Simultaneous Effects of Carboxyl Group-Containing Hyperbranched Polymers on Glass Fiber-Reinforced Polyamide 6/Hollow Glass Microsphere Syntactic Foams
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Sample Preparation
2.2.2. Characterization
3. Results and Discussion
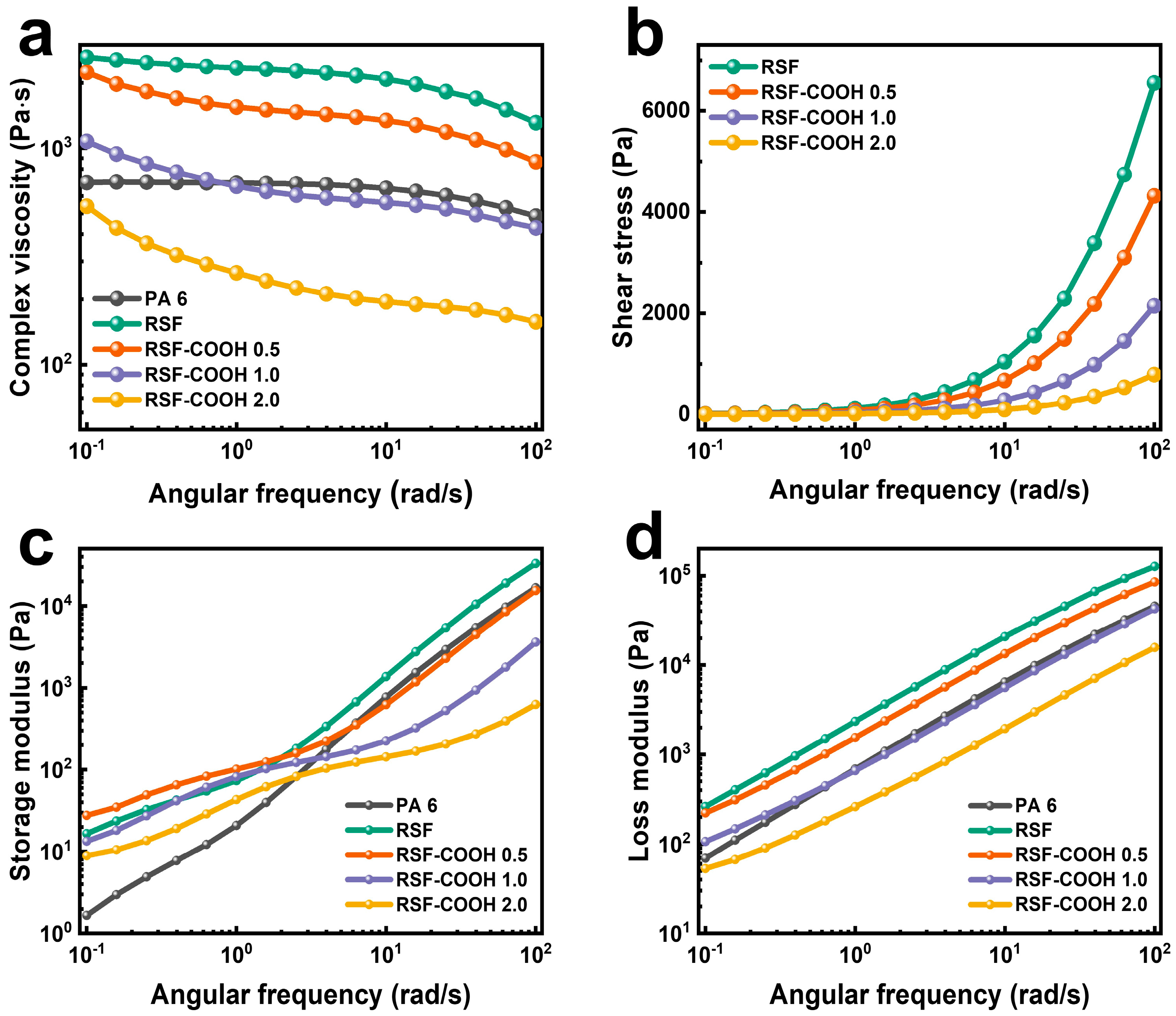
3.1. Rheological Properties
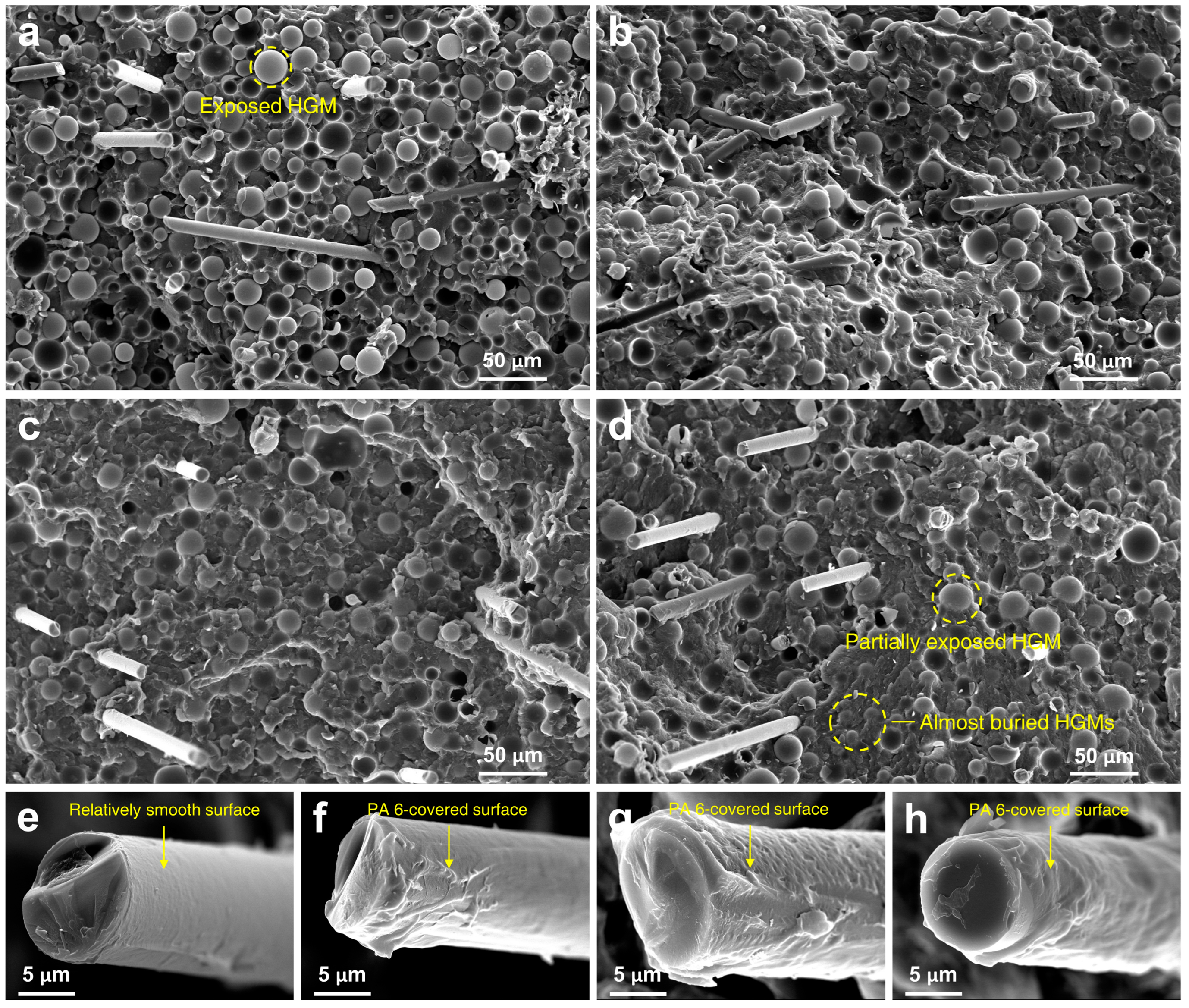
3.2. Morphology
