Hybrid Macrocyclic Polymers: Self-Assembly Containing Cucurbit[m]uril-pillar[n]arene
Abstract
1. Introduction
2. The Competition and Cooperation between CB[m] and Pillar[n]arene as the Host
3. The Preparation of Mechanically Interlocked Molecules Containing CB[m] and Pillar[n]arene
4. The Preparation of Supramolecular Polymer containing CB[m] and pillar[n]arene
5. Overview and Outlook
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CB[m] | Cucurbit[m]uril |
| Ka | Associate constants |
| LCST | Lower critical solution temperature |
| MIM | Mechanically interlocked molecules |
| Tcloud | Clouding point |
References
- Ji, T.; Li, Y.; Deng, X.; Rwei, A.Y.; Offen, A.; Hall, S.; Zhang, W.; Zhao, C.; Mehta, M.; Kohane, D.S. Delivery of local anaesthetics by a self-assembled supramolecular system mimicking their interactions with a sodium channel. Nat. Biomed. Eng. 2021, 5, 1099–1109. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.L.; Lin, W.; Chen, Y.; Dai, X.Y.; Liu, Z.; Liu, Y. Multivalent supramolecular assembly with ultralong organic room temperature phosphorescence, high transfer efficiency and ultrahigh antenna effect in water. Chem. Sci. 2022, 13, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Khmelinskaia, A.; Wargacki, A.; King, N.P. Structure-based design of novel polyhedral protein nanomaterials. Curr. Opin. Microbiol. 2021, 61, 51–57. [Google Scholar] [CrossRef]
- Hendrikse, S.I.S.; Wijnands, S.P.W.; Lafleur, R.P.M.; Pouderoijen, M.J.; Janssen, H.M.; Dankers, P.Y.W.; Meijer, E.W. Controlling and tuning the dynamic nature of supramolecular polymers in aqueous solutions. Chem. Commun. 2017, 53, 2279–2282. [Google Scholar] [CrossRef] [PubMed]
- Yin, G.Q.; Wang, H.; Wang, X.Q.; Song, B.; Chen, L.J.; Wang, L.; Hao, X.Q.; Yang, H.B.; Li, X. Self-assembly of emissive supramolecular rosettes with increasing complexity using multitopic terpyridine ligands. Nat. Commun. 2018, 9, 567. [Google Scholar] [CrossRef] [PubMed]
- Thosar, A.U.; Patel, A.J. Hydration determines anion accumulation. Nat. Chem. 2022, 14, 8–10. [Google Scholar] [CrossRef] [PubMed]
- Guillemeney, L.; Lermusiaux, L.; Landaburu, G.; Wagnon, B.; Abécassis, B. Curvature and self-assembly of semi-conducting nanoplatelets. Commun. Chem. 2022, 5, 7. [Google Scholar] [CrossRef]
- Dong, S.; Zheng, B.; Wang, F.; Huang, F. Supramolecular polymers constructed from macrocycle-based host-guest molecular recognition motifs. Acc. Chem. Res. 2014, 47, 1982–1994. [Google Scholar] [CrossRef]
- Kakuta, T.; Yamagishi, T.a.; Ogoshi, T. Stimuli-responsive supramolecular assemblies constructed from pillar[n]arenes. Acc. Chem. Res. 2018, 51, 1656–1666. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, T.; Yan, X.Y.; Guo, Q.Y.; Lei, H.; Huang, Z.; Zhang, R.; Wang, Y.; Wang, J.; Liu, F.; et al. Expanding quasiperiodicity in soft matter: Supramolecular decagonal quasicrystals by binary giant molecule blends. Proc. Natl. Acad. Sci. USA 2022, 119, e2115304119. [Google Scholar] [CrossRef]
