Structure and Properties of Composite Fibers Based on Chitosan and Single-Walled Carbon Nanotubes for Peripheral Nerve Regeneration
Abstract
:1. Introduction
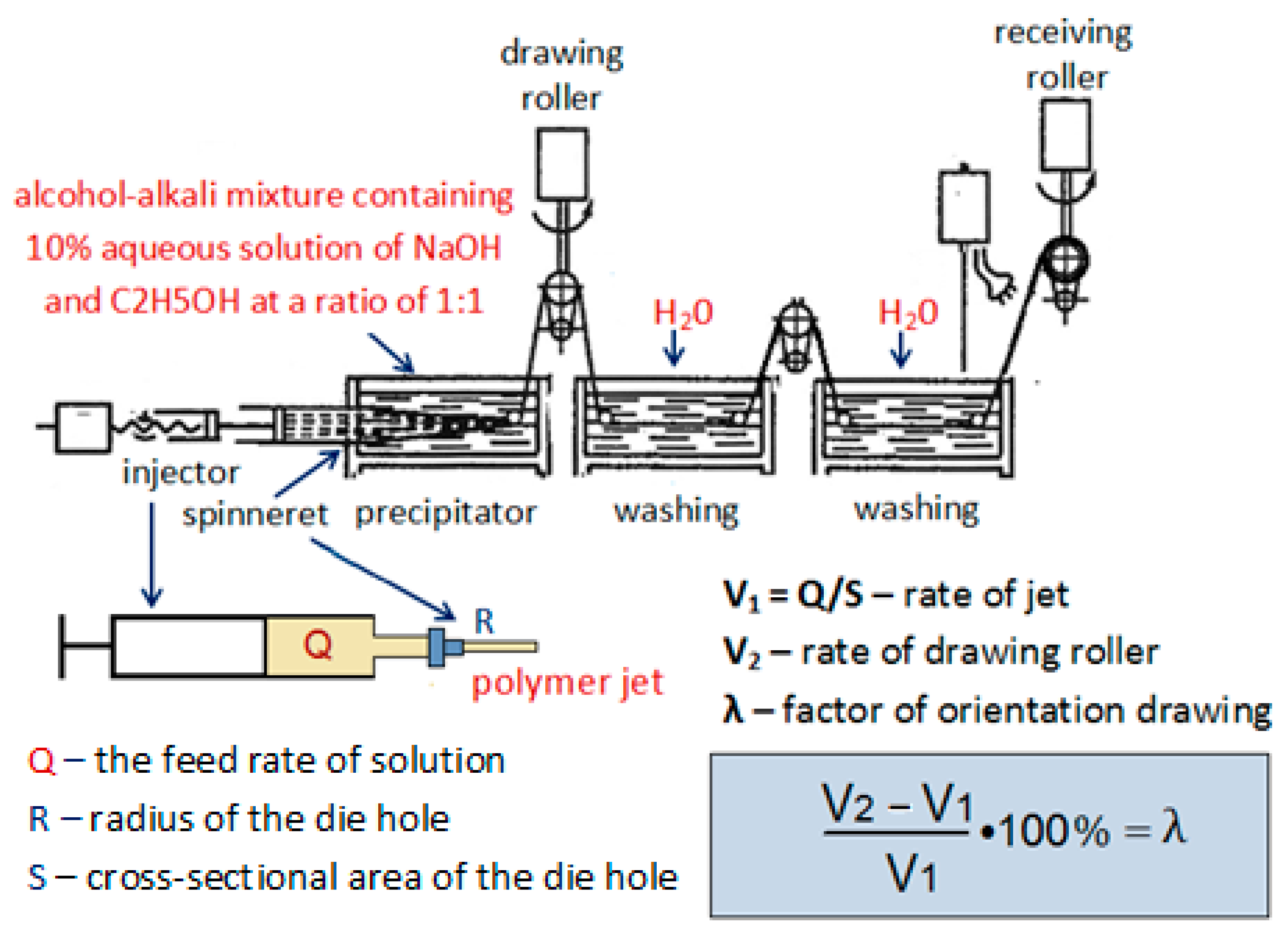
2. Materials and Methods
3. Results
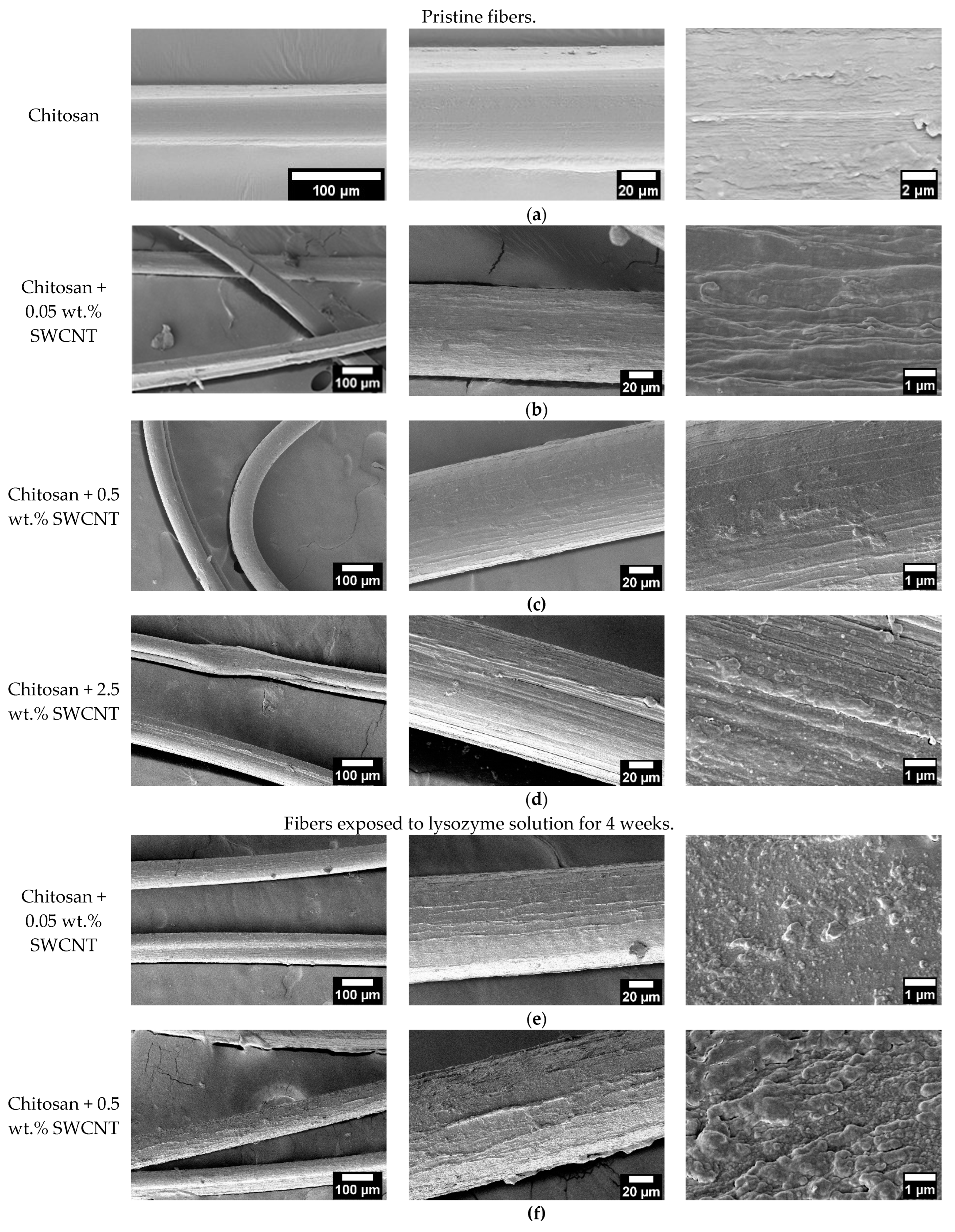
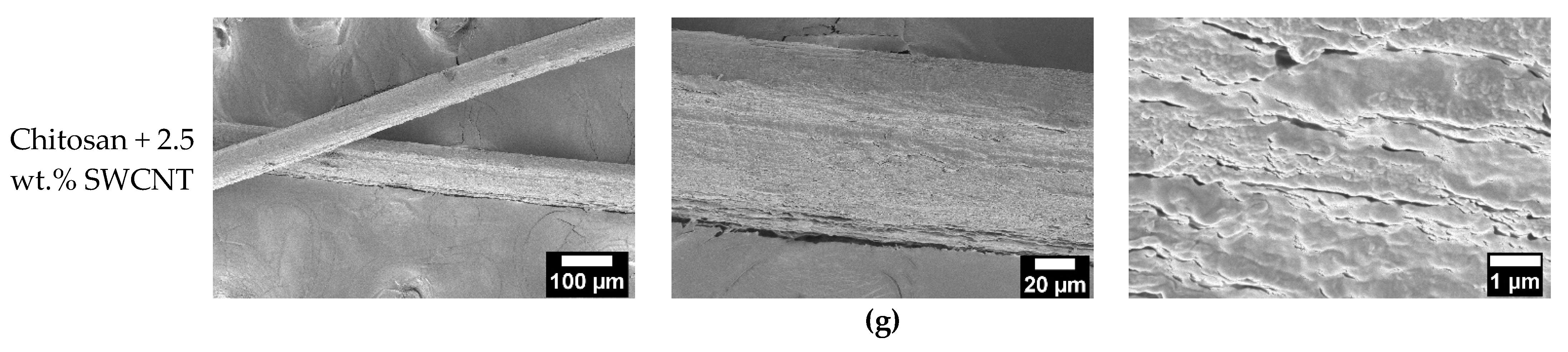
3.1. Scanning Electron Microscopy
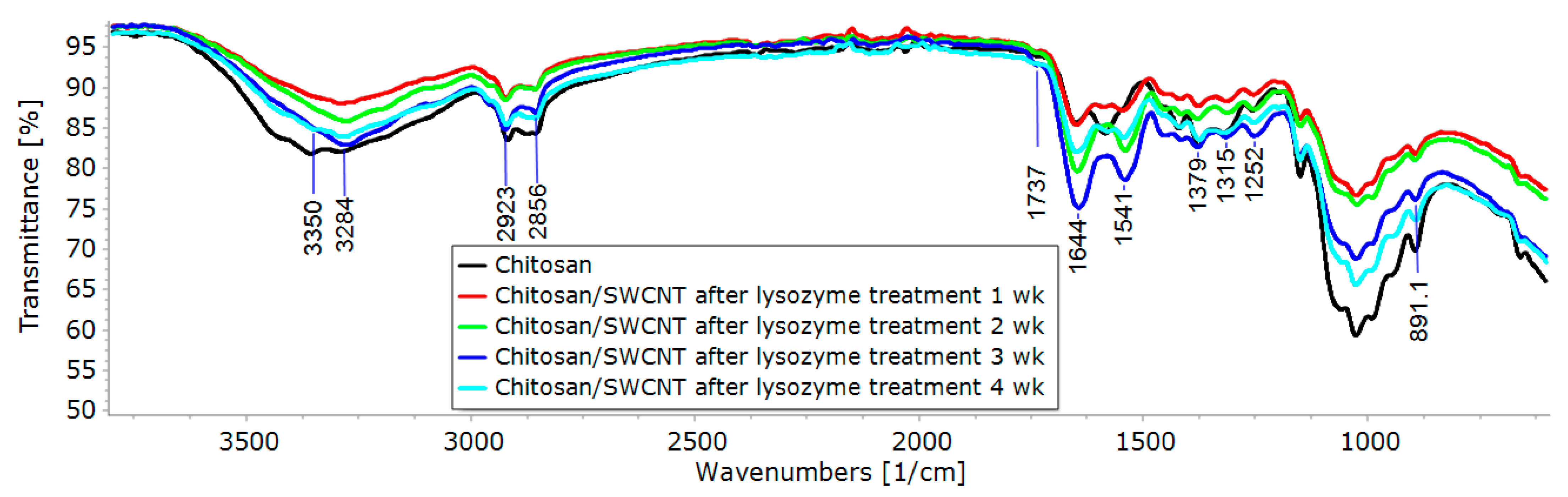
3.2. IR Spectroscopy
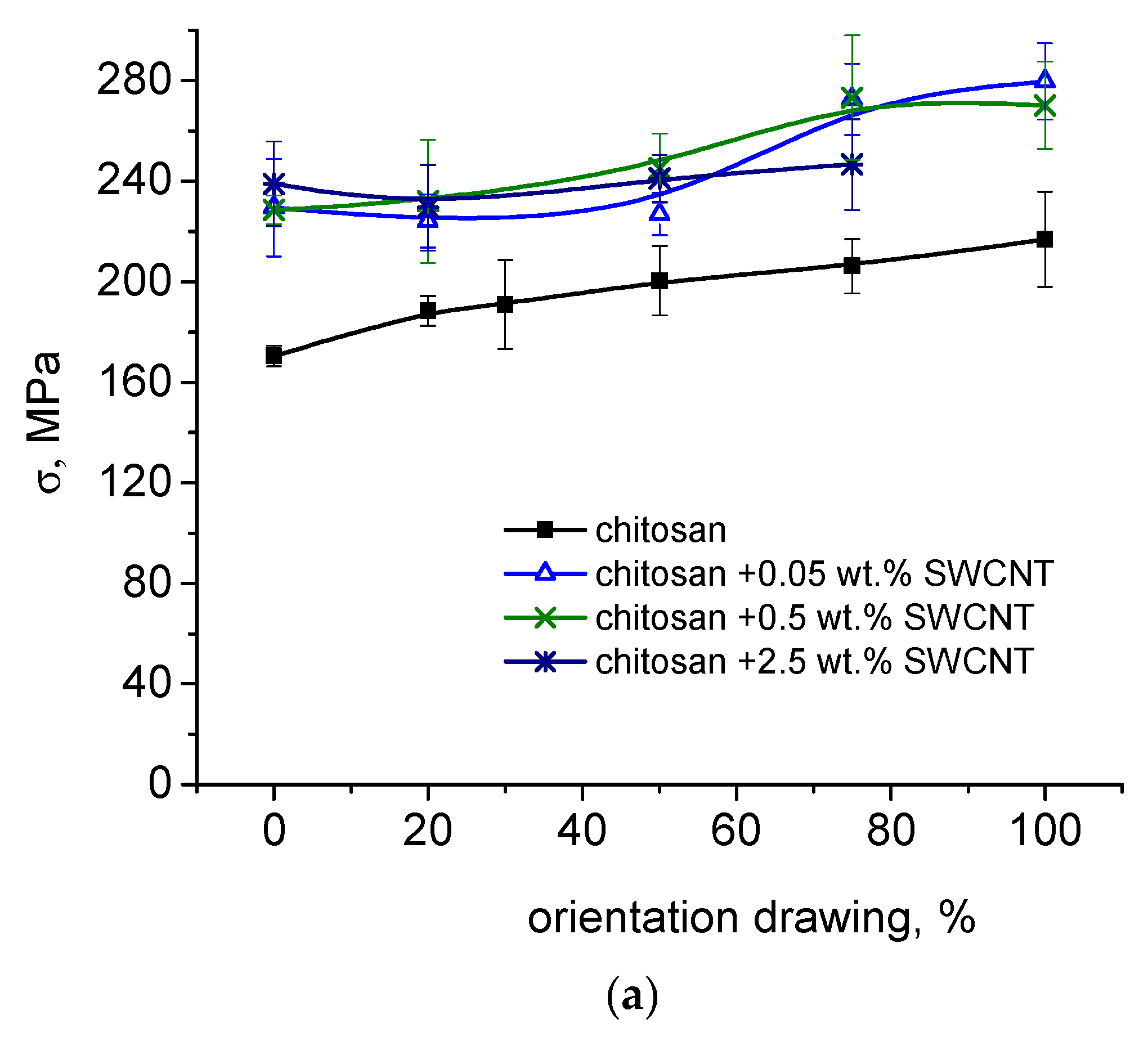
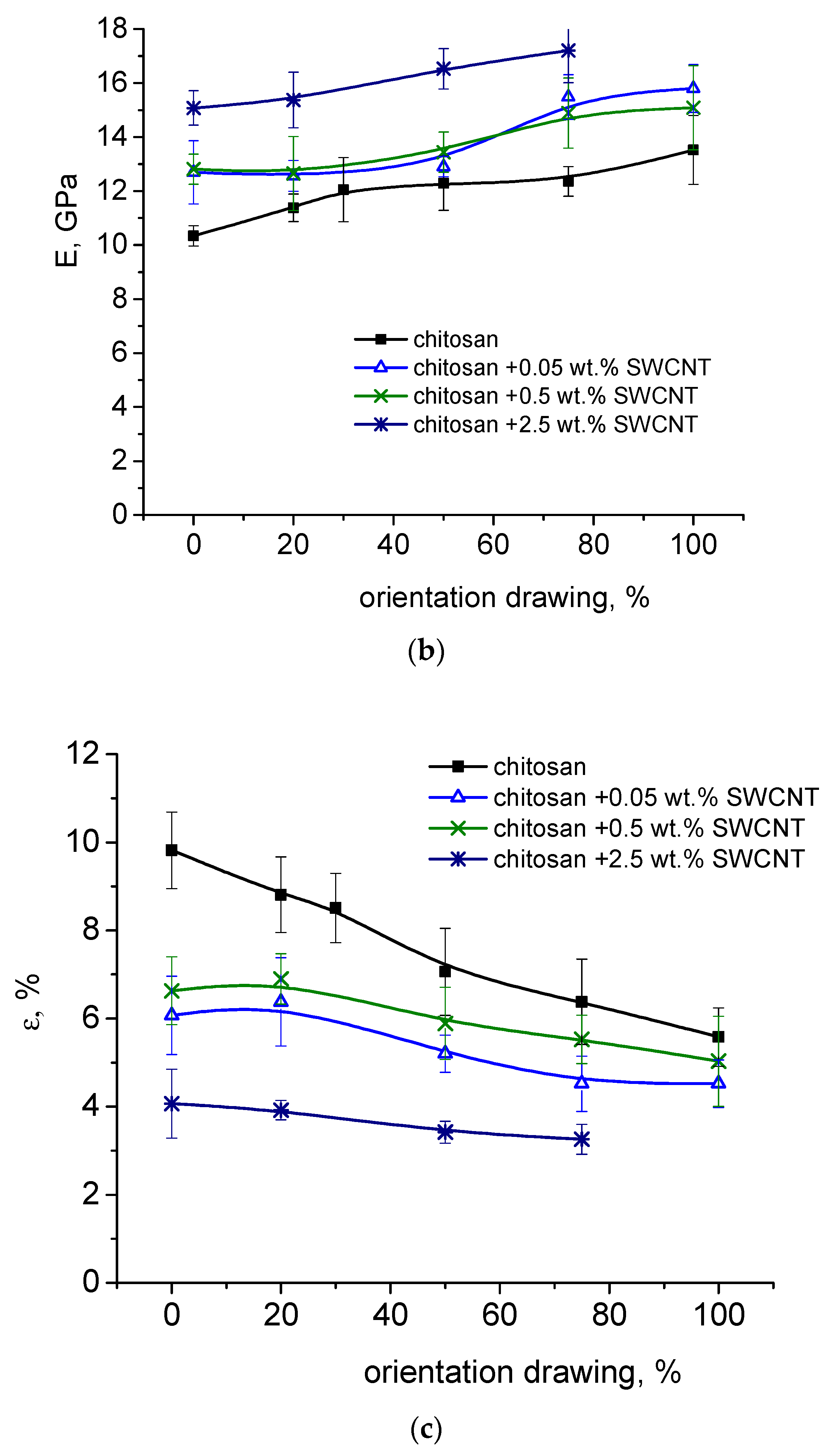
3.3. Measurements of Strength and Deformation Characteristics
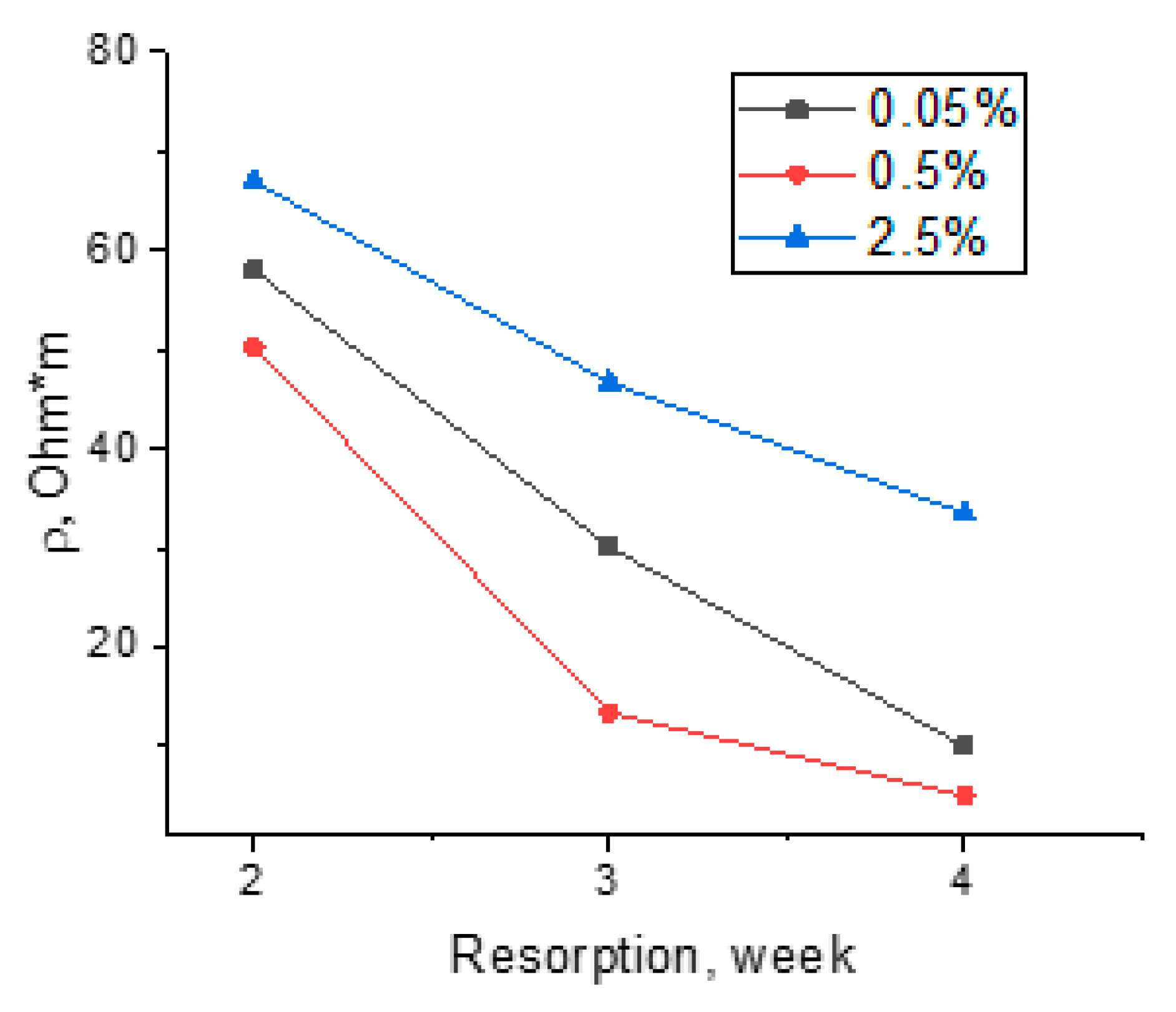
3.4. Measurements of Electrical Resistivity of Fibers
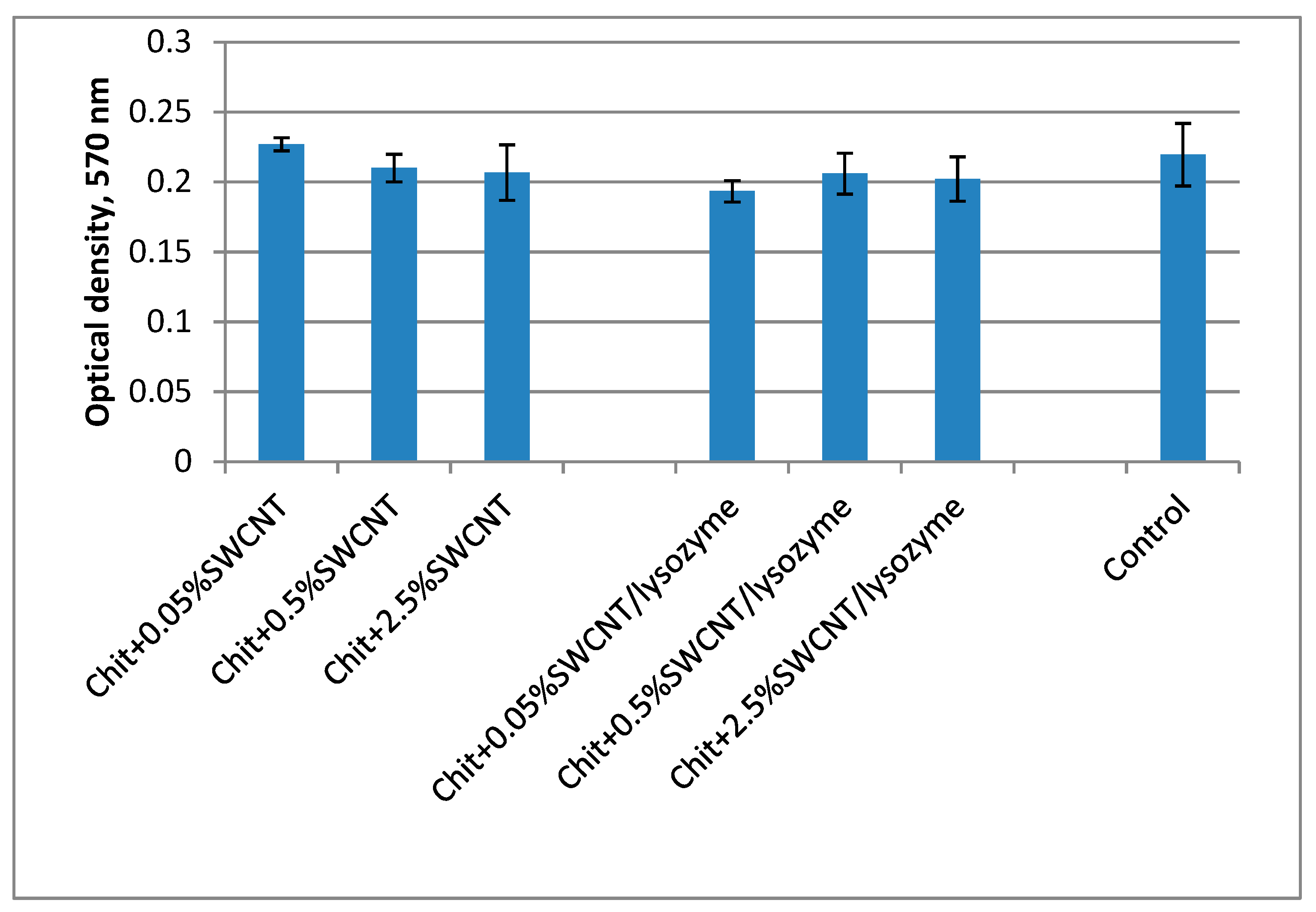
3.5. Investigation of the Biocompatibility of the Fibers via the MTT Technique
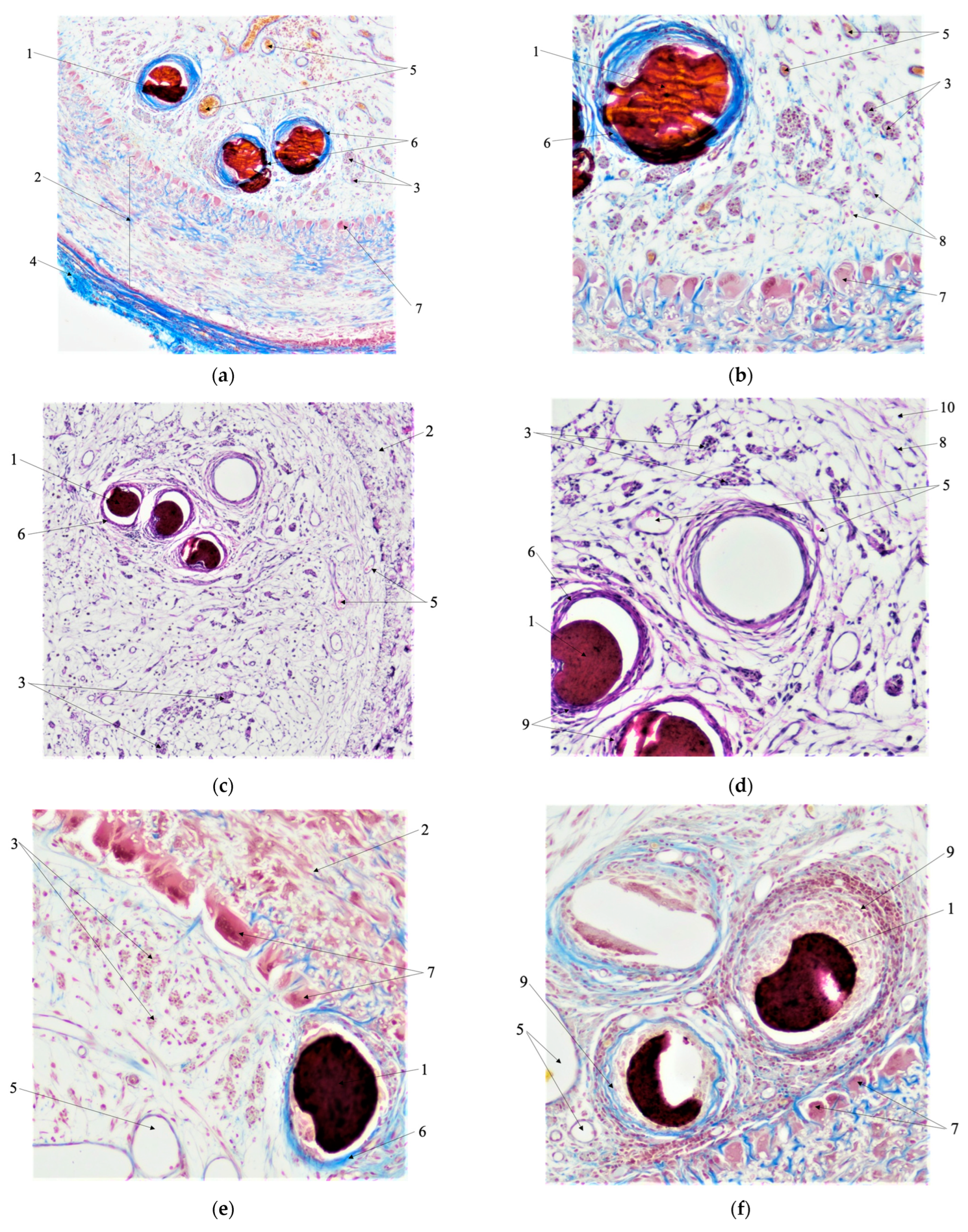
3.6. Morphological Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schmidt, C.E.; Leach, J.B. Neural Tissue Engineering: Strategies for Repair and Regeneration. Annu. Rev. Biomed. Eng. 2003, 5, 293–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gunatillake, P.A.; Adhikari, R. Biodegradable Synthetic Polymers for Tissue Engineering. Eur. Cell. Mater. 2003, 5, 1–16. discussion 16. [Google Scholar] [CrossRef] [PubMed]
- Dash, T.K.; Konkimalla, V.B. Poly-ϵ-Caprolactone Based Formulations for Drug Delivery and Tissue Engineering: A Review. J. Control. Release 2012, 158, 15–33. [Google Scholar] [CrossRef] [PubMed]
- Balint, R.; Cassidy, N.J.; Cartmell, S.H. Conductive Polymers: Towards a Smart Biomaterial for Tissue Engineering. Acta Biomater. 2014, 10, 2341–2353. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.L.; Santoro, M. Processing and Production of Bioresorbable Polymer Scaffolds for Tissue Engineering. In Bioresorbable Polymers for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2017; pp. 181–203. [Google Scholar]
- Stewart, E.; Kobayashi, N.R.; Higgins, M.J.; Quigley, A.F.; Jamali, S.; Moulton, S.E.; Kapsa, R.M.I.; Wallace, G.G.; Crook, J.M. Electrical Stimulation Using Conductive Polymer Polypyrrole Promotes Differentiation of Human Neural Stem Cells: A Biocompatible Platform for Translational Neural Tissue Engineering. Tissue Eng. Part. C. Methods 2015, 21, 385–393. [Google Scholar] [CrossRef]
