, 170–179. [Google Scholar] [CrossRef]
- Janik, W.; Ledniowska, K.; Nowotarski, M.; Kudła, S.; Knapczyk-Korczak, J.; Stachewicz, U.; Nowakowska-Bogdan, E.; Sabura, E.; Nosal-Kovalenko, H.; Turczyn, R.; et al. Chitosan-based films with alternative eco-friendly plasticizers: Preparation, physicochemical properties and stability. Carbohydr. Polym. 2023, 301, 120277. [Google Scholar] [CrossRef]
- Movaffagh, J.; Khatib, M.; Bazzaz, B.S.F.; Taherzadeh, Z.; Hashemi, M.; Moghaddam, A.S.; Tabatabaee, S.A.; Azizzadeh, M.; Jirofti, N. Evaluation of wound-healing efficiency of a functional Chitosan/Aloe vera hydrogel on the improvement of re-epithelialization in full thickness wound model of rat. J. Tissue Viability 2022, 31, 649–656. [Google Scholar] [CrossRef]
- Shagdarova, B.; Konovalova, M.; Zhuikova, Y.; Lunkov, A.; Zhuikov, V.; Khaydapova, D.; Il’Ina, A.; Svirshchevskaya, E.; Varlamov, V. Collagen/Chitosan Gels Cross-Linked with Genipin for Wound Healing in Mice with Induced Diabetes. Materials 2021, 15, 15. [Google Scholar] [CrossRef]
- Li, X.; Hetjens, L.; Wolter, N.; Li, H.; Shi, X.; Pich, A. Charge-reversible and biodegradable chitosan-based microgels for lysozyme-triggered release of vancomycin. J. Adv. Res. 2022. [Google Scholar] [CrossRef]
- Huang, Y.; Huang, Z.; Liu, H.; Zhang, X.; Cai, Q.; Yang, X. Photoluminescent biodegradable polyorganophosphazene: A promising scaffold material for in vivo application to promote bone regeneration. Bioact. Mater. 2020, 5, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Kedir, W.M.; Abdi, G.F.; Goro, M.M.; Tolesa, L.D. Pharmaceutical and drug delivery applications of chitosan biopolymer and its modified nanocomposite: A review. Heliyon 2022, 8, e10196. [Google Scholar] [CrossRef] [PubMed]
- Singha, I.; Basu, A. Chitosan based injectable hydrogels for smart drug delivery applications. Sensors Int. 2022, 3, 100168. [Google Scholar] [CrossRef]
- Popova, E.V.; Tikhomirova, V.E.; Beznos, O.V.; Chesnokova, N.B.; Grigoriev, Y.V.; Klyachko, N.L.; Kost, O.A. Chitosan-covered calcium phosphate particles as a drug vehicle for delivery to the eye. Nanomed. Nanotechnol. Biol. Med. 2022, 40, 102493. [Google Scholar] [CrossRef] [PubMed]
- Drozd, N.N.; Lunkov, A.P.; Shagdarova, B.T.; Zhuikova, Y.V.; Il’ina, A.V.; Varlamov, V.P. Chitosan/heparin layer-by-layer coatings for improving thromboresistance of polyurethane. Surf. Interfaces 2022, 28, 101674. [Google Scholar] [CrossRef]
- Yang, C.; Wang, M.; Wang, W.; Liu, H.; Deng, H.; Du, Y.; Shi, X. Electrodeposition induced covalent cross-linking of chitosan for electrofabrication of hydrogel contact lenses. Carbohydr. Polym. 2022, 292, 119678. [Google Scholar] [CrossRef]
- Lin, X.; Liu, J.; Zhou, F.; Ou, Y.; Rong, J.; Zhao, J. Poly(2-hydroxyethyl methacrylate-co-quaternary ammonium salt chitosan) hydrogel: A potential contact lens material with tear protein deposition resistance and antimicrobial activity. Biomater. Adv. 2022, 136, 212787. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.-H.; Wu, G.-J.; Wu, C.-H.; Huang, C.-H.; Tsai, G.-J. Oral administration with chitosan hydrolytic products modulates mitogen-induced and antigen-specific immune responses in BALB/c mice. Int. J. Biol. Macromol. 2019, 131, 158–166. [Google Scholar] [CrossRef]
- Chirkov, S.N. The Antiviral Activity of Chitosan (Review). Appl. Biochem. Microbiol. 2002, 38, 1–8. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Q.; Chen, J.; Dong, H.; Cui, A.; Sun, L.; Wang, N.; Li, J.; Qu, Z. Cost-effective chitosan thermal bonded nonwovens serving as an anti-viral inhibitor layer in face mask. Mater. Lett. 2022, 318, 132203. [Google Scholar] [CrossRef]
- Pardeshi, C.V.; Belgamwar, V.S. Controlled synthesis of N,N,N-trimethyl chitosan for modulated bioadhesion and nasal membrane permeability. Int. J. Biol. Macromol. 2016, 82, 933–944. [Google Scholar] [CrossRef] [PubMed]