- Deng, J.H.; Luo, J.; Mao, Y.L.; Lai, S.; Gong, Y.N.; Zhong, D.C.; Lu, T.B. π-π Stacking interactions: Non-negligible forces for stabilizing porous supramolecular frameworks. Sci. Adv. 2020, 6, eaax9976. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Bai, H.; Yang, Y.; Yoon, J.; Wang, S.; Zhang, X. Supramolecular antibacterial materials for combatting antibiotic resistance. Adv. Mater. 2019, 31, 1805092. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.M.S.; Amiri, M.; Martin, N.P.; Lulich, A.; Palys, L.N.; Zhu, G.; De Yoreo, J.J.; Nyman, M. Solvent-driven transformation of Zn/Cd2+-deoxycholate assemblies. Inorg. Chem. 2022, 61, 1275–1286. [Google Scholar] [CrossRef] [PubMed]
- He, A.; Jiang, Z.; Wu, Y.; Hussain, H.; Rawle, J.; Briggs, M.E.; Little, M.A.; Livingston, A.G.; Cooper, A.I. A smart and responsive crystalline porous organic cage membrane with switchable pore apertures for graded molecular sieving. Nat. Mater. 2022, 21, 463–470. [Google Scholar] [CrossRef]
- Wang, S.; Lee, S.; Du, J.S.; Partridge, B.E.; Cheng, H.F.; Zhou, W.; Dravid, V.P.; Lee, B.; Glotzer, S.C.; Mirkin, C.A. The emergence of valency in colloidal crystals through electron equivalents. Nat. Mater. 2022, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Jiang, M. Cyclodextrin-based inclusion complexation bridging supramolecular chemistry and macromolecular self-assembly. Chem. Soc. Rev. 2011, 40, 2254–2266. [Google Scholar] [CrossRef]
- Resconi, L.; Cavallo, L.; Fait, A.; Piemontesi, F. Selectivity in propene polymerization with metallocene catalysts. Chem. Rev. 2000, 100, 1253–1346. [Google Scholar] [CrossRef]
- Zhang, J.; Pesak, D.J.; Ludwick, J.L.; Moore, J.S. Geometrically-controlled and site-specifically-functionalized phenylacetylene macrocycles. J. Am. Chem. Soc. 1994, 116, 4227–4239. [Google Scholar] [CrossRef]
- Lehn, J.M. Constitutional dynamic chemistry: Bridge from supramolecular chemistry to adaptive chemistry. In Constitutional Dynamic Chemistry; Barboiu, M., Ed.; Springer: Berlin/Heidelberg Germany, 2012; pp. 1–32. [Google Scholar]
- Zhang, H.; Zhao, Y. Pillararene-based assemblies: Design principle, preparation and applications. Chem. Eur. J. 2013, 19, 16862–16879. [Google Scholar] [CrossRef]
- Lehn, J.M. Toward complex matter: Supramolecular chemistry and self-organization. Proc. Natl. Acad. Sci. USA 2002, 99, 4763–4768. [Google Scholar] [CrossRef]
- Liu, Z.; Qiao, J.; Niu, Z.; Wang, Q. Natural supramolecular building blocks: From virus coat proteins to viral nanoparticles. Chem. Soc. Rev. 2012, 41, 6178–6194. [Google Scholar] [CrossRef] [PubMed]
- Sieber, J.J.; Willig, K.I.; Kutzner, C.; Reimers, C.G.; Harke, B.; Donnert, G.; Rammner, B.; Eggeling, C.; Hell, S.W.; Grubmüller, H.; et al. Anatomy and dynamics of a supramolecular membrane protein cluster. Science 2007, 317, 1072–1076. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Nalluri, S.K.M.; Stoddart, J.F. Surveying macrocyclic chemistry: From flexible crown ethers to rigid cyclophanes. Chem. Soc. Rev. 2017, 46, 2459–2478. [Google Scholar] [CrossRef] [PubMed]
