Abstract
Natural products have proven their value as drugs that can be therapeutically beneficial in the treatment of various diseases. However, most natural products have low solubility and poor bioavailability, which pose significant challenges. To solve these issues, several drug nanocarriers have been developed. Among these methods, dendrimers have emerged as vectors for natural products due to their superior advantages, such as a controlled molecular structure, narrow polydispersity index, and the availability of multiple functional groups. This review summarizes current knowledge on the structures of dendrimer-based nanocarriers for natural compounds, with a particular focus on applications in alkaloids and polyphenols. Additionally, it highlights the challenges and perspectives for future development in clinical therapy.
1. Introduction
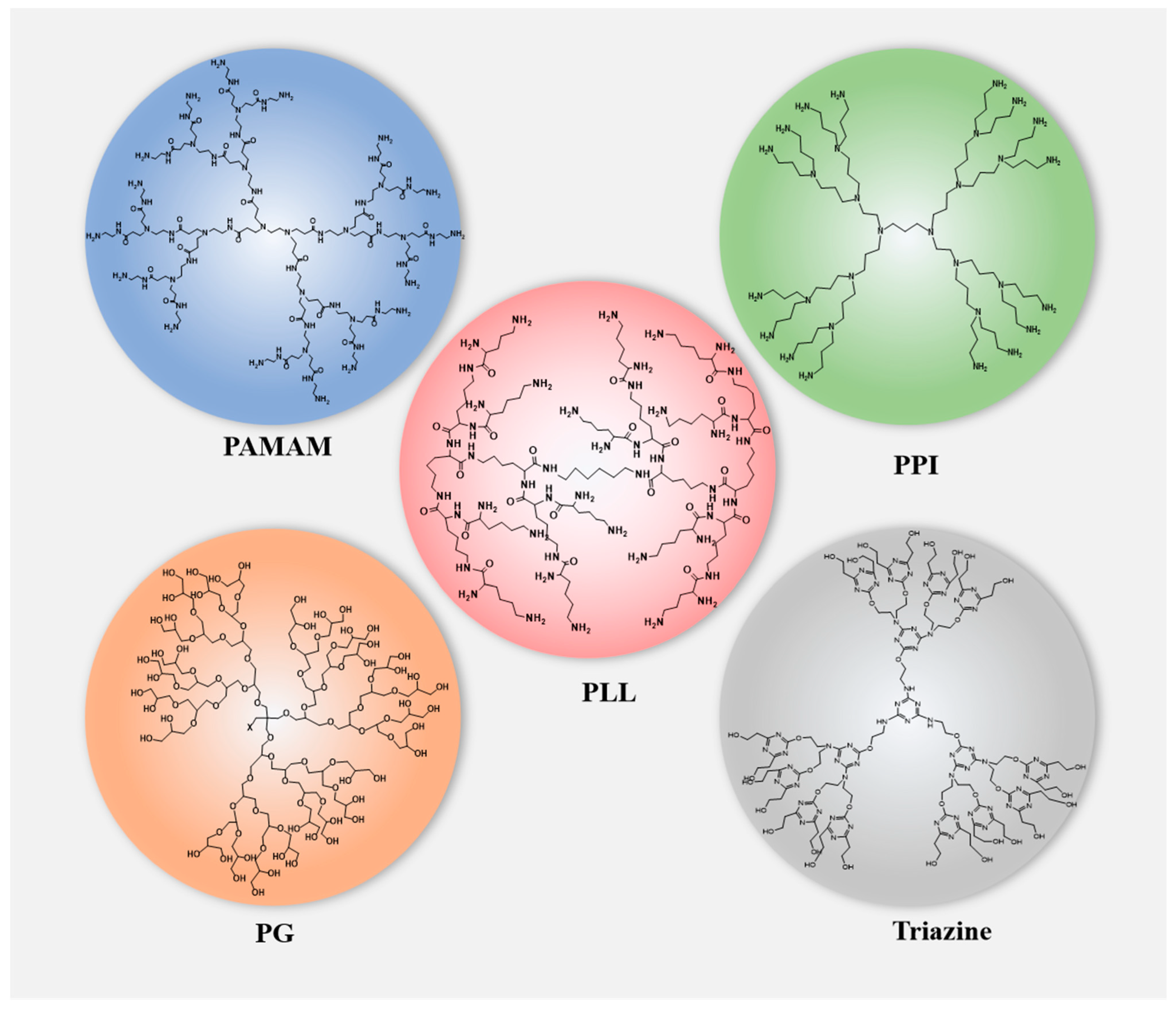
Natural products possess diverse pharmacological activities and low toxicity [1]. However, most drugs have poor bioavailability due to their hydrophobicity, making them unable to develop into ideal dosage forms. The development of dendrimer molecules can largely solve this. Dendrimers are three-dimensional, hyperbranched, monodispersed polymers composed of a central core, branches, and terminal functional groups attached to the branches [2,3]. They are considered ideal carriers for drug delivery due to their rich internal cavities, functionalized surfaces that introduce various chemical groups to achieve high customization, and good biocompatibility [4,5,6]. Compared with traditional polymers, dendrimers have the advantages of high water solubility, polyvalency, biocompatibility, and a precise molecular weight [7]. Various dendrimers have been developed and applied as drug delivery vehicles for natural products (Figure 1) such as polyamidoamine (PAMAM), polylysine (PLL), polypropylene (PPI), and polyglycerol (PG) [8]. The above dendrimers can be used for targeting drug delivery through various methods, such as intravenous, subcutaneous, intraperitoneal injection, oral, and ocular delivery [9].
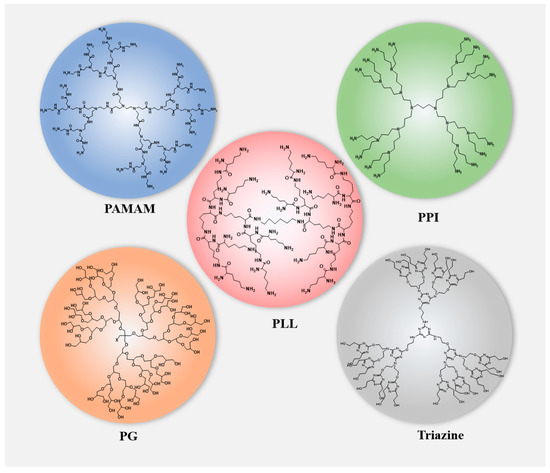
Figure 1.
Structures of dendrimers that are used in drug delivery for natural products.
Drugs are transported by dendrimers in two ways: (1) non-covalent interactions—dendrimers wrap drug molecules within the interior of dendrimers and protect them from being metabolized by the body when they reach their target location, thereby increasing their bioavailability; (2) covalent interactions—drugs are covalently linked to dendritic polymers, and the covalent bonds typically select cleavable functional groups such as esters, amines, and carbamates to effectively control drug release [10,11,12]. This review introduces the current status of dendrimer delivery systems for natural products, especially alkaloids and polyphenolic compounds (Table 1), and provides challenges and perspectives for the clinical development of the above dendrimers in the future.
2. Alkaloids Compound
Alkaloids are nitrogen-containing organic compounds which are divided into quinoline alkaloids, quinolizidine alkaloids, indole alkaloids, etc. [13]. These molecules have a wide range of biological activities, including antitumor, antibacterial, antiviral activities, etc. [14,15,16,17]. However, there are characteristics such as low solubility, instability, and low bioavailability in vivo. Typical bioactive alkaloids include camptothecin, paclitaxel, and berberine.
2.1. Camptothecin
Camptothecin (CPT) is an alkaloid isolated from Camptotheca acuminata which exhibits effective antitumor activity by targeting intracellular topoisomerase I enzyme and which is used to treat different types of cancer [18]. However, the bioavailability of CPT is unsatisfactory. The area under curve (AUC) and half-life of CPT (1 mg/kg, intravenous injection) were 2 × 10−4 mg h/mL and 1.291 h, respectively [19]. Additionally, the low water solubility, poor stability, and certain toxicity to normal cells limit the clinical use of CPT [20].
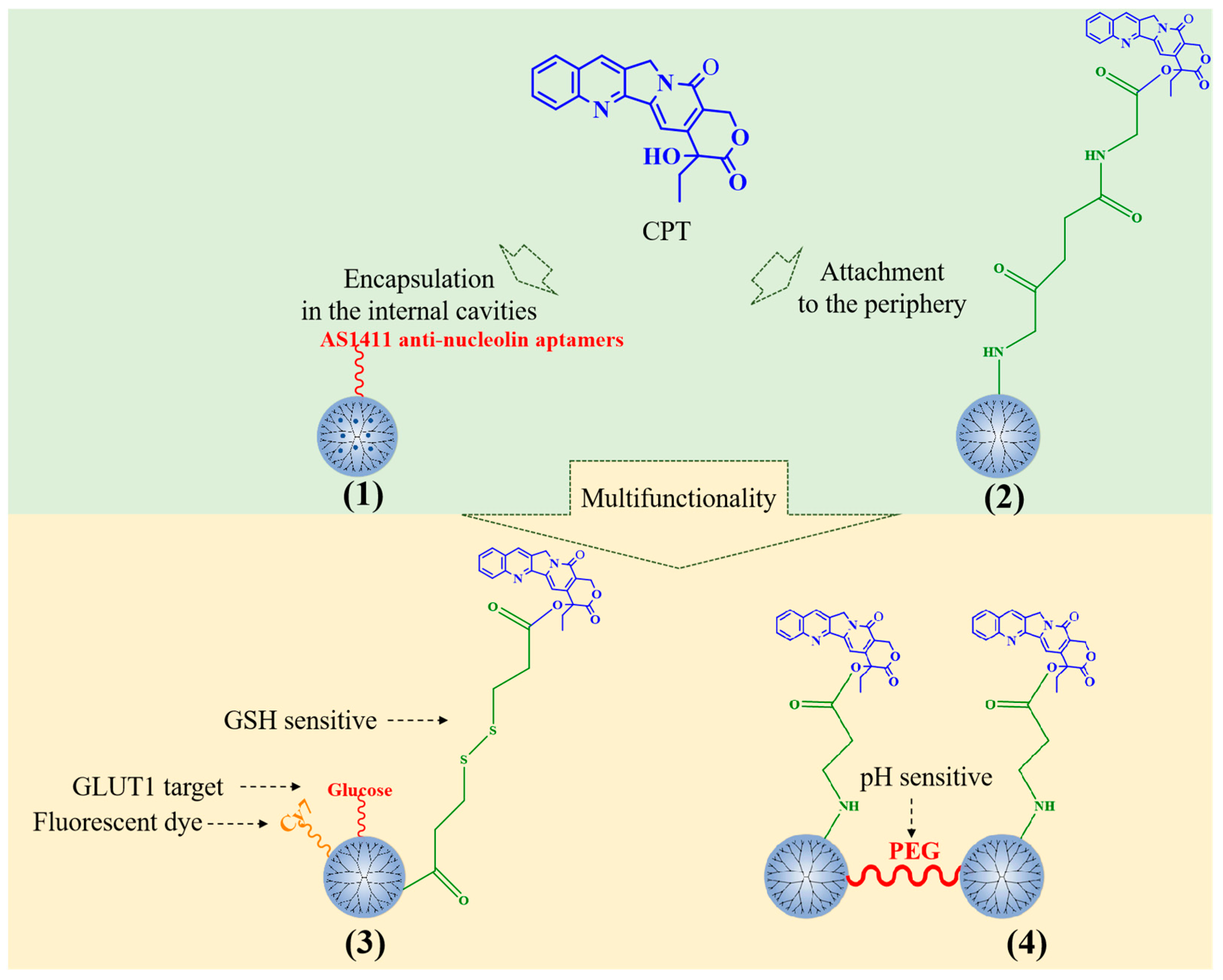
CPT can be encapsulated in the dendriform interior of PAMAM through hydrophobic interaction and can also be chemically combined with PAMAM dendrimers to achieve sustained drug release (Figure 2). Alibolandi et al. prepared a CPT-loaded PEGylated PAMAM G5 dendrimer and functionalized the carrier with AS1411 antinucleolin aptamers (1) to enhance the specific targeting to tumor cells and improve endocytosis [21]. N-acetyl-D-glucosamine has also been applied to increase the targeting of CPT-loaded PAMAM [22].