- Huang, J.; Hu, X.; Lu, L.; Ye, Z.; Zhang, Q.; Luo, Z. Electrical Regulation of Schwann Cells Using Conductive Polypyrrole/Chitosan Polymers. J. Biomed. Mater. Res. A 2010, 93, 164–174. [Google Scholar] [CrossRef]
- Forciniti, L.; Ybarra, J.; Zaman, M.H.; Schmidt, C.E. Schwann Cell Response on Polypyrrole Substrates upon Electrical Stimulation. Acta Biomater. 2014, 10, 2423–2433. [Google Scholar] [CrossRef]
- Gordon, T.; Brushart, T.M.; Chan, K.M. Augmenting Nerve Regeneration with Electrical Stimulation. Neurol. Res. 2008, 30, 1012–1022. [Google Scholar] [CrossRef]
- Lu, M.-C.; Ho, C.-Y.; Hsu, S.-F.; Lee, H.-C.; Lin, J.-H.; Yao, C.-H.; Chen, Y.-S. Effects of Electrical Stimulation at Different Frequencies on Regeneration of Transected Peripheral Nerve. Neurorehabil. Neural Repair 2008, 22, 367–373. [Google Scholar] [CrossRef]
- Hing, K.A. Bone Repair in the Twenty–First Century: Biology, Chemistry or Engineering? Philos. Trans. R. Soc. Lond. Ser. A Math. Phys. Eng. Sci. 2004, 362, 2821–2850. [Google Scholar] [CrossRef]
- Ghasemi-Mobarakeh, L.; Prabhakaran, M.P.; Morshed, M.; Nasr-Esfahani, M.H.; Baharvand, H.; Kiani, S.; Al-Deyab, S.S.; Ramakrishna, S. Application of Conductive Polymers, Scaffolds and Electrical Stimulation for Nerve Tissue Engineering. J. Tissue Eng. Regen. Med. 2011, 5, e17–e35. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Bashur, C.A.; Goldstein, A.S.; Schmidt, C.E. Polypyrrole-Coated Electrospun PLGA Nanofibers for Neural Tissue Applications. Biomaterials 2009, 30, 4325–4335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, X.-H.; Xu, Q.; Feng, Z.-Q.; Xiao, J.-Q.; Li, Q.; Sun, X.-T.; Cao, Y.; Ding, Y.-T. In Vitro Biocompatibility of Polypyrrole/PLGA Conductive Nanofiber Scaffold with Cultured Rat Hepatocytes. Mater. Res. Express 2014, 1, 035402. [Google Scholar] [CrossRef]
- Wang, Z.; Roberge, C.; Dao, L.H.; Wan, Y.; Shi, G.; Rouabhia, M.; Guidoin, R.; Zhang, Z. In Vivo Evaluation of a Novel Electrically Conductive Polypyrrole/Poly(d, l-Lactide) Composite and Polypyrrole-Coated Poly(d,l-Lactide-Co-Glycolide) Membranes. J. Biomed. Mater. Res. A 2004, 70, 28–38. [Google Scholar] [CrossRef]