3.3. Crystallization Behavior
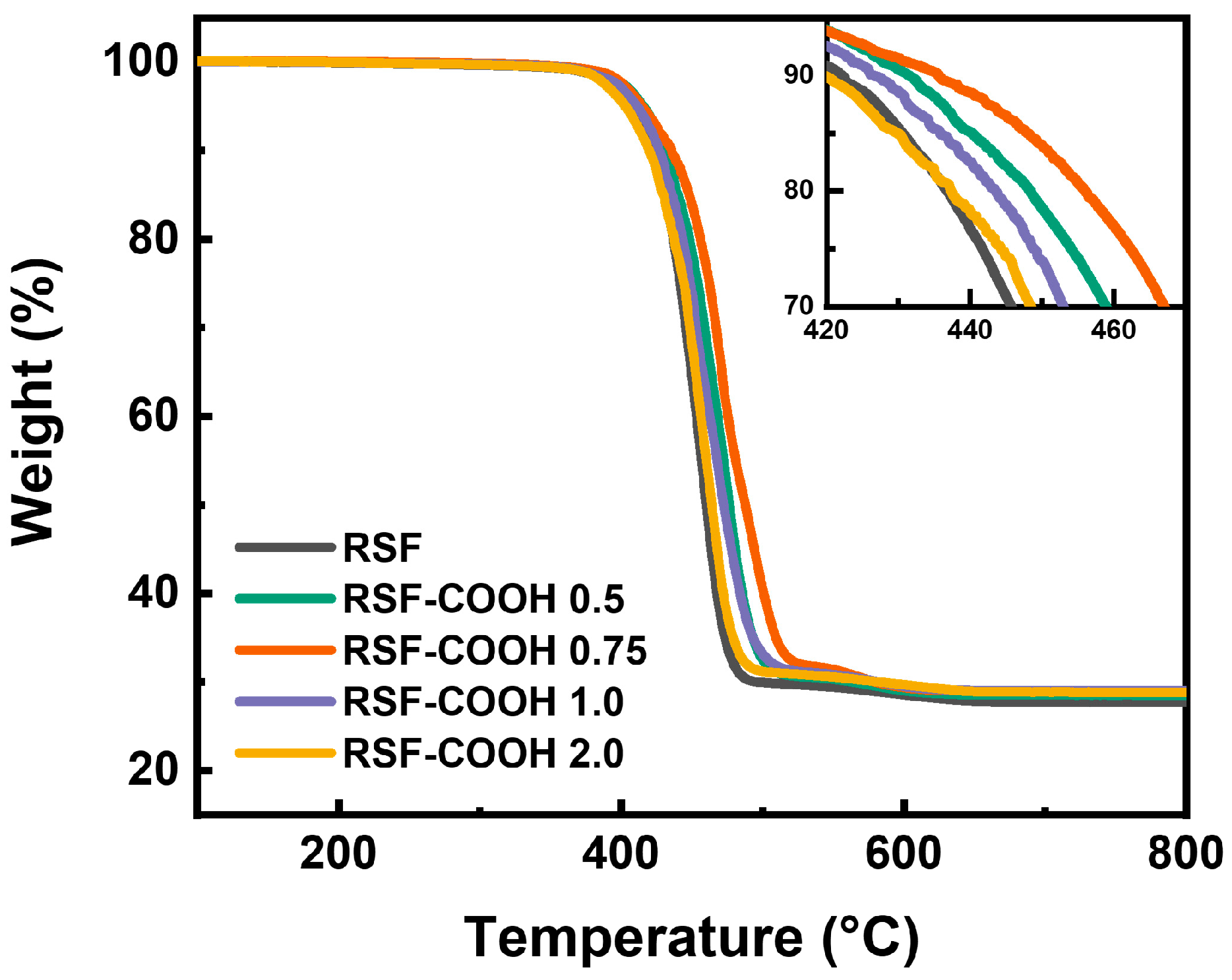
3.4. Thermogravimetric Analysis
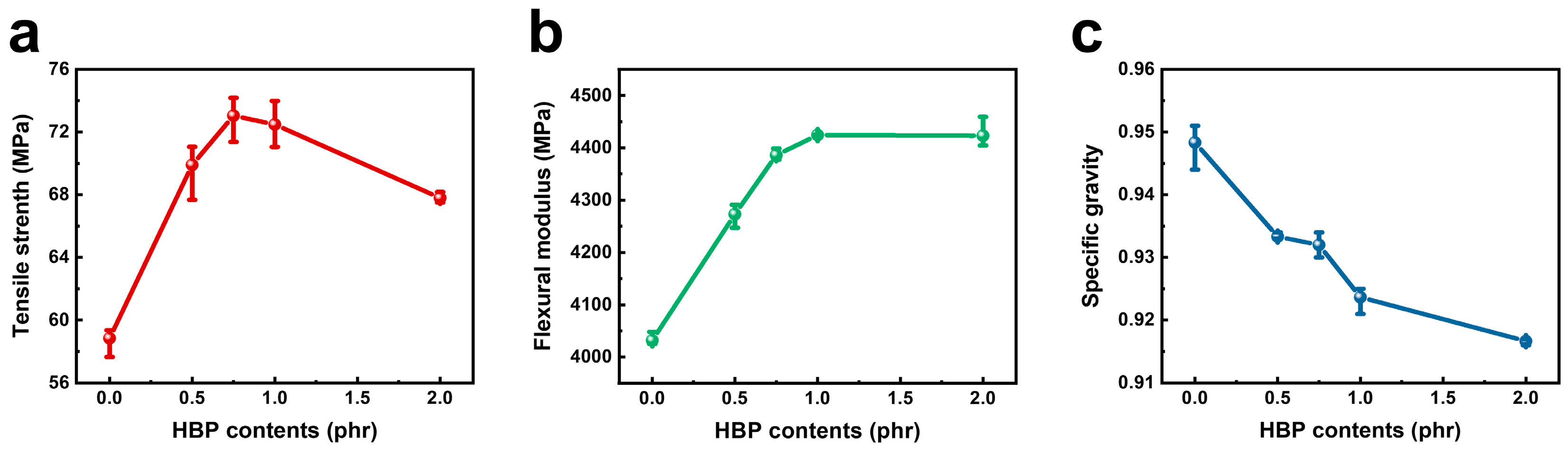
3.5. Mechanical Properties and Specific Gravity of the Composite
4. Conclusions
- (1)
- In the rheological characterization, the complex viscosity and the shear stress of the RSF-COOH composites decreased across the entire frequency range compared to the neat RSF composite without HBP. The storage modulus and loss modulus tended to decrease with increasing content of HBP in the composite; however, the slope of the storage modulus at low frequency tended to decrease. These results are evidence that the lubricating effect due to the dendritic structure of HBP, in addition to hydrogen bonding, were functioning in the RSF-COOH composite at the same time.