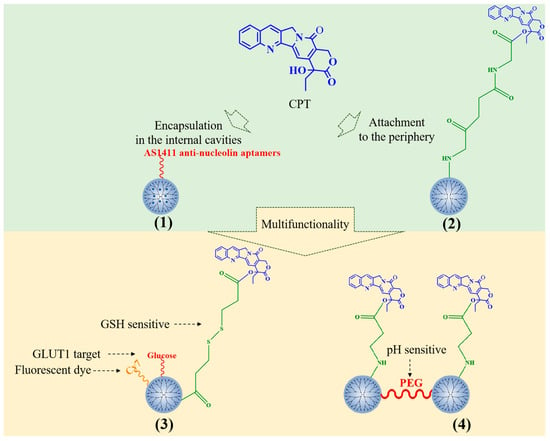
Figure 2.
Typical CPT-loaded PAMAM dendrimers.
CPT has also been covalently conjugated to the surface of PAMAM dendrimers. CPT-conjugated PAMAM G4 dendrimers (2) can inhibit the growth of colorectal cancer cell line HCT-116 and induced nuclear fission [23]. Ma et al. selected a glucose transporter 1 (GLUT1)-specific ligand and glutathione (GSH)-sensitive junction to prepare a glucose-polyethylene glycol(PEG)-PAMAM-S-CPT-Cy7 conjugate to deliver CPT to GLUT1 overexpressed HepG2 liver tumor cells (3) [24]. CPT could be covalently linked to PAMAM G3 dendrimers by an acrylate end group. The conjugates are linked by PEG (4). CPT can be cleaved from dendrimers through the ammonolysis of ester bonds, and the rate of cleavage can be adjusted by pH. The drug delivery system prolongs the release time of the drug, and has injectability, which has a significant tumor inhibitory effect in head and neck cancer [25]. Furthermore, PLL dendrimers can be covalently linked to CPT. Fox et al. [26] prepared two CPT-bonded PLL dendrimers with glycines as the linker. In summary, PAMAM is the main carrier of CPT and has been used to develop several multifunctional nanomedicines.
2.2. Paclitaxel
Paclitaxel (PTX) is a kind of taxane diterpenoid compound used to treat breast cancer, ovarian cancer, pancreatic cancer, lymphatic cancer, etc. [27]. However, PTX is a hydrophobic substance and almost insoluble in water (3 × 10−4 mg/mL) [28]. PTX also has dose-dependent toxicity, including neurotoxicity, cardiovascular toxicity, gastrointestinal toxicity, and cutaneous toxicity [29].
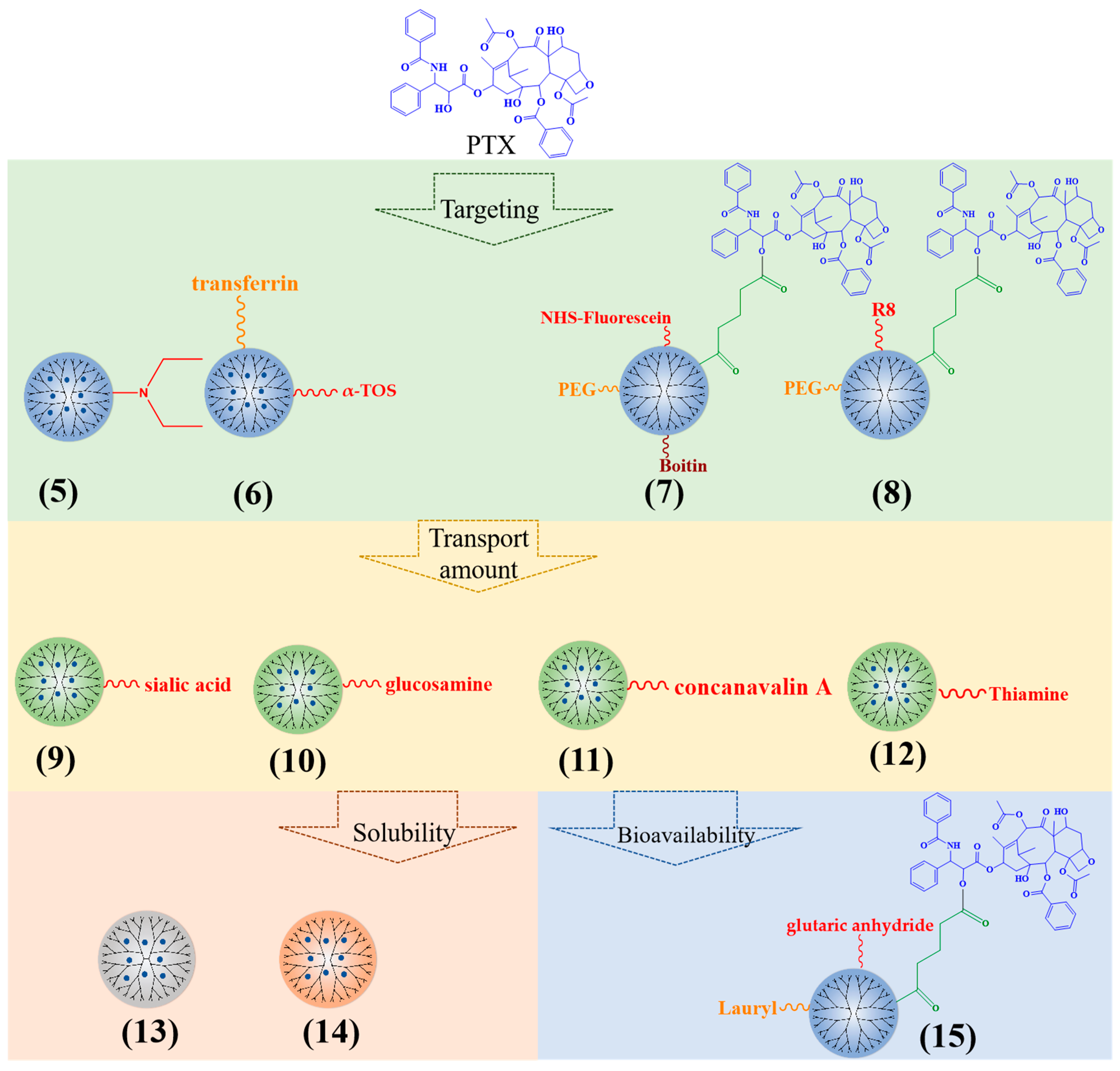
In order to increase the specificity of PTX, the PTX-loaded PAMAM dendrimers are modified by ligands or active substances (Figure 3). The cationic PAMAM G3 dendrimers with dodecyl groups and diethylethanolamine (5) improved the effect of PTX on the inhibition of primary tumor growth and reduced tumor metastasis [30]. α-tocopheryl succinate (α-TOS) (6) can increase the targeting of PTX-PAMAM dendrimers [31]. Biotin (7), omega-3 fatty acid, alkali blue, and octa-arginine (R8) (8) can also be applied to increase the targeting of PTX-loaded PAMAM, subsequently enhancing the potential for cell uptake, cytotoxicity, and apoptosis [32,33,34,35,36].
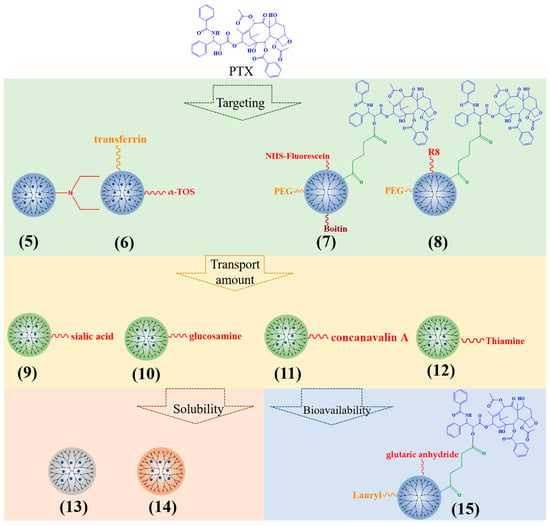
Figure 3.
Typical PTX-loaded dendrimers.
Additionally, sialic acid (9), glucosamine (10), concanavalin A (11), and thiamine (12)-modified PPI dendrimers can significantly increase the transport amount of PTX, resulting in a higher biological distribution of PTX in brain tumors [37,38].
With regard to solubility enhancement, the triazine dendrimers had abundant hydroxyl groups (13) and increased solubility of PTX, from 0.0003 mg/mL to 0.562 mg/mL [39]. PG dendrimers enhanced the solubility of PTX by surrounding the aromatic ring of PTX and some methylene groups (14). The solubility increases with the increase in PG dendrimer generation [40].
The connection between drugs and carriers can affect the bioavailability of drug delivery platforms. Teow et al. selected a glutaric anhydride linker (15) to connect PTX to PAMAM G3 dendrimers; the permeability was 12 times that of PTX alone [41]. When the PTX was linked to PAMAM G4 dendrimers with succinic acid, the cytotoxicity of the conjugate was 10 times higher than that of PTX alone in A2780 human ovarian cancer cells [42]. Using the enzyme-sensitive linker glycylphenylalanylleucoglycine (GFLG) to connect PTX with dendrimers, the conjugate can specifically target cancer cells, causing significant cancer cell toxicity and apoptosis [43,44].