- Başaran, E.; Yenilmez, E.; Berkman, M.S.; Büyükköroğlu, G.; Yazan, Y. Chitosan nanoparticles for ocular delivery of cyclosporine A. J. Microencapsul. 2014, 31, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhuang, C.; Wang, M.; Sun, X.; Nie, S.; Pan, W. Liposome coated with low molecular weight chitosan and its potential use in ocular drug delivery. Int. J. Pharm. 2009, 379, 131–138. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, S.-B.; Wang, Y.-G.; Zhang, S.-H.; Yu, Z.-F.; Tang, T.-T. Bacterial inhibition potential of quaternised chitosan-coated VICRYL absorbable suture: An in vitro and in vivo study. J. Orthop. Transl. 2017, 8, 49–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jayakumar, R.; Prabaharan, M.; Kumar, P.T.S.; Nair, S.V.; Tamura, H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol. Adv. 2011, 29, 322–337. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, D.; Chen, X.; Xu, Q.; Lu, F.; Nie, J. Electrospun water-soluble carboxyethyl chitosan/poly(vinyl alcohol) nanofibrous membrane as potential wound dressing for skin regeneration. Biomacromolecules 2008, 9, 349–354. [Google Scholar] [CrossRef]
- Ribeiro, M.P.; Espiga, A.; Silva, D.; Baptista, P.; Henriques, J.; Ferreira, C.; Silva, J.C.; Borges, J.P.; Pires, E.; Chaves, P.; et al. Development of a new chitosan hydrogel for wound dressing. Wound Repair Regen. 2009, 17, 817–824. [Google Scholar] [CrossRef] [Green Version]
- Boucard, N.; Viton, C.; Agay, D.; Mari, E.; Roger, T.; Chancerelle, Y.; Domard, A. The use of physical hydrogels of chitosan for skin regeneration following third-degree burns. Biomaterials 2007, 28, 3478–3488. [Google Scholar] [CrossRef]
- Mi, F.-L.; Shyu, S.-S.; Wu, Y.-B.; Lee, S.-T.; Shyong, J.-Y.; Huang, R.-N. Fabrication and characterization of a sponge-like asymmetric chitosan membrane as a wound dressing. Biomaterials 2000, 22, 165–173. [Google Scholar] [CrossRef]
- Silva, S.S.; Luna, S.M.; Gomes, M.E.; Benesch, J.; Pashkuleva, I.; Mano, J.F.; Reis, R.L. Plasma surface modification of chitosan membranes: Characterization and preliminary cell response studies. Macromol. Biosci. 2008, 8, 568–576. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, D.; Nie, J. Chitosan/polyethylene glycol diacrylate films as potential wound dressing material. Int. J. Biol. Macromol. 2008, 43, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; He, H.; Gao, W.-J.; Lu, S.-Y.; Liu, Y.; Gu, H.-Y. Rapid adhesion and proliferation of keratinocytes on the gold colloid/chitosan film scaffold. Mater. Sci. Eng. C 2009, 29, 908–912. [Google Scholar] [CrossRef]
- Bonartsev, A.P.; Zharkova, I.I.; Yakovlev, S.G.; Myshkina, V.L.; Mahina, T.K.; Voinova, V.V.; Zernov, A.L.; Zhuikov, V.A.; Akoulina, E.A.; Ivanova, E.V.; et al. Biosynthesis of poly(3-hydroxybutyrate) copolymers by Azotobacter chroococcum 7B: A precursor feeding strategy. Prep. Biochem. Biotechnol. 2017, 47, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Jose, A.A.; Hazeena, S.H.; Lakshmi, N.M.; B, A.K.; Madhavan, A.; Sirohi, R.; Tarafdar, A.; Sindhu, R.; Awasthi, M.K.; Pandey, A.; et al. Bacterial biopolymers: From production to applications in biomedicine. Sustain. Chem. Pharm. 2022, 25, 100582. [Google Scholar] [CrossRef]
- Kourmentza, C.; Plácido, J.; Venetsaneas, N.; Burniol-Figols, A.; Varrone, C.; Gavala, H.N.; Reis, M.A.M. Recent Advances and Challenges towards Sustainable Polyhydroxyalkanoate (PHA) Production. Bioengineering 2017, 4, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anburajan, P.; Kumar, A.N.; Sabapathy, P.C.; Kim, G.-B.; Cayetano, R.D.; Yoon, J.-J.; Kumar, G.; Kim, S.-H. Polyhydroxy butyrate production by Acinetobacter junii BP25, Aeromonas hydrophila ATCC 7966, and their co-culture using a feast and famine strategy. Bioresour. Technol. 2019, 293, 122062. [Google Scholar] [CrossRef]
- García, A.; Segura, D.; Espín, G.; Galindo, E.; Castillo, T.; Peña, C. High production of poly-β-hydroxybutyrate (PHB) by an Azotobacter vinelandii mutant altered in PHB regulation using a fed-batch fermentation process. Biochem. Eng. J. 2014, 82, 117–123. [Google Scholar] [CrossRef]
- Myshkina, V.L.; Nikolaeva, D.A.; Makhina, T.K.; Bonartsev, A.; Bonartseva, G. Effect of growth conditions on the molecular weight of poly-3-hydroxybutyrate produced by Azotobacter chroococcum 7B. Appl. Biochem. Microbiol. 2008, 44, 482–486. [Google Scholar] [CrossRef]
- Bonartsev, A.; Yakovlev, S.; Boskhomdzhiev, A.; Zharkova, I.; Bagrov, D.; Myshkina, V.; Mahina, T.; Kharitonova, E.; Samsonova, O.; Zernov, A.; et al. The Terpolymer Produced by Azotobacter Chroococcum 7B: Effect of Surface Properties on Cell Attachment. PLoS ONE 2013, 8, e57200. [Google Scholar] [CrossRef]
- Lee, H.-J.; Kim, S.-G.; Cho, D.-H.; Bhatia, S.K.; Gurav, R.; Yang, S.-Y.; Yang, J.; Jeon, J.-M.; Yoon, J.-J.; Choi, K.-Y.; et al. Finding of novel lactate utilizing Bacillus sp. YHY22 and its evaluation for polyhydroxybutyrate (PHB) production. Int. J. Biol. Macromol. 2022, 201, 653–661. [Google Scholar] [CrossRef]
