Development of an Immunoassay Method for the Sensitive Detection of Histamine and Tryptamine in Foods Based on a CuO@Au Nanoenzyme Label and Molecularly Imprinted Biomimetic Antibody
Abstract
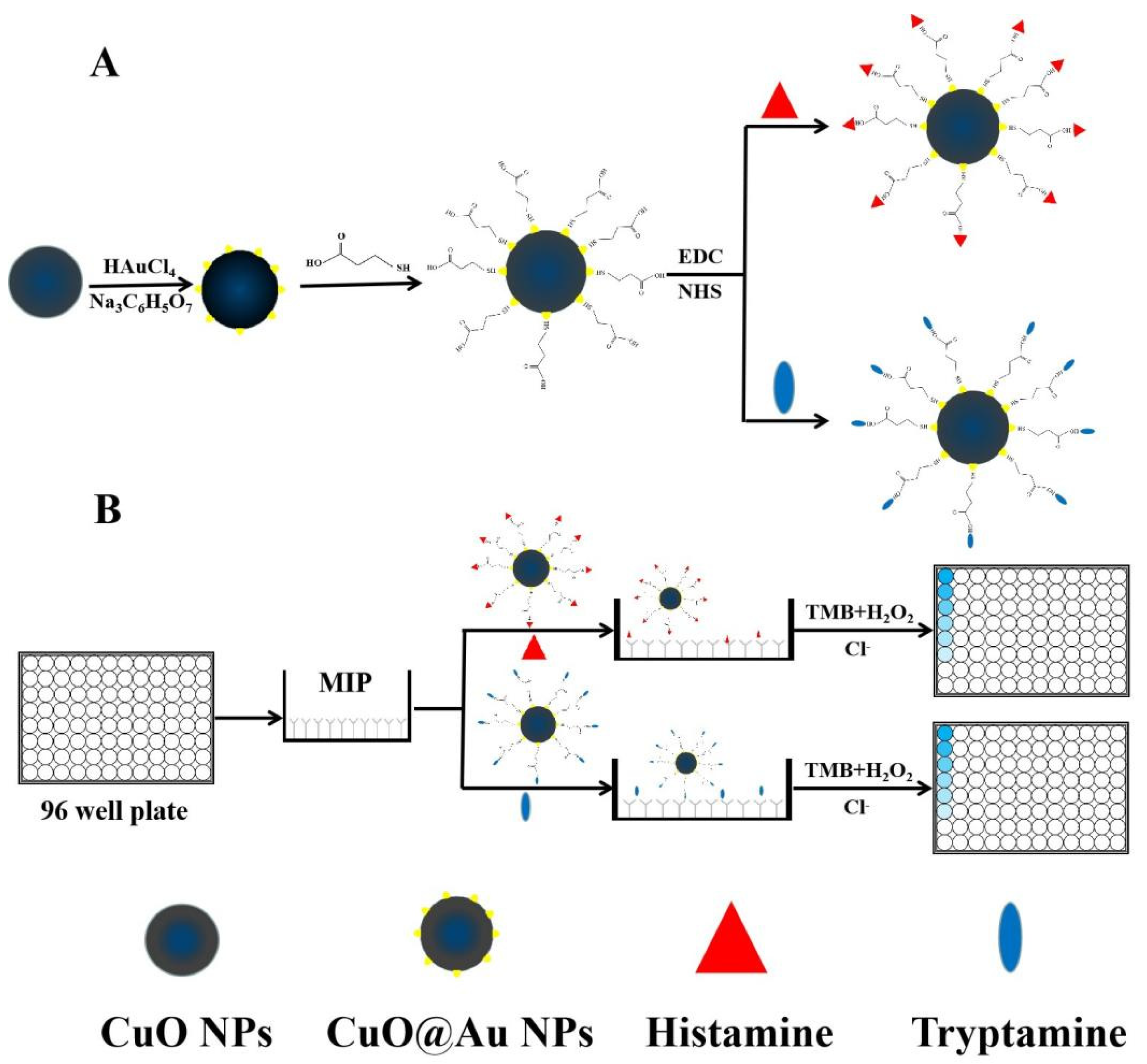
:1. Introduction
2. Results and Discussions
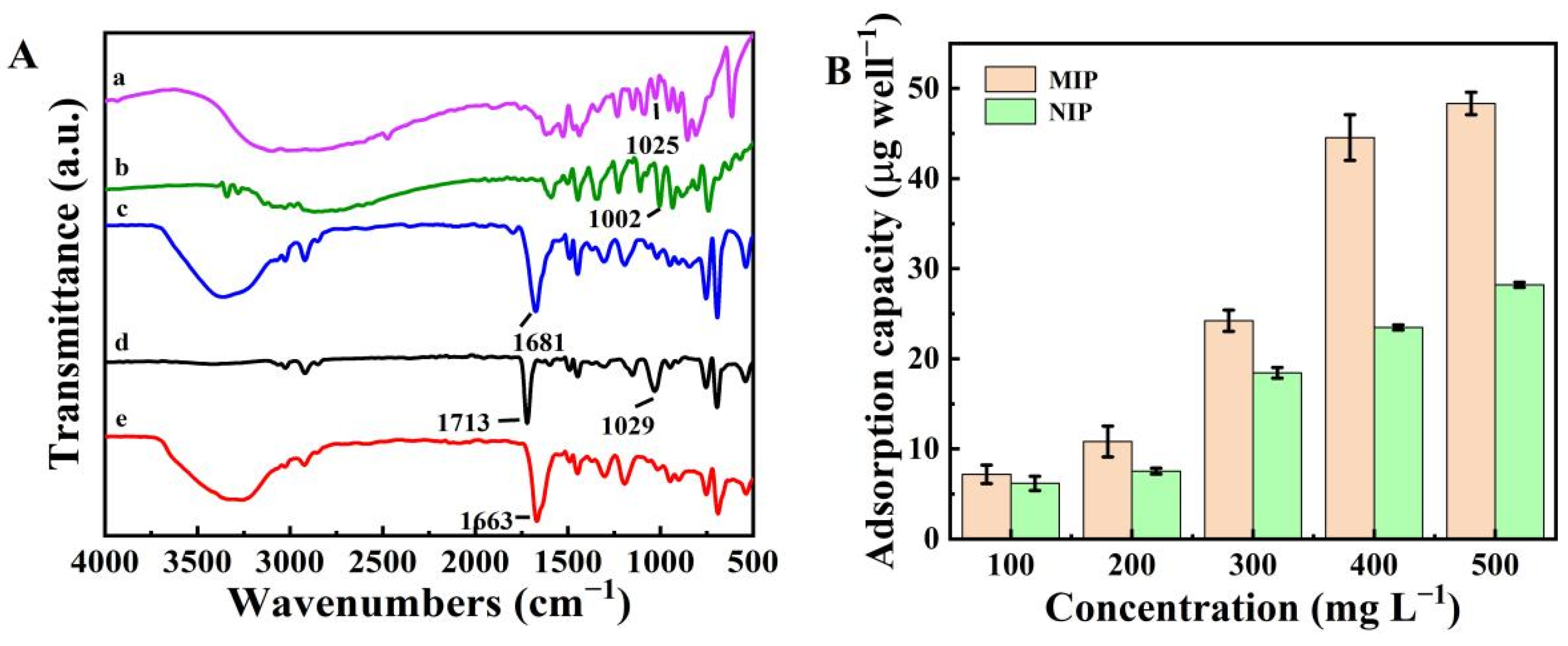
2.1. Characterization of the MIP
2.2. Characterization of CuO@Au NPs
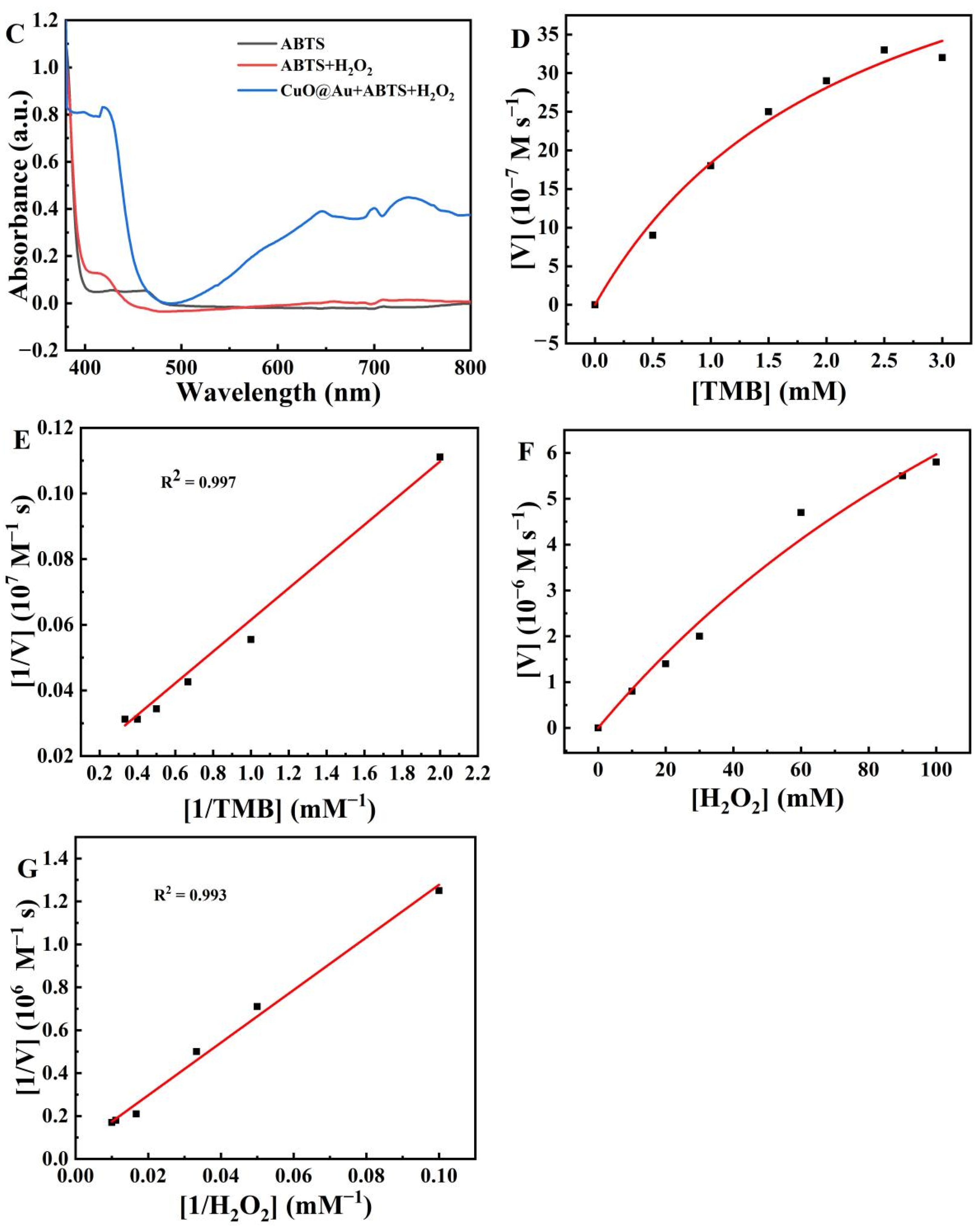
2.3. Evaluation of the Catalytic Ability of the CuO@Au NPs
2.4. Optimization of BELISA Conditions
2.5. Standard Curve of the BELISA Method
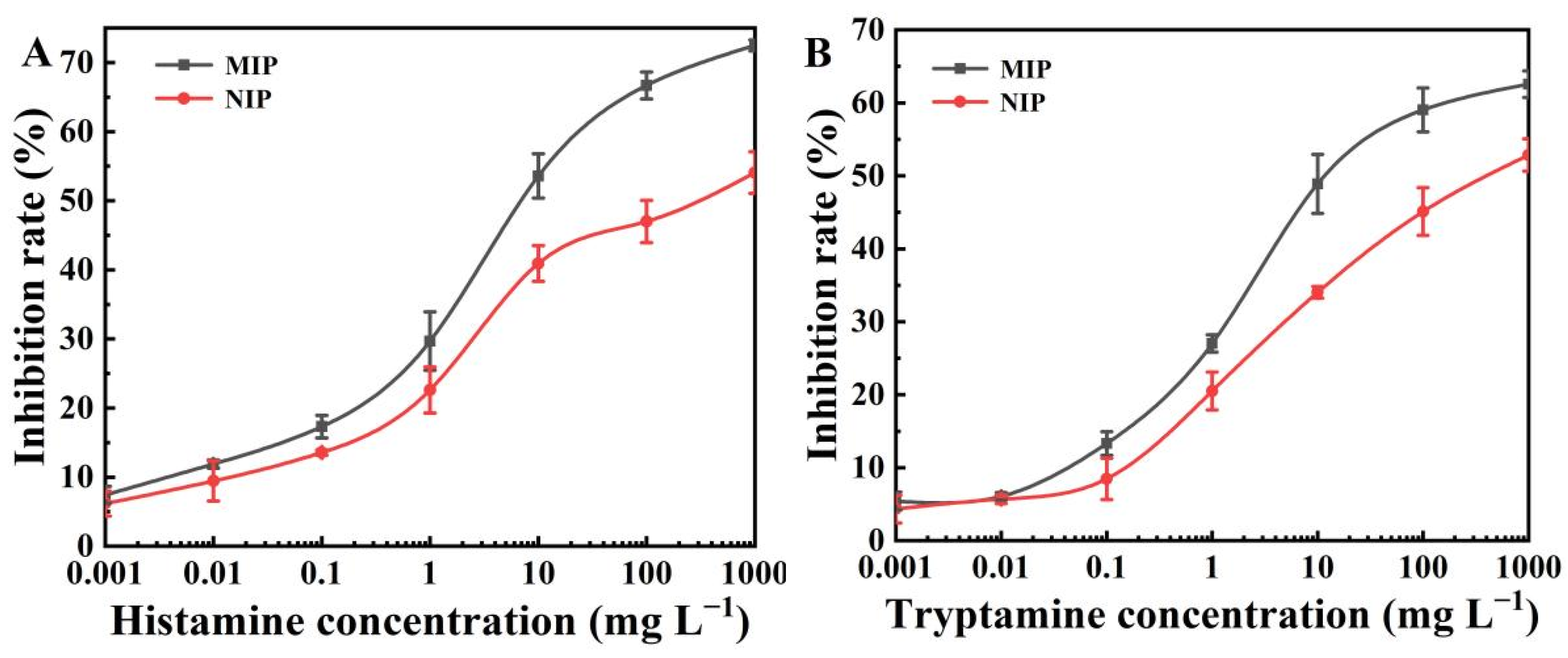
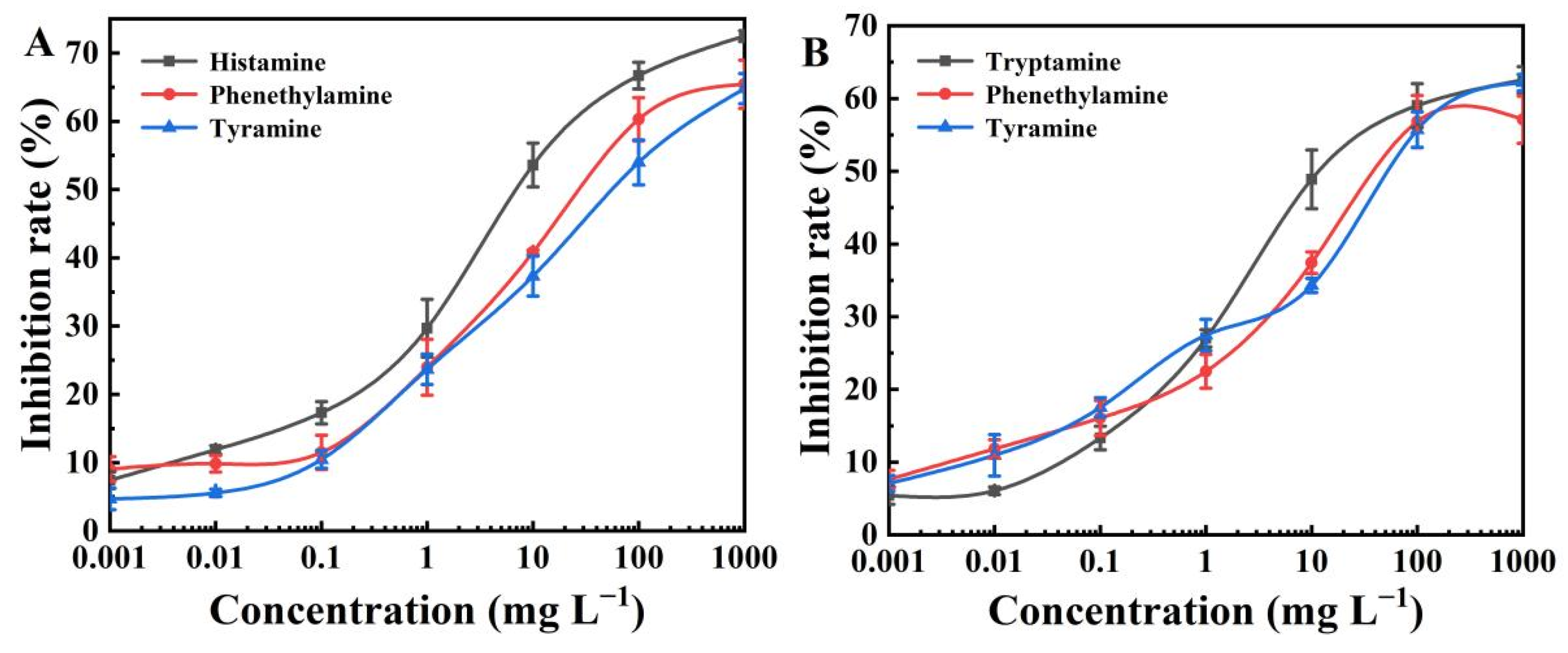
2.6. Selectivity of the BELISA Method
2.7. Accuracy and Applicability of the BELISA Method
2.8. Advantages and Disadvantages of the BELISA Method
3. Material and Methods
3.1. Materials, Chemicals, and Apparatus
3.2. Synthesis of CuO@Au NPs
3.3. Preparation of Nanozyme Conjugates
3.4. Preparation of the MIP Film on the Surface of the 96-Well Plate
3.5. BELISA Procedure
3.6. Sample Preparation
3.7. Statistical Analysis of Data
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leng, P.Q.; Zhao, F.L.; Yin, B.C.; Ye, B.C. A novel, colorimetric method for biogenic amine detection based on arylalkylamine N-acetyltransferase. Chem. Commun. 2015, 51, 8712–8714. [Google Scholar] [CrossRef] [PubMed]
