Comparison of the Properties of Epoxy Resins Containing Various Trifluoromethyl Groups with Low Dielectric Constant
Abstract
:1. Introduction
2. Experimental
2.1. Materials
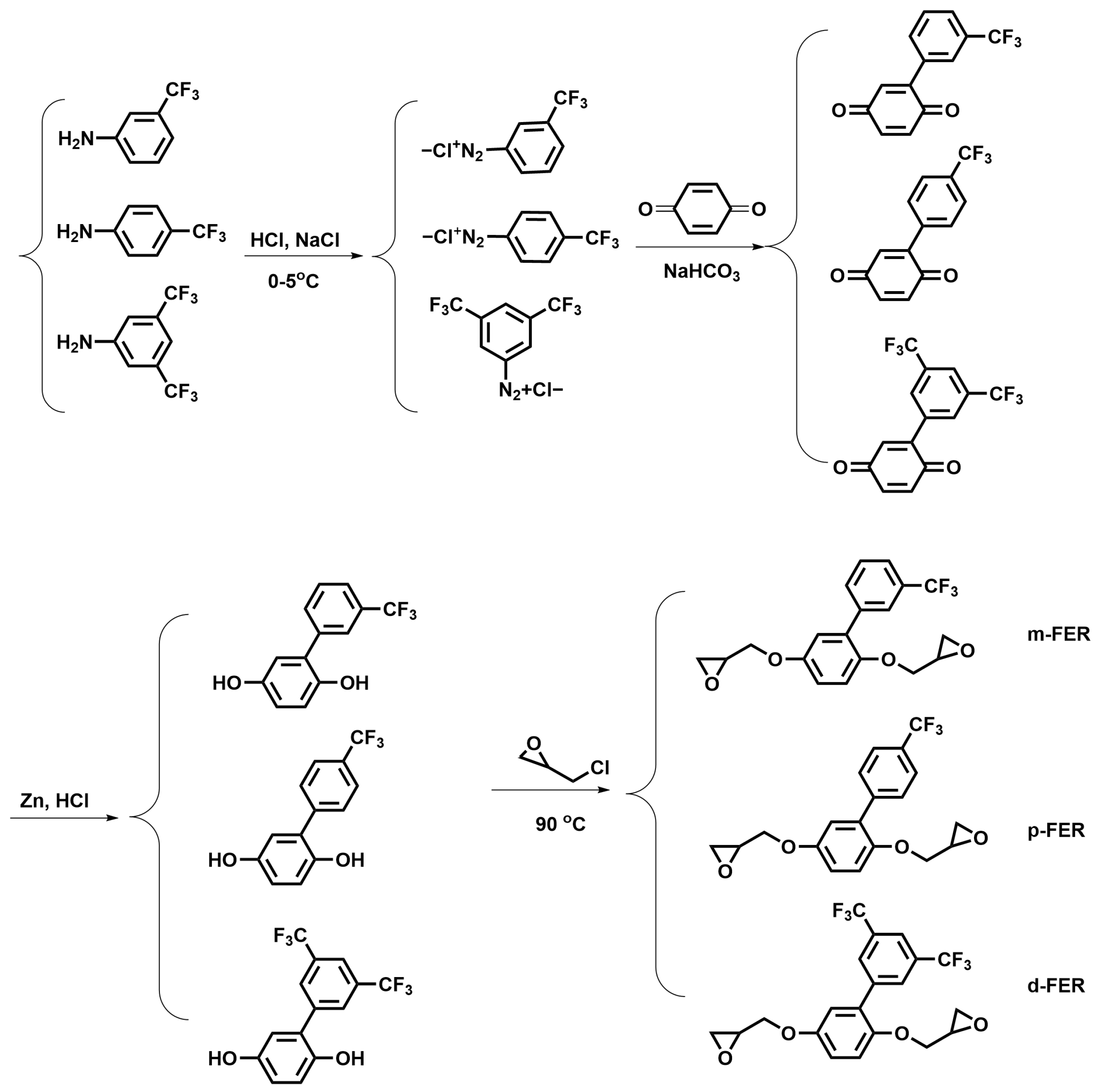
2.2. Synthesis of Phenylhydroquinones Containing Trifluoromethyl Groups (FPQs)
2.3. Synthesis of Epoxy Resin Monomers Containing Trifluoromethyl Groups (FERs)
2.4. Preparation of the Cured Epoxy Resins
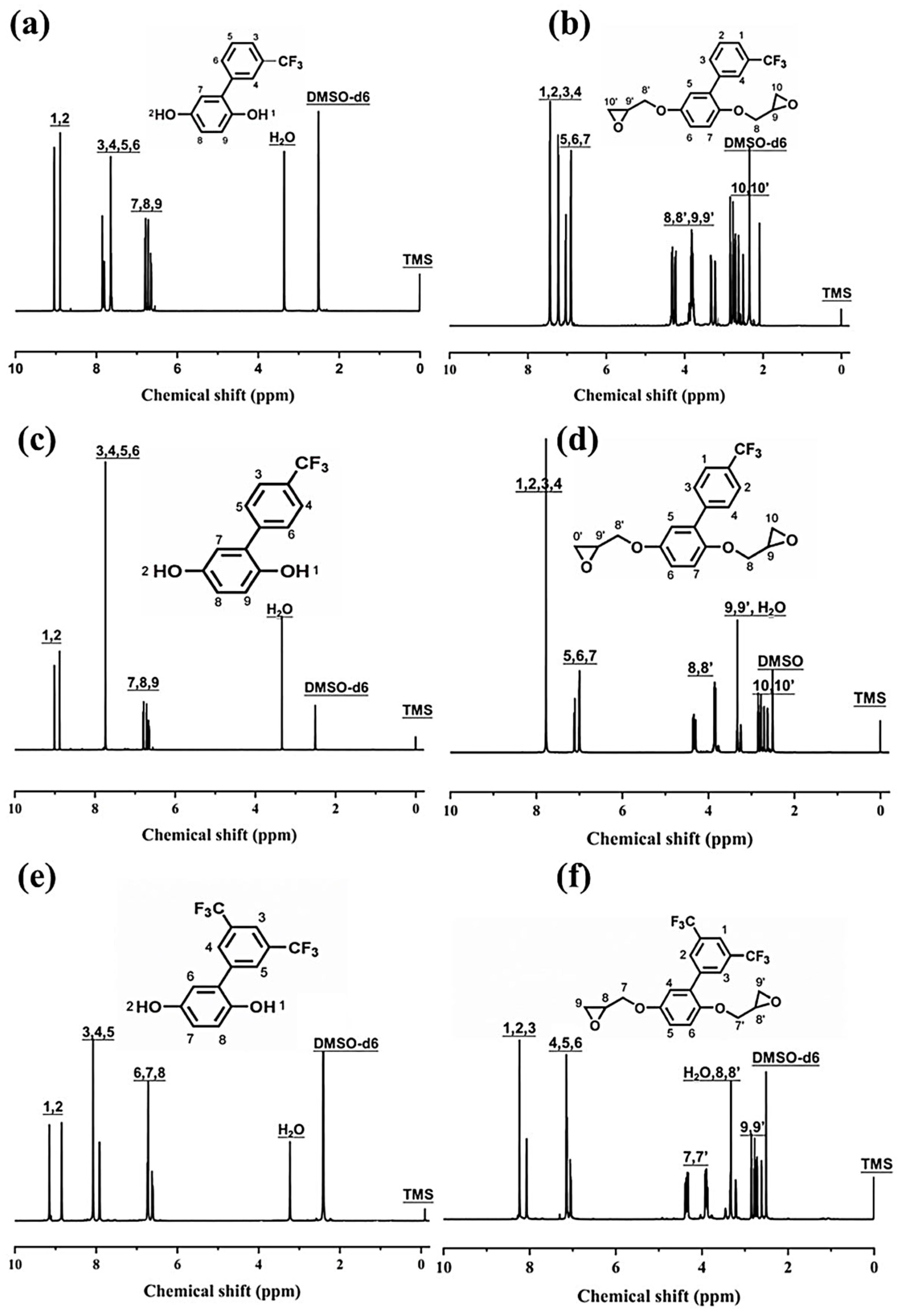
2.5. Characterization
3. Results and Discussion
3.1. Characteristics of Epoxy Resins Containing Trifluoromethyl Groups
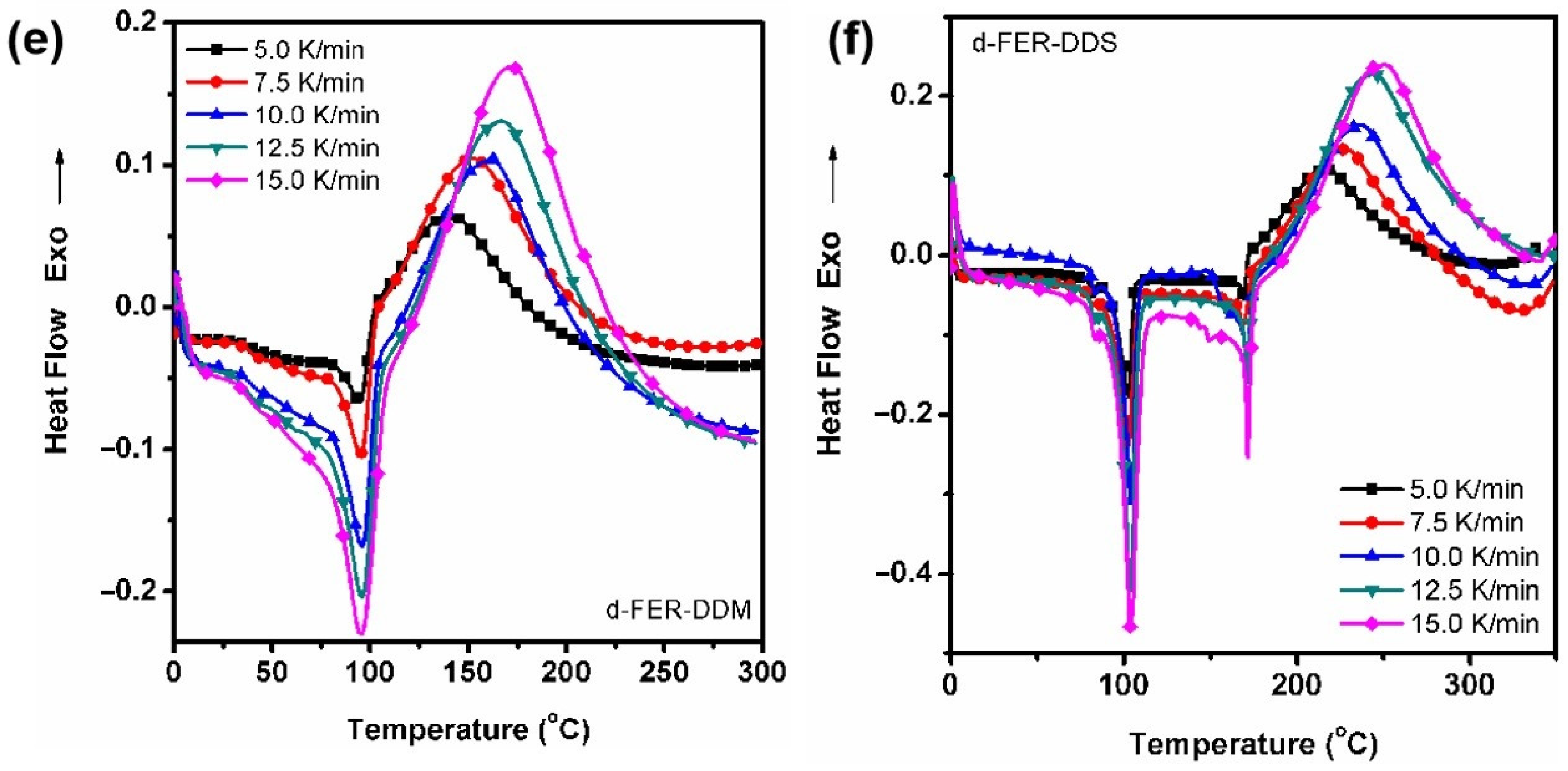
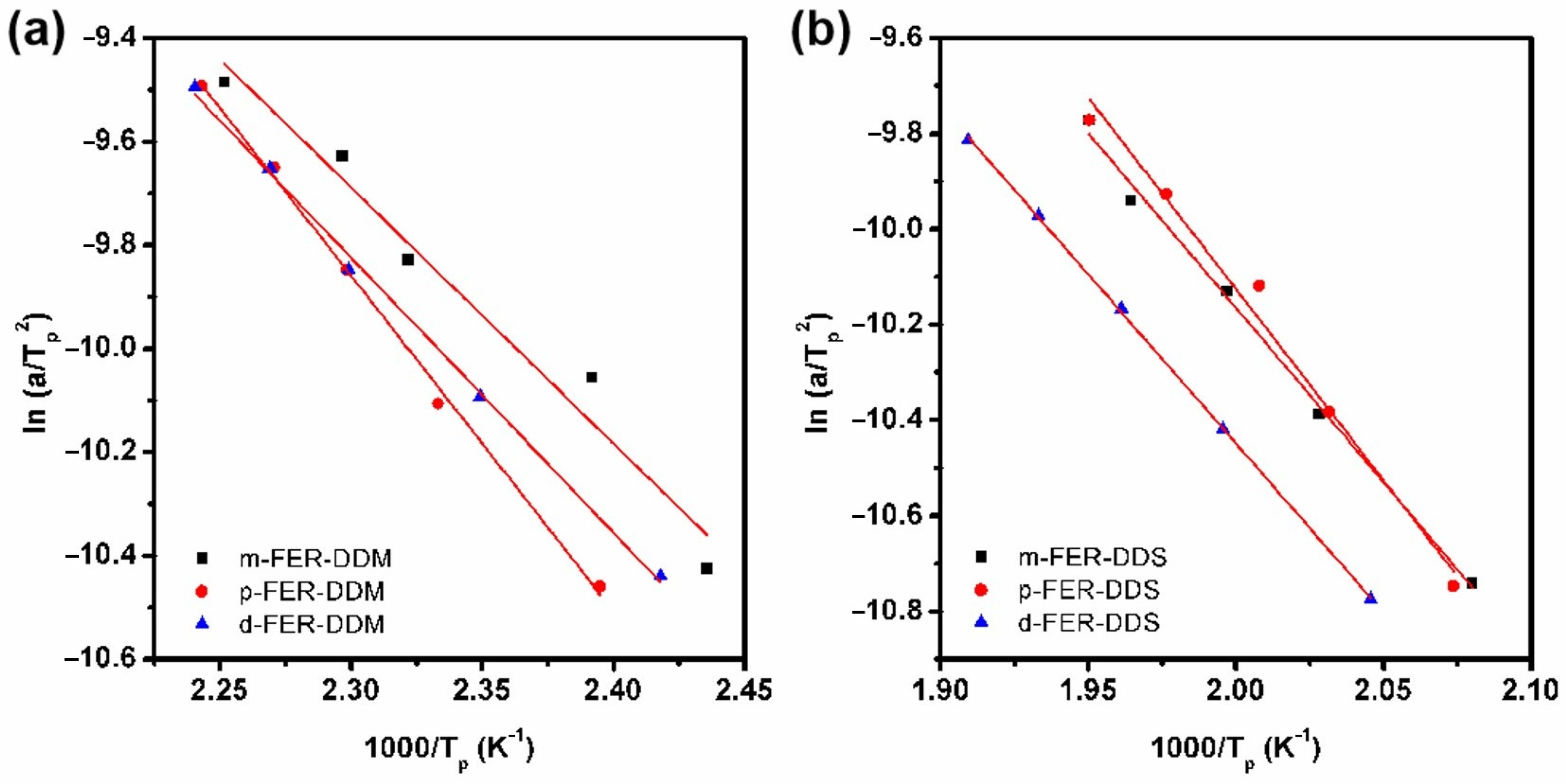
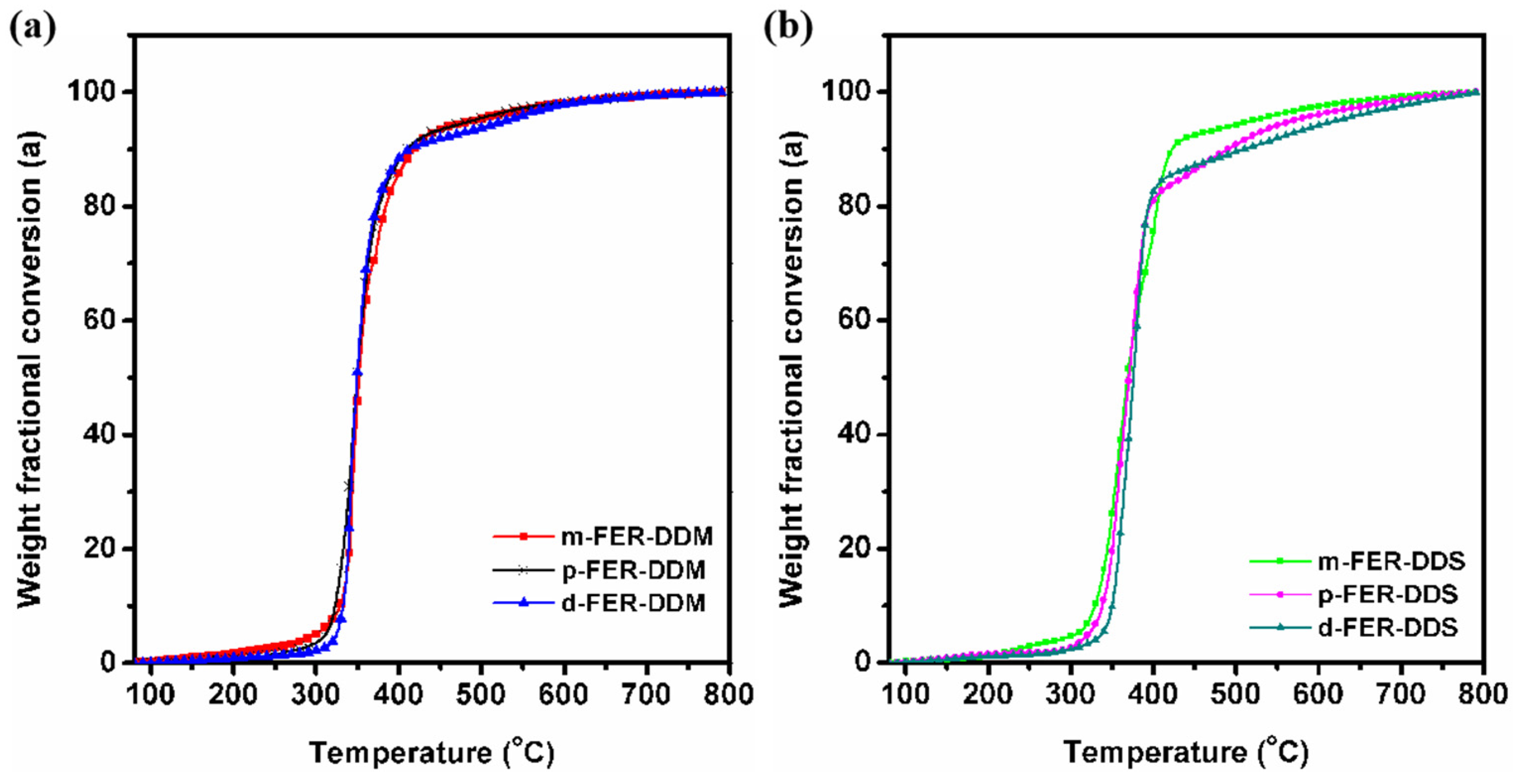
3.2. Curing Kinetic Analysis
3.3. Thermal Properties of Epoxy Resins
3.4. Flexural Properties
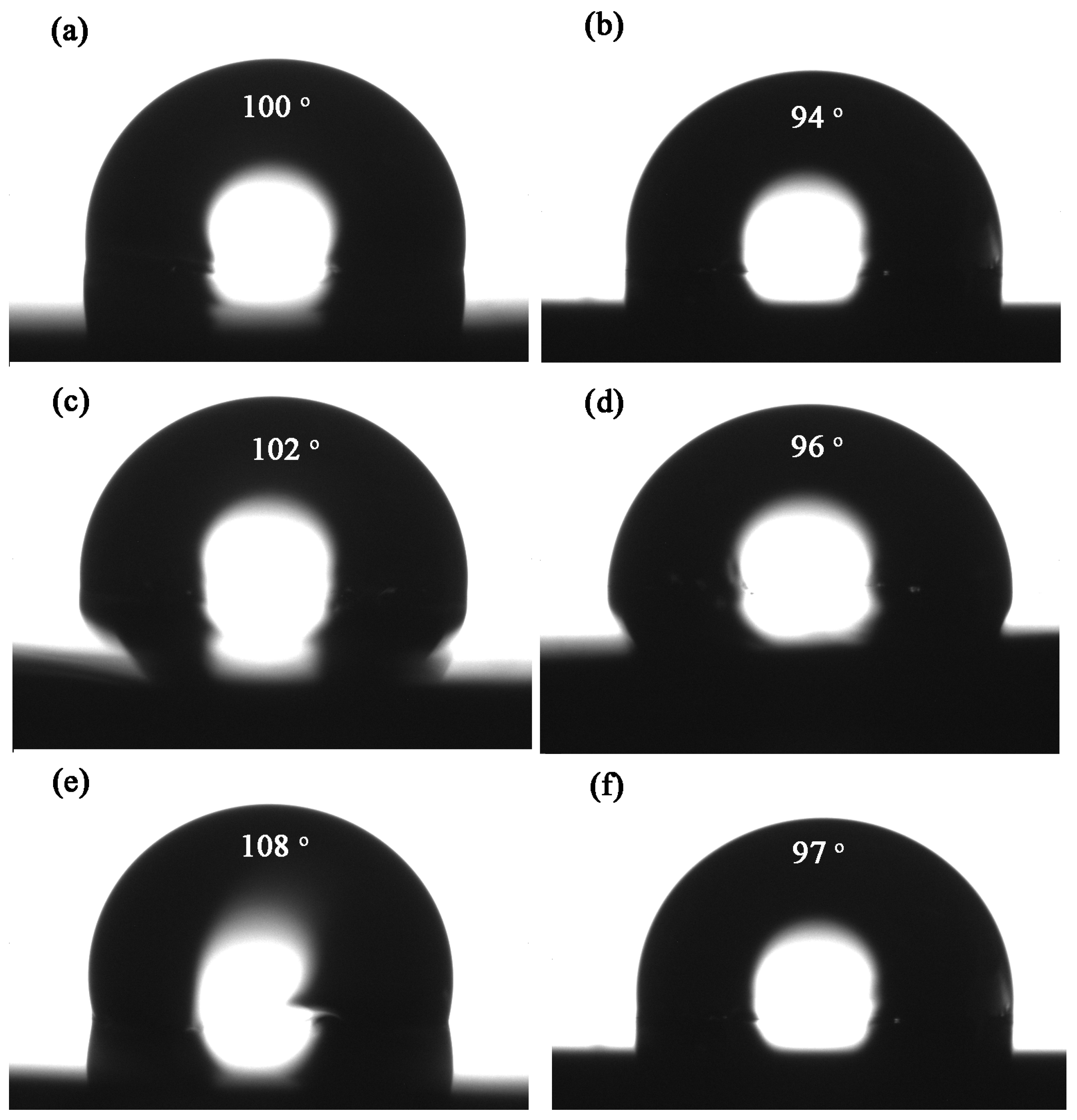
3.5. Water Absorption and Contact Angle
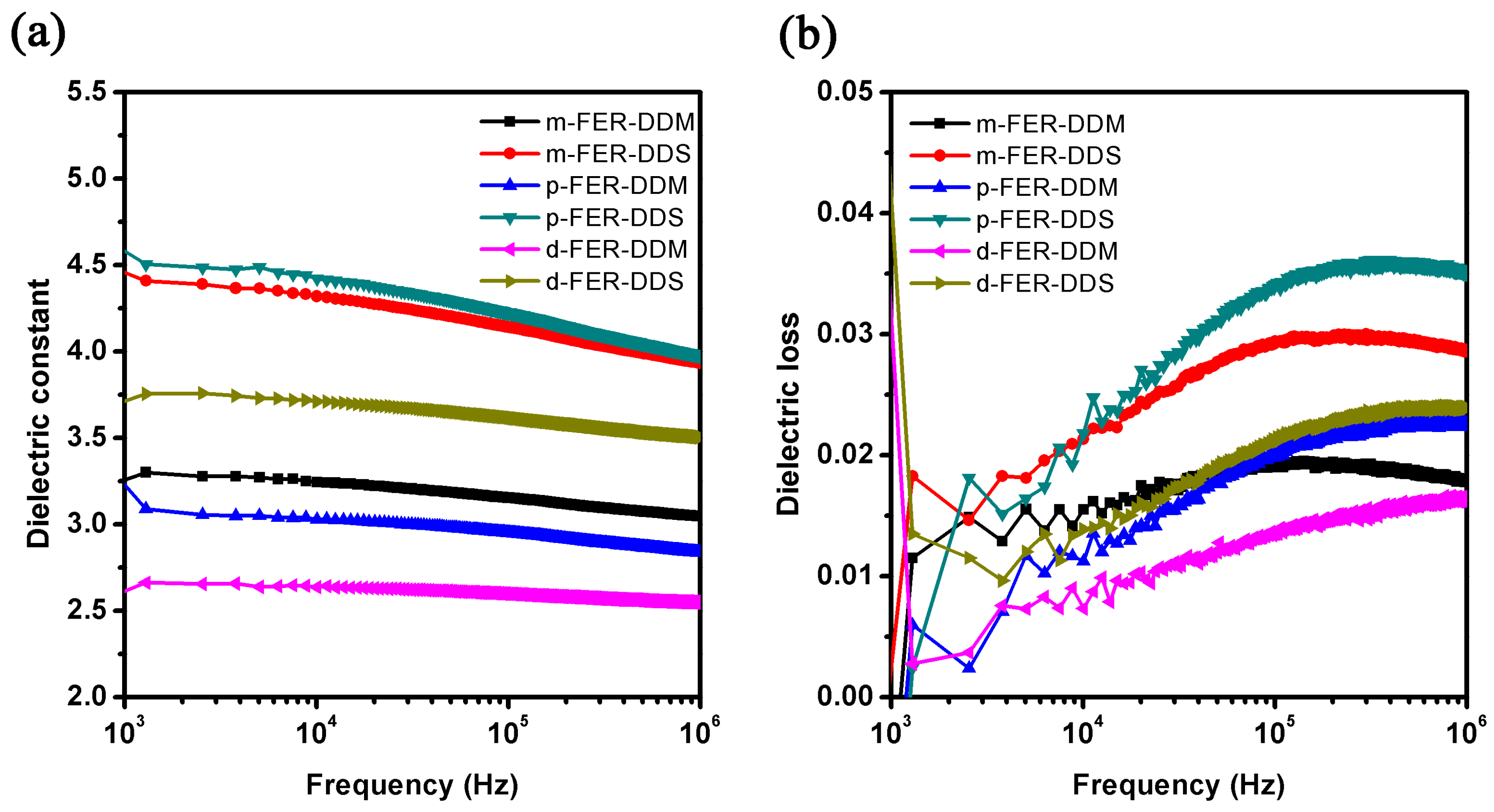
3.6. Dielectric Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Jin, F.-L.; Li, X.; Park, S.-J. Synthesis and application of epoxy resins: A review. J. Ind. Eng. Chem. 2015, 29, 1–11. [Google Scholar] [CrossRef]
- Hong, Y.; Goh, M. Advances in liquid crystalline epoxy resins for high thermal conductivity. Polymers 2021, 13, 1302. [Google Scholar] [CrossRef]
