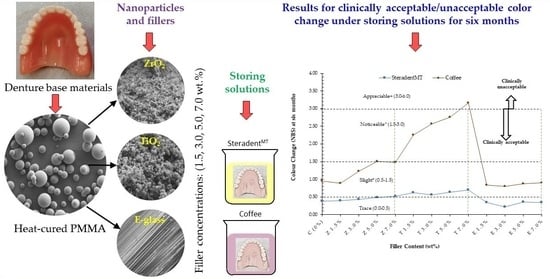
Effect of Different Solutions on the Colour Stability of Nanoparticles or Fibre Reinforced PMMA
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.2.1. Modification of Nano-ZrO2 and Nano-TiO2
2.2.2. Combining Fillers with PMMA/MMA
2.2.3. Colour Measurement Process
2.3. Statistical Analysis
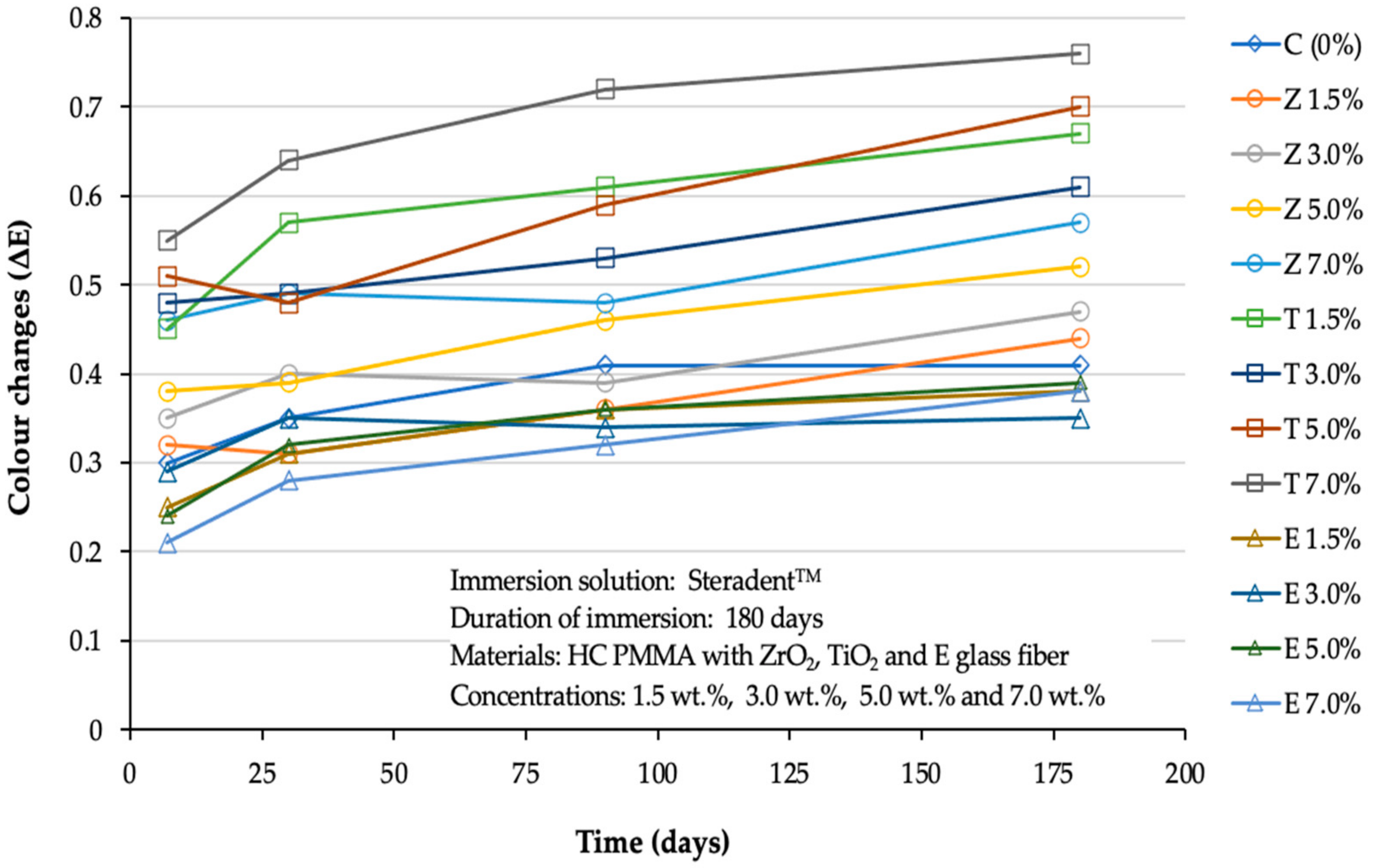
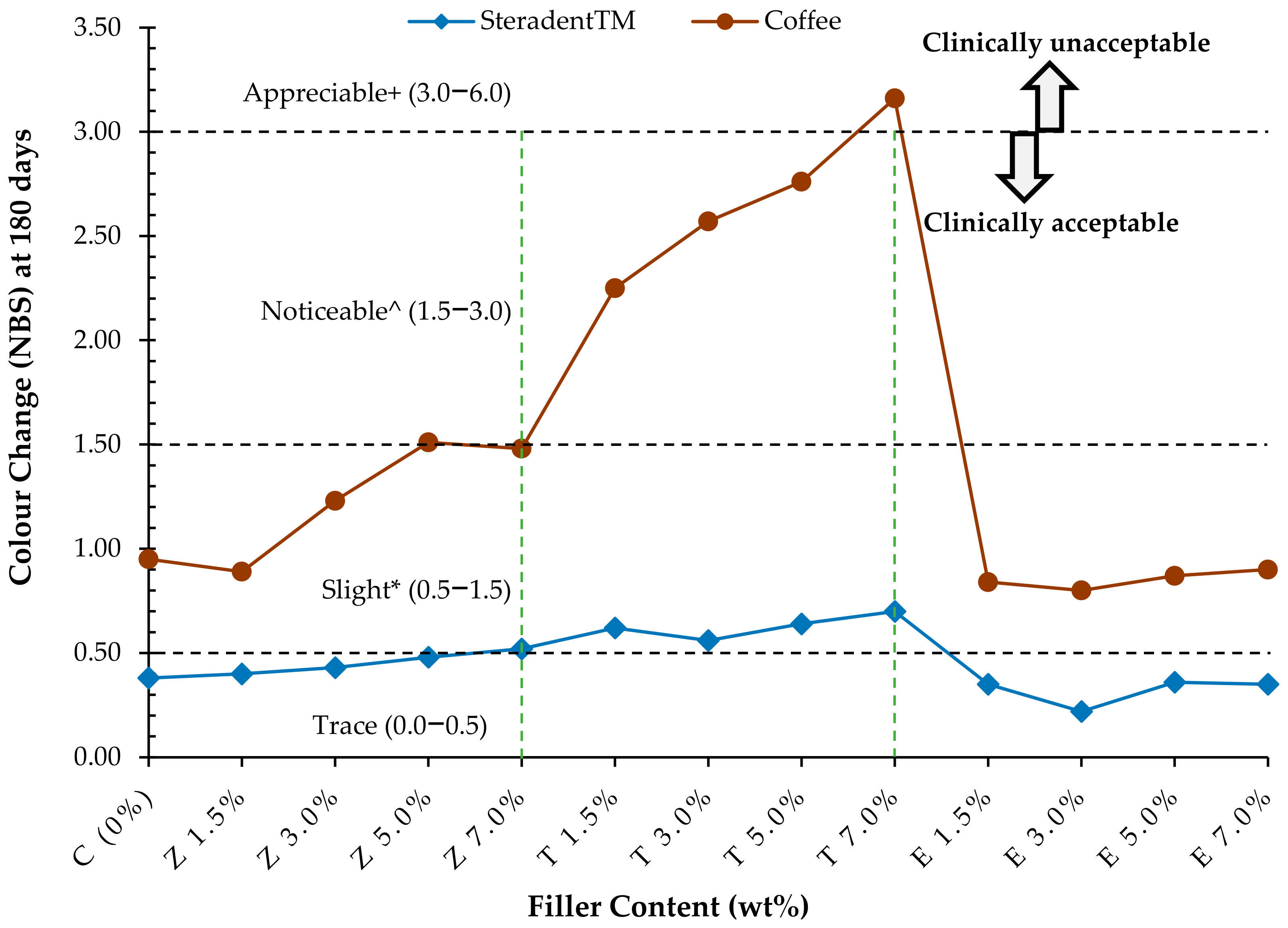
3. Results
3.1. Steradent™ Solution
3.2. Coffee Solution
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karci, M.; Demir, N.; Yazman, S. Evaluation of Flexural Strength of Different Denture Base Materials Reinforced with Different Nanoparticles. J. Prosthodont. 2019, 28, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Sahin, O.; Koroglu, A.; Dede, D.O.; Yilmaz, B. Effect of surface sealant agents on the surface roughness and color stability of denture base materials. J. Prosteth. Dent. 2016, 116, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Salim, N.; Satterthwaite, J.; Rautemaa, R.; Silikas, N. Impregnation with antimicrobials has an impact on degree of conversion and colour stability of acrylic liner. Dent. Mater. J. 2012, 31, 1008–1013. [Google Scholar] [CrossRef] [PubMed]
- Heintze, S.D.; Ilie, N.; Hickel, R.; Reis, A.; Loguercio, A.; Rousson, V. Laboratory mechanical parameters of composite resins and their relation to fractures and wear in clinical trials—A systematic review. Dent. Mater. 2017, 33, e101–e114. [Google Scholar] [CrossRef]
- Tuncdemir, A.R.; Aykent, F. Effects of fibers on the color change and stability of resin composites after accelerated aging. Dent. Mater. J. 2012, 31, 872–878. [Google Scholar] [CrossRef]
- Goiato, M.C.; dos Santos, D.M.; Baptista, G.T.; Moreno, A.; Andreotti, A.M.; Bannwart, L.C.; Dekon, S.F.J.G. Effect of thermal cycling and disinfection on colour stability of denture base acrylic resin. Gerodontolgy 2013, 30, 276–282. [Google Scholar] [CrossRef]