- Đorđević, Đ.; Buchtova, H.; Borkovcova, I. Estimation of amino acids profile and escolar fish consumption risks due to biogenic amines content fluctuations in vacuum skin packaging/VSP during cold storage. LWT-Food Sci. Technol. 2016, 66, 657–663. [Google Scholar] [CrossRef]
- Costa, D.J.E.; Martínez, A.M.; Ribeiro, W.F.; Bichinho, K.M.; Di Nezio, M.S.; Pistonesi, M.F.; Araujo, M.C.U. Determination of tryptamine in foods using square wave adsorptive stripping voltammetry. Talanta 2016, 154, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.J.; Feng, S.H.; Park, C.Y.; Park, K.Y.; Song, J.; Park, J.P.; Chun, H.S.; Park, T.J. Fluorescence detection of histamine based on specific binding bioreceptors and carbon quantum dots. Biosens. Bioelectron. 2020, 167, 112519. [Google Scholar] [CrossRef] [PubMed]
- Gil, R.L.; Amorim, C.G.; Montenegro, M.C.; Araújo, A.N. HPLC-potentiometric method for determination of biogenic amines in alcoholic beverages: A reliable approach for food quality control. Food Chem. 2022, 372, 131288. [Google Scholar] [CrossRef] [PubMed]
- Munir, M.A.; Mackeen, M.M.M.; Heng, L.Y.; Badri, K.H. Study of histamine detection using liquid chromatography and gas chromatography. ASM Sci. J. 2021, 16, 1–9. [Google Scholar] [CrossRef]
- Altieri, I.; Semeraro, A.; Scalise, F.; Calderari, I.; Stacchini, P. European official control of food: Determination of histamine in fish products by a HPLC-UV-DAD method. Food Chem. 2016, 211, 694–699. [Google Scholar] [CrossRef]
- Kivirand, K.; Rinken, T. Biosensors for biogenic amines: The present state of art mini-review. Anal. Lett. 2011, 44, 2821–2833. [Google Scholar] [CrossRef]
