Application of Perovskite Nanocrystals as Fluorescent Probes in the Detection of Agriculture- and Food-Related Hazardous Substances
Abstract
:1. Introduction
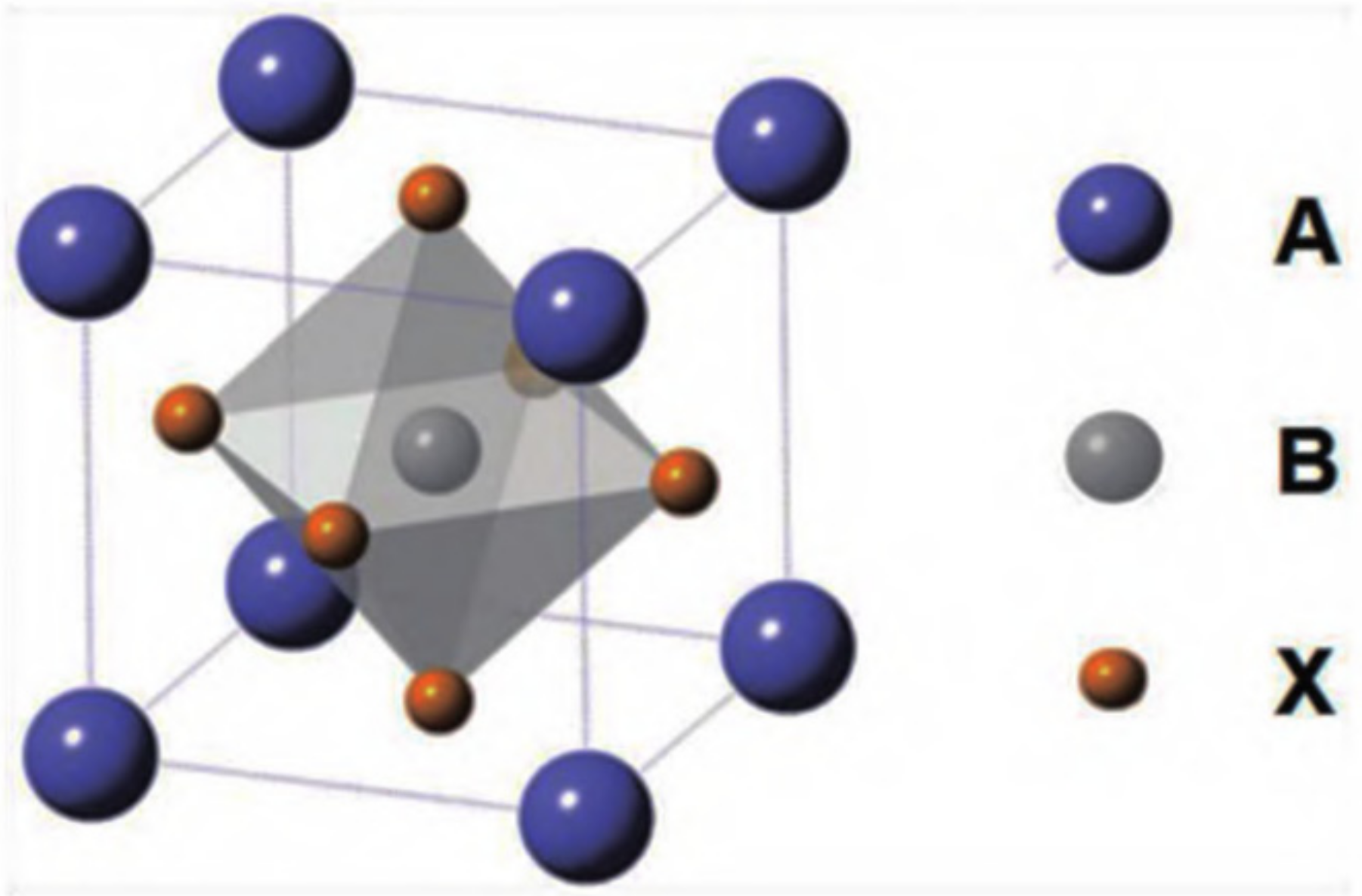
1.1. Structure and Basic Properties of PNCs
1.2. Methods to Improve the Stability of PNCs
1.2.1. Surface Coating by Polymer, Inorganic Oxide, and Porous Materials
1.2.2. Ion Doping
1.2.3. Surface Passivation
2. Application of PNCs as Fluorescent Probes in the Detection of Agriculture- and Food-Related Hazardous Substances
2.1. The Detection of Agriculture-Related Hazardous Substances
2.1.1. Pesticide Residue Detection
Organophosphorus Pesticide
Triazine Herbicides
Organochlorine Pesticides
Other Pesticides
2.1.2. Environmental Pollutant Detection
2.1.3. Ion Detection
Cation
Anion
2.2. The Detection of Food-Related Hazardous Substances
2.2.1. Antibiotic Detection
2.2.2. Detection of Microbial Toxins, Pathogens, and Carcinogens
2.2.3. Detection of Food Spoilage Gas
2.2.4. Detection of Harmful Additives in Food
2.2.5. Edible oil Quality Inspection
3. Summary and Prospects
- (1)
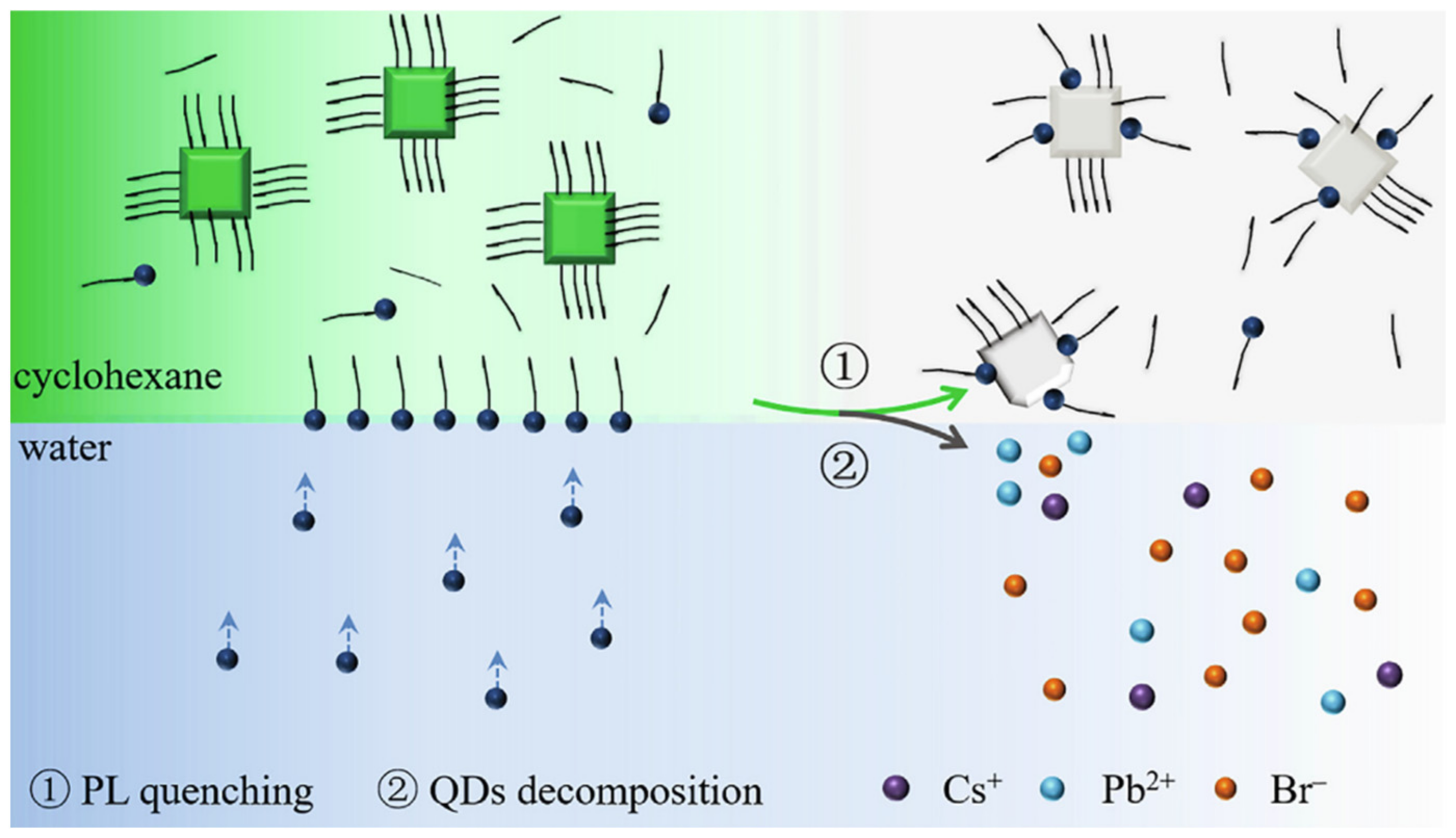
- The external environment (light, humidity, temperature, etc.) is easy to affect photoelectric properties of PNCs. Although, many modification methods have been proved to be effective to achieve PNCs in aqueous solutions, some of these methods are complex or cannot be used as fluorescent probes in the field of agriculture and food. Simple and novel water-stabilized method that is suitable for detection in agriculture and food should be further developed in the future. For example, the exploration of organic semiconductor ligands to prepare PNCs materials with good optoelectrical properties and heterostructures.
- (2)
- Lead is an important component of PNCs structure, and it is a huge obstacle, which hinders PNCs in practice in agriculture and food. Non-contact detection (gas or separated liquid samples) may be more suitable for the lead-based PNCs fluorescent probe. The development of novel and efficient lead-free PNCs fluorescent probe is more preferred and should be the future trend in this area.
- (3)
- The specificity of the fluorescent probe based on PNCs can be further developed to selectively detect specific analyte. Combined with molecular imprinting techniques, it has been proved to be an effective way to improve its selectivity. Especially, the novel characterizing methods should be paid more attention, such as MIECL platform, PEC immunosensing platform, etc.