Small interfering RNA (siRNA) is widely used to silence malignant genes and has shown great prospects in cancer treatment [45]. In addition to their use in delivering siRNA, dendrimers have also been utilized to improve the delivery of PTX for the treatment of pancreatic and ovarian cancer. Studies have shown that these dendrimer–PTX complexes exhibit excellent loading capacity and targeting, leading to significant inhibition of tumor growth and cell apoptosis [46,47]. Overall, the use of dendrimers offers promising opportunities to enhance the targeting, transferability, solubility, and bioavailability of PTX for cancer therapy.
2.3. Berberine
Berberine (BBR) is a nitrogen-containing cyclic natural alkaloid [48,49]. Modern pharmacological studies have confirmed that BBR is used to treat various cancers, including breast cancer, lung cancer, liver cancer, ovarian cancer, cervical cancer, prostate cancer, etc. [50,51]. However, BBR has a very low absolute bioavailability (0.68%) [52]. The half-life and AUC of BBR in mice (0.5 mg/mL, intravenous injection) are 6.7 h and 1.424 mg/mL/h, respectively [53].
PAMAM dendrimers could increase the permeability of BBR and prevent its outflow from cancer cells [54]. The PAMAM dendrimer of BBR conjugation could increase the half-life and AUC by 2.1 and 1.7 times, respectively, and reduce the elimination rate constant [53]. BBR was delivered through PAMAM G4 dendrimer conjugation or encapsulation, and both formulations showed hemolytic toxicity of less than 5%, demonstrating safety and biocompatibility. Compared with encapsulation, conjugated PAMAM-BBR has stronger anticancer activity against MCF-7 and MDA-MB-468 breast cancer cells and a slower drug release rate. It is believed that using conjugation to deliver BBRs through dendrimers is better than using encapsulation [53].
3. Polyphenolic Compounds
Polyphenol compounds are widely found in natural plants, vegetables, and fruits. They mainly include phenolic acids, flavonoids, tannins, stilbene, and lignans [55,56]. Polyphenol compounds have antioxidant, anti-inflammatory, and antitumor activities as well as outstanding performance in the treatment of cancer, metabolic diseases, and other aspects. Their specificity, low toxicity, or non-toxicity are key advantages of dendrimers as anticancer agents, especially in the field of oncology [57,58,59,60,61]. However, their low bioavailability in humans limits their clinical applications [62,63,64].
3.1. Quercetin
Quercetin has antioxidant, anti-inflammatory, and antitumor pharmacological activities. The antitumor aspect involves breast cancer, liver cancer, colon cancer, etc. [65,66,67,68,69,70]. However, there are application limitations related to the stability of use in vivo, which limits the use of quercetin [71]. The aqueous solubility of quercetin is 0.000171 mg/mL [72].
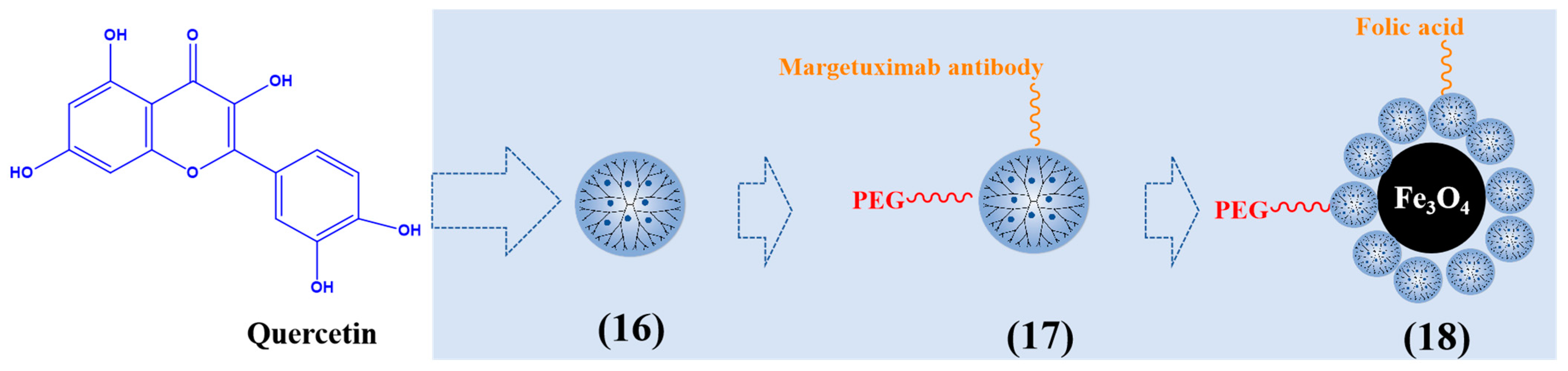
PAMAM dendrimers can promote the solubility of quercetin. The main reason is that the amine groups of PAMAM dendrimers interact with quercetin. Another reason is that PAMAM dendrimers have sufficiently large internal cavities to capture the guest molecules of quercetin, and hydrophobic molecules dissolve in the aqueous medium. Pharmacodynamic studies showed that quercetin PAMAM G3 dendrimer (16) increased the anti-inflammatory activity of quercetin and extended the biological half-life of quercetin [73].
Targeted drug delivery can improve the therapeutic effect of cancer. Margetuximab can attach to the extracellular domain of the HER2 receptor on the surface of breast cancer, promote the effective internalization of nanocarriers, and be used as a targeting agent in drug delivery systems [74]. Yasaman et al. synthesized margetuximab and PEG conjugated PAMAM G4 dendrimer to deliver quercetin to MDA-MB-231 breast cancer cells (17). It has an obvious inhibitory effect on breast cancer cells by enhancing the expression of apoptosis genes bax and caspase 9 [75]. Seyed et al. composed hyperbranched PAMAM-PEG-folic acid-modified Fe3O4-nanoparticle-loaded quercetin (18). Due to the intracellular endocytosis mediated by the folic acid receptor, nanoparticles have significant targeting, selectively enter cancer cells to release drugs, and improve the anticancer effect [76]. Figure 4 describes typical quercetin-loaded dendrimers.
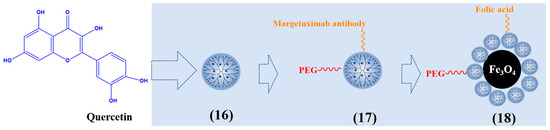
Figure 4.
Typical quercetin-loaded dendrimers.
3.2. Gallic Acid
Gallic acid (GA) is a kind of phenolic acid which shows obvious anticancer activity in various cancers, such as colon cancer, breast cancer, lung cancer, stomach cancer, liver cancer, etc. GA has been recognized as a potential anticancer agent [77,78].
PAMAM G4 dendrimers can increase the bioavailability of GA and its antitumor activity against colon cancer cells HCT116. Its mechanism is to increase the uptake of GA [79]. At the same time, dendrimers target the release of GA and play a synergistic role with anticancer drugs [80]. After oral administration of the conjugation to a CCl4-induced oxidative damage rat model, the conjugation significantly reduced liver marker enzymes and enhanced the protection of GA to affect the liver. It may be related to dendrimers controlling the release rate of GA, allowing GA to be continuously released and maintaining the minimum effective concentration of the drug for a longer period of time, thereby improving the bioavailability [81].
3.3. Resveratrol
Resveratrol (RSV) is a kind of stilbene compound and an obvious antioxidant. RSV’s pharmacological applications include anticancer, diabetes, vascular metabolic diseases, etc. [82,83,84]. Due to its low water solubility, short half-life (9.2 ± 0.6 h), and low bioavailability, the current use of RSV is insufficient [85,86,87].
Due to electrostatic interactions and hydrogen bonding, RSV can be encapsulated by dendritic macromolecules, which can solve the shortcomings of RSV. PAMAM G4 dendrimers can improve the solubility and stability of RSV in water and cream formulations and enhance its penetration in the skin [88]. Sugary maze dendrimer-like glucan (SMDG) increased the solubility of RSV; thus, the antioxidant activity and cell uptake ability of RSV were also significantly enhanced [89]. Octenylsuccinate hydroxypropyl phosphoglycogen (OHPP) dendrimer decreased the crystallinity of RSV and increased the solubility in a dose-dependent manner. This was mainly due to hydrophobicity and hydrogen bonding between OHPP and RSV. Specifically, the high-density distributed hydroxypropyl and octenylsuccinate groups on the surface of OHPP have strong interactions with RSV, providing a favorable environment for this compound [90].
3.4. Silybin
Silybin (SIL) is a natural flavonoid lignan isolated from the plant of Silybin. SIL has antioxidant and anti-inflammatory properties as well as antitumor effects [91]. Unfortunately, it has poor water solubility (0.4 mg/mL). The oral bioavailability of SIL in rats is approximately 0.73% [92,93].
PAMAM dendrimers can significantly improve the water solubility and bioavailability through the electrostatic interaction between the external amines and the phenolic hydroxyl groups of the SIL [94]. PEGylated PAMAM-G4 dendrimers could increase the solubility of insoluble drug SIL. The results showed that the PEGylated system with a 2.0 kDa chain increased the solubility of SIL by 5 times [95]. Shetty et al. found that peptide dendrimers can enhance the skin penetration and deposition of antioxidant SIL [96].