- Wu, L.; Li, G.H.; Xu, X.; Zhu, L.; Huang, R.M.; Chen, X.Q. Application of nano-ELISA in food analysis: Recent advances and challenges. TrAC Trends Anal. Chem. 2019, 113, 140–156. [Google Scholar] [CrossRef]
- Maryška, M.; Fojtíková, L.; Jurok, R.; Holubová, B.; Lapčík, O.; Kuchař, M. Use of novel haptens in the production of antibodies for the detection of tryptamines. RSC Adv. 2018, 8, 16243–16250. [Google Scholar] [CrossRef]
- Xie, J.; Tang, M.Q.; Chen, J.; Zhu, Y.H.; Lei, C.B.; He, H.W.; Xu, X.H. A sandwich ELISA-like detection of C-reactive protein in blood by citicoline-bovine serum albumin conjugate and aptamer-functionalized gold nanoparticles nanozyme. Talanta 2020, 217, 121070. [Google Scholar] [CrossRef] [PubMed]
- Ali, G.K.; Omer, K.M. Molecular imprinted polymer combined with aptamer (MIP-aptamer) as a hybrid dual recognition element for bio (chemical) sensing applications. Review. Talanta 2022, 236, 122878. [Google Scholar] [CrossRef] [PubMed]
- Moczko, E.; Díaz, R.; Rivas, B.; García, C.; Pereira, E.; Piletsky, S.; Cáceres, C. Molecularly imprinted nanoparticles assay (MINA) in pseudo ELISA: An alternative to detect and quantify octopamine in water and human urine samples. Polymers 2019, 11, 1497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, J.B.; Zhang, L.; Xu, L.H.; Kong, F.F.; Xu, Z.X. Development of nanozyme-labeled biomimetic immunoassay for determination of sulfadiazine residue in foods. Adv. Polym. Technol. 2020, 2020, 7647580. [Google Scholar] [CrossRef]