- Garoushi, S.; Vallittu, P.K.; Lassila, L. Effect of short fiber fillers on the optical properties of composite resins. J. Mater. Sci. Res. 2012, 1, 174. [Google Scholar] [CrossRef][Green Version]
- Alp, G.; Johnston, W.M.; Yilmaz, B. Optical properties and surface roughness of prepolymerized poly (methyl methacrylate) denture base materials. J. Prosthet. Dent. 2019, 121, 347–352. [Google Scholar] [CrossRef]
- Hollis, S.; Eisenbeisz, E.; Versluis, A. Color stability of denture resins after staining and exposure to cleansing agents. J. Prosthet. Dent. 2015, 114, 709–714. [Google Scholar] [CrossRef]
- Durkan, R.; Ayaz, E.A.; Bagis, B.; Gurbuz, A.; Ozturk, N.; Korkmaz, F.M. Comparative effects of denture cleansers on physical properties of polyamide and polymethyl methacrylate base polymers. Dent. Mater. J. 2013, 32, 367–375. [Google Scholar] [CrossRef]
- Zuo, W.; Feng, D.; Song, A.; Gong, H.; Zhu, S. Effects of organic-inorganic hybrid coating on the color stability of denture base resins. J. Prosthet. Dent. 2016, 115, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Sepulveda-Navarro, W.F.; Arana-Correa, B.E.; Ferreira Borges, C.P.; Habib Jorge, J.; Urban, V.M.; Campanha, N.H. Color stability of resins and nylon as denture base material in beverages. J. Prosthodont. Implant. Esthet. Reconstr. Dent. 2011, 20, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Imirzalioglu, P.; Karacaer, O.; Yilmaz, B.; Ozmen, I. Color stability of denture acrylic resins and a soft lining material against tea, coffee, and nicotine. J. Prosthodont. Implant. Esthet. Reconstr. Dent. 2010, 19, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Zidan, S.; Silikas, N.; Haider, J.; Yates, J. Effect of cleansers on the colour stability of zirconia impregnated PMMA bio-nanocomposite. Nanomaterials 2020, 10, 1757. [Google Scholar] [CrossRef]
- Dietschi, D.; Campanile, G.; Holz, J.; Meyer, J.-M. Comparison of the color stability of ten new-generation composites: An in vitro study. Dent. Mater. 1994, 10, 353–362. [Google Scholar] [CrossRef]