3.5. Curcumin
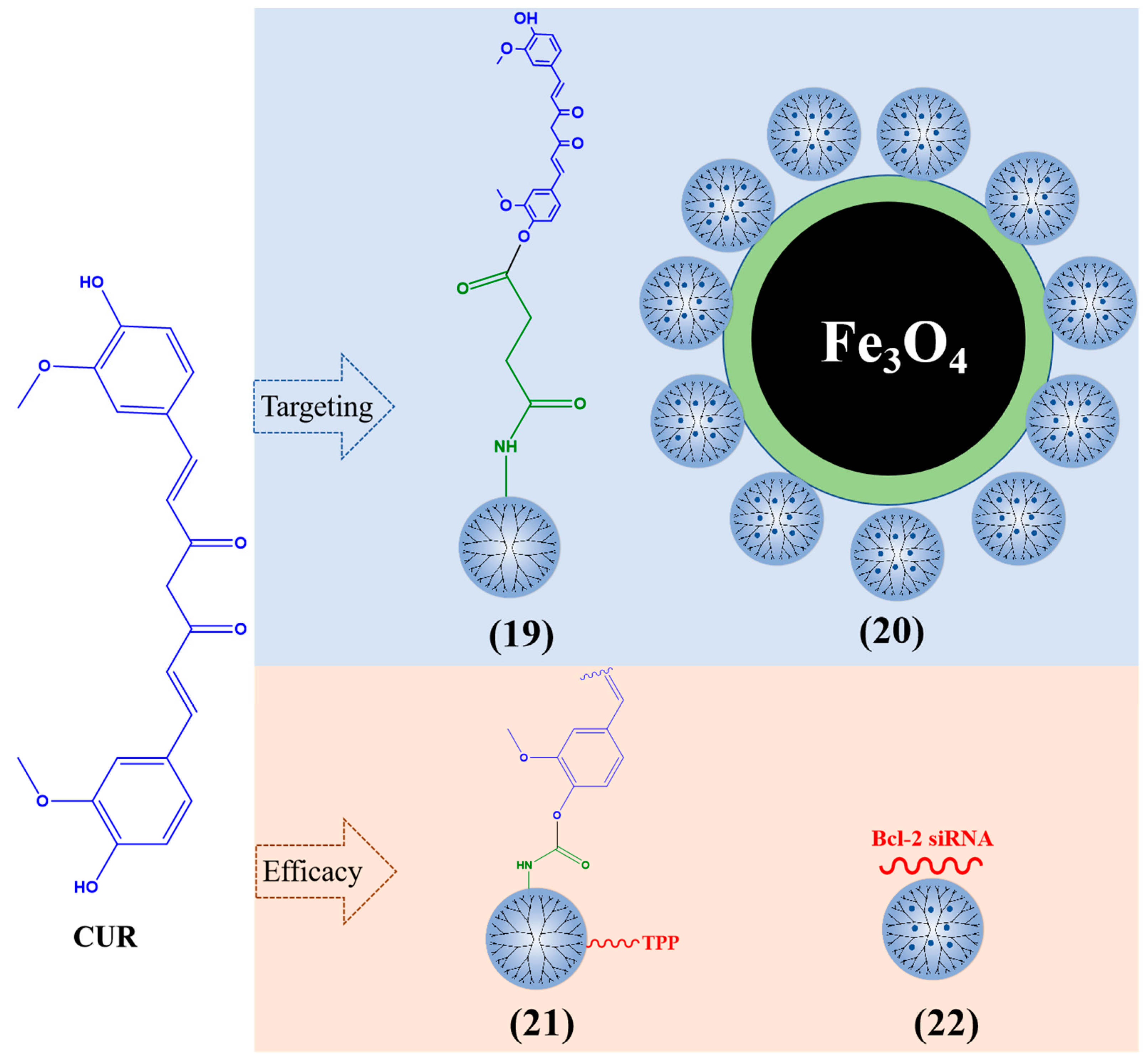
Curcumin (CUR) is a natural polyphenol compound derived from the rhizome of Curcuma longa [97,98,99]. CUR can treat various diseases including cancer, metabolic diseases, orthopedic diseases, cardiovascular diseases, etc. [100,101,102,103]. Several clinical experiments have proved that CUR is non-toxic and safe for the human body [104,105,106]. CUR (2 g/kg) administered orally to healthy humans produced a maximum serum concentration (Cmax) of only 6 × 10−6 mg/mL within 1 h, an AUC0−t of 4 × 10−6 mg/h/mL, and no serum concentration of CUR detected in the serum [107]. CUR has rapid metabolism, a short half-life, and poor bioavailability [108].
PAMAM dendrimers are considered a suitable carrier for encapsulating CUR, which protects CUR and has low toxicity through their surface-located amine groups, resulting in a significant inhibitory effect on cancer cells [109]. When the PAMAM dendrimers are loaded with CUR (Figure 5), the solubility of CUR is 415 times that of free Cur [110]. PAMAM G3 dendrimer-CUR (19) enhanced the toxicity of CUR on different types of cancer cells (MDA-MB-231 breast cancer cells, U-251 human malignant glioma, head and neck squamous cell carcinoma cells, etc.) [111]. PAMAM G5 dendrimers with acetyl terminal groups increased the solubility of CUR by 200 times and promoted the production of reactive oxygen species (ROS) in human lung adenocarcinoma A549 cells [112]. CUR was encapsulated by dendrimers (10% amine and 90% hydroxy) and can reduce the cell activity of three glioblastoma cell lines (mouse GL261, rat F98, and human U87). By decreasing the amount of amine on the surface of PAMAM dendrimers, the effective utilization rate of CUR can be improved [113]. Nosrati et al. modified PAMAM G5 dendrimers with Fe3O4 nanoparticles and loaded CUR on the surface of the nanocarrier (20). The inhibitory effect of this composite on MCF7 human breast cancer cells was stronger than that of free CUR [114].
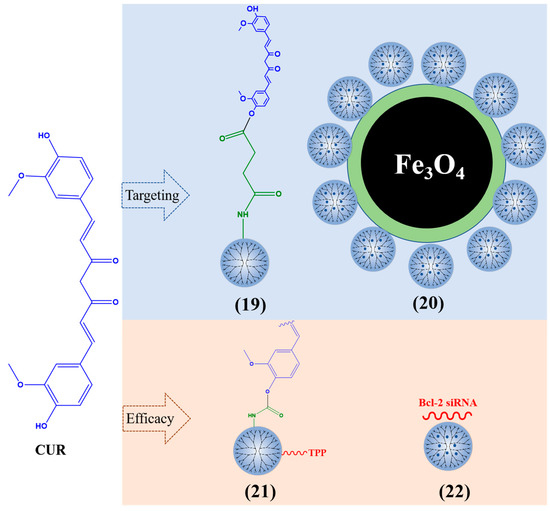
Figure 5.
Typical CUR-loaded dendrimers.
Mitochondrial function is crucial for the occurrence and development of cancer cells [115]. Kianamiri et al. prepared a CUR mitochondrial delivery system using triphenylphosphonium (TPP)–PAMAM G4 dendrimer conjugation CUR (21) and found that CUR co-located with the mitochondria of cancer cells, inducing apoptosis of liver cancer cells without affecting normal cells. It can resist tumors by inducing ROS and lipid peroxidation, thereby activating the signaling pathway of apoptosis [116]. CUR–PAMAM G4 dendrimers grafted Bcl-2 siRNA onto amine groups, These nanoparticles (22) were delivered to human cervical cancer (HeLa) cells and increased the solubility and stability of CUR [117].
In addition, CUR can improve osteoporosis by acting on multiple steps of osteoclast activation and differentiation [118,119]. Yang studied the use of hexachlorocyclotriphosphazene (HCCP) as a linker to couple CUR and PAMAM to make nanoparticles, and the loading capacity of CUR could reach 27.2%. This nanoparticle effectively inhibited the differentiation of osteoclasts in a dose-dependent manner and promoted osteogenesis [120].
3.6. Puerarin
Puerarin (PUE) is an isoflavone compound extracted from pueraria lobata which has been widely used in the treatment of eye diseases, but there is a problem of low bioavailability. PAMAM dendrimers could interact with PUE through a weak hydrogen bond. The slow release of PUE from the complex results in prolonged ocular retention time, thereby increasing the bioavailability of PUE [121], in Table 1. Liu et al. prepared PUE liposomes coated with PAMAM dendrimers, and complex can improve the corneal permeability of rabbit eyes [122]. The pharmacokinetic parameters Cmax, AUC, and elimination half-life of PUE-PAMAM G3 dendrimer complex after ocular infusion were 1.3, 2, and 2.7 times higher than those of the PUE solution, respectively [123]. Dendrimers can also improve the oral bioavailability of PUE. The main reason is that the electrostatic interaction between the amine groups on the surface of the PAMAM dendrimers and the phenolic hydroxy groups on PUE increases the solubility of PUE. Additionally, higher generations of PAMAM dendrimers are more likely to interact with the cornea than lower generations [124,125], and the solubility of the whole generation of PAMAM dendrimers (G2/G3) is much stronger than that of the half-generation (G1.5/G2.5) [126].

Table 1.
Characteristics of dendrimers for different applications.
4. Conclusions
The most ideal characteristics for the development of natural product pharmaceuticals are high stability, good bioavailability, and specific targeting. Dendrimers can be used in promising strategies due to the size and structure of nanospheres, high water solubility, and multivalent surface properties, which can effectively deliver drugs through encapsu-lation or covalent methods.
This review found that the most commonly used dendrimers for natural products are the relatively mature PAMAM dendrimers, followed by PPI dendrimers, PPL dendrimers, PG dendrimers, and triazine dendrimers, which are used to treat various cancers (breast cancer, glioma, lung cancer, cervical cancer, ovarian cancer, pancreatic cancer, etc.), eye diseases, and osteoporosis. The above dendritic macromolecules have active groups such as amino and hydroxyl groups, which are suitable for drug covalent binding and physical adsorption and also provide rich modification sites for targeted therapy. The linkers between drugs and carriers can also affect the bioavailability of drug delivery platforms, such as GFLG, modified Fe3O4, succinic acid-glycine, and HCCP linkers, to achieve a pH response and enzyme-triggered transcytosis. In order to increase the specific targeting of natural product delivery platforms, researchers have developed various conjugated ligands and active substances to co-deliver drugs such as siRNA, N-acetyl-D-glucosamine, α-TOS, folic acid, Biotin, AS1411 antinucleolin aptamers, alkali blue, sialic acid, glucosa-mine, concanavalin A, thiamine, R8, transferrin, etc. Thus, dendrimers have been used as a platform for natural products and multiple ligands. However, the dendrimers currently used in natural products have a single structure, often only amino groups or hydroxyl groups, and there is no mixed-carrier matrix with multiple functional groups. Although these single-structured dendrimers are currently used for the delivery of some natural products, we need to specifically design and synthesize dendrimers with amino, carboxyl, and hydroxyl groups based on different drug solubility and targeting requirements. For example, the solubility and cell internalization of PEGylated dendrimers is increased by the change of the ratio between surface amino and hydroxyl groups. Thus, these multifunctional drug delivery systems will be able to better utilize the pharmacological effects of natural products.
Another limitation of dendrimers in the application of natural products is that there are currently only a few natural compounds used. This may be one reason why dendritic macromolecule-modified natural product complexes have not yet been used in clinical practice. Natural products have low toxicity and good safety, and have unique advantages in many chronic diseases, such as diabetes, obesity, hypertension, digestive diseases, etc. The natural products modified by dendritic macromolecules may provide better therapeutic effects in the treatment of these diseases. In addition, current research on natural products loaded with dendrimers mainly focuses on their pharmacological effects, and further exploration of their pharmacological mechanisms is needed.
In summary, dendrimers can effectively overcome the drawbacks of low water solubility, low bioavailability, and poor targeting of natural products. In order to develop more perfect drug delivery dendrimers for natural products in the future, it is necessary to design and synthesize multifunctional surfaces of dendrimers, select suitable ligands, and couple specific targeted substances to construct a drug delivery system to meet the needs of natural products with different structures and the targeted treatments of different diseases.