- Liu, Q.R.; Tian, J.X.; Jiang, M.D.; Qiao, X.G.; Xu, Z.X. Direct competitive biomimetic immunoassay based on quantum dot label for simultaneous determination of two pesticide residues in fruit and vegetable samples. Food Anal. Methods 2018, 11, 3015–3022. [Google Scholar] [CrossRef]
- Wang, X.F.; Chen, Y.F.; Yu, R.Z.; Wang, R.Q.; Xu, Z.X. A sensitive biomimetic enzyme-linked immunoassay method based on Au@Pt@Au composite nanozyme label and molecularly imprinted biomimetic antibody for histamine detection. Food Agric. Immunol. 2021, 32, 592–605. [Google Scholar] [CrossRef]
- Jin, S.R.; Wu, C.; Ye, Z.Z.; Ying, Y.B. Designed inorganic nanomaterials for intrinsic peroxidase mimics: A review. Sens. Actuators B Chem. 2019, 283, 18–34. [Google Scholar] [CrossRef]
- Karakuş, S.; Baytemir, G.; Özeroğlu, C.; Taşaltın, N. An ultra-sensitive smartphone-integrated digital colorimetric and electrochemical Camellia sinensis polyphenols encapsulated CuO nanoparticles-based ammonia biosensor. Inorg. Chem. Commun. 2022, 143, 109733. [Google Scholar] [CrossRef]
- Velsankar, K.; Suganya, S.; Muthumari, P.; Mohandoss, S.; Sudhahar, S. Ecofriendly green synthesis, characterization and biomedical applications of CuO nanoparticles synthesized using leaf extract of capsicum frutescens. J. Environ. Chem. Eng. 2021, 9, 106299. [Google Scholar]