- Runge, M.B.; Dadsetan, M.; Baltrusaitis, J.; Knight, A.M.; Ruesink, T.; Lazcano, E.A.; Lu, L.; Windebank, A.J.; Yaszemski, M.J. The Development of Electrically Conductive Polycaprolactone Fumarate-Polypyrrole Composite Materials for Nerve Regeneration. Biomaterials 2010, 31, 5916–5926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jing, W.; Ao, Q.; Wang, L.; Huang, Z.; Cai, Q.; Chen, G.; Yang, X.; Zhong, W. Constructing Conductive Conduit with Conductive Fibrous Infilling for Peripheral Nerve Regeneration. Chem. Eng. J. 2018, 345, 566–577. [Google Scholar] [CrossRef]
- Xu, H.; Holzwarth, J.M.; Yan, Y.; Xu, P.; Zheng, H.; Yin, Y.; Li, S.; Ma, P.X. Conductive PPY/PDLLA Conduit for Peripheral Nerve Regeneration. Biomaterials 2014, 35, 225–235. [Google Scholar] [CrossRef] [Green Version]
- Pooshidani, Y.; Zoghi, N.; Rajabi, M.; Haghbin Nazarpak, M.; Hassannejad, Z. Fabrication and Evaluation of Porous and Conductive Nanofibrous Scaffolds for Nerve Tissue Engineering. J. Mater. Sci. Mater. Med. 2021, 32, 31. [Google Scholar] [CrossRef]
- Meek, M.F.; Coert, J.H. US Food and Drug Administration/Conformit Europe-Approved Absorbable Nerve Conduits for Clinical Repair of Peripheral and Cranial Nerves. Ann. Plast. Surg. 2008, 60, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Lundborg, G. A 25-Year Perspective of Peripheral Nerve Surgery: Evolving Neuroscientific Concepts and Clinical Significance. J. Hand Surg. Am. 2000, 25, 391–414. [Google Scholar] [CrossRef]
- Hodgkin, A.L.; Huxley, A.F. Action Potentials Recorded from Inside a Nerve Fibre. Nature 1939, 144, 710–711. [Google Scholar] [CrossRef]
- Millesi, H. Bridging Defects: Autologous Nerve Grafts. Acta Neurochir. Suppl. 2007, 100, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Babu, P.; Behl, A.; Chakravarty, B.; Bhandari, P.; Bhatti, T.; Maurya, S. Entubulation Techniques in Peripheral Nerve Repair. Indian J. Neurotrauma 2008, 5, 15–20. [Google Scholar] [CrossRef]
- Dahlin, L.B.; Lundborg, G. Use of Tubes in Peripheral Nerve Repair. Neurosurg. Clin. North Am. 2001, 12, 341–352. [Google Scholar] [CrossRef]
- de Ruiter, G.C.W.; Malessy, M.J.A.; Yaszemski, M.J.; Windebank, A.J.; Spinner, R.J. Designing Ideal Conduits for Peripheral Nerve Repair. Neurosurg. Focus. 2009, 26, E5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hudson, A.R.; Morris, J.; Weddell, G.; Drury, A. Peripheral Nerve Autografts. J. Surg. Res. 1972, 12, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Martini, R. Expression and Functional Roles of Neural Cell Surface Molecules and Extracellular Matrix Components during Development and Regeneration of Peripheral Nerves. J. Neurocytol. 1994, 23, 1–28. [Google Scholar] [CrossRef]
