Abstract
Plant viruses are a global concern for sustainable crop production. Among the currently available antiviral approaches, nanotechnology has been overwhelmingly playing an effective role in circumventing plant viruses. Alfalfa mosaic virus (AMV) was isolated and identified from symptomatic pepper plants in Egypt using symptomatology, serological tests using the direct ELISA technique, differential hosts and electron microscopy. The virus was biologically purified from a single local lesion that developed on Chenopodium amaranticolor. The AMV infection was further confirmed using an AMV coat protein-specific primer RT-PCR. We further evaluated the antiviral potential of chitosan nanoparticles (CS-NPs) and chitosan silver nanocomposites (CS-Ag NC) in different concentrations against AMV infections in pepper plants. All tested concentrations of CS-NPs and CS-Ag NC induced the inhibition of AMV systemically infected pepper plants when applied 24 h after virus inoculation. The foliar application of 400 ppm CS-NPs or 200 ppm CS-Ag NC produced the highest AMV inhibitory effect (90 and 91%) when applied 24 h after virus inoculation. Treatment with CS-NPs and CS-Ag NC considerably increased the phenol, proline and capsaicin contents compared to the infected plants. Moreover, the agronomic metrics (plant height, fresh and dry pod weights and number of pods per plant) were also significantly improved. According to our results, the potential applications of CS-NPs and CS-Ag NC may provide an effective therapeutic measure for better AMV and other related plant virus management.
1. Introduction
Hot peppers (Capsicum annum), a member of the Solanaceae family, are considered an essential and seasoning vegetable due to their distinctive taste, color, flavor, spice, aroma and medicinal value. Furthermore, they are a good source of antioxidants, including phenols, flavonoids, anthocyanin, vitamins, B-carotene and capsaicin, which are involved in defense mechanisms and are important for human health [1]. Viral diseases are one of the main threats to pepper production, causing significant economic losses by reducing the quality and quantity of the marketable yield [2]. Peppers have been known to host diverse plant viruses of the Bromoviridae, Bunyaviridae, Geminiviridae, Luteoviridae, Potyviridae and Virgaviridae families. Among these viruses, alfalfa mosaic virus (AMV) (family Bromoviridae, genus Alfamovirus) is an economically significant and widespread virus in Egypt [3]. It has been reported in Australia, France, Greece, Iran, Italy, New Zealand, Saudi Arabia and North America [4]. Besides the main crop hosts of the Fabaceae and Solanaceae families, AMV has a wide host range, infecting ~430 herbaceous and woody plant species in 51 families with a wide range of symptoms [5]. Aphids play a vital role in the biological transmission of AMV non-persistently [6]. In addition, AMV can also be transmitted mechanically or through seeds [7]. Moreover, uncultivated plants and weeds are also crucial for the prevalence and persistence of AMV as secondary plant hosts [8].
The viral architecture of AMV is built on icosahedral particles 30–57 nm in length and a diameter of 18 nm. The RNA-based AMV genome is divided into three single-stranded positive-sense RNA components (RNA1, RNA2 and RNA3). The replicase subunits P1 and P2 are encoded on RNA1 and RNA2 molecules, respectively. The movement protein (MP) and coat protein (CP) are encoded on the RNA3 molecule. The CP is translated via the additional sub-genomic component RNA4 and is critical in the translation and binding of RNAs, viral shuttling in the nucleus and cytoplasm, virion assembly and systemic viral movements [9].
Plant viruses are among the most critical pathogens jeopardizing global agriculture and food security. Using ecofriendly antiviral agents can be an alternate approach to elicit a plant innate resistance development against viruses. Chitosan is a natural biopolymer with enormous biological benefits due to its unique characteristics, positive charge and the presence of reactive hydroxyl and amino groups. Due to its bioadhesive, biodegradable and biocompatible properties, chitosan is a safe and cost-effective biocontrol agent [10]. Although the antiviral activity of chitosan nanoparticles (or their derivatives) has been shown in some studies, its full potential has yet to be explored. For example, silver nanoparticles (AgNPs) used in controlling viral infections are still limited. The antiviral activity of AgNPs/chitosan composites has been tested against the influenza A virus. It was shown that the inhibitory effect against H1N1 improved as the concentration of AgNPs increased [11]. Chitosan/dextran nanoparticles (CDNPs) at a conc. of 100 mg L−1 reduced the disease severity of AMV in treated Nicotiana glutinosa plants [12]. Similarly, chitosan nanoparticles at a conc. of 300 and 400 mg/L inhibited bean yellow mosaic virus (BYMV) infection and substantially reduced viral accumulation [13].
Pepper (Capsicum annum L.) plants treated with chitosan silver nanoparticles (CS-Ag NPs) and chitosan nanoparticles (CS-NPs) significantly increased the vegetative plant growth and the total phenol, proline and capsaicin accumulation [14,15]. The use of chitosan nanoparticles as a biostimulant has been explored in many crop plants [16,17]. Nevertheless, studies on the antiviral activities of chitosan (particularly CS-AgNPs) are still limited. Therefore, this study was designed to synthesize and characterize CS-AgNPs and CS-Ag NC to explore their role in inducing plant defenses in pepper plants against AMV infection.
2. Materials and Methods
2.1. Virus Isolation and Identification
Ten pepper (Capsicum annum L.) plant samples with suspected AMV-like symptoms were collected from a pepper field in the Ismailia Governorate, Egypt. The observed symptoms included yellow mosaic on the leaves, mottling, curling, chlorosis, yellow blotching and chlorotic sectors. The isolated virus was maintained on ten pepper plants for the propagation of the virus and used as a source for subsequent studies. The leaf samples of healthy and symptomatic pepper plants were tested for the presence of the known viruses from peppers, viz., the potato virus Y (PVY), cucumber mosaic virus (CMV), AMV and tomato spotted wilt virus (TSWV), using the Double Antibody Sandwich-Enzyme-Linked Immunosorbent Assay (DAS-ELISA), as described earlier [18]. PVY, CMV and AMV were tested using the LOEWE Biochemica GmbH Kit (Biochemica, Mühlweg, Germany), while TSWV was tested using the Bioreba kit (Bioreba, Reinach, Switzerland). The positive samples for AMV were used as a source of virus inoculum, and the diagnostic host plants were mechanically inoculated: Phaseolus vulgaris, Vigna unguculata, Vicia faba, Datura stramonium, Nicotiana glutinosa, Chenopodium amaranticolor, C. quinoa, C. murlae, Catharanthus roseus and Ocimum basilicum. For biological purification, C. amaranticolor plants grown under natural lighting with day/night temperatures of approx. (23 ± 2 °C) were inoculated against the virus as a local lesion host for three consecutive passages [19], then transmitted mechanically to pepper plants.