- Wang, L.; Hou, J.J.; Liu, S.Z.; Carrier, A.J.; Guo, T.; Liang, Q.S.; Oakley, D.; Zhang, X. CuO nanoparticles as haloperoxidase-mimics: Chloride-accelerated heterogeneous Cu-Fenton chemistry for H2O2 and glucose sensing. Sens. Actuators B Chem. 2019, 287, 180–184. [Google Scholar] [CrossRef]
- Tian, S.; Zeng, K.; Yang, A.; Wang, Q.; Yang, M. A copper based enzyme-free fluorescence ELISA for HER2 detection. J. Immunol. Methods 2017, 451, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Qayyum, H.; Amin, S.; Ahmed, W.; Mohamed, T.; Rehman, Z.U.; Hussain, S. Laser-based two-step synthesis of Au-Ag alloy nanoparticles and their application for surface-enhanced Raman spectroscopy (SERS) based detection of rhodamine 6G and urea nitrate. J. Mol. Liq. 2022, 365, 120120. [Google Scholar] [CrossRef]
- Zulfikar, M.A.; Utami, A.R.; Handayani, N.; Wahyuningrum, D.; Setiyanto, H.; Azis, M.Y. Removal of phthalate ester compound from PVC plastic samples using magnetic molecularly imprinted polymer on the surface of superparamagnetic Fe3O4 (Fe3O4@MIPs). Environ. Nanotechnol. Monit. Manag. 2022, 17, 100646. [Google Scholar] [CrossRef]
- Nithiyavathi, R.; Sundaram, S.J.; Anand, G.T.; Kumar, D.R.; Raj, A.D.; Al Farraj, D.A.; Aljowaie, R.M.; AbdelGawwad, M.R.; Samson, Y.; Kaviyarasu, K. Gum mediated synthesis and characterization of CuO nanoparticles towards infectious disease-causing antimicrobial resistance microbial pathogens. J. Infect. Public Health 2021, 14, 1893–1902. [Google Scholar] [CrossRef]
