Electrospinning and Electrospraying: Emerging Techniques for Probiotic Stabilization and Application
Abstract
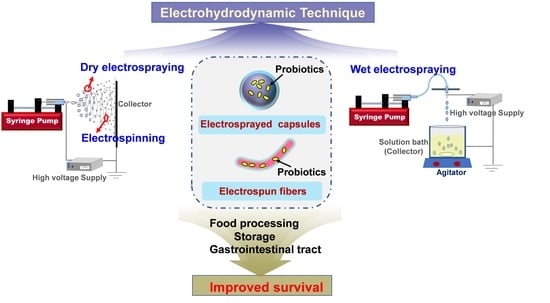
:1. Introduction
2. Electrospinning and Electrospraying
3. Electrospinning for Probiotic Stabilization
3.1. Electrospinning for Probiotic Encapsulation
3.1.1. Feasibility of Electrospinning for Encapsulating Probiotics
3.1.2. Modified Electrospinning Protocols for Probiotic Encapsulation
3.1.3. Potential of Electrospun Fiber Mat for Delivering Probiotics to the Colon
3.2. Electrospinning for Probiotics Immobilization
4. Electrospraying for Probiotic Stabilization
4.1. Dry Electrospraying for Probiotic Encapsulation
4.2. Wet Electrospraying for Probiotic Encapsulation
5. Applications of Electrospun/Electrosprayed Probiotic Formulations
5.1. Tissue Healing
5.2. Food Preservation
5.3. Food Processing
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document: The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastro. Hepat. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Fang, K.L.; Jin, X.; Hong, S.H. Probiotic Escherichia coli inhibits biofilm formation of pathogenic E. coli via extracellular activity of DegP. Sci. Rep. 2018, 8, 4939. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Mansell, T.J. Yeasts as probiotics: Mechanisms, outcomes, and future potential. Fungal Genet. Biol. 2020, 137, 103333. [Google Scholar] [CrossRef] [PubMed]
- Bommasamudram, J.; Kumar, P.; Kapur, S.; Sharma, D.; Devappa, S. Development of thermotolerant Lactobacilli cultures with improved probiotic properties using adaptive laboratory evolution method. Probiotics Antimicro. 2022, 21, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Farhangfar, A.; Gandomi, H.; Basti, A.A.; Misaghi, A.; Noori, N. Study of growth kinetic and gastrointestinal stability of acid-bile resistant Lactobacillus plantarum strains isolated from Siahmazgi traditional cheese. Vet. Res. Forum 2021, 12, 235–240. [Google Scholar] [PubMed]
- Yao, M.F.; Xie, J.J.; Du, H.J.; Mcclements, D.J.; Xiao, H.; Li, L.J. Progress in microencapsulation of probiotics: A review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 857–874. [Google Scholar] [CrossRef]
- Juodeikiene, G.; Zadeike, D.; Bartkiene, E.; Lėlė, V.; Bernatoniene, J.; Jakštas, V. A new delivery system based on apple pomace–pectin gels to encourage the viability of antimicrobial strains. Food Sci. Technol. Int. 2019, 26, 242–253. [Google Scholar] [CrossRef]
- Frakolaki, G.; Giannou, V.; Kekos, D.; Tzia, C. A review of the microencapsulation techniques for the incorporation of probiotic bacteria in functional foods. Crit. Rev. Food. Sci. 2020, 61, 1515–1536. [Google Scholar] [CrossRef]
- Rodrigues, F.J.; Cedran, M.F.; Bicas, J.L.; Sato, H.H. Encapsulated probiotic cells: Relevant techniques, natural sources as encapsulating materials and food applications-a narrative review. Food Res. Int. 2020, 137, 109682. [Google Scholar] [CrossRef]
- Zare, M.; Dziemidowicz, K.; Williams, G.R.; Ramakrishna, S. Encapsulation of pharmaceutical and nutraceutical active ingredients using electrospinning processes. Nanomaterials 2021, 11, 1968. [Google Scholar] [CrossRef]
- Rostamabadi, H.; Assadpour, E.; Tabarestani, H.S.; Falsafi, S.R.; Jafari, S.M. Electrospinning approach for nanoencapsulation of bioactive compounds; recent advances and innovations. Trends Food Sci. Tech. 2020, 100, 190–209. [Google Scholar] [CrossRef]
- Coelho, S.C.; Estevinho, B.N.; Rocha, F. Encapsulation in food industry with emerging electrohydrodynamic techniques: Electrospinning and electrospraying–A review. Food Chem. 2021, 339, 127850. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.; Zong, M.H.; Linhardt, R.J.; Feng, K.; Wu, H. Electrospinning: A novel nano-encapsulation approach for bioactive compounds. Trends Food Sci. Technol. 2017, 70, 56–68. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.Q.; Xia, Y.N. Electrospinning and electrospun nanofibers: Methods, materials, and applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Lim, L.T.; Mendes, A.C.; Chronakis, I.S. Electrospinning and electrospraying technologies for food applications. Adv. Food Nutr. Res. 2019, 88, 167–234. [Google Scholar]
- Jacobsen, C.; García-Moreno, P.J.; Mendes, A.C.; Mateiu, R.V.; Chronakis, I.S. Use of electrohydrodynamic processing for encapsulation of sensitive bioactive compounds and applications in food. Annu. Rev. Food Sci. Technol. 2018, 9, 525–549. [Google Scholar] [CrossRef]
- Rostami, M.; Yousefi, M.; Khezerlou, A.; Mohammadi, M.A.; Jafari, S.M. Application of different biopolymers for nanoencapsulation of antioxidants via electrohydrodynamic processes. Food Hydrocoll. 2019, 97, 105170. [Google Scholar] [CrossRef]
- Moreira, A.; Lawson, D.; Onyekuru, L.; Dziemidowicz, K.; Angkawinitwong, U.; Costa, P.F.; Radasci, N.; Williams, G.R. Protein encapsulation by electrospinning and electrospraying. J. Control. Release 2020, 329, 1172–1197. [Google Scholar] [CrossRef]
- Chui, C.Y.; Odeleye, A.; Nguyen, L.; Kasoju, N.; Soliman, E.; Ye, H. Electrosprayed genipin cross-linked alginate-chitosan microcarriers for ex vivo expansion of mesenchymal stem cells. J. Biomed. Mater. Res. A 2018, 107, 122–133. [Google Scholar] [CrossRef]