- Seddon, H.J. Three Types of Nerve Injury. Brain 1943, 66, 237–288. [Google Scholar] [CrossRef]
- Kehoe, S.; Zhang, X.F.; Boyd, D. FDA Approved Guidance Conduits and Wraps for Peripheral Nerve Injury: A Review of Materials and Efficacy. Injury 2012, 43, 553–572. [Google Scholar] [CrossRef]
- Miller, C.; Jeftinija, S.; Mallapragada, S. Micropatterned Schwann Cell-Seeded Biodegradable Polymer Substrates Significantly Enhance Neurite Alignment and Outgrowth. Tissue Eng. 2001, 7, 705–715. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.; Lim, S.H.; Mao, H.-Q.; Chew, S.Y. Current Applications and Future Perspectives of Artificial Nerve Conduits. Exp. Neurol. 2010, 223, 86–101. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Yu, Y.; Wang, S.; Xu, H.; He, Q.; Wen, J.; Xu, J.; Li, K.; Huang, Z.; Xu, P. Preparation and Characterization of Conductive Nerve Guide Conduit Filled with Dual Drug-Loaded Nanofibers. J. Bioact. Compat. Polym. 2021, 36, 531–547. [Google Scholar] [CrossRef]
- Ito, T.; Nakamura, T.; Suzuki, K.; Takagi, T.; Toba, T.; Hagiwara, A.; Kihara, K.; Miki, T.; Yamagishi, H.; Shimizu, Y. Regeneration of Hypogastric Nerve Using a Polyglycolic Acid (PGA)-Collagen Nerve Conduit Filled with Collagen Sponge Proved Electrophysiologically in a Canine Model. Int. J. Artif. Organs 2003, 26, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Saeki, M.; Tanaka, K.; Imatani, J.; Okamoto, H.; Watanabe, K.; Nakamura, T.; Gotani, H.; Ohi, H.; Nakamura, R.; Hirata, H. Efficacy and Safety of Novel Collagen Conduits Filled with Collagen Filaments to Treat Patients with Peripheral Nerve Injury: A Multicenter, Controlled, Open-Label Clinical Trial. Injury 2018, 49, 766–774. [Google Scholar] [CrossRef]
- Matsumoto, K.; Ohnishi, K.; Kiyotani, T.; Sekine, T.; Ueda, H.; Nakamura, T.; Endo, K.; Shimizu, Y. Peripheral Nerve Regeneration across an 80-Mm Gap Bridged by a Polyglycolic Acid (PGA)–Collagen Tube Filled with Laminin-Coated Collagen Fibers: A Histological and Electrophysiological Evaluation of Regenerated Nerves. Brain Res. 2000, 868, 315–328. [Google Scholar] [CrossRef] [Green Version]