Author Contributions
Conceptualization, N.W.; methodology, H.A.; investigation, F.W. and P.X.; formal analysis, X.D.; writing—original draft preparation, H.A.; writing—review and editing, N.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Zhejiang Provincial Natural Science Foundation of China (LY21H280001, 2021C03047), National Natural Science Foundation of China (81973447), China Postdoctoral Science Foundation (2020M681364), and Zhejiang Provincial Medicine Foundation (2021ZQ018, 2022ZX002, 2021ZYY28, GZY-ZJ-KJ-23006).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
α-tocopheryl succinate (α-TOS), berberine (BBR), camptothecin (CPT), curcumin (CUR), dimethyl sulfoxide (DMSO), encapsulation efficiency% (EE), gallic acid (GA), glucose transporter 1 (GLUT1), glutathione (GSH), glycylphenylalanylleucoglycine (GFLG), hexachlorocyclotriphosphazene (HCCP), loading content% (LC), maximum serum concentration (Cmax), octa-arginine (R8), octenylsuccinate hydroxypropyl phosphoglycogen (OHPP), paclitaxel (PTX), polyamidoamine (PAMAM), polyethylene glycol (PEG), polyglycerol (PG), polylysine (PLL), polypropylene (PPI), puerarin (PUE), reactive oxygen species (ROS), resveratrol (RSV), silybin (SIL), small interfering RNA (siRNA), sugary maze dendrimer-like glucan (SMDG), triphenylphosphonium (TPP).
References
- Razavi, M.S.; Ebrahimnejad, P.; Fatahi, Y.; D’Emanuele, A.; Dinarvand, R. Recent Developments of Nanostructures for the Ocular Delivery of Natural Compounds. Front. Chem. 2022, 10, 850757. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.; Veiga, F.; Figueiras, A. Dendrimers as Pharmaceutical Excipients: Synthesis, Properties, Toxicity and Biomedical Applications. Materials 2019, 13, 65. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Alvarez, F.; Martinez-Garcia, M. Click Reaction in the Synthesis of Dendrimer Drug-delivery Systems. Curr. Med. Chem. 2022, 29, 3445–3470. [Google Scholar] [CrossRef]
- Mignani, S.; Shi, X.; Guidolin, K.; Zheng, G.; Karpus, A.; Majoral, J.P. Clinical diagonal translation of nanoparticles: Case studies in dendrimer nanomedicine. J. Control. Release 2021, 337, 356–370. [Google Scholar] [CrossRef]
- Li, H.; Zha, S.; Li, H.; Liu, H.; Wong, K.L.; All, A.H. Polymeric Dendrimers as Nanocarrier Vectors for Neurotheranostics. Small 2022, 18, e2203629. [Google Scholar] [CrossRef] [PubMed]
- Kesharwani, P.; Jain, K.; Jain, N.K. Dendrimer as nanocarrier for drug delivery. Prog. Polym. Sci. 2014, 39, 268–307. [Google Scholar] [CrossRef]
- Wang, J.; Li, B.; Qiu, L.; Qiao, X.; Yang, H. Dendrimer-based drug delivery systems: History, challenges, and latest developments. J. Biol. Eng. 2022, 16, 18. [Google Scholar] [CrossRef]
- Mignani, S.; Shi, X.; Rodrigues, J.; Tomás, H.; Majoral, J.P. Dendrimer nanoplatforms for veterinary medicine applications: A concise overview. Drug Discov. Today 2022, 27, 1251–1260. [Google Scholar] [CrossRef]
- Sathe, R.Y.; Bharatam, P.V. Drug-dendrimer complexes and conjugates: Detailed furtherance through theory and experiments. Adv. Colloid Interface Sci. 2022, 303, 102639. [Google Scholar] [CrossRef]
- Chis, A.A.; Dobrea, C.; Morgovan, C.; Arseniu, A.M.; Rus, L.L.; Butuca, A.; Juncan, A.M.; Totan, M.; Vonica-Tincu, A.L.; Cormos, G.; et al. Applications and Limitations of Dendrimers in Biomedicine. Molecules 2020, 25, 3982. [Google Scholar] [CrossRef]
- Choudhary, S.; Gupta, L.; Rani, S.; Dave, K.; Gupta, U. Impact of Dendrimers on Solubility of Hydrophobic Drug Molecules. Front. Pharmacol. 2017, 8, 261. [Google Scholar] [CrossRef] [PubMed]
- Caminade, A.M.; Turrin, C.O. Dendrimers for drug delivery. J. Mater. Chem. B 2014, 2, 4055–4066. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Wu, F.; Lin, X.; Shen, L.; Feng, Y. Developments in drug delivery of bioactive alkaloids derived from traditional Chinese medicine. Drug Deliv. 2018, 25, 398–416. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Zhang, B.; Li, M.; Zhang, J. The current scenario of naturally occurring indole alkaloids with anticancer potential. Fitoterapia 2023, 165, 105430. [Google Scholar] [CrossRef] [PubMed]
- Sindhoor, M.S.; Naveen, N.R.; Rao, G.K.; Gopan, G.; Chopra, H.; Park, M.N.; Alshahrani, M.M.; Jose, J.; Emran, T.B.; Kim, B. A spotlight on alkaloid nanoformulations for the treatment of lung cancer. Front. Oncol. 2022, 12, 994155. [Google Scholar]
- Wang, Y.; Xu, Y.; Liu, Z. A review of plant antipathogenic constituents: Source, activity and mechanism. Pestic. Biochem. Physiol. 2022, 188, 105225. [Google Scholar] [CrossRef]
- Faisal, S.; Badshah, S.L.; Kubra, B.; Emwas, A.H.; Jaremko, M. Alkaloids as potential antivirals. A comprehensive review. Nat. Prod. Bioprospect. 2023, 13, 4. [Google Scholar] [CrossRef]
- Ghanbari-Movahed, M.; Kaceli, T.; Mondal, A.; Farzaei, M.H.; Bishayee, A. Recent Advances in Improved Anticancer Efficacies of Camptothecin Nano-Formulations: A Systematic Review. Biomedicines 2021, 9, 480. [Google Scholar] [CrossRef]
- Schluep, T.; Cheng, J.; Khin, K.T.; Davis, M.E. Pharmacokinetics and biodistribution of the camptothecin-polymer conjugate IT-101 in rats and tumor-bearing mice. Cancer Chemother. Pharmacol. 2006, 57, 654–662. [Google Scholar] [CrossRef]
- Khaiwa, N.; Maarouf, N.R.; Darwish, M.H.; Alhamad, D.W.M.; Sebastian, A.; Hamad, M.; Omar, H.A.; Orive, G.; Al-Tel, T.H. Camptothecin’s journey from discovery to WHO Essential Medicine: Fifty years of promise. Eur. J. Med. Chem. 2021, 223, 113639. [Google Scholar] [CrossRef]
- Alibolandi, M.; Taghdisi, S.M.; Ramezani, P.; Hosseini Shamili, F.; Farzad, S.A.; Abnous, K.; Ramezani, M. Smart AS1411-aptamer conjugated pegylated PAMAM dendrimer for the superior delivery of camptothecin to colon adenocarcinoma in vitro and in vivo. Int. J. Pharm. 2017, 519, 352–364. [Google Scholar] [CrossRef] [PubMed]
- Pooja, D.; Srinivasa Reddy, T.; Kulhari, H.; Kadari, A.; Adams, D.J.; Bansal, V.; Sistla, R. N-acetyl-d-glucosamine-conjugated PAMAM dendrimers as dual receptor-targeting nanocarriers for anticancer drug delivery. Eur. J. Pharm. Biopharm. 2020, 154, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Thiagarajan, G.; Ray, A.; Malugin, A.; Ghandehari, H. PAMAM-camptothecin conjugate inhibits proliferation and induces nuclear fragmentation in colorectal carcinoma cells. Pharm. Res. 2010, 27, 2307–2316. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.; Sun, Y.; Chen, J.; Li, H.; Zhu, H.; Gao, X.; Bi, X.; Zhang, Y. Enhanced anti-hepatocarcinoma efficacy by GLUT1 targeting and cellular microenvironment-responsive PAMAM–camptothecin conjugate. Drug Deliv. 2017, 25, 153–165. [Google Scholar] [CrossRef]
- Wang, J.; He, H.; Cooper, R.C.; Gui, Q.; Yang, H. Drug-Conjugated Dendrimer Hydrogel Enables Sustained Drug Release via a Self-Cleaving Mechanism. Mol. Pharm. 2019, 16, 1874–1880. [Google Scholar] [CrossRef]
- Fox, M.E.; Guillaudeu, S.; Frechet, J.M.; Jerger, K.; Macaraeg, N.; Szoka, F.C. Synthesis and in vivo antitumor efficacy of PEGylated poly(l-lysine) dendrimer-camptothecin conjugates. Mol. Pharm. 2009, 6, 1562–1572. [Google Scholar] [CrossRef]
- Min, L.; Han, J.-C.; Zhang, W.; Gu, C.-C.; Zou, Y.-P.; Li, C.-C. Strategies and Lessons Learned from Total Synthesis of Taxol. Chem. Rev. 2023, 123, 4934–4971. [Google Scholar] [CrossRef] [PubMed]
- Stage, T.B.; Bergmann, T.K.; Kroetz, D.L. Clinical Pharmacokinetics of Paclitaxel Monotherapy: An Updated Literature Review. Clin. Pharmacokinet. 2018, 57, 7–19. [Google Scholar] [CrossRef]
- Niznansky, L.; Osinova, D.; Kuruc, R.; Hengerics Szabo, A.; Szoradova, A.; Masar, M.; Niznanska, Z. Natural Taxanes: From Plant Composition to Human Pharmacology and Toxicity. Int. J. Mol. Sci. 2022, 23, 15619. [Google Scholar] [CrossRef]
- Li, T.; Akinade, T.; Zhou, J.; Wang, H.; Tong, Q.; He, S.; Rinebold, E.; Valencia Salazar, L.E.; Bhansali, D.; Zhong, Y.; et al. Therapeutic Nanocarriers Inhibit Chemotherapy-Induced Breast Cancer Metastasis. Adv. Sci. 2022, 9, e2203949. [Google Scholar] [CrossRef]
- Bhatt, H.; Kiran Rompicharla, S.V.; Ghosh, B.; Torchilin, V.; Biswas, S. Transferrin/α-tocopherol modified poly(amidoamine) dendrimers for improved tumor targeting and anticancer activity of paclitaxel. Nanomedicine 2019, 14, 3159–3176. [Google Scholar] [CrossRef] [PubMed]