- (2)
- The tensile strength and flexural modulus were increased by 24.3 and 9.7%, respectively, in RSF-COOH composite compared to the neat RSF. In the FE-SEM microphotographs, it was shown that the compatibility of the filler improved, and that filler-polymer adhesion enhanced when HBP was added. In addition, in DSC data, the crystallinity of the composite (Xc) increased to 22.54% with the addition of HBP in the composite. The enhanced compatibility of the filler with the polymer matrix, and crystallization behavior contributed to the increased mechanical strength of the RSF-COOH composite material.
- (3)
- As a result of the reduced shear stress of the RSF-COOH composite in the high shear-applied extrusion process, HGM breakage considerably decreased from 9.14 to 2.84%. As a result, the specific gravity of the composite significantly decreased from 0.948 to 0.917 when HBP was introduced to the RSF composite.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Regulations for Emissions from Vehicles and Engines. Available online: https://www.epa.gov/regulations-emissions-vehicles-and-engines (accessed on 14 February 2022).
- Sun, Q.; Zhou, G.; Meng, Z.; Jain, M.; Su, X. An integrated computational materials engineering framework to analyze the failure behaviors of carbon fiber reinforced polymer composites for lightweight vehicle applications. Compos. Sci. Technol. 2021, 202, 108560. [Google Scholar] [CrossRef] [PubMed]
- Mallick, P.K. Materials, design and manufacturing for lightweight vehicles; Woodhead Publishing: Sawston, UK, 2020. [Google Scholar]
- Graziano, A.; Garcia, C.; Jaffer, S.; Tjong, J.; Yang, W.; Sain, M. Functionally tuned nanolayered graphene as reinforcement of polyethylene nanocomposites for lightweight transportation industry. Carbon 2020, 169, 99–110. [Google Scholar] [CrossRef]
- Du, Y.; Keller, T.; Song, C.; Wu, L.; Xiong, J. Origami-inspired carbon fiber-reinforced composite sandwich materials—Fabrication and mechanical behavior. Compos. Sci. Technol. 2021, 205, 108667. [Google Scholar] [CrossRef]
- Ozden, S.; Dutta, N.S.; Randazzo, K.; Tsafack, T.; Arnold, C.B.; Priestley, R.D. Interfacial engineering to tailor the properties of multifunctional ultralight weight hBN-polymer composite aerogels. ACS Appl. Mater. Interf. 2021, 13, 13620–13628. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.; Kil, H.-S.; Lee, S. Fabrication of low-cost carbon fibers using economical precursors and advanced processing technologies. Carbon 2019, 142, 610–649. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, P.; Zhou, Y.; Li, X.; Yang, E.-H.; Yu, T.X.; Yang, J. The effect of strain rate and filler volume fraction on the mechanical properties of hollow glass microsphere modified polymer. Compos. Part B Eng. 2016, 101, 53–63. [Google Scholar] [CrossRef]
