Synergistic Effect of Metal Oxide and Carbon Nanoparticles on the Thermal and Mechanical Properties of Polyimide Composite Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis and Treatment of Nanoparticles
2.3. Synthesis of R-BAPS Prepolymer
2.4. Preparation of Pristine and Nanocomposite Films
2.5. Characterization Techniques
3. Results and Discussion
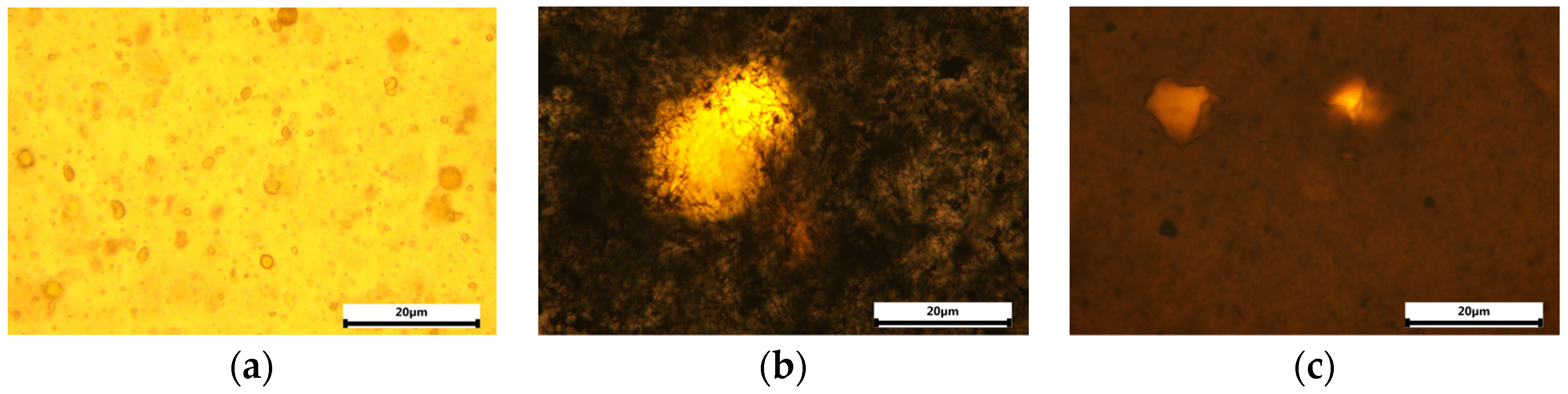
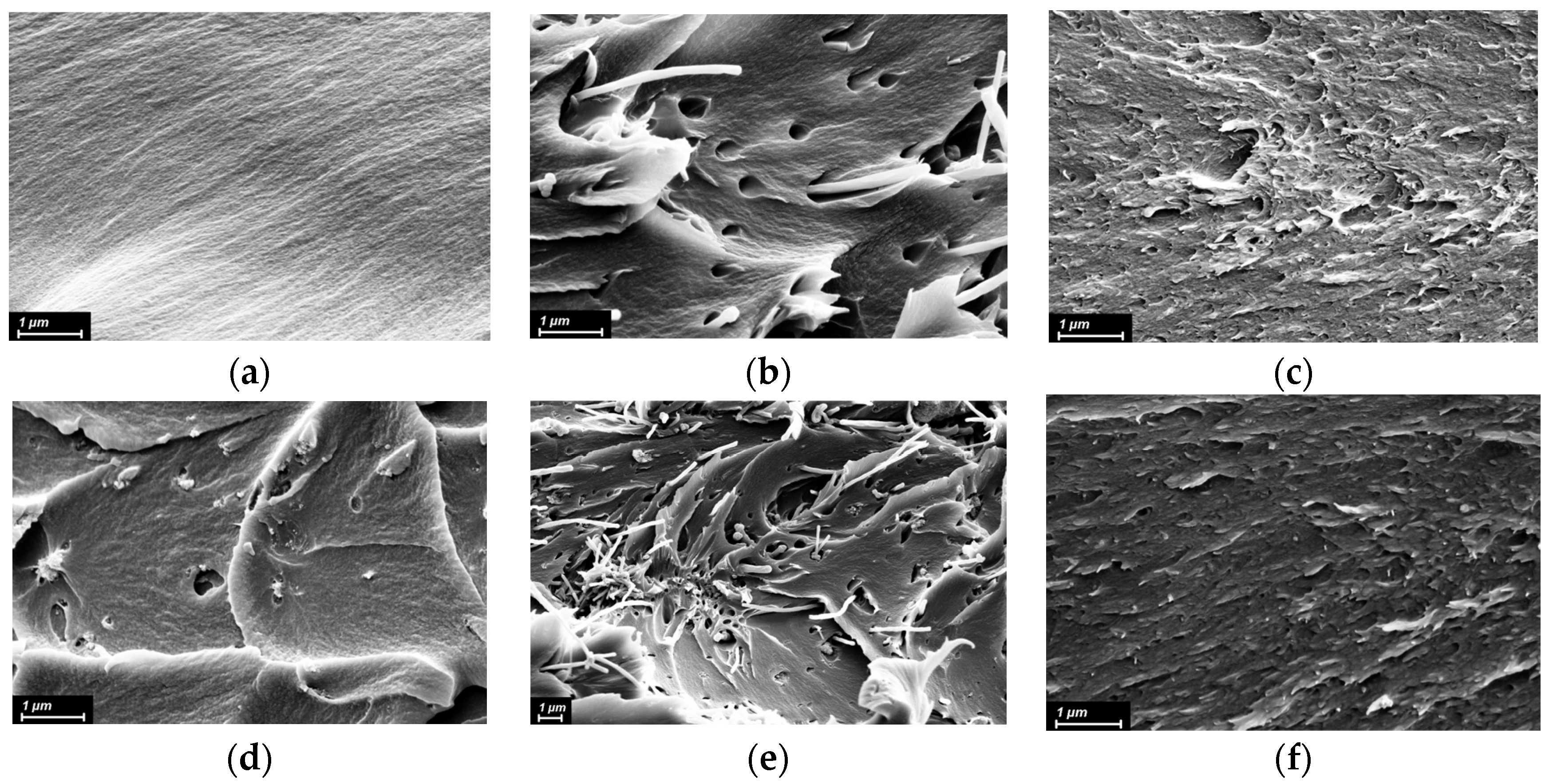
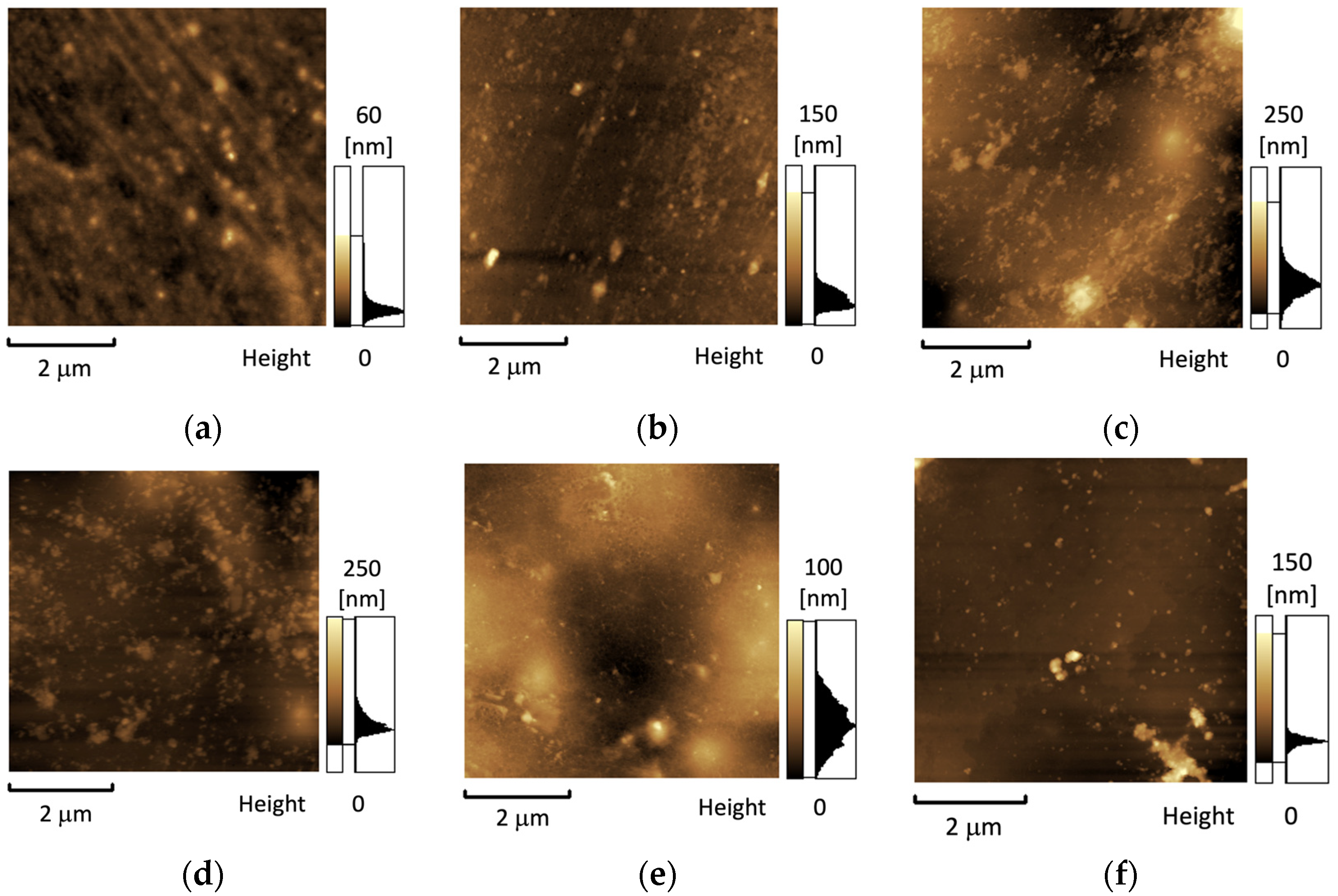
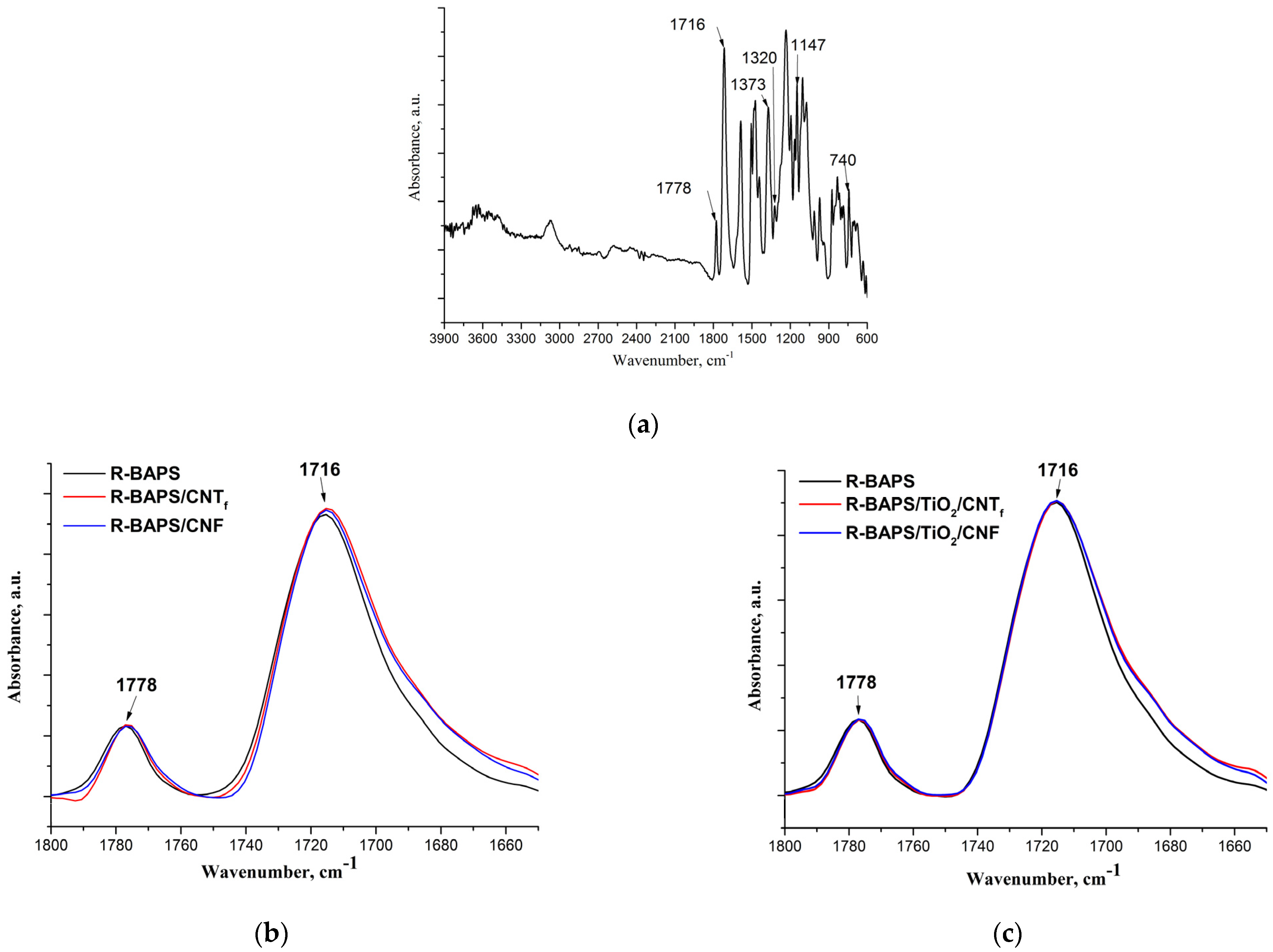
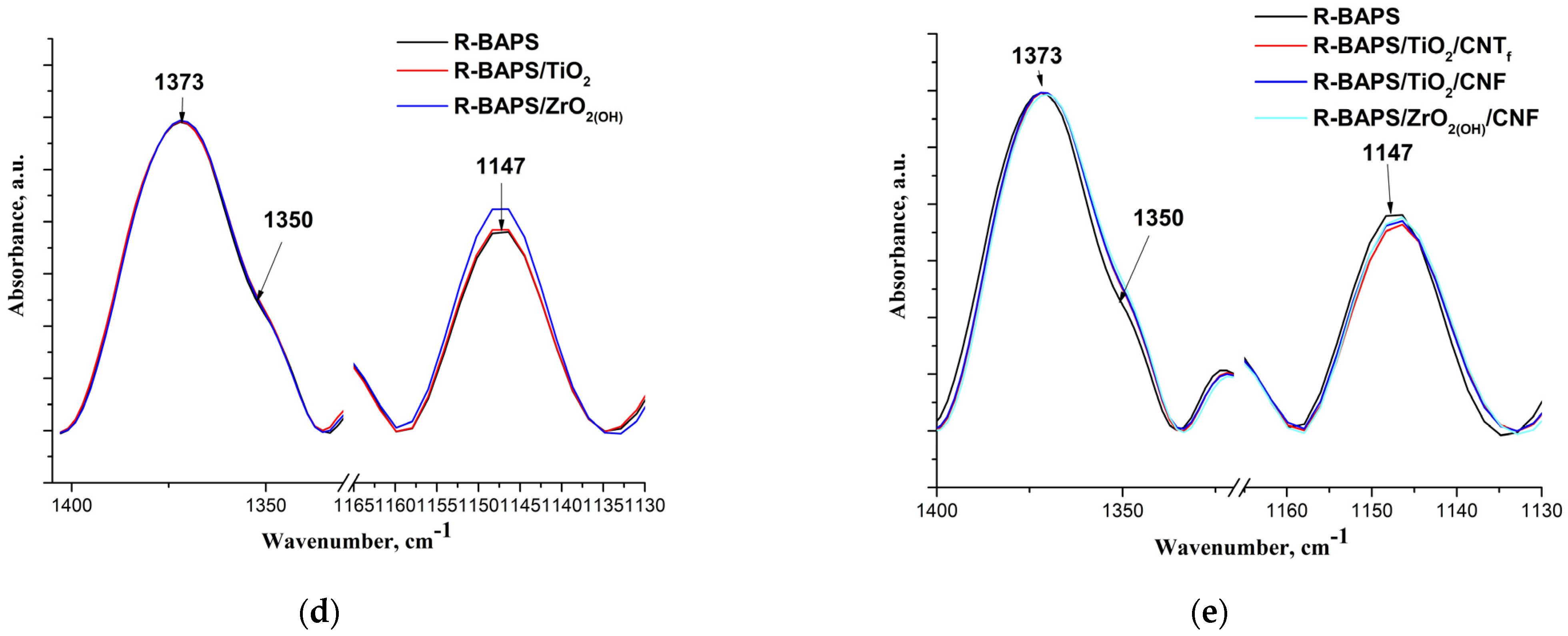
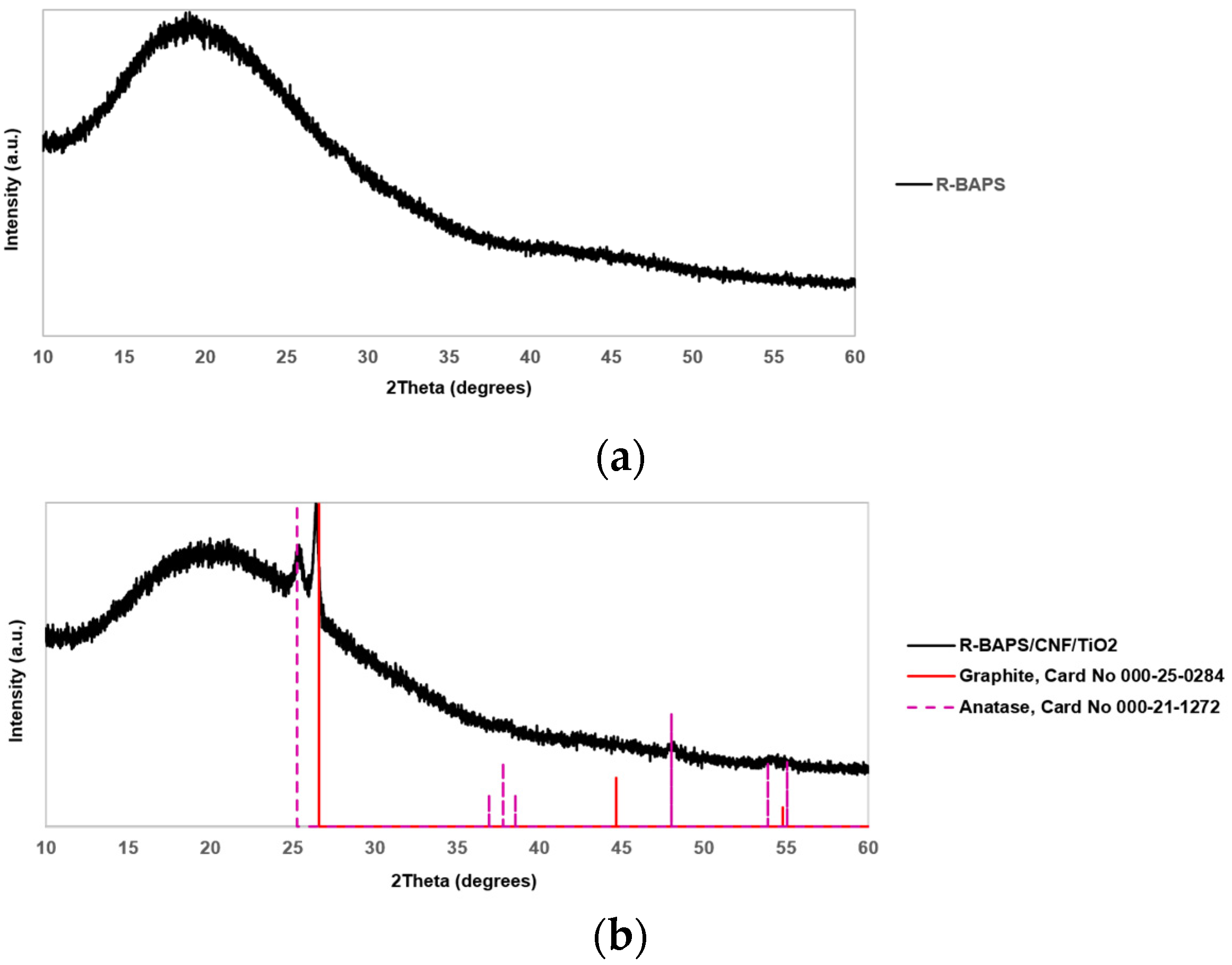
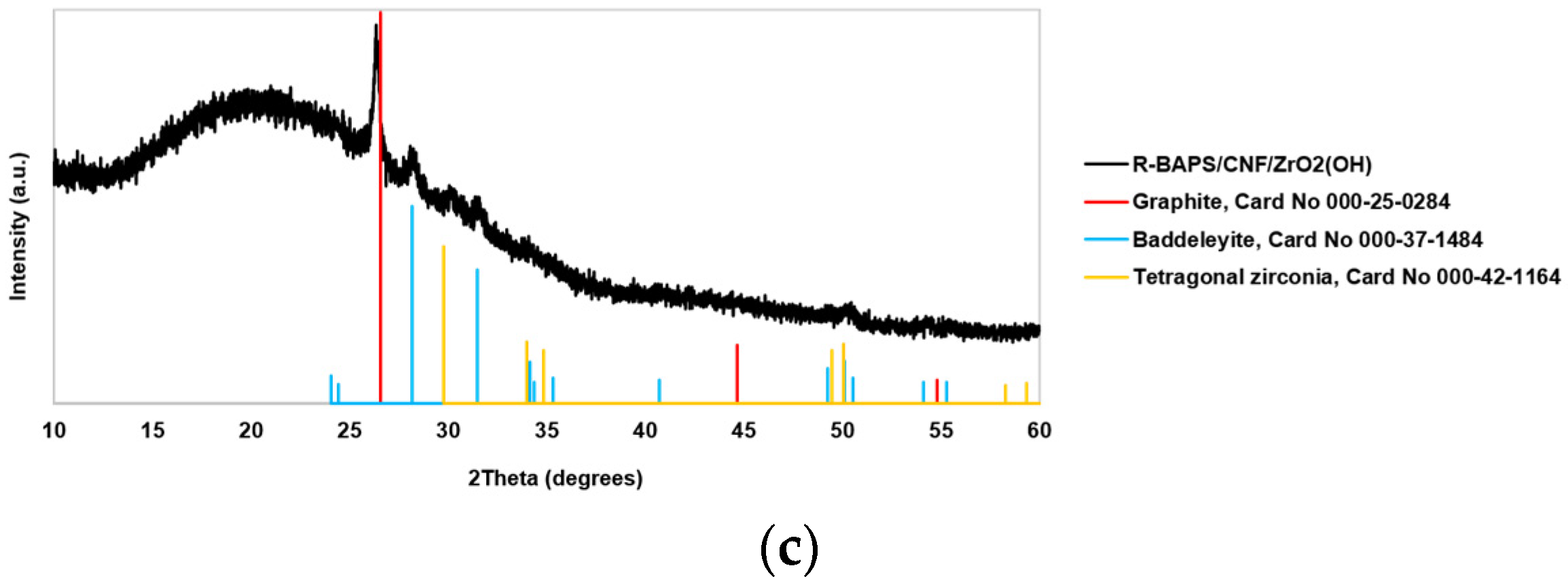
3.1. Structure and Morphology
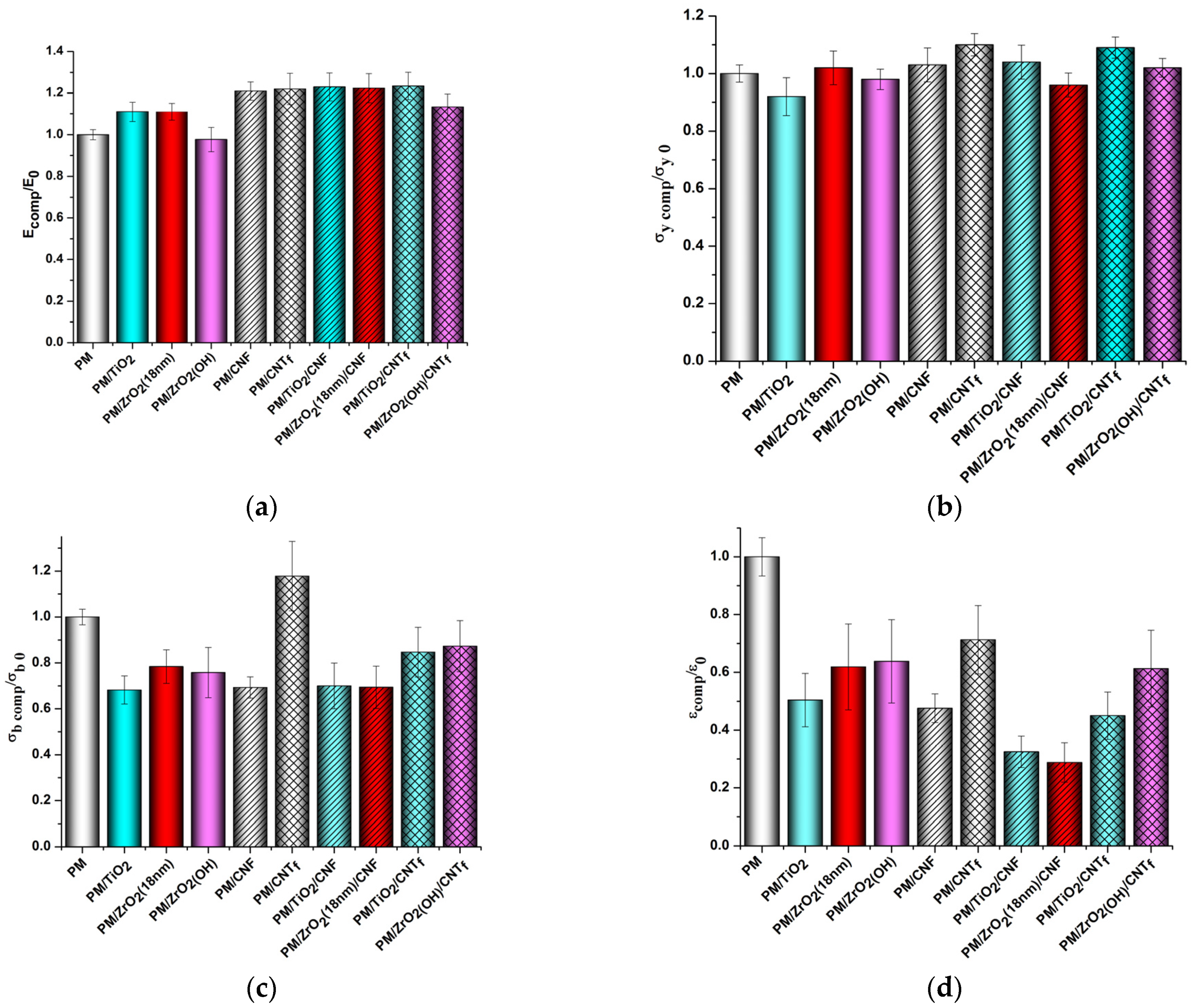
3.2. Mechanical Properties
3.3. Thermal Properties
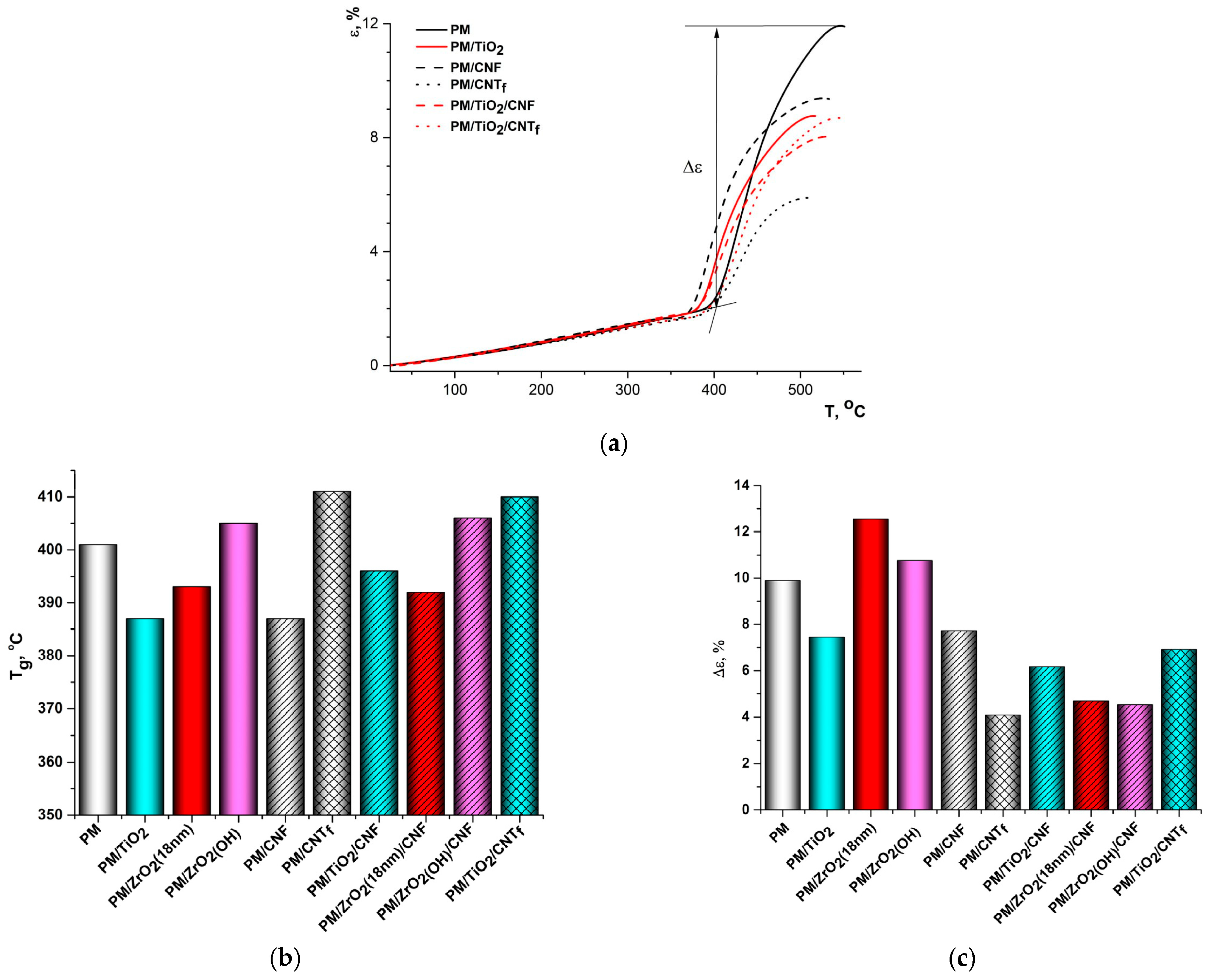
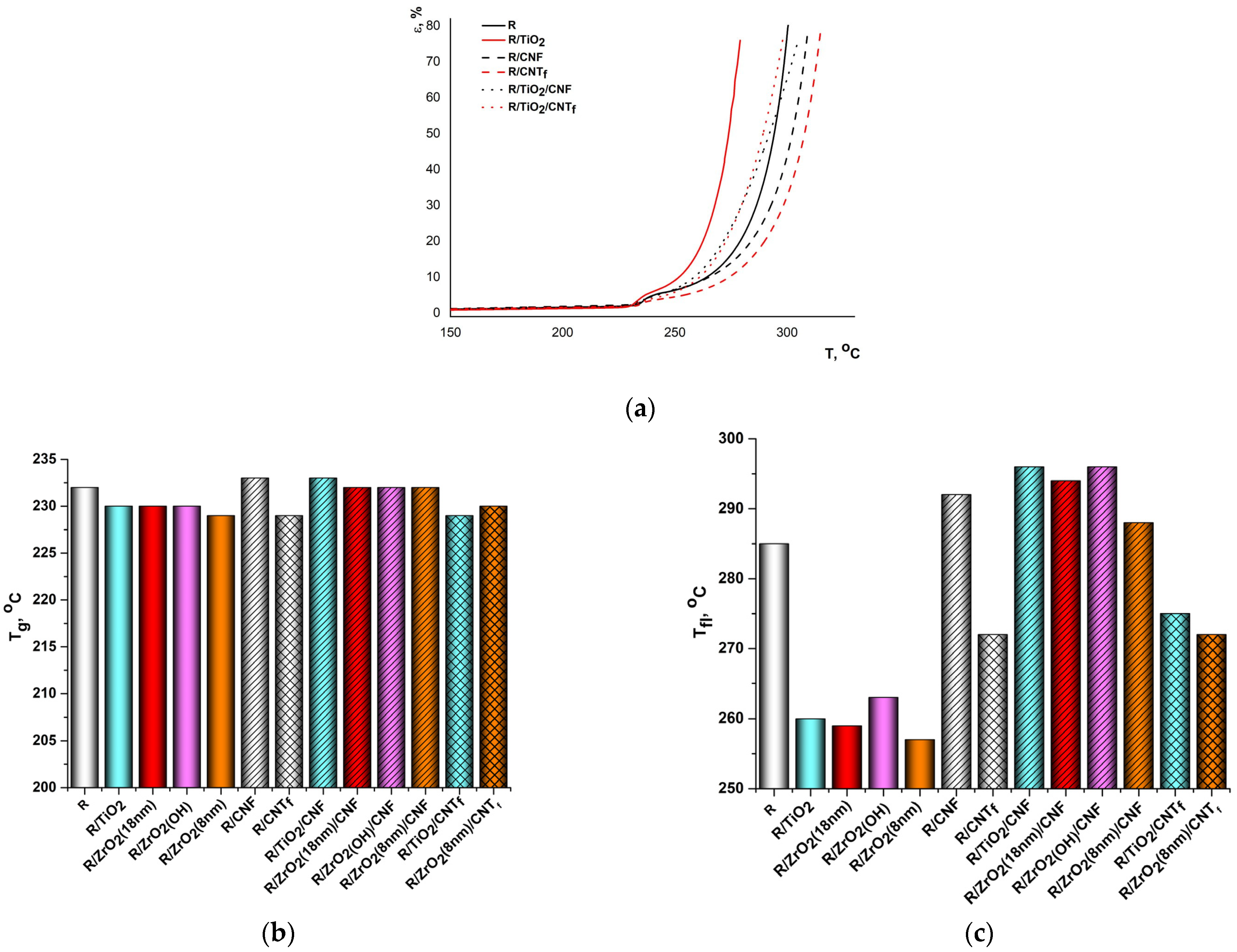
3.4. Thermomechanical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Sezer Hicyilmaz, A.; Celik Bedeloglu, A. Applications of Polyimide Coatings: A Review. SN Appl. Sci. 2021, 3, 363. [Google Scholar] [CrossRef]
- Wu, Z.; He, J.; Yang, H.; Yang, S. Progress in Aromatic Polyimide Films for Electronic Applications: Preparation, Structure and Properties. Polymers 2022, 14, 1269. [Google Scholar] [CrossRef]
- Gouzman, I.; Grossman, E.; Verker, R.; Atar, N.; Bolker, A.; Eliaz, N. Advances in Polyimide-Based Materials for Space Applications. Adv. Mater. 2019, 31, 1807738. [Google Scholar] [CrossRef] [PubMed]
- Ji, D.; Li, T.; Hu, W.; Fuchs, H. Recent Progress in Aromatic Polyimide Dielectrics for Organic Electronic Devices and Circuits. Adv. Mater. 2019, 31, 1806070. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zhang, K.; Chen, X.; Dou, S.; Zhao, J.; Li, Y. Mechanical, Dielectric and Thermal Properties of Polyimide Films with Sandwich Structure. Compos. Struct. 2021, 261, 113305. [Google Scholar] [CrossRef]
- Ogbonna, V.E.; Popoola, A.P.I.; Popoola, O.M.; Adeosun, S.O. A Review on Polyimide Reinforced Nanocomposites for Mechanical, Thermal, and Electrical Insulation Application: Challenges and Recommendations for Future Improvement. Polym. Bull. 2022, 79, 663–695. [Google Scholar] [CrossRef]
- Yoonessi, M.; Shi, Y.; Scheiman, D.A.; Lebron-Colon, M.; Tigelaar, D.M.; Weiss, R.A.; Meador, M.A. Graphene Polyimide Nanocomposites; Thermal, Mechanical, and High-Temperature Shape Memory Effects. ACS Nano 2012, 6, 7644–7655. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Liu, X.; Wang, R.; Lu, C.; Wen, X.; Tu, G. Research Progress and Application of Polyimide-Based Nanocomposites. Nanomaterials 2023, 13, 656. [Google Scholar] [CrossRef]