- Huang, R.M.; Feng, K.; Li, S.F.; Zong, M.H.; Han, S.Y. Enhanced survival of probiotics in the electrosprayed microcapsule by addition of fish oil. J. Food Eng. 2021, 307, 110650. [Google Scholar] [CrossRef]
- Suksamran, T.; Opanasopit, P.; Rojanarata, T.; Ngawhirunpat, T.; Ruktanonchai, U.P. Biodegradable alginate microparticles developed by electrohydrodynamic spraying techniques for oral delivery of protein. J. Microencapsul. 2009, 26, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Ghayempour, S.; Mortazavi, S.M. Fabrication of micro–nanocapsules by a new electrospraying method using coaxial jets and examination of effective parameters on their production. J. Electrostat. 2013, 71, 717–727. [Google Scholar] [CrossRef]
- Yao, Z.C.; Jin, L.J.; Ahamad, Z.; Huang, J.; Chang, M.W.; Li, J.S. Ganoderma lucidum polysaccharide loaded sodium alginate micro-particles prepared via electrospraying in controlled deposition environments. Int. J. Pharmaceut. 2017, 524, 148–158. [Google Scholar] [CrossRef]
- Pankongadisak, P.; Ruktanonchai, R.U.; Supaphol, P.O. Preparation and characterization of silver nanoparticles-loaded calcium alginate beads embedded in gelatin scaffolds. AAPS PharmSciTech 2014, 15, 1105–1115. [Google Scholar] [CrossRef]
- Gryshkov, O.; Pogozhykh, D.; Zernetsch, H.; Hofmann, N.; Mueller, T.; Glasmacher, B. Process engineering of high voltage alginate encapsulation of mesenchymal stem cells. Mat. Sci. Eng. C 2014, 36, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Azad, A.K.; Al-Mahmood SM, A.; Chatterjee, B.; Sulaiman WM, A.W.; Elsayed, T.M.; Doolaanea, A.A. Encapsulation of black seed oil in alginate beads as a pH-sensitive carrier for intestine-targeted drug delivery: In vitro, in vivo and ex vivo Study. Pharmaceutics 2020, 12, 219. [Google Scholar] [CrossRef]
- Zhao, D.; Li, J.S.; Suen, W.; Chang, M.W.; Huang, J. Preparation and characterization of Ganoderma lucidum spores-loaded alginate microspheres by electrospraying. Mat. Sci. Eng. C 2016, 62, 835–842. [Google Scholar] [CrossRef]
- Klokk, T.I.; Melvik, J.E. Controlling the size of alginate gel beads by use of a high electrostatic potential. J. Microencapsul. 2002, 19, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.; Cho, G.Y.; Bai, S.J. Alginate Hydrogel Beads for Immobilizing Single Photosynthetic Cells. Int. J. Precis. Eng. Manuf. 2020, 21, 739–745. [Google Scholar] [CrossRef]
- Qayyum, A.S.; Jain, E.; Kolar, G.; Kim, Y.; Sell, S.A.; Zustiak, S.P. Design of electrohydrodynamic sprayed polyethylene glycol hydrogel microspheres for cell encapsulation. Biofabrication 2017, 9, 025019. [Google Scholar] [CrossRef]
- Panahi, A.; Pishevar, A.R.; Tavakoli, M.R. Experimental investigation of electrohydrodynamic modes in electrospraying of viscoelastic polymeric solutions. Phys. Fluids 2020, 32, 012116. [Google Scholar] [CrossRef]
- Khorram, S.M. Electro-spray of high viscous liquids for producing mono-sized spherical alginate beads. Particuology 2008, 6, 271–275. [Google Scholar]
- Ma, Y.T.; Björnmalm, M.; Wise, A.K.; Cortez-Jugo, C.; Revalor, E.; Ju, Y.; Feeney, O.M.; Richardson, R.T.; Hanssen, E.; Shepherd, R.K.; et al. Gel-mediated electrospray assembly of silica supraparticles for sustained drug delivery. ACS Appl. Mater. Interfaces 2018, 10, 31019–31031. [Google Scholar] [CrossRef] [PubMed]
- Celebioglu, A.; Uyar, T. Fast-dissolving antioxidant curcumin/cyclodextrin inclusion complex electrospun nanofibrous webs. Food Chem. 2020, 317, 126397. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.; Zhu, D.H.; Feng, K.; Liu, F.J.; Lou, W.Y.; Li, N.; Zong, M.H.; Wu, H. Fabrication of electrospun polylactic acid nanofilm incorporating cinnamon essential oil/β-cyclodextrin inclusion complex for antimicrobial packaging. Food Chem. 2016, 196, 996–1004. [Google Scholar] [CrossRef]
- Wen, P.; Hu, T.G.; Li, L.; Zong, M.H.; Wu, H. A colon-specific delivery system for quercetin with enhanced cancer prevention based on co-axial electrospinning. Food Funct. 2018, 9, 5999. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Feng, K.; Wen, P.; Zong, M.H.; Lou, W.Y.; Wu, H. Enhancing oxidative stability of encapsulated fish oil by incorporation of ferulic acid into electrospun zein mat. LWT-Food Sci. Technol. 2017, 84, 82–90. [Google Scholar] [CrossRef]
- Feng, K.; Wen, P.; Yang, H.; Lou, W.Y.; Li, N.; Zong, M.H.; Wu, H. Enhancement of the antimicrobial activity of cinnamon essential oil-based electrospun nanofilm by incorporation of lysozyme. RSC Adv. 2017, 7, 1572–1580. [Google Scholar] [CrossRef]
- Feng, K.; Li, S.H.; Wei, Y.S.; Zong, M.H.; Hu, T.G.; Wu, H.; Han, S.Y. Fabrication of nanostructured multi-unit vehicle for intestinal-specific delivery and controlled release of peptide. Nanotechnology 2021, 32, 245101. [Google Scholar] [CrossRef]
- Li, C.; Wei, Y.S.; Wen, P.; Feng, K.; Zong, M.H.; Wu, H. Preparation and characterization of an electrospun colon-specific delivery system for salmon calcitonin. RSC Adv. 2018, 8, 9762–9769. [Google Scholar] [CrossRef]