- Mohammed, S.; Panda, A.N.; Ray, L. An investigation for recovery of polyhydroxyalkanoates (PHA) from Bacillus sp. BPPI-14 and Bacillus sp. BPPI-19 isolated from plastic waste landfill. Int. J. Biol. Macromol. 2019, 134, 1085–1096. [Google Scholar] [CrossRef] [PubMed]
- Lemgruber, R.D.S.P.; Valgepea, K.; Tappel, R.; Behrendorff, J.B.; Palfreyman, R.W.; Plan, M.; Hodson, M.P.; Simpson, S.D.; Nielsen, L.K.; Köpke, M.; et al. Systems-level engineering and characterisation of Clostridium autoethanogenum through heterologous production of poly-3-hydroxybutyrate (PHB). Metab. Eng. 2019, 53, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.-R.; Choi, T.-R.; Han, Y.H.; Park, Y.-L.; Park, J.Y.; Song, H.-S.; Yang, S.-Y.; Bhatia, S.K.; Gurav, R.; Park, H.; et al. Production of blue-colored polyhydroxybutyrate (PHB) by one-pot production and coextraction of indigo and PHB from recombinant Escherichia coli. Dye. Pigment. 2020, 173, 107889. [Google Scholar] [CrossRef]
- Kirk, R.G.; Ginzburg, M. Ultrastructure of two species of halobacterium. J. Ultrastruct. Res. 1972, 41, 80–94. [Google Scholar] [CrossRef]
- Tyagi, B.; Gupta, B.; Khatak, D.; Meena, R.; Thakur, I.S. Genomic analysis, simultaneous production, and process optimization of extracellular polymeric substances and polyhydroxyalkanoates by Methylobacterium sp. ISTM1 by utilizing molasses. Bioresour. Technol. 2022, 354, 127204. [Google Scholar] [CrossRef]
- Höfer, P.; Vermette, P.; Groleau, D. Production and characterization of polyhydroxyalkanoates by recombinant Methylobacterium extorquens: Combining desirable thermal properties with functionality. Biochem. Eng. J. 2011, 54, 26–33. [Google Scholar] [CrossRef] [Green Version]
- Mohanrasu, K.; Rao, R.G.R.; Dinesh, G.; Zhang, K.; Sudhakar, M.; Pugazhendhi, A.; Jeyakanthan, J.; Ponnuchamy, K.; Govarthanan, M.; Arun, A. Production and characterization of biodegradable polyhydroxybutyrate by Micrococcus luteus isolated from marine environment. Int. J. Biol. Macromol. 2021, 186, 125–134. [Google Scholar] [CrossRef]
- Zhang, M.; Zhu, C.; Gao, J.; Fan, Y.; He, L.; He, C.; Wu, J. Deep-level nutrient removal and denitrifying phosphorus removal (DPR) potential assessment in a continuous two-sludge system treating low-strength wastewater: The transition from nitration to nitritation. Sci. Total Environ. 2020, 744, 140940. [Google Scholar] [CrossRef]
- Tyagi, B.; Takkar, S.; Meena, R.; Thakur, I.S. Production of polyhydroxybutyrate (PHB) by Parapedobacter sp. ISTM3 isolated from Mawsmai cave utilizing molasses as carbon source. Environ. Technol. Innov. 2021, 24, 101854. [Google Scholar] [CrossRef]
- Nitschke, M.; Costa, S.G.; Contiero, J. Rhamnolipids and PHAs: Recent reports on Pseudomonas-derived molecules of increasing industrial interest. Process. Biochem. 2011, 46, 621–630. [Google Scholar] [CrossRef]
- Aloui, H.; Khomlaem, C.; Torres, C.A.; Freitas, F.; Reis, M.A.; Kim, B.S. Enhanced co-production of medium-chain-length polyhydroxyalkanoates and phenazines from crude glycerol by high cell density cultivation of Pseudomonas chlororaphis in membrane bioreactor. Int. J. Biol. Macromol. 2022, 211, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Lakshman, K.; Rastogi, N.; Shamala, T. Simultaneous and comparative assessment of parent and mutant strain of Rhizobium meliloti for nutrient limitation and enhanced polyhydroxyalkanoate (PHA) production using optimization studies. Process Biochem. 2004, 39, 1977–1983. [Google Scholar] [CrossRef]
- Ramachander, T.; Rawal, S. PHB synthase from Streptomyces aureofaciens NRRL 2209. FEMS Microbiol. Lett. 2005, 242, 13–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krishnan, S.; Chinnadurai, G.S.; Perumal, P. Polyhydroxybutyrate by Streptomyces sp.: Production and characterization. Int. J. Biol. Macromol. 2017, 104, 1165–1171. [Google Scholar] [CrossRef] [PubMed]
- Brandl, H.; Gross, R.A.; Lenz, R.W.; Fuller, R.C. Plastics from Bacteria and for Bacteria: Poly(β-hydroxyalkanoates) as Natural, Biocompatible, and Biodegradable Polyesters. In Microbial Bioproducts; Springer: Berlin/Heidelberg, Germany, 1990; Volume 41, pp. 77–93. [Google Scholar]
- Birley, C.; Briddon, J.; Sykes, K.E.; Barker, P.A.; Organ, S.J.; Barham, P.J. Morphology of single crystals of poly (hydroxybutyrate) and copolymers of hydroxybuty rate and hydroxyvalerate. J. Mater. Sci. 1995, 30, 633–638. [Google Scholar] [CrossRef]