- Serenko, O.; Andropova, U.; Tebeneva, N.; Buzin, M.; Afanasyev, E.; Tarasenkov, A.; Bukalov, S.; Leites, L.; Aysin, R.; Novikov, L.; et al. Influence of the Composition of the Hybrid Filler on the Atomic Oxygen Erosion Resistance of Polyimide Nanocomposites. Materials 2020, 13, 3204. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.; Dai, C.; Wang, H.; Li, Z.; Liu, H.; Li, W.; Yang, C. A Review on High Temperature Resistant Polyimide Films: Heterocyclic Structures and Nanocomposites. Compos. Commun. 2019, 16, 84–93. [Google Scholar] [CrossRef]
- Wang, C.W.; Cao, X.D.; Tian, J.Y.; Yu, F.; Jiang, F.Y.; Ren, K.L. Significantly Enhanced Energy Density of Nanodiamond/Polyimide Composites at High Temperatures with Ultralow Nanodiamond Contents. Sci. China Technol. Sci. 2023, 66, 956–965. [Google Scholar] [CrossRef]
- Chavali, M.S.; Nikolova, M.P. Metal Oxide Nanoparticles and Their Applications in Nanotechnology. SN Appl. Sci. 2019, 1, 607. [Google Scholar] [CrossRef] [Green Version]
- Van Tran, V.; Nu, T.T.V.; Jung, H.-R.; Chang, M. Advanced Photocatalysts Based on Conducting Polymer/Metal Oxide Composites for Environmental Applications. Polymers 2021, 13, 3031. [Google Scholar] [CrossRef] [PubMed]
- Prasanna, S.R.V.R.V.S.; Pandey, S.; Balaji, K.; Rana, S. Metal Oxide Based Nanomaterials and Their Polymer Nanocomposites. In Nanomaterials and Polymer Nanocomposites: Raw Materials to Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 123–144. ISBN 9780128146156. [Google Scholar]
- Dontsova, T.A.; Nahirniak, S.V.; Astrelin, I.M. Metaloxide Nanomaterials and Nanocomposites of Ecological Purpose. J. Nanomater. 2019, 2019, 5942194. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Yang, Y.; Han, D.; Li, Y. Oxygen Defective Metal Oxides for Energy Conversion and Storage. Nano Today 2017, 13, 23–39. [Google Scholar] [CrossRef]
- Ganduglia-Pirovano, M.V.; Hofmann, A.; Sauer, J. Oxygen Vacancies in Transition Metal and Rare Earth Oxides: Current State of Understanding and Remaining Challenges. Surf. Sci. Rep. 2007, 62, 219–270. [Google Scholar] [CrossRef]
- Yudin, V.E.; Bugrov, A.N.; Didenko, A.L.; Smirnova, V.E.; Gofman, I.V.; Kononova, S.V.; Kremnev, R.V.; Popova, E.N.; Svetlichnyi, V.M.; Kudryavtsev, V.V. Composites of Multiblock (Segmented) Aliphatic Poly(Ester Imide) with Zirconia Nanoparticles: Synthesis, Mechanical Properties, and Pervaporation Behavior. Polym. Sci.-Ser. B 2014, 56, 919–926. [Google Scholar] [CrossRef]
