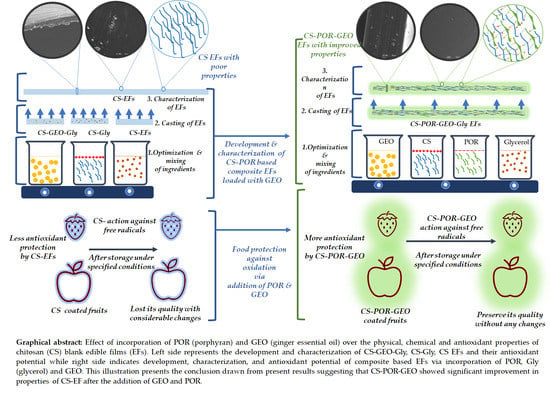
Development and Characterization of Chitosan and Porphyran Based Composite Edible Films Containing Ginger Essential Oil
Abstract
1. Introduction
2. Results and Discussion
2.1. Visual Appearance of the EFs
2.2. Film Thickness
2.3. Mechanical Properties
2.4. Water Vapor Permeability (WVP)
2.5. Oxygen Barrier Properties
2.6. Water Solubility (WS) and Swelling Degree (SD)
2.7. Moisture Content
2.8. Optical Transmittance Analysis
2.9. Color Parameters of the EFs
2.10. TGA Analysis
2.11. X-ray Diffraction (XRD) Analysis
2.12. SEM
2.13. FTIR Analysis
2.14. Antioxidant Assays
3. Materials and Methods
3.1. Chemicals
3.2. Isolation of Porphyran from Pyropia vietnamensis
3.3. Edible Films (EFs) Production
3.4. Thickness of the EFs
3.5. Mechanical Testing
3.6. Water Vapor Permeability (WVP)
3.7. Oxygen Barrier Property
3.8. Water Solubility and Swelling Degree
3.9. Moisture Content
3.10. Optical Transmittance Analysis
3.11. Color Measurement of the EFs
3.12. Thermogravimetric (TGA) Analysis
3.13. X-ray Diffraction (XRD) Studies
3.14. SEM (Scanning Electron Microscopy) Analysis
3.15. FTIR Spectra Analysis
3.16. Antioxidant Assays
3.16.1. Sample Preparation
3.16.2. Assessment of Total Phenolic Content
3.16.3. Determination of Scavenging Capacity against DPPH Radical
3.16.4. Analysis of Trolox Equivalent Antioxidant Capacity (TEAC)
3.16.5. Determination of FRAP (Ferric Antioxidant Reducing Power)
3.16.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Zhou, Y.; Wu, X.; Chen, J.; He, J. Effects of Cinnamon Essential Oil on the Physical, Mechanical, Structural and Thermal Properties of Cassava Starch-Based Edible Films. Int. J. Biol. Macromol. 2021, 184, 574–583. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.; Kumar, V.; Sharma, K.; Nagpal, K.; Bera, T. Significance of Algal Polymer in Designing Amphotericin B Nanoparticles. Sci. World J. 2014, 2014, 564573. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.; Namdeo, A.G.; Nanda, S. Factors Affecting the Gelling and Emulsifying Property of Natural Polymer. Syst. Rev. Pharm. 2010, 9, 93–101. [Google Scholar] [CrossRef]
- Jeena, K.; Liju, V.B.; Kuttan, R. Antioxidant, Anti-Inflammatory, and Antinociceptive Activities of Essential Oil from Ginger. Indian J. Physiol. Pharmacol. 2013, 57, 51–62. [Google Scholar]
- Kanatt, S.R.; Chander, R.; Sharma, A. Chitosan and mint mixture: A new preservative for meat and meat products. Food Chem. 2008, 107, 845–852. [Google Scholar] [CrossRef]
- Hazrati, K.Z.; Sapuan, S.M.; Zuhri, M.; Jumaidin, R. Effect of plasticizers on physical, thermal, and tensile properties of thermoplastic films based on Dioscorea hispida starch. Int. J. Biol. Macromol. 2021, 185, 219–228. [Google Scholar] [CrossRef]