- Girvin, Z.C.; Andrews, M.K.; Liu, X.; Gellman, S.H. Foldamer-templated catalysis of macrocycle formation. Science 2019, 366, 1528–1531. [Google Scholar] [CrossRef]
- Park, J.; Park, J.; Lee, J.; Lim, C.; Lee, D.W. Size compatibility and concentration dependent supramolecular host-guest interactions at interfaces. Nat. Commun. 2022, 13, 112. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, S.T.; Gin, D.L.; Hupp, J.T.; Zhang, X. Supramolecular chemistry: Functional structures on the mesoscale. Proc. Natl. Acad. Sci. USA 2001, 98, 11849–11850. [Google Scholar] [CrossRef]
- Yudin, A.K. Macrocycles: Lessons from the distant past, recent developments, and future directions. Chem. Sci. 2015, 6, 30–49. [Google Scholar] [CrossRef]
- Chen, W.; Chen, P.; Zhang, G.; Xing, G.; Feng, Y.; Yang, Y.W.; Chen, L. Macrocycle-derived hierarchical porous organic polymers: Synthesis and applications. Chem. Soc. Rev. 2021, 50, 11684–11714. [Google Scholar] [CrossRef]
- Surur, A.S.; Sun, D. Macrocycle-antibiotic hybrids: A path to clinical candidates. Front. Chem. 2021, 9, 659845. [Google Scholar] [CrossRef]
- Smith, J.M.; Fasan, R. Synthesis of macrocyclic organo-peptide hybrids from ribosomal polypeptide precursors via CuAAC-/hydrazide-mediated cyclization. In Peptide Libraries: Methods and Protocols; Derda, R., Ed.; Springer: New York, NY, USA, 2015; pp. 23–38. [Google Scholar]
- Liu, Z.; Zhang, H.; Han, J. Crown ether-pillararene hybrid macrocyclic systems. Org. Biomol. Chem. 2021, 19, 3287–3302. [Google Scholar] [CrossRef]
- Boehm, M.; Beaumont, K.; Jones, R.; Kalgutkar, A.S.; Zhang, L.; Atkinson, K.; Bai, G.; Brown, J.A.; Eng, H.; Goetz, G.H.; et al. Discovery of potent and orally bioavailable macrocyclic peptide-peptoid hybrid CXCR7 modulators. J. Med. Chem. 2017, 60, 9653–9663. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Rajeswara Rao, M.; Lee, W.Z.; Ravikanth, M. Hybrid macrocycles of subporphyrins and triphyrins. Org. Lett. 2017, 19, 5924–5927. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Zhang, Y.; Liu, M.; Gao, G.K.; Ji, W.; Jiang, C.; Huang, X.; Chen, Y.; Li, S.L.; Lan, Y.Q. Single-metal site-embedded conjugated macrocyclic hybrid catalysts enable boosted CO2 reduction and evolution kinetics in Li-CO2 batteries. Cell Rep. Phys. Sci. 2021, 2, 100583. [Google Scholar] [CrossRef]
- Frost, J.R.; Fasan, R. Macrocyclic organo-peptide hybrids by intein-mediated ligation: Synthesis and applications. In Chemical Ligation; Wiley: New York, NY, USA, 2017; pp. 391–419. [Google Scholar]
- Strutt, N.L.; Zhang, H.; Schneebeli, S.T.; Stoddart, J.F. Functionalizing pillar[n]arenes. Acc. Chem. Res. 2014, 47, 2631–2642. [Google Scholar] [CrossRef]
- Sessler, J.L.; Zimmerman, R.S.; Bucher, C.; Král, V.; Andrioletti, B. Calixphyrins. Hybrid macrocycles at the structural crossroads between porphyrins and calixpyrroles. Pure Appl. Chem. 2001, 73, 1041–1057. [Google Scholar] [CrossRef]
- Zhang, H.; Han, J. The synthesis and applications of porphyrin-containing pillararenes. Org. Biomol. Chem. 2020, 18, 4894–4905. [Google Scholar] [CrossRef]