- Ngo, T.-T.B.; Waggoner, P.J.; Romero, A.A.; Nelson, K.D.; Eberhart, R.C.; Smith, G.M. Poly(L-Lactide) Microfilaments Enhance Peripheral Nerve Regeneration across Extended Nerve Lesions. J. Neurosci. Res. 2003, 72, 227–238. [Google Scholar] [CrossRef]
- Navissano, M.; Malan, F.; Carnino, R.; Battiston, B. Neurotube for Facial Nerve Repair. Microsurgery 2005, 25, 268–271. [Google Scholar] [CrossRef]
- Sundback, C.; Hadlock, T.; Cheney, M.; Vacanti, J. Manufacture of Porous Polymer Nerve Conduits by a Novel Low-Pressure Injection Molding Process. Biomaterials 2003, 24, 819–830. [Google Scholar] [CrossRef]
- Stang, F.; Fansa, H.; Wolf, G.; Reppin, M.; Keilhoff, G. Structural Parameters of Collagen Nerve Grafts Influence Peripheral Nerve Regeneration. Biomaterials 2005, 26, 3083–3091. [Google Scholar] [CrossRef]
- Allodi, I.; Udina, E.; Navarro, X. Specificity of Peripheral Nerve Regeneration: Interactions at the Axon Level. Prog. Neurobiol. 2012, 98, 16–37. [Google Scholar] [CrossRef]
- Popryadukhin, P.V.; Popov, G.I.; Yukina, G.Y.; Dobrovolskaya, I.P.; Ivan’kova, E.M.; Vavilov, V.N.; Yudin, V.E. Tissue-Engineered Vascular Graft of Small Diameter Based on Electrospun Polylactide Microfibers. Int. J. Biomater. 2017, 2017, 9034186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dresvyanina, E.; Yudenko, A.; Yevlampieva, N.; Maevskaya, E.; Yudin, V.; Gubarev, A.; Slyusarenko, M.; Heppe, K. The Molecular Mass Effect on Mechanical Properties of Chitosan Fibers. Vlak. Text. 2018, 25, 27–31. [Google Scholar]
- Maevskaia, E.N.; Dresvyanina, E.N.; Yudenko, A.N.; Yudin, V.E. The Mechanical Properties of Chitosan Fibers Obtained in Different Spinning Conditions by Coagulation Method. Tech. Phys. 2018, 63, 1323–1327. [Google Scholar] [CrossRef]
- Maevskaia, E.N.; Kirichuk, O.P.; Kuznetzov, S.I.; Dresvyanina, E.N.; Yudin, V.V.; Morganti, P. Hemocompatible Chitin-Chitosan Composite Fibers. Cosmetics 2020, 7, 28. [Google Scholar] [CrossRef] [Green Version]
- Dobrovolskaya, I.P.; Yudin, V.E.; Popryadukhin, P.V.; Ivan’kova, E.M. Polymer Scaffolds for Tissue Engineering; Mediapapir: St. Petersburg, Russia, 2018. [Google Scholar]