- Zhuikov, V.A.; Bonartsev, A.P.; Zharkova, I.; Bykova, G.S.; Taraskin, N.Y.; Kireynov, A.V.; Kopitsyna, M.N.; Bonartseva, G.A.; Shaitan, K.V. Effect of Poly(ethylene glycol) on the Ultrastructure and Physicochemical Properties of the Poly(3-hydroxybutyrate). Macromol. Symp. 2017, 375, 1600189. [Google Scholar] [CrossRef]
- Zhuikov, V.A.; Bonartsev, A.P.; Bagrov, D.; Yakovlev, S.; Myshkina, V.L.; Makhina, T.K.; Bessonov, I.V.; Kopitsyna, M.N.; Morozov, A.S.; Rusakov, A.A.; et al. Mechanics and surface ultrastructure changes of poly(3-hydroxybutyrate) films during enzymatic degradation in pancreatic lipase solution. Mol. Cryst. Liq. Cryst. 2017, 648, 236–243. [Google Scholar] [CrossRef]
- Barham, P.J.; Keller, A.; Otun, E.L.; Holmes, P.A. Crystallization and morphology of a bacterial thermoplastic: Poly-3-hydroxybutyrate. J. Mater. Sci. 1984, 19, 2781–2794. [Google Scholar] [CrossRef]
- Vakhrusheva, A.; Endzhievskaya, S.; Zhuikov, V.; Nekrasova, T.; Parshina, E.; Ovsiannikova, N.; Popov, V.; Bagrov, D.; Minin, A.; Sokolova, O.S. The role of vimentin in directional migration of rat fibroblasts. Cytoskeleton 2019, 76, 467–476. [Google Scholar] [CrossRef]
- Zhuikov, V.A.; Zhuikova, Y.V.; Makhina, T.K.; Myshkina, V.L.; Rusakov, A.; Useinov, A.; Voinova, V.V.; Bonartseva, G.A.; Berlin, A.A.; Bonartsev, A.P.; et al. Comparative Structure-Property Characterization of Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate)s Films under Hydrolytic and Enzymatic Degradation: Finding a Transition Point in 3-Hydroxyvalerate Content. Polymers 2020, 12, 728. [Google Scholar] [CrossRef]
- Li, X.; Liu, K.L.; Wang, M.; Wong, S.Y.; Tjiu, W.C.; He, C.; Goh, S.H.; Li, J. Improving hydrophilicity, mechanical properties and biocompatibility of poly[(R)-3-hydroxybutyrate-co-(R)-3-hydroxyvalerate] through blending with poly[(R)-3-hydroxybutyrate]-alt-poly(ethylene oxide). Acta Biomater. 2009, 5, 2002–2012. [Google Scholar] [CrossRef] [PubMed]
- Shih, W.-J.; Chen, Y.-H.; Shih, C.-J.; Hon, M.-H.; Wang, M.-C. Structural and morphological studies on poly(3-hydroxybutyrate acid) (PHB)/chitosan drug releasing microspheres prepared by both single and double emulsion processes. J. Alloys Compd. 2007, 434–435, 826–829. [Google Scholar] [CrossRef]
- Degli Esposti, M.; Chiellini, F.; Bondioli, F.; Morselli, D.; Fabbri, P. Highly porous PHB-based bioactive scaffolds for bone tissue engineering by in situ synthesis of hydroxyapatite. Mater. Sci. Eng. C 2019, 100, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, M.D.P.; de Menezes, L.R.; Rodrigues, E.J.D.R.; Tavares, M.I.B. In vitro characterization of a biocompatible composite based on poly(3-hydroxybutyrate)/hydroxyapatite nanoparticles as a potential scaffold for tissue engineering. J. Mech. Behav. Biomed. Mater. 2022, 128, 105138. [Google Scholar] [CrossRef] [PubMed]
- Akoulina, E.A.; Demianova, I.V.; Zharkova, I.I.; Voinova, V.V.; Zhuikov, V.A.; Khaydapova, D.D.; Chesnokova, D.V.; Menshikh, K.A.; Dudun, A.A.; Makhina, T.K.; et al. Growth of Mesenchymal Stem Cells on Poly(3-Hydroxybutyrate) Scaffolds Loaded with Simvastatin. Bull. Exp. Biol. Med. 2021, 171, 172–177. [Google Scholar] [CrossRef]
- Volkov, A.V.; Muraev, A.A.; Zharkova, I.I.; Voinova, V.V.; Akoulina, E.A.; Zhuikov, V.A.; Khaydapova, D.D.; Chesnokova, D.V.; Menshikh, K.A.; Dudun, A.A.; et al. Poly(3-hydroxybutyrate)/hydroxyapatite/alginate scaffolds seeded with mesenchymal stem cells enhance the regeneration of critical-sized bone defect. Mater. Sci. Eng. C 2020, 114, 110991. [Google Scholar] [CrossRef]
- Parvizifard, M.; Karbasi, S. Physical, mechanical and biological performance of PHB-Chitosan/MWCNTs nanocomposite coating deposited on bioglass based scaffold: Potential application in bone tissue engineering. Int. J. Biol. Macromol. 2020, 152, 645–662. [Google Scholar] [CrossRef]
- Parvizifard, M.; Karbasi, S.; Salehi, H.; Bakhtiari, S.S.E. Evaluation of physical, mechanical and biological properties of bioglass/titania scaffold coated with poly (3-hydroxybutyrate)-chitosan for bone tissue engineering applications. Mater. Technol. 2019, 35, 75–91. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.; Li, J.; Wang, B.; Liu, J.; Chen, P.; Miao, M.; Gu, Q. Chitin nanocrystals grafted with poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and their effects on thermal behavior of PHBV. Carbohydr. Polym. 2012, 87, 784–789. [Google Scholar] [CrossRef]