- Mohan, P. A Critical Review: The modification, properties, and applications of epoxy resins. Polym. Plast. Technol. Eng. 2013, 52, 107–125. [Google Scholar] [CrossRef]
- Liu, J.; Wang, S.; Peng, Y.; Zhu, J.; Zhao, W.; Liu, X. Advances in sustainable thermosetting resins: From renewable feedstock to high performance and recyclability. Prog. Polym. Sci. 2021, 113, 101353. [Google Scholar] [CrossRef]
- Prolongo, S.G.; del Rosario, G.; Urena, A. Comparative study on the adhesive properties of different epoxy resins. Int. J. Adhes. Adhes. 2006, 26, 125–132. [Google Scholar] [CrossRef]
- Sun, Z.; Li, J.; Yu, M.; Kathaperumal, M.; Wong, C.-P. A review of the thermal conductivity of silver-epoxy nanocomposites as encapsulation material for packaging applications. Chem. Eng. J. 2022, 446, 137319. [Google Scholar] [CrossRef]
- Alim, M.A.; Abdullah, M.Z.; Aziz, M.S.A.; Kamarudin, R.; Gunnasegaran, P. Recent advances on thermally conductive adhesive in electronic packaging: A review. Polymers 2021, 13, 3337. [Google Scholar] [CrossRef]
- Na, T.; Jiang, H.; Liu, X.; Zhao, C. Preparation and properties of novel fluorinated epoxy resins cured with 4-trifluoromethyl phenylbenzimidazole for application in electronic materials. Eur. Polym. J. 2018, 100, 96–102. [Google Scholar] [CrossRef]