2.2. RNA Isolation and Reverse Transcription Polymerase Chain Reaction RT-PCR Amplification of AMV Coat Protein Gene
According to the manufacturer’s manual, the total RNAs were extracted from newly emerged leaves of naturally infected, mechanically inoculated and healthy pepper plants using a Gene jetTM plant RNA purification mini kit (Thermo Fisher Scientific, Carlsbad, CA, USA). RT-PCR was conducted using the versoTM one-step RT-PCR kit (Thermo Fisher Scientific Co., Waltham, MA, USA), according to the manufacturer’s instructions. The oligonucleotide primers AMVcoat-F/AMVcoat-R [20] were used to amplify the conserved region in the CP gene of AMV by one-step RT-PCR. The RT-PCR reaction was optimized to be performed in a final volume of 25 µL containing 3 µL RNA (4 ng/µL), 12.5 µL of one-step PCR master mix (2×), 3 µL of 10 µM of each primer, 0.5 µL of Verso enzyme mix, 1.25 µL of RTEnhancer and 4.75 µL of nuclease-free water. Amplification was performed in an automated T Gradient Biometra (Biometra, Jena, Germany) thermal cycler. Samples were amplified using the following cycling parameters: hold at 50 °C for 30 min (RT step) and hold at 95 °C for 15 min (hot start to PCR); the tubes were heated at 94 °C for 2 min and then subjected to 35 cycles of amplification: 30 s at 94 °C for denaturation, 30 s at 58 °C for annealing and 30 s at 74 °C for extension, followed by a final hold at 72 °C for 10 min. RT-PCR products were analyzed by 1% agarose gel electrophoresis, stained with gel star (Lonza, Carlsbad, CA, USA) and visualized by UV illumination (Gel Doc 2000, Bio-Rad, Carlsbad, CA, USA). The DNA ladder (1 kb) was used for comparison.
2.3. Preparation of Chitosan Nanoparticles (CS-NPs)
Chitosan nanoparticles (CS-NPs) were prepared by the ionic gelation method according to Calvo et al. [21]. The technique utilized the electrostatic interaction between the amine group of chitosan and a negatively charged group of polyanion, such as trisodium polyphosphate (TPP) and CS aqueous solution (0.2% w/v) (molecular weight 50,000–190,000 Da, degree of deacetylation 75–85% and viscosity: 20–300 cP, Sigma-Aldrich, Missouri, USA), prepared by dissolving CS in acetic acid solution (1% v/v) (99–100%, Riedel-de Haën) at room temperature. Subsequently, the TPP solution (0.06% w/v) (Sigma-Aldrich, St. Louis, MO, USA) was added dropwise to the CS solution under vigorous stirring for 30 min. The CS-NPs suspension was freeze-dried before further use or analysis.
2.4. Preparation of Chitosan Silver Nanocomposites (CS-Ag NC)
Chitosan silver nanocomposites (CS-Ag NC) were prepared by the chitosan reduction of silver nitrate, according to Babu et al. [22]. The polymeric chain’s amino groups coordinated the silver ions in an acidic chitosan solution. Ion reduction to metallic silver nanoparticles was coupled with the chitosan hydroxyl group’s oxidation. Briefly, chitosan aqueous solution (1% w/v) (molecular weight 50,000–190,000 Da, degree of deacetylation 75–85% and viscosity: 20–300 cP, Sigma-Aldrich, St. Louis, MO, USA) was prepared by dissolving chitosan in an acetic acid solution (1% v/v) (99–100%, Riedel-de Haën, Chapel Hill, NC, USA) at room temperature. Subsequently, the silver nitrate solution (0.01 M) (Sigma-Aldrich, St. Louis, MO, USA) was added immediately into the suspension under continuous stirring for two hours. Sodium borohydride (20 mL, 0.04 M) (Sigma-Aldrich, St. Louis, MO, USA) was added to the previous suspension, and an immediate color change from pale yellow to brown was observed. The resulting CS-Ag NC suspension was centrifuged at 20,000× g for 30 min. The pellet was resuspended in deionized water. The CS-Ag NC suspension was freeze-dried before further use or analysis.
2.5. Characterization of Chitosan Nanoparticles and Chitosan Silver Nanocomposites
The exact morphology of the prepared CS-NPs and CS-Ag NC was examined using a high-resolution transmission electron microscope (HR-TEM) JEOL (JEM-1400 TEM, Tokyo, Japan) operating at an accelerating voltage of 200 kV (Tecnai G2, FEI, Amsterdam, The Netherlands). The diluted CS-Ag NC solution was ultra-sonicated for 5 min to reduce particle aggregation. Three droplets of the sonicated solution were applied with a micropipette on a copper grid coated with carbon, and they were then allowed to dry at room temperature. The HR-TEM images of the CS-NPs and CS-Ag NC deposited on the grid were captured for morphological evaluation. Using photon correlation, the nanoparticles’ size (Z-average mean) and zeta potential were examined using zetasizer 3000 HS to perform the spectroscopy and a laser Doppler anemometer in triplicate, respectively (Malvern Instruments, Zs Nano, Almelo, The Netherlands). The chemical structure of the prepared CS-NPs and CS-Ag NC was assessed using the X-ray diffraction (XRD) technique. The corresponding XRD pattern was recorded in the scanning mode (X ‘pert PRO, PAN analytical, Almelo, The Netherlands) operated by a Cu K radiation tube (=1.54 A˚) at 40 kV and 30 mA. The default ICCD library in PDF4 was used to analyze the resulting diffraction pattern. Qualitative and quantitative measurements of the applied silver concentrations in CS-Ag NC and a sample of pepper fruits after drying were determined by the inductivity coupled plasma (ICP) technique (PerkinElmer ICP-OES: Optima 2000, Rodgau, Germany). The synthesis and characterization of the CS-NPs and CS-Ag NC were performed in the Nanotechnology & Advanced Materials Central Laboratory (NAMCL), Agricultural Research Center, Egypt.
2.6. Effect of Foliar Application of Chitosan Nanoparticles and Chitosan Silver Nanocomposites on Virus Infectivity and Plant Vegetative Growth
The effect of CS-NPs at conc. of 400, 200, 150 and 100 ppm and CS-Ag NC at conc. of 200, 150, 100 and 50 ppm in controlling AMV was evaluated on pepper plant seedlings to prevent AMV infection under greenhouse conditions. Pepper seeds were grown in plastic pots packed with sand and clay (1:2 v/v) and under natural lighting and in day/night temperatures of approx. (23 ± 2 °C). After three weeks of growth, the seedlings were transferred to 40 × 40 cm pots (4 seedlings per pot). The seedlings were divided into four groups. In group I, the seedlings were first treated with CS-NP or CS-Ag NC and then mechanically inoculated with AMV inoculum (1 mL/plant) after 24 h. In group II, the seedlings were first inoculated with AMV inoculum and then treated with CS-NPs or CS-Ag NC after 24 h. In group III, the seedlings were treated either with CS-NPs or CS-Ag NC immediately after virus inoculation. In group IV (the control group), the seedlings were sub-grouped as C1—seedlings inoculated only with the AMV inoculum (positive control), C2—untreated seedlings (healthy control), C3—seedlings treated with CS-AgNC and C4—seedlings treated with CS-NPs. Four plant seedlings were used for each treatment in each group and sub-group. All the plants received the recommended agronomic practices and were observed routinely for symptom development. The virus inhibition percentage (%) of the tested plants was determined through DAS-ELISA three weeks after inoculation, as described by Devi et al. [23] using the following equation: Inhibition % = (A − B/A) × 100, where A is the number of plants in the positive control experiment (untreated), and B is the number of treated inoculated plants. The newly emerged plant leaves were used for RNA extraction and RT-PCR analysis, as described above.
Plant height (cm), fresh and dry weights of pods (g) and number of pods per plant were recorded 7 months after planting.
2.7. Effect of Various Concentrations of Chitosan Nanoparticles and Chitosan Silver Nanocomposites on Active Ingredients in Pepper Pods
The determination of capsaicin was achieved according to Peng and Wang [24] as mg/kg DW. The determination of the proline content was achieved according to Bates et al. (1973) as mg gFW−1.
