Benzylfentanyl as a Surrogate Template for Fentanyl-Selective Imprinted Polymers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
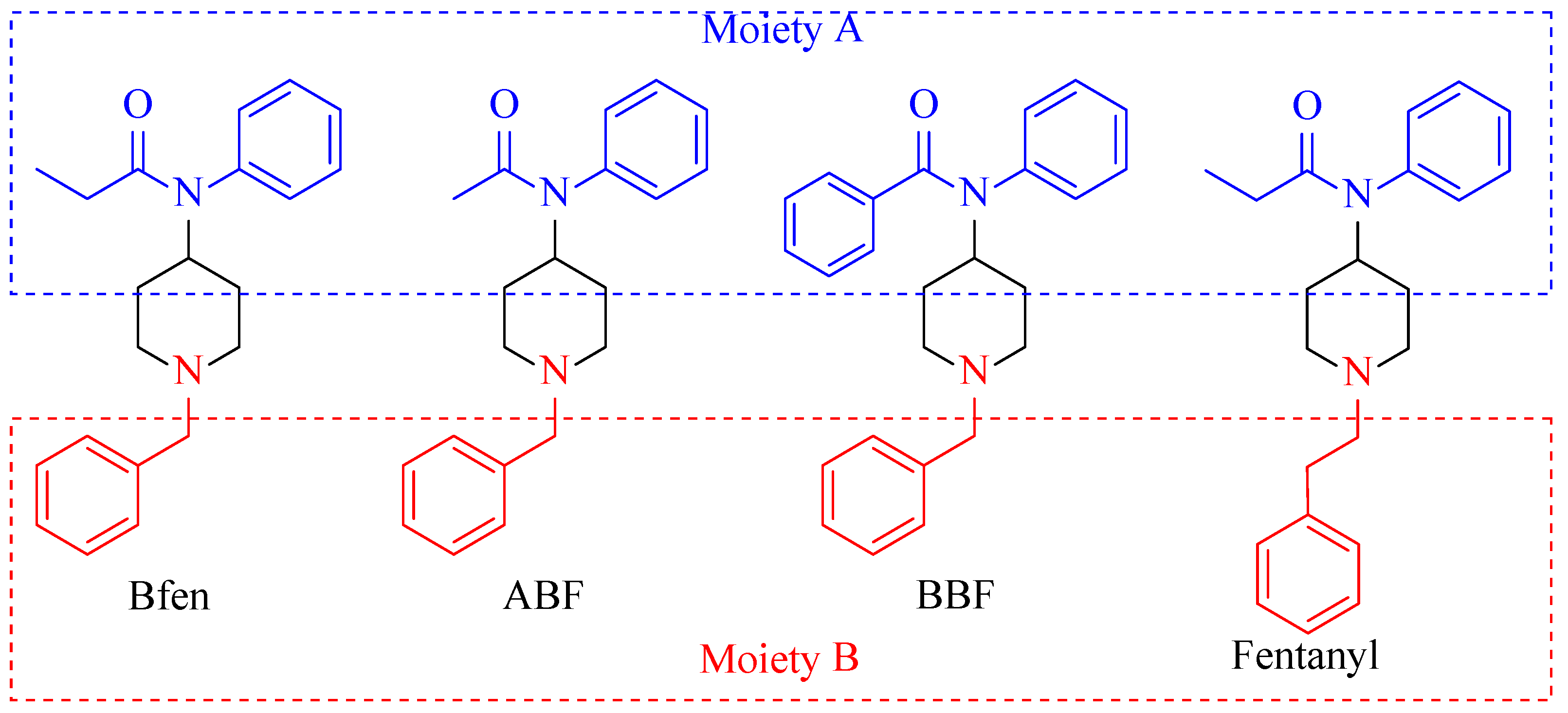
2.2. Synthesis of Benzylfentanyl, Acetyl-Benzylfentanyl, and Benzoyl-Benzylfentanyl