- Tsai, C.L.; Liou, G.S. Highly Transparent and Flexible Polyimide/ZrO2 Nanocomposite Optical Films with a Tunable Refractive Index and Abbe Number. Chem. Commun. 2015, 51, 13523–13526. [Google Scholar] [CrossRef]
- Ahmadizadegan, H. Synthesis and Gas Transport Properties of Novel Functional Polyimide/ZnO Nanocomposite Thin Film Membranes. RSC Adv. 2016, 6, 106778. [Google Scholar] [CrossRef]
- Sokolova, M.P.; Smirnov, M.A.; Geydt, P.; Bugrov, A.N.; Ovaska, S.S.; Lahderanta, E.; Toikka, A.M. Structure and Transport Properties of Mixed-Matrix Membranes Based on Polyimides with ZrO2 Nanostars. Polymers 2016, 8, 403. [Google Scholar] [CrossRef] [Green Version]
- Dong, N.; Wang, J.; Chen, N.; Liu, B.; Tian, G.; Qi, S.; Sun, G.; Wu, D. In Situ Reinforcing: ZrO2-Armored Hybrid Polyimide Separators for Advanced and Safe Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2021, 9, 6250–6257. [Google Scholar] [CrossRef]
- Kizilkaya, C.; Dumludaǧ, F.; Karataş, S.; Apohan, N.K.; Altindal, A.; Güngör, A. The Effect of Titania Content on the Physical Properties of Polyimide/Titania Nanohybrid Films. J. Appl. Polym. Sci. 2012, 125, 3802–3810. [Google Scholar] [CrossRef]
- Atabaki, F.; Ahmadizadegan, H. Fabrication of a New Polyimide/Titania (TiO2) Nanocomposite Thin Film by the Sol-Gel Route. Polym.-Plast. Technol. Eng. 2015, 54, 523–531. [Google Scholar] [CrossRef]
- Gao, X.; Sheng, L.; Li, M.; Xie, X.; Yang, L.; Gong, Y.; Cao, M.; Bai, Y.; Dong, H.; Liu, G.; et al. Flame-Retardant Nano-TiO2/Polyimide Composite Separator for the Safety of a Lithium-Ion Battery. ACS Appl. Polym. Mater. 2022, 4, 5125. [Google Scholar] [CrossRef]
- Aframehr, W.M.; Molki, B.; Bagheri, R.; Heidarian, P.; Davodi, S.M. Characterization and Enhancement of the Gas Separation Properties of Mixed Matrix Membranes: Polyimide with Nickel Oxide Nanoparticles. Chem. Eng. Res. Des. 2020, 153, 789–805. [Google Scholar] [CrossRef]
- Nikolaeva, A.L.; Gofman, I.V.; Yakimansky, A.V.; Ivan’kova, E.M.; Gulii, N.S.; Teplonogova, M.A.; Ivanova, O.S.; Baranchikov, A.E.; Ivanov, V.K. Interplay of Polymer Matrix and Nanosized Redox Dopant with Regard to Thermo-Oxidative and Pyrolytic Stability: CeO2 Nanoparticles in a Milieu of Aromatic Polyimides. Mater. Today Commun. 2020, 22, 100803. [Google Scholar] [CrossRef]
- Hsu, S.C.; Whang, W.T.; Hung, C.H.; Chiang, P.C.; Hsiao, Y.N. Effect of the Polyimide Structure and ZnO Concentration on the Morphology and Characteristics of Polyimide/ZnO Nanohybrid Films. Macromol. Chem. Phys. 2005, 206, 291–298. [Google Scholar] [CrossRef]