- Sun, H.; Ren, J.; Qu, X. Carbon nanomaterials and DNA: From molecular recognition to applications. Acc. Chem. Res. 2016, 49, 461–470. [Google Scholar] [CrossRef]
- Whyte, G.F.; Vilar, R.; Woscholski, R. Molecular recognition with boronic acids-applications in chemical biology. J. Chem. Biol. 2013, 6, 161–174. [Google Scholar] [CrossRef]
- Marsh, D. Thermodynamics of phospholipid self-assembly. Biophys. J. 2012, 102, 1079–1087. [Google Scholar] [CrossRef]
- Kirschner, J.; Wang, Z.; Eigler, S.; Steinrück, H.P.; Jäger, C.M.; Clark, T.; Hirsch, A.; Halik, M. Driving forces for the self-assembly of graphene oxide on organic monolayers. Nanoscale 2014, 6, 11344–11350. [Google Scholar] [CrossRef]
- Sherje, A.P.; Dravyakar, B.R.; Kadam, D.; Jadhav, M. Cyclodextrin-based nanosponges: A critical review. Carbohydr. Polym. 2017, 173, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Gidwani, B.; Vyas, A. A comprehensive review on cyclodextrin-based carriers for delivery of chemotherapeutic cytotoxic anticancer drugs. BioMed Res. Int. 2015, 2015, 198268. [Google Scholar] [CrossRef] [PubMed]
- Challa, R.; Ahuja, A.; Ali, J.; Khar, R.K. Cyclodextrins in drug delivery: An updated review. AAPS PharmSciTech. 2005, 6, E329–E357. [Google Scholar] [CrossRef] [PubMed]
- Del Valle, E.M.M. Cyclodextrins and their uses: A review. Process Biochem. 2004, 39, 1033–1046. [Google Scholar] [CrossRef]
- Shang, J.; Liu, Y.; Pan, T. Macrocycles in bioinspired catalysis: From molecules to materials. Front. Chem. 2021, 9. [Google Scholar] [CrossRef]
- Jökel, J.; Schwer, F.; von Delius, M.; Apfel, U.P. A dinuclear porphyrin-macrocycle as efficient catalyst for the hydrogen evolution reaction. Chem. Commun. 2020, 56, 14179–14182. [Google Scholar] [CrossRef]
- Ning, R.; Ao, Y.F.; Wang, D.X.; Wang, Q.Q. Macrocycle-enabled counteranion trapping for improved catalytic efficiency. Chemistry 2018, 24, 4268–4272. [Google Scholar] [CrossRef]
- Ogoshi, T.; Kanai, S.; Fujinami, S.; Yamagishi, T.A.; Nakamoto, Y. Para-bridged symmetrical pillar[5]arenes: Their lewis acid catalyzed synthesis and host-guest property. J. Am. Chem. Soc. 2008, 130, 5022–5023. [Google Scholar] [CrossRef]
- Cao, D.; Kou, Y.; Liang, J.; Chen, Z.; Wang, L.; Meier, H. A facile and efficient preparation of pillararenes and a pillarquinone. Angew. Chem. Int. Ed. 2009, 48, 9721–9723. [Google Scholar] [CrossRef]
- Li, H.; Yang, Y.; Xu, F.; Liang, T.; Wen, H.; Tian, W. Pillararene-based supramolecular polymers. Chem. Commun. 2019, 55, 271–285. [Google Scholar] [CrossRef]
- Cao, S.; Zhang, H.; Zhao, Y.; Zhao, Y. Pillararene/Calixarene-based systems for battery and supercapacitor applications. eScience 2021, 1, 28. [Google Scholar] [CrossRef]
- Chen, L.; Cai, Y.; Feng, W.; Yuan, L. Pillararenes as macrocyclic hosts: A rising star in metal ion separation. Chem. Commun. 2019, 55, 7883–7898. [Google Scholar] [CrossRef] [PubMed]
- Li, M.H.; Lou, X.Y.; Yang, Y.W. Pillararene-based molecular-scale porous materials. Chem. Commun. 2021, 57, 13429–13447. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wang, P.; Turner, P.; Lewis, W.; Catal, O.; Thomas, D.S.; Gale, P.A. Tetraurea macrocycles: Aggregation-driven binding of chloride in aqueous solutions. Chemistry 2019, 5, 1210–1222. [Google Scholar] [CrossRef]