- (4)
- The integration of fluorescent probe based on PNCs, excitation light source, and fluorescence detector into a portable instrument is still a big challenge. The future practice of fluorescent probe based on PNCs in the field of agriculture and food should tend to portable, real-time, and visualized detection. Fluorescent probe based on PNCs prefer to be developed into the form of test strip. Ratio fluorescence is a good method to get visual sensing with high accuracy. In addition, with the help of the color analysis software in smart phones, qualitative and semi-quantitative visual sensing can be easily realized, which has a good application prospect.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Vajubhai, G.N.; Chetti, P.; Kailasa, S.K. Perovskite quantum dots for fluorescence turn-off detection of the clodinafop pesticide in food samples via liquid–liquid microextraction. ACS Appl. Nano Mater. 2022, 5, 18220–18228. [Google Scholar] [CrossRef]
- He, J.; Yu, L.; Jiang, Y.; Lü, L.; Han, Z.; Zhao, X.; Xu, Z. Encoding CsPbX3 perovskite quantum dots with different colors in molecularly imprinted polymers as fluorescent probes for the quantitative detection of Sudan I in food matrices. Food Chem. 2023, 402, 134499. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Mehta, S.K.; Kunal, K.; Mukhopadhyay, K.; Singh, S. Quantum dots as promising nanomaterials in agriculture. In Agricultural Nanobiotechnology; Woodhead Publishing: Southton, UK, 2022; pp. 243–296. [Google Scholar]
- Huang, S.; Guo, M.; Tan, J.; Geng, Y.; Wu, J.; Tang, Y.; Liang, Y. Novel fluorescent probe based on all-inorganic perovskite quantum dots coated with molecularly imprinted polymers for highly selective and sensitive detection of omethoate. ACS Appl. Mater. Interfaces 2018, 10, 39056–39063. [Google Scholar] [CrossRef] [PubMed]
- Shangguan, Z.; Zheng, X.; Zhang, J.; Lin, W.; Guo, W.; Li, C.; Wu, T.; Lin, Y.; Chen, Z. The stability of metal halide perovskite nanocrystals—A key issue for the application on quantum-dot-based micro light-emitting diodes display. Nanomaterials 2020, 10, 1375. [Google Scholar] [CrossRef]
- Jia, D.; Xu, M.; Mu, S.; Ren, W.; Liu, C. Recent progress of perovskite nanocrystals in chem/bio sensing. Biosensors 2022, 12, 754. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Cheng, Z.; Lin, J. An overview on enhancing the stability of lead halide perovskite quantum dots and their applications in phosphor-converted LEDs. Chem. Soc. Rev. 2019, 48, 310–350. [Google Scholar] [CrossRef] [PubMed]
- Stoumpos, C.C.; Malliakas, C.D.; Kanatzidis, M.G. Semiconducting tin and lead iodide perovskites with organic cations: Phase transitions, high mobilities, and near-infrared photoluminescent properties. Inorg. Chem. 2013, 52, 9019–9038. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, G.; Wang, R.; Shao, H.; Wang, H.; Xu, W.; Song, H. Considerably enhanced exciton emission of CsPbCl3 perovskite quantum dots by the introduction of potassium and lanthanide ions. Nanoscale 2018, 10, 14067–14072. [Google Scholar] [CrossRef]
- Huang, S.; Wang, B.; Zhang, Q.; Li, Z.; Shan, A.; Li, L. Postsynthesis potassium modification method to improve stability of CsPbBr3 perovskite nanocrystals. Adv. Opt. Mater. 2018, 6, 1701106. [Google Scholar] [CrossRef]
- Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Krieg, F.; Caputo, R.; Hendon, C.H.; Kovalenko, M.V. Nanocrystals of cesium lead halide perovskites (CsPbX(3), X = Cl, Br, and I): Novel optoelectronic materials showing bright emission with wide color gamut. Nano Lett. 2015, 15, 3692–3696. [Google Scholar] [CrossRef] [Green Version]
- Di Stasio, F.; Christodoulou, S.; Huo, N.; Konstantatos, G. Near-unity photoluminescence quantum yield in cspbbr3 nanocrystal solid-state films via postsynthesis treatment with lead bromide. Chem. Mater. 2017, 29, 7663–7667. [Google Scholar] [CrossRef]
- Chen, M.; Zou, Y.; Wu, L.; Pan, Q.; Yang, D.; Hu, H.; Zhang, Q. Solvothermal synthesis of high-quality all-inorganic cesium lead halide perovskite nanocrystals: From nanocube to ultrathin nanowire. Adv. Funct. Mater. 2017, 27, 1701121. [Google Scholar] [CrossRef]
- Pan, Q.; Hu, H.; Zou, Y.; Chen, M.; Wu, L.; Yang, D.; Zhang, Q. Microwave-assisted synthesis of high-quality “all-inorganic” CsPbX3 (X = Cl, Br, I) perovskite nanocrystals and their application in light emitting diodes. J. Mater. Chem. C 2017, 5, 10947–10954. [Google Scholar] [CrossRef]
