Thermoset/Thermoplastic Interphases: The Role of Initiator Concentration in Polymer Interdiffusion
Abstract
:1. Introduction
2. Experiments, Methodology and Background
2.1. Materials
2.2. Cure Kinetics (DSC) Measurements
2.3. Gelation Time Measurements
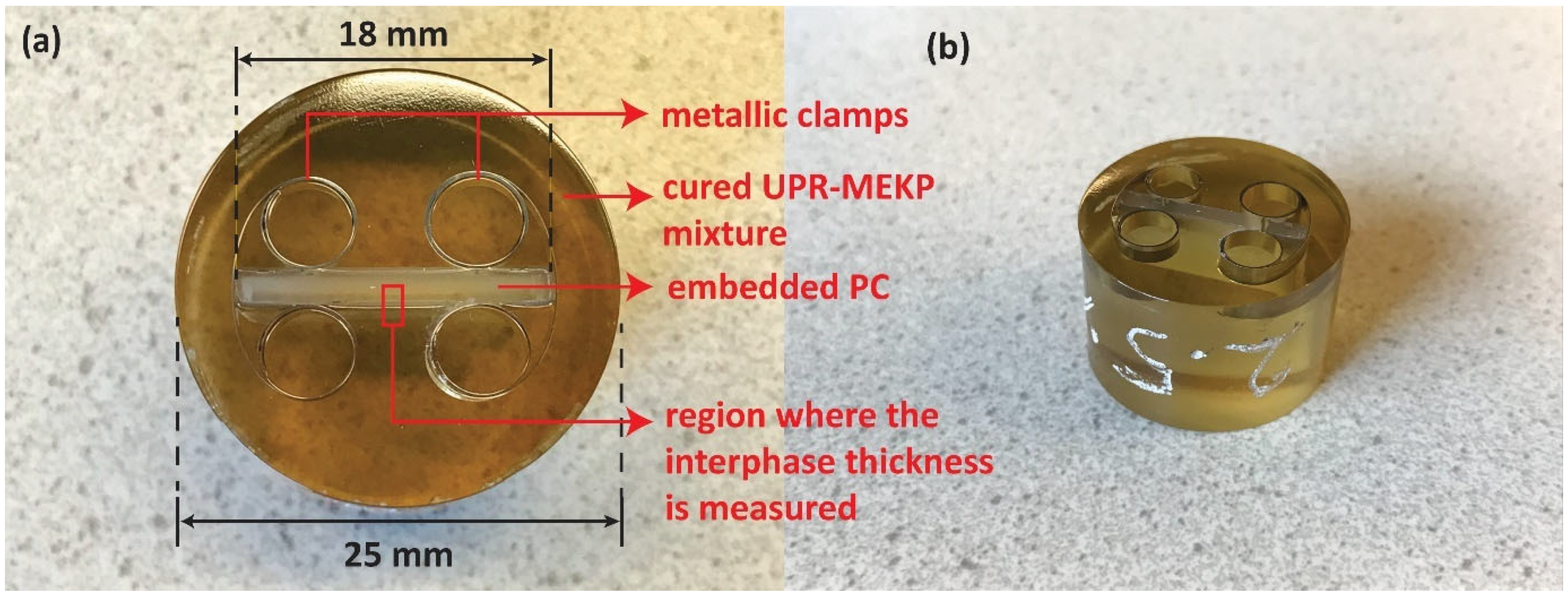
2.4. Co-Bonding
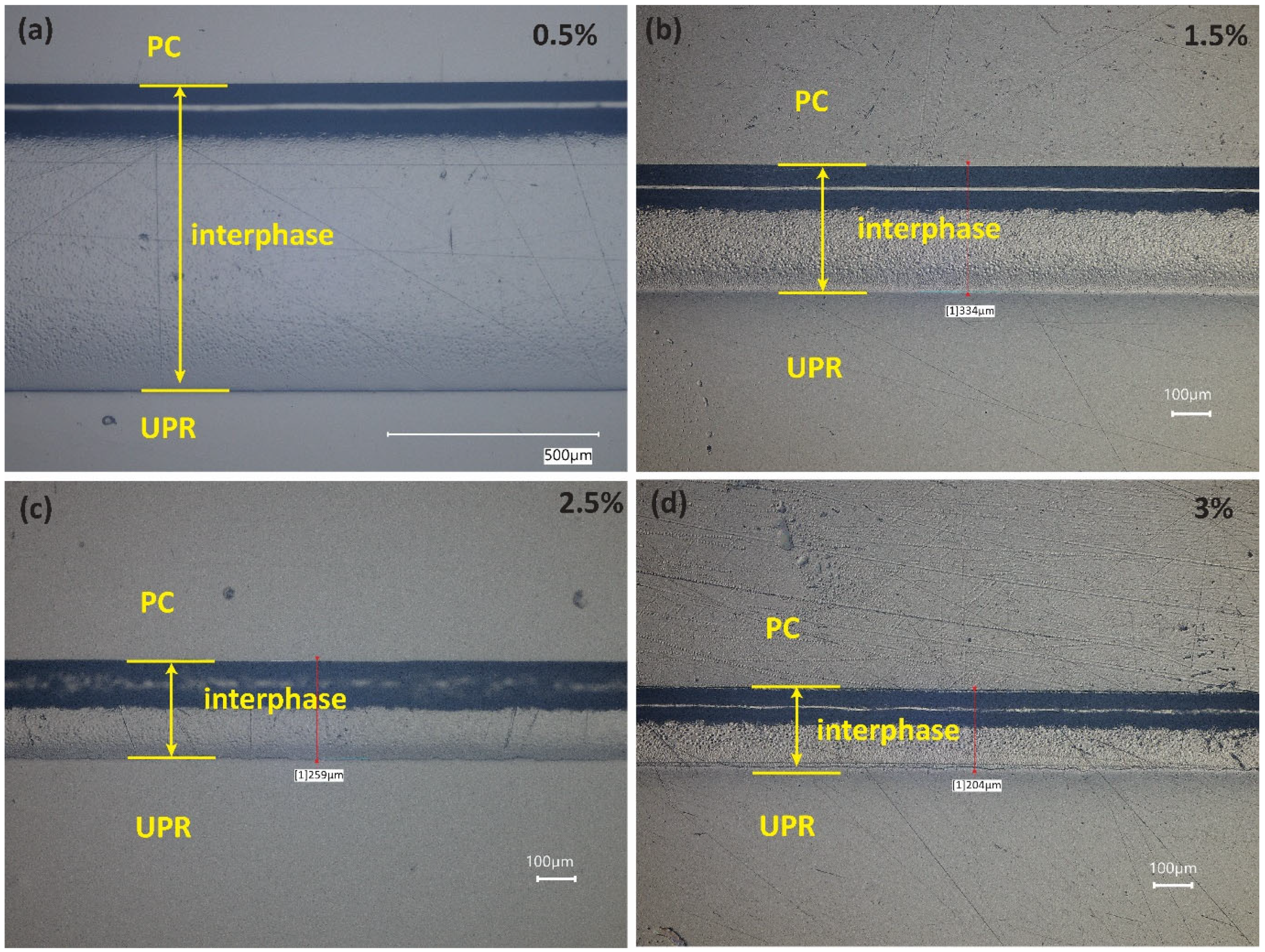
2.5. Interphase Thickness Measurements
2.6. Diffusivity of UPR into Thermoplastic Polymers
3. Results and Discussion
3.1. Cure Kinetics and Gelation Time
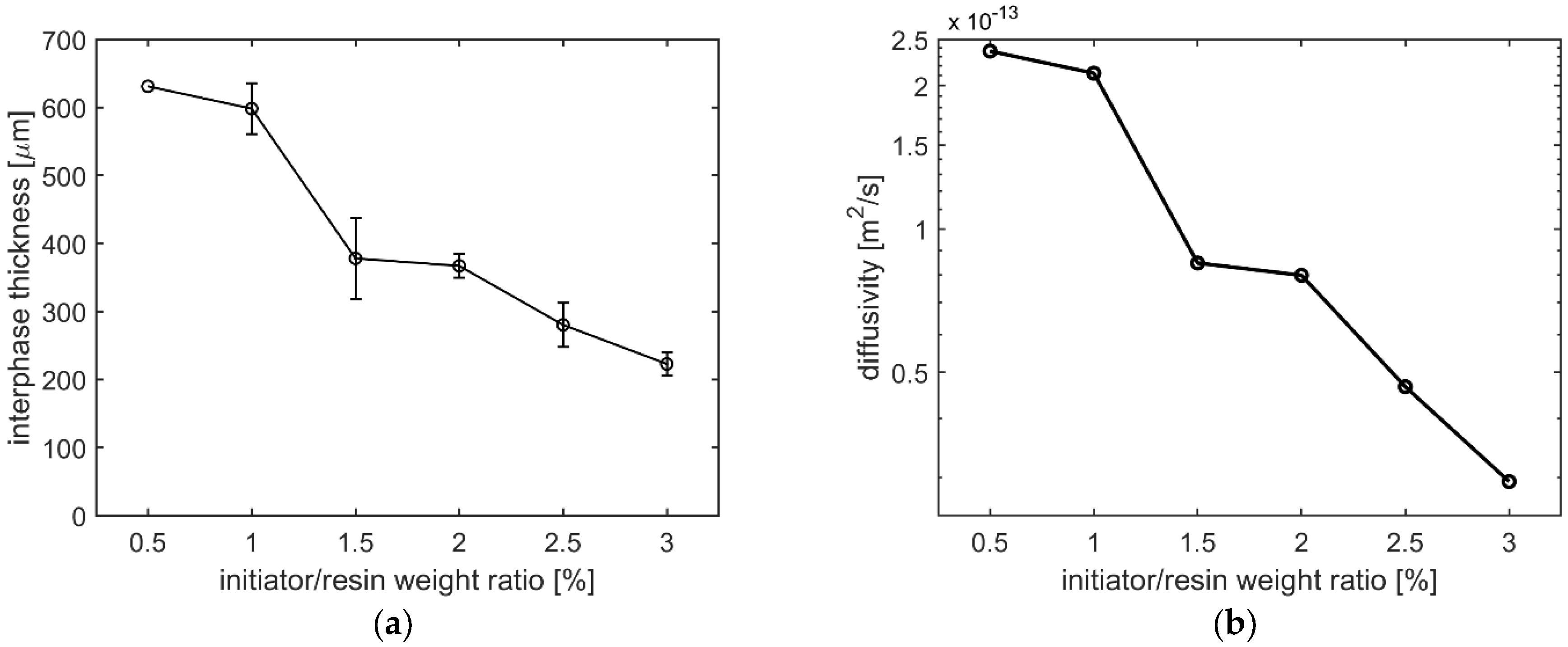
3.2. Interphase Morphology
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baran, I. Warpage prediction in over-infusion process of glass/polyester composite laminates. In Proceedings of the 21st ICCM International Conference on Composite Materials, Xi’an, China, 20–25 August 2017. [Google Scholar]
- Zanjani, J.S.M.; Baran, I.; Akkerman, R. Characterization of interdiffusion mechanisms during co-bonding of unsaturated polyester resin to thermoplastics with different thermodynamic affinities. Polymer 2020, 209, 122991. [Google Scholar] [CrossRef]
- Erartsın, O.; Zanjani, J.S.M.; Baran, I. Warpage of fiber-reinforced thermoset polymers co-bonded to thermoplastics. In Proceedings of the SAMPE EUROPE Conference and Exhibition 2021, Baden, Switzerland, 29–30 September 2021. [Google Scholar]
- Zanjani, J.S.M.; Baran, I. Co-bonded hybrid thermoplastic-thermoset composite interphase: Process-microstructure-property correlation. Materials 2021, 14, 291. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.W. Prediction of Process Induced Shape Distortions and Residual Stresses in Large—With Application to Wind Turbine Blades. Ph.D. Thesis, Technical University of Denmark, Lyngby, Denmark, 2013. [Google Scholar]