2.2.1. Synthesis of 1-Benzyl-4-(phenylamino) Piperidine (2)
2.2.2. Acylation of 1-Benzyl-4-(phenylamino) Piperidine (2)
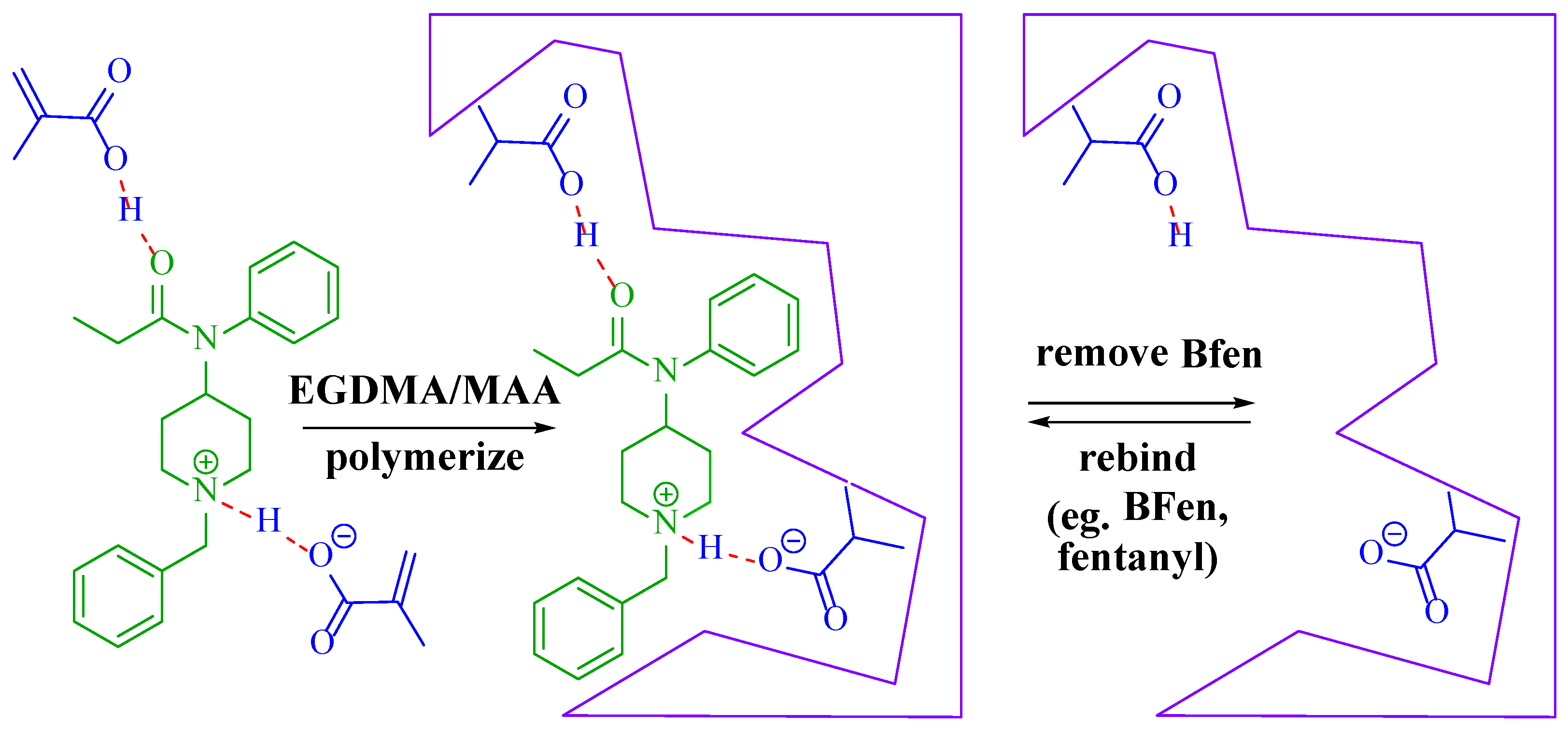
2.3. Polymer Preparation
2.4. Particle Sizing
2.5. Chromatographic Tests
3. Results and Discussion