- Kodolova-Chukhontseva, V.V.; Shishov, M.A.; Kolbe, K.A.; Smirnova, N.V.; Dobrovol’skaya, I.P.; Dresvyanina, E.N.; Bystrov, S.G.; Terebova, N.S.; Kamalov, A.M.; Bursian, A.E.; et al. Conducting Composite Material Based on Chitosan and Single-Wall Carbon Nanotubes for Cellular Technologies. Polymers 2022, 14, 3287. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A Fascinating Fiber Fabrication Technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef]
- Beachley, V.; Wen, X. Effect of Electrospinning Parameters on the Nanofiber Diameter and Length. Mater. Sci. Eng. C 2009, 29, 663–668. [Google Scholar] [CrossRef] [Green Version]
- Yang, F.; Murugan, R.; Wang, S.; Ramakrishna, S. Electrospinning of Nano/Micro Scale Poly(L-Lactic Acid) Aligned Fibers and Their Potential in Neural Tissue Engineering. Biomaterials 2005, 26, 2603–2610. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Touny, A.H.; Bhaduri, S.B. Fabrication of Novel PLA/CDHA Bionanocomposite Fibers for Tissue Engineering Applications via Electrospinning. J. Mater. Sci. Mater. Med. 2011, 22, 1183–1193. [Google Scholar] [CrossRef]
- Kodolova-Chukhontseva, V.V.; Dresvyanina, E.N.; Maevskaia, E.N.; Dobrovolskaya, I.P.; Koroleva, M.R.; Vlasova, E.N.; Ivan’kova, E.M.; Elokhovskii, V.Y.; Yudin, V.E.; Morganti, P. Influence of Chitin Nanofibrils Ultrasonic Treatment on Structure and Properties of Chitosan-Based Composite Materials. Carbohydr. Polym. 2022, 285, 119194. [Google Scholar] [CrossRef]
- Freier, T.; Koh, H.S.; Kazazian, K.; Shoichet, M.S. Controlling Cell Adhesion and Degradation of Chitosan Films by N-Acetylation. Biomaterials 2005, 26, 5872–5878. [Google Scholar] [CrossRef]
- Lončarević, A.; Ivanković, M.; Rogina, A. Lysozyme-Induced Degradation of Chitosan: The Characterisation of Degraded Chitosan Scaffolds. J. Tissue Repair Regen. 2017, 1, 12–22. [Google Scholar] [CrossRef] [Green Version]
- Dresvyanina, E.N.; Dobrovol’skaya, I.P.; Smirnov, V.E.; Popova, E.N.; Vlasova, E.N.; Yudin, V.E. Thermal Properties of Salt and Base Forms of Chitosan. Polym. Sci. Ser. A 2018, 60, 179–183. [Google Scholar] [CrossRef]