The determination of the total phenols in the pods was conducted according to the method described by Diaz and Martin [25] as (mg/100 g of fresh matter).
2.8. Data Analysis
The mean value was calculated by performing 2-way or 1-way analyses of variance (ANOVA) using Statgraphics Centurion XVI (Statpoint Technologies, Inc., Warrenton, VA, USA). When appropriate, the means were separated by Fisher’s Protected LSD test (p < 0.05) [26].
3. Results
3.1. Collection of Field-Infected Pepper Plants and Preparation of Virus Inoculum
The symptomatic pepper plants showed yellow mosaic on leaves, mottling, curling, chlorosis, yellow blotching and chlorotic sectors symptoms compared to the non-symptomatic plants (Figure 1A,B). The young leaf samples were detached from each plant and tested for the presence of common pepper-infecting plant viruses using DAS-ELISA and RT-PCR (Supplementary Figure S1). The results showed that all ten plants tested negative for the presence of PVY, CMV and TSWV, while these were positive for only AMV infection.
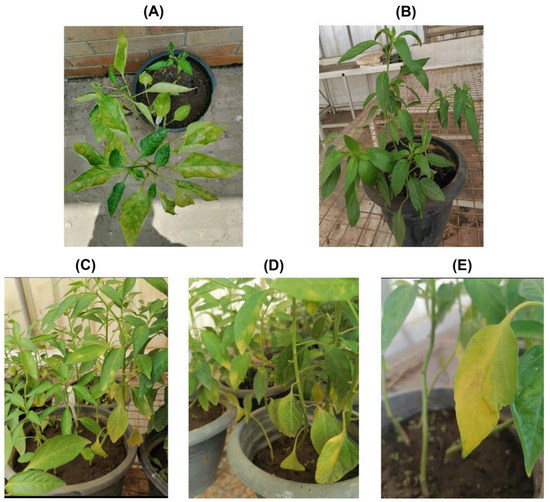
Figure 1.
Field-infected symptomatic (A) and symptomless or healthy pepper plants (B). The sap from symptomatic AMV-infected pepper plants was used to mechanically inoculate greenhouse-grown pepper plants for further confirmation. The mechanically inoculated pepper plants were showing typical AMV symptoms of mosaic (C) and leaf curling, yellow blotching (D) and chlorosis symptoms (E).
For further propagation, the sap from each pepper plant was inoculated onto ten pepper plants under greenhouse conditions (Figure 1C–E). The plants started showing typical AMV symptoms 21 days post-inoculation (DPI). All the plants were tested for the presence/absence of AMV using DAS-ELISA and RT-PCR. It was found that all the plants tested positive for the presence of AMV.
The AMV inoculum from pepper plants was used to inoculate ten differential host plant species belonging to six families (Supplementary Figure S2). The symptoms induced in the tested hosts ranged between mosaic, chlorotic and necrotic local lesions; necrotic spots and yellowing (Supplementary Figure S2 and Table 1). The results grouped all differential host plants into two groups. The plants C. amaranticolor, C. quinoa, C. mural, Phaseolus vulgaris and Vigna unguiculata were grouped together to show only the local lesions that developed 5–7 DPI, whereas Catharanthus roseus, Vicia faba, Ocimum basilicum, Datura stramonium and Nicotiana tabacum were grouped separately for showing systemic infections 21 DPI. All plants were tested with DAS-ELISA and RT-PCR for AMV infection.

Table 1.
Differential hosts of the alfalfa mosaic virus (AMV) tested by mechanical inoculation.
3.2. Characterization of Chitosan Nanoparticles and Chitosan Silver Nanocomposites
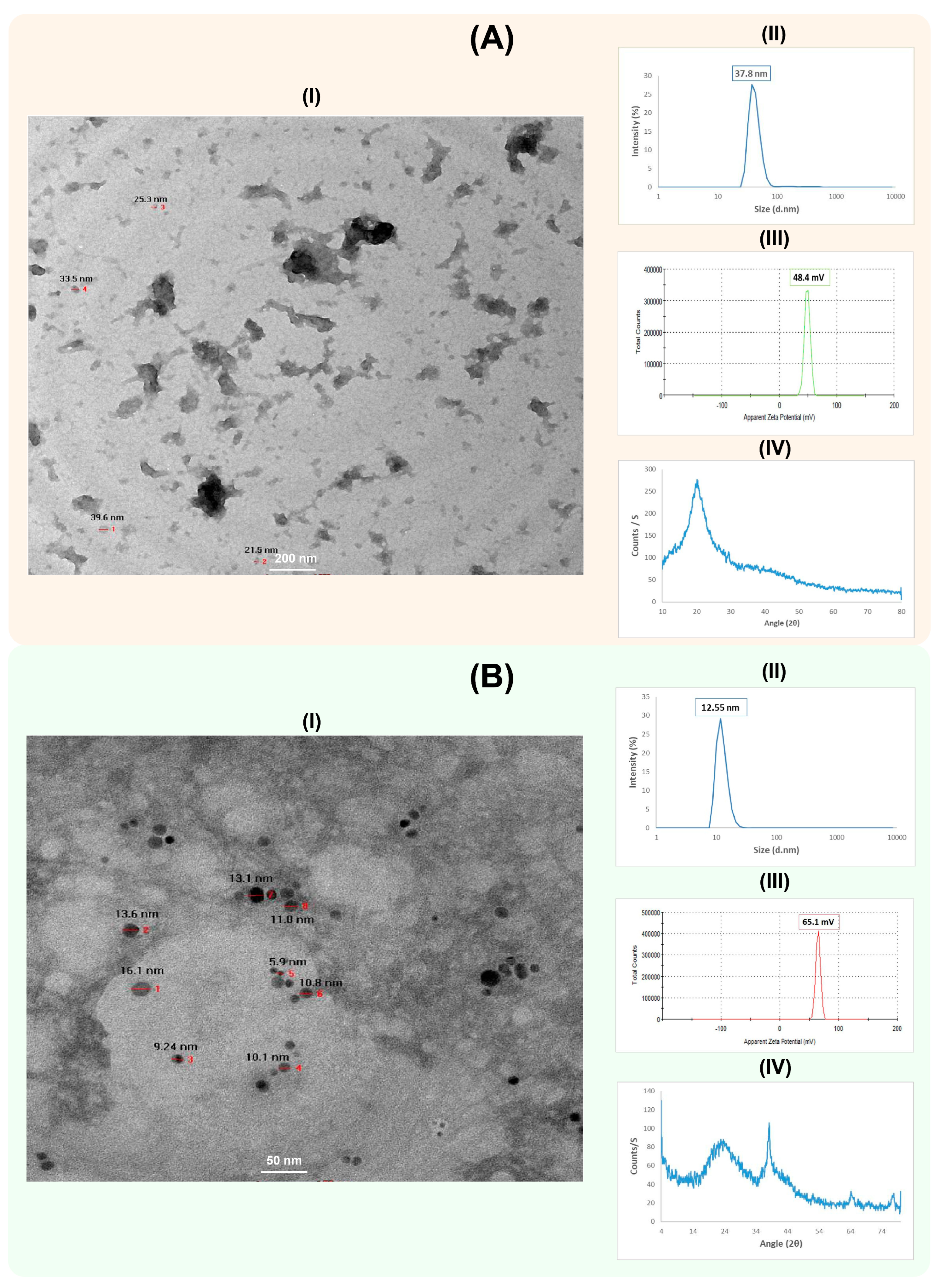
The synthesized CS-NPs and CS-Ag NC were characterized for physiochemical properties using HR-TEM (Figure 2A,B). The CS-NPs had a nearly spherical shape, smooth surface and size range of about 30 nm. The hydrodynamic diameter of the CS-NPs was 37.8 nm, with a zeta potential +48.4 mV (Figure 2A). The crosslinking between chitosan and silver in CS-Ag NCs was spherical in shape, with a smooth surface and size range of about 11.33 nm. The hydrodynamic diameter of CS-Ag NC was 12.55 nm, with a zeta potential +65.1 mV (Figure 2B). The X-Ray diffraction patterns of CS-NPs and CS-Ag NC were also determined (Figure 2A,B). The XRD pattern of the CS-NPs showed a broad typical hump peak start from 2θ = 10° to 2θ = 30°. The main peak of the CS-NPs pattern was observed at 2θ = 20°. The peaks at 2θ = 38.13°, 64.46° and 77.42° were assigned to (111), (220) and (311) of CS-Ag NC.