- Xu, W.; Na, H.; Zhao, C. Hollow-glass-microsphere-based biphenyl epoxy resin composite with low dielectric constant. Chem. Res. Chin. Univ. 2018, 34, 862–866. [Google Scholar] [CrossRef]
- Rimdusit, S.; Ishida, H. Development of new class of electronic packaging materials based on ternary systems of benzoxazine, epoxy, and phenolic resins. Polymer 2000, 41, 7941–7949. [Google Scholar] [CrossRef]
- Jin, B.H.; Jang, J.; Kang, D.J.; Yoon, S.; Im, H.-G. Epoxy-based siloxane composites for electronic packaging: Effect of composition and molecular structure of siloxane matrix on their properties. Compos. Sci. Technol. 2022, 224, 109456. [Google Scholar] [CrossRef]
- Maier, G. Low dielectric constant polymers for microelectronics. Prog. Polym. Sci. 2001, 26, 3–65. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Liu, H.J. Review of polymer materials with low dielectric constant. Polym. Int. 2010, 59, 597–606. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, C.; Liu, J.; Na, H. Preparation and characterization of ultralow dielectric and fibrous epoxy thermoset cured with poly(arylene ether ketone) containing phenolic hydroxyl groups. Eur. Polym. J. 2019, 109, 110–116. [Google Scholar] [CrossRef]