- Rompicharla, S.V.K.; Kumari, P.; Bhatt, H.; Ghosh, B.; Biswas, S. Biotin functionalized PEGylated poly(amidoamine) dendrimer conjugate for active targeting of paclitaxel in cancer. Int. J. Pharm. 2019, 557, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Dichwalkar, T.; Patel, S.; Bapat, S.; Pancholi, P.; Jasani, N.; Desai, B.; Yellepeddi, V.K.; Sehdev, V. Omega-3 Fatty Acid Grafted PAMAM-Paclitaxel Conjugate Exhibits Enhanced Anticancer Activity in Upper Gastrointestinal Cancer Cells. Macromol. Biosci. 2017, 17, 201600457. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Mao, Y.; Ye, T.; Xia, S.; Wang, S.; Wang, S. Study on enhanced lymphatic exposure of polyamidoamin-alkali blue dendrimer for paclitaxel delivery and influence of the osmotic pressure on the lymphatic targeting. Drug Deliv. 2016, 23, 2617–2629. [Google Scholar] [CrossRef]
- Rompicharla, S.V.K.; Kumari, P.; Ghosh, B.; Biswas, S. Octa-arginine modified poly(amidoamine) dendrimers for improved delivery and cytotoxic effect of paclitaxel in cancer. Artif. Cells Nanomed. Biotechnol. 2018, 46, 847–859. [Google Scholar] [CrossRef]
- Bhatt, H.; Ghosh, B.; Biswas, S. Cell-Penetrating Peptide and alpha-Tocopherol-Conjugated Poly(amidoamine) Dendrimers for Improved Delivery and Anticancer Activity of Loaded Paclitaxel. ACS Appl. Bio Mater. 2020, 3, 3157–3169. [Google Scholar] [CrossRef]
- Patel, H.K.; Gajbhiye, V.; Kesharwani, P.; Jain, N.K. Ligand anchored poly(propyleneimine) dendrimers for brain targeting: Comparative in vitro and in vivo assessment. J. Colloid Interface Sci. 2016, 482, 142–150. [Google Scholar] [CrossRef]
- Patel, S.K.; Gajbhiye, V.; Jain, N.K. Synthesis, characterization and brain targeting potential of paclitaxel loaded thiamine-PPI nanoconjugates. J. Drug Target 2012, 20, 841–849. [Google Scholar] [CrossRef]
- Bansal, K.K.; Kakde, D.; Gupta, U.; Jain, N.K. Development and characterization of triazine based dendrimers for delivery of antitumor agent. J. Nanosci. Nanotechnol. 2010, 10, 8395–8404. [Google Scholar] [CrossRef]
- Ooya, T.; Lee, J.; Park, K. Hydrotropic dendrimers of generations 4 and 5: Synthesis, characterization, and hydrotropic solubilization of paclitaxel. Bioconjug. Chem. 2004, 15, 1221–1229. [Google Scholar] [CrossRef]
- Teow, H.M.; Zhou, Z.; Najlah, M.; Yusof, S.R.; Abbott, N.J.; D’Emanuele, A. Delivery of paclitaxel across cellular barriers using a dendrimer-based nanocarrier. Int. J. Pharm. 2013, 441, 701–711. [Google Scholar] [CrossRef] [PubMed]
- Khandare, J.J.; Jayant, S.; Singh, A.; Chandna, P.; Wang, Y.; Vorsa, N.; Minko, T. Dendrimer versus linear conjugate: Influence of polymeric architecture on the delivery and anticancer effect of paclitaxel. Bioconjug. Chem. 2006, 17, 1464–1472. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Cai, H.; Jiang, L.; Hu, J.; Bains, A.; Hu, J.; Gong, Q.; Luo, K.; Gu, Z. Enzyme-Sensitive and Amphiphilic PEGylated Dendrimer-Paclitaxel Prodrug-Based Nanoparticles for Enhanced Stability and Anticancer Efficacy. ACS Appl. Mater. Interfaces 2017, 9, 6865–6877. [Google Scholar] [CrossRef] [PubMed]
- Satsangi, A.; Roy, S.S.; Satsangi, R.K.; Vadlamudi, R.K.; Ong, J.L. Design of a paclitaxel prodrug conjugate for active targeting of an enzyme upregulated in breast cancer cells. Mol. Pharm. 2014, 11, 1906–1918. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Sun, T.; Ferrari, M. Nanovector delivery of siRNA for cancer therapy. Cancer Gene Ther. 2012, 19, 367–373. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H.; Wang, K.; Hu, Q.; Yao, Q.; Shen, Y.; Yu, G.; Tang, G. Targeted Co-delivery of PTX and TR3 siRNA by PTP Peptide Modified Dendrimer for the Treatment of Pancreatic Cancer. Small 2017, 13, 201602697. [Google Scholar] [CrossRef]
- Kala, S.; Mak, A.S.; Liu, X.; Posocco, P.; Pricl, S.; Peng, L.; Wong, A.S. Combination of dendrimer-nanovector-mediated small interfering RNA delivery to target Akt with the clinical anticancer drug paclitaxel for effective and potent anticancer activity in treating ovarian cancer. J. Med. Chem. 2014, 57, 2634–2642. [Google Scholar] [CrossRef]
- Xia, Q.; Wang, W.; Liu, Z.; Xiao, J.; Qiao, C.; Zhao, Y.; Li, B.; Liu, Y.; Peng, Y.; Yang, X.; et al. New insights into mechanisms of berberine in alleviating reproductive disorders of polycystic ovary syndrome: Anti-inflammatory properties. Eur. J. Pharmacol. 2023, 939, 175433. [Google Scholar] [CrossRef]
- Li, D.D.; Yu, P.; Xiao, W.; Wang, Z.Z.; Zhao, L.G. Berberine: A Promising Natural Isoquinoline Alkaloid for the Development of Hypolipidemic Drugs. Curr. Top. Med. Chem. 2020, 20, 2634–2647. [Google Scholar] [CrossRef]
- Xiong, R.-G.; Huang, S.-Y.; Wu, S.-X.; Zhou, D.-D.; Yang, Z.-J.; Saimaiti, A.; Zhao, C.-N.; Shang, A.; Zhang, Y.-J.; Gan, R.-Y.; et al. Anticancer Effects and Mechanisms of Berberine from Medicinal Herbs: An Update Review. Molecules. 2022, 27, 4523. [Google Scholar] [CrossRef]
- Goel, A. Current understanding and future prospects on Berberine for anticancer therapy. Chem. Biol. Drug Des. 2023. (Online ahead of print. early view). [Google Scholar] [CrossRef]
- Chen, W.; Miao, Y.Q.; Fan, D.J.; Yang, S.S.; Lin, X.; Meng, L.K.; Tang, X. Bioavailability study of berberine and the enhancing effects of TPGS on intestinal absorption in rats. AAPS PharmSciTech 2011, 12, 705–711. [Google Scholar] [CrossRef]
- Gupta, L.; Sharma, A.K.; Gothwal, A.; Khan, M.S.; Khinchi, M.P.; Qayum, A.; Singh, S.K.; Gupta, U. Dendrimer encapsulated and conjugated delivery of berberine: A novel approach mitigating toxicity and improving in vivo pharmacokinetics. Int. J. Pharm. 2017, 528, 88–99. [Google Scholar] [CrossRef]
- Yadav, D.; Semwal, B.C.; Dewangan, H.K. Grafting, characterization and enhancement of therapeutic activity of berberine loaded PEGylated PAMAM dendrimer for cancerous cell. J. Biomater. Sci. Polym. Ed. 2022. (Online ahead of print). [Google Scholar] [CrossRef]
- Kyriakoudi, A.; Spanidi, E.; Mourtzinos, I.; Gardikis, K. Innovative Delivery Systems Loaded with Plant Bioactive Ingredients: Formulation Approaches and Applications. Plants 2021, 10, 1238. [Google Scholar] [CrossRef]
- Albuquerque, B.R.; Heleno, S.A.; Oliveira, M.; Barros, L.; Ferreira, I. Phenolic compounds: Current industrial applications, limitations and future challenges. Food Funct. 2021, 12, 14–29. [Google Scholar] [CrossRef]
- Liu, H.M.; Cheng, M.Y.; Xun, M.H.; Zhao, Z.W.; Zhang, Y.; Tang, W.; Cheng, J.; Ni, J.; Wang, W. Possible Mechanisms of Oxidative Stress-Induced Skin Cellular Senescence, Inflammation, and Cancer and the Therapeutic Potential of Plant Polyphenols. Int. J. Mol. Sci. 2023, 24, 3755. [Google Scholar] [CrossRef]
- Dominguez-Avila, J.A.; Wall-Medrano, A.; Velderrain-Rodriguez, G.R.; Chen, C.O.; Salazar-Lopez, N.J.; Robles-Sanchez, M.; Gonzalez-Aguilar, G.A. Gastrointestinal interactions, absorption, splanchnic metabolism and pharmacokinetics of orally ingested phenolic compounds. Food Funct. 2017, 8, 15–38. [Google Scholar] [CrossRef]
- Fantini, M.; Benvenuto, M.; Masuelli, L.; Frajese, G.V.; Tresoldi, I.; Modesti, A.; Bei, R. In vitro and in vivo antitumoral effects of combinations of polyphenols, or polyphenols and anticancer drugs: Perspectives on cancer treatment. Int. J. Mol. Sci. 2015, 16, 9236–9282. [Google Scholar] [CrossRef]
- Dini, I.; Grumetto, L. Recent Advances in Natural Polyphenol Research. Molecules 2022, 27, 8777. [Google Scholar] [CrossRef]
- Brglez Mojzer, E.; Knez Hrncic, M.; Skerget, M.; Knez, Z.; Bren, U. Polyphenols: Extraction Methods, Antioxidative Action, Bioavailability and Anticarcinogenic Effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef]
- Rakotondrabe, T.F.; Fan, M.X.; Muema, F.W.; Guo, M.Q. Modulating Inflammation-Mediated Diseases via Natural Phenolic Compounds Loaded in Nanocarrier Systems. Pharmaceutics 2023, 15, 699. [Google Scholar] [CrossRef]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Dietary phenolics: Chemistry, bioavailability and effects on health. Nat. Prod. Rep. 2009, 26, 1001–1043. [Google Scholar] [CrossRef]
- Williamson, G.; Manach, C. Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. Am. J. Clin. Nutr. 2005, 81, 243s–255s. [Google Scholar] [CrossRef]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, Inflammation and Immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef]
- Chen, S.; Jiang, H.; Wu, X.; Fang, J. Therapeutic Effects of Quercetin on Inflammation, Obesity, and Type 2 Diabetes. Mediat. Inflamm. 2016, 2016, 9340637. [Google Scholar] [CrossRef]