- Cosse, R.L.; Araújo, F.H.; Pinto, F.A.N.C.; de Carvalho, L.H.; de Morais, A.C.L.; Barbosa, R.; Alves, T.S. Effects of the type of processing on thermal, morphological and acoustic properties of syntactic foams. Compos. Part B Eng. 2019, 173, 106933. [Google Scholar] [CrossRef]
- Jayavardhan, M.L.; Doddamani, M. Quasi-static compressive response of compression molded glass microballoon/HDPE syntactic foam. Compos. Part B Eng. 2018, 149, 165–177. [Google Scholar] [CrossRef]
- Jayavardhan, M.L.; Bharath Kumar, B.R.; Doddamani, M.; Singh, A.K.; Zeltmann, S.E.; Gupta, N. Development of glass microballoon/HDPE syntactic foams by compression molding. Compos. Part B Eng. 2017, 130, 119–131. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, J. Effect of coupling agent on mechanical properties of hollow carbon microsphere/phenolic resin syntactic foam. Compos. Sci. Technol. 2010, 70, 1265–1271. [Google Scholar] [CrossRef]
- Tagliavia, G.; Porfiri, M.; Gupta, N. Analysis of flexural properties of hollow-particle filled composites. Compos. Part B Eng. 2010, 41, 86–93. [Google Scholar] [CrossRef]
- Wang, L.; Yang, X.; Zhang, J.; Zhang, C.; He, L. The compressive properties of expandable microspheres/epoxy foams. Compos. Part B Eng. 2014, 56, 724–732. [Google Scholar] [CrossRef]
- Gupta, N.; Zeltmann, S.E.; Shunmugasamy, V.C.; Pinisetty, D. Applications of polymer matrix syntactic foams. Jom 2013, 66, 245–254. [Google Scholar] [CrossRef]
- Swetha, C.; Kumar, R. Quasi-static uni-axial compression behaviour of hollow glass microspheres/epoxy based syntactic foams. Mater. Des. 2011, 32, 4152–4163. [Google Scholar] [CrossRef]
- He, S.; Carolan, D.; Fergusson, A.; Taylor, A.C. Toughening epoxy syntactic foams with milled carbon fibres: Mechanical properties and toughening mechanisms. Mater. Des. 2019, 169, 107654. [Google Scholar] [CrossRef]
- Gogoi, R.; Manik, G.; Arun, B. High specific strength hybrid polypropylene composites using carbon fibre and hollow glass microspheres: Development, characterization and comparison with empirical models. Compos. Part B Eng. 2019, 173, 106875. [Google Scholar] [CrossRef]
- Kumar, N.; Mireja, S.; Khandelwal, V.; Arun, B.; Manik, G. Light-weight high-strength hollow glass microspheres and bamboo fiber based hybrid polypropylene composite: A strength analysis and morphological study. Compos. Part B Eng. 2017, 109, 277–285. [Google Scholar] [CrossRef]
- Hu, Y.; Mei, R.; An, Z.; Zhang, J. Silicon rubber/hollow glass microsphere composites: Influence of broken hollow glass microsphere on mechanical and thermal insulation property. Compos. Sci. Technol. 2013, 79, 64–69. [Google Scholar] [CrossRef]
- Lin, C.-A.; Ku, T.-H. Shear and elongational flow properties of thermoplastic polyvinyl alcohol melts with different plasticizer contents and degrees of polymerization. J. Mater. Process. Technol. 2008, 200, 331–338. [Google Scholar] [CrossRef]