3.1. Optimization of MIP Parameters
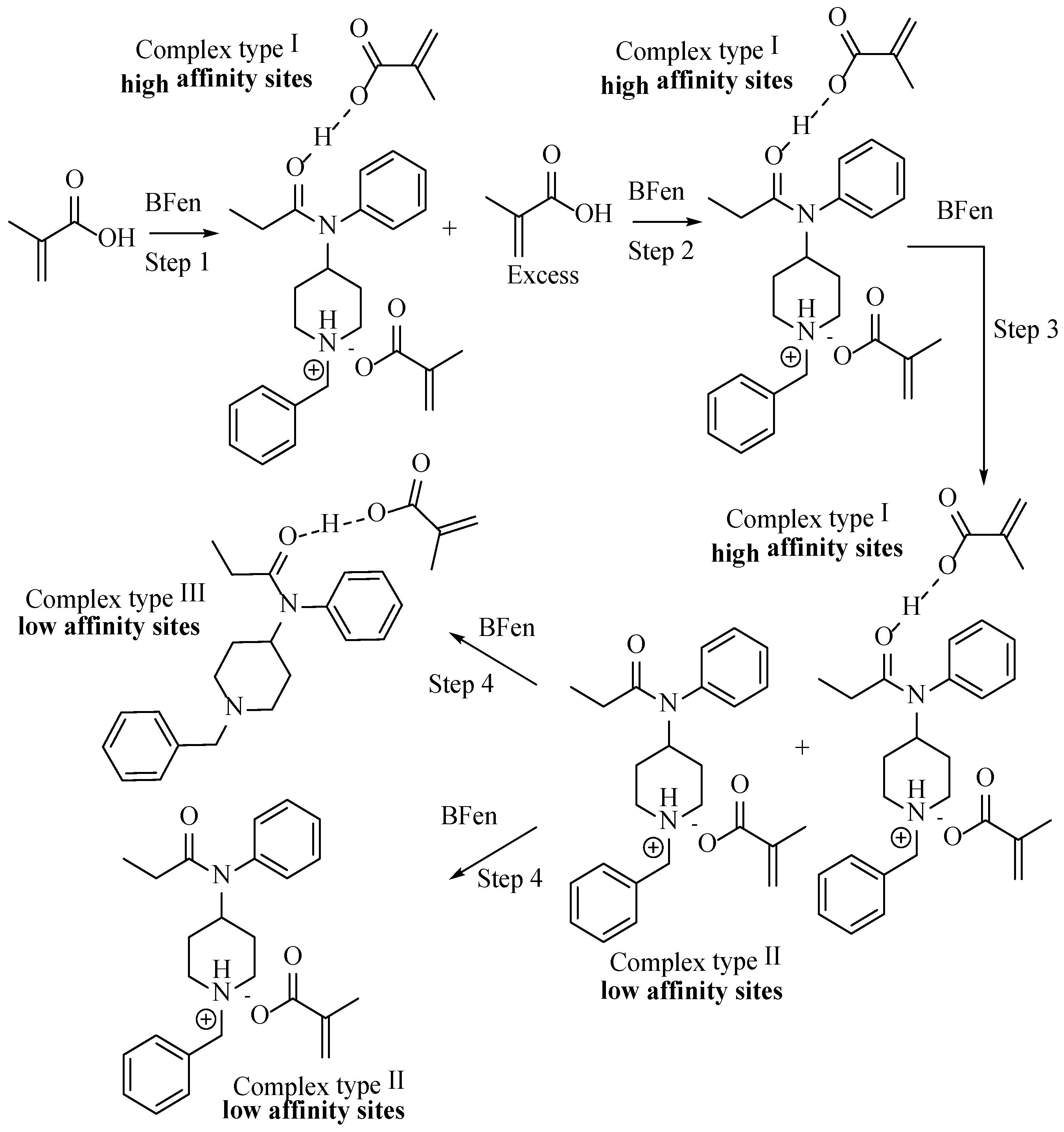
3.1.1. Optimization of Template/Functional Monomer Ratio
3.1.2. Particle Size Optimization
3.1.3. Mobile Phase Composition and Flow Rate
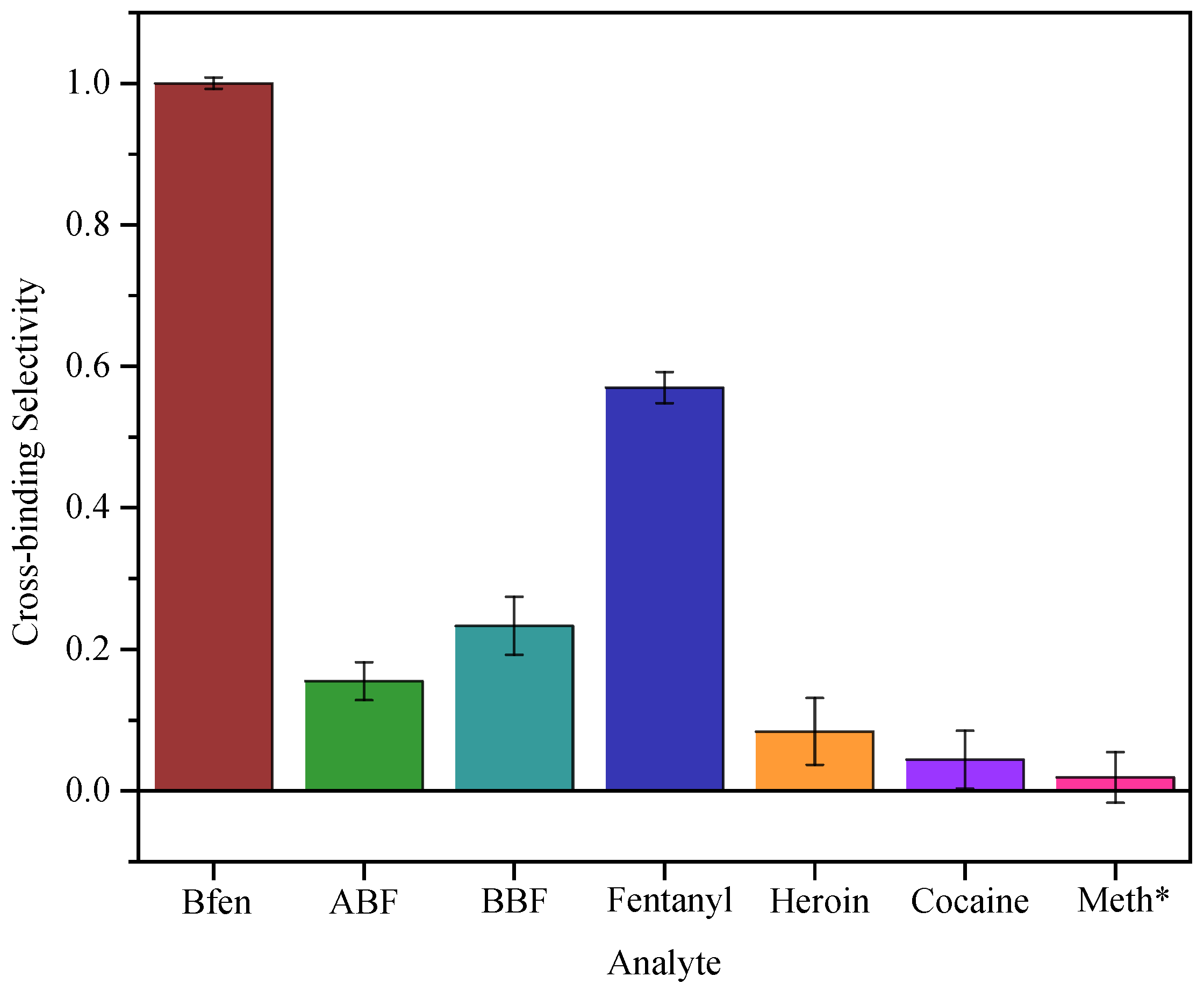
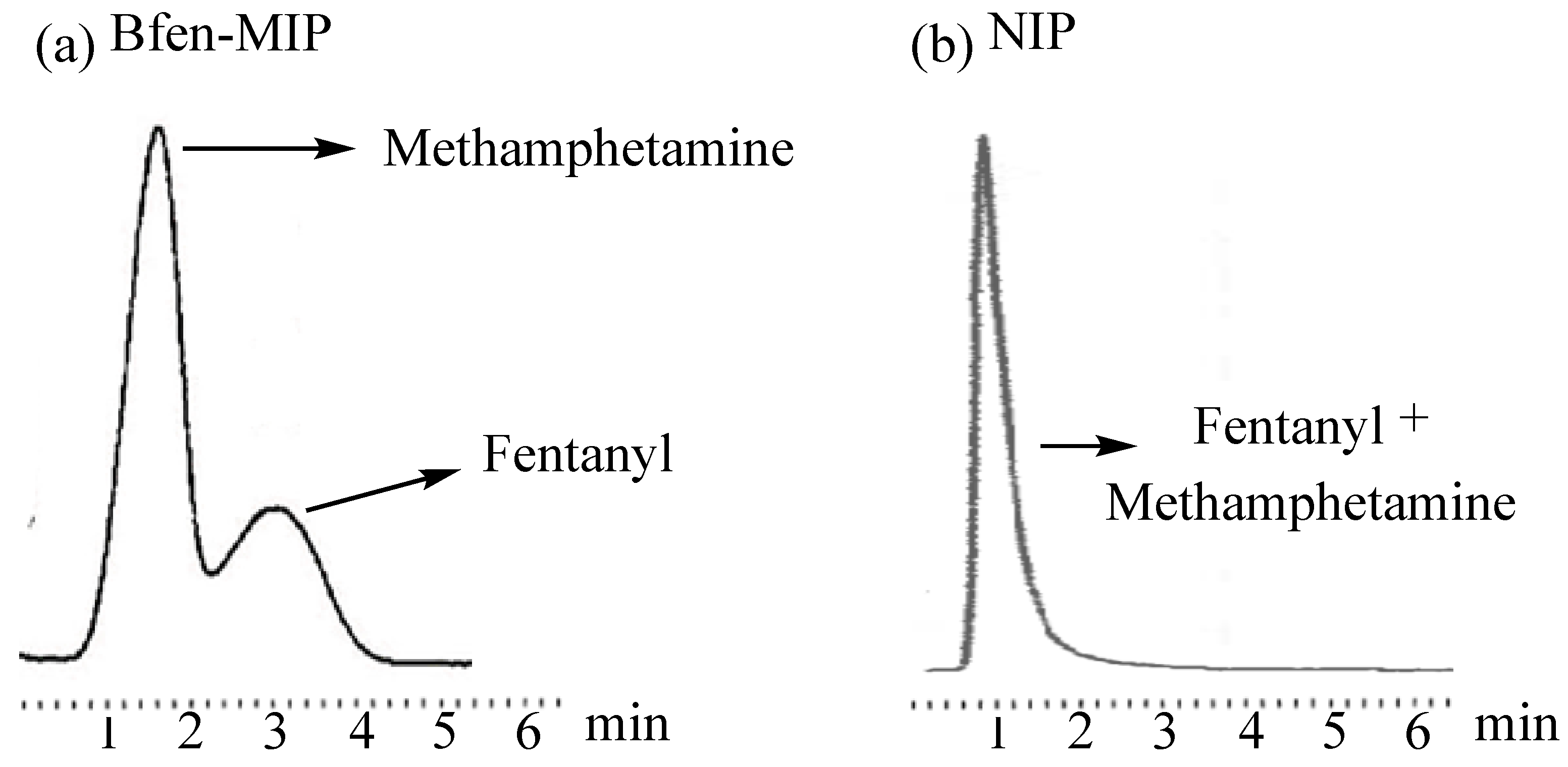
3.2. Evaluation of Selectivity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Drug Enforcement Administration 2019 National Drug Threat Assessment. Available online: https://www.dea.gov/sites/default/files/2020-01/2019-NDTA-final-01-14-2020_Low_Web-DIR-007-20_2019.pdf (accessed on 28 July 2023).
- Sharp Increase in Fake Prescription Pills Containing Fentanyl and Meth. Available online: https://www.dea.gov/alert/sharp-increase-fake-prescription-pills-containing-fentanyl-and-meth (accessed on 28 July 2023).