- Al-Thobity, A.M.; Gad, M.; ArRejaie, A.; Alnassar, T.; Al-Khalifa, K.S. Impact of denture cleansing solution immersion on some properties of different denture base materials: An in vitro study. J. Prosthodont. 2019, 28, 913–919. [Google Scholar] [CrossRef]
- Polyzois, G.; Niarchou, A.; Ntala, P.; Pantopoulos, A.; Frangou, M. The effect of immersion cleansers on gloss, colour and sorption of acetal denture base material. Gerodontology 2013, 30, 150–156. [Google Scholar] [CrossRef]
- Hong, G.; Murata, H.; Li, Y.; Sadamori, S.; Hamada, T. Influence of denture cleansers on the color stability of three types of denture base acrylic resin. J. Prosthet. Dent. 2009, 101, 205–213. [Google Scholar] [CrossRef]
- Mallakpour, S.; Madani, M. A review of current coupling agents for modification of metal oxide nanoparticles. Prog. Org. Coat. 2015, 86, 194–207. [Google Scholar] [CrossRef]
- Ho, B.J.; Tsoi, J.K.-H.; Liu, D.; Lung, C.Y.-K.; Wong, H.-M.; Matinlinna, J.P. Effects of sandblasting distance and angles on resin cement bonding to zirconia and titanium. Int. J. Adhes. Adhes. 2015, 62, 25–31. [Google Scholar] [CrossRef]
- Xia, Y.; Zhang, F.; Xie, H.; Gu, N. Nanoparticle-reinforced resin-based dental composites. J. Dent. 2008, 36, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.-H.; Lee, Y.; Oh, S.; Cho, H.-W.; Oda, Y.; Bae, J.-M. Reinforcing effects of different fibers on denture base resin based on the fiber type, concentration, and combination. Dent. Mater. J. 2012, 31, 1039–1046. [Google Scholar] [CrossRef] [PubMed]
- Alhotan, A.; Yates, J.; Zidan, S.; Haider, J.; Silikas, N. Flexural Strength and Hardness of Filler-Reinforced PMMA Targeted for Denture Base Application. Materials 2021, 14, 2659. [Google Scholar] [CrossRef] [PubMed]
- Jordan, J.; Jacob, K.I.; Tannenbaum, R.; Sharaf, M.A.; Jasiuk, I. Experimental trends in polymer nanocomposites—A review. Mater. Sci. Eng. A 2005, 393, 1–11. [Google Scholar] [CrossRef]
- Alhotan, A.; Yates, J.; Zidan, S.; Haider, J.; Silikas, N. Assessing Fracture Toughness and Impact Strength of PMMA Reinforced with Nano-Particles and Fibre as Advanced Denture Base Materials. Materials 2021, 14, 4127. [Google Scholar] [CrossRef]