- Padrão, J.R.; Casal, M.; Lanceros-Méndez, S.; Rodrigues, L.R.; Dourado, F.; Sencadas, V. Antibacterial performance of bovine lactoferrin-fish gelatin electrospun membranes. Int. J. Biol. Macromol. 2015, 81, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.; Hu, T.G.; Wen, Y.; Linhardt, R.J.; Zong, M.H.; Zou, Y.X.; Wu, H. Targeted delivery of phycocyanin for the prevention of colon cancer using electrospun fibers. Food Funct. 2019, 10, 1816–1825. [Google Scholar] [CrossRef] [PubMed]
- Feng, K.; Li, C.; Wei, Y.S.; Zong, M.H.; Wu, H. Development of a polysaccharide based multi-unit nanofiber mat for colon-targeted sustained release of salmon calcitonin. J. Colloid Interface Sci. 2019, 552, 186–195. [Google Scholar] [CrossRef]
- Wang, C.; Ma Chao Wu, Z.K.; He, L.; Yan, P.; Song, J.; Ma, N.; Zhao, Q.H. Enhanced bioavailability and anticancer effect of curcumin-loaded electrospun nanofiber: In vitro and in vivo study. Nanoscale Res. Lett. 2015, 10, 439. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wen, P.; Feng, K.; Zong, M.H.; Lou, W.Y.; Wu, H. Encapsulation of fish oil in a coaxial electrospun nanofibrous mat and its properties. RSC Adv. 2017, 7, 14939–14946. [Google Scholar] [CrossRef]
- Feng, K.; Zhai, M.Y.; Wei, Y.S.; Zong, M.H.; Wu, H.; Han, S.Y. Fabrication of nano/micro-structured electrospun detection card for the detection of pesticide residues. Foods 2021, 10, 889. [Google Scholar] [CrossRef]
- Khan, R.S.; Rather, A.H.; Wani, T.U.; Rather, S.U.; Amna, T.; Hassan, M.S.; Sheikh, F.A. Recent trends using natural polymeric nanofibers as supports for enzyme immobilization and catalysis. Biotechnol. Bioeng. 2023, 120, 22–40. [Google Scholar] [CrossRef]
- Fung, W.Y.; Yuen, K.H.; Liong, M.T. Agrowaste-based nanofibers as a probiotic encapsulant: Fabrication and characterization. J. Agr. Food Chem. 2011, 59, 8140–8147. [Google Scholar] [CrossRef]
- Feng, K.; Huang, R.M.; Wu, R.Q.; Wei, Y.S.; Zong, M.H.; Linhardt, R.J.; Wu, H. A novel route for double-layered encapsulation of probiotics with improved viability under adverse conditions. Food Chem. 2020, 310, 125977. [Google Scholar] [CrossRef]
- Škrlec, K.; Zupančič, Š.; Mihevc, S.P.; Kocbek, P.; Kristl, J.; Berlec, A. Development of electrospun nanofibers that enable high loading and long-term viability of probiotics. Eur. J. Pharm. Biopharm. 2019, 136, 108–119. [Google Scholar] [CrossRef]
- López-Rubio, A.; Sanchez, E.; Wilkanowicz, S.; Sanz, Y.; Lagaron, J.M. Electrospinning as a useful technique for the encapsulation of living Bifidobacteria in Food hydrocolloid. Food Hydrocoll. 2012, 28, 159–167. [Google Scholar] [CrossRef]
- Alehosseini, A.; Sarabi-Jamab, M.; Ghorani, B.; Kadkhodaee, R. Electro-encapsulation of Lactobacillus casei in high-resistant capsules of whey protein containing transglutaminase enzyme. LWT-Food Sci. Technol. 2019, 102, 150–158. [Google Scholar] [CrossRef]
- Moreno, J.S.; Dima, P.; Chronakis, I.S.; Mendes, A.C. Electrosprayed ethyl cellulose core-shell microcapsules for the encapsulation of probiotics. Pharmaceutics 2022, 14, 7. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Belcher, A.M. Virus-based fabrication of micro- and nanofibers using electrospinning. Nano Lett. 2004, 4, 387–390. [Google Scholar] [CrossRef]
- Salalha, W.; Kuhn, J.; Dror, Y.; Zussman, E. Encapsulation of bacteria and viruses in electrospun nanofibres. Nanotechnology 2006, 17, 4675–4681. [Google Scholar] [CrossRef] [PubMed]
- Heunis, T.D.; Botes, J.M.; Dicks LM, T. Encapsulation of Lactobacillus plantarum 423 and its bacteriocin in nanofibers. Probiotics Antimicrob. Proteins 2010, 2, 46–51. [Google Scholar]
- Gensheimer, M.; Becker, M.; Brandis-Heep, A.; Wendorff, J.H.; Thauer, R.K.; Greiner, A. Novel biohybrid materials by electrospinning: Nanofibers of poly(ethylene oxide) and living bacteria. Adv. Mater. 2007, 19, 2480–2482. [Google Scholar] [CrossRef]
- Zupančič, Š.; Škrlec, K.; Kocbek, P.; Kristl, J.; Berlec, A. Effects of electrospinning on the viability of ten species of Lactic acid bacteria in poly(ethylene oxide) nanofibers. Pharmaceutics 2019, 11, 483. [Google Scholar] [CrossRef]
- Krasowska, A.; Sigler, K. How microorganisms use hydrophobicity and what does this mean for human needs? Front. Cell. Infect. Microbiol. 2014, 4, 112. [Google Scholar] [CrossRef]
- Feng, K.; Zhai, M.Y.; Zhang, Y.; Linhardt, R.J.; Zong, M.H.; Li, L.; Wu, H. Improved viability and thermal stability of the probiotics encapsulated in a novel electrospun fiber mat. J. Agr. Food Chem. 2018, 66, 10890–10897. [Google Scholar] [CrossRef]
- Ceylan, Z.; Uslu, E.; Spirli, H.; Meral, R.; Gavgali, M.; Yilmaz, M.T.; Dertli, E. A novel perspective for Lactobacillus reuteri: Nanoencapsulation to obtain functional fish fillets. LWT-Food Sci. Technol. 2019, 115, 108427. [Google Scholar] [CrossRef]
- Atraki, R.; Azizkhani, M. Survival of probiotic bacteria nanoencapsulated within biopolymers in a simulated gastrointestinal model. Innov. Food Sci. Emerg. Technol. 2021, 72, 102750. [Google Scholar] [CrossRef]