- Wu, C.; McGinity, J.W. Influence of methylparaben as a solid-state plasticizer on the physicochemical properties of Eudragit® RS PO hot-melt extrudates. Eur. J. Pharm. Biopharm. 2003, 56, 95–100. [Google Scholar] [CrossRef]
- Belous, A.; Tchoudakov, R.; Tzur, A.; Narkis, M.; Alperstein, D. Development and characterization of plasticized polyamides by fluid and solid plasticizers. Polym. Adv. Technol. 2012, 23, 938–945. [Google Scholar] [CrossRef]
- Wang, J.; Ye, Z.; Zhu, S. Topology-engineered hyperbranched high-molecular-weight polyethylenes as lubricant viscosity-index improvers of high shear stability. Ind. Eng. Chem. Res. 2007, 46, 1174–1178. [Google Scholar] [CrossRef]
- Wang, F.C.-Y.; Buzanowski, W.C. Polymer additive analysis by pyrolysis–gas chromatography. J. Chromatogr. A 2000, 891, 313–324. [Google Scholar] [CrossRef]
- Bettini, S.H.; de Miranda Josefovich, M.P.; Munoz, P.A.; Lotti, C.; Mattoso, L.H. Effect of lubricant on mechanical and rheological properties of compatibilized PP/sawdust composites. Carbohydr. Polym. 2013, 94, 800–806. [Google Scholar] [CrossRef] [Green Version]
- Jacobsen, S.; Fritz, H.-G. Plasticizing polylactide—the effect of different plasticizers on the mechanical properties. Polym. Eng. Sci. 1999, 39, 1303–1310. [Google Scholar] [CrossRef]
- Cosimbescu, L.; Robinson, J.W.; Zhou, Y.; Qu, J. Dual functional star polymers for lubricants. RSC Adv. 2016, 6, 86259–86268. [Google Scholar] [CrossRef]
- Robinson, J.W.; Zhou, Y.; Bhattacharya, P.; Erck, R.; Qu, J.; Bays, J.T.; Cosimbescu, L. Probing the molecular design of hyper-branched aryl polyesters towards lubricant applications. Sci. Rep. 2016, 6, 18624. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Li, S.; Weng, Z.; Gao, C. Hyperbranched polymers: Advances from synthesis to applications. Chem. Soc. Rev. 2015, 44, 4091–4130. [Google Scholar] [CrossRef]
- Hawker, C.J.; Farrington, P.J.; Mackay, M.E.; Wooley, K.L.; Frechet, J.M. Molecular ball bearings: The unusual melt viscosity behavior of dendritic macromolecules. J. Am. Chem. Soc. 1995, 117, 4409–4410. [Google Scholar] [CrossRef]
- Chen, L.; Qin, Y.; Wang, X.; Li, Y.; Zhao, X.; Wang, F. Toughening of poly(propylene carbonate) by hyperbranched poly(ester-amide) via hydrogen bonding interaction. Polym. Inter. 2011, 60, 1697–1704. [Google Scholar] [CrossRef]
- Wu, C.; Huang, X.; Wang, G.; Wu, X.; Yang, K.; Li, S.; Jiang, P. Hyperbranched-polymer functionalization of graphene sheets for enhanced mechanical and dielectric properties of polyurethane composites. J. Mater. Chem. 2012, 22, 7010–7019. [Google Scholar] [CrossRef]
- Gu, W.; Liu, X.; Gao, Q.; Gong, S.; Li, J.; Shi, S.Q. Multiple hydrogen bonding enables strong, tough, and recyclable soy protein films. ACS Sustain. Chem. Eng. 2020, 8, 7680–7689. [Google Scholar] [CrossRef]