- Alfouzan, A.F.; Alotiabi, H.M.; Labban, N.; Al-Otaibi, H.N.; Al Taweel, S.M.; AlShehri, H.A. Color stability of 3D-printed denture resins: Effect of aging, mechanical brushing and immersion in staining medium. J. Adv. Prosthodont. 2021, 13, 160. [Google Scholar] [CrossRef]
- Hersek, N.; Uzun, G.; Yildiz, P. Color stability of denture base acrylic resins in three food colorants. J. Prosthet. Dent. 1999, 81, 375–379. [Google Scholar] [CrossRef]
- Sham, A.S.; Chu, F.C.; Chai, J.; Chow, T.W. Color stability of provisional prosthodontic materials. J. Prosthet. Dent. 2004, 91, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Sampath, S.; Ramachandra, G.S. Effects of glass fibers on light transmittance and color of fiber-reinforced composite. Dent. Mater. 2008, 24, 34–38. [Google Scholar] [CrossRef]
- Vichi, A.; Ferrari, M.; Davidson, C.L. Color and opacity variations in three different resin-based composite products after water aging. Dent. Mater. 2004, 20, 530–534. [Google Scholar] [CrossRef] [PubMed]
- Ferracane, J.L. Hygroscopic and hydrolytic effects in dental polymer networks. Dent. Mater. 2006, 22, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Sarac, D.; Saraç, Y.S.; Kurt, M.; Yuzbasioglu, E. The effectiveness of denture cleansers on soft denture liners colored by food colorant solutions. J. Prosthodont. 2007, 16, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Villalta, P.; Lu, H.; Okte, Z.; Garcia-Godoy, F.; Powers, J.M. Effects of staining and bleaching on color change of dental composite resins. J. Prosthet. Dent. 2006, 95, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Hosoya, Y.; Shiraishi, T.; Odatsu, T.; Ogata, T.; Miyazaki, M.; Powers, J.M. Effects of specular component and polishing on color of resin composites. J. Oral Sci. 2010, 52, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Mutlu-Sagesen, L.; Ergun, G.; Ozkan, Y.; Semiz, M. Color stability of a dental composite