- Poveda, R.L.; Gupta, N. Carbon Nanofiber Reinforced Polymer Composites; SpringerBriefs in Materials; Springer International Publishing: Cham, Switzerland, 2016; ISBN 978-3-319-23786-2. [Google Scholar]
- Zhu, J.; Mu, L.; Chen, L.; Shi, Y.; Wang, H.; Feng, X.; Lu, X. Interface-Strengthened Polyimide/Carbon Nanofibers Nanocomposites with Superior Mechanical and Tribological Properties. Macromol. Chem. Phys. 2014, 215, 1407–1414. [Google Scholar] [CrossRef]
- So, H.H.; Cho, J.W.; Sahoo, N.G. Effect of Carbon Nanotubes on Mechanical and Electrical Properties of Polyimide/Carbon Nanotubes Nanocomposites. Eur. Polym. J. 2007, 43, 3750–3756. [Google Scholar] [CrossRef]
- Xu, W.; Feng, Y.; Ding, Y.; Jiang, S.; Fang, H.; Hou, H. Short Electrospun Carbon Nanofiber Reinforced Polyimide Composite with High Dielectric Permittivity. Mater. Lett. 2015, 161, 431–434. [Google Scholar] [CrossRef]
- Yudin, V.E.; Svetlichnyi, V.M.; Shumakov, A.N.; Schechter, R.; Harel, H.; Marom, G. Morphology and Mechanical Properties of Carbon Fiber Reinforced Composites Based on Semicrystalline Polyimides Modified by Carbon Nanofibers. Compos. Part A Appl. Sci. Manuf. 2008, 39, 85–90. [Google Scholar] [CrossRef]
- Singh, B.P.; Singh, D.; Mathur, R.B.; Dhami, T.L. Influence of Surface Modified MWCNTs on the Mechanical, Electrical and Thermal Properties of Polyimide Nanocomposites. Nanoscale Res. Lett. 2008, 3, 444. [Google Scholar] [CrossRef] [Green Version]
- Ogasawara, T.; Ishida, Y.; Ishikawa, T.; Yokota, R. Characterization of Multi-Walled Carbon Nanotube/Phenylethynyl Terminated Polyimide Composites. Compos. Part A Appl. Sci. Manuf. 2004, 35, 67–74. [Google Scholar] [CrossRef]
- Smirnova, V.E.; Gofman, I.V.; Ivan’Kova, E.M.; Didenko, A.L.; Krestinin, A.V.; Zvereva, G.I.; Svetlichnyi, V.M.; Yudin, V.E. Effect of Single-Walled Carbon Nanotubes and Carbon Nanofibers on the Structure and Mechanical Properties of Thermoplastic Polyimide Matrix Films. Polym. Sci.-Ser. A 2013, 55, 268–278. [Google Scholar] [CrossRef]
- Chao, M.; Li, Y.; Wu, G.; Zhou, Z.; Yan, L. Functionalized Multiwalled Carbon Nanotube-Reinforced Polyimide Composite Films with Enhanced Mechanical and Thermal Properties. Int. J. Polym. Sci. 2019, 2019, 9302803. [Google Scholar] [CrossRef] [Green Version]
- Su, C.; Xue, F.; Li, T.; Xin, Y.; Wang, M.; Tang, J.; Ma, Y. Fabrication and Multifunctional Properties of Polyimide Based Hierarchical Composites with in Situ Grown Carbon Nanotubes. RSC Adv. 2017, 7, 29686. [Google Scholar] [CrossRef] [Green Version]