- Tong, Y.; Bladt, E.; Aygüler, M.F.; Manzi, A.; Milowska, K.Z.; Hintermayr, V.A.; Feldmann, J. Highly luminescent cesium lead halide perovskite nanocrystals with tunable composition and thickness by ultrasonication. Angew. Chem. Int. Ed. 2016, 55, 13887–13892. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Bai, J.; Chen, R.; Li, H. Synthesis and properties of B-site doped all-inorganic perovskite quantum dots. Chin. J. Appl. Chem. 2021, 38, 1541. [Google Scholar]
- Wei, Y.; Deng, X.; Xie, Z. Enhancing the stability of perovskite quantum dots by encapsulation in crosslinked polystyrene beads via a swelling–shrinking strategy toward superior water resistance. Adv. Funct. Mater. 2017, 27, 1703535. [Google Scholar] [CrossRef]
- An, H.; Kim, W.K.; Wu, C.; Kim, T.W. Highly-stable memristive devices based on poly (methylmethacrylate): CsPbCl3 perovskite quantum dot hybrid nanocomposites. Org. Electron. 2018, 56, 41–45. [Google Scholar] [CrossRef]
- Xuan, T.; Huang, J.; Liu, H.; Lou, S.; Cao, L.; Gan, W.; Wang, J. Super-hydrophobic cesium lead halide perovskite quantum dot-polymer composites with high stability and luminescent efficiency for wide color gamut white light-emitting diodes. Chem. Mater. 2019, 31, 1042–1047. [Google Scholar] [CrossRef]
- Avugadda, S.K.; Castelli, A.; Dhanabalan, B.; Fernandez, T.; Silvestri, N.; Collantes, C.; Arciniegas, M.P. Highly emitting perovskite nanocrystals with 2-year stability in water through an automated polymer encapsulation for bioimaging. ACS Nano 2022, 16, 13657–13666. [Google Scholar] [CrossRef]
- Li, S.; Lei, D.; Ren, W.; Guo, X.; Wu, S.; Zhu, Y.; Jen, A.K.Y. Water-resistant perovskite nanodots enable robust two-photon lasing in aqueous environment. Nat. Commun. 2020, 11, 1192. [Google Scholar] [CrossRef] [Green Version]
- You, C.Y.; Li, F.M.; Lin, L.H.; Lin, J.S.; Chen, Q.Q.; Radjenovic, P.M.; Li, J.F. Ultrastable monodispersed lead halide perovskite nanocrystals derived from interfacial compatibility. Nano Energy 2020, 71, 104554. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, B.; Zheng, W.; Kong, L.; Wan, Q.; Zhang, C.; Li, L. Ceramic-like stable CsPbBr3 nanocrystals encapsulated in silica derived from molecular sieve templates. Nat. Commun. 2020, 11, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiao, G.Y.; Guan, D.; Yuan, S.; Rao, H.; Chen, X.; Wang, J.A.; Yu, J. Perovskite quantum dots encapsulated in a mesoporous metal–organic framework as synergistic photocathode materials. J. Am. Chem. Soc. 2021, 143, 14253–14260. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.Y.; Mu, Y.F.; Guo, X.X.; Zhang, W.; Zhang, Z.M.; Zhang, M.; Lu, T.B. Encapsulating perovskite quantum dots in iron-based metal-organic frameworks (MOFs) for efficient photocatalytic CO2 reduction. Angew. Chem. Int. Ed. 2019, 58, 9491–9495. [Google Scholar] [CrossRef]
- Liu, B.; Guo, X.; Gao, D. Research progress on the improvement of the stability of perovskite quantum dots. Chem. Ind. Eng. Prog. 2021, 40, 247–258. [Google Scholar] [CrossRef]
- Pering, S.R.; Deng, W.; Troughton, J.R.; Kubiak, P.S.; Ghosh, D.; Niemann, R.G.; Cameron, P.J. Azetidinium lead iodide for perovskite solar cells. J. Mater. Chem. A 2017, 5, 20658–20665. [Google Scholar] [CrossRef] [Green Version]
- Filip, M.R.; Eperon, G.E.; Snaith, H.J.; Giustino, F. Steric engineering of metal-halide perovskites with tunable optical band gaps. Nat. Commun. 2014, 5, 5757. [Google Scholar] [CrossRef] [Green Version]
- Protesescu, L.; Yakunin, S.; Kumar, S.; Bär, J.; Bertolotti, F.; Masciocchi, N.; Kovalenko, M.V. Dismantling the “red wall” of colloidal perovskites: Highly luminescent formamidinium and formamidinium–cesium lead iodide nanocrystals. ACS Nano 2017, 11, 3119–3134. [Google Scholar] [CrossRef]
- Wu, X.; Jiang, Y.; Chen, C.; Guo, J.; Kong, X.; Feng, Y.; Gao, J. Stable triple cation perovskite precursor for highly efficient perovskite solar cells enabled by interaction with 18C6 stabilizer. Adv. Funct. Mater. 2020, 30, 1908613. [Google Scholar] [CrossRef]