- Haag, M.D. A Wind Turbine Blade Having an Erosion Shield. U.S. Patent 20180209400A1, 26 July 2018. [Google Scholar]
- Li, J.; Yao, X.; Liu, Y.; Cen, Z.; Kou, Z.; Dai, D. A study of the integrated composite material structures under different fabrication processing. Compos. Part A Appl. Sci. Manuf. 2009, 40, 455–462. [Google Scholar] [CrossRef]
- Deng, S.; Djukic, L.; Paton, R.; Ye, L. Thermoplastic—Epoxy interactions and their potential applications in joining composite structures—A review. Compos. Part A Appl. Sci. Manuf. 2015, 68, 121–132. [Google Scholar] [CrossRef]
- Vandi, L.J.; Hou, M.; Veidt, M.; Truss, R.; Heitzmann, M.; Paton, R. Interface diffusion and morphology of aerospace grade epoxy co-cured with thermoplastic polymers. In Proceedings of the 28th Congress of the International Council of the Aeronautical Sciences 2012 (ICAS 2012), Brisbane, Australia, 23–28 September 2012; pp. 1–9. [Google Scholar]
- Zweifel, L.; Brauner, C. Investigation of the interphase mechanisms and welding behaviour of fast-curing epoxy based composites with co-cured thermoplastic boundary layers. Compos. Part A Appl. Sci. Manuf. 2020, 139, 106120. [Google Scholar] [CrossRef]
- Farooq, U.; Heuer, S.; Teuwen, J.; Dransfeld, C. Effect of a Dwell Stage in the Cure Cycle on the Interphase Formation in a Poly(ether imide)/High Tg Epoxy System. ACS Appl. Polym. Mater. 2021, 3, 6111–6119. [Google Scholar] [CrossRef]
- Zweifel, L.; Brauner, C.; Teuwen, J.; Dransfeld, C. In Situ Characterization of the Reaction-Diffusion Behavior during the Gradient Interphase Formation of Polyetherimide with a High-Temperature Epoxy System. Polymers 2022, 14, 435. [Google Scholar] [CrossRef] [PubMed]
- Twigg, G.; Poursartip, A.; Fernlund, G. Tool-part interaction in composites processing. Part II: Numerical modelling. Compos. Part A Appl. Sci. Manuf. 2004, 35, 135–141. [Google Scholar] [CrossRef]