- Drubetski, M.; Siegmann, A.; Narkis, M. Electrical Properties of Hybrid Carbon Black/Carbon Fiber Polypropylene Composites. J. Mater. Sci. 2007, 42, 1–8. [Google Scholar] [CrossRef]
- Belashov, A.V.; Zhikhoreva, A.A.; Moskalyuk, O.A.; Beltukov, Y.M.; Semenova, I.V. Linear and Nonlinear Elastic Properties of Polystyrene-Based Nanocomposites with Allotropic Carbon Fillers and Binary Mixtures. Polymers 2022, 14, 5462. [Google Scholar] [CrossRef]
- Wei, T.; Song, L.; Zheng, C.; Wang, K.; Yan, J.; Shao, B.; Fan, Z.J. The Synergy of a Three Filler Combination in the Conductivity of Epoxy Composites. Mater. Lett. 2010, 64, 2376–2379. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, S.; Ruan, K.; Zhang, H.; Gu, J. Highly Thermally Conductive Carbon Nanotubes Pillared Exfoliated Graphite/Polyimide Composites. NPJ Flex. Electron. 2021, 5, 16. [Google Scholar] [CrossRef]
- Wan, H.; Fan, L.; Jia, J.; Han, Q.; Abdollahzadeh Jamalabadi, M.Y. Micromechanical Modeling over Two Length-Scales for Elastic Properties of Graphene Nanoplatelet/Graphite Fiber/Polyimide Composites. Mater. Chem. Phys. 2021, 262, 124255. [Google Scholar] [CrossRef]
- Hussin, F.N.N.M.; Wahab, R.A.; Attan, N. Nanocellulose and Nanoclay as Reinforcement Materials in Polymer Composites: A Review. Malaysian J. Fundam. Appl. Sci. 2020, 16, 145–153. [Google Scholar] [CrossRef]
- Praveen, S.; Chattopadhyay, P.K.; Albert, P.; Dalvi, V.G.; Chakraborty, B.C.; Chattopadhyay, S. Synergistic Effect of Carbon Black and Nanoclay Fillers in Styrene Butadiene Rubber Matrix: Development of Dual Structure. Compos. Part A Appl. Sci. Manuf. 2009, 40, 309–316. [Google Scholar] [CrossRef]
- Salehi, M.M.; Khalkhali, T.; Davoodi, A.A. The Physical and Mechanical Properties and Cure Characteristics of NBR/Silica/MWCNT Hybrid Composites. Polym. Sci.-Ser. A 2016, 58, 567–577. [Google Scholar] [CrossRef]
- Nuruddin, M.; Gupta, R.; Tcherbi-Narteh, A.; Hosur, M.; Jeelani, S. Synergistic Effect of Graphene Nanoplatelets and Nanoclay on Epoxy Polymer Nanocomposites. Adv. Mater. Res. 2015, 1119, 155–159. [Google Scholar] [CrossRef]
- Chen, S.; Yu, H.; Ren, W.; Zhang, Y. Thermal Degradation Behavior of Hydrogenated Nitrile-Butadiene Rubber (HNBR)/Clay Nanocomposite and HNBR/Clay/Carbon Nanotubes Nanocomposites. Thermochim. Acta 2009, 491, 103–108. [Google Scholar] [CrossRef]