- Hajikhani, M.; Lin, M.S. A review on designing nanofibers with high porous and rough surface via electrospinning technology for rapid detection of food quality and safety attributes. Trends Food Sci. Technol. 2022, 128, 118–128. [Google Scholar] [CrossRef]
- Diep, E.; Schiffman, J.D. Encapsulating bacteria in alginate-based electrospun nanofibers. Biomater. Sci. 2021, 9, 4364. [Google Scholar] [CrossRef]
- Ma, J.G.; Xu, C.; Yu, H.L.; Feng, Z.B.; Yu, W.; Gu, L.Y.; Liu, Z.J.; Chen, L.J.; Jiang, Z.M.; Hou, J.C. Electro-encapsulation of probiotics in gum Arabic-pullulan blend nanofibres using electrospinning technology. Food Hydrocoll. 2021, 111, 106381. [Google Scholar] [CrossRef]
- Stojanov, S.; Plavec, T.V.; Kristl, J.; Zupančič, Š.; Berlec, A. Engineering of vaginal Lactobacilli to express fluorescent proteins enables the analysis of their mixture in nanofibers. Int. J. Mol. Sci. 2021, 22, 13631. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Zare, E.N.; Torres-Mendieta, R.; Wacawek, S.; Makvandi, P.; Černík, M.; Padil VV, T.; Varma, R.S. Electrospun fibers based on botanical, seaweed, microbial, and animal sourced Biomacromolecules and their multidimensional applications. Int. J. Biol. Macromol. 2021, 171, 130–149. [Google Scholar] [CrossRef]
- Kumar, T.S.M.; Kumar, K.S.; Rajini, N.; Siengchin, S.; Ayrilmis, N.; Rajulu, A.V. A comprehensive review of electrospun nanofibers: Food and packaging perspective. Compos. Part B Eng. 2019, 175, 107074. [Google Scholar] [CrossRef]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.M.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef]
- Ta, L.P.; Bujna, E.; Antal, O.; Ladányi, M.; Juhász, R.; Szécsi, A.; Kun, S.; Sudheer, S.; Gupta, V.K.; Nguyen, Q.D. Effects of various polysaccharides (alginate, carrageenan, gums, chitosan) and their combination with prebiotic saccharides (resistant starch, lactosucrose, lactulose) on the encapsulation of probiotic bacteria Lactobacillus casei 01 strain. Int. J. Biol. Macromol. 2021, 183, 1136–1144. [Google Scholar] [CrossRef]
- Luca, L.; Oroian, M. Influence of different prebiotics on viability of Lactobacillus casei, Lactobacillus plantarum and Lactobacillus rhamnosus encapsulated in alginate microcapsules. Foods 2021, 10, 710. [Google Scholar] [CrossRef] [PubMed]
- Duman, D.; Karadag, A. Inulin added electrospun composite nanofibres by electrospinning for the encapsulation of probiotics: Characterisation and assessment of viability during storage and simulated gastrointestinal digestion. Int. J. Food Sci. Technol. 2021, 56, 927–935. [Google Scholar] [CrossRef]
- Liu, S.C.; Li, R.; Tomasula, P.M.; Sousa AM, M.; Liu, L.S. Electrospun food-grade ultrafine fibers from pectin and pullulan blends. Food Nutr. Sci. 2016, 7, 636–646. [Google Scholar] [CrossRef]
- Ghorbani, S.; Maryam, A. Encapsulation of lactic acid bacteria and Bifidobacteria using starch-sodium alginate nanofibers to enhance viability in food model. J. Food Process. Pres. 2021, 45, e16048. [Google Scholar] [CrossRef]
- Nagy, Z.K.; Wagner, I.; Suhajda, Á.; Tobak, T.; Harasztos, A.H.; Vigh, T.; Sóti, P.L.; Pataki, H.; Molnár, K.; Marosi, G. Nanofibrous solid dosage form of living bacteria prepared by electrospinning. Express Polym. Lett. 2014, 8, 352–361. [Google Scholar] [CrossRef]
- Ceylan, Z.; Meral, R.; Karakas, C.Y.; Dertli, E.; Yilmaz, M.T. A novel strategy for probiotic bacteria: Ensuring microbial stability of fish fillets using characterized probiotic bacteria-loaded nanofibers. Innov. Food Sci. Emerg. Technol. 2018, 48, 212–218. [Google Scholar] [CrossRef]
- Karakas, C.K.; Duman, D.; Yilmaz, M.T. Encapsulation of probiotic living cells in alginate-PVA based electrospun nanofibers: Evaluation of viability and survival in simulated gastrointestinal conditions. J. Biotechnol. 2018, 280, S12–S31. [Google Scholar] [CrossRef]
- Ceylan, Z.; Meral, R.; Cavidoglu, I.; Karakas, C.Y.; Yilmaz, M.T. A new application on fatty acid stability of fish fillets: Coating with probiotic bacteria-loaded polymer-based characterized nanofibers. J. Food Saf. 2018, 38, e12547. [Google Scholar] [CrossRef]
- Zupančič, Š.; Rijavec, T.; Lapanje, A.; Petelin, M.; Kristl, J.; Kocbek, P. Nanofibers with incorporated autochthonous bacteria as potential probiotics for local treatment of periodontal disease. Biomacromolecules 2018, 19, 4299–4306. [Google Scholar] [CrossRef]
- Khan, M.A.; Hussain, Z.; Ali, S.; Qamar, Z.; Imran, M.; Hafeez, Y. Fabrication of electrospun probiotic functionalized nanocomposite scaffolds for infection control and dermal burn healing in a mice model. ACS Biomater. Sci. Eng. 2019, 5, 6109–6116. [Google Scholar] [CrossRef]
- Mojaveri, S.J.; Hosseini, S.F.; Gharsallaoui, A. Viability improvement of Bifidobacterium animalis bb12 by encapsulation in chitosan/poly(vinyl alcohol) hybrid electrospun fiber mats. Carbohydr. Polym. 2020, 241, 116278. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, M.T.; Taylan, O.; Karakas, C.Y.; Dertli, E. An alternative way to encapsulate probiotics within electrospun alginate nanofibers as monitored under simulated gastrointestinal conditions and in kefir. Carbohydr. Polym. 2020, 244, 116447. [Google Scholar] [CrossRef] [PubMed]