- Fischer, B. The continuous opioid death crisis in Canada: Changing characteristics and implications for path options forward. Lancet Reg. Health Am. 2023, 19, 100437. [Google Scholar] [CrossRef]
- O’Donnell, J.K.; Halpin, J.; Mattson, C.L.; Goldberger, B.A.; Gladden, R.M. Deaths involving fentanyl, fentanyl analogs, and U-47700—10 states, July–December 2016. Morb. Mortal. Wkly. Rep. 2017, 66, 1197. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Halifax, J.C.; Tangsombatvisit, C.; Yun, C.; Pang, S.; Hooshfar, S.; Wu, A.H.; Lynch, K.L. Development and application of a High-Resolution mass spectrometry method for the detection of fentanyl analogs in urine and serum. J. Mass Spectrom. Adv. Clin. Lab 2022, 26, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, S.R.; Fulton, A.C.; DeGreeff, L.E. Comparative analysis of vapor profiles of fentalogs and illicit fentanyl. Anal. Bioanal. Chem. 2021, 413, 7055–7062. [Google Scholar] [CrossRef]
- Suzuki, S. Studies on fentanyl and related compounds: II. Spectrometric discrimination of five monomethylated fentanyl isomers by gas chromatography/Fourier transform-infrared spectrometry. Forensic Sci. Int. 1989, 43, 15–19. [Google Scholar] [CrossRef]
- Moore, J.M.; Allen, A.C.; Cooper, D.A.; Carr, S.M. Determination of fentanyl and related compounds by capillary gas chromatography with electron capture detection. Anal. Chem. 1986, 58, 1656–1660. [Google Scholar] [CrossRef] [PubMed]
- Lurie, I.; Allen, A. Reversed-phase high-performance liquid chromatographic separation of fentanyl homologues and analogues: II. Variables affecting hydrophobic group contribution. J. Chromatogr. A 1984, 292, 283–294. [Google Scholar] [CrossRef]
- Smith, C.D.; Fulton, A.C.; Romanczyk, M.; Giordano, B.C.; Katilie, C.J.; DeGreeff, L.E. Detection of N-phenylpropanamide vapor from fentanyl materials by secondary electrospray ionization-ion mobility spectrometry (SESI-IMS). Talanta Open 2022, 5, 100114. [Google Scholar] [CrossRef]
- Kang, M.; Lian, R.; Zhang, X.; Li, Y.; Zhang, Y.; Zhang, Y.; Zhang, W.; Ouyang, Z. Rapid and on-site detection of multiple fentanyl compounds by dual-ion trap miniature mass spectrometry system. Talanta 2020, 217, 121057. [Google Scholar] [CrossRef]
- Rittgen, J.; Pütz, M.; Zimmermann, R. Identification of fentanyl derivatives at trace levels with nonaqueous capillary electrophoresis-electrospray-tandem mass spectrometry (MS n, n= 2, 3): Analytical method and forensic applications. Electrophoresis 2012, 33, 1595–1605. [Google Scholar] [CrossRef]
- Sisco, E.; Verkouteren, J.; Staymates, J.; Lawrence, J. Rapid detection of fentanyl, fentanyl analogues, and opioids for on-site or laboratory based drug seizure screening using thermal desorption DART-MS and ion mobility spectrometry. Forensic Chem. 2017, 4, 108–115. [Google Scholar] [CrossRef]
- Lin, Y.; Sun, J.; Xiang, X.; Yu, H.; Shao, B.; He, Y. Surfactants directly participate in the molecular recognition for visual and sensitive detection of fentanyl. Sens. Actuators B Chem. 2022, 354, 131215. [Google Scholar] [CrossRef]
- He, Y.; Liang, J.; Sun, J.; Zhao, X.; Lin, Y.; Shao, B. A Chemically Initiated Electron Exchange Chromogenic Reaction System for Colorimetric Detection of Fentanyl and Norfentanyl. 2022; preprint. [Google Scholar]
- Glasscott, M.W.; Vannoy, K.J.; Fernando, P.A.I.; Kosgei, G.K.; Moores, L.C.; Dick, J.E. Electrochemical sensors for the detection of fentanyl and its analogs: Foundations and recent advances. TrAC Trends Anal. Chem. 2020, 132, 116037. [Google Scholar] [CrossRef]