- Liu, D.; Ma, C.; Chi, H.; Li, S.; Zhang, P.; Dai, P. Enhancing Thermal Conductivity of Polyimide Composite Film by Electrostatic Self-Assembly and Two-Step Synergism of Al2O3microspheres and BN Nanosheets. RSC Adv. 2020, 10, 42584–42595. [Google Scholar] [CrossRef]
- Liu, L.; Cao, C.; Ma, X.; Zhang, X.; Lv, T. Thermal Conductivity of Polyimide/AlN and Polyimide/(AlN + BN) Composite Films Prepared by in-Situ Polymerization. J. Macromol. Sci. Part A Pure Appl. Chem. 2020, 57, 398–407. [Google Scholar] [CrossRef]
- Deepak, A.; Shankar, P. Exploring the Properties of Lead Oxide and Tungsten Oxide Based Graphene Mixed Nanocomposite Films. Nanosyst. Phys. Chem. Math. 2016, 7, 502–505. [Google Scholar] [CrossRef]
- Li, R.; Ding, C.; Yu, J.; Wang, X.; Huang, P. Enhanced Thermal Conductivity of Polyimide Composite Film Filled with Hybrid Fillers. High Perform. Polym. 2021, 33, 905–913. [Google Scholar] [CrossRef]
- Cheng, Y.K.; Campéon, B.D.L.; Obata, S.; Nishina, Y. Synergic Effect of Graphene Oxide and Boron Nitride on the Mechanical Properties of Polyimide Composite Films. Nanoscale Adv. 2022, 4, 2339–2345. [Google Scholar] [CrossRef] [PubMed]
- Nikolaeva, A.L.; Gofman, I.V.; Yakimansky, A.V.; Ivan’kova, E.M.; Abalov, I.V.; Baranchikov, A.E.; Ivanov, V.K. Polyimide-Based Nanocomposites with Binary CeO2/Nanocarbon Fillers: Conjointly Enhanced Thermal and Mechanical Properties. Polymers 2020, 12, 1952. [Google Scholar] [CrossRef] [PubMed]
- Hołyńska, M.; Tighe, A.; Semprimoschnig, C. Coatings and Thin Films for Spacecraft Thermo-Optical and Related Functional Applications. Adv. Mater. Interfaces 2018, 5, 1701644. [Google Scholar] [CrossRef]
- Chiang, P.-C.; Whang, W.-T.; Tsai, M.-H.; Wu, S.-C. Physical and Mechanical Properties of Polyimide/Titania Hybrid Films. Thin Solid Films 2004, 447–448, 359–364. [Google Scholar] [CrossRef]
- Yudin, V.E.; Otaigbe, J.U.; Svetlichnyi, V.M.; Korytkova, E.N.; Almjasheva, O.V.; Gusarov, V.V. Effects of Nanofiller Morphology and Aspect Ratio on the Rheo-Mechanical Properties of Polyimide Nanocomposites. Express Polym. Lett. 2008, 2, 485–493. [Google Scholar] [CrossRef]
- Nikolaeva, A.L.; Bugrov, A.N.; Sokolova, M.P.; Ivan’kova, E.M.; Abalov, I.V.; Vlasova, E.N.; Gofman, I.V. Metal Oxide Nanoparticles: An Effective Tool to Modify the Functional Properties of Thermally Stable Polyimide Films. Polymers 2022, 14, 2580. [Google Scholar] [CrossRef]
- Rahman, A.; Ali, I.; Al Zahrani, S.M.; Eleithy, R.H. A review of the applications of nanocarbon polymer composites. Nano 2011, 6, 185–203. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, N.; He, C. The Superior Mechanical and Physical Properties of Nanocarbon Reinforced Bulk Composites Achieved by Architecture Design—A Review. Prog. Mater. Sci. 2020, 113, 100672. [Google Scholar] [CrossRef]