- Li, C.C.; Shuford, K.L.; Park, Q.H.; Cai, W.P.; Li, Y.; Lee, E.J.; Cho, S.O. High-yield synthesis of single-crystalline gold nano-octahedra. Angew. Chem. 2007, 119, 3328–3332. [Google Scholar] [CrossRef]
- Veseli, A.; Vasjari, M.; Arbneshi, T.; Hajrizi, A.; Švorc, Ľ.; Samphao, A.; Kalcher, K. Electrochemical determination of histamine in fish sauce using heterogeneous carbon electrodes modified with rhenium (IV) oxide. Sens. Actuators B Chem. 2016, 228, 774–781. [Google Scholar] [CrossRef]
- Li, Y.F.; Lin, Z.Z.; Hong, C.Y.; Huang, Z.Y. Histamine detection in fish samples based on indirect competitive ELISA method using iron-cobalt co-doped carbon dots labeled histamine antibody. Food Chem. 2021, 345, 128812. [Google Scholar] [CrossRef]
- Chen, C.; Wang, X.M.; Zhang, Y.F.; Li, X.Y.; Gao, H.J.; Waterhouse, G.I.; Qiao, X.G.; Xu, Z.X. A molecularly-imprinted SERS sensor based on a TiO2@Ag substrate for the selective capture and sensitive detection of tryptamine in foods. Food Chem. 2022, 394, 133536. [Google Scholar] [CrossRef]
- Wang, M.J.; Liu, J.T.; Chen, H.M.; Lin, J.J.; Lin, C.H. Comparison of the separation of nine tryptamine standards based on gas chromatography, high performance liquid chromatography and capillary electrophoresis methods. J. Chromatogr. A 2008, 1181, 131–136. [Google Scholar] [CrossRef]
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S.; Yan, X. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar]