- Xu, L.; Yuan, S.; Zeng, H.; Song, J.J. A comprehensive review of doping in perovskite nanocrystals/quantum dots: Evolution of structure, electronics, optics, and light-emitting diodes. Mater. Today Nano 2019, 6, 100036. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Zhou, L.; Shen, W.; Li, M.; He, R. Highly luminescent and stable quasi-2D perovskite quantum dots by introducing large organic cations. Nanoscale Adv. 2021, 3, 5393–5398. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, J.; Bakr, O.M.; Sun, H.T. Metal-doped lead halide perovskites: Synthesis, properties, and optoelectronic applications. Chem. Mater. 2018, 30, 6589–6613. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, X.; Sun, R.; Zhou, D.; Liu, S.; Wu, Y.; Shi, Z.; Song, H. Synergistic effects of multifunctional lanthanides doped CsPbBrCl2 quantum dots for efficient and stable MAPbI3 perovskite solar cells. Adv. Funct. Mater. 2022, 32, 2110346. [Google Scholar] [CrossRef]
- Shen, X.; Zhang, Y.; Kershaw, S.V.; Li, T.; Wang, C.; Zhang, X.; Rogach, A.L. Zn-alloyed CsPbI3 nanocrystals for highly efficient perovskite light-emitting devices. Nano Lett. 2019, 19, 1552–1559. [Google Scholar] [CrossRef]
- Song, P.; Qiao, B.; Song, D.; Cao, J.; Shen, Z.; Xu, Z.; Al-Ghamdi, A. Modifying the crystal field of CsPbCl3: Mn2+ nanocrystals by co-doping to enhance its red emission by a hundredfold. ACS Appl. Mater. Interfaces 2020, 12, 30711–30719. [Google Scholar] [CrossRef]
- Kim, H.; Bae, S.R.; Lee, T.H.; Lee, H.; Kang, H.; Park, S.; Kim, S.Y. Enhanced optical properties and stability of CsPbBr3 nanocrystals through nickel doping. Adv. Funct. Mater. 2021, 31, 2102770. [Google Scholar] [CrossRef]
- Zeng, Q.; Zhang, X.; Bing, Q.; Xiong, Y.; Yang, F.; Liu, H.; Yang, B. Surface Stabilization of Colloidal Perovskite Nanocrystals via Multi-amine Chelating Ligands. ACS Energy Lett. 2022, 7, 1963–1970. [Google Scholar] [CrossRef]
- Bi, C.; Kershaw, S.V.; Rogach, A.L.; Tian, J. Improved stability and photodetector performance of CsPbI3 perovskite quantum dots by ligand exchange with aminoethanethiol. Adv. Funct. Mater. 2019, 29, 1902446. [Google Scholar] [CrossRef]
- Krieg, F.; Ochsenbein, S.T.; Yakunin, S.; Ten Brinck, S.; Aellen, P.; Süess, A.; Kovalenko, M.V. Colloidal CsPbX3 (X = Cl, Br, I) nanocrystals 2.0: Zwitterionic capping ligands for improved durability and stability. ACS Energy Lett. 2018, 3, 641–646. [Google Scholar] [CrossRef] [Green Version]
- Krieg, F.; Ong, Q.K.; Burian, M.; Rainò, G.; Naumenko, D.; Amenitsch, H.; Kovalenko, M.V. Stable ultraconcentrated and ultradilute colloids of CsPbX3 (X= Cl, Br) nanocrystals using natural lecithin as a capping ligand. J. Am. Chem. Soc. 2019, 141, 19839–19849. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Du, L.; Jin, Z.; Xin, Y.; Mattoussi, H. Enhanced stabilization and easy phase transfer of CsPbBr3 perovskite quantum dots promoted by high-affinity polyzwitterionic ligands. J. Am. Chem. Soc. 2020, 142, 12669–12680. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Hu, J.; Fu, J.; Lin, Y.; Zou, C.; Di, D.; Cao, M. Ultrahigh stability of perovskite nanocrystals by using semiconducting molecular species for displays. ACS Nano 2022, 16, 12253–12261. [Google Scholar] [CrossRef] [PubMed]
- Rajput, S.; Sharma, R.; Kumari, A.; Kaur, R.; Sharma, G.; Arora, S.; Kaur, R. Pesticide residues in various environmental and biological matrices: Distribution, extraction, and analytical procedures. Environ. Dev. Sustain. 2022, 24, 6032–6052. [Google Scholar] [CrossRef]
- Damalas, C.A.; Eleftherohorinos, I.G. Pesticide exposure, safety issues, and risk assessment indicators. Int. J. Environ. Res. Public Health 2011, 8, 1402–1419. [Google Scholar] [CrossRef]
- Huang, S.; Tan, L.; Zhang, L.; Wu, J.; Zhang, L.; Tang, Y.; Liang, Y. Molecularly imprinted mesoporous silica embedded with perovskite CsPbBr3 quantum dots for the fluorescence sensing of 2, 2-dichlorovinyl dimethyl phosphate. Sens. Actuators B Chem. 2020, 325, 128751. [Google Scholar] [CrossRef]
- Tan, L.; Guo, M.; Tan, J.; Geng, Y.; Huang, S.; Tang, Y.; Liang, Y. Development of high-luminescence perovskite quantum dots coated with molecularly imprinted polymers for pesticide detection by slowly hydrolysing the organosilicon monomers in situ. Sens. Actuators B Chem. 2019, 291, 226–234. [Google Scholar] [CrossRef]
- Chen, D.; Guan, J.; Zhang, G. Research status of microbial degradation of pesticides. J. Henan Inst. Sci. Technol. (Nat. Sci. Ed.) 2020, 48, 39–46. [Google Scholar]
- Zhang, R.R.; Gan, X.T.; Xu, J.J.; Pan, Q.F.; Liu, H.; Sun, A.L.; Zhang, Z.M. Ultrasensitive electrochemiluminescence sensor based on perovskite quantum dots coated with molecularly imprinted polymer for prometryn determination. Food Chem. 2022, 370, 131353. [Google Scholar] [CrossRef]