- Wang, B.; Li, J.; Zhang, J.; Li, H.; Chen, P.; Gu, Q.; Wang, Z. Thermo-mechanical properties of the composite made of poly (3-hydroxybutyrate-co-3-hydroxyvalerate) and acetylated chitin nanocrystals. Carbohydr. Polym. 2013, 95, 100–106. [Google Scholar] [CrossRef]
- Karbasi, S.; Khorasani, S.N.; Ebrahimi, S.; Khalili, S.; Fekrat, F.; Sadeghi, D. Preparation and characterization of poly (hydroxy butyrate)/chitosan blend scaffolds for tissue engineering applications. Adv. Biomed. Res. 2016, 5, 177. [Google Scholar] [CrossRef] [PubMed]
- Rajan, R.; Sreekumar, P.A.; Joseph, K.; Skrifvars, M. Thermal and mechanical properties of chitosan reinforced polyhydroxybutyrate composites. J. Appl. Polym. Sci. 2012, 124, 3357–3362. [Google Scholar] [CrossRef]
- Holappa, J.; Hjálmarsdóttir, M.; Másson, M.; Rúnarsson, Ö.; Asplund, T.; Soininen, P.; Nevalainen, T.; Järvinen, T. Antimicrobial activity of chitosan N-betainates. Carbohydr. Polym. 2006, 65, 114–118. [Google Scholar] [CrossRef]
- Makuška, R.; Gorochovceva, N. Regioselective grafting of poly(ethylene glycol) onto chitosan through C-6 position of glucosamine units. Carbohydr. Polym. 2006, 64, 319–327. [Google Scholar] [CrossRef]
- Li, G.; Zhuang, Y.; Mu, Q.; Wang, M.; Fang, Y. Preparation, characterization and aggregation behavior of amphiphilic chitosan derivative having poly (l-lactic acid) side chains. Carbohydr. Polym. 2008, 72, 60–66. [Google Scholar] [CrossRef]
- Salama, H.E.; Saad, G.R.; Sabaa, M.W. Synthesis, characterization and antimicrobial activity of biguanidinylated chitosan- g -poly[( R )-3-hydroxybutyrate]. Int. J. Biol. Macromol. 2017, 101, 438–447. [Google Scholar] [CrossRef]
- Arslan, H.; Hazer, B.; Yoon, S.C. Grafting of poly(3-hydroxyalkanoate) and linoleic acid onto chitosan. J. Appl. Polym. Sci. 2007, 103, 81–89. [Google Scholar] [CrossRef]
- Salama, H.E.; Aziz, M.S.A.; Saad, G.R. Thermal properties, crystallization and antimicrobial activity of chitosan biguanidine grafted poly(3-hydroxybutyrate) containing silver nanoparticles. Int. J. Biol. Macromol. 2018, 111, 19–27. [Google Scholar] [CrossRef]
- Vernaez, O.; Neubert, K.J.; Kopitzky, R.; Kabasci, S. Compatibility of Chitosan in Polymer Blends by Chemical Modification of Bio-based Polyesters. Polymers 2019, 11, 1939. [Google Scholar] [CrossRef] [Green Version]
- Hu, S.-G.; Jou, C.-H.; Yang, M. Protein adsorption, fibroblast activity and antibacterial properties of poly(3-hydroxybutyric acid-co-3-hydroxyvaleric acid) grafted with chitosan and chitooligosaccharide after immobilized with hyaluronic acid. Biomaterials 2003, 24, 2685–2693. [Google Scholar] [CrossRef]
- Loeffler, A.P.; Ma, P.X. Bioinspired Nanomaterials for Tissue Engineering. In Nanotechnologies for the Life Sciences; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2012. [Google Scholar]
- Surmenev, R.A.; Ivanov, A.N.; Cecilia, A.; Baumbach, T.; Chernozem, R.V.; Mathur, S.; Surmeneva, M.A. Electrospun composites of poly-3-hydroxybutyrate reinforced with conductive fillers for in vivo bone regeneration. Open Ceram. 2022, 9, 100237. [Google Scholar] [CrossRef]
- Matsumoto, H.; Tanioka, A. Functionality in Electrospun Nanofibrous Membranes Based on Fiber’s Size, Surface Area, and Molecular Orientation. Membranes 2011, 1, 249–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadeghi, D.; Karbasi, S.; Razavi, S.; Mohammadi, S.; Shokrgozar, M.A.; Bonakdar, S. Electrospun poly(hydroxybutyrate)/chitosan blend fibrous scaffolds for cartilage tissue engineering. J. Appl. Polym. Sci. 2016, 133, 44171. [Google Scholar] [CrossRef]
- Khoroushi, M.; Foroughi, M.R.; Karbasi, S.; Hashemibeni, B.; Khademi, A.A. Effect of Polyhydroxybutyrate/Chitosan/Bioglass nanofiber scaffold on proliferation and differentiation of stem cells from human exfoliated deciduous teeth into odontoblast-like cells. Mater. Sci. Eng. C 2018, 89, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, Y.; Li, D.; Yin, Y.; Zhou, F. Electrospun PHB/Chitosan Composite Fibrous Membrane and Its Degradation Behaviours in Different pH Conditions. J. Funct. Biomater. 2022, 13, 58. [Google Scholar] [CrossRef] [PubMed]
- Veleirinho, B.; Ribeiro-do-Valle, R.M.; Lopes-da-Silva, J.A. Processing conditions and characterization of novel electrospun poly (3-hydroxybutyrate-co-hydroxyvalerate)/chitosan blend fibers. Mater. Lett. 2011, 65, 2216–2219. [Google Scholar] [CrossRef]