- Shang, J.; Li, B.; Shen, X.; Pan, T.; Cui, Z.; Wang, Y.; Ge, Y.; Qi, Z. Selenacrown macrocycle in aqueous medium: Synthesis, redox-responsive self-assembly, and enhanced disulfide formation reaction. J. Org. Chem. 2021, 86, 1430–1436. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, F.; Yang, F.; Ma, D. Carboxylated pillar[n]arene (n = 5–7) host molecules: High affinity and selective binding in water. Org. Biomol. Chem. 2019, 17, 5106–5111. [Google Scholar] [CrossRef] [PubMed]
- Kosiorek, S.; Rad, N.; Sashuk, V. Supramolecular catalysis by carboxylated pillar[n]arenes. Chem. Cat. Chem. 2020, 12, 2776–2782. [Google Scholar] [CrossRef]
- Chen, J.F.; Lin, Q.; Yao, H.; Zhang, Y.M.; Wei, T.B. Pillar[5] arene-based multifunctional supramolecular hydrogel: Multistimuli responsiveness, self-healing, fluorescence sensing, and conductivity. Mater. Chem. Front. 2018, 2, 999–1003. [Google Scholar] [CrossRef]
- Xue, M.; Yang, Y.; Chi, X.; Zhang, Z.; Huang, F. Pillararenes, a new class of macrocycles for supramolecular chemistry. Acc. Chem. Res. 2012, 45, 1294–1308. [Google Scholar] [CrossRef]
- Freeman, W.A.; Mock, W.L.; Shih, N.Y. Cucurbituril. J. Am. Chem. Soc. 1981, 103, 7367–7368. [Google Scholar] [CrossRef]
- Lü, J.; Lin, J.X.; Cao, M.N.; Cao, R. Cucurbituril: A promising organic building block for the design of coordination compounds and beyond. Coord. Chem. Rev. 2013, 257, 1334–1356. [Google Scholar] [CrossRef]
- Zhang, H.; Li, C. Pillararene-functionalised graphene nanomaterials. RSC Adv. 2020, 10, 18502–18511. [Google Scholar] [CrossRef]
- Gürbüz, S.; Idris, M.; Tuncel, D. Cucurbituril-based supramolecular engineered nanostructured materials. Org. Biomol. Chem. 2015, 13, 330–347. [Google Scholar] [CrossRef] [PubMed]
- Stoddart, J.F. Mechanically interlocked molecules (mims)-molecular shuttles, switches, and machines (nobel lecture). Angew. Chem. Int. Ed. 2017, 56, 11094–11125. [Google Scholar] [CrossRef]
- Schröder, H.V.; Zhang, Y.; Link, A.J. Dynamic covalent self-assembly of mechanically interlocked molecules solely made from peptides. Nat. Chem. 2021, 13, 850–857. [Google Scholar] [CrossRef]
- Sluysmans, D.; Stoddart, J.F. The burgeoning of mechanically interlocked molecules in chemistry. Trends Chem. 2019, 1, 185–197. [Google Scholar] [CrossRef]
- Davis, J.J.; Orlowski, G.A.; Rahman, H.; Beer, P.D. Mechanically interlocked and switchable molecules at surfaces. Chem. Commun. 2010, 46, 54–63. [Google Scholar] [CrossRef]
- Riebe, J.; Niemeyer, J. Mechanically interlocked molecules for biomedical applications. Eur. J. Org. Chem. 2021, 2021, 5106–5116. [Google Scholar] [CrossRef]
- Weyandt, E.; Leanza, L.; Capelli, R.; Pavan, G.M.; Vantomme, G.; Meijer, E.W. Controlling the length of porphyrin supramolecular polymers via coupled equilibria and dilution-induced supramolecular polymerization. Nat. Commun. 2022, 13, 248. [Google Scholar] [CrossRef]