- Pan, Q.F.; Jiao, H.F.; Liu, H.; You, J.J.; Sun, A.L.; Zhang, Z.M.; Shi, X.Z. Highly selective molecularly imprinted-electrochemiluminescence sensor based on perovskite/Ru (bpy)32+ for simazine detection in aquatic products. Sci. Total Environ. 2022, 843, 156925. [Google Scholar] [CrossRef]
- Yang, Y.; Han, A.; Hao, S.; Li, X.; Luo, X.; Fang, G.; Liu, J.; Wang, S. Fluorescent methylammonium lead halide perovskite quantum dots as a sensing material for the detection of polar organochlorine pesticide residues. Analyst 2020, 145, 6683–6690. [Google Scholar] [CrossRef]
- Liu, C.X.; Zhao, J.; Zhang, R.R.; Zhang, Z.M.; Xu, J.J.; Sun, A.L.; Shi, X.Z. Development and application of fluorescent probe and test strip based on molecularly imprinted quantum dots for the selective and sensitive detection of propanil in fish and seawater samples. J. Hazard. Mater. 2020, 389, 121884. [Google Scholar] [CrossRef] [PubMed]
- Halali, V.V.; Rani, R.S.; Balakrishna, R.G.; Budagumpi, S. Ultra-trace level chemosensing of uranyl ions; scuffle between electron and energy transfer from perovskite quantum dots to adsorbed uranyl ions. Microchem. J. 2020, 156, 104808. [Google Scholar] [CrossRef]
- Deng, P.; Wang, W.; Liu, X.; Wang, L.; Yan, Y. A hydrophobic polymer stabilized CsPbBr3 sensor for environmental pollutant detection. New J. Chem. 2021, 45, 930–938. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, X.; Zhu, T.; Deng, M.; Ikechukwu, I.P.; Huang, W.; Qiu, F. All-inorganic CsPbBr3 perovskite quantum dots as a photoluminescent probe for ultrasensitive Cu 2+ detection. J. Mater. Chem. C 2018, 6, 4793–4799. [Google Scholar] [CrossRef]
- Li, Q.; Zhou, W.; Yu, L.; Lian, S.; Xie, Q. Perovskite quantum dots as a fluorescent probe for metal ion detection in aqueous solution via phase transfer. Mater. Lett. 2021, 282, 128654. [Google Scholar] [CrossRef]
- Lu, L.Q.; Tan, T.; Tian, X.K.; Li, Y.; Deng, P. Visual and sensitive fluorescent sensing for ultratrace mercury ions by perovskite quantum dots. Anal. Chim. Acta 2017, 986, 109–114. [Google Scholar] [CrossRef]
- Chen, M.; An, J.; Hu, Y.; Chen, R.; Lyu, Y.; Hu, N.; Liu, Y. Swelling-shrinking modified hyperstatic hydrophilic perovskite polymer fluorescent beads for Fe (III) detection. Sens. Actuators B Chem. 2020, 325, 128809. [Google Scholar] [CrossRef]
- Wang, J.; Li, M.; Shen, W.; Su, W.; He, R. Ultrastable carbon quantum dots-doped MAPbBr3 perovskite with silica encapsulation. ACS Appl. Mater. Interfaces 2019, 11, 34348–34354. [Google Scholar] [CrossRef]
- Yan, J.; He, Y.; Chen, Y.; Zhang, Y.; Yan, H. CH3NH3Br solution as a novel platform for the selective fluorescence detection of Pb2+ ions. Sci. Rep. 2019, 9, 15840. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Huang, Y.; Zhang, L.; Li, F.; Lin, F.; Wang, Y.; Chen, X. Highly selective fluorescence turn-on determination of Pb (II) in Water by in-situ enrichment of Pb (II) and MAPbBr3 perovskite growth in sulfydryl functionalized mesoporous alumina film. Sens. Actuators B Chem. 2021, 326, 128975. [Google Scholar] [CrossRef]
- Park, B.; Kang, S.M.; Lee, G.W.; Kwak, C.H.; Rethinasabapathy, M.; Huh, Y.S. Fabrication of CsPbBr3 perovskite quantum dots/cellulose-based colorimetric sensor: Dual-responsive on-site detection of chloride and iodide ions. Ind. Eng. Chem. Res. 2019, 59, 793–801. [Google Scholar] [CrossRef]
- Han, J.; Sharipov, M.; Hwang, S.; Lee, Y.; Huy, B.T.; Lee, Y.I. Water-stable perovskite-loaded nanogels containing antioxidant property for highly sensitive and selective detection of roxithromycin in animal-derived food products. Sci. Rep. 2022, 12, 3147. [Google Scholar] [CrossRef]
- Jia, L.; Xu, Z.; Zhang, L.; Li, Y.; Zhao, T.; Xu, J. The fabrication of water-stable perovskite-europium hybrid polychromatic fluorescence nanosensor for fast visual sensing of tetracycline. Appl. Surf. Sci. 2022, 592, 153170. [Google Scholar] [CrossRef]
- Thuy, T.T.; Huy, B.T.; Kumar, A.P.; Lee, Y.I. Highly stable Cs4PbBr6/CsPbBr3 perovskite nanoparticles as a new fluorescence nanosensor for selective detection of trace tetracycline in food samples. J. Ind. Eng. Chem. 2021, 104, 437–444. [Google Scholar] [CrossRef]
- Wang, W.; Deng, P.; Liu, X.; Ma, Y.; Yan, Y. A CsPbBr3 quantum dots/ultra-thin BN fluorescent probe for stability and highly sensitive detection of tetracycline. Microchem. J. 2021, 162, 105876. [Google Scholar] [CrossRef]