- Sanyang, M.L.; Sapuan, S.M.; Jawaid, M.; Ishak, M.R.; Sahari, J. Effect of Plasticizer Type and Concentration on Physical Properties of Biodegradable Films Based on Sugar Palm (Arenga pinnata) Starch for Food Packaging. J. Food Sci. Technol. 2016, 53, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Valizadeh, S.; Naseri, M.; Babaei, S.; Hosseini, S.M.H.; Imani, A. Development of Bioactive Composite Films from Chitosan and Carboxymethyl Cellulose Using Glutaraldehyde, Cinnamon Essential Oil and Oleic Acid. Int. J. Biol. Macromol. 2019, 134, 604–612. [Google Scholar] [CrossRef]
- Jamróz, E.; Konieczna-Molenda, A.; Para, A. Ternary Potato Starch-Furcellaran-Gelatin Film-A New Generation of Biodegradable Foils. Polimery 2017, 62, 673–679. [Google Scholar] [CrossRef]
- Jahani, S.; Shakiba, A.; Azami, M. Functional Properties, Antibacterial and Antioxidant Activities of Zataria multiflora Encapsulated in Geltin Nanofilms. J. Microbiol. Biotechnol. Food Sci. 2014, 4, 88–92. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Rezaei, M.; Zandi, M.; Farahmandghavi, F. Bio-based Composite Edible Films containing Origanum vulgare L. Essential Oil. Ind. Crops Prod. 2015, 67, 403–413. [Google Scholar] [CrossRef]
- Antares, L.; Chiralt, A. Essential oils As Additives in Biodegradable Films and Coatings for Active Food Packaging. Trends Food Sci. Technol. 2016, 48, 51–62. [Google Scholar] [CrossRef]
- Shaw, N.; Monahan, F.; O’riordan, E.; O’sullivan, M. Physical Properties of WPI Films Plasticized with Glycerol, Xylitol, or Sorbitol. Food Eng. Phys. Prop. 2002, 67, 164–167. [Google Scholar] [CrossRef]
- Silva-Weiss, A.; Bifani, V.; Ihl, M.; Sobral, P.J.A.; Gómez-Guillén, M.C. Structural Properties of Films and Rheology of Film-Forming Solutions Based on Chitosan and Chitosan-Starch Blend Enriched with Murta Leaf Extract. Food Hydrocoll. 2013, 31, 458–466. [Google Scholar] [CrossRef]
- Mohammadi, M.; Mirabzadeh, S.; Shahvalizadeh, R.; Hamishehkar, H. Development of Novel Active Packaging Films Based on Whey Protein Isolate Incorporated with Chitosan Nanofiber and Nano-Formulated Cinnamon Oil. Int. J. Biol. Macromol. 2020, 149, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Arezoo, E.; Mohammadreza, E.; Maryam, M.; Abdorreza, M.N. The Synergistic Effects of Cinnamon Essential Oil and Nano Tio2 on Antimicrobial and Functional Properties of Sago Starch Films. Int. J. Biol. Macromol. 2020, 157, 743–751. [Google Scholar] [CrossRef]
- Hong, P.Z.; Li, S.D.; Ou, C.Y.; Li, C.P.; Yang, L.; Zhang, C.H. Thermogravimetric analysis of chitosan. J. Appl. Polym. Sci. 2007, 105, 547–551. [Google Scholar] [CrossRef]
- Martins, J.T.; Cerqueira, M.A.; Bourbon, A.I.; Pinheiro, A.C.; Souza, B.W.; Vicente, A.A. Synergistic Effects Between k-carrageenan and Locust Bean Gum on Physicochemical Properties of Edible Films Made there-of. Food Hydrocoll. 2012, 29, 280–289. [Google Scholar] [CrossRef]