- Qin, B.; Yin, Z.; Tang, X.; Zhang, S.; Wu, Y.; Xu, J.F.; Zhang, X. Supramolecular polymer chemistry: From structural control to functional assembly. Prog. Polym. Sci. 2020, 100, 101167. [Google Scholar] [CrossRef]
- Brunsveld, L.; Folmer, B.J.B.; Meijer, E.W.; Sijbesma, R.P. Supramolecular polymers. Chem. Rev. 2001, 101, 4071–4098. [Google Scholar] [CrossRef] [PubMed]
- de Greef, T.F.A.; Meijer, E.W. Supramolecular polymers. Nature 2008, 453, 171–173. [Google Scholar] [CrossRef] [PubMed]
- Klöck, C.; Dsouza, R.N.; Nau, W.M. Cucurbituril-mediated supramolecular acid catalysis. Org. Lett. 2009, 11, 2595–2598. [Google Scholar] [CrossRef] [PubMed]
- Masoud, S.N. Nanodimensional microreactor-encapsulation of 18-membered decaaza macrocycle copper(ii) complexes. Chem. Lett. 2005, 34, 244–245. [Google Scholar]
- Ohto, K.; Kim, J.; Morisada, S.; Maeki, M.; Yamashita, K.; Miyazaki, M. Microreactor extraction system with macrocyclic host compounds for rare metal recovery. Int. J. Soc. Mater. Eng. Resour. 2014, 20, 92–96. [Google Scholar] [CrossRef][Green Version]
- Ogoshi, T.; Shiga, R.; Yamagishi, T.A. Reversibly tunable lower critical solution temperature utilizing host-guest complexation of pillar[5] arene with triethylene oxide substituents. J. Am. Chem. Soc. 2012, 134, 4577–4580. [Google Scholar] [CrossRef]
- Behr, J.P. Perspectives in Supramolecular Chemistry: The Lock-and-Key Principle; John Wiley & Sons, Ltd.: Chichester, UK, 2007; Volume 1. [Google Scholar]
- Wei, P.; Cook, T.R.; Yan, X.; Huang, F.; Stang, P.J. A discrete amphiphilic organoplatinum(ii) metallacycle with tunable lower critical solution temperature behavior. J. Am. Chem. Soc. 2014, 136, 15497–15500. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Y.M.; Li, S.H.; Cui, Y.L.; Yu, J.; Liu, Y. Tunable nanosupramolecular aggregates mediated by host-guest complexation. Angew. Chem. Int. Ed. 2016, 55, 11452–11456. [Google Scholar] [CrossRef]
- Cao, M.; Hu, F.; Han, X.; Zhang, Y.; Wu, D.; Liu, S.H.; Yin, J. Aggregation control of hemicyanine fluorescent dye by using of cucurbit[7] uril and pillar[6] arene. Chin. J. Chem. 2015, 33, 351–355. [Google Scholar] [CrossRef]
- Zhang, D.; Tang, H.; Zhang, G.; Wang, L.; Cao, D. A cucurbituril-pillararene ring-on-ring complex. Chem. Commun. 2021, 57, 6562–6565. [Google Scholar] [CrossRef]
- Ling, S.; Chen, W.; Fan, Y.; Zheng, K.; Jin, K.; Yu, H.; Buehler, M.J.; Kaplan, D.L. Biopolymer nanofibrils: Structure, modeling, preparation, and applications. Prog. Polym. Sci. 2018, 85, 1–56. [Google Scholar] [CrossRef] [PubMed]
- Bew, S.P.; Brimage, R.A.; L’Hermit, N.; Sharma, S.V. Upper rim appended hybrid calixarenes via click chemistry. Org. Lett. 2007, 9, 3713–3716. [Google Scholar] [CrossRef] [PubMed]
- Ogoshi, T.; Yamafuji, D.; Kotera, D.; Aoki, T.; Fujinami, S.; Yamagishi, T.A. Clickable di- and tetrafunctionalized pillar[n] arenes (n = 5,6) by oxidation-reduction of pillar[n]arene units. J. Org. Chem. 2012, 77, 11146–11152. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Ke, C.; Fraser Stoddart, J. Cooperative capture synthesis: Yet another playground for copper-free click chemistry. Chem. Soc. Rev. 2016, 45, 3766–3780. [Google Scholar] [CrossRef]