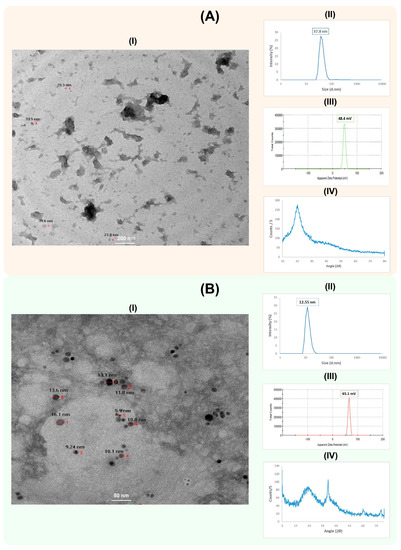
Figure 2.
Characterization of chitosan nanoparticles (CS-NPs) (A) and chitosan silver nanocomposites (CS-Ag NC) (B). The HR-TEM image, particle size distribution, zeta potential and XRD pattern analysis of CS-NPs and CS-Ag NC are shown as I–IV in each panel, respectively. The HR-TEM images of CS-NPs and CS-Ag NC were captured at 200 nm and 50 nm magnifications, respectively. The numbers in the images are showing the respective sizes of the CS-NPs and CS-Ag NCs.
3.3. Effect of Chitosan Nanoparticles and Chitosan Silver Nanocomposites on Virus Infectivity
Based on the DAS-ELISA analysis, the treated pepper plant seedlings showed significant variations in inhibiting viral proliferation compared to the control treatments (Table 2). However, spraying 400 ppm CS-NPs and 200 ppm CS-Ag NC on all groups showed the most significant results. The inhibitory effect of 200 ppm CS-Ag NC and 400 ppm CS-NPs in group II showed the highest inhibitory effect against AMV infection in pepper plants (91% and 90%). Similarly, in group III, 200 ppm CS-Ag NC and 400 ppm CS-NPs produced a higher inhibitory effect (78% and 76%, respectively) than all the other treatments. In addition, the seedlings treated with 200 ppm CS-Ag NC and 400 ppm CS-NPs in group I also showed significantly high inhibitory effects (67% and 60%, respectively) compared to the control treatments. However, amongst all the treatments, the 100 ppm CS-NPs pre-inoculation treatment showed the lowest inhibitory effect (47%; Table 2).

Table 2.
Effect of chitosan silver nanocomposites and chitosan nanoparticles on virus infectivity.
3.4. Effect of Various Concentrations of Chitosan Silver Nanocomposites and Chitosan Nanoparticles on Active Ingredients in Pepper Pods
The results (Table 3) showed an increase in the total phenols in all treatments compared to the healthy control. The highest significant phenol contents were produced in group II pepper plant pods treated with 200 ppm CS-Ag NC (1.83) and 400 ppm CS-NPs (1.80), respectively, whereas the pepper plants in group I treated with 200 ppm CS-Ag NC and 400 ppm CS-NPs produced low phenol contents (1.62 and 1.58), respectively. The plants infected with AMV gave a higher value of the phenol content (1.22) than the healthy control (1.12).

Table 3.
Effect of various concentrations of chitosan silver nanocomposites and chitosan nanoparticles on active ingredients in pepper pods.
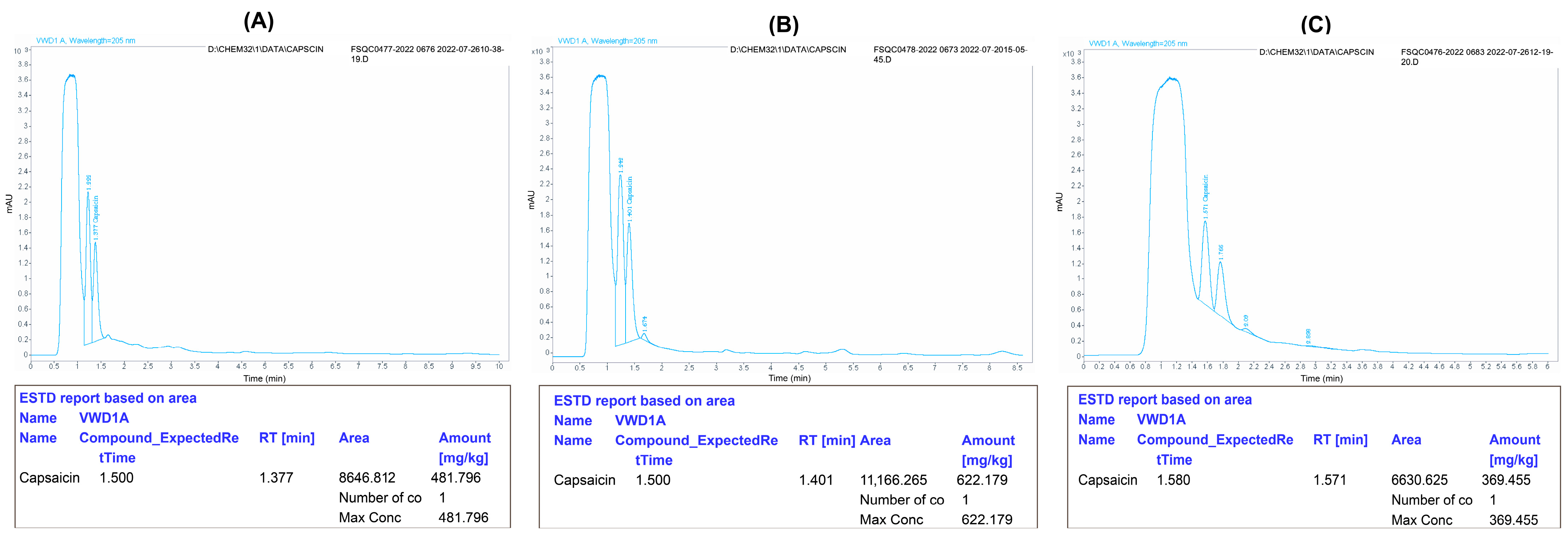
The capsaicin content was recorded as the highest in the healthy control treatments (622.17) compared to the AMV-positive control (369.45), whereas the capsaicin content was improved in the plants treated with 400 ppm CS-NPs and 200 ppm CS-Ag NC in all the treatment groups. It was found that, in group II, the treatment of 200 ppm CS-Ag NC and 400 ppm CS-NPs increased the capsaicin content to 481.79 and 478.83, respectively (Figure 3). Similarly, the treatment of plants with 400 ppm CS-NPs and 200 ppm CS-Ag NC in group III also improved the capsaicin content to 200 ppm CS-Ag NC and 472.33 and 470.47, respectively. Similarly, in group I, the capsaicin content was also enhanced in plants treated with 200 ppm CS-Ag NC (401.20) and 400 ppm CS-NPs (390.73), respectively.