- Wang, G.; Wang, Y.; Yao, L.; Gu, W.; Zhao, S.; Shen, Z.; Lin, Z.; Liu, W.; Yan, T.; Cohen, G. Pharmacological Activity of Quercetin: An Updated Review. Evid.-Based Complement. Altern. Med. 2022, 2022, 3997190. [Google Scholar] [CrossRef]
- Azizi, E.; Fouladdel, S.; Komeili Movahhed, T.; Modaresi, F.; Barzegar, E.; Ghahremani, M.H.; Ostad, S.N.; Atashpour, S. Quercetin Effects on Cell Cycle Arrest and Apoptosis and Doxorubicin Activity in T47D Cancer Stem Cells. Asian Pac. J. Cancer Prev. 2022, 23, 4145–4154. [Google Scholar] [CrossRef]
- Sethi, G.; Rath, P.; Chauhan, A.; Ranjan, A.; Choudhary, R.; Ramniwas, S.; Sak, K.; Aggarwal, D.; Rani, I.; Tuli, H.S. Apoptotic Mechanisms of Quercetin in Liver Cancer: Recent Trends and Advancements. Pharmaceutics 2023, 15, 712. [Google Scholar] [CrossRef]
- Bhatiya, M.; Pathak, S.; Jothimani, G.; Duttaroy, A.K.; Banerjee, A. A Comprehensive Study on the Anti-cancer Effects of Quercetin and Its Epigenetic Modifications in Arresting Progression of Colon Cancer Cell Proliferation. Arch. Immunol. Ther. Exp. 2023, 71, 6. [Google Scholar] [CrossRef]
- Maugeri, A.; Calderaro, A.; Patanè, G.T.; Navarra, M.; Barreca, D.; Cirmi, S.; Felice, M.R. Targets Involved in the Anti-Cancer Activity of Quercetin in Breast, Colorectal and Liver Neoplasms. Int. J. Mol. Sci. 2023, 24, 2952. [Google Scholar] [CrossRef]
- Moghimipour, E.; Farsimadan, N.; Salimi, A. Ocular Delivery of Quercetin Using Microemulsion System: Design, Characterization, and Ex-vivo Transcorneal Permeation. Iran. J. Pharm. Res. 2022, 21, e127486. [Google Scholar] [CrossRef]
- Madaan, K.; Lather, V.; Pandita, D. Evaluation of polyamidoamine dendrimers as potential carriers for quercetin, a versatile flavonoid. Drug Deliv. 2016, 23, 254–262. [Google Scholar] [CrossRef]
- Mitri, Z.; Constantine, T.; O’Regan, R. The HER2 Receptor in Breast Cancer: Pathophysiology, Clinical Use, and New Advances in Therapy. Chemother. Res. Pract. 2012, 2012, 743193. [Google Scholar] [CrossRef]
- Khakinahad, Y.; Sohrabi, S.; Razi, S.; Narmani, A.; Khaleghi, S.; Asadiyun, M.; Jafari, H.; Mohammadnejad, J. Margetuximab conjugated-PEG-PAMAM G4 nano-complex: A smart nano-device for suppression of breast cancer. Biomed. Eng. Lett. 2022, 12, 317–329. [Google Scholar] [CrossRef]
- Rezaei, S.J.T.; Malekzadeh, A.M.; Ramazani, A.; Niknejad, H. pH-Sensitive Magnetite Nanoparticles Modified with Hyperbranched Polymers and Folic Acid for Targeted Imaging and Therapy. Curr. Drug Deliv. 2019, 16, 839–848. [Google Scholar] [CrossRef]
- Jiang, Y.; Pei, J.; Zheng, Y.; Miao, Y.J.; Duan, B.Z.; Huang, L.F. Gallic Acid: A Potential Anti-Cancer Agent. Chin. J. Integr. Med. 2022, 28, 661–671. [Google Scholar] [CrossRef]
- Tuli, H.S.; Mistry, H.; Kaur, G.; Aggarwal, D.; Garg, V.K.; Mittal, S.; Yerer, M.B.; Sak, K.; Khan, M.A. Gallic Acid: A Dietary Polyphenol that Exhibits Anti-neoplastic Activities by Modulating Multiple Oncogenic Targets. Anticancer Agents Med. Chem. 2022, 22, 499–514. [Google Scholar] [CrossRef]
- Priyadarshi, K.; Shirsath, K.; Waghela, N.B.; Sharma, A.; Kumar, A.; Pathak, C. Surface modified PAMAM dendrimers with gallic acid inhibit, cell proliferation, cell migration and inflammatory response to augment apoptotic cell death in human colon carcinoma cells. J. Biomol. Struct. Dyn. 2021, 39, 6853–6869. [Google Scholar] [CrossRef]
- Sharma, A.; Gautam, S.P.; Gupta, A.K. Surface modified dendrimers: Synthesis and characterization for cancer targeted drug delivery. Bioorg. Med. Chem. 2011, 19, 3341–3346. [Google Scholar] [CrossRef]
- Abdou, E.M.; Masoud, M.M. Gallic acid-PAMAM and gallic acid-phospholipid conjugates, physicochemical characterization and in vivo evaluation. Pharm. Dev. Technol. 2018, 23, 55–66. [Google Scholar] [CrossRef]
- Lalani, A.R.; Fakhari, F.; Radgoudarzi, S.; Rastegar-Pouyani, N.; Moloudi, K.; Khodamoradi, E.; Taeb, S.; Najafi, M. Immunoregulation by resveratrol; implications for normal tissue protection and tumour suppression. Clin. Exp. Pharmacol. Physiol. 2023, 50, 353–368. [Google Scholar] [CrossRef]
- Fernandez-Quintela, A.; Macarulla, M.T.; Gomez-Zorita, S.; Gonzalez, M.; Milton-Laskibar, I.; Portillo, M.P. Relationship between changes in microbiota induced by resveratrol and its anti-diabetic effect on type 2 diabetes. Front. Nutr. 2022, 9, 1084702. [Google Scholar] [CrossRef]
- Fan, D.; Liu, C.; Zhang, Z.; Huang, K.; Wang, T.; Chen, S.; Li, Z. Progress in the Preclinical and Clinical Study of Resveratrol for Vascular Metabolic Disease. Molecules 2022, 27, 7524. [Google Scholar] [CrossRef]
- Singh, G. Resveratrol: Nanocarrier-based delivery systems to enhance its therapeutic potential. Nanomedicine 2020, 15, 2801–2817. [Google Scholar] [CrossRef]
- Walle, T.; Hsieh, F.; DeLegge, M.H.; Oatis, J.E., Jr.; Walle, U.K. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab. Dispos. 2004, 32, 1377–1382. [Google Scholar] [CrossRef]
- Gambini, J.; López-Grueso, R.; Olaso-González, G.; Inglés, M.; Abdelazid, K.; El Alami, M.; Bonet-Costa, V.; Borrás, C.; Viña, J. [Resveratrol: Distribution, properties and perspectives]. Rev. Esp. Geriatr. Gerontol. 2013, 48, 79–88. [Google Scholar] [CrossRef]
- Pentek, T.; Newenhouse, E.; O’Brien, B.; Chauhan, A.S. Development of a Topical Resveratrol Formulation for Commercial Applications Using Dendrimer Nanotechnology. Molecules 2017, 22, 137. [Google Scholar] [CrossRef]
- Shi, Y.; Ye, F.; Lu, K.; Hui, Q.; Miao, M. Characterizations and Bioavailability of Dendrimer-like Glucan Nanoparticulate System Containing Resveratrol. J. Agric. Food. Chem. 2020, 68, 6420–6429. [Google Scholar] [CrossRef]
- Xie, Y.; Yao, Y. Octenylsuccinate hydroxypropyl phytoglycogen, a dendrimer-like biopolymer, solubilizes poorly water-soluble active pharmaceutical ingredients. Carbohydr. Polym. 2018, 180, 29–37. [Google Scholar] [CrossRef]
- Islam, A.; Mishra, A.; Siddiqui, M.A.; Siddiquie, S. Recapitulation of Evidence of Phytochemical, Pharmacokinetic and Biomedical Application of Silybin. Drug Res. 2021, 71, 489–503. [Google Scholar] [CrossRef]
- Křen, V.; Marhol, P.; Purchartová, K.; Gabrielová, E.; Modrianský, M. Biotransformation of silybin and its congeners. Curr. Drug Metab. 2013, 14, 1009–1021. [Google Scholar] [CrossRef]
- Wu, J.W.; Lin, L.C.; Hung, S.C.; Chi, C.W.; Tsai, T.H. Analysis of silibinin in rat plasma and bile for hepatobiliary excretion and oral bioavailability application. J. Pharm. Biomed. Anal. 2007, 45, 635–641. [Google Scholar] [CrossRef]
- Huang, X.; Wu, Z.; Gao, W.; Chen, Q.; Yu, B. Polyamidoamine dendrimers as potential drug carriers for enhanced aqueous solubility and oral bioavailability of silybin. Drug Dev. Ind. Pharm. 2011, 37, 419–427. [Google Scholar] [CrossRef]
- Diaz, C.; Guzman, J.; Jimenez, V.A.; Alderete, J.B. Partially PEGylated PAMAM dendrimers as solubility enhancers of Silybin. Pharm. Dev. Technol. 2018, 23, 689–696. [Google Scholar] [CrossRef]
- Shetty, P.K.; Manikkath, J.; Tupally, K.; Kokil, G.; Hegde, A.R.; Raut, S.Y.; Parekh, H.S.; Mutalik, S. Skin Delivery of EGCG and Silibinin: Potential of Peptide Dendrimers for Enhanced Skin Permeation and Deposition. AAPS PharmSciTech 2017, 18, 2346–2357. [Google Scholar] [CrossRef]
- Dehzad, M.J.; Ghalandari, H.; Nouri, M.; Askarpour, M. Antioxidant and anti-inflammatory effects of curcumin/turmeric supplementation in adults: A GRADE-assessed systematic review and dose-response meta-analysis of randomized controlled trials. Cytokine. 2023, 164, 156144. [Google Scholar] [CrossRef]
- Shakeri, A.; Ward, N.; Panahi, Y.; Sahebkar, A. Anti-Angiogenic Activity of Curcumin in Cancer Therapy: A Narrative Review. Curr. Vasc. Pharmacol. 2019, 17, 262–269. [Google Scholar] [CrossRef]
- Hussain, Y.; Alam, W.; Ullah, H.; Dacrema, M.; Daglia, M.; Khan, H.; Arciola, C.R. Antimicrobial Potential of Curcumin: Therapeutic Potential and Challenges to Clinical Applications. Antibiotics 2022, 11, 322. [Google Scholar] [CrossRef]
- PDQ Integrative, Alternative; Complementary Therapies Editorial Board. Curcumin (Curcuma, Turmeric) and Cancer (PDQ®): Health Professional Version. In PDQ Cancer Information Summaries; National Cancer Institute (US): Bethesda, MD, USA, 2002. [Google Scholar]
- Zeng, Y.; Luo, Y.; Wang, L.; Zhang, K.; Peng, J.; Fan, G. Therapeutic Effect of Curcumin on Metabolic Diseases: Evidence from Clinical Studies. Int. J. Mol. Sci. 2023, 24, 3323. [Google Scholar] [CrossRef]