- Dhara, M.G.; Banerjee, S. Fluorinated high-performance polymers: Poly(arylene ether)s and aromatic polyimides containing trifluoromethyl groups. Prog. Polym. Sci. 2010, 35, 1022–1077. [Google Scholar] [CrossRef]
- Zhou, Y.; Peng, Z.; Ji, J.; Wu, Y.; Chen, Z.; Huang, H.; Liu, S.; Zhao, J. Fluorinated low molecular weight poly(phenylene oxide): Synthesis, characterization, and application in epoxy resin toward improved thermal and dielectric properties. Eur. Polym. J. 2021, 157, 110674. [Google Scholar] [CrossRef]
- Xing, A.; Bao, F.; Fu, J.; Miao, X.; Liu, T.; Zhai, H.; Cao, X.; Meng, Y.; Li, X. Tertiary-amine-free, non-planar, fluorine-containing tetrafunctional epoxy and its application as high performance matrix. Polym. Test. 2018, 71, 38–48. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, G.; Liu, Z.; Sun, H.; Zhao, C.; Wang, S.; Li, G.; Na, H. Synthesis and properties of an epoxy resin containing trifluoromethyl side chains and its cross-linking networks with different curing agents. Polym. Degrad. Stabil. 2012, 97, 691–697. [Google Scholar] [CrossRef]