- Veleirinho, B.; Coelho, D.S.; Dias, P.F.; Maraschin, M.; Ribeiro-do-Valle, R.M.; Lopes-da-Silva, J.A. Nanofibrous poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/chitosan scaffolds for skin regeneration. Int. J. Biol. Macromol. 2012, 51, 343–350. [Google Scholar] [CrossRef]
- Toloue, E.B.; Karbasi, S.; Salehi, H.; Rafienia, M. Potential of an electrospun composite scaffold of poly (3-hydroxybutyrate)-chitosan/alumina nanowires in bone tissue engineering applications. Mater. Sci. Eng. C 2019, 99, 1075–1091. [Google Scholar] [CrossRef]
- Pavlova, E.; Nikishin, I.; Bogdanova, A.; Klinov, D.; Bagrov, D. The miscibility and spatial distribution of the components in electrospun polymer–protein mats. RSC Adv. 2020, 10, 4672–4680. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Dong, L. Biodegradable Blends Based on Microbial Poly(3-Hydroxybutyrate) and Natural Chitosan. In Biodegradable Polymer Blends and Composites from Renewable Resources; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; pp. 227–237. [Google Scholar]
- Ignatova, M.; Manolova, N.; Rashkov, I.; Markova, N. Quaternized chitosan/κ-carrageenan/caffeic acid–coated poly(3-hydroxybutyrate) fibrous materials: Preparation, antibacterial and antioxidant activity. Int. J. Pharm. 2016, 513, 528–537. [Google Scholar] [CrossRef]
- Ikejima, T.; Yagi, K.; Inoue, Y. Thermal properties and crystallization behavior of poly(3-hydroxybutyric acid) in blends with chitin and chitosan. Macromol. Chem. Phys. 1999, 200, 413–421. [Google Scholar] [CrossRef]
- Chen, C.; Zhou, X.; Zhuang, Y.; Dong, L. Thermal behavior and intermolecular interactions in blends of poly(3-hydroxybutyrate) and maleated poly(3-hydroxybutyrate) with chitosan. J. Polym. Sci. Part B Polym. Phys. 2005, 43, 35–47. [Google Scholar] [CrossRef]
- Kolhe, P.; Kannan, R.M. Improvement in Ductility of Chitosan through Blending and Copolymerization with PEG: FTIR Investigation of Molecular Interactions. Biomacromolecules 2002, 4, 173–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khasanah; Reddy, K.R.; Sato, H.; Takahashi, I.; Ozaki, Y. Intermolecular hydrogen bondings in the poly(3-hydroxybutyrate) and chitin blends: Their effects on the crystallization behavior and crystal structure of poly(3-hydroxybutyrate). Polymer 2015, 75, 141–150. [Google Scholar] [CrossRef]
- Suttiwijitpukdee, N.; Sato, H.; Zhang, J.; Hashimoto, T.; Ozaki, Y. Intermolecular interactions and crystallization behaviors of biodegradable polymer blends between poly (3-hydroxybutyrate) and cellulose acetate butyrate studied by DSC, FT-IR, and WAXD. Polymer 2011, 52, 461–471. [Google Scholar] [CrossRef]
- Medvecky, L. Microstructure and Properties of Polyhydroxybutyrate-Chitosan-Nanohydroxyapatite Composite Scaffolds. Sci. World J. 2012, 2012, 537973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, W.; Wang, A.; Jing, D.; Gong, Y.; Zhao, N.; Zhang, X. Novel biodegradable films and scaffolds of chitosan blended with poly(3-hydroxybutyrate). J. Biomater. Sci. Polym. Ed. 2005, 16, 1379–1394. [Google Scholar] [CrossRef]
- Ivantsova, E.L.; Iordanskii, A.L.; Kosenko, R.Y.; Rogovina, S.Z.; Grachev, A.V.; Prut, É.V. Poly(3-hydroxybutyrate)-chitosan: A new biodegradable composition for prolonged delivery of biologically active substances. Pharm. Chem. J. 2011, 45, 51–55. [Google Scholar] [CrossRef]
- Peschel, G.; Dahse, H.-M.; Konrad, A.; Wieland, G.D.; Mueller, P.-J.; Martin, D.P.; Roth, M. Growth of keratinocytes on porous films of poly(3-hydroxybutyrate) and poly(4-hydroxybutyrate) blended with hyaluronic acid and chitosan. J. Biomed. Mater. Res. Part A 2008, 85A, 1072–1081. [Google Scholar] [CrossRef]
- Iordanskii, A.L.; Ivantsova, E.L.; Kosenko, R.Y.; Zernova, Y.N.; Rogovina, S.Z.; Filatova, A.G.; Gumargalieva, K.Z.; Rusanova, S.N.; Stoyanov, O.V.; Zaikov, G.E. Diffusion and structural characteristics of compositions based on polyhydroxybutyrate and chitosan for directed transport of medicinal substances. part 2. Bull. Kazan Technol. Univ. 2013, 16, 162–164. (In Russian) [Google Scholar]
- Iordanskii, A.L.; Ivantsova, E.L.; Kosenko, R.Y.; Zernova, Y.N.; Rogovina, S.Z.; Filatova, A.G.; Gumargalieva, K.Z.; Rusanova, S.N.; Stoyanov, O.V.; Zaikov, G.E. Diffusion and structural characteristics of compositions based on polyhydroxybutyrate and chitosan for directed transport of medicinal substances. part 3. Bull. Kazan Technol. Univ. 2014, 17, 145–148. (In Russian) [Google Scholar]