Figure 3.
Effect of chitosan 200 ppm silver nanocomposites (CS-AgNC) in post-inoculation treatment on the capsaicin content in pepper pods. The capsaicin content in pepper pods of the treated plants (A) can be compared to the healthy control (B) and infected control (C). The original images were edited using Adobe Illustrator 2020 software for more clarity and have been provided as Supplementary Materials.
The data also showed that AMV infection increased the proline content (0.95) in pods compared to the healthy control (0.75). The proline content was significantly highest in plants treated with 200 ppm CS-Ag NC (1.23) and 400 ppm CS-NPs (1.20) in group II compared to the other concentrations. The proline contents were recorded as 1.17 and 1.16 in plants treated with 200 ppm CS-Ag NC and 400 ppm CS-NPs in group III, while, in group I, the treatments with 200 ppm CS-Ag NC and 400 ppm CS-NPs produced the lowest values for the proline content (1.14 and 1.13), respectively.
3.5. Effect of Chitosan Silver Nanocomposites and Chitosan Nanoparticles on Growth and Yield of Pepper Plants Inoculated with AMV
The results showed that AMV infection significantly affected the vegetative metrics (Table 4). A significant reduction was observed in plant height (78.1%), fresh (54.0%) and dry weights of pods (35.4%) and number of pods per plant (57.28%) for AMV-infected plants compared to the untreated healthy controls. Amongst all the treatments, the treatments with 400 ppm CS-NPs and 200 ppm CS-Ag NC in group II significantly improved all the vegetative parameters compared to the AMV-infected plants. It was found that 200 ppm CS-Ag NC and 400 ppm CS-NPs showed significantly increased plant heights (60.6 cm and 58.9 cm), fresh weights (21.2 g and 20.6 g) and dry weights of pods (10.3 g and 10.1 g ) and number of pods per plant (24.7 and 23.5), respectively.

Table 4.
Effect of chitosan silver nanocomposites and chitosan nanoparticles on the growth and yield of pepper plants inoculated with AMV.
4. Discussion
Plant viral infections have a significant negative economic impact on sustainable agriculture. Among various cutting-edge approaches, using nanoparticles has been proven as a novel approach to withstand viral infections in different crop plants [27,28,29]. AMV is an emerging threat to crop production in Egypt [30,31]. During a survey in the Ismailia Governorate of Egypt, symptomatic pepper plants showed typical AMV symptoms, as previously described [31]. RT-PCR produced an amplicon size of ~700 bp, equivalent to the AMV CP gene. AMV CP is the most critical region in the viral genome and is an essential criterion for describing the identification and taxonomy of the virus [32]. After confirmation, the field-collected plants were maintained in a greenhouse and were used as a source for AMV inoculum to inoculate ten differential plant hosts representing six different plant families. Based on the type of symptoms and infection, these plants were grouped into two major categories, i.e., those developing only local lesions and others showing systemic infections as well. The results showed that AMV can potentially threaten C. roseus, V. faba, O. basilicum, D. stramonium and N. tabacum hosts, because these plants showed a systemic development of the symptoms. The virus was easily transmitted mechanically to other plant hosts, as has been previously reported [20,31,33]. AMV is known to cause infections in broad host plants of Solanaceae and Leguminosae families in Egypt, India, Turkey and Saudi Arabia [34,35]. As previously described [12], C. amaranticolor leaves showed single local lesion development in 5–7 DPI and were further used for biological purification through three consecutive passages [19], then transmitted mechanically to pepper plants for further antiviral studies.
Chitosan is a natural polymer used to synthesize nanoparticles and is generally considered safe by the US Food and Drug Administration (U.S. FDA). It has been a new therapeutic method in controlling human [36] and plant infection viruses [12,37,38]. Our study investigated the antiviral ability of CS-NPs and their silver nanocomposites (CS-Ag NCs) against AMV infection in peppers. The TEM analysis revealed that the synthesized CS-NPs were spherical, with a smooth surface and ~30 nm in size, while the hydrodynamic diameter of the CS-NPs was 37.8 nm. In the CS-Ag NCs, the crosslinking between chitosan and silver was spherical in shape, with a smooth surface and size range of 11.3 nm and a hydrodynamic diameter of 12.55 nm. The X-ray diffraction of the CS-Ag NCs indicated that the crystalline structure of synthesized CS-Ag NC presented a cubic-phase structure of silver (JCPDS 04-004-8730). No silver residues were found in the pepper fruit samples treated with CS-NPs and CS-Ag NCs.
The foliar application of CS-NPs and CS-Ag NCs significantly improved plant immunity to inhibit viral proliferation and disease severity. Among all the treatments, 400 ppm CS-NPs and 200 ppm CS-Ag NC applied after 24 h of AMV inoculation showed the most significant results for virus inhibition. Chitosan nanoparticles are known to enhance plant growth by stimulating nutrient uptake, photosynthesis, cell division and the production of plant hormones [39]. These physiological processes are involved in plants’ defense against plant pathogens [37]. Therefore, it is likely that CS-NPs and CS-Ag NCs helped pepper plants to withstand AMV infection in this study. According to the previous studies, chitosan nanoparticles have the potential to attach to virus particles, inhibit viral replication inside infected cells and boost plant immunity and antioxidant defense systems [40,41]. Chitosan increases plant resistance by increasing the activity of ribonucleases and proteases [40]. In turn, it makes it difficult for viruses to efficiently invade plant cells. These findings suggest that chitosan or chitosan-based nanoparticles could be a promising tool to control plant viruses. Nevertheless, the antiviral activity of CS-Ag NCs has been confirmed against human viruses, such as the H1N1 influenza A virus [11]; however, based on our knowledge, this is the first comprehensive study on the antiviral potential of CS-Ag NCs against plant viruses. Our results are per El Gamal et al. [13], who showed that using 300 and 400 mg/L of chitosan nanoparticles after 48 h of bean yellow mosaic virus infection entirely inhibited the viral infection in beans. Furthermore, Abdelkhalek et al. [12] also found that using chitosan/dextran nanoparticles significantly inactivated and suppressed the AMV accumulation in N. glutinosa plants by promoting plant growth. Contrary to our results, Abdelkhalek et al. [12] found that the application of nanoparticles 24 h after AMV inoculation (curative treatment) produced the least significant AMV inhibition compared to the protective treatment (24 h pre-inoculation of AMV). Such differences can be related to the formulation of nanoparticles and/or their derivatives.