- Wang, T.; Wei, X.; Zong, Y.; Zhang, S.; Guan, W. An efficient and stable fluorescent sensor based on APTES-functionalized CsPbBr3 perovskite quantum dots for ultrasensitive tetracycline detection in ethanol. J. Mater. Chem. C 2020, 8, 12196–12203. [Google Scholar] [CrossRef]
- Salari, R.; Amjadi, M.; Hallaj, T. Perovskite quantum dots as a chemiluminescence platform for highly sensitive assay of cefazolin. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 285, 121845. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Kralj, M.; Zhang, Y. Colorimetric test strips based on cesium lead bromide perovskite nanocrystals for rapid detection of ciprofloxacin hydrochloride. J. Phys. Condens. Matter 2022, 34, 304002. [Google Scholar] [CrossRef]
- Su, L.; Tong, P.; Zhang, L.; Luo, Z.; Fu, C.; Tang, D.; Zhang, Y. Photoelectrochemical immunoassay of aflatoxin B1 in foodstuff based on amorphous TiO2 and CsPbBr3 perovskite nanocrystals. Analyst 2019, 144, 4880–4886. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, Q.; Xiong, C.; Deng, Q.; Zhang, X.; Wang, S.; Chen, M.M. An ultrasensitive CH3NH3PbBr3 quantum dots@ SiO2-based electrochemiluminescence sensing platform using an organic electrolyte for aflatoxin B1 detection in corn oil. Food Chem. 2022, 390, 133200. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Shi, L.; Jing, X.; Miao, H.; Zhao, Y. SERS-active composites with Au–Ag Janus nanoparticles/perovskite in immunoassays for Staphylococcus aureus enterotoxins. ACS Appl. Mater. Interfaces 2022, 14, 3293–3301. [Google Scholar] [CrossRef]
- Ye, W.; Cao, Q.; Cheng, X.F.; Yu, C.; He, J.H.; Lu, J.M. Lead-free Cs2PdBr6 perovskite-based humidity sensor for artificial fruit waxing detection. J. Mater. Chem. A 2020, 8, 17675–17682. [Google Scholar] [CrossRef]
- Huang, H.; Hao, M.; Song, Y.; Dang, S.; Liu, X.; Dong, Q. Dynamic passivation in perovskite quantum dots for specific ammonia detection at room temperature. Small 2020, 16, 1904462. [Google Scholar] [CrossRef] [PubMed]
- Luo, F.; Li, S.; Cui, L.; Zu, Y.; Chen, Y.; Huang, D.; Lin, Z. Biocompatible perovskite quantum dots with superior water resistance enable long-term monitoring of the H2S level in vivo. Nanoscale 2021, 13, 14297–14303. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Y.; Zu, Y.; Chen, Y.; Luo, F.; Huang, D.; Lin, Z. Photothermal sensor based on water-soluble CsPbBr3@ sulfobutylether-β-cyclodextrins nanocomposite using a thermometer as readout. Sens. Actuators B Chem. 2022, 355, 131301. [Google Scholar] [CrossRef]
- Luo, F.; Zhang, Y.; Zu, Y.; Li, S.; Chen, Y.; Chen, Z.; Lin, Z. Quick preparation of water-soluble perovskite nanocomposite via cetyltrimethylammonium bromide and its application. Microchim. Acta 2022, 189, 68. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Lu, Q.; Miu, X.; Fang, A.; Li, H.; Zhang, Y. A simple assay platform for sensitive detection of Sudan I–IV in chilli powder based on CsPbBr3 quantum dots. J. Food Sci. Technol. 2018, 55, 2497–2503. [Google Scholar] [CrossRef]
- Chan, K.K.; Yap, S.H.K.; Giovanni, D.; Sum, T.C.; Yong, K.T. Water-stable perovskite quantum dots-based FRET nanosensor for the detection of rhodamine 6G in water, food, and biological samples. Microchem. J. 2022, 180, 107624. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, Y.; Huang, J.; Cai, J.; Zhu, J.; Yang, X.; Li, C. CsPbBr3 perovskite quantum dots-based monolithic electrospun fiber membrane as an ultrastable and ultrasensitive fluorescent sensor in aqueous medium. J. Phys. Chem. Lett. 2016, 7, 4253–4258. [Google Scholar] [CrossRef]
- Ni, D.J.; Zhang, J.; Cao, Z.K.; Li, R.; Xu, T.F.; Sang, H.W.; Long, Y.Z. Supersensitive and reusable perovskite nanocomposite fiber paper for time-resolved single-droplet detection. J. Hazard. Mater. 2021, 403, 123959. [Google Scholar] [CrossRef]
- Shi, L.; Xu, C.; Fan, Y. Determination of basic yellow in food by fluorescence colorimetry based on perovskite nanomaterials. J. Food Saf. Qual. 2021, 12, 3658–3664. [Google Scholar]