- Ragavan, M.L.; Das, N. Nanoencapsulation of Saccharomycopsis fibuligera VIT-MN04 using electrospinning technique for easy gastrointestinal transit. IET Nanobiotechnol. 2020, 14, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, E.; Pantea, E.; Vass, P.; Domján, J.; Molnár, M.; Suhajda, Á.; Andersen, S.K.; Vigh, T.; Verreck, M.; Marosi, G.; et al. Probiotic bacteria stabilized in orally dissolving nanofibers prepared by high-speed electrospinning. Food Bioprod. Process. 2021, 128, 84–94. [Google Scholar] [CrossRef]
- Wei, L.L.; Zhou, D.; Kang, X.J. Electrospinning as a novel strategy for the encapsulation of living probiotics in polyvinyl alcohol/silk fibroin. Innov. Food Sci. Emerg. Technol. 2021, 71, 102726. [Google Scholar] [CrossRef]
- Xu, C.; Ma, J.; Wang, W.; Liu, Z.J.; Gu, L.Y.; Qian, S.S.; Hou, J.C. Preparation of pectin-based nanofibers encapsulating Lactobacillus rhamnosus 1.0320 by electrospinning. Food Hydrocoll. 2022, 124, 107216. [Google Scholar] [CrossRef]
- Fareed, F.; Saeed, F.; Afzaal, M.; Imran, A.; Ahmad, A.; Mahmood, K.; Shah, Y.A.; Hussain, M.; Ateeq, H. Fabrication of electrospun gum Arabic–polyvinyl alcohol blend nanofibers for improved viability of the probiotic. J. Food Sci. Technol. 2022, 59, 4812–4821. [Google Scholar] [CrossRef]
- Grilc, N.K.; Zidar, A.; Kocbek, P.; Rijavec, T.; Colja, T.; Lapanje, A.; Jeras, M.; Gobec, M.; Mlinarič-Raščan, I.; Gašperlin, M.; et al. Nanofibers with genotyped Bacillus strains exhibiting antibacterial and immunomodulatory activity. J. Control. Release 2023, 355, 371–384. [Google Scholar] [CrossRef]
- Simonič, M.; Slapničar, Š.; Trček, J.; Matijašić, B.B.; Lorbeg, P.M.; Vesel, A.; Zemljič, L.F.; Fratnik, Z.P. Probiotic Lactobacillus paragasseri K7 nanofiber encapsulation using nozzle-free electrospinning. Appl. Biochem. Biotechnol. 2023, 15, 1–22. [Google Scholar] [CrossRef]
- Ghalehjooghi, H.D.; Tajik, H.; Shahbazi, Y. Development and characterization of active packaging nanofiber mats based on gelatin-sodium alginate containing probiotic microorganisms to improve the shelf-life and safety quality of silver carp fillets. Int. J. Food Microbiol. 2023, 384, 109984. [Google Scholar] [CrossRef]
- López-Rubio, A.; Sanchez, E.; Sanz, Y.; Lagaron, J.M. Encapsulation of living Bifidobacteria in ultrathin PVOH electrospun fibers. Biomacromolecules 2009, 10, 2823–2829. [Google Scholar] [CrossRef] [PubMed]
- Lancuški, A.; Ammar, A.A.; Avrahami, R.; Vilensky, R.; Vasilyev, G.; Zussman, E. Design of starch-formate compound fibers as encapsulation platform for biotherapeutics. Carbohydr. Polym. 2017, 158, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Feng, K.; Wei, Y.S.; Hu, T.G.; Linhardt, R.J.; Zong, M.H.; Wu, H. Colon-targeted delivery systems for nutraceuticals: A review of current vehicles, evaluation methods and future prospects. Trends Food Sci. Technol. 2020, 102, 203–222. [Google Scholar] [CrossRef]
- Cui, M.X.; Zhang, M.; Liu, K.H. Colon-targeted drug delivery of polysaccharide-based nanocarriers for the synergistic treatment of inflammatory bowel disease:A review. Carbohydr. Polym. 2021, 272, 118530. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.A.; Pereira, E.D.; Raphaelli, C.D.; Radünz, M.; Camargo, T.M.; Cocenco, F.I.G.D.; Radunz, M.; Camargo, T.M.; Cantillano, R.F.F.; Fiorentini, Â.M.; et al. Application of prebiotics in apple products and potential health benefits. J. Food Sci. Technol. 2021, 59, 1249–1262. [Google Scholar] [CrossRef]
- Yu, H.L.; Liu, W.H.; Li, D.M.; Liu, C.C.; Feng, Z.B.; Jiang, B. Targeting delivery system for Lactobacillus plantarum based on functionalized electrospun nanofibers. Polymers 2020, 12, 1565. [Google Scholar] [CrossRef]
- Xu, C.; Ma, J.G.; Liu, Z.J.; Wang, W.; Liu, X.; Qian, S.S.; Chen, L.J.; Gu, L.Y.; Sun, C.Q.; Hou, J.C.; et al. Preparation of shell-core fiber-encapsulated Lactobacillus rhamnosus 1.0320 using coaxial electrospinning. Food Chem. 2023, 402, 134253. [Google Scholar] [CrossRef]
- Çanga, E.M.; Dudak, F.C. Improved digestive stability of probiotics encapsulated within poly(vinyl alcohol)/cellulose acetate hybrid fibers. Carbohydr. Polym. 2021, 264, 117990. [Google Scholar] [CrossRef]
- Ajalloueian, F.; Guerra, P.R.; Bahl, M.I.; Trop, A.M.; Hwu, E.T.; Licht, T.R.; Boisen, A. Multi-layer PLGA-pullulan-PLGA electrospun nanofibers for probiotic delivery. Food Hydrocoll. 2022, 123, 107112. [Google Scholar] [CrossRef]
- Jun, I.; Han, H.S.; Edwards, J.R.; Jeon, H. Electrospun fibrous scaffolds for tissue engineering: Viewpoints on architecture and fabrication. Int. J. Mol. Sci. 2018, 19, 745. [Google Scholar] [CrossRef]
- San, N.O.; Celebioglu, A.; Tümtaş, Y.; Uyar, T.; Tekinay, T. Reusable bacteria immobilized electrospun nanofibrous webs for decolorization of methylene blue dye in wastewater treatment. RSC Adv. 2014, 4, 32249–32255. [Google Scholar] [CrossRef]
- Sarioglu, O.F.; Keskin, N.; Celebioglu, A.; Tekinay, T.; Uyar, T. Bacteria immobilized electrospun polycaprolactone and polylactic acid fibrous webs for remediation of textile dyes in water. Chemosphere 2017, 184, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.X.; Li, J.N.; Guo, Q.; Zhu, Y.Q.; Niu, H.M. Probiotics biofilm-integrated electrospun nanofiber membranes: A new starter culture for fermented milk production. J. Agr. Food Chem. 2019, 67, 3198–3208. [Google Scholar] [CrossRef] [PubMed]