Hot peppers’ active component, capsaicin, is responsible for their hotness and spiciness. The AMV infection significantly reduced the capsaicin content compared to healthy pepper plants. However, using 400 ppm CS-NPs and 200 ppm CS-Ag NC resulted in a significant increase in the capsaicin content when applied after 24 h of AMV inoculation. These results were per those obtained by El-Shazly et al. [42]. They found that tomato plants infected with (TSWV) had reduced levels of lycopene compared to healthy plants. This may be due to the ability of the virus to affect fruit metabolism [43]. The improved capsaicin content was the effect of CS-NPs and CS-Ag NC to circumvent the deleterious effects of AMV and increase the secondary metabolites in the infected pepper plants. Proline has been shown to play a critical protective role in plant cells under abiotic or biotic stress. The proline metabolic pathway was suggested to play a regulatory role in oxidation–reduction balance and cell survival, which may help to explain these effects [44]. The production of phenols was reported to be enhanced in both virus-infected plants and plants treated with nanoparticles, which may function as an antioxidant to scavenge ROS (reactive oxygen species) and increase the quantities of the antioxidants in plant tissues [45,46]. Biotic and abiotic stresses may cause an increase in phenolic metabolism and antioxidant capacity, which may cause an increase in phenolic compounds. Treatments with CS-AgNC and CS-NPs still produce higher phenols and proline contents, because these nanoparticles improve plant resistance to abiotic stress through mechanisms such as (1) triggering plant cell signals due to the increased production of ROS and/or reactive nitrogen species (RNS) and (2) stimulating the plant protection mechanism, which includes enzymatic and non-enzymatic antioxidants [47]. Phenolic compounds can scavenge ROS and prevent cellular oxidation. The higher proline amount might be attributed to a decrease in proline oxidation to glutamate, an increase in protein turnover or a reduction in protein usage. Furthermore, it may activate the proline biosynthesis genes that produce proline from glutamate [48].
Applying chitosan-based nanoparticles has been shown to affect vegetative growth and yield positively and improve plant mineral contents [49,50,51]. Similarly, the foliar application of silver nanoparticles improved the vegetative growth in plants due to increased photosynthetic pigments, indole acetic acid and stimulated protein and carbohydrate biosynthesis pathways [52,53]. Per the previous studies, it was found that applying 400 ppm CS-NPs and 200 ppm CS-Ag NC 24 h post-inoculation improved the plant height, fresh and dry weights of pods and number of pods per plant significantly. The vegetative metrics of the inoculated pepper plants were negatively affected by AMV infection, as shown in various studies [31,54]. However, applying 400 ppm CS-NPs and 200 ppm CS-Ag NC significantly improved the plant height, fresh and dry weights of pods and number of pods per plant compared to all other treatments during this study.
A probable route of action of silver nanoparticles is the cell periphery, where they commence their antiviral activity after their first contact with the glycoprotein of the virus surface [55]. Due to their smaller size, they enter the host plant cells, disrupting the cellular factors and/or viral proteins that aid in viral replication. They disrupt the viral polymerase activity (in RNA viruses) and prevent the development of new virions [56,57].
It is hypothesized that chitosan nanoparticles may have a robust bioreactivity to bind viral RNA, which has negatively charged phosphate groups in its main chain [58,59]. Furthermore, the positively charged nanoparticles could also target the virus coat protein, because all viral proteins contain a negatively charged cluster of glycol proteins. It further supports our speculation that the role of CS-NPs in controlling plant viruses may be substantially influenced by their nanophysiochemical characteristics, particle chemical nature and bioreactivity, where nano-sized chitosan is essential for their antiviral capabilities.
5. Conclusions
The current study empirically evaluated the antiviral potential of synthesized CS-NPs and CS-Ag NC in AMV-infected pepper plants. The electron microscopy analysis revealed that the CS-NPs and CS-Ag NC particles were uniform in size and spherical in shape. It was found that, among all the treatments, the foliar application of 400 ppm CS-NPs and 200 ppm CS-Ag NC was significantly effective in controlling AMV infection. Thus, the application of 400 ppm CS-NPs and 200 ppm CS-Ag NC can provide a long-term and viable control not only for AMV but other similar viral infections in crop plants. However, other parameters associated with these nanoparticles and/or their derivatives, viz., size, effective concentration, biocompatibility and environmental safety (if any), must be investigated explicitly before considering their many applications in crop plants.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/polym15132961/s1, Figure S1: RT-PCR amplified product analysis using agarose gel electrophoresis; Figure S2: AMV infection symptoms on some differential host plants after mechanical inoculation.
Author Contributions
Conceptualization, R.M.S., A.M.S. and S.M.E.-G.; methodology, R.M.S. and K.Y.F.; software, A.M.S. and R.M.S.; writing—original draft preparation, R.M.S. and A.M.I. and writing—review and editing, M.N.S., R.M.S., K.Y.F. and S.M.E.-G. All authors have read and agreed to the published version of the manuscript.
Funding
Deanship of Scientific Research, Vice Presidency for Graduate Studies and Scientific Research, King Faisal University, Saudi Arabia, financially supported this research (GRANT2625).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hamed, M.; Kalita, D.; Bartolo, M.E.; Jayanty, S.S. Capsaicinoids, polyphenols and antioxidant activities of Capsicum annuum: Comparative study of the effect of ripening stage and cooking methods. Antioxidants 2019, 8, 364. [Google Scholar] [CrossRef] [PubMed]
- Waweru, B.; Kilalo, D.; Miano, D.; Kimenju, J.; Rukundo, P. Diversity and economic importance of viral diseases of pepper (Capsicum spp.) in Eastern Africa. J. Appl. Hortic. 2019, 21, 70–76. [Google Scholar] [CrossRef]
- Abdelkhalek, A.; Al-Askar, A.A.; Behiry, S.I. Bacillus licheniformis strain POT1 mediated polyphenol biosynthetic pathways genes activation and systemic resistance in potato plants against Alfalfa mosaic virus. Sci. Rep. 2020, 10, 16120. [Google Scholar] [CrossRef]