- Wu, J.; Lv, M.; Zhou, Y. Efficacy and side effect of curcumin for the treatment of osteoarthritis: A meta-analysis of randomized controlled trials. Pak. J. Pharm. Sci. 2019, 32, 43–51. [Google Scholar]
- Salehi, B.; Del Prado-Audelo, M.L.; Cortes, H.; Leyva-Gomez, G.; Stojanovic-Radic, Z.; Singh, Y.D.; Patra, J.K.; Das, G.; Martins, N.; Martorell, M.; et al. Therapeutic Applications of Curcumin Nanomedicine Formulations in Cardiovascular Diseases. J. Clin. Med. 2020, 9, 746. [Google Scholar] [CrossRef]
- Cheng, A.L.; Hsu, C.H.; Lin, J.K.; Hsu, M.M.; Ho, Y.F.; Shen, T.S.; Ko, J.Y.; Lin, J.T.; Lin, B.R.; Ming-Shiang, W.; et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001, 21, 2895–2900. [Google Scholar]
- Soni, K.B.; Kuttan, R. Effect of oral curcumin administration on serum peroxides and cholesterol levels in human volunteers. Indian J. Physiol. Pharmacol. 1992, 36, 273–275. [Google Scholar]
- Lao, C.D.; Ruffin, M.T.t.; Normolle, D.; Heath, D.D.; Murray, S.I.; Bailey, J.M.; Boggs, M.E.; Crowell, J.; Rock, C.L.; Brenner, D.E. Dose escalation of a curcuminoid formulation. BMC Complement. Altern. Med. 2006, 6, 10. [Google Scholar] [CrossRef]
- Shoba, G.; Joy, D.; Joseph, T.; Majeed, M.; Rajendran, R.; Srinivas, P.S. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998, 64, 353–356. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef]
- Mollazade, M.; Nejati-Koshki, K.; Akbarzadeh, A.; Zarghami, N.; Nasiri, M.; Jahanban-Esfahlan, R.; Alibakhshi, A. PAMAM dendrimers augment inhibitory effects of curcumin on cancer cell proliferation: Possible inhibition of telomerase. Asian Pac. J. Cancer Prev. 2013, 14, 6925–6928. [Google Scholar] [CrossRef]
- Falconieri, M.C.; Adamo, M.; Monasterolo, C.; Bergonzi, M.C.; Coronnello, M.; Bilia, A.R. New Dendrimer-Based Nanoparticles Enhance Curcumin Solubility. Planta Med. 2017, 83, 420–425. [Google Scholar] [CrossRef]
- Gamage, N.H.; Jing, L.; Worsham, M.J.; Ali, M.M. Targeted Theranostic Approach for Glioma Using Dendrimer-Based Curcumin Nanoparticle. J. Nanomed. Nanotechnol. 2016, 7, 393. [Google Scholar]
- Wang, L.; Xu, X.; Zhang, Y.; Zhang, Y.; Zhu, Y.; Shi, J.; Sun, Y.; Huang, Q. Encapsulation of curcumin within poly(amidoamine) dendrimers for delivery to cancer cells. J. Mater. Sci. Mater. Med. 2013, 24, 2137–2144. [Google Scholar] [CrossRef]
- Gallien, J.; Srinageshwar, B.; Gallo, K.; Holtgrefe, G.; Koneru, S.; Otero, P.S.; Bueno, C.A.; Mosher, J.; Roh, A.; Kohtz, D.S.; et al. Curcumin Loaded Dendrimers Specifically Reduce Viability of Glioblastoma Cell Lines. Molecules 2021, 26, 6050. [Google Scholar] [CrossRef]
- Nosrati, H.; Adibtabar, M.; Sharafi, A.; Danafar, H.; Hamidreza Kheiri, M. PAMAM-modified citric acid-coated magnetic nanoparticles as pH sensitive biocompatible carrier against human breast cancer cells. Drug Dev. Ind. Pharm. 2018, 44, 1377–1384. [Google Scholar] [CrossRef]
- Wallace, D.C. Mitochondria and cancer. Nat. Rev. Cancer. 2012, 12, 685–698. [Google Scholar] [CrossRef]
- Kianamiri, S.; Dinari, A.; Sadeghizadeh, M.; Rezaei, M.; Daraei, B.; Bahsoun, N.E.; Nomani, A. Mitochondria-Targeted Polyamidoamine Dendrimer-Curcumin Construct for Hepatocellular Cancer Treatment. Mol. Pharm. 2020, 17, 4483–4498. [Google Scholar] [CrossRef]
- Ghaffari, M.; Dehghan, G.; Baradaran, B.; Zarebkohan, A.; Mansoori, B.; Soleymani, J.; Ezzati Nazhad Dolatabadi, J.; Hamblin, M.R. Co-delivery of curcumin and Bcl-2 siRNA by PAMAM dendrimers for enhancement of the therapeutic efficacy in HeLa cancer cells. Colloids Surf. B Biointerfaces 2020, 188, 110762. [Google Scholar] [CrossRef]
- Peddada, K.V.; Peddada, K.V.; Shukla, S.K.; Mishra, A.; Verma, V. Role of Curcumin in Common Musculoskeletal Disorders: A Review of Current Laboratory, Translational, and Clinical Data. Orthop. Surg. 2015, 7, 222–231. [Google Scholar] [CrossRef]
- Oh, S.; Kyung, T.W.; Choi, H.S. Curcumin inhibits osteoclastogenesis by decreasing receptor activator of nuclear factor-kappaB ligand (RANKL) in bone marrow stromal cells. Mol. Cells 2008, 26, 486–489. [Google Scholar]
- Yang, X.; Kuang, Z.; Yang, X.; Hu, X.; Luo, P.; Lai, Q.; Zhang, B.; Zhang, X.; Wei, Y. Facile synthesis of curcumin-containing poly(amidoamine) dendrimers as pH-responsive delivery system for osteoporosis treatment. Colloids Surf. B 2023, 222, 113029. [Google Scholar] [CrossRef]
- Yao, W.; Sun, K.; Mu, H.; Liang, N.; Liu, Y.; Yao, C.; Liang, R.; Wang, A. Preparation and characterization of puerarin-dendrimer complexes as an ocular drug delivery system. Drug Dev. Ind. Pharm. 2010, 36, 1027–1035. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, K.; Yao, W.; Liang, N.; Mu, H.; Liang, R.; Yao, C. Corneal penetration of PAMAM dendrimers-coated puerarin liposomes. Zhongguo Zhong Yao Za Zhi 2010, 35, 30–34. [Google Scholar]
- Wang, W.Y.; Yao, C.; Shao, Y.F.; Mu, H.J.; Sun, K.X. Determination of puerarin in rabbit aqueous humor by liquid chromatography tandem mass spectrometry using microdialysis sampling after topical administration of puerarin PAMAM dendrimer complex. J. Pharm. Biomed. Anal. 2011, 56, 825–829. [Google Scholar] [CrossRef]
- Yao, W.J.; Sun, K.X.; Liu, Y.; Liang, N.; Mu, H.J.; Yao, C.; Liang, R.C.; Wang, A.P. Effect of poly(amidoamine) dendrimers on corneal penetration of puerarin. Biol. Pharm. Bull. 2010, 33, 1371–1377. [Google Scholar] [CrossRef]
- Yao, C.; Wang, W.; Zhou, X.; Qu, T.; Mu, H.; Liang, R.; Wang, A.; Sun, K. Effects of poly(amidoamine) dendrimers on ocular absorption of puerarin using microdialysis. J. Ocul. Pharmacol. Ther. 2011, 27, 565–569. [Google Scholar] [CrossRef]
- Gu, L.; Wu, Z.h.; Qi, X.; He, H.; Ma, X.; Chou, X.; Wen, X.; Zhang, M.; Jiao, F. Polyamidomine dendrimers: An excellent drug carrier for improving the solubility and bioavailability of puerarin. Pharm. Dev. Technol. 2012, 18, 1051–1057. [Google Scholar] [CrossRef]
- Wang, G.; Zhou, Z.; Zhao, Z.; Li, Q.; Wu, Y.; Yan, S.; Shen, Y.; Huang, P. Enzyme-Triggered Transcytosis of Dendrimer–Drug Conjugate for Deep Penetration into Pancreatic Tumors. ACS Nano 2020, 14, 4890–4904. [Google Scholar] [CrossRef]
- Bhatt, H.; Kiran Rompicharla, S.V.; Ghosh, B.; Biswas, S. Alpha-Tocopherol Succinate-Anchored PEGylated Poly(amidoamine) Dendrimer for the Delivery of Paclitaxel: Assessment of In Vitro and In Vivo Therapeutic Efficacy. Mol. Pharm. 2019, 16, 1541–1554. [Google Scholar] [CrossRef]
- Liu, J.; Li, J.; Liu, N.; Guo, N.; Gao, C.; Hao, Y.; Chen, L.; Zhang, X. In vitro studies of phospholipid-modified PAMAM-siMDR1 complexes for the reversal of multidrug resistance in human breast cancer cells. Int. J. Pharm. 2017, 530, 291–299. [Google Scholar] [CrossRef]
- Mei, M.; Ren, Y.; Zhou, X.; Yuan, X.B.; Han, L.; Wang, G.X.; Jia, Z.; Pu, P.Y.; Kang, C.S.; Yao, Z. Downregulation of miR-21 enhances chemotherapeutic effect of taxol in breast carcinoma cells. Technol. Cancer Res. Treat. 2010, 9, 77–86. [Google Scholar] [CrossRef]
- Sharma, R.; Kambhampati, S.P.; Zhang, Z.; Sharma, A.; Chen, S.; Duh, E.I.; Kannan, S.; Tso, M.O.M.; Kannan, R.M. Dendrimer mediated targeted delivery of sinomenine for the treatment of acute neuroinflammation in traumatic brain injury. J. Control. Release 2020, 323, 361–375. [Google Scholar] [CrossRef]
- Tripathi, P.K.; Gupta, S.; Rai, S.; Shrivatava, A.; Tripathi, S.; Singh, S.; Khopade, A.J.; Kesharwani, P. Curcumin loaded poly (amidoamine) dendrimer-plamitic acid core-shell nanoparticles as anti-stress therapeutics. Drug Dev. Ind. Pharm. 2020, 46, 412–426. [Google Scholar] [CrossRef]
- Lv, T.; Yu, T.; Fang, Y.; Zhang, S.; Jiang, M.; Zhang, H.; Zhang, Y.; Li, Z.; Chen, H.; Gao, Y. Role of generation on folic acid-modified poly(amidoamine) dendrimers for targeted delivery of baicalin to cancer cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 75, 182–190. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, Y.; Su, Y.; Zhang, H.; Ding, L.; Yan, X.; Zhao, D.; Shao, N.; Ye, X.; Cheng, Y. Inclusion complexes of isoflavones with two commercially available dendrimers: Solubility, stability, structures, release behaviors, cytotoxicity, and anti-oxidant activities. Int. J. Pharm. 2011, 421, 301–309. [Google Scholar] [CrossRef]
- Yesil-Celiktas, O.; Pala, C.; Cetin-Uyanikgil, E.O.; Sevimli-Gur, C. Synthesis of silica-PAMAM dendrimer nanoparticles as promising carriers in Neuro blastoma cells. Anal. Biochem. 2017, 519, 1–7. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).