after immersion in various media. Dent. Mater. J. 2005, 24, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Polychronakis, N.C.; Polyzois, G.L.; Lagouvardos, P.E.; Papadopoulos, T.D. Effects of cleansing methods on 3-D surface roughness, gloss and color of a polyamide denture base material. Acta Odontol. Scand. 2015, 73, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Altıncı, P.; Durkaya, P. Effects of thermocycling and various drinks on color stability of heat-polymerized acrylic resin. J. Istanb. Univ. Fac. Dent. 2016, 50, 15–20. [Google Scholar] [CrossRef]
- Goiato, M.C.; Sonego, M.V.; Carneiro, D.D.B.; Da Silva, E.V.F.; Bonatto, L.D.R.; Rangel, E.C.; Dos Santos, D.M. Evaluation of a glaze polishing technique for pigmented denture acrylic resin submitted to thermocycling and disinfection. J. Int. Oral Health 2017, 9, 213–221. [Google Scholar] [CrossRef]
- Devlin, H.; Kaushik, P. The effect of water absorption on acrylic surface properties. J. Prosthodont. Implant. Esthet. Reconstr. Dent. 2005, 14, 233–238. [Google Scholar] [CrossRef]
- Gad, M.M.; Abualsaud, R.; Fouda, S.M.; Rahoma, A.; Al-Thobity, A.M.; Khan, S.Q.; Akhtar, S.; Al-Abidi, K.S.; Ali, M.S.; Al-Harbi, F.A. Color Stability and Surface Properties of PMMA/ZrO2 Nanocomposite Denture Base Material after Using Denture Cleanser. Int. J. Biomater. 2021, 2021, 1–10. [Google Scholar] [CrossRef]
- Buyukyilmaz, S.; Ruyter, I. Color stability of denture base polymers. Int. J. Prosthodont. 1994, 7, 372–378. [Google Scholar] [PubMed]
- Guler, A.U.; Yilmaz, F.; Kulunk, T.; Guler, E.; Kurt, S. Effects of different drinks on stainability of resin composite provisional restorative materials. J. Prosthet. Dent. 2005, 94, 118–124. [Google Scholar] [CrossRef] [PubMed]

| Material | Composition and Specifications | Manufacturer |
|---|---|---|
| Lucitone-199™ | Heat-polymerised acrylic resin powder: PMMA; monomer: MMA | Dentsply International, York, PA, USA |
| Zirconium oxide | Zirconium (IV) oxide-yttria stabilised, nanopowder, <100 nm particle size | Sigma Aldrich, Gillingham, UK |