- Voleppe, Q.; Ballout, W.; Van Velthem, P.; Bailly, C.; Pardoen, T. Enhanced fracture resistance of thermoset/thermoplastic interfaces through crack trapping in a morphology gradient. Polymer 2021, 218, 123497. [Google Scholar] [CrossRef]
- Kuppusamy, R.R.P.; Neogi, S. Influence of curing agents on gelation and exotherm behaviour of an unsaturated polyester resin. Bull. Mater. Sci. 2013, 36, 1217–1224. [Google Scholar] [CrossRef]
- Ramis, X.; Salla, J.M. Effect of the initiator content and temperature on the curing of an unsaturated polyester resin. J. Polym. Sci. Part B Polym. Phys. 1999, 37, 751–768. [Google Scholar] [CrossRef]
- Vilas, J.L.; Laza, J.M.; Garay, M.T.; Rodríguez, M.; León, L.M. Unsaturated polyester resins cure: Kinetic, rheologic, and mechanical-dynamical analysis. 1. Cure kinetics by DSC and TSR. J. Appl. Polym. Sci. 2001, 79, 447–457. [Google Scholar] [CrossRef]
- Cook, W.D.; Lau, M.; Mehrabi, M.; Dean, K.; Zipper, M. Control of gel time and exotherm behaviour during cure of unsaturated polyester resins. Polym. Int. 2001, 50, 129–134. [Google Scholar] [CrossRef]
- Ballout, W.; Coulon, B.; Janssens, Y.-A.; Van Velthem, P.; Sclavons, M.; Magnin, D.; Pardoen, T.; Bailly, C. Quantitative characterization of interdiffusion at the resin–resin and resin–prepreg interphases of epoxy systems processed by model SQ-RTM. Polym. Eng. Sci. 2016, 56, 1061–1069. [Google Scholar] [CrossRef]
- Campbell, F.C. Thermoset resins: The glue that holds the strings together. Manuf. Processes Adv. Compos. 2004, 63–101. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion, 2nd ed.; Oxford University Press: Oxford, UK, 1979; ISBN 0-19-853344-6. [Google Scholar]