- Ke, C.; Strutt, N.L.; Li, H.; Hou, X.; Hartlieb, K.J.; McGonigal, P.R.; Ma, Z.; Iehl, J.; Stern, C.L.; Cheng, C.; et al. Pillar[5] arene as a Co-factor in templating rotaxane formation. J. Am. Chem. Soc. 2013, 135, 17019–17030. [Google Scholar] [CrossRef]
- Hou, X.; Ke, C.; Cheng, C.; Song, N.; Blackburn, A.K.; Sarjeant, A.A.; Botros, Y.Y.; Yang, Y.W.; Stoddart, J.F. Efficient syntheses of pillar[6]arene-based hetero[4]rotaxanes using a cooperative capture strategy. Chem. Commun. 2014, 50, 6196–6199. [Google Scholar] [CrossRef]
- Chen, J.F.; Ding, J.D.; Wei, T.B. Pillararenes: Fascinating planar chiral macrocyclic arenes. Chem. Commun. 2021, 57, 9029–9039. [Google Scholar] [CrossRef]
- Zhu, H.; Li, Q.; Gao, Z.; Wang, H.; Shi, B.; Wu, Y.; Shangguan, L.; Hong, X.; Wang, F.; Huang, F. Pillararene host-guest complexation induced chirality amplification: A new way to detect cryptochiral compounds. Angew. Chem. Int. Ed. 2020, 59, 10868–10872. [Google Scholar] [CrossRef]
- Yang, J.; Shao, L.; Yuan, J.; Huang, F. Construction of a pillar[6] arene based water-soluble supramolecular pseudopolyrotaxane driven by cucurbit[8] uril-enhanced π-π interaction. Chem. Commun. 2016, 52, 12510–12512. [Google Scholar] [CrossRef]
- Ding, J.D.; Jin, W.J.; Pei, Z.; Pei, Y. Morphology transformation of pillararene-based supramolecular nanostructures. Chem. Commun. 2020, 56, 10113–10126. [Google Scholar] [CrossRef]
- Chen, Y.; Tao, H.Q.; Kou, Y.H.; Meier, H.; Fu, J.L.; Cao, D.R. Synthesis of pillar[7] arene. Chin. Chem. Lett. 2012, 23, 509–511. [Google Scholar] [CrossRef]
- Zhang, Z.; Shao, L.; Li, Z.; Yu, G.; Yang, J. A cationic water-soluble pillar[7] arene: Synthesis and its fluorescent host-guest complex in water. Tetrahedron Lett. 2017, 58, 2736–2739. [Google Scholar] [CrossRef]
- Shao, L.; Hua, B.; Yang, J.; Yu, G. Pillar[7] arene-based host-guest complex in water: Dual-responsiveness and application in controllable self-assembly. RSC Adv. 2016, 6, 60029–60033. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Z.; Zhao, Y. Pillararene-based self-assembled amphiphiles. Chem. Soc. Rev. 2018, 47, 5491–5528. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Z.; Xin, F.; Zhao, Y. Metal-ligated pillararene materials: From chemosensors to multidimensional self-assembled architectures. Coord. Chem. Rev. 2020, 420, 213425. [Google Scholar] [CrossRef]
- Feng, W.; Jin, M.; Yang, K.; Pei, Y.; Pei, Z. Supramolecular delivery systems based on pillararenes. Chem. Commun. 2018, 54, 13626–13640. [Google Scholar] [CrossRef]
- Yu, G.; Yu, W.; Mao, Z.; Gao, C.; Huang, F. A pillararene-based ternary drug-delivery system with photocontrolled anticancer drug release. Small 2015, 11, 919–925. [Google Scholar] [CrossRef]
- Collier, C.P.; Mattersteig, G.; Wong, E.W.; Luo, Y.; Beverly, K.; Sampaio, J.; Raymo, F.M.; Stoddart, J.F.; Heath, J.R. A[2]catenane-based solid state electronically reconfigurable switch. Science 2000, 289, 1172–1175. [Google Scholar] [CrossRef]