| Titanium oxide | Titanium (IV) oxide, anatase, nanopowder, <25 nm particle size | Sigma Aldrich, Gillingham, UK |
| Silanised E-glass fibre | 3 mm in length, 15 μm in diameter | Hebei Yuniu Fibreglass, Xingtai, China |
| Ethanol | Ethanol, absolute (C2H6O, EtOH) | Fisher Scientific, Loughborough, UK |
| Silane coupling agent | 3-(Trimethoxysilyl) propyl methacrylate, assay 98% | Sigma Aldrich, Gillingham, UK |
| Steradent™ | Sodium bicarbonate, potassium carbonate peroxide, sodium sulfate, citric acid | Reckitt Benckiser Healthcare, Dansom Lane, Limited, UK |
| Nescafe original instant coffee™ | Arabica and Robusta coffee beans | Nestle Ltd., York, UK |
| Critical Levels of Colour Changes | ∆E NBS Units |
|---|---|
| Trace | 0.0–0.5 |
| Slight | 0.5–1.5 |
| Noticeable | 1.5–3.0 |
| Appreciable | 3.0–6.0 |
| Much | 6.0–12.0 |
| Very much | 12.0+ |
| Tested Groups | L* Mean (SD) | a* Mean (SD) | b* Mean (SD) |
|---|---|---|---|
| C (0%) | 6.02 (0.76) | 0.32 (0.00) | 0.31 (0.00) |
| Z 1.5% | 10.14 (0.20) | 0.34 (0.00) | 0.33 (0.00) |
| Z 3.0% | 15.61 (0.46) | 0.35 (0.00) | 0.34 (0.00) |
| Z 5.0% | 20.86 (2.28) | 0.35 (0.00) | 0.34 (0.00) |
| Z 7.0% | 25.61 (0.39) | 0.36 (0.00) | 0.35 (0.00) |
| T 1.5% | 19.80 (1.55) | 0.35 (0.00) | 0.35 (0.00) |
| T 3.0% | 24.90 (1.98) | 0.36 (0.00) | 0.36 (0.00) |
| T 5.0% | 35.73 (1.42) | 0.37 (0.00) | 0.37 (0.00) |
| T 7.0% | 38.80 (2.31) | 0.37 (0.00) | 0.38 (0.00) |
| E 1.5% | 8.49 (4.5) | 0.33 (0.00) | 0.32 (0.00) |
| E 3.0% | 6.22 (0.23) | 0.33 (0.00) | 0.31 (0.00) |
| E 5.0% | 6.41 (0.60) | 0.33 (0.00) | 0.31 (0.00) |
| E 7.0% | 6.10 (0.32) | 0.33 (0.00) | 0.31 (0.00) |
| Tested Groups | Steradent™ | |||
|---|---|---|---|---|
| Day 1 ΔE (SD) NBS | Day 30 ΔE (SD) NBS | Day 90 ΔE (SD) NBS | Day 180 ΔE (SD) NBS | |
| C (0%) | 0.30 Aa (0.02) 0.29 | 0.35 Aab (0.03) 0.32 | 0.41 Bab (0.03) 0.38 | 0.41 Ba (0.04) 0.38 |
| Z 1.5% | 0.32 Aa (0.03) 0.29 | 0.31 Aa (0.02) 0.30 | 0.36 Aa (0.03) 0.33 | 0.44 Ba (0.03) 0.40 |