- Karpova, S.G.; Jordan, A.L.; Olkhov, A.A.; Povov, A.A.; Lomakin, S.M.; Shilkina, N.S.; Zaikov, G.E. Effect of rolling on the structure of fibrous materials based on poly(3-hydroxybutyrate) with chitosan obtained by electroforming. Bull. Univ. Technol. 2015, 18, 109–115. (In Russian) [Google Scholar]
- Karpova, S.G.; Iordanskii, A.L.; Klenina, N.S.; Popov, A.A.; Lomakin, S.M.; Shilkina, N.G.; Rebrov, A.V. Changes in the structural parameters and molecular dynamics of polyhydroxybutyrate-chitosan mixed compositions under external influences. Russ. J. Phys. Chem. B 2013, 7, 225–231. [Google Scholar] [CrossRef]
- Ikejima, T.; Inoue, Y. Crystallization behavior and environmental biodegradability of the blend films of poly(3-hydroxybutyric acid) with chitin and chitosan. Carbohydr. Polym. 2000, 41, 351–356. [Google Scholar] [CrossRef]
- Cheung, M.K.; Wan, K.P.; Yu, P.H. Miscibility and morphology of chiral semicrystalline poly-(R)-(3-hydroxybutyrate)/chitosan and poly-(R)-(3-hydroxybutyrate-co-3-hydroxyvalerate)/chitosan blends studied with DSC,1HT1 andT1? CRAMPS. J. Appl. Polym. Sci. 2002, 86, 1253–1258. [Google Scholar] [CrossRef]
- Silvestri, D.; Wacławek, S.; Sobel, B.; Torres-Mendieta, R.; Novotný, V.; Nguyen, N.H.A.; Ševců, A.; Padil, V.V.T.; Müllerová, J.; Stuchlík, M.; et al. A poly(3-hydroxybutyrate)–chitosan polymer conjugate for the synthesis of safer gold nanoparticles and their applications. Green Chem. 2018, 20, 4975–4982. [Google Scholar] [CrossRef]
- Yalpani, M.; Marchessault, R.H.; Morin, F.G.; Monasterios, C.J. Synthesis of poly(3-hydroxyalkanoate) (PHA) conjugates: PHA-carbohydrate and PHA-synthetic polymer conjugates. Macromolecules 1991, 24, 6046–6049. [Google Scholar] [CrossRef]
- Choonut, A.; Prasertsan, P.; Klomklao, S.; Sangkharak, K. An Environmentally Friendly Process for Textile Wastewater Treatment with a Medium-Chain-Length Polyhydroxyalkanoate Film. J. Polym. Environ. 2021, 29, 3335–3346. [Google Scholar] [CrossRef]
- Anbukarasu, P.; Sauvageau, D.; Elias, A. Tuning the properties of polyhydroxybutyrate films using acetic acid via solvent casting. Sci. Rep. 2015, 5, 17884. [Google Scholar] [CrossRef] [Green Version]
- Mukheem, A.; Shahabuddin, S.; Akbar, N.; Miskon, A.; Sarih, N.M.; Sudesh, K.; Khan, N.A.; Saidur, R.; Sridewi, N. Boron nitride doped polyhydroxyalkanoate/chitosan nanocomposite for antibacterial and biological applications. Nanomaterials 2019, 9, 645. [Google Scholar] [CrossRef] [Green Version]
- Zaccone, M.; Patel, M.K.; De Brauwer, L.; Nair, R.; Montalbano, M.L.; Monti, M.; Oksman, K. Influence of Chitin Nanocrystals on the Crystallinity and Mechanical Properties of Poly(hydroxybutyrate) Biopolymer. Polymers 2022, 14, 562. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.K.; Zaccone, M.; De Brauwer, L.; Nair, R.; Monti, M.; Martinez-Nogues, V.; Frache, A.; Oksman, K. Improvement of Poly(lactic acid)-Poly(hydroxy butyrate) Blend Properties for Use in Food Packaging: Processing, Structure Relationships. Polymers 2022, 14, 5104. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Yun, S.I. Chitosan-reinforced PHB hydrogel and aerogel monoliths fabricated by phase separation with the solvent-exchange method. Carbohydr. Polym. 2022, 284, 119184. [Google Scholar] [CrossRef]
- Bazzo, G.; Lemos-Senna, E.; Pires, A. Poly(3-hydroxybutyrate)/chitosan/ketoprofen or piroxicam composite microparticles: Preparation and controlled drug release evaluation. Carbohydr. Polym. 2009, 77, 839–844. [Google Scholar] [CrossRef]
- Bazzo, G.C.; Lemos-Senna, E.; Gonçalves, M.C.; Pires, A.T.N. Effect of preparation conditions on morphology, drug content and release profiles of poly(hydroxybutyrate) microparticles containing piroxicam. J. Braz. Chem. Soc. 2008, 19, 914–921. [Google Scholar] [CrossRef]
- Hall, A.R.; Geoghegan, M. Polymers and biopolymers at interfaces. Rep. Prog. Phys. 2018, 81, 036601. [Google Scholar] [CrossRef]
- Garavand, F.; Cacciotti, I.; Vahedikia, N.; Rehman, A.; Tarhan, Ö.; Akbari-Alavijeh, S.; Shaddel, R.; Rashidinejad, A.; Nejatian, M.; Jafarzadeh, S.; et al. A comprehensive review on the nanocomposites loaded with chitosan nanoparticles for food packaging. Crit. Rev. Food Sci. Nutr. 2020, 62, 1383–1416. [Google Scholar] [CrossRef]
- Boura-Theodoridou, O.; Giannakas, A.; Katapodis, P.; Stamatis, H.; Ladavos, A.; Barkoula, N.-M. Performance of ZnO/chitosan nanocomposite films for antimicrobial packaging applications as a function of NaOH treatment and glycerol/PVOH blending. Food Packag. Shelf Life 2020, 23, 100456. [Google Scholar] [CrossRef]