- Wester, N.; Mynttinen, E.; Etula, J.; Lilius, T.; Kalso, E.; Mikladal, B.F.; Zhang, Q.; Jiang, H.; Sainio, S.; Nordlund, D. Single-walled carbon nanotube network electrodes for the detection of fentanyl citrate. ACS Appl. Nano Mater. 2020, 3, 1203–1212. [Google Scholar] [CrossRef]
- Ruangyuttikarn, W.; Law, M.Y.; Rollins, D.E.; Moody, D.E. Detection of fentanyl and its analogs by enzyme-linked immunosorbent assay. J. Anal. Toxicol. 1990, 14, 160–164. [Google Scholar] [CrossRef]
- Bagheri, H.; Piri-Moghadam, H.; Bayat, P.; Balalaie, S. Application of sol–gel based molecularly imprinted xerogel for on-line capillary microextraction of fentanyl from urine and plasma samples. Anal. Methods 2013, 5, 7096–7101. [Google Scholar] [CrossRef]
- Liu, L.; Grillo, F.; Canfarotta, F.; Whitcombe, M.; Morgan, S.P.; Piletsky, S.; Correia, R.; He, C.; Norris, A.; Korposh, S. Carboxyl-fentanyl detection using optical fibre grating-based sensors functionalised with molecularly imprinted nanoparticles. Biosens. Bioelectron. 2021, 177, 113002. [Google Scholar] [CrossRef]
- Vardanyan, R.S.; Hruby, V.J. Fentanyl-related compounds and derivatives: Current status and future prospects for pharmaceutical applications. Future Med. Chem. 2014, 6, 385–412. [Google Scholar] [CrossRef]
- Henderson, J.L. Designer drugs: Past history and future prospects. J. Forensic Sci. 1988, 33, 569–575. [Google Scholar] [CrossRef]
- Gupta, P.K.; Yadav, S.K.; Bhutia, Y.D.; Singh, P.; Rao, P.; Gujar, N.L.; Ganesan, K.; Bhattacharya, R. Synthesis and comparative bioefficacy of N-(1-phenethyl-4-piperidinyl) propionanilide (fentanyl) and its 1-substituted analogs in Swiss albino mice. Med. Chem. Res. 2013, 22, 3888–3896. [Google Scholar] [CrossRef]
- Qin, Y.; Ni, L.; Shi, J.; Zhu, Z.; Shi, S.; Lam, A.-l.; Magiera, J.; Sekar, S.; Kuo, A.; Smith, M.T. Synthesis and biological evaluation of fentanyl analogues modified at phenyl groups with alkyls. ACS Chem. Neurosci. 2018, 10, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Spivak, D.A. New insight into modeling non-covalently imprinted polymers. J. Am. Chem. Soc. 2003, 125, 11269–11275. [Google Scholar] [CrossRef] [PubMed]
- Simon, R.; Houck, S.; Spivak, D.A. Comparison of particle size and flow rate optimization for chromatography using one-monomer molecularly imprinted polymers versus traditional non-covalent molecularly imprinted polymers. Anal. Chim. Acta 2005, 542, 104–110. [Google Scholar] [CrossRef]
- Cheong, S.H.; Rachkov, A.E.; Park, J.K.; Yano, K.; Karube, I. Synthesis and binding properties of a noncovalent molecularly imprinted testosterone-specific polymer. J. Polym. Sci. Part A Polym. Chem. 1998, 36, 1725–1732. [Google Scholar] [CrossRef]
- Spivak, D.A.; Simon, R.; Campbell, J. Evidence for shape selectivity in non-covalently imprinted polymers. Anal. Chim. Acta 2004, 504, 23–30. [Google Scholar] [CrossRef]
- Hasan, M.R. Polymer Mimetics for Soil Modeling and Detection of Biomarkers. Ph.D. Thesis, Louisiana State University and Agricultural & Mechanical College, Baton Rouge, LA, USA, 2022. [Google Scholar]


| Entry # | Particle Size (µm) | IF |
|---|---|---|
| 1 | 25–37 | 4.26 (±0.03) |
| 2 | 11–24 | 2.48 (±0.04) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasan, M.R.; Spivak, D.A. Benzylfentanyl as a Surrogate Template for Fentanyl-Selective Imprinted Polymers. Polymers 2023, 15, 3669. https://doi.org/10.3390/polym15183669
Hasan MR, Spivak DA. Benzylfentanyl as a Surrogate Template for Fentanyl-Selective Imprinted Polymers. Polymers. 2023; 15(18):3669. https://doi.org/10.3390/polym15183669
Chicago/Turabian StyleHasan, Md. Ragib, and David A. Spivak. 2023. "Benzylfentanyl as a Surrogate Template for Fentanyl-Selective Imprinted Polymers" Polymers 15, no. 18: 3669. https://doi.org/10.3390/polym15183669