- Rajagopalan, G.; Narayanan, C.; Gillespie, J.W.; McKnight, S.H. Diffusion and reaction of epoxy and amine in polysulfone-transport modeling and experimental validation. Polymer 2000, 41, 8543–8556. [Google Scholar] [CrossRef]
- Lestriez, B.; Chapel, J.P.; Gérard, J.F. Gradient interphase between reactive epoxy and glassy thermoplastic from dissolution process, reaction kinetics, and phase separation thermodynamics. Macromolecules 2001, 34, 1204–1213. [Google Scholar] [CrossRef]
- Baran, I.; Akkerman, R.; Hattel, J.H. Material characterization of a polyester resin system for the pultrusion process. Compos. Part B Eng. 2014, 64, 194–201. [Google Scholar] [CrossRef]
- Ramis, X.; Salla, J.M. Time-temperature transformation (TTT) cure diagram of an unsaturated polyester resin. J. Polym. Sci. Part B Polym. Phys. 1997, 35, 371–388. [Google Scholar] [CrossRef]


| Initiator/Resin Weight Ratio | Heat of Cure [J/g] | Normalized Degree of Cure [-] |
|---|---|---|
| 1% | 149.7 | 0.58 |
| 1.5% | 233.4 ± 2.9 | 0.90 ± 0.01 |
| 2.5% | 249.1 | 0.96 |
| 3% | 259.9 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erartsın, O.; Zanjani, J.S.M.; Baran, I. Thermoset/Thermoplastic Interphases: The Role of Initiator Concentration in Polymer Interdiffusion. Polymers 2022, 14, 1493. https://doi.org/10.3390/polym14071493
Erartsın O, Zanjani JSM, Baran I. Thermoset/Thermoplastic Interphases: The Role of Initiator Concentration in Polymer Interdiffusion. Polymers. 2022; 14(7):1493. https://doi.org/10.3390/polym14071493
Chicago/Turabian StyleErartsın, Ozan, Jamal Sayyed Monfared Zanjani, and Ismet Baran. 2022. "Thermoset/Thermoplastic Interphases: The Role of Initiator Concentration in Polymer Interdiffusion" Polymers 14, no. 7: 1493. https://doi.org/10.3390/polym14071493