- Valamehr, B.; Jonas, S.J.; Polleux, J.; Qiao, R.; Guo, S.; Gschweng, E.H.; Stiles, B.; Kam, K.; Luo, T.J.; Witte, O.N.; et al. Hydrophobic surfaces for enhanced differentiation of embryonic stem cell-derived embryoid bodies. Proc. Natl. Acad. Sci. USA 2008, 105, 14459–14464. [Google Scholar] [CrossRef] [PubMed]
- Anselme, K.; Davidson, P.; Popa, A.M.; Giazzon, M.; Liley, M.; Ploux, L. The interaction of cells and bacteria with surfaces structured at the nanometre scale. Acta Biomater. 2010, 6, 3824–3846. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Li, S.F.; Feng, K.; Han, S.Y.; Hu, T.G.; Wu, H. Improving the viability of probiotics under harsh conditions by the formation of biofilm on electrospun nanofiber mat. Foods 2022, 11, 1203. [Google Scholar] [CrossRef]
- Berezina, O.Y.; Vasilyeva, A.V.; Sidorova, N.A.; Savushkin, A.I.; Marcova, N.P. The effect of polyvinylpyrrolidone nanowires on the metabolic activity of Lactobacillus acidophilus. IOP Conf. Ser. Mater. Sci. Eng. 2019, 525, 012058. [Google Scholar] [CrossRef]
- Jayani, T.; Sanjeev, B.; Marimuthu, S.; Uthandi, S. Bacterial cellulose nano fiber (BCNF) as carrier support for the immobilization of probiotic, Lactobacillus acidophilus 016. Carbohydr. Polym. 2020, 250, 116965. [Google Scholar] [CrossRef]
- Grzywaczyk, A.; Zdarta, A.; Jankowska, K.; Biadasz, A.; Zdarta, J.; Jesionowski, T.; Kaczorek, E.; Smułek, W. New biocomposite electrospun fiber/alginate hydrogel for probiotic bacteria immobilization. Materials 2021, 14, 3861. [Google Scholar] [CrossRef]
- Harandi, F.N.; Khorasani, A.C.; Shojaosadati, S.A.; Hashemi-Najafabadi, S. Surface modification of electrospun wound dressing material by Fe2O3 nanoparticles incorporating Lactobacillus strains for enhanced antimicrobial and antibiofilm activity. Surf. Interfaces 2022, 28, 101592. [Google Scholar] [CrossRef]
- Amiri, S.; Teymorlouei, M.J.; Bari, M.R.; Khaledabad, M.A. Development of Lactobacillus acidophilus LA5-loaded whey protein isolate/lactose bionanocomposite powder by electrospraying: A strategy for entrapment. Food Biosci. 2021, 43, 101222. [Google Scholar] [CrossRef]
- Gómez-Mascaraque, L.G.; Morfin, R.C.; Pérez-Masiá, R.; Sanchez, G.; López-Rubio, A. Optimization of electrospraying conditions for the microencapsulation of probiotics and evaluation of their resistance during storage and in-vitro digestion. LWT-Food Sci. Technol. 2016, 69, 438–446. [Google Scholar] [CrossRef]
- Gómez-Mascaraque, L.G.; Ambrosio-Martín, J.; Perez-Masiá, R.; López-Rubio, A. Impact of acetic acid on the survival of L. plantarum upon microencapsulation by coaxial electrospraying. J. Healthc. Eng. 2017, 4698079. [Google Scholar]
- Librán, C.M.; Castro, S.; Lagaron, J.M. Encapsulation by electrospray coating atomization of probiotic strains. Innov. Food. Sci. Emerg. Technol. 2017, 39, 216–222. [Google Scholar] [CrossRef]
- Moayyedi, M.; Eskandari, M.H.; Rad AH, E.; Ziaee, E.; Khodaparast MH, H.; Golmakani, M.T. Effect of drying methods (electrospraying, freeze drying and spray drying) on survival and viability of microencapsulated Lactobacillus rhamnosus ATCC 7469. J. Funct. Foods 2018, 40, 391–399. [Google Scholar] [CrossRef]
- Premjit, Y.; Mitra, J. Optimization of electrospray-assisted microencapsulation of probiotics (Leuconostoc lactis) in soy protein isolate-oil particles using Box-Behnken experimental design. Food Bioprocess Technol. 2021, 14, 1712–1729. [Google Scholar] [CrossRef]
- Laelorspoen, N.; Wongsasulak, S.; Yoovidhya, T.; Devahastin, S. Microencapsulation of lactobacillus acidophilus in zein-alginate core-shell microcapsules via electrospraying. J. Funct. Foods 2014, 7, 342–349. [Google Scholar] [CrossRef]
- Coghetto, C.C.; Brinques, G.B.; Siqueira, N.M.; Pletsch, J.; Soares RM, D.; Ayub MA, Z. Electrospraying microencapsulation of Lactobacillus plantarum enhances cell viability under refrigeration storage and simulated gastric and intestinal fluids. J. Funct. Foods 2016, 24, 316–326. [Google Scholar] [CrossRef]
- Coghetto, C.C.; Flores, S.H.; Brinques, G.B.; Ayub MA, Z. Viability and alternative uses of a dried powder, microencapsulated Lactobacillus plantarum without the use of cold chain or dairy products. LWT-Food Sci. Technol. 2016, 71, 54–59. [Google Scholar] [CrossRef]
- Zaeim, D.; Sarabi-Jamab, M.; Ghorani, B.; Kadkhoda, R.; Tromp, R.H. Electrospray assisted fabrication of hydrogel microcapsules by single-and double-stage procedures for encapsulation of probiotics. Food Bioprod. Process. 2017, 102, 250–259. [Google Scholar] [CrossRef]
- Zaeim, D.; Sarabi-Jamab, M.; Ghorani, B.; Kadkhodaee, R. Double layer co-encapsulation of probiotics and prebiotics by electro-hydrodynamic atomization. LWT-Food Sci. Technol. 2019, 110, 102–109. [Google Scholar] [CrossRef]
- Ta, L.P.; Bujna, E.; Kun, S.; Charalampopoulos, D.; Khutoryanskiy, V.V. Electrosprayed mucoadhesive alginate-chitosan microcapsules for gastrointestinal delivery of probiotics. Int. J. Pharmaceut. 2021, 597, 120342. [Google Scholar]