- Li, Q.; Wang, H.; Yue, X.; Du, J. Perovskite nanocrystals fluorescence nanosensor for ultrasensitive detection of trace melamine in dairy products by the manipulation of inner filter effect of gold nanoparticles. Talanta 2020, 211, 120705. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Yu, X.; Chen, J.; Zhang, W.; Huang, Y.; Zheng, J.; Chi, Y. Highly Electrochemiluminescent Cs4PbBr6@ CsPbBr3 perovskite nanoacanthospheres and their application for sensing bisphenol A. Anal. Chem. 2022, 94, 17142–17150. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Li, F.; Huang, Y.; Lin, F.; Chen, X. Wavelength-shift-based colorimetric sensing for peroxide number of edible oil using CsPbBr3 perovskite nanocrystals. Anal. Chem. 2019, 91, 14183–14187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Xu, Y.; Shi, L.; Fan, Y. Perovskite nanomaterial-engineered multiplex-mode fluorescence sensing of edible oil quality. Anal. Chem. 2021, 93, 11033–11042. [Google Scholar] [CrossRef]
- Wang, S.; Yu, L.; Wei, Z.; Xu, Q.; Zhou, W.; Xiao, Y. Dual-response ratiometric fluorescence based ligand-functionalized CsPbBr3 perovskite quantum dots for sensitive detection of trace water in edible oils. Sens. Actuators B Chem. 2022, 366, 132010. [Google Scholar] [CrossRef]
- Huangfu, C.; Feng, L. High-performance fluorescent sensor based on CsPbBr3 quantum dots for rapid analysis of total polar materials in edible oils. Sens. Actuators B Chem. 2021, 344, 130193. [Google Scholar] [CrossRef]



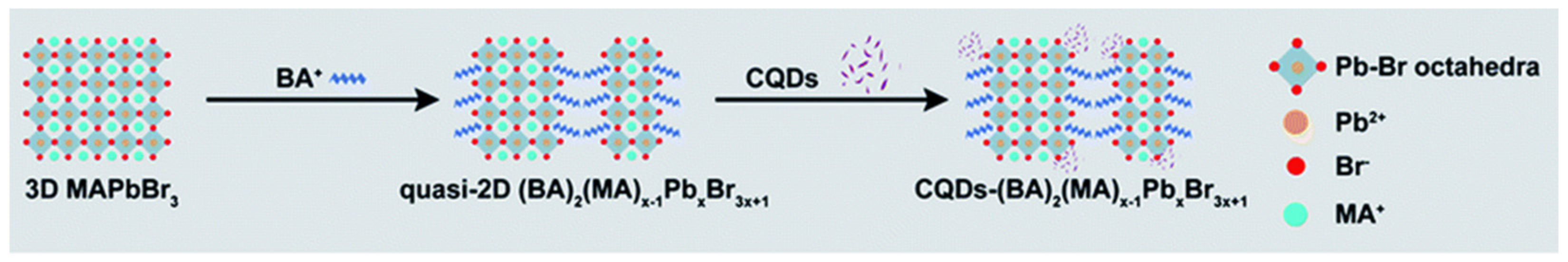


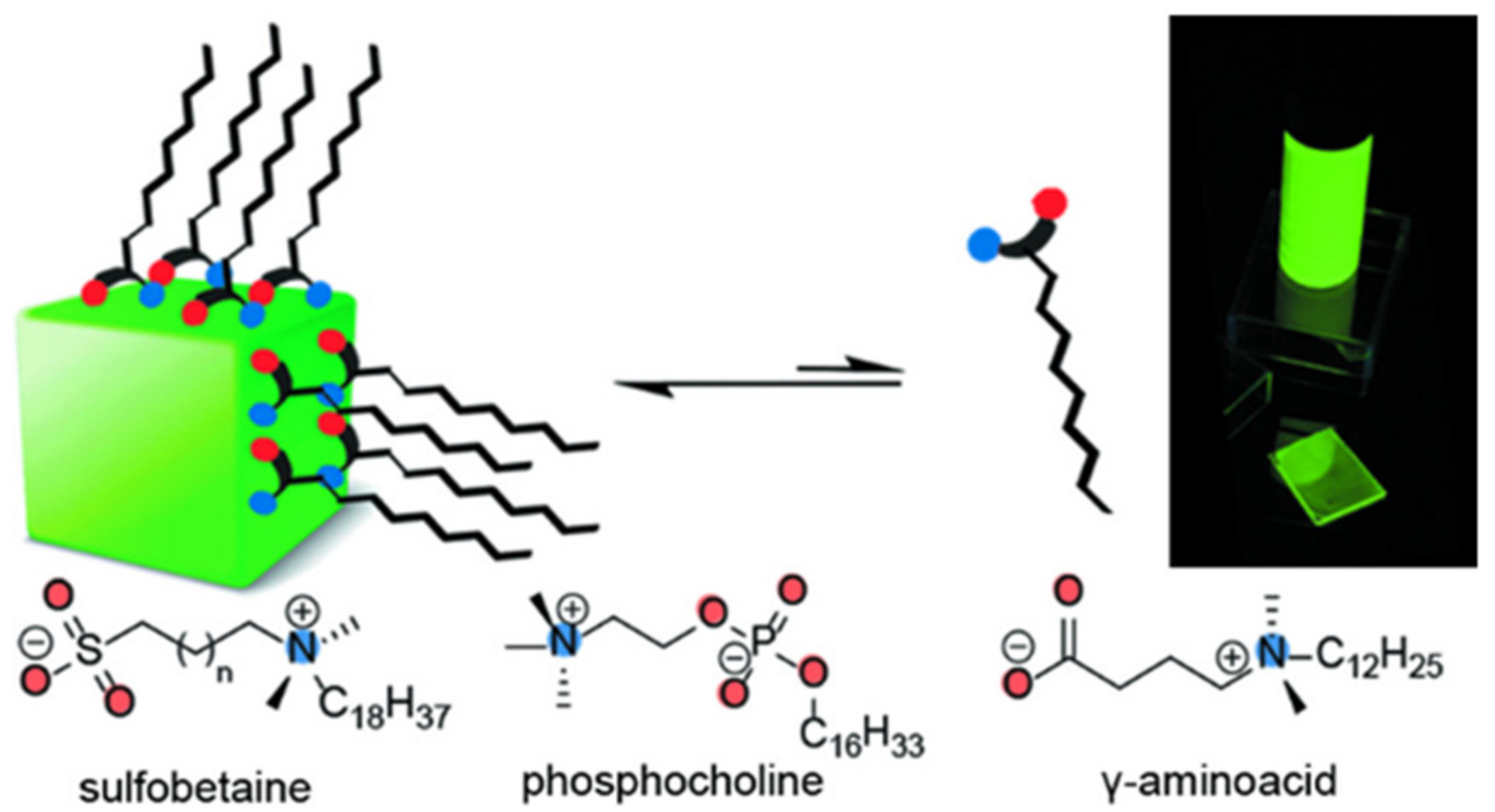

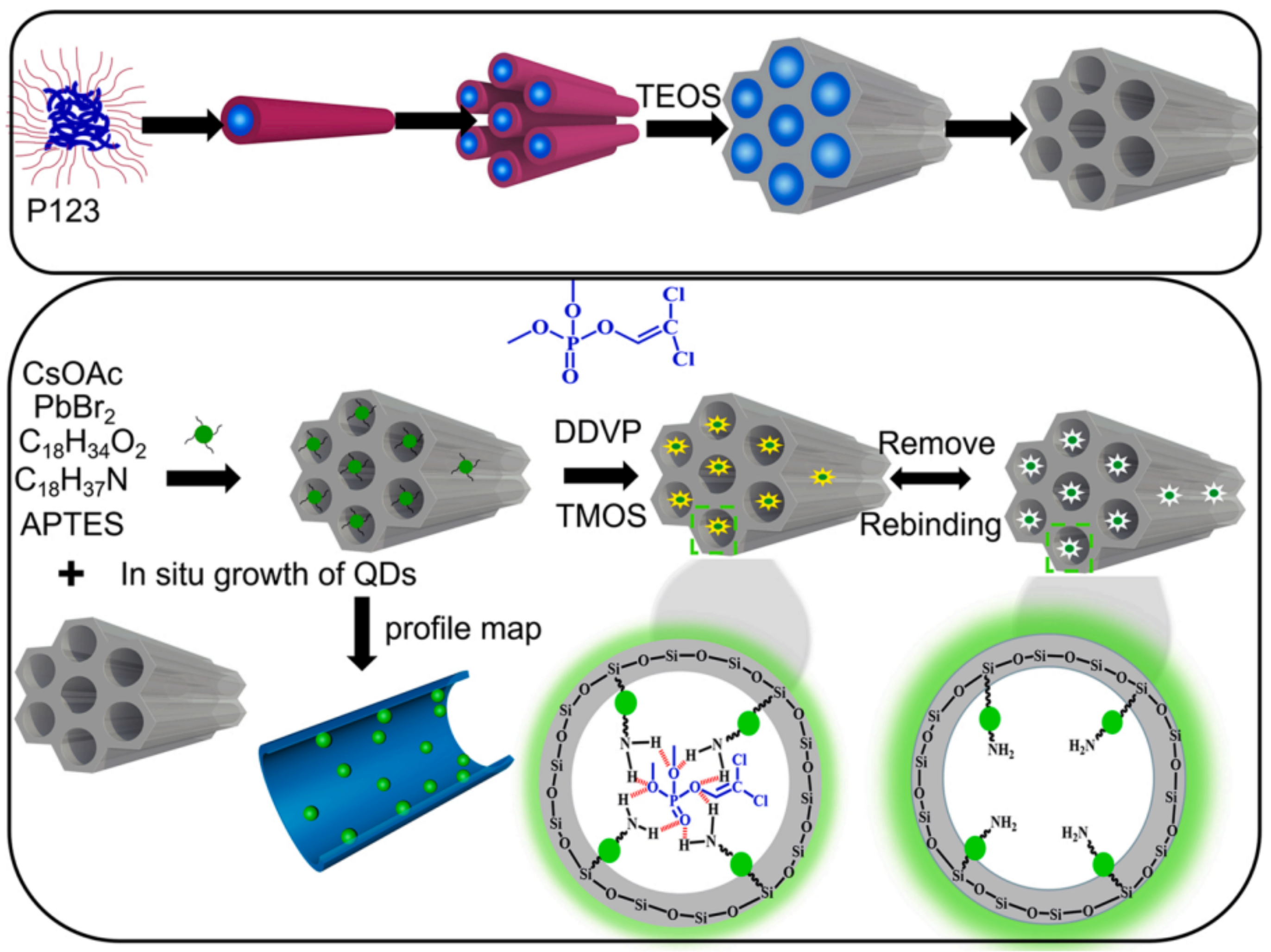


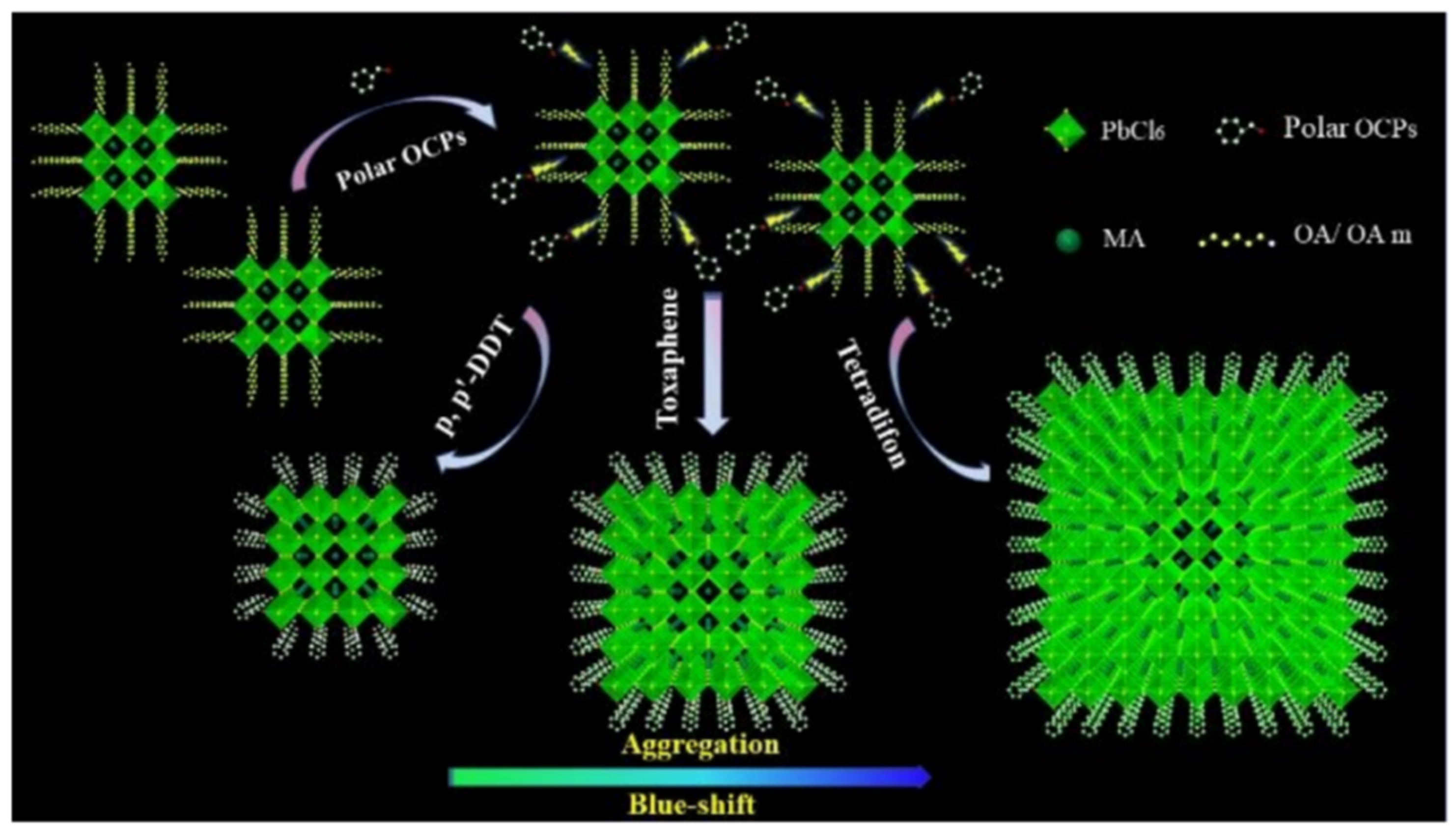


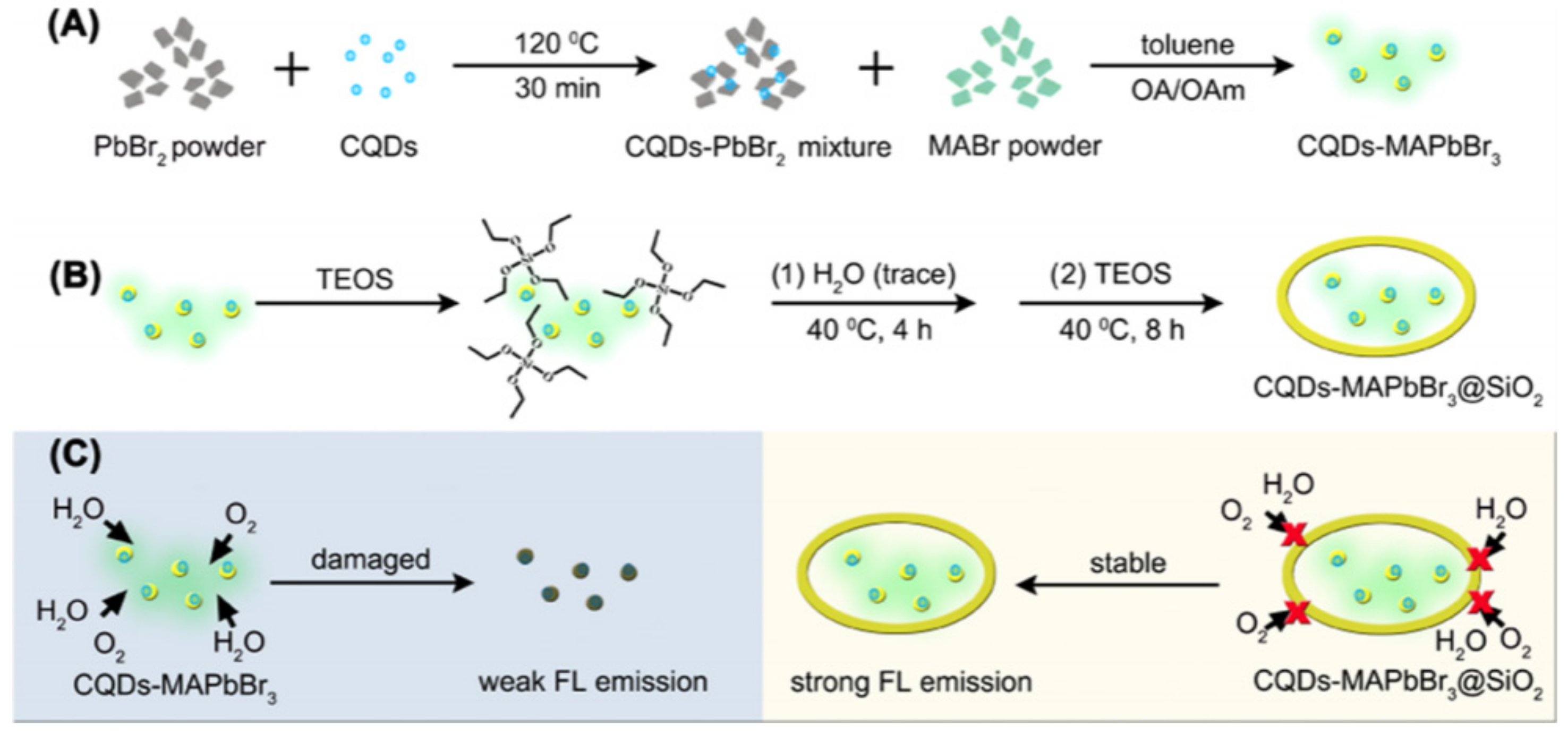

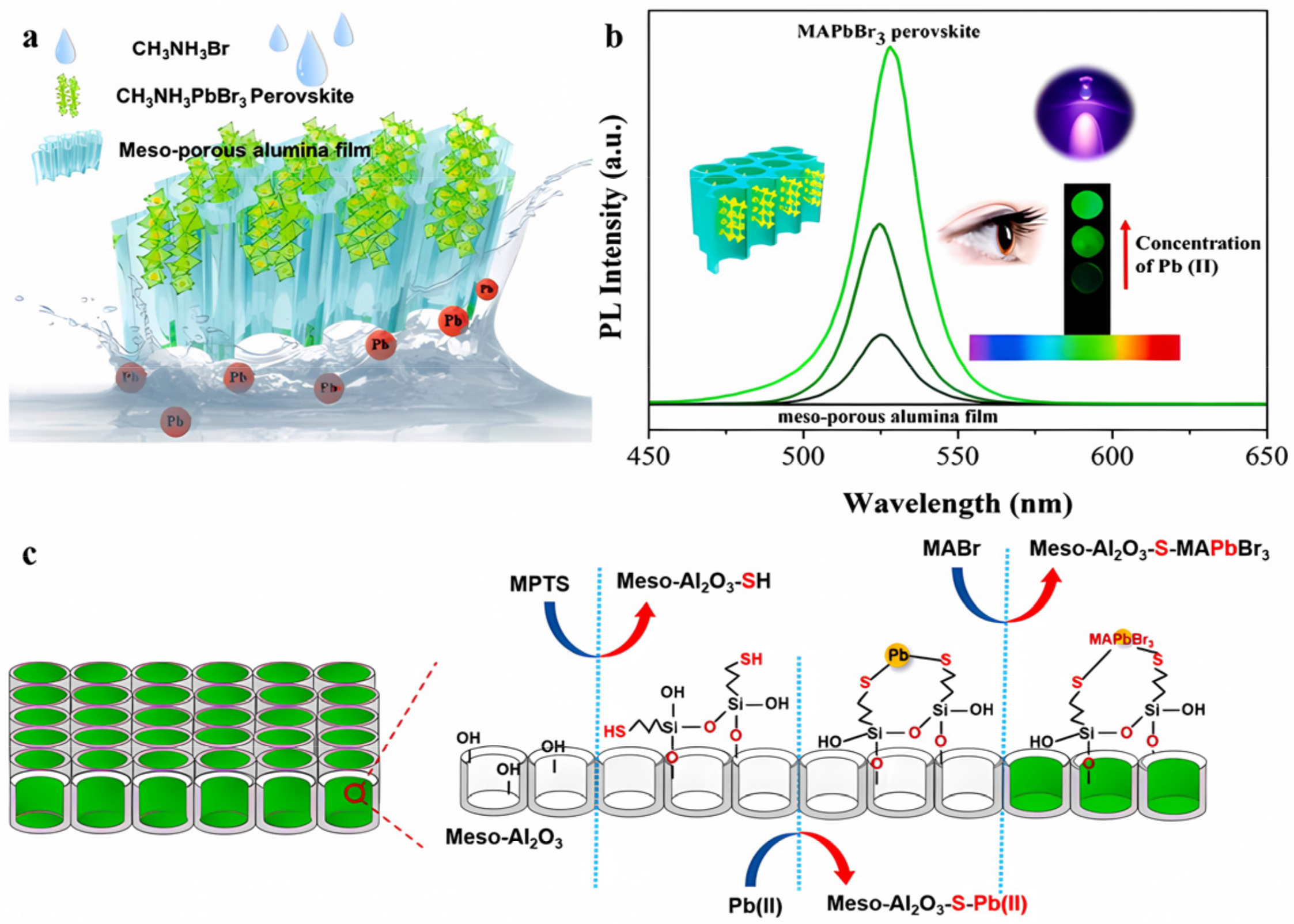
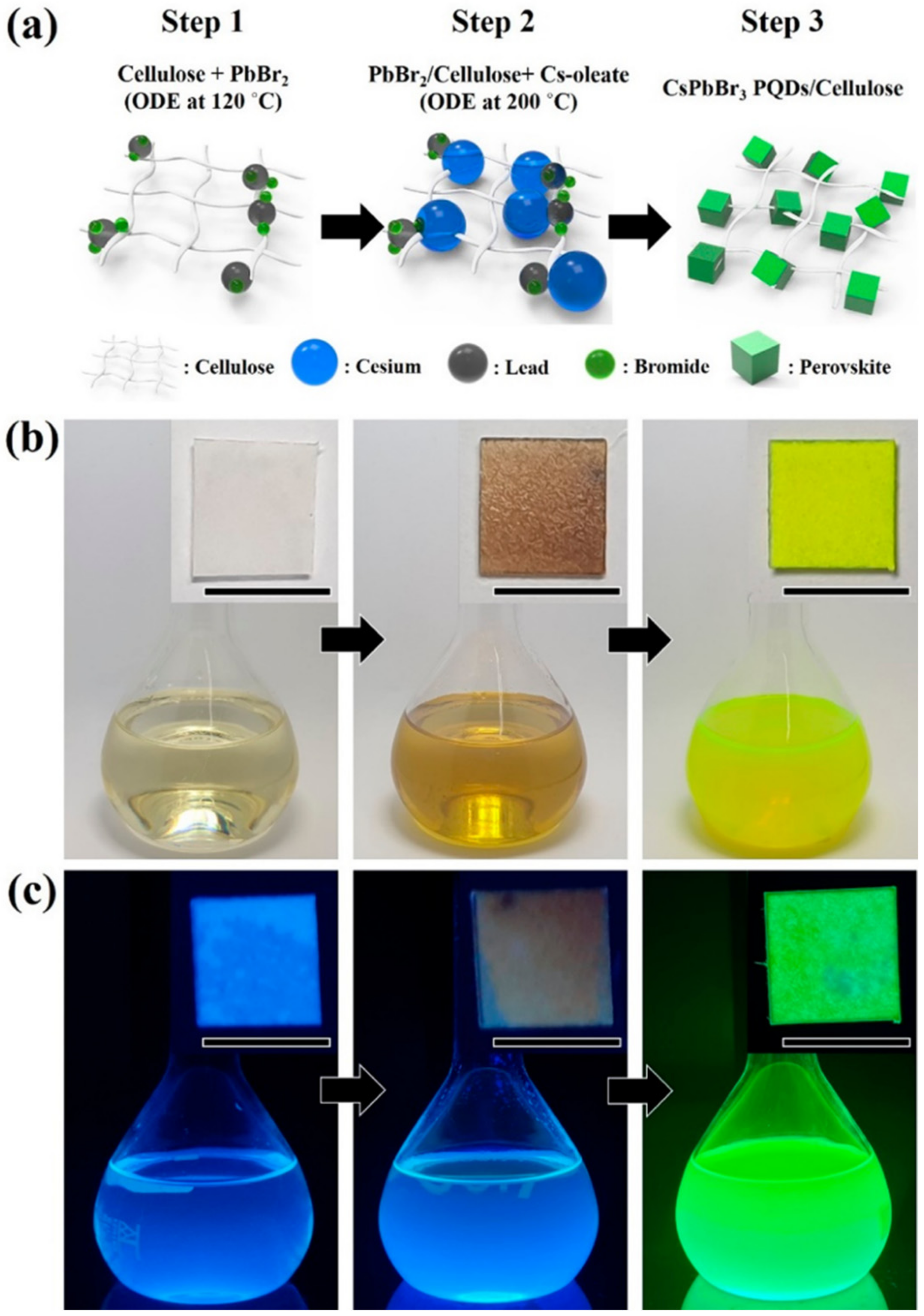







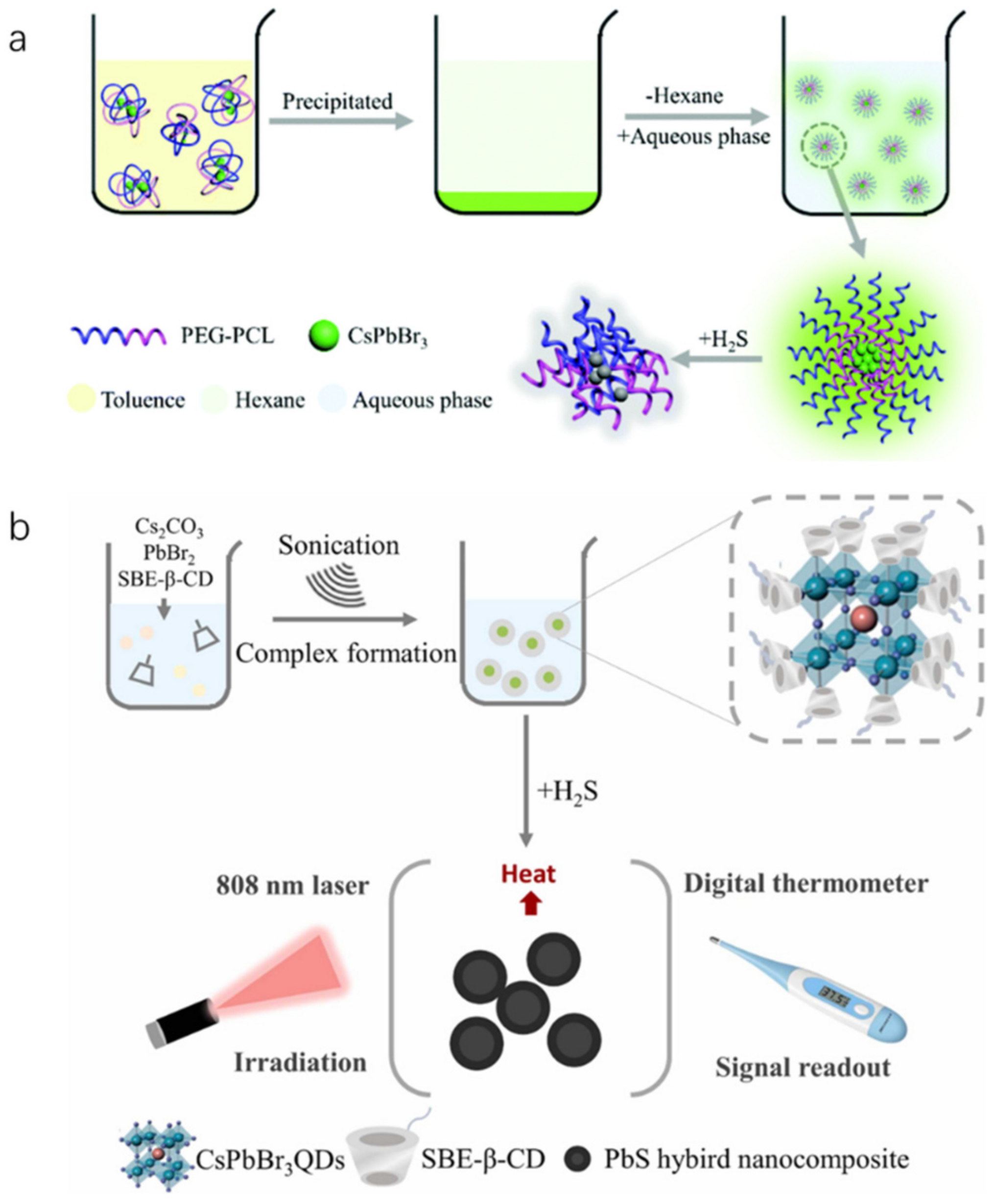





| Methods | Modification Material | Water-Stabilized PNCs | Stability of PNCs | Ref. | |
|---|---|---|---|---|---|
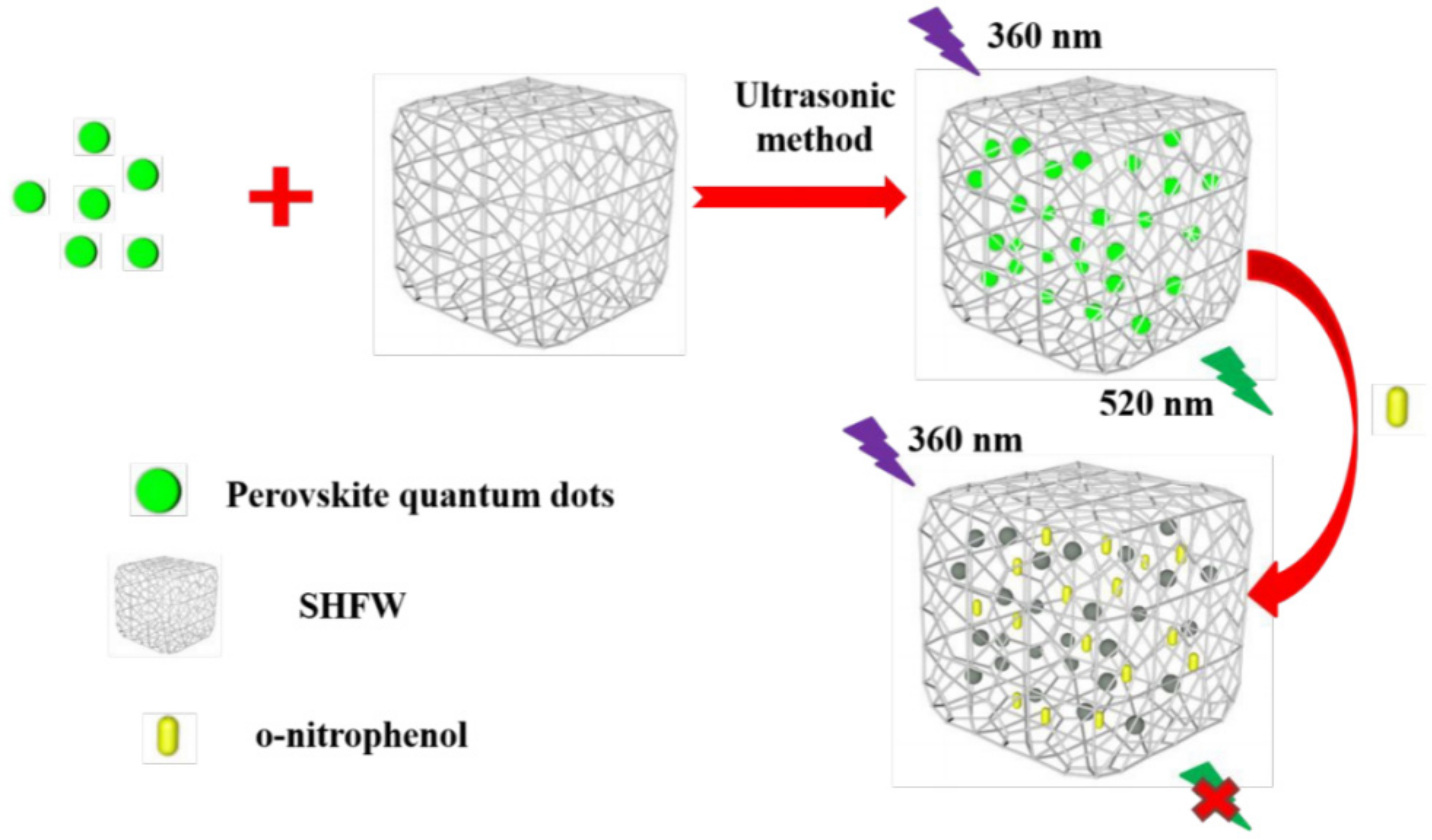
| Surface coating | Polymer | Divinylbenzene, ethyl acetate, and AIBN | CPB@SHFW | PLQY remained at 91%, 1 month in water | [19] |
| PS-b-PAA | CsPbBr3@PS-b-PAA | PLQY remained at 60%, 2 years in water | [20] | ||
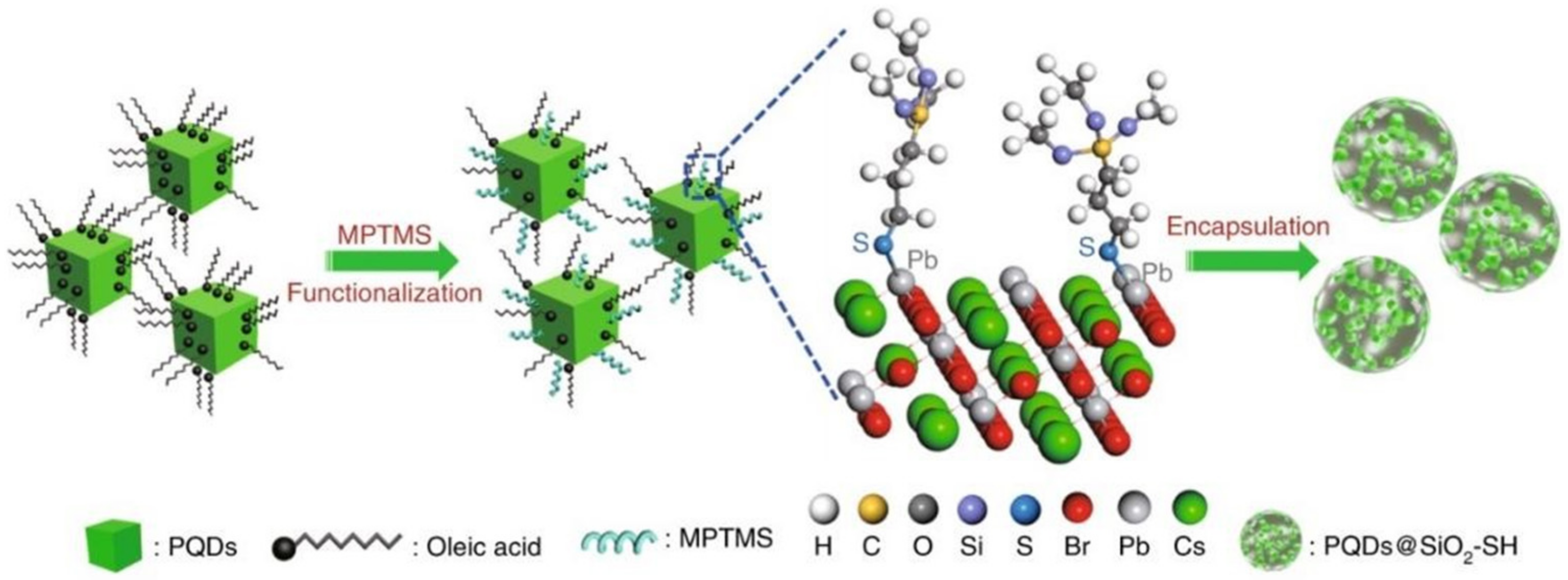
| Inorganic oxide | C6H15O3Si-SH | PQDs-Pb-S-SiO2-SH | PLQY remained at 80%, 13 h in water | [21] | |
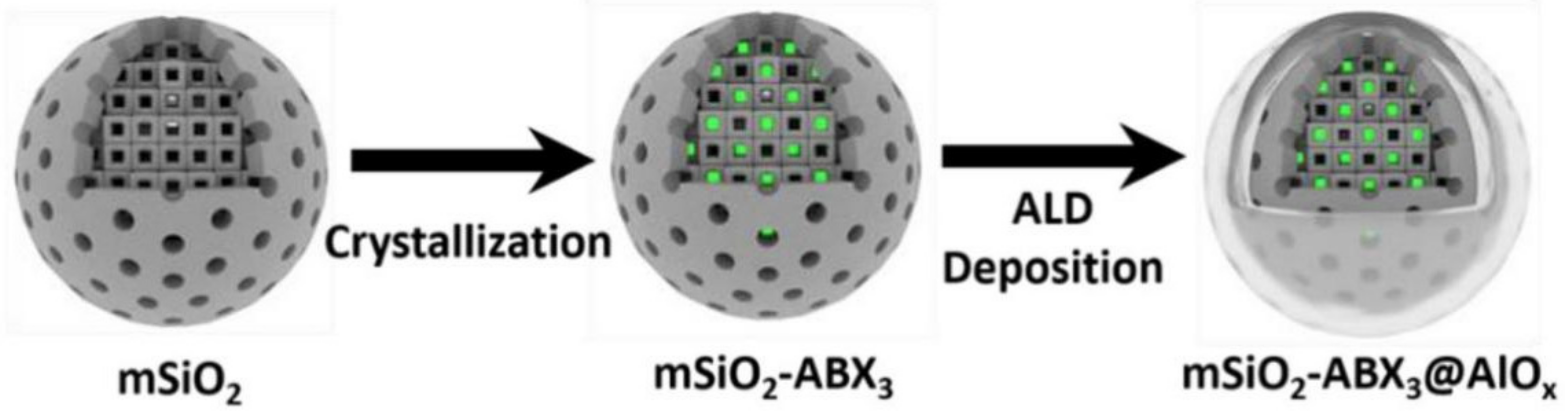
| Porous materials | Mesoporous silicon | mSiO2-CsPbBr3@AlOx | Over 20% PL emission intensity, 90 days in water | [22] | |
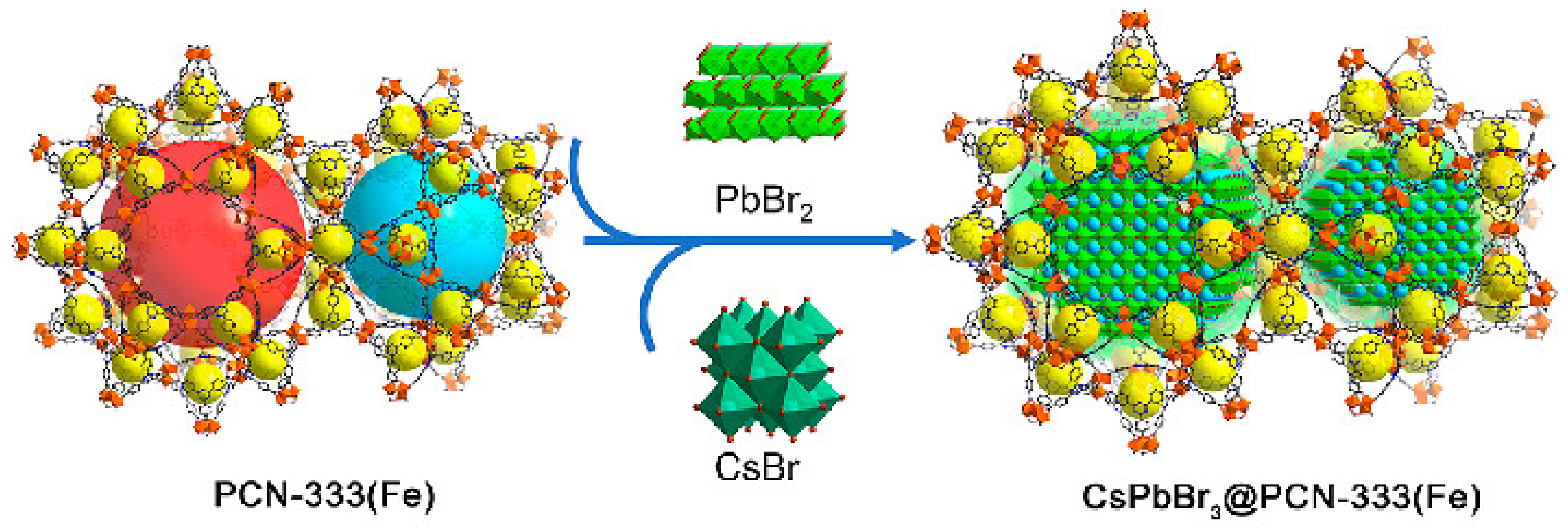
| Ferric organic skeleton MOFs | CsPbBr3@PN-333 (Fe) | The Li–O2 battery can be cycled stably for more than 200 h at 0.01 mA cm−2 under illumination | [24] | ||
| MAPbI3@PCN-221(Fex) | The catalytic system can operate continuously in water for more than 80 h | [25] | |||
| Metal ion doping | A site | Cs+ ions with FA+ | FA0.1Cs0.9PbI3 PQDs | PLQY remained at 95%, several months in solution | [29] |
| B site | Ni2+ | Ni:CsPbBr3 PNCs | The stability of Ni:CsPbBr3 PNCs are higher than CsPbBr3 PNCs | [37] | |
| Surface passivation | Strong chelating ligands | The polyamine chelating ligand: AHDA | AHDA-CsPbI3 PNCs | PLQY remained at 60%, after 15 purification cycles | [38] |
| SH ligands: 2-amino-ethylmercaptan (AET) | AET-CsPbI3-QDs | PLQY remained at 95%, 1 h in water | [39] | ||
| Zwitterionic organic ligands | Sulfobetaine or phosphocholine | Sulfobetaine-capped CsPbBr3 NCs | PLQY remained at 70–90% for 28–50 days under ambient conditions | [40] | |
| Organic semiconductor ligands | Rhodamine B derivative (COM) | COM-CsPbBr3 NCs | 84% of the initial PL intensity, 300 h under HT 85 °C and HH 85% | [43] | |
| Pesticide Type | Target Analyte | Molecular Structure | Fluorescent Probe | Linearity Range | LOD | Recovery Rate | Ref. |
|---|---|---|---|---|---|---|---|
| Organophosphorus | OMT | 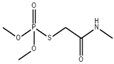 | MIPs@CsPbBr3 | 50–400 ng/mL | 18.8 ng/mL | [4] | |
| DDVP |  | CsPbBr3 QDs | 5–25 μg/L | 1.27 μg/L | 87.4–101% | [46] | |
| Phoxim | 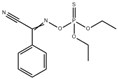 | CsPbBr3 QDs | 5–100 ng/mL | 1.45 ng/mL | [47] | ||
| Triazine herbicides | Prometryn |  | MIP/CsPbBr3- QDs | 0.010 μg/kg, 0.050 μg/L | 88.0–106.0% | [49] | |
| Simazine | 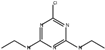 | MIP-CsPbBr3 | 0.1–500.0 μg/L | 0.06 μg/L | 86.5–103.9% | [50] | |
| Organochlorine | OCPs | MAPB-QDs | [51] | ||||
| Other pesticides | Clodinafop |  | CsPbI3 PQDs | 0.1–5.0 μM | 34.70 nM | 97–100% | [1] |
| Propanil | 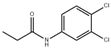 | MIP-QDs | 1.0 μg/L– 2 × 104 μg/L | 0.42 μg/kg, 0.38 μg/L | 87.2–112.2% | [52] |
| Target Analyte | Molecular Structure | Fluorescent Probe | Linearity Range | LOD | Ref. |
|---|---|---|---|---|---|
| U | U | CsPbBr3 PQD | 0–3300 nM (3.3 μM) | 0–3300 nM (3.3 μM) | [53] |