- Sun, L.; Sun, J.; Chen, L.; Niu, P.; Yang, X.; Guo, Y. Preparation and Characterization of Chitosan Film Incorporated with Thinned Young Apple Polyphenols as an Active Packaging Material. Carbohydr. Polym. 2017, 163, 81–91. [Google Scholar] [CrossRef]
- Ngo, D.H.; Kim, S.K. Antioxidant Effects of Chitin, Chitosan, and their Derivatives. Adv. Food Nutr. Res. 2014, 73, 15–31. [Google Scholar] [CrossRef]
- Yanai, N.; Shiotani, S.; Hagiwara, S.; Nabetani, H.; Nakajima, M. Antioxidant Combination Inhibits Reactive Oxygen Species Mediated Damage. Biosci. Biotechnol. Biochem. 2008, 72, 3100–3106. [Google Scholar] [CrossRef] [PubMed][Green Version]
- ASTM. Standard test methods for tensile properties of thin plastic sheeting D882-10. In Annual Book of ASTM; American Society for Testing and Materials: Philadelphia, PA, USA, 2010. [Google Scholar]
- Kurt, A.; Kahyaoglu, T. Characterization of a New Biodegradable Edible Film Made from Salep Glucomannan. Carbohydr. Polym. 2014, 104, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Souza, V.G.L.; Fernando, A.L.; Pires, J.R.A.; Rodrigues, P.F.; Lopes, A.A.S.; Fernandes, F.M.B. Physical Properties of Chitosan Films Incorporated with Natural Antioxidants. Ind. Crops Prod. 2017, 107, 565–572. [Google Scholar] [CrossRef]
- Shiku, Y.; Hamaguchi, P.Y.; Benjakul, S.; Visessanguan, W.; Tanaka, M. Effect of Surimi Quality on Properties of Edible Films Based on Alaska pollack. Food Chem. 2004, 86, 493–499. [Google Scholar] [CrossRef]
- Braca, A.; Tommasi, N.D.; Bari, L.D. Antioxidant Principles from Bauhinia terapotensis. J. Nat. Prod. 2001, 64, 892–895. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.; Strain, J. The Ferric Reducing Ability of Plasma as a Measure of Antioxidant. Anal. Biochem. 2000, 239, 70–76. [Google Scholar] [CrossRef]








| EF Codes | Composition | Appearance of Films |
|---|---|---|
| S1 | CS | Transparent, fragile, brittle, stiff, inflexible, difficult to peel, non-sticky |
| S2 | CS-Gly | Less transparent than S1, brittle and fragile, flexible than S1, easy to peel and handle |
| S3 | CS-S-EO | Less transparent than S1 and S2, sticky, brittle and fragile, more flexible than S1 and S3, difficult to peel and handle, |
| S4 | CS-POR-Gly | Less transparent than S1 and S2, not brittle and fragile, not rigid, flexible, easy to peel, less sticky |
| S5 | CS-POR-Gly-EO | Less transparent than S1–S4, not fragile and brittle, easy to peel off from the surface, more flexible than S1–S4, not adhesive |
| Formulations | WVP (×10−12 g⋅cm/cm2⋅s⋅Pa) | Thickness (μm) | EB (%) | TS (MPa) | Young Modulus (MPa) | OP (g/100 g) |
|---|---|---|---|---|---|---|
