One-Step Method for Direct Acrylation of Vegetable Oils: A Biobased Material for 3D Printing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Acrylated Epoxidized Soybean Oil (AESO)
2.3. Synthesis of Acrylated Soybean Oil (ASO)
2.4. Characterization of Acrylated Oils by Epoxy Index (E.I.), Acidity Index and Iodine Value
2.5. Fourier Transformed Infrared Spectroscopy (FTIR)
2.6. Nuclear Magnetic Resonance (1H-NMR)
2.7. Preparation of 3D Printable Formulations and Solid Samples
2.8. Characterization of 3D Printable Formulations
2.9. Characterization of Solid Samples
3. Results
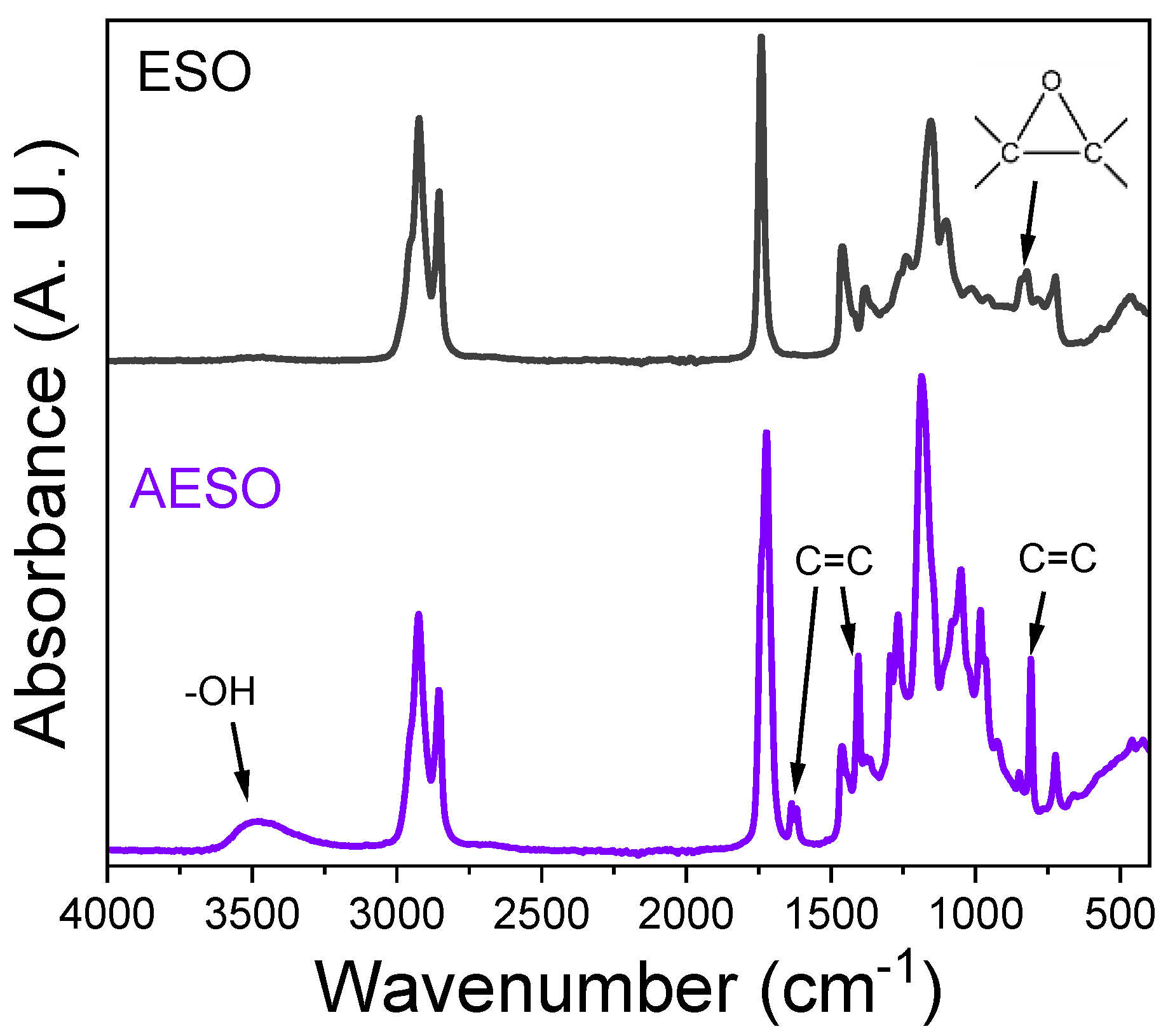
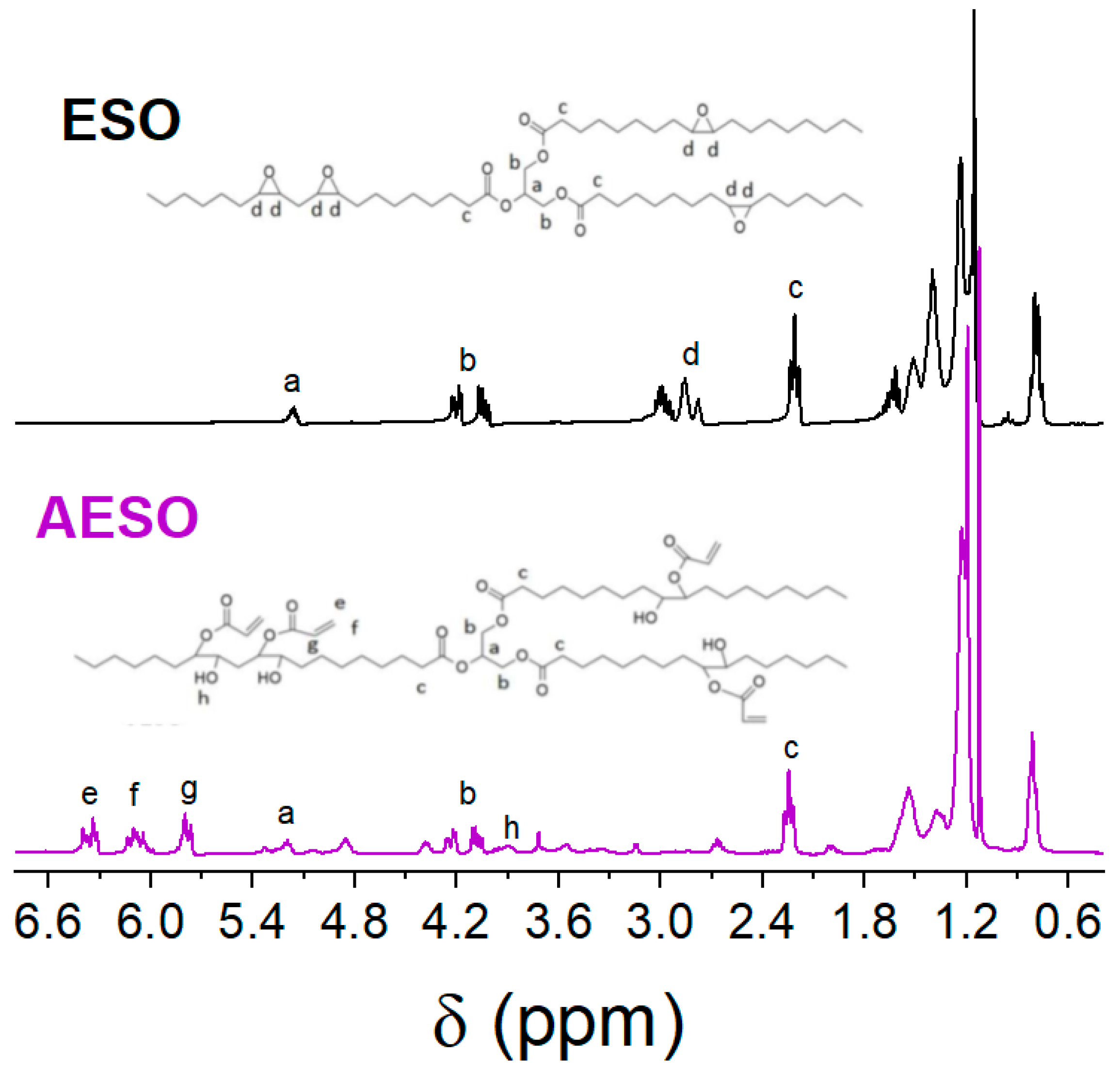