- Fritzen-Freire, C.B.; Prudencio, E.S.; Amboni RD, M.C.; Pinto, S.S.; Negrao-Murakami, A.N.; Murakami, F.S. Microencapsulation of Bifidobacteria by spray drying in the presence of prebiotics. Food Res. Int. 2012, 45, 306–312. [Google Scholar] [CrossRef]
- Raddatz, G.C.; Poletto, G.; Deus, C.D.; Codevilla, C.F.; Menezes, C. Use of prebiotic sources to increase probiotic viability in pectin microparticles obtained by emulsification/internal gelation followed by freeze-drying. Food Res. Int. 2019, 130, 108902. [Google Scholar] [CrossRef]
- Gandomi, H.; Abbaszadeh, S.; Misaghi, A.; Bokaie, S.; Noori, N. Effect of chitosan-alginate encapsulation with inulin on survival of Lactobacillus rhamnosus GG during apple juice storage and under simulated gastrointestinal conditions. LWT-Food Sci. Technol. 2016, 69, 365–371. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Eratte, D.; Dowling, K.; Barrow, C.J.; Adhikari, B.P. In-vitro digestion of probiotic bacteria and omega-3 oil co-microencapsulated in whey protein isolate-gum Arabic complex coacervates. Food Chem. 2017, 227, 129–136. [Google Scholar] [CrossRef]
- Harandi, F.N.; Khorasani, A.C.; Shojaosadati, S.A.; Hashemi-Najafabadi, S. Living Lactobacillus-ZnO nanoparticles hybrids as antimicrobial and antibiofilm coatings for wound dressing application. Mat. Sci. Eng. C-Mater. 2021, 130, 112457. [Google Scholar] [CrossRef]
- Iglesias, M.B.; Echeverría, G.; Viñas, I.; López, M.L.; Abadias, M. Biopreservation of fresh-cut pear using Lactobacillus rhamnosus GG and effect on quality and volatile compounds. LWT-Food Sci. Technol. 2018, 87, 581–588. [Google Scholar] [CrossRef]







| Probiotic | Polymer | Additive | Diameter (nm) | Key Point | Ref. |
|---|---|---|---|---|---|
| L. acidophilus | Soluble dietary fiber (SDF), poly(vinyl alcohol) (PVA) | - | 229–703 | The addition of SDF improved the melting temperature of nanofibers, suggesting possible protection of probiotics in thermally processed foods. | [50] |
| L. acidophilus | PVA and polyvinylpyrrolidone (PVP) | - | 142–934 | Probiotic cells encapsulated in the nanofibers exhibited long term stability when stored under the temperature below 7 °C. | [75] |
| L. rhamnosus | Pectin (PEC), pullulan (PUL) | - | - | The development of PEC/PUL fiber mat excludes the usage of synthetic PEO and organic solvents, and is therefore promising in the food industry. | [73] |
| L. plantarum | PVA | Fructo-oligosaccharide (FOS) | 410 ± 150 | The addition of FOS during electrospinning improved the viability and thermal stability of L. plantarum. | [49] |
| L. rhamnosus | PVA, sodium alginate (SA) | - | 60–580 | The probiotic cells’ loaded nanofiber would be a promising coating material for fish fillets to prevent the rapid proliferation of total bacteria. | [76] |
| L. brevis, L. reuteri, L. rhamnosus | PVA, SA | - | - | The encapsulation within the SA/PVA fiber mat could prevent the pepsin-induced degradation of the carriers in simulated gastric juice. | [77] |
| L. rhamnosus | PVA, SA | - | 60.09–522.1 | The L. rhamnosus-loaded PVA/SA nanofibers provided better stable in terms of fatty acids in fish fillets. | [78] |
| Strain 25.2.M | PEO, chitosan (CS) | - | 105 ± 30 | The viability of probiotics in the carriers was preserved after 12 months of storage at room temperature, and release could be controlled by selecting the polymers. | [79] |
| Enterococcus mundtii | PVA, PVP | Glycerol | 318 ± 12 | The shelf-life test supported that the survival of probiotics encapsulated in the scaffold was increased by 2.78 ± 0.10 log10 CFU compared to the bio-dispersion. | [80] |
| L. plantarum | PEO | Lyo-protectant (i.e., sucrose, trehalose) | 492 ± 35 | The viability was not vitally influenced by the voltage and relative humidity used during the electrospinning. The addition of lyo-protectant in the fibers is beneficial for the survival due to the interactions between the lyo-protectant and probiotic cells. | [53] |
| B. animalis | PVA, CS | Inulin | 117.5–217.6 | The survival of probiotics loaded in CS/PVA/Inulin fibers were significantly increased under simulated gastric and intestinal fluids. | [81] |
| L. paracasei | PVA, SA | - | 305 | The encapsulated probiotics exhibited enhanced tolerance to simulated gastric juice and improved viability/survival in kefir, respectively. Incorporation of probiotics in kefir has no obvious influence on the characteristic pseudoplastic flow behavior and viscoelastic nature. | [82] |
| Saccharomycopsis fibuligera (S. fibuligera) | Wheat bran fiber, exopolysaccharide, PVP | - | 250–300 | The survival of encapsulated probiotics increased in comparison to the free cells during the in vitro digestion. The encapsulated cells could maintain their viability during 56 days of storage at 4 °C. | [83] |
| L. fermentum | PVA, SA | Inulin (P95, GR and HPX) | 200–400 | The survival of cells especially encapsulated in the fiber mat containing inulin showed higher viability against SGF and SIF. | [72] |