- Peng, Q.; Li, Y.; He, X.; Lv, H.; Hu, P.; Shang, Y.; Wang, C.; Wang, R.; Sritharan, T.; Du, S. Interfacial enhancement of carbon fiber composites by poly(amido amine) functionalization. Compos. Sci. Technol. 2013, 74, 37–42. [Google Scholar] [CrossRef]
- Avrami, M. Granulation, Phase change, and microstructure kinetics of phase change. III. J. Chem. Phy. 1941, 9, 177–184. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of phase change. I General theory. J. Chem. Phy. 1939, 7, 1103–1112. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of phase change. II Transformation-Time relations for random distribution of nuclei. J. Chem. Phy. 1940, 8, 212–224. [Google Scholar] [CrossRef]
- Fornes, T.D.; Paul, D.R. Crystallization behavior of nylon 6 nanocomposites. Polymer 2003, 44, 3945–3961. [Google Scholar] [CrossRef]
- Fan, Z.; Jaehnichen, K.; Desbois, P.; Haeussler, L.; Vogel, R.; Voit, B. Blends of different linear polyamides with hyperbranched aromatic AB2 and A2+ B3 polyesters. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 3558–3572. [Google Scholar] [CrossRef]
- Kang, D.; Hwang, S.W.; Jung, B.N.; Shim, J.K. Effect of hollow glass microsphere (HGM) on the dispersion state of single-walled carbon nanotube (SWNT). Compos. Part B Eng. 2017, 117, 35–42. [Google Scholar] [CrossRef]
- Goldansaz, H.; Goharpey, F.; Afshar-Taromi, F.; Kim, I.; Stadler, F.J.; van Ruymbeke, E.; Karimkhani, V. Anomalous Rheological Behavior of Dendritic Nanoparticle/Linear Polymer Nanocomposites. Macromolecules 2015, 48, 3368–3375. [Google Scholar] [CrossRef]
- Bhardwaj, R.; Mohanty, A.K. Modification of brittle polylactide by novel hyperbranched polymer-based nanostructures. Biomacromolecules 2007, 8, 2476–2484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carolan, D.; Mayall, A.; Dear, J.P.; Fergusson, A.D. Micromechanical modelling of syntactic foam. Compos. Part B Eng. 2020, 183, 107701. [Google Scholar] [CrossRef]
- Privalko, V.; Kawai, T.; Lipalov, Y.S. Crystallization of filled nylon 6 III. Non-isothermal crystallization. Colloid Polym. Sci. 1979, 257, 1042–1048. [Google Scholar] [CrossRef]
- Jang, K.-S. Low-density polycarbonate composites with robust hollow glass microspheres by tailorable processing variables. Polym. Test. 2020, 84, 106408. [Google Scholar] [CrossRef]
- Zhang, J.-F.; Sun, X. Mechanical properties and crystallization behavior of poly(lactic acid) blended with dendritic hyperbranched polymer. Polym. Inter. 2004, 53, 716–722. [Google Scholar] [CrossRef]
- Lu, S.; Li, S.; Yu, J.; Guo, D.; Ling, R.; Huang, B. The effect of hyperbranched polymer lubricant as a compatibilizer on the structure and properties of lignin/polypropylene composites. Wood Mater. Sci. Eng. 2013, 8, 159–165. [Google Scholar] [CrossRef]
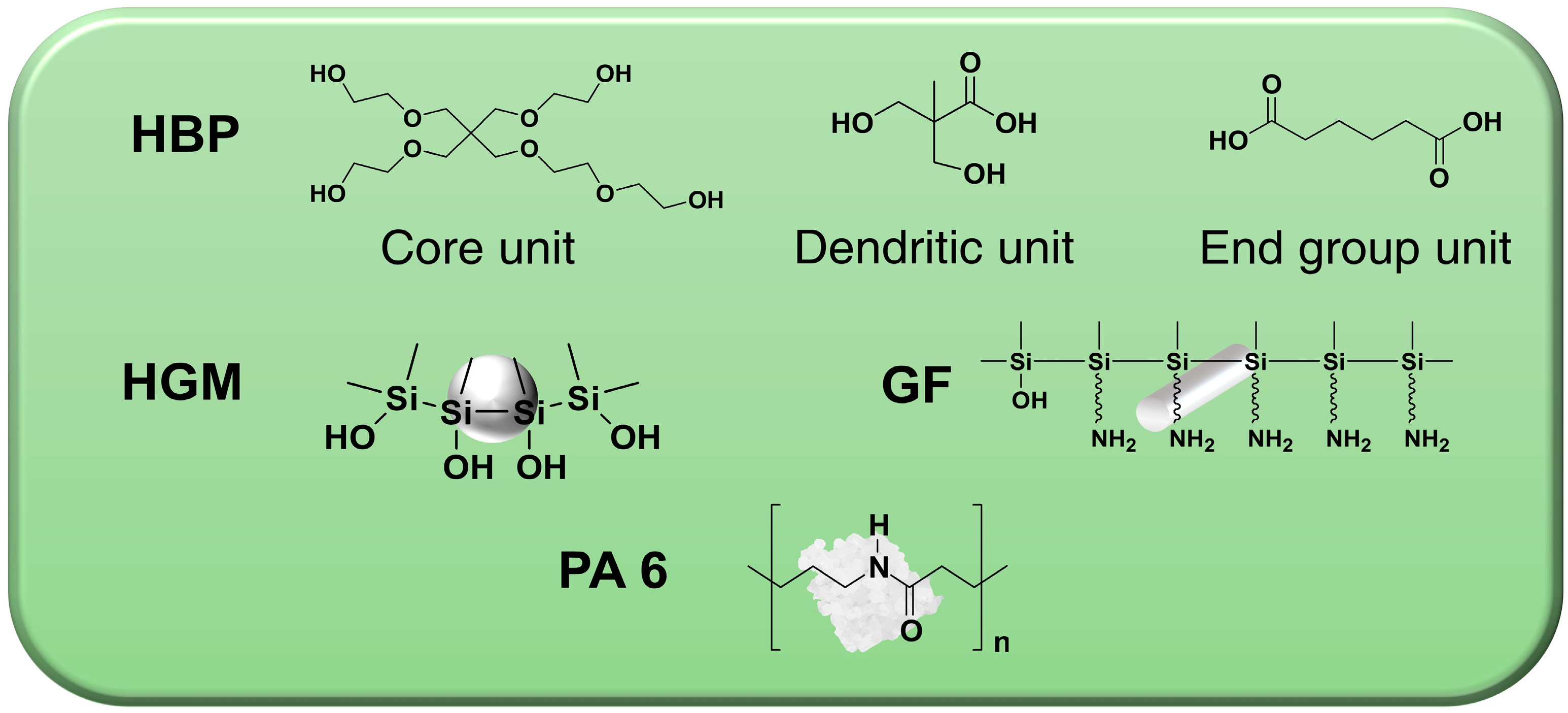

| Sample Label | PA 6 (wt%) | HGM (wt%) | GF (wt%) | HBP (phr a) |
|---|---|---|---|---|
| RSF | 72 | 23 | 5 | - |
| RSF-COOH 0.5 | 71.6 | 23 | 5 | 0.5 |