- Yudin, V.E.; Dobrovolskaya, I.P.; Neelov, I.M.; Dresvyanina, E.N.; Popryadukhin, P.V.; Ivan’kova, E.M.; Elokhovskii, V.Y.; Kasatkin, I.A.; Okrugin, B.M.; Morganti, P. Wet Spinning of Fibers Made of Chitosan and Chitin Nanofibrils. Carbohydr. Polym. 2014, 108, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.M.; Eng, G.; Caridade, S.G.; Mano, J.F.; Reis, R.L.; Vunjak-Novakovic, G. Electrically Conductive Chitosan/Carbon Scaffolds for Cardiac Tissue Engineering. Biomacromolecules 2014, 15, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Matrenichev, V.V.; Popryadukhin, P.V.; Kryukov, A.E.; Smirnova, N.V.; Ivan’kova, E.M.; Dobrovol’skaya, I.P.; Yudin, V.E. Properties of Film Materials Based on Composite Nanofibers from Aliphatic Copolyamide and Carbon Nanotubes for Tissue Engineering. Polym. Sci. Ser. A 2018, 60, 215–221. [Google Scholar] [CrossRef]
- Moskalyuk, O.A.; Malafeev, K.V.; Yudin, V.E.; Kamalov, A.M.; Ivankova, E.M. Electric Conductive and Mechanical Properties of Fibers Based on Polylactide and Carbon Nanofiber. Fibre Chem. 2020, 52, 191–195. [Google Scholar] [CrossRef]
- Dresvyanina, E.N.; Kodolova-Chukhontseva, V.V.; Bystrov, S.G.; Dobrovolskaya, I.P.; Vaganov, G.V.; Smirnova, N.V.; Kolbe, K.A.; Kamalov, A.M.; Ivan’kova, E.M.; Morganti, P.; et al. Influence of Surface Morphology of Chitosan Films Modified by Chitin Nanofibrils on Their Biological Properties. Carbohydr. Polym. 2021, 262, 117917. [Google Scholar] [CrossRef]
- Iijima, S. Helical Microtubules of Graphitic Carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Shokrieh, M.M.; Rafiee, R. A Review of the Mechanical Properties of Isolated Carbon Nanotubes and Carbon Nanotube Composites. Mech. Compos. Mater. 2010, 46, 155–172. [Google Scholar] [CrossRef]
- Balandin, A.A. Thermal Properties of Graphene and Nanostructured Carbon Materials. Nat. Mater. 2011, 10, 569–581. [Google Scholar] [CrossRef] [Green Version]
- MacDonald, R.A.; Voge, C.M.; Kariolis, M.; Stegemann, J.P. Carbon Nanotubes Increase the Electrical Conductivity of Fibroblast-Seeded Collagen Hydrogels. Acta Biomater. 2008, 4, 1583–1592. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Zhou, X.; Ye, F.; He, Q.; Chen, G.C.K.; Soo, J.; Boey, F.; Zhang, H.; Chen, P. Interfacing Live Cells with Nanocarbon Substrates. Langmuir 2010, 26, 2244–2247. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.K.; Ohashi, F.; Valappil, S.P.; Knowles, J.C.; Roy, I.; Silva, S.R.P.; Salih, V.; Boccaccini, A.R. Characterization of Carbon Nanotube (MWCNT) Containing P(3HB)/Bioactive Glass Composites for Tissue Engineering Applications. Acta Biomater. 