- Gil-Ramírez, G.; Leigh, D.A.; Stephens, A.J. Catenanes: Fifty years of molecular links. Angew. Chem. Int. Ed. 2015, 54, 6110–6150. [Google Scholar] [CrossRef]
- Evans, N.H. Chiral catenanes and rotaxanes: Fundamentals and emerging applications. Chem. Eur. J. 2018, 24, 3101–3112. [Google Scholar] [CrossRef]
- Wu, Q.; Rauscher, P.M.; Lang, X.; Wojtecki, R.J.; Pablo, J.J.d.; Hore, M.J.A.; Rowan, S.J. Poly[n] catenanes: Synthesis of molecular interlocked chains. Science 2017, 358, 1434–1439. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Zhou, L.; Liu, C.; Zhang, H.; Zhao, Y.; Zhao, Y. Pillararene-based self-assemblies for electrochemical biosensors. Biosens. Bioelectron. 2021, 181, 113164. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhu, H.; Huang, F. Pillararene-based supramolecular functional materials. Trends Chem. 2020, 2, 850–864. [Google Scholar] [CrossRef]
- Liang, J.; Gvilava, V.; Jansen, C.; Öztürk, S.; Spieß, A.; Lin, J.; Xing, S.; Sun, Y.; Wang, H.; Janiak, C. Cucurbituril-encapsulating metal-organic framework via mechanochemistry: Adsorbents with enhanced performance. Angew. Chem. Int. Ed. 2021, 60, 15365–15370. [Google Scholar] [CrossRef] [PubMed]
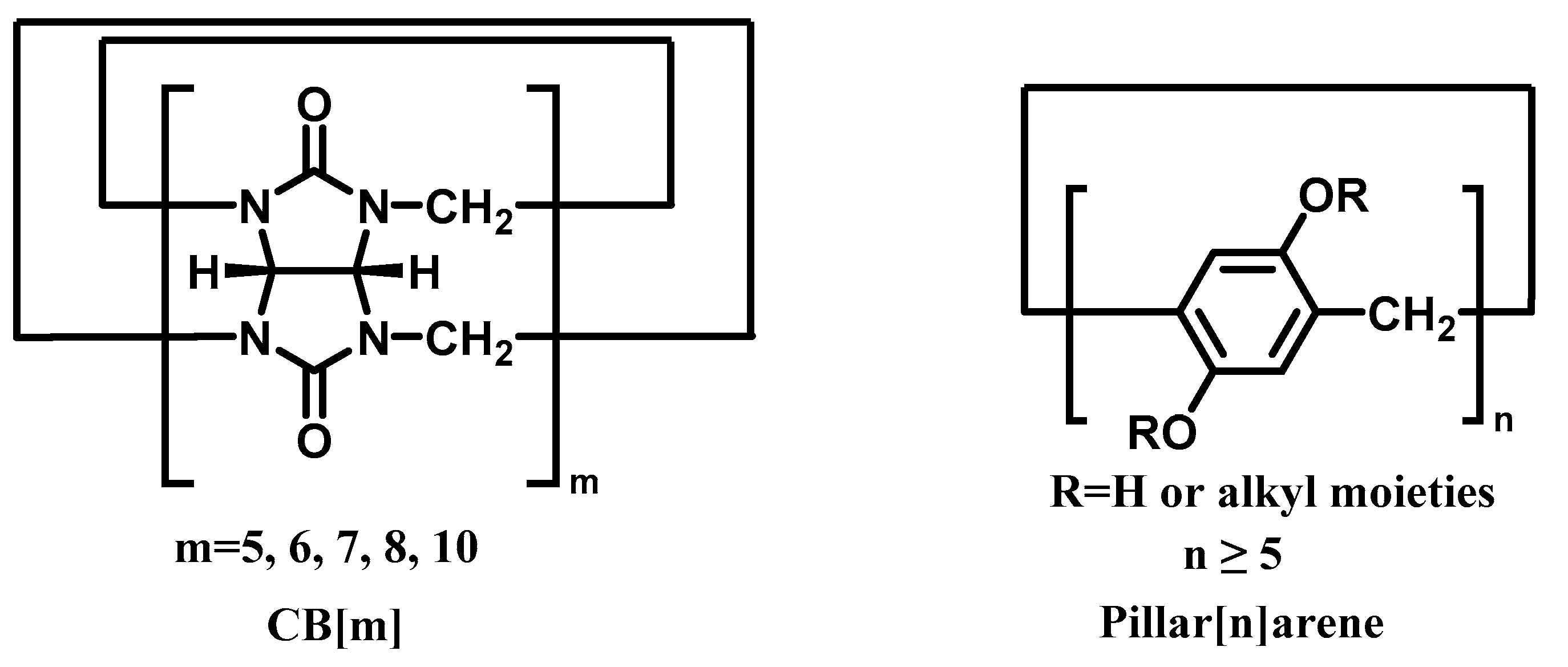
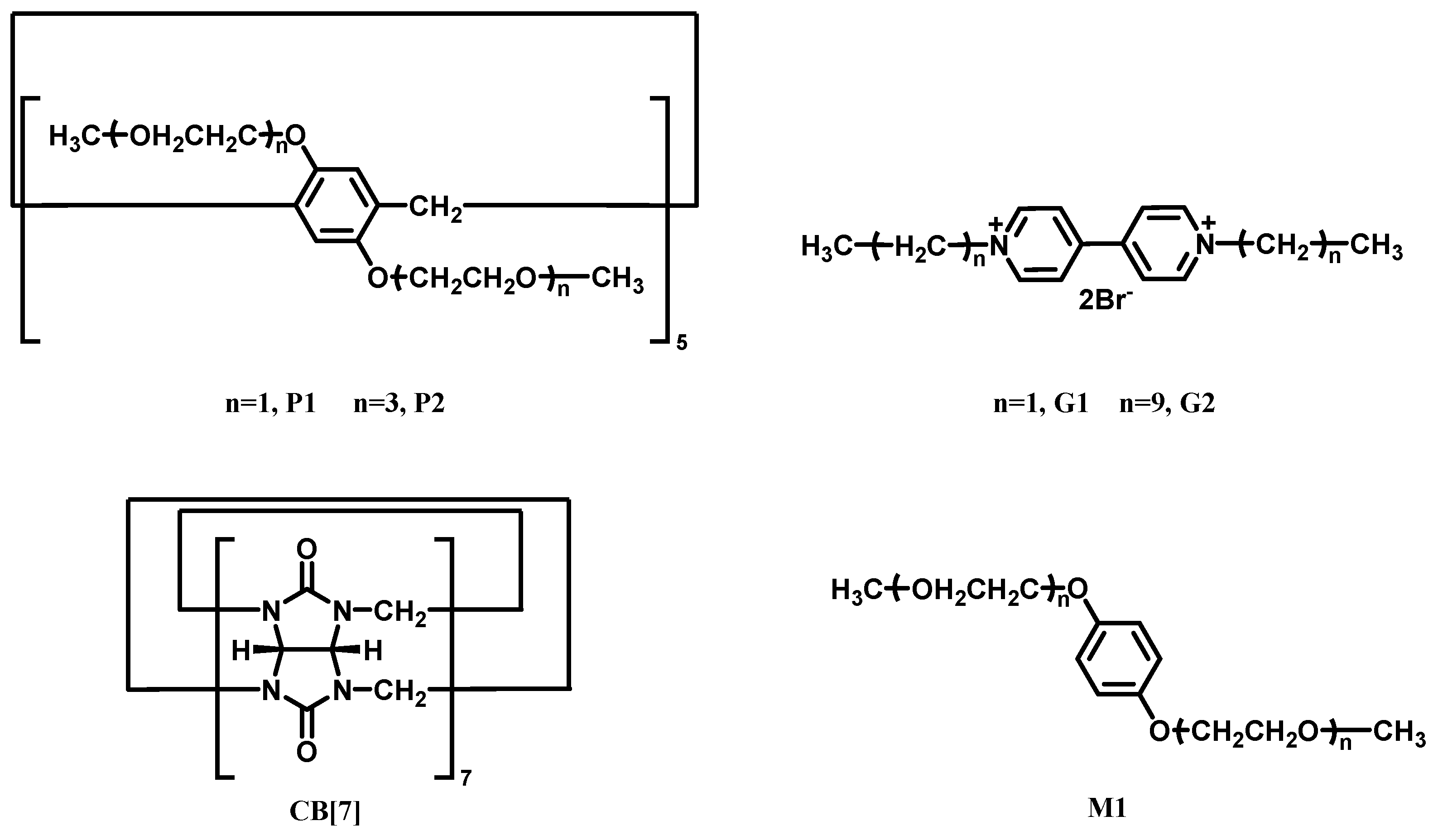
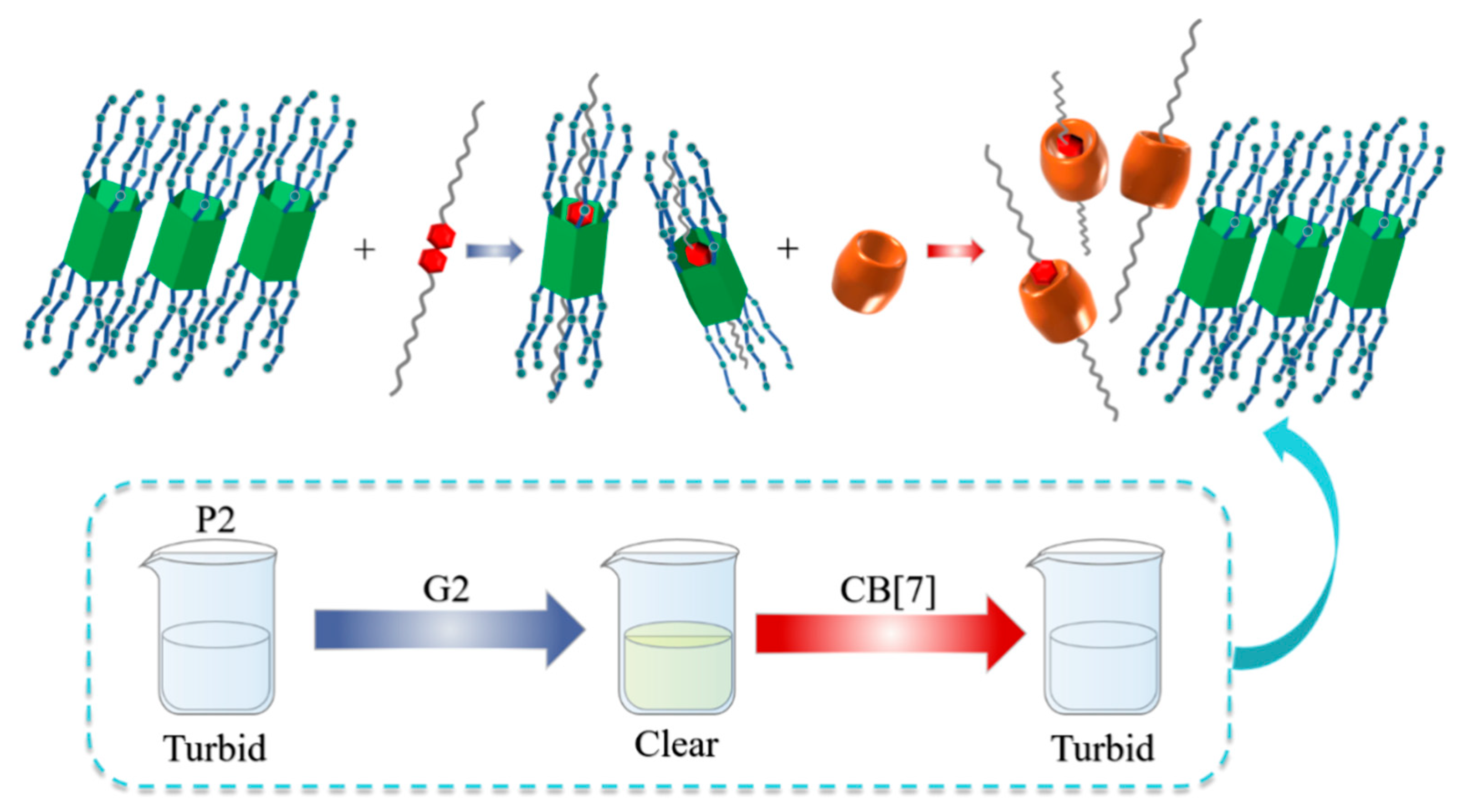

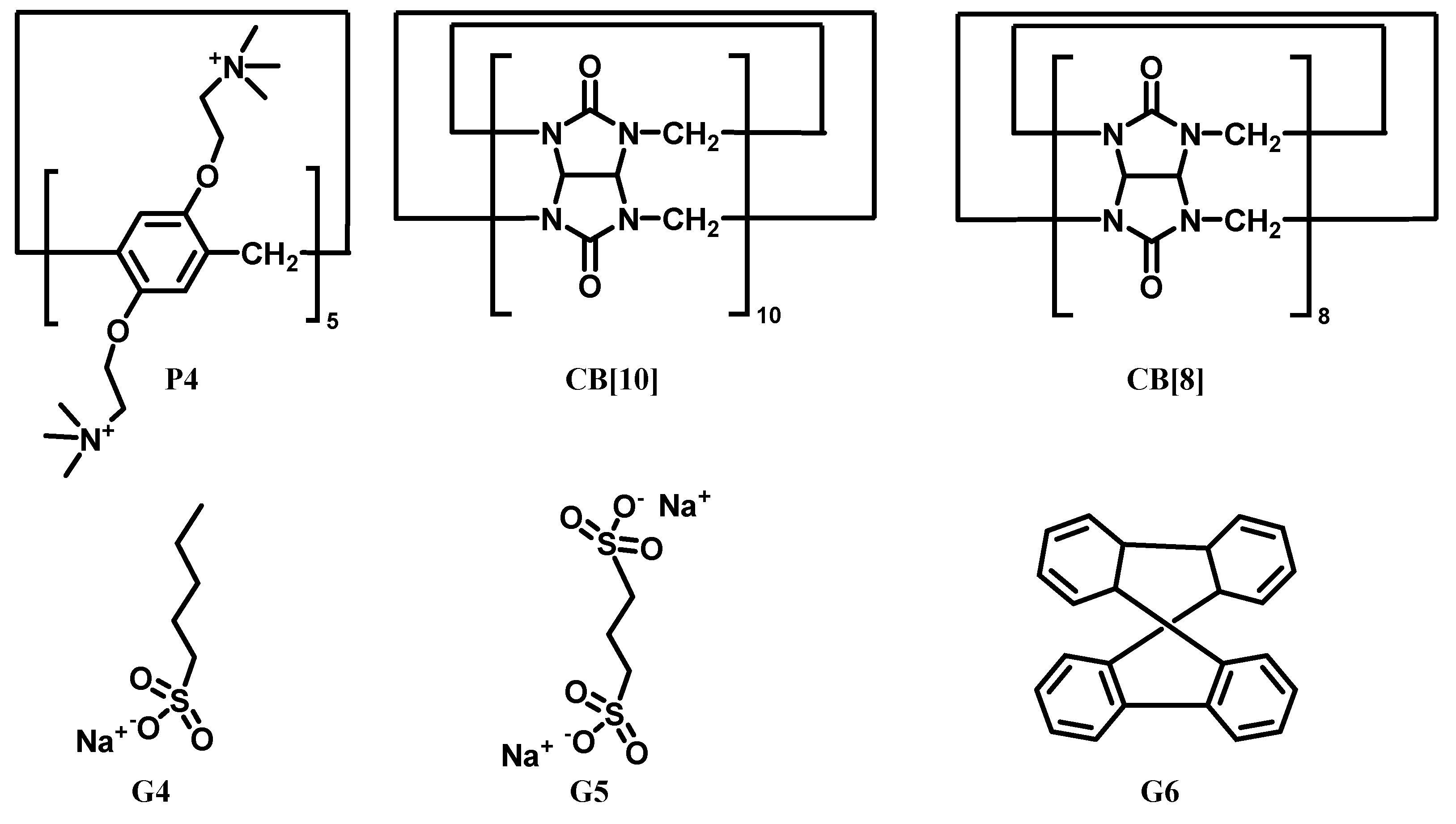



| CB[m] | Pillar[n]arene | Relationship | Guests | Ref |
|---|---|---|---|---|
| CB[7] | Pillar[5]arene P2 | Competition of hosts (Ka of P2⸧G2 ~103 M−1) vs. (Ka of CB[7]⸧G2 ~105 M−1) | G2 | [79] |
| CB[10] | Pillar[5]arene P4 | Forming hybrid macrocyclic system CB[10]•P4 presenting integrated Ka towards G4 and G6 as ~106 and ~105 M−1 | G4-G6 | [84] |
| CB[m] | Pillar[n]arene | Self-Assembly | Guests as Building Blocks | Ref |
|---|---|---|---|---|
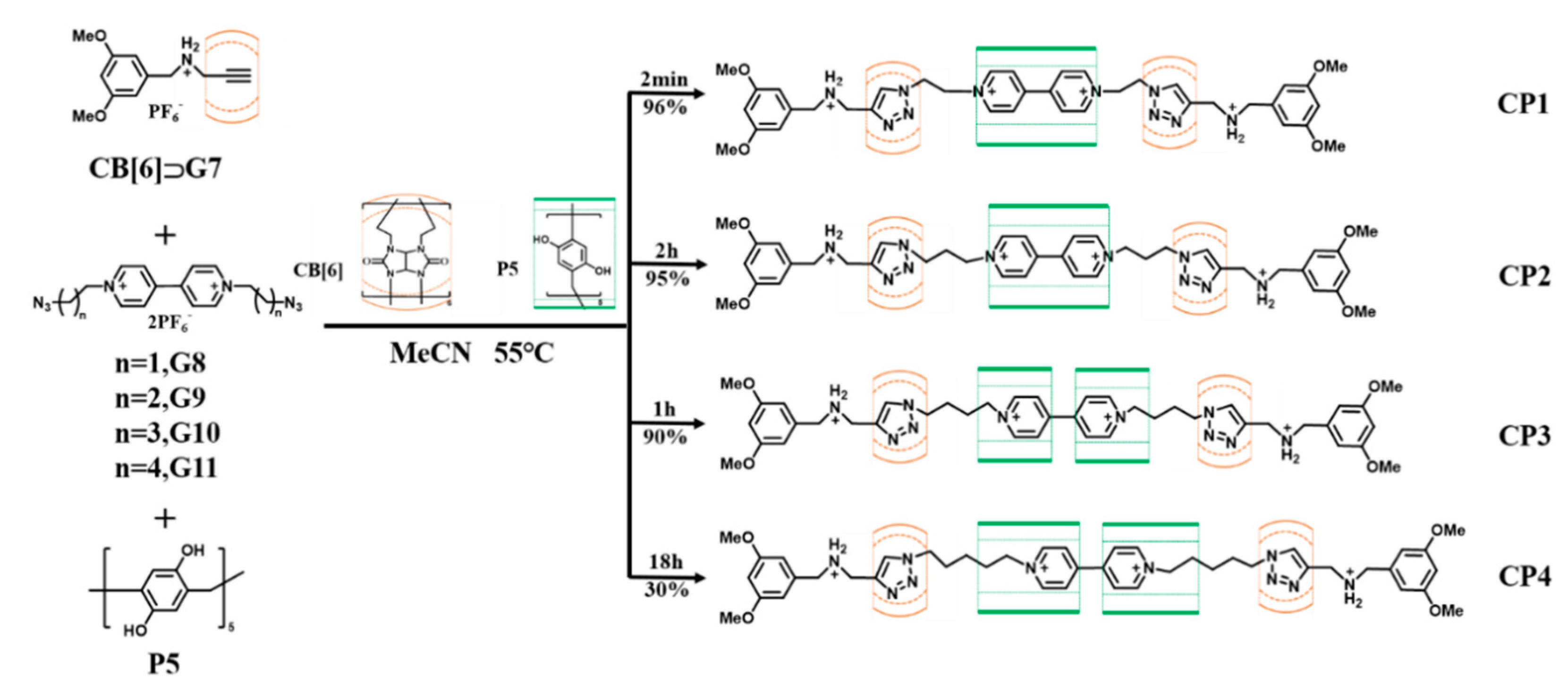
| CB[6] | Pillar[5]arene P5 | n]Rotaxane CP1-CP4 | G7-G11 | [89] |
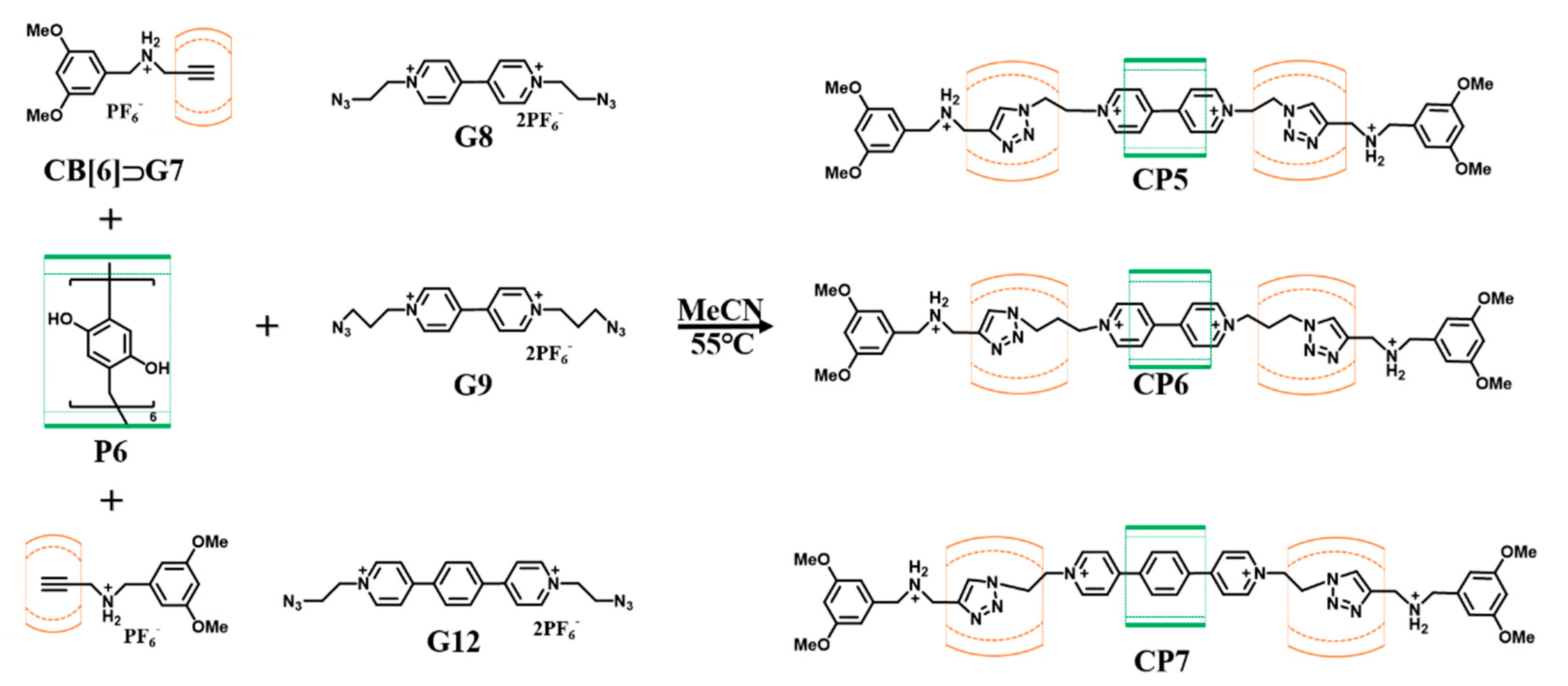
| CB[6] | Pillar[6]arene P6 | [n]Rotaxane CP5-CP7 | G8, G9 and G12 | [90] |
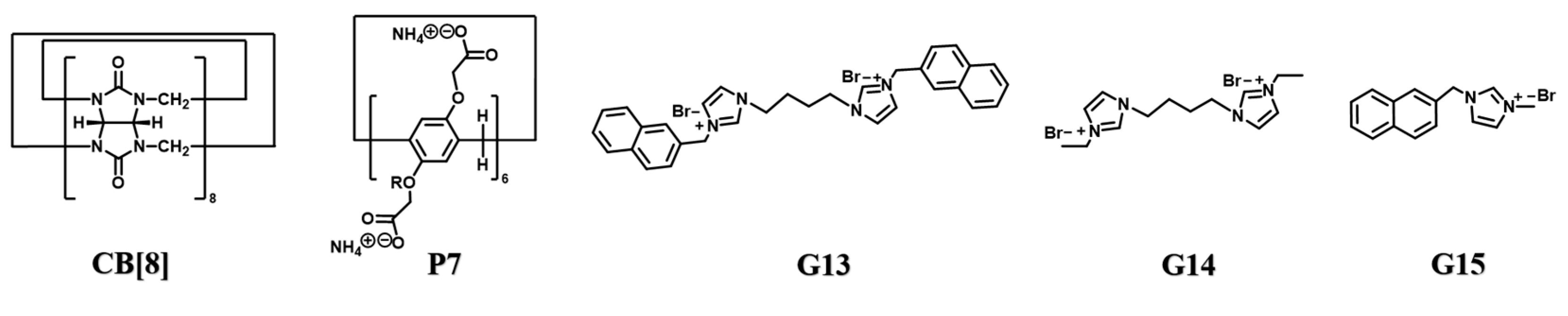
| CB[8] | Pillar[6]arene P7 | Supramolecular polymer P7⸧G13⊂CB[8] | G13 | [93] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Li, Z.; Li, B.; Zhou, L.; Zhang, H.; Han, J. Hybrid Macrocyclic Polymers: Self-Assembly Containing Cucurbit[m]uril-pillar[n]arene. Polymers 2022, 14, 1777. https://doi.org/10.3390/polym14091777
Liu Z, Li Z, Li B, Zhou L, Zhang H, Han J. Hybrid Macrocyclic Polymers: Self-Assembly Containing Cucurbit[m]uril-pillar[n]arene. Polymers. 2022; 14(9):1777. https://doi.org/10.3390/polym14091777
Chicago/Turabian StyleLiu, Zhaona, Zhizheng Li, Bing Li, Le Zhou, Huacheng Zhang, and Jie Han. 2022. "Hybrid Macrocyclic Polymers: Self-Assembly Containing Cucurbit[m]uril-pillar[n]arene" Polymers 14, no. 9: 1777. https://doi.org/10.3390/polym14091777
APA StyleLiu, Z., Li, Z., Li, B., Zhou, L., Zhang, H., & Han, J. (2022). Hybrid Macrocyclic Polymers: Self-Assembly Containing Cucurbit[m]uril-pillar[n]arene. Polymers, 14(9), 1777. https://doi.org/10.3390/polym14091777