- Lee, J.R.; Jin, F.L.; Park, S.J. Substitution effects of methyl and trifluoromethyl groups on the physicochemical properties of epoxy resins. J. Appl. Polym. Sci. 2005, 98, 1860–1864. [Google Scholar] [CrossRef]
- Tao, Z.; Yang, S.; Ge, Z.; Chen, J.; Fan, L. Synthesis and properties of novel fluorinated epoxy resins based on 1,1-bis(4-glycidylesterphenyl)1-(3′-trifluoromethylphenyl)-2,2,2-trifluoroethane. Eur. Polym. J. 2007, 43, 550–560. [Google Scholar] [CrossRef]
- Ge, Z.; Tao, Z.; Li, G.; Ding, J.; Fan, L.; Yang, S. Synthesis and properties of novel fluorinated epoxy resins. J. Appl. Polym. Sci. 2011, 120, 148–155. [Google Scholar] [CrossRef]
- Yin, M.; Yang, L.; Li, X.; Ma, H. Synthesis and properties of methyl hexahydrophthalic anhydride-cured fluorinated epoxy resin 2,2-bisphenol hexafluoropropane diglycidyl ether. J. Appl. Polym. Sci. 2013, 130, 2801–2808. [Google Scholar] [CrossRef]
- Na, T.; Jiang, H.; Zhao, L.; Zhao, C. Preparation and characterization of novel naphthyl epoxy resin containing 4-fluorobenzoyl side chains for low-k dielectrics application. RSC Adv. 2017, 7, 53970–53976. [Google Scholar] [CrossRef] [Green Version]