- Gao, Y.; Fan, G.; Cheng, S.; Zhang, W.; Bai, Y. Evolutionary history and global spatiotemporal pattern of alfalfa mosaic virus. Front. Microbiol. 2022, 13, 1051834. [Google Scholar] [CrossRef]
- Mangeli, F.; Massumi, H.; Alipour, F.; Maddahian, M.; Heydarnejad, J.; Hosseinipour, A.; Amid-Motlagh, M.H.; Azizizadeh, M.; Varsani, A. Molecular and partial biological characterization of the coat protein sequences of Iranian alfalfa mosaic virus isolates. J. Plant Pathol. 2019, 101, 735–742. [Google Scholar] [CrossRef]
- van Leur, J.; Duric, Z.; George, J.; Boschma, S. Alfalfa mosaic virus infects the tropical legume Desmanthus virgatus in Australia and the potential role of the cowpea aphid (Aphis craccivora) as the virus vector. Aust. Plant. Dis. Notes 2019, 14, 3. [Google Scholar] [CrossRef]
- Fidan, H.; Adak, N.; Konuksal, A.; Akerzurumlu, E.; Yılmaz, M. Occurrence of Alfalfa Mosaic Virus (AMV) diseases on potato crops in northern Cyprus. In Proceedings of the V Balkan Symposium on Vegetables and Potatoes 960, Tirana, Albania, 9–12 October 2011; pp. 341–346. [Google Scholar]
- Abdalla, O.; Al-Shahwan, I.; Al-Saleh, M.; Amer, M. Molecular characterization of Alfalfa mosaic virus (AMV) isolates in alfalfa and other plant species in different regions in Saudi Arabia. Eur. J. Plant Pathol. 2020, 156, 603–613. [Google Scholar] [CrossRef]
- Herranz, M.C.; Pallas, V.; Aparicio, F. Multifunctional roles for the N-terminal basic motif of Alfalfa mosaic virus coat protein: Nucleolar/cytoplasmic shuttling, modulation of RNA-binding activity, and virion formation. Mol. Plant Microbe Interact. 2012, 25, 1093–1103. [Google Scholar] [CrossRef]
- Hassan, O.; Chang, T. Chitosan for eco-friendly control of plant disease. Asian J. Plant Pathol 2017, 11, 53–70. [Google Scholar] [CrossRef]
- Mori, Y.; Ono, T.; Miyahira, Y.; Nguyen, V.Q.; Matsui, T.; Ishihara, M. Antiviral activity of silver nanoparticle/chitosan composites against H1N1 influenza A virus. Nanoscale Res. Lett. 2013, 8, 93. [Google Scholar] [CrossRef]
- Abdelkhalek, A.; Qari, S.H.; Abu-Saied, M.A.A.-R.; Khalil, A.M.; Younes, H.A.; Nehela, Y.; Behiry, S.I. Chitosan Nanoparticles Inactivate Alfalfa Mosaic Virus Replication and Boost Innate Immunity in Nicotiana glutinosa Plants. Plants 2021, 10, 2701. [Google Scholar] [CrossRef]
- El Gamal, A.Y.; Tohamy, M.R.; Abou-Zaid, M.I.; Atia, M.M.; El Sayed, T.; Farroh, K.Y. Silver nanoparticles as a viricidal agent to inhibit plant-infecting viruses and disrupt their acquisition and transmission by their aphid vector. Arch. Virol. 2022, 165, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Sangwan, S.; Sharma, P.; Wati, L.; Mehta, S. Chapter 4—Effect of chitosan nanoparticles on growth and physiology of crop plants. In Engineered Nanomaterials for Sustainable Agricultural Production, Soil Improvement and Stress Management; Husen, A., Ed.; Academic Press: Cambridge, MA, USA, 2023; pp. 99–123. [Google Scholar]
- Lala, S. Nanoparticles as elicitors and harvesters of economically important secondary metabolites in higher plants: A review. IET Nanobiotechnol. 2021, 15, 28–57. [Google Scholar] [CrossRef] [PubMed]
- Saharan, V.; Kumaraswamy, R.; Choudhary, R.C.; Kumari, S.; Pal, A.; Raliya, R.; Biswas, P. Cu-chitosan nanoparticle mediated sustainable approach to enhance seedling growth in maize by mobilizing reserved food. J. Agric. Food Chem. 2016, 64, 6148–6155. [Google Scholar] [CrossRef] [PubMed]
- Stasińska-Jakubas, M.; Hawrylak-Nowak, B. Protective, Biostimulating, and Eliciting Effects of Chitosan and Its Derivatives on Crop Plants. Molecules 2022, 27, 2801. [Google Scholar] [CrossRef] [PubMed]
- Clark, M.F.; Adams, A. Characteristics of the microplate method of enzyme-linked immunosorbent assay for the detection of plant viruses. J. Gen. Virol. 1977, 34, 475–483. [Google Scholar] [CrossRef]
- Kuhn, C.W. Separation of cowpea virus mixtures. Phytopathology 1964, 54, 739. [Google Scholar]
- Xu, H.; Nie, J. Identification, characterization, and molecular detection of Alfalfa mosaic virus in potato. Phytopathology 2006, 96, 1237–1242. [Google Scholar] [CrossRef]
- Calvo, P.; Remunan-Lopez, C.; Vila-Jato, J.L.; Alonso, M. Novel hydrophilic chitosan-polyethylene oxide nanoparticles as protein carriers. J. Appl. Polym. Sci. 1997, 63, 125–132. [Google Scholar] [CrossRef]
- Babu, B.; Nair, R.S.; Angelo, J.M.C.; Mathai, V.; Vineet, R. Evaluation of efficacy of chitosan-silver nanocomposite on Candida albicans when compared to three different antifungal agents in combination with standard irrigation protocol: An: Ex vivo: Study. Saudi Endod. J. 2017, 7, 87–91. [Google Scholar]
- Devi, P.R.; Doraiswamy, S.; Nakkeeran, S.; Rabindran, R.; Ganapathy, T.; Ramiah, M.; Mathiyazhagan, S. Antiviral action of Harpulia Cupanioides and Mirabilis Jalapa against tomato spotted wilt virus (TSWV) infecting tomato. Arch. Phytopathol. Plant Protect. 2004, 37, 245–259. [Google Scholar] [CrossRef]
- Guo, C.-L.; Chen, H.-Y.; Cui, B.-L.; Chen, Y.-H.; Zhou, Y.-F.; Peng, X.-S.; Wang, Q. Development of a HPLC method for the quantitative determination of capsaicin in collagen sponge. Int. J. Anal. Chem. 2015, 2015, 912631. [Google Scholar] [CrossRef] [PubMed]
- Diaz, D.H.; Martin, G.C. Peach seed dormancy in relation to endogenous inhibitors and applied growth substances1. J. Am. Soc. Hortic. Sci. 1972, 97, 651–654. [Google Scholar] [CrossRef]
- Soudani, S.; Poza-Carrión, C.; De la Cruz Gómez, N.; González-Coloma, A.; Andrés, M.F.; Berrocal-Lobo, M. Essential oils prime epigenetic and metabolomic changes in tomato defense against Fusarium oxysporum. Front. Plant Sci. 2022, 13, 4104. [Google Scholar] [CrossRef]
- Awasthi, A.; Saxena, K.K.; Arun, V. Sustainable and smart metal forming manufacturing process. Mater. Today Proc. 2021, 44, 2069–2079. [Google Scholar] [CrossRef]
- Kanakari, E.; Dendrinou-Samara, C. Fighting Phytopathogens with Engineered Inorganic-Based Nanoparticles. Materials 2023, 16, 2388. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.-R.; Jiang, R.; Chen, J.-F.; Xiao, Y.; Lv, Z.-Y.; Chen, W.-S. Strategic nanoparticle-mediated plant disease resistance. Crit. Rev. Biotechnol. 2023, 43, 22–37. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.; Behiry, S.; Abdelkhalek, A.; Younes, H. Isolation and purification of Alfalfa mosaic virus-infecting potato (Solanum tuberosum L.) in Beheira governorate. Middle East J. 2020, 9, 617–623. [Google Scholar]
- Sofy, A.R.; Sofy, M.R.; Hmed, A.A.; Dawoud, R.A.; Refaey, E.E.; Mohamed, H.I.; El-Dougdoug, N.K. Molecular Characterization of the Alfalfa mosaic virus Infecting Solanum melongena in Egypt and the Control of Its Deleterious Effects with Melatonin and Salicylic Acid. Plants 2021, 10, 459. [Google Scholar] [CrossRef]