| Z 3.0% | 0.35 Aa (0.03) 0.32 | 0.40 Ab (0.03) 0.37 | 0.39 Aa (0.03) 0.36 | 0.47 Ba (0.02) 0.43 |
| Z 5.0% | 0.38 Aa (0.04) 0.35 | 0.39 Ab (0.02) 0.36 | 0.46 Bb (0.03) 0.42 | 0.52 Cb (0.04) 0.48 |
| Z 7.0% | 0.46 Ab (0.10) 0.42 | 0.49 Ac (0.02) 0.45 | 0.48 Abc (0.02) 0.44 | 0.57 Bb (0.05) 0.52 |
| T 1.5% | 0.45 Abc (0.05) 0.41 | 0.57 Bd (0.04) 0.52 | 0.61 BCd (0.03) 0.56 | 0.67 Cc (0.05) 0.62 |
| T 3.0% | 0.48 Abc (0.08) 0.44 | 0.49 ABc (0.03) 0.45 | 0.53 Bce (0.03) 0.49 | 0.61 Cbc (0.03) 0.56 |
| T 5.0% | 0.51 Abc (0.04) 0.47 | 0.48 Ac (0.05) 0.44 | 0.59 Be (0.05) 0.54 | 0.70 Cd (0.05) 0.64 |
| T 7.0% | 0.55 Ac (0.04) 0.51 | 0.64 Bd (0.06) 0.59 | 0.72 Cf (0.04) 0.66 | 0.76 Cd (0.03) 0.70 |
| E 1.5% | 0.25 Aad (0.03) 0.23 | 0.31 ABa (0.04) 0.30 | 0.36 BCa (0.05) 0.33 | 0.38 Ca (0.06) 0.35 |
| E 3.0% | 0.29 Aad (0.02) 0.27 | 0.35 Aab (0.03) 0.32 | 0.34 Aa (0.03) 0.31 | 0.35 Aa (0.05) 0.22 |
| E 5.0% | 0.24 Aad (0.01) 0.22 | 0.32 ABa (0.02) 0.29 | 0.36 BCa (0.05) 0.33 | 0.39 Ca (0.02) 0.36 |
| E 7.0% | 0.21 Ad (0.01) 0.19 | 0.28 ABa (0.03) 0.26 | 0.32 BCa (0.03) 0.29 | 0.38 Ca (0.03) 0.35 |
| Tested Groups | Coffee | |||
|---|---|---|---|---|
| Day 7 ΔE (SD) NBS | Day 30 ΔE (SD) NBS | Day 90 ΔE (SD) NBS | Day 180 ΔE (SD) NBS | |
| C (0%) | 0.51 Aa (0.03) 0.47 | 0.81 Ba (0.02) 0.74 | 0.83 BCa (0.06) 0.76 | 1.03 Ca (0.09) 0.95 |
| Z 1.5% | 0.49 Aa (0.02) 0.45 | 0.71 Ba (0.06) 0.65 | 0.86 Ca (0.04) 0.79 | 0.97 Ca (0.05) 0.89 |
| Z 3.0% | 0.56 Aa (0.02) 0.52 | 0.87 Ba (0.05) 0.8 | 1.26 Cb (0.17) 1.16 | 1.34 Ca (0.13) 1.23 |
| Z 5.0% | 0.68 Ab (0.05) 0.63 | 1.16 Bb (0.11) 1.07 | 1.52 Cc (0.07) 1.40 | 1.64 Cb (0.06) 1.51 |
| Z 7.0% | 0.71 Ab (0.05) 0.65 | 1.45 Bc (0.04) 1.33 | 1.49 Bc (0.07) 1.37 | 1.61 Bb (0.06) 1.48 |
| T 1.5% | 0.71 Ab (0.03) 0.65 | 1.56 Bcd (0.11) 1.44 | 1.87 Cd (0.09) 1.72 | 2.45 Dc (0.12) 2.25 |
| T 3.0% | 0.88 Ac (0.12) 0.81 | 1.68 Bde (0.08) 1.55 | 2.09 Ce (0.17) 1.92 | 2.79 Dd (0.18) 2.57 |
| T 5.0% | 1.10 Ad (0.06) 1.01 | 1.74 Be (0.08) 1.60 | 2.21 Ce (0.11) 2.03 | 3.00 Dd (0.19) 2.76 |