- Na, T.; Liu, X.; Jiang, H.; Zhao, L.; Zhao, C. Enhanced thermal conductivity of fluorinated epoxy resins by incorporating inorganic filler, React. Funct. Polym. 2018, 128, 84–90. [Google Scholar] [CrossRef]
- Khalafi, H.R.; Ehsani, M.; Khonakdar, H.A. Investigation of the cure kinetics and thermal stability of an epoxy system containing cystamine as curing agent. Polym. Adv. Technol. 2021, 32, 1251–1261. [Google Scholar] [CrossRef]
- Lee, J.Y.; Choi, H.K.; Shim, M.J.; Kim, S.W. Kinetic studies of an epoxy cure reaction by isothermal DSC analysis. Thermochim. Acta 2000, 343, 111–117. [Google Scholar] [CrossRef]
- Vinnik, R.M.; Roznyatovsky, V.A. Kinetic method by using calorimetry to mechanism of epoxy-amine cure reaction. Part VIII. A comparative study of some epoxy-amine reactions. J. Therm. Anal. Calorim. 2006, 85, 455–461. [Google Scholar] [CrossRef]
- Kissinger, H.E. Reaction kinetics in differential thermal analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, Z.; Yue, S.; Huang, H. Preparation and curing behavior study of primary amine-functional benzoxazine/epoxy copolymer. J. Polym. Mater. 2014, 31, 477–489. [Google Scholar]
- Zhang, X.; Wang, Z.; Xie, J. Preparation and properties of p-coumaric acid epoxy resin co-cured by a novel fluorinated diamine curing agent based on bisphenol AF in electronic materials. Mater. Lett. 2023, 339, 134120. [Google Scholar] [CrossRef]