2010, 6, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Tercero, J.E.; Namin, S.; Lahiri, D.; Balani, K.; Tsoukias, N.; Agarwal, A. Effect of Carbon Nanotube and Aluminum Oxide Addition on Plasma-Sprayed Hydroxyapatite Coating’s Mechanical Properties and Biocompatibility. Mater. Sci. Eng. C 2009, 29, 2195–2202. [Google Scholar] [CrossRef]
- Lobo, A.O.; Antunes, E.F.; Machado, A.H.A.; Pacheco-Soares, C.; Trava-Airoldi, V.J.; Corat, E.J. Cell Viability and Adhesion on as Grown Multi-Wall Carbon Nanotube Films. Mater. Sci. Eng. C 2008, 28, 264–269. [Google Scholar] [CrossRef]
- Aoki, N.; Akasaka, T.; Watari, F.; Yokoyama, A. Carbon Nanotubes as Scaffolds for Cell Culture and Effect on Cellular Functions. Dent. Mater. J. 2007, 26, 178–185. [Google Scholar] [CrossRef] [Green Version]
- Edwards, L.S.; Werkmeister, J.A.; Ramshaw, J. Carbon nanotubes in scaffolds for tissue engineering. Expert. Rev. Med. Devices 2009, 6, 499–505. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dresvyanina, E.N.; Tagandurdyyeva, N.A.; Kodolova-Chukhontseva, V.V.; Dobrovol’skaya, I.P.; Kamalov, A.M.; Nashchekina, Y.A.; Nashchekin, A.V.; Ivanov, A.G.; Yukina, G.Y.; Yudin, V.E. Structure and Properties of Composite Fibers Based on Chitosan and Single-Walled Carbon Nanotubes for Peripheral Nerve Regeneration. Polymers 2023, 15, 2860. https://doi.org/10.3390/polym15132860
Dresvyanina EN, Tagandurdyyeva NA, Kodolova-Chukhontseva VV, Dobrovol’skaya IP, Kamalov AM, Nashchekina YA, Nashchekin AV, Ivanov AG, Yukina GY, Yudin VE. Structure and Properties of Composite Fibers Based on Chitosan and Single-Walled Carbon Nanotubes for Peripheral Nerve Regeneration. Polymers. 2023; 15(13):2860. https://doi.org/10.3390/polym15132860
Chicago/Turabian StyleDresvyanina, Elena N., Nurjemal A. Tagandurdyyeva, Vera V. Kodolova-Chukhontseva, Irina P. Dobrovol’skaya, Almaz M. Kamalov, Yulia A. Nashchekina, Alexey V. Nashchekin, Alexey G. Ivanov, Galina Yu. Yukina, and Vladimir E. Yudin. 2023. "Structure and Properties of Composite Fibers Based on Chitosan and Single-Walled Carbon Nanotubes for Peripheral Nerve Regeneration" Polymers 15, no. 13: 2860. https://doi.org/10.3390/polym15132860