| O-nitrophenol (ONP) | 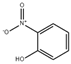 | CsPbBr3@SHFW | 0–280 μM | 7.69 × 10−3 μM | [54] |
| Ion Type | Target Analyte | Fluorescent Probe | Linearity Range | LOD | Ref. |
|---|---|---|---|---|---|
| Cation | Cu2+ | CsPbBr3 (PQD) | 0–100 nM | 0.1 nM | [55] |
| Cu2+ | CsPbBr3 (CPB) | 106 M–102 M | [56] | ||
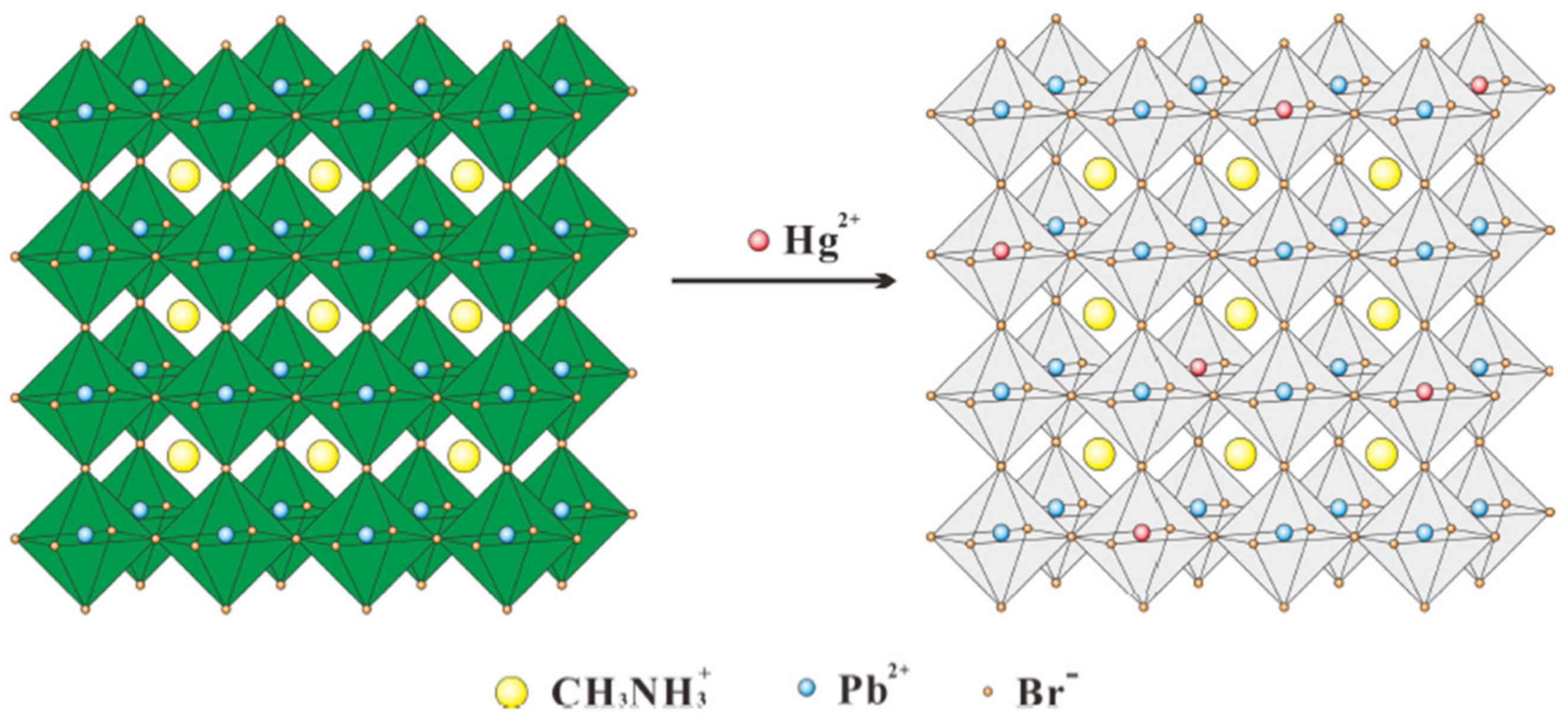
| Hg2+ | CH3NH3PbBr3 (QDs) | 0–100 nM | 0.124 nM (24.87 ppt) | [57] | |
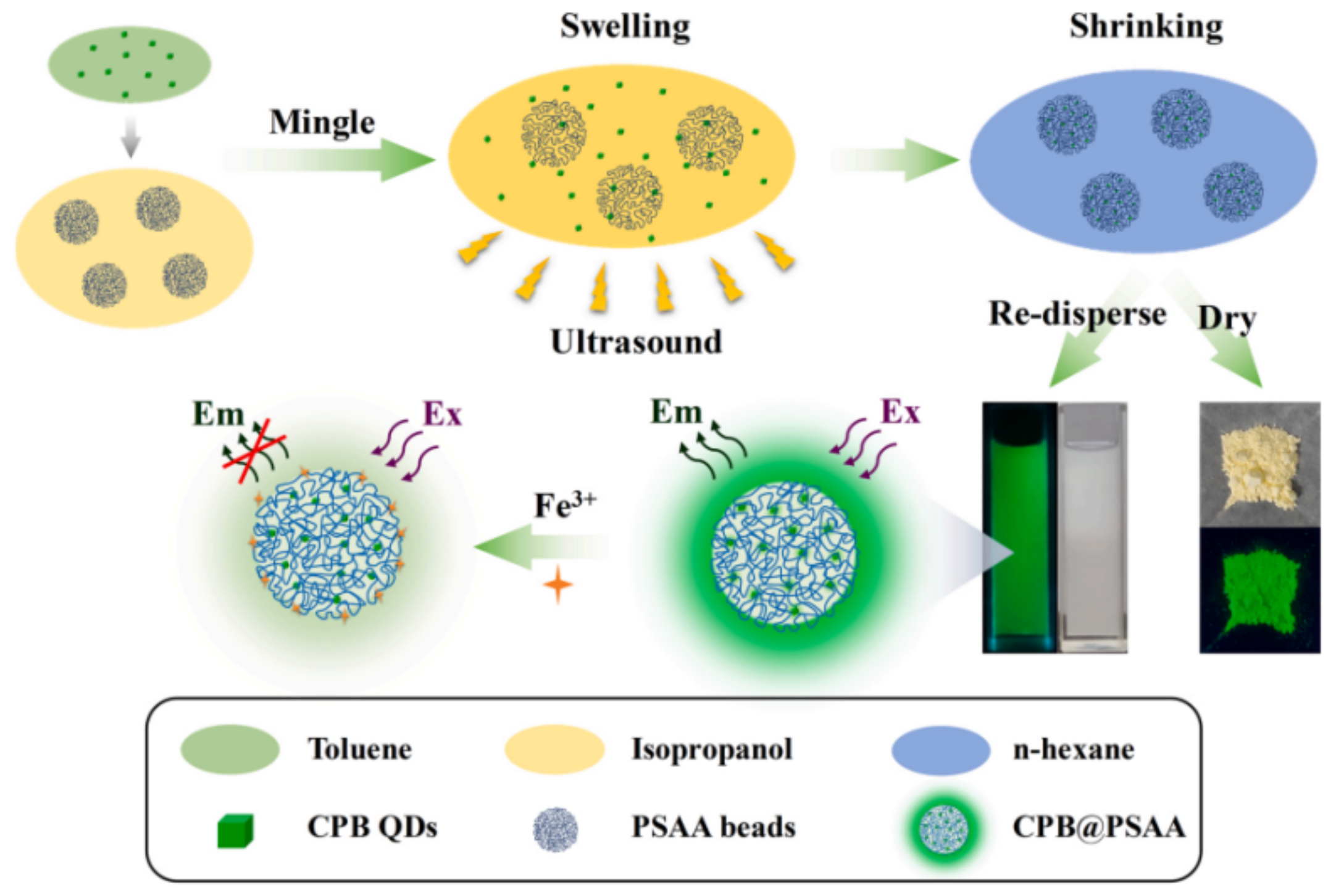
| Fe(III) | CPB@PSAA | 5–150 μM | 2.2 μM | [58] | |
| Zn2+, Ag+ | CQD-MAPbBr3@SiO2 | [59] | |||
| Pb2+ | CH3NH3PbBr3 (MAPbBr3) | [60] | |||
| Pb2+ | MAPbBr3 | 5 × 10−3 μg/mL | [61] | ||
| Anion | I−, Cl− | CsPbBr3 PQDs | 2.56 mM, 4.11 mM | [62] |
| Target Analyte | Molecular Structure of the Target Analyte | Fluorescent Probe | Linearity Range | LOD | Recovery Rate | Ref. |
|---|---|---|---|---|---|---|
| Roxithromycin | 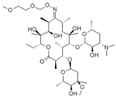 | MIP-CsPbBr3 | 1.7 × 105μg/mL (20.6 pM) | [63] | ||
| Tetracycline | 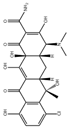 | MAPbBr3 @PbBr(OH)@SiO2-Cit-Eu | 0–25 μM | 11.15 nM | [64] | |
| Cs4PbBr6/CsPbBr3 | 0.4–10 μM | 76 nM | [65] | |||
| CsPbBr3@BN | 0–0.44 mg/L | 6.5 μg/L | [66] | |||
| APTES@IPQDs | [67] | |||||
| Cefazolin |  | CsPbBr3 QDs | 25–300 nM | 9.6 nM | 94–106% | [68] |
| Ciprofloxacin hydrochloride |  | CsPbBr(3−x)Clx NCs | [69] |
| Target Analyte | Molecular Structure | Fluorescent Probe | Linearity Range | LOD | Ref. |
|---|---|---|---|---|---|
| B1 (AFB1) | 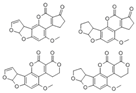 | CsPbBr3/a-TiO2 | 0.01~15 ng/mL | 2.8 pg/mL | [70] |
| MAPB QDs@SiO2 and MAPB | 8.5 fg/mL | [71] | |||
| SEs | Staphylococcal enterotoxin | CsPb2Br5 @MSN | [72] | ||
| Artificial wax on fruit |  | Cs2PdBr6 | [73] |
| Target Analyte | Fluorescent Probe | Linearity Range | LOD | Ref. |
|---|---|---|---|---|
| NH3 | CsPbBr3 QDs | 25–350 ppm | 8.85 ppm | [74] |
| H2S | CsPbBr3@PEG-PCL | [75] | ||
| H2S | CsPbBr3@SBE-β-CD | 0.5–6000.0 μM | 0.19 μM | [76] |
| H2S | CsPbBr3@CO | 0.15–105.0 μM | 53.0 nM | [77] |
| Target Analyte | Molecular Structure | Fluorescent Probe | Linearity Range | LOD | Recovery Rate | Ref. |
|---|---|---|---|---|---|---|
| Sudan red I | 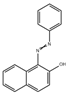 | CsPbX3 | 0.5–150 μg/L | 0.3 μg/L | 95.27–105.96% | [2] |
| Sudan red I–IV |  | CsPbBr3 | 3.33, 0.03, 0.03, 0.04 ng/mL | [78] | ||
| Rhodamine 6G |  | CsPbBr3/SiO2 QDs | 0–10 mg/mL | 0.01 mg/mL | [79] | |
| CPBQDs/PSFM | 0.01 ppm | [80] | ||||
| RhoB |  | CsPbBr3-PVDF | 0.01 ppm | [81] | ||
| Basic yellow dye | 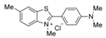 | CsPbBrI2 QDs | 1–500 μg/mL | 0.78 μg/mL | 95.27–98.84% | [82] |
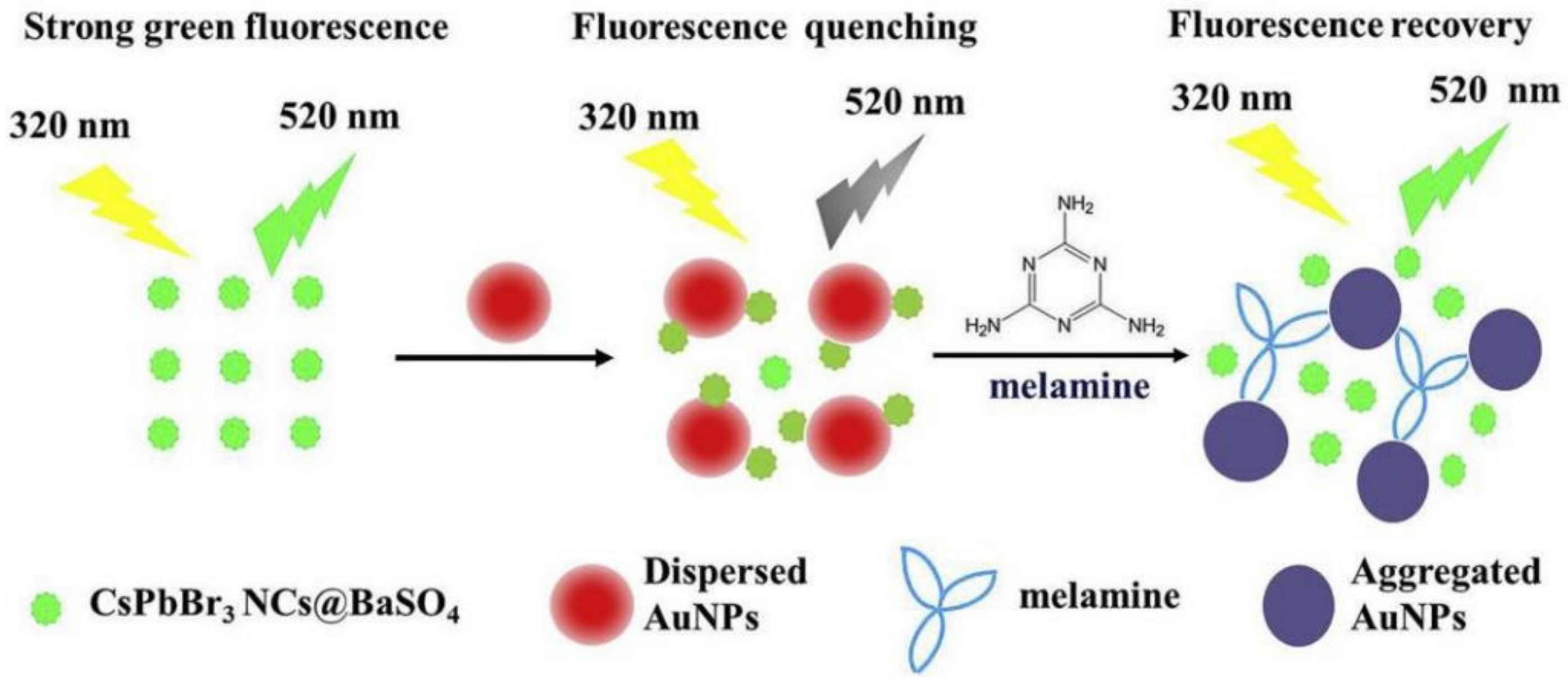
| Melamine |  | CsPbBr3NCs@BaSO4 | 0.42 nmol/L | [83] | ||
| Bisphenol A |  | Cs4PbBr6@CsPbBr3 PQD | [84] |
| Target Analyte | Molecular Structure | Fluorescent Probe | Linearity Range | LOD | Recovery Rate | Ref. |
|---|---|---|---|---|---|---|
| Peroxide value | CH3(CH2)7CH=CH(CH2)7CH2NH3I | CsPbBr3 NCs | [85] | |||
| AN, 3-MCPD, MC | Excessive acid number , H2O , H2O | CsPbBr1.5I1.5@MSNs | 0.71 mg KOH/g, 39.8 μg/mL 3-MCPD, 0.45% MC | [86] | ||
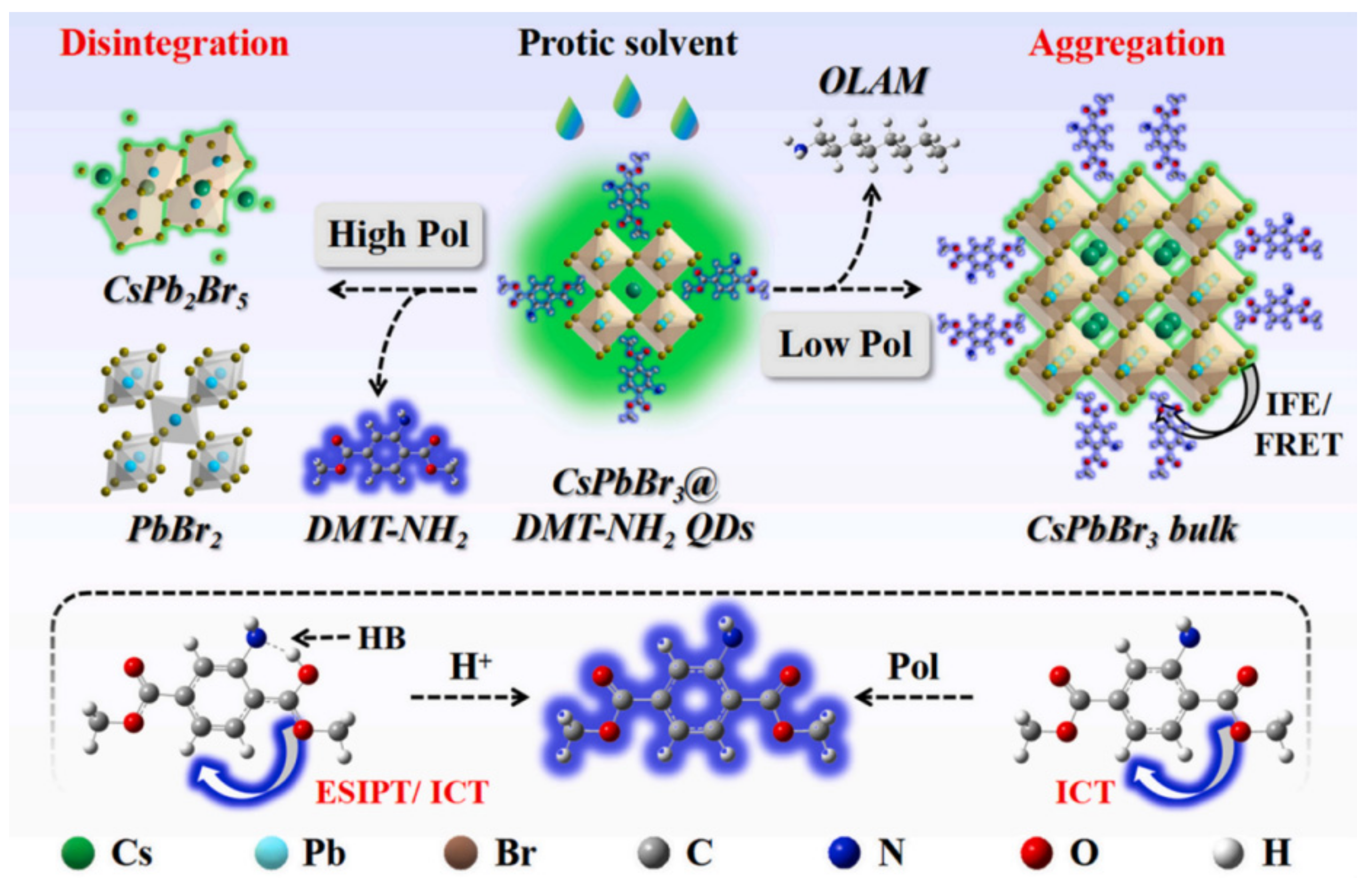
| H2O | H2O | CsPbBr3@DMT-NH2 QDs | 0.006% (v/v), 0.01% (v/v) | 93.0~108.0% | [87] | |
| TPM |  | CsPbBr3 QD | 17–31.5%, 25–31.5%, 21.5–33% | [88] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, W.; Zhang, J.; Kong, F.; Ye, T. Application of Perovskite Nanocrystals as Fluorescent Probes in the Detection of Agriculture- and Food-Related Hazardous Substances. Polymers 2023, 15, 2873. https://doi.org/10.3390/polym15132873
Zhao W, Zhang J, Kong F, Ye T. Application of Perovskite Nanocrystals as Fluorescent Probes in the Detection of Agriculture- and Food-Related Hazardous Substances. Polymers. 2023; 15(13):2873. https://doi.org/10.3390/polym15132873
Chicago/Turabian StyleZhao, Wei, Jianguo Zhang, Fanjun Kong, and Tengling Ye. 2023. "Application of Perovskite Nanocrystals as Fluorescent Probes in the Detection of Agriculture- and Food-Related Hazardous Substances" Polymers 15, no. 13: 2873. https://doi.org/10.3390/polym15132873
APA StyleZhao, W., Zhang, J., Kong, F., & Ye, T. (2023). Application of Perovskite Nanocrystals as Fluorescent Probes in the Detection of Agriculture- and Food-Related Hazardous Substances. Polymers, 15(13), 2873. https://doi.org/10.3390/polym15132873