- Zou, C.; Fothergill, J.C. The effect of water absorption on the dielectric properties of epoxy nanocomposites. IEEE Trans. Dielectr. Electr. Insul. 2008, 15, 106–117. [Google Scholar] [CrossRef]
- Chen, M.; Li, J.; Chen, L.; Qin, Y.; Xiao, M.; Wang, Y. Methyl-substitution affects dielectric, thermal, mechanical properties, and shrinkage of fluorinated epoxy. Polymer 2022, 253, 125012. [Google Scholar] [CrossRef]
- Miller, R.D. In search of low-k dielectrics. Science 1999, 286, 421–423. [Google Scholar] [CrossRef]

| Sample | Tg (°C) | Td5 (°C) | Td10 (°C) |
|---|---|---|---|
| m-FER-DDM | 128 | 307 | 332 |
| m-FER-DDS | 160 | 316 | 337 |
| p-FER-DDM | 147 | 315 | 325 |
| p-FER-DDS | 185 | 324 | 342 |
| d-FER-DDM | 133 | 326 | 334 |
| d-FER-DDS | 178 | 341 | 352 |
| Samples | Flexural Strength (MPa) | Flexural Modulus (GPa) | Water Absorption (%) | Dielectric Constant (1 MHz) | Dielectric Loss (1 MHz) |
|---|---|---|---|---|---|
| m-FER-DDM | 151.36 ± 13.86 | 2.65 ± 0.23 | 0.95 | 3.05 | 0.018 |
| m-FER-DDS | 56.46 ± 2.55 | 1.47 ± 0.31 | 1.25 | 3.93 | 0.029 |
| p-FER-DDM | 95.55 ± 6.23 | 1.71 ± 0.34 | 0.87 | 2.84 | 0.023 |