- Lu, C.; Lin, F.; Shao, H.; Bi, S.; Chen, N.; Shao, G.; Jiang, J. Carboxylated Carbon Nanotube/Polyimide Films with Low Thermal Expansion Coefficient and Excellent Mechanical Properties. Polymers 2022, 14, 4565. [Google Scholar] [CrossRef]
- Bugrov, A.N.; Almjasheva, O. V Effect of Hydrothermal Synthesis Conditions on the Morphology of ZrO 2nanoparticles. Nanosyst. Phys. Chem. Math. 2013, 4, 810–815. [Google Scholar]
- Bugrov, A.N.; Smyslov, R.Y.; Zavialova, A.Y.; Kopitsa, G.P. The Influence of Chemical Prehistory on the Structure, Photoluminescent Properties, Surface and Biological Characteristics of Zr0.98Eu0.02O1.99 Nanophosphors. Nanosyst. Phys. Chem. Math. 2019, 10, 164–175. [Google Scholar] [CrossRef] [Green Version]
- Zavialova, A.Y.; Bugrov, A.N.; Smyslov, R.Y.; Kirilenko, D.A.; Khamova, T.V.; Kopitsa, G.P.; Licitra, C.; Rouchon, D. Structure and Photoluminescent Properties of TiO2:Eu3+ Nanoparticles Synthesized under Hydro and Solvothermal Conditions from Different Precursors. Nanosyst. Phys. Chem. Math. 2019, 10, 361–373. [Google Scholar] [CrossRef]
- Gofman, I.V.; Abalov, I.V.; Vlasova, E.N.; Goikhman, M.J.; Zhang, B. Comparative Evaluation of Different Methods of Carboxylation of Carbon Nanotubes as a Modifier of Mechanical Properties of Heat-Resistant Polyimide Based Nanocomposites. Fibre Chem. 2015, 47, 236–243. [Google Scholar] [CrossRef]
- Gofman, I.V.; Abalov, I.V.; Gladchenko, S.V.; Afanas’eva, N. V Carbon Nanocones/Discs—A New Type of Filler to Improve the Thermal and Mechanical Properties of Polymer Films. Polym. Adv. Technol. 2012, 23, 408–413. [Google Scholar] [CrossRef]
- Bessonov, M.I.; Koton, M.M.; Kudryavtsev, V.V.; Laius, L.A. Polyimides–Thermally Stable Polymers; Plenum Publishing Corp.: New York, NY, USA, 1987; ISBN 978-1-4615-7636-5. [Google Scholar]
- Calleja, E.; Balta; Vonk, C.G. X-ray Scattering of Synthetic Polymers; Elsevier: Amsterdam, The Netherlands, 1989; Volume 8, p. 317. ISBN 9780444873859. [Google Scholar]
- Gofman, I.V.; Abalov, I.V.; Tiranov, V.G.; Yudin, V.E. Effect of Carbon Nanoparticles of Different Shapes on Mechanical Properties of Aromatic Polyimide-Based Composite Films. Polym. Sci. Ser. A 2013, 55, 313–319. [Google Scholar] [CrossRef]
- Yudin, V.E.; Svetlichnyi, V.M. Effect of the Structure and Shape of Filler Nanoparticles on the Physical Properties of Polyimide Composites. Russ. J. Gen. Chem. 2010, 80, 2157–2169. [Google Scholar] [CrossRef]