| S1 | 2.3 ± 0.06 a | 52.12 ± 2.3 a | 2.17 ± 1.71 a | 21.23 ± 7.11 a | 77.21 ± 0.51 a | 4.17 ± 0.034 a |
| S2 | 2.9 ± 0.02 b | 55.67 ± 1.7 b | 17.12 ± 4.21 b | 16.24 ± 2.71 b | 52.12 ± 0.54 c | 3.21 ± 0.030 b |
| S3 | 2.1 ± 0.03 a | 58.12 ± 2.1 c | 37.24 ± 9.31 c | 11.24 ± 3.16 c | 42.16 ± 0.71 b | 2.11 ± 0.037 c |
| S4 | 1.6 ± 0.01 c | 46.21 ± 3.7 d | 33.27 ± 6.81 c | 41.32 ± 1.24 d | 38.42 ± 0.42 b | 1.78 ± 0.026 c |
| S5 | 1.1 ± 0.05 d | 49.78 ± 1.8 e | 71.12 ± 12.3 d | 32.77 ± 6.71 e | 27.21 ± 0.21 d | 0.77 ± 0.011 d |
| Codes of the Samples | WS (%) | Swelling Degree (%) | MC (%) |
|---|---|---|---|
| S1 | 26.1 ± 0.7 a | 167 ± 12.2 a | 12.71 ± 0.12 a |
| S2 | 34.2 ± 1.1 b | 198 ± 17.7 b | 18.46 ± 0.11 b |
| S3 | 23.2 ± 0.6 c | 112 ± 9.1 c | 15.62 ± 0.17 c |
| S4 | 21.4 ± 3.2 c | 79 ± 4.2 d | 15.27 ± 0.11 c |
| S5 | 12.2 ± 0.9 d | 37 ± 7.1 e | 14.81 ± 0.12 c |
| L | a* | b* | △E* | CI* | Transparency (%) | |
|---|---|---|---|---|---|---|
| S1 | 67.22 ± 3.01 a | 1.17 ± 0.03 a | 0.31 ± 0.02 a | 6.81 ± 3.17 a | 1.20 ± 0.21 a | 42.81 ± 1.24 a |
| S2 | 62.43 ± 2.84 b | 1.64 ± 0.01 b | 0.77 ± 0.01 b | 12.6 ± 1.34 b | 1.81 ± 0.13 b | 33.23 ± 2.24 b |
| S3 | 41.18 ± 5.22 c | −5.24 ± 0.32 c | 4.23 ± 0.22 c | 33.4 ± 7.31 c | 6.72 ± 0.34 c | 30.22 ± 1.67 c |
| S4 | 63.56 ± 4.32 b | 1.62 ± 0.07 b | 0.71 ± 0 07 b | 10.5 ± 2.88 b | 1.76 ± 0.52 b | 26.75 ± 1.07 d |
| S5 | 38.30 ± 6.21 d | −6.78 ± 0.55 d | 4.77 ± 1.13 c | 36.5 ± 6.24 d | 8.21 ± 0.64 d | 28.34 ± 3.21 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Harrasi, A.; Bhtaia, S.; Al-Azri, M.S.; Makeen, H.A.; Albratty, M.; Alhazmi, H.A.; Mohan, S.; Sharma, A.; Behl, T. Development and Characterization of Chitosan and Porphyran Based Composite Edible Films Containing Ginger Essential Oil. Polymers 2022, 14, 1782. https://doi.org/10.3390/polym14091782
Al-Harrasi A, Bhtaia S, Al-Azri MS, Makeen HA, Albratty M, Alhazmi HA, Mohan S, Sharma A, Behl T. Development and Characterization of Chitosan and Porphyran Based Composite Edible Films Containing Ginger Essential Oil. Polymers. 2022; 14(9):1782. https://doi.org/10.3390/polym14091782
Chicago/Turabian StyleAl-Harrasi, Ahmed, Saurabh Bhtaia, Mohammed Said Al-Azri, Hafiz A. Makeen, Mohammed Albratty, Hassan A. Alhazmi, Syam Mohan, Ajay Sharma, and Tapan Behl. 2022. "Development and Characterization of Chitosan and Porphyran Based Composite Edible Films Containing Ginger Essential Oil" Polymers 14, no. 9: 1782. https://doi.org/10.3390/polym14091782
APA StyleAl-Harrasi, A., Bhtaia, S., Al-Azri, M. S., Makeen, H. A., Albratty, M., Alhazmi, H. A., Mohan, S., Sharma, A., & Behl, T. (2022). Development and Characterization of Chitosan and Porphyran Based Composite Edible Films Containing Ginger Essential Oil. Polymers, 14(9), 1782. https://doi.org/10.3390/polym14091782