- Ghosh, T.; Mondal, K.; Giri, B.S.; Katiyar, V. Silk nanodisc based edible chitosan nanocomposite coating for fresh produces: A candidate with superior thermal, hydrophobic, optical, mechanical and food properties. Food Chem. 2021, 360, 130048. [Google Scholar] [CrossRef]
- Serafim, L.S.; Lemos, P.C.; Oliveira, R.; Reis, M.A.M. Optimization of polyhydroxybutyrate production by mixed cultures submitted to aerobic dynamic feeding conditions. Biotechnol. Bioeng. 2004, 87, 145–160. [Google Scholar] [CrossRef]
- Xu, P.; Yang, W.; Niu, D.; Yu, M.; Du, M.; Dong, W.; Chen, M.; Jan Lemstra, P.; Ma, P. Multifunctional and robust polyhydroxyalkanoate nanocomposites with superior gas barrier, heat resistant and inherent antibacterial performances. Chem. Eng. J. 2020, 382, 122864. [Google Scholar] [CrossRef]
- Manikandan, N.A.; Pakshirajan, K.; Pugazhenthi, G. Preparation and characterization of environmentally safe and highly biodegradable microbial polyhydroxybutyrate (PHB) based graphene nanocomposites for potential food packaging applications. Int. J. Biol. Macromol. 2020, 154, 866–877. [Google Scholar] [CrossRef] [PubMed]
- Zare, M.; Namratha, K.; Ilyas, S.; Hezam, A.; Mathur, S.; Byrappa, K. Smart Fortified PHBV-CS Biopolymer with ZnO–Ag Nanocomposites for Enhanced Shelf Life of Food Packaging. ACS Appl. Mater. Interfaces 2019, 11, 48309–48320. [Google Scholar] [CrossRef] [PubMed]




| Acinetobacter | [96] |
| Azotobacter | [93,97,98,99] |
| Bacillus | [100,101] |
| Clostridium | [102] |
| Escherichia | [103] |
| Halobacterium | [104] |
| Methylobacterium | [105,106] |
| Micrococcus | [107] |
| Nitrobacter | [108] |
| Parapedobacter | [109] |
| Pseudomonas | [110,111] |
| Rhizobium | [112] |
| Streptomyces | [113,114] |
| Sample | Inhibition Zone (mm) | ||
|---|---|---|---|
| S. pneumonia | E. coli | A. fumigatus | |
| ChG-g-PHB | 21.30 ± 2.10 | 21.80 ± 2.10 | 19.40 ± 1.5 |
| Ampicillin | 23.80 ± 0.20 | - | - |
| Gentamicin | - | 19.90 ±0.30 | - |
| Amphotericin | - | - | 23.70 ± 0.10 |
| Sample | Young’s Modulus (MPa) | Elongation at Break (%) | Tensile Strength (MPa) | Contact Angle (Degrees) | Proliferation of HaCaT Cells (% vs. Control) |
|---|---|---|---|---|---|
| PHB | 1640 | 1.4 | 12.9 | 84 | 22 ± 19 |
| PHB/Cs | 334 | 1.6 | 3.3 | 93 | 90 ± 10 |
| Methods | References | Advantages | Disadvantages |
|---|---|---|---|
| Extrusion, melt functionalization | [140,174,175] | There is no need to use expensive and toxic solvents. | Melting temperatures of PHB and chitosan are different. Thermal degradation of polymers and reduction in molecular weight can occur. Expensive specific equipment is required. |
| Copolymerization | [137,138,139,140] | Covalent bonding ensures the creation of a single branched structure. | Difficulty in synthesis, selection of optimal conditions is necessary, in some cases it is not easy to determine the degree of grafting |
| Electrospinning | [143,144,145,146,147,148,149,150] | Creating products with a variety of structures (controlled fiber size, porosity) | Requires expensive equipment. Difficult to manufacture. Limited choice of solvents |
| Blending in different solutions | [152,153,154,155,156,157,158,159,160,161] | The relative ease of creating the material. No specific equipment is required. | It is necessary to carefully select the mixing conditions, the ratio of polymers due to the problem of stability |
| Blending in a common solution | [132,154,167,168,169,170,171,172,173] | Relative stability, simplicity of manufacturing | The most widely used common solvents are expensive and toxic. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhuikova, Y.; Zhuikov, V.; Varlamov, V. Biocomposite Materials Based on Poly(3-hydroxybutyrate) and Chitosan: A Review. Polymers 2022, 14, 5549. https://doi.org/10.3390/polym14245549
Zhuikova Y, Zhuikov V, Varlamov V. Biocomposite Materials Based on Poly(3-hydroxybutyrate) and Chitosan: A Review. Polymers. 2022; 14(24):5549. https://doi.org/10.3390/polym14245549
Chicago/Turabian StyleZhuikova, Yuliya, Vsevolod Zhuikov, and Valery Varlamov. 2022. "Biocomposite Materials Based on Poly(3-hydroxybutyrate) and Chitosan: A Review" Polymers 14, no. 24: 5549. https://doi.org/10.3390/polym14245549
APA StyleZhuikova, Y., Zhuikov, V., & Varlamov, V. (2022). Biocomposite Materials Based on Poly(3-hydroxybutyrate) and Chitosan: A Review. Polymers, 14(24), 5549. https://doi.org/10.3390/polym14245549