- Gofman, I.V.; Balik, K.; Cerny, M.; Zaloudkova, M.; Goikhman, M.J.; Yudin, V.E. Peculiarities of the Initial Stages of Carbonization Processes in Polyimide-Based Nanocomposite Films Containing Carbon Nanoparticles. Cogent Chem. 2015, 1, 1076712. [Google Scholar] [CrossRef]
- Sidorovich, A.V.; Kallistov, O.V.; Kydryavtsev, V.V.; Lavrent’ev, V.K.; Svetlichnyi, V.M. Nature of Fluidity of Some Polyimides. Vysokomol. Soedin. Seriya B 1983, 25, 565–568. [Google Scholar]



| Sample | PI | Nanofiller | Weight Content of Nanofiller, wt.% |
|---|---|---|---|
| PMDA-ODA | PMDA-ODA | - | 0 |
| PMDA-ODA/ZrO2(18nm) | ZrO2 | 5 | |
| PMDA-ODA/ZrO2(OH) | 3 | ||
| PMDA-ODA/TiO2 | TiO2 | 3 | |
| PMDA-ODA/CNF | CNF | 4.5 | |
| PMDA-ODA/CNTf | CNTf | 3 | |
| PMDA-ODA/TiO2/CNF | TiO2/CNF | 3/4.5 | |
| PMDA-ODA/ZrO2(18nm)/CNF | ZrO2(18nm)/CNF | 5/4.5 | |
| PMDA-ODA/TiO2/CNTf | TiO2/CNTf | 3/3 | |
| PMDA-ODA/ZrO2(OH)/CNTf | ZrO2(OH)/CNTf | 3/3 | |
| R-BAPS | R-BAPS | - | 0 |
| R-BAPS/ZrO2(8nm) | ZrO2 | 3 | |
| R-BAPS/ZrO2(18nm) | 3 | ||
| R-BAPS/ZrO2(OH) | 3 | ||
| R-BAPS/TiO2 | TiO2 | 3 | |
| R-BAPS/CNF | CNF | 4.5 | |
| R-BAPS/CNTf | CNTf | 3 | |
| R-BAPS/TiO2/CNF | TiO2/CNF | 3/4.5 | |
| R-BAPS/ZrO2(18nm)/CNF | ZrO2(18nm)/CNF | 3/4.5 | |
| R-BAPS/ZrO2(OH)/CNF | ZrO2(OH)/CNF | 3/4.5 | |
| R-BAPS/TiO2/CNTf | TiO2/CNTf | 3/3 | |
| R-BAPS/ZrO2(OH)/CNTf | ZrO2(OH)/CNTf | 3/3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikolaeva, A.L.; Bugrov, A.N.; Sokolova, M.P.; Kuntsman, I.V.; Vlasova, E.N.; Ivan’kova, E.M.; Abalov, I.V.; Gofman, I.V. Synergistic Effect of Metal Oxide and Carbon Nanoparticles on the Thermal and Mechanical Properties of Polyimide Composite Films. Polymers 2023, 15, 2298. https://doi.org/10.3390/polym15102298
Nikolaeva AL, Bugrov AN, Sokolova MP, Kuntsman IV, Vlasova EN, Ivan’kova EM, Abalov IV, Gofman IV. Synergistic Effect of Metal Oxide and Carbon Nanoparticles on the Thermal and Mechanical Properties of Polyimide Composite Films. Polymers. 2023; 15(10):2298. https://doi.org/10.3390/polym15102298
Chicago/Turabian StyleNikolaeva, Alexandra L., Alexander N. Bugrov, Maria P. Sokolova, Igor V. Kuntsman, Elena N. Vlasova, Elena M. Ivan’kova, Ivan V. Abalov, and Iosif V. Gofman. 2023. "Synergistic Effect of Metal Oxide and Carbon Nanoparticles on the Thermal and Mechanical Properties of Polyimide Composite Films" Polymers 15, no. 10: 2298. https://doi.org/10.3390/polym15102298