| E. coli | PEO, SA | Polysorbate 80 | 167 ± 23 | 2.74 × 105 CFU/g of viable E. coli could be encapsulated in the fibers formed from the electrospun solution of 2.5/1.5/3 wt% SA/PEO/PS80. | [64] |
| L. paracasei | PVA, PEO | Glucose, lactose, mannitol, saccharose, trehalose, inulin, and skim milk | 856–969 | The use of excipients could reduce osmotic and dehydration stress during electrospinning and long-term storage, while increasing the survival of encapsulated cells. | [84] |
| L. plantarum | PVA, silk fibroin | - | 190 ± 70 | The encapsulated probiotics exhibited increased survival rates, after being treated in SGF for 2 h. | [85] |
| L. acidophilus, L. rhamnosus, B. bifdum, B. animalis | Corn starch, SA | - | ~797 | 81–100% of the initial population retained viability after treatment in SGF. | [62] |
| L. rhamnosus, L. acidophilus, B. bifidum, and B. animalis | Corn starch, SA | - | ~797 | The encapsulation of probiotics in the SA/starch nanofiber mats had higher protective effects compared to the encapsulation method with a single biopolymer. | [74] |
| L. rhamnosus, L. acidophilus, L. plantarum and L. casei | Gum Arabic (GA), PUL | - | - | The GA/PUL (20:80) fibers ensure higher cell survivability than freeze-drying samples, and the encapsulated cells maintained viability during 28 days of storage at 4 °C. | [65] |
| L. rhamnosus | PVA, PEC | - | - | The survival rate of encapsulated L. rhamnosus 1.0320 encapsulated in the PVA/PEC nanofibers was 84.63% after 21 days of storage at 4 °C. | [86] |
| L. acidophilus | PVA, GA | - | ~617 | Free cells lost their vitality, while encapsulated cells maintained a viability count above the recommended level (107 CFU) under simulated gastrointestinal conditions. | [87] |
| Bacillus strains | PEO, SA | - | 200–300 | Probiotics loaded in the nanofibers maintained good viability during the electrospinning and 6 months of storage at room temperature. Spores could be rapidly released from the PEO nanofibers, while presence of SA in the nanofiber prolonged their release. | [88] |
| L. paragasseri | PEO, SA | inulin | 300–600 | The probiotic form used in the electrospun samples influenced the release amount. | [89] |
| L. acidophilus, Limosilactobacillus reuteri, Lacticaseibacillus casei, Lacticaseibacillus rhamnosus | Gelatin (GE), SA | - | 423–429 | GE/SA nanofiber is a good platform for protecting live bacteria, inhibiting the growth of pathogenic bacteria, and extending the shelf life of fresh carp fillets under refrigerated conditions. | [90] |
| Probiotic | Polymer | Nozzle | Type | Size (μm) | Voltage (kV) | Distance (cm) | Flow Rate (mL/h) | Ref. |
|---|---|---|---|---|---|---|---|---|
| B. animalis | Whey protein concentrate (WPS), PUL | uniaxial | DE | 0.259–0.658 | 12–14 | 7 | 0.3 | [51] |
| L. plantarum | WPC, resistant starch (RS) | uniaxial | DE | 20–40 | 10–14 | 10 | 0.15 | [112] |
| L. plantarum | Shell: gelatin, Core: WPC | coaxial | DE | 0.6 ± 0.39 | 17 | 10 | Shell: 0.15, Core: 0.05 | [113] |
| B. longum subsp. infantis | WPC | uniaxial | DE | 2.47 ± 1.15 | - | - | - | [114] |
| L. rhamnosus | Whey protein isolate (WPI), inulin, gum | uniaxial | DE | 0.359–0.596 | 14 | 7 | 0.7 | [115] |
| L. casei | WPC, WPI | uniaxial | DE | 3.09 ± 1.04 | 14 | 10 | 0.5 | [52] |
| L. acidophilus | WPI, lactose | uniaxial | DE | 0.435 | 6–12 | 10 | 1 | [111] |
| Leuconostoc lactis | Soy protein isolate (SPI) | uniaxial | DE | 4.11 | 10–15 | 10 | 0.4 | [116] |
| B. animalis subsp. lactis | Shell: Ethyl cellulose, Core: maltodextrin | coaxial | DE | 3.33 ± 1.18 | 35 | 10 | Shell: 0.42, core: 0.21 | [53] |
| L. acidophilus | SA | uniaxial | WE | 315 ± 56 | 4–10 | 6 | 10 | [117] |
| L. plantarum | SA, PEC | uniaxial | WE | 111–116 | 24 | 15 | 2 | [118] |
| L. plantarum | SA | uniaxial | WE | 100–300 | 24 | 15 | 2 | [119] |
| L. plantarum | SA, CS | uniaxial | WE | 300–450 | 9.5 | 10 | 5 | [120] |
| L. plantarum and B. lactis | SA, CS, inulin, RS | uniaxial | WE | 710–1040 | 9.5 | 10 | 5 | [121] |
| L. plantarum | SA, CS, RS | uniaxial | WE | 30–1300 | 7–16 | 10 | - | [122] |
| L. plantarum | SA, PEC, SPI | coaxial | WE | ~1400 | 12 | 10 | Shell: 7, core: 3 | [20] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, K.; Huangfu, L.; Liu, C.; Bonfili, L.; Xiang, Q.; Wu, H.; Bai, Y. Electrospinning and Electrospraying: Emerging Techniques for Probiotic Stabilization and Application. Polymers 2023, 15, 2402. https://doi.org/10.3390/polym15102402
Feng K, Huangfu L, Liu C, Bonfili L, Xiang Q, Wu H, Bai Y. Electrospinning and Electrospraying: Emerging Techniques for Probiotic Stabilization and Application. Polymers. 2023; 15(10):2402. https://doi.org/10.3390/polym15102402
Chicago/Turabian StyleFeng, Kun, Lulu Huangfu, Chuanduo Liu, Laura Bonfili, Qisen Xiang, Hong Wu, and Yanhong Bai. 2023. "Electrospinning and Electrospraying: Emerging Techniques for Probiotic Stabilization and Application" Polymers 15, no. 10: 2402. https://doi.org/10.3390/polym15102402
APA StyleFeng, K., Huangfu, L., Liu, C., Bonfili, L., Xiang, Q., Wu, H., & Bai, Y. (2023). Electrospinning and Electrospraying: Emerging Techniques for Probiotic Stabilization and Application. Polymers, 15(10), 2402. https://doi.org/10.3390/polym15102402