- Aleem, E.E.A.; Taha, R.M.; Fattouh, F.A. Biodiversity and full genome sequence of potato viruses Alfalfa mosaic virus and potato leaf roll virus in Egypt. Z. Naturforsch. C J. Biosci. 2018, 73, 423–438. [Google Scholar] [CrossRef]
- Parrella, G.; Acanfora, N.; Bellardi, M.G. First Record and Complete Nucleotide Sequence of Alfalfa mosaic virus from Lavandula stoechas in Italy. Plant Dis. 2010, 94, 924. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, S.; Erilmez, S.; Paylan, I. First report of Alfalfa mosaic virus in eggplant in Turkey. J. Plant Pathol. 2011, 93, 4–82. [Google Scholar]
- Al-Shahwan, I.; Abdalla, O.; Al-Saleh, M.; Amer, M. Detection of new viruses in alfalfa, weeds and cultivated plants growing adjacent to alfalfa fields in Saudi Arabia. Saudi J. Biol. Sci. 2017, 24, 1336–1343. [Google Scholar] [CrossRef] [PubMed]
- Tortella, G.R.; Rubilar, O.; Diez, M.C.; Padrão, J.; Zille, A.; Pieretti, J.C.; Seabra, A.B. Advanced Material Against Human (Including COVID-19) and Plant Viruses: Nanoparticles As a Feasible Strategy. Glob. Chall. 2021, 5, 2000049. [Google Scholar] [CrossRef] [PubMed]
- El Gamal, A.Y.; Atia, M.M.; Sayed, T.E.; Abou-Zaid, M.I.; Tohamy, M.R. Antiviral activity of chitosan nanoparticles for controlling plant-infecting viruses. S. Afr. J. Sci. 2022, 118, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Boroumand, H.; Badie, F.; Mazaheri, S.; Seyedi, Z.S.; Nahand, J.S.; Nejati, M.; Baghi, H.B.; Abbasi-Kolli, M.; Badehnoosh, B.; Ghandali, M. Chitosan-based nanoparticles against viral infections. Front. Cell. Infect. Microbiol. 2021, 11, 643953. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, R.C.; Kumaraswamy, R.; Kumari, S.; Sharma, S.; Pal, A.; Raliya, R.; Biswas, P.; Saharan, V. Cu-chitosan nanoparticle boost defense responses and plant growth in maize (Zea mays L.). Sci. Rep. 2017, 7, 9754. [Google Scholar] [CrossRef]
- Rendina, N.; Nuzzaci, M.; Scopa, A.; Cuypers, A.; Sofo, A. Chitosan-elicited defense responses in Cucumber mosaic virus (CMV)-infected tomato plants. J. Plant Physiol. 2019, 234–235, 9–17. [Google Scholar] [CrossRef]
- Nagorskaya, V.; Reunov, A.; Lapshina, L.; Davydova, V.; Yermak, I. Effect of chitosan on tobacco mosaic virus (TMV) accumulation, hydrolase activity, and morphological abnormalities of the viral particles in leaves of N. tabacum L. cv. Samsun. Virol. Sin. 2014, 29, 250–256. [Google Scholar] [CrossRef]
- El-Shazly, M.A.; Kobeasy, M.I.; Altalhi, S.H. Biological, serological and inhibition activity of Tomato spotted wilt virus isolated from tomato plants in Taif region, Saudi Arabia. J. Vir. Sci. 2016, 13, 52–73. [Google Scholar]
- Kobayashi, S.; Ishimaru, M.; Hiraoka, K.; Honda, C. Myb-related genes of the Kyoho grape (Vitis labruscana) regulate anthocyanin biosynthesis. Planta 2002, 215, 924–933. [Google Scholar]
- Phang, J.M. The regulatory functions of proline and pyrroline-5-carboxylic acid. Curr. Top. Dev. Biol. 1985, 25, 91–132. [Google Scholar]
- Ibrahim, M.; Abd El-Gawad, H.; Bondok, A. Physiological impacts of potassium citrate and folic acid on growth, yield and some viral diseases of potato plants. Middle East J. Agric. Res. 2015, 4, 577–589. [Google Scholar]
- Da Costa, M.; Sharma, P. Effect of copper oxide nanoparticles on growth, morphology, photosynthesis, and antioxidant response in Oryza sativa. Photosynthetica 2016, 54, 110–119. [Google Scholar] [CrossRef]
- Khan, M.N.; Mobin, M.; Abbas, Z.K.; AlMutairi, K.A.; Siddiqui, Z.H. Role of nanomaterials in plants under challenging environments. Plant Physiol. Biochem. 2017, 110, 194–209. [Google Scholar] [CrossRef] [PubMed]
- Ali, E.; El-Shehawi, A.; Ibrahim, O.; Abdul-Hafeez, E.; Moussa, M.; Hassan, F. A vital role of chitosan nanoparticles in improvisation the drought stress tolerance in Catharanthus roseus (L.) through biochemical and gene expression modulation. Plant Physiol. Biochem. 2021, 161, 166–175. [Google Scholar] [CrossRef]
- Mahdi, A.; Ibrahim, H.; Farroh, K.; Saleh, E.; Ghaly, O. Chitosan nanoparticles and its impact on growth, yield, some biochemical and molecular markers in Silybium marianum. Egypt. J. Desert Res. 2021, 71, 163–190. [Google Scholar] [CrossRef]
- Ismail, A.M.; Mosa, M.A.; El-Ganainy, S.M. Chitosan-Decorated Copper Oxide Nanocomposite: Investigation of Its Antifungal Activity against Tomato Gray Mold Caused by Botrytis cinerea. Polymers 2023, 15, 1099. [Google Scholar] [CrossRef]
- Sathiyabama, M.; Manikandan, A. Foliar application of chitosan nanoparticle improves yield, mineral content and boost innate immunity in finger millet plants. Carbohydr. Polym. 2021, 258, 117691. [Google Scholar] [CrossRef] [PubMed]
- Krishnaraj, C.; Jagan, E.; Ramachandran, R.; Abirami, S.; Mohan, N.; Kalaichelvan, P. Effect of biologically synthesized silver nanoparticles on Bacopa monnieri (Linn.) Wettst. plant growth metabolism. Process Biochem. 2012, 47, 651–658. [Google Scholar] [CrossRef]
- Sadak, M.S. Impact of silver nanoparticles on plant growth, some biochemical aspects, and yield of fenugreek plant (Trigonella foenum-graecum). Bull. Natl. Res. Cent. 2019, 43, 38. [Google Scholar] [CrossRef]
- Trucco, V.; Castellanos Collazo, O.; Vaghi Medina, C.G.; Cabrera Mederos, D.; Lenardon, S.; Giolitti, F. Alfalfa mosaic virus (AMV): Genetic diversity and a new natural host. J. Plant Pathol. 2022, 104, 349–356. [Google Scholar] [CrossRef]
- Galdiero, S.; Falanga, A.; Vitiello, M.; Cantisani, M.; Marra, V.; Galdiero, M. Silver nanoparticles as potential antiviral agents. Molecules 2011, 16, 8894–8918. [Google Scholar] [CrossRef] [PubMed]
- Khandelwal, N.; Kaur, G.; Kumar, N.; Tiwari, A. Application of silver nanoparticles in viral inhibition: A new hope for antivirals. Dig. J. Nanomater. Biostruct. 2014, 9, 175–186. [Google Scholar]
- Narasimha, G.; Khadri, H.; Alzohairy, M. Antiviral properties of silver nanoparticles synthesized by Aspergillus spp. Der Pharm. Lett. 2012, 4, 649–651. [Google Scholar]
- Xing, K.; Chen, X.G.; Liu, C.S.; Cha, D.S.; Park, H.J. Oleoyl-chitosan nanoparticles inhibits Escherichia coli and Staphylococcus aureus by damaging the cell membrane and putative binding to extracellular or intracellular targets. Int. J. Food Microbiol. 2009, 132, 127–133. [Google Scholar] [CrossRef]
- Mansilla, A.Y.; Albertengo, L.; Rodríguez, M.S.; Debbaudt, A.; Zúñiga, A.; Casalongué, C. Evidence on antimicrobial properties and mode of action of a chitosan obtained from crustacean exoskeletons on Pseudomonas syringae pv. tomato DC3000. Appl. Microbiol. Biotechnol. 2013, 97, 6957–6966. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).