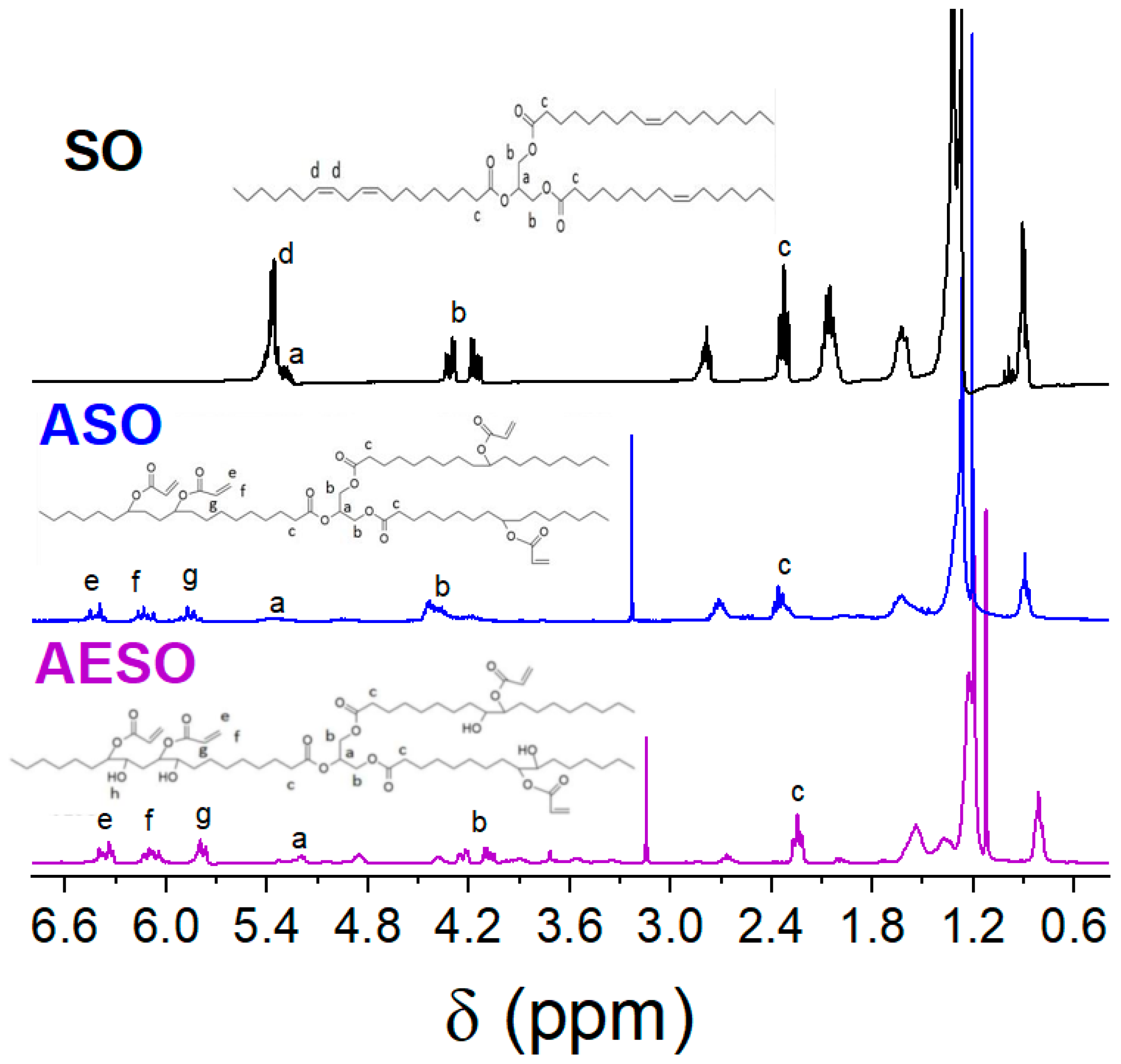
3.1. Synthesis and Characterization of Acrylated Epoxidized Soybean Oil (AESO), Effect of the Catalyst Nature
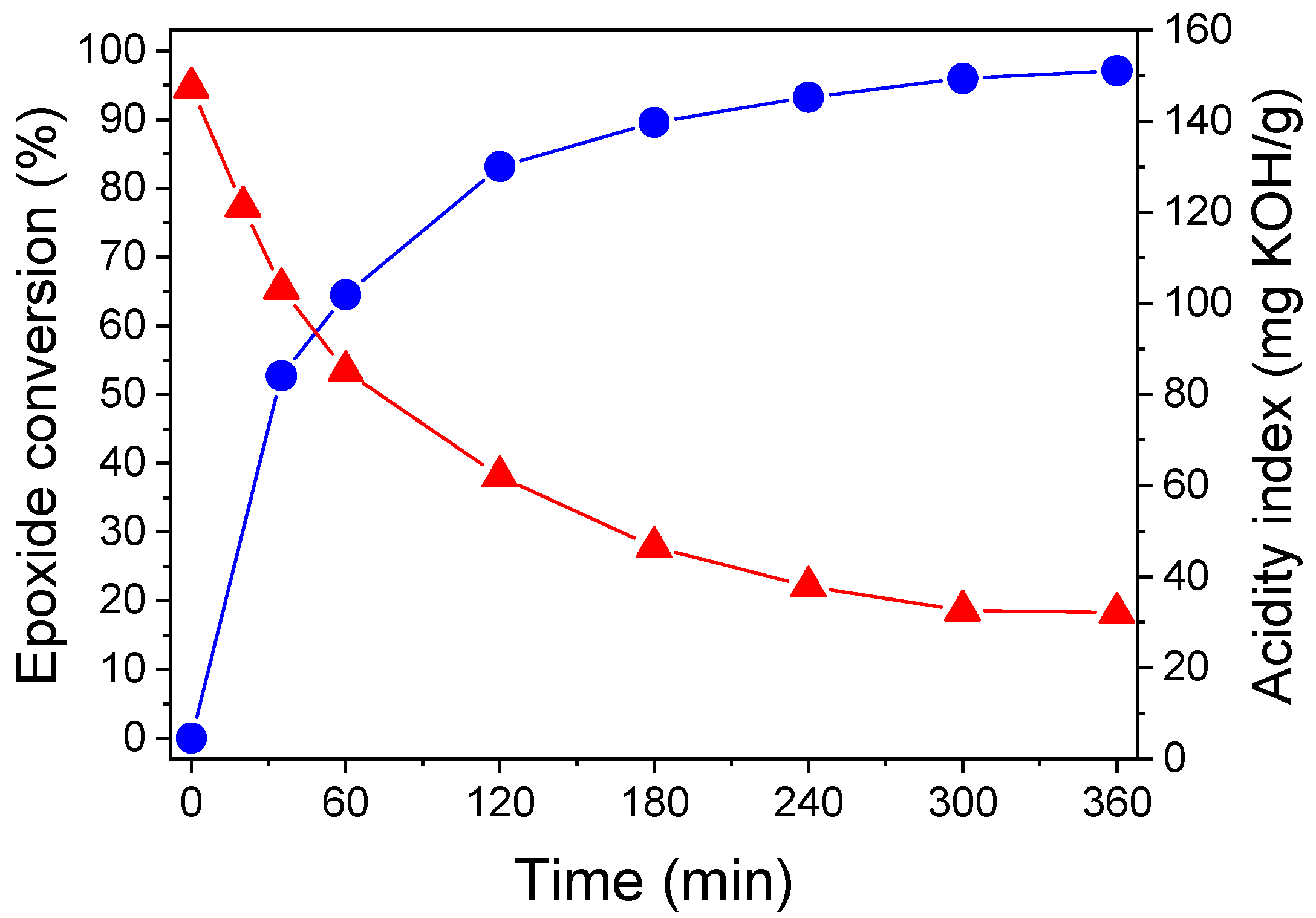
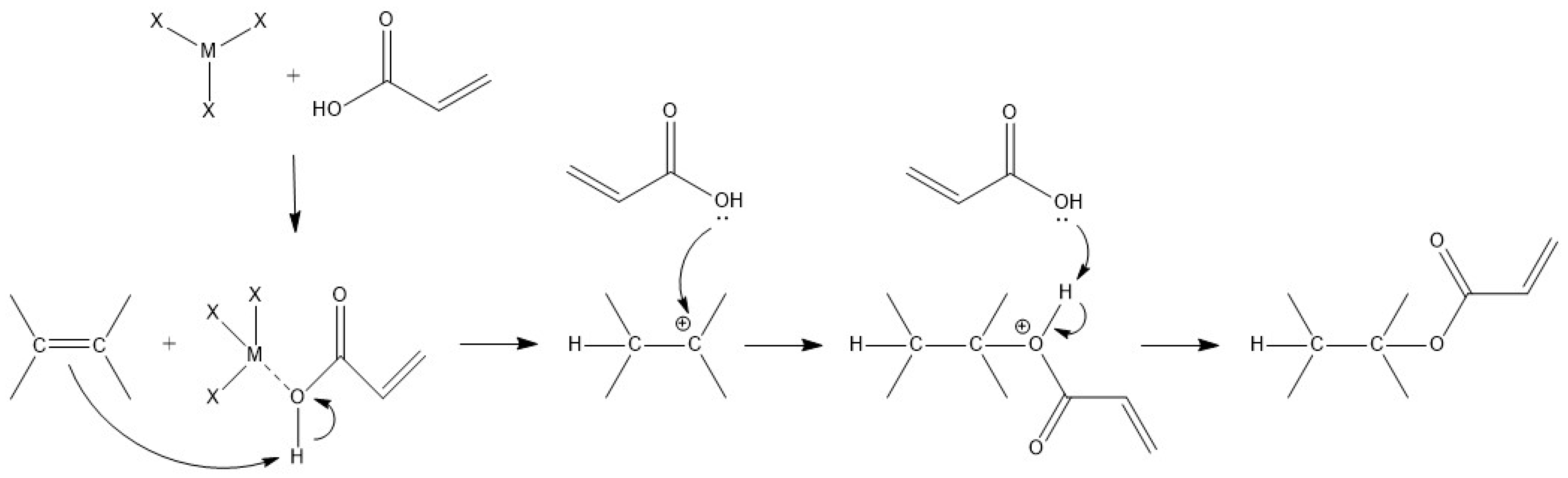