| RSF-COOH 0.75 | 71.5 | 23 | 5 | 0.75 |
| RSF-COOH 1.0 | 71 | 23 | 5 | 1.0 |
| RSF-COOH 2.0 | 70.6 | 23 | 5 | 2.0 |
| Sample Label | (°C) | (J/g) | (%) | (°C) | (°C) | (J/g) | (J/g) |
|---|---|---|---|---|---|---|---|
| RSF | 221.3 | 34.68 | 20.00 | 190.8 | 195.1 | 40.98 | 56.70 |
| RSF-COOH 0.5 | 220.8 | 35.00 | 20.40 | 191.3 | 195.4 | 41.16 | 57.57 |
| RSF-COOH 1.0 | 221.1 | 38.39 | 22.54 | 191.7 | 195.7 | 43.30 | 61.01 |
| RSF-COOH 2.0 | 221.0 | 37.34 | 21.86 | 192.4 | 196.2 | 42.36 | 59.51 |
| Sample Label | n | t1/2 (min) | Adj. R-Square | ||
|---|---|---|---|---|---|
| RSF | 3.8 | 1.376 | 1.032 | 0.84 | 0.9997 |
| RSF-COOH 0.5 | 4.1 | 1.337 | 1.029 | 0.85 | 0.9997 |
| RSF-COOH 1.0 | 3.8 | 1.633 | 1.050 | 0.80 | 0.9997 |
| RSF-COOH 2.0 | 3.9 | 1.972 | 1.070 | 0.76 | 0.9997 |
| Sample Code | Specific Gravity | Tensile Strength (MPa) | Elongation (%) | Flexural Modulus (MPa) | Flexural Strength (MPa) |
|---|---|---|---|---|---|
| PA 6 | 1.125 | 80.2 2.6 | 13.6 13.8 | 2843 73 | 35.3 0.9 |
| RSF | 0.948 | 58.8 1.2 | 1.4 0.2 | 4032 16 | 50.0 0.2 |
| RSF-COOH 0.5 | 0.933 | 69.9 2.2 | 1.4 0.6 | 4273 26 | 53.0 0.3 |
| RSF-COOH 0.75 | 0.932 | 73.1 1.7 | 1.3 0.3 | 4386 13 | 54.6 0.2 |
| RSF-COOH 1.0 | 0.924 | 72.5 1.5 | 1.8 0.6 | 4424 0 | 54.9 0 |
| RSF-COOH 2.0 | 0.917 | 70.7 1.5 | 1.3 0.2 | 4423 36 | 54.6 0.4 |
| Sample Code | Ash Density a (g/cm3) | Residual HGM Density (g/cm3) | HGM Breakage (vol%) |
|---|---|---|---|
| RSF | 0.5829 | 0.4772 | 9.14 |
| RSF-COOH 0.5 | 0.5590 | 0.4596 | 6.07 |
| RSF-COOH 1.0 | 0.5463 | 0.4504 | 3.73 |
| RSF-COOH 2.0 | 0.5431 | 0.4470 | 2.84 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Lee, J.; Hwang, S.; Park, K.; Hong, S.; Lee, S.; Shim, S.E.; Qian, Y. Simultaneous Effects of Carboxyl Group-Containing Hyperbranched Polymers on Glass Fiber-Reinforced Polyamide 6/Hollow Glass Microsphere Syntactic Foams. Polymers 2022, 14, 1915. https://doi.org/10.3390/polym14091915
Kim J, Lee J, Hwang S, Park K, Hong S, Lee S, Shim SE, Qian Y. Simultaneous Effects of Carboxyl Group-Containing Hyperbranched Polymers on Glass Fiber-Reinforced Polyamide 6/Hollow Glass Microsphere Syntactic Foams. Polymers. 2022; 14(9):1915. https://doi.org/10.3390/polym14091915
Chicago/Turabian StyleKim, Jincheol, Jaewon Lee, Sosan Hwang, Kyungjun Park, Sanghyun Hong, Seojin Lee, Sang Eun Shim, and Yingjie Qian. 2022. "Simultaneous Effects of Carboxyl Group-Containing Hyperbranched Polymers on Glass Fiber-Reinforced Polyamide 6/Hollow Glass Microsphere Syntactic Foams" Polymers 14, no. 9: 1915. https://doi.org/10.3390/polym14091915
APA StyleKim, J., Lee, J., Hwang, S., Park, K., Hong, S., Lee, S., Shim, S. E., & Qian, Y. (2022). Simultaneous Effects of Carboxyl Group-Containing Hyperbranched Polymers on Glass Fiber-Reinforced Polyamide 6/Hollow Glass Microsphere Syntactic Foams. Polymers, 14(9), 1915. https://doi.org/10.3390/polym14091915