| Sample | Original Level (mg L−1) | Added Level (mg L−1) | Found Level (mg L−1 ±SD) | Recovery (%, ±RSD) | |||
|---|---|---|---|---|---|---|---|
| Histamine | Tryptamine | Histamine | Tryptamine | Histamine | Tryptamine | ||
| Liquor | 1.64 | 1.72 | 2 | 3.76 ± 0.07 | 4.02 ± 0.16 | 106.00 ± 3.51 | 115.00 ± 5.35 |
| 5 | 6.50 ± 0.22 | 7.26 ± 0.20 | 97.20 ± 4.58 | 110.80 ± 3.55 | |||
| 10 | 10.63 ± 0.29 | 11.56 ± 0.13 | 89.90 ± 3.20 | 98.40 ± 1.28 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, X.; Chen, Y.; Gao, C.; Sun, Y.; Waterhouse, G.I.N.; Xu, Z. Development of an Immunoassay Method for the Sensitive Detection of Histamine and Tryptamine in Foods Based on a CuO@Au Nanoenzyme Label and Molecularly Imprinted Biomimetic Antibody. Polymers 2023, 15, 21. https://doi.org/10.3390/polym15010021
Peng X, Chen Y, Gao C, Sun Y, Waterhouse GIN, Xu Z. Development of an Immunoassay Method for the Sensitive Detection of Histamine and Tryptamine in Foods Based on a CuO@Au Nanoenzyme Label and Molecularly Imprinted Biomimetic Antibody. Polymers. 2023; 15(1):21. https://doi.org/10.3390/polym15010021
Chicago/Turabian StylePeng, Xinli, Yongfeng Chen, Chunhui Gao, Yufeng Sun, Geoffrey I. N. Waterhouse, and Zhixiang Xu. 2023. "Development of an Immunoassay Method for the Sensitive Detection of Histamine and Tryptamine in Foods Based on a CuO@Au Nanoenzyme Label and Molecularly Imprinted Biomimetic Antibody" Polymers 15, no. 1: 21. https://doi.org/10.3390/polym15010021