| T 7.0% | 1.21 Ad (0.18) 1.11 | 1.89 Bf (0.08) 1.74 | 2.67 Cf (0.12) 2.46 | 3.44 De (0.22) 3.16 |
| E 1.5% | 0.36 Ae (0.02) 0.33 | 0.53 Bg (0.06) 0.17 | 0.75 Cag (0.05) 0.69 | 0.91 Caf (0.06) 0.84 |
| E 3.0% | 0.39 Ae (0.03) 0.36 | 0.55 Bg (0.03) 0.49 | 0.69 Cg (0.04) 0.63 | 0.87 Cf (0.06) 0.80 |
| E 5.0% | 0.40 Aae (0.03) 0.37 | 0.63 Bg (0.03) 0.58 | 0.81 Cag (0.02) 0.75 | 0.95 Caf (0.04) 0.87 |
| E 7.0% | 0.42 Aae (0.02) 0.39 | 0.58 Bg (0.03) 0.53 | 0.87 Cag (0.03) 0.80 | 0.98 Caf (0.08) 0.90 |
| Tested Groups | NBS Values after 180 Days in Solutions | |
|---|---|---|
| Steradent™ | Coffee | |
| C (0%) | 0.38 | 0.95 * |
| Z 1.5% | 0.40 | 0.89 * |
| Z 3.0% | 0.43 | 1.23 * |
| Z 5.0% | 0.48 | 1.51 ^ |
| Z 7.0% | 0.52 * | 1.48 * |
| T 1.5% | 0.62 * | 2.25 ^ |
| T 3.0% | 0.56 * | 2.57 ^ |
| T 5.0% | 0.64 * | 2.76^ |
| T 7.0% | 0.70 * | 3.16+ |
| E 1.5% | 0.35 | 0.84 * |
| E 3.0% | 0.22 | 0.80 * |
| E 5.0% | 0.36 | 0.87 * |
| E 7.0% | 0.35 | 0.90 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhotan, A.; Elraggal, A.; Yates, J.; Haider, J.; Jurado, C.A.; Silikas, N. Effect of Different Solutions on the Colour Stability of Nanoparticles or Fibre Reinforced PMMA. Polymers 2022, 14, 1521. https://doi.org/10.3390/polym14081521
Alhotan A, Elraggal A, Yates J, Haider J, Jurado CA, Silikas N. Effect of Different Solutions on the Colour Stability of Nanoparticles or Fibre Reinforced PMMA. Polymers. 2022; 14(8):1521. https://doi.org/10.3390/polym14081521
Chicago/Turabian StyleAlhotan, Abdulaziz, Alaaeldin Elraggal, Julian Yates, Julfikar Haider, Carlos Alberto Jurado, and Nikolaos Silikas. 2022. "Effect of Different Solutions on the Colour Stability of Nanoparticles or Fibre Reinforced PMMA" Polymers 14, no. 8: 1521. https://doi.org/10.3390/polym14081521
APA StyleAlhotan, A., Elraggal, A., Yates, J., Haider, J., Jurado, C. A., & Silikas, N. (2022). Effect of Different Solutions on the Colour Stability of Nanoparticles or Fibre Reinforced PMMA. Polymers, 14(8), 1521. https://doi.org/10.3390/polym14081521