| p-FER-DDS | 42.30 ± 9.90 | 1.98 ± 0.06 | 1.18 | 3.98 | 0.035 |
| d-FER-DDM | 127.00 ± 2.08 | 2.28 ± 0.37 | 0.49 | 2.55 | 0.016 |
| d-FER-DDS | 118.85 ± 2.52 | 1.54 ± 0.27 | 0.99 | 3.51 | 0.024 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Lin, H.; Dong, K.; Tang, S.; Zhao, C. Comparison of the Properties of Epoxy Resins Containing Various Trifluoromethyl Groups with Low Dielectric Constant. Polymers 2023, 15, 2853. https://doi.org/10.3390/polym15132853
Zhang Y, Lin H, Dong K, Tang S, Zhao C. Comparison of the Properties of Epoxy Resins Containing Various Trifluoromethyl Groups with Low Dielectric Constant. Polymers. 2023; 15(13):2853. https://doi.org/10.3390/polym15132853
Chicago/Turabian StyleZhang, Yurong, Haidan Lin, Kai Dong, Shasha Tang, and Chengji Zhao. 2023. "Comparison of the Properties of Epoxy Resins Containing Various Trifluoromethyl Groups with Low Dielectric Constant" Polymers 15, no. 13: 2853. https://doi.org/10.3390/polym15132853
APA StyleZhang, Y., Lin, H., Dong, K., Tang, S., & Zhao, C. (2023). Comparison of the Properties of Epoxy Resins Containing Various Trifluoromethyl Groups with Low Dielectric Constant. Polymers, 15(13), 2853. https://doi.org/10.3390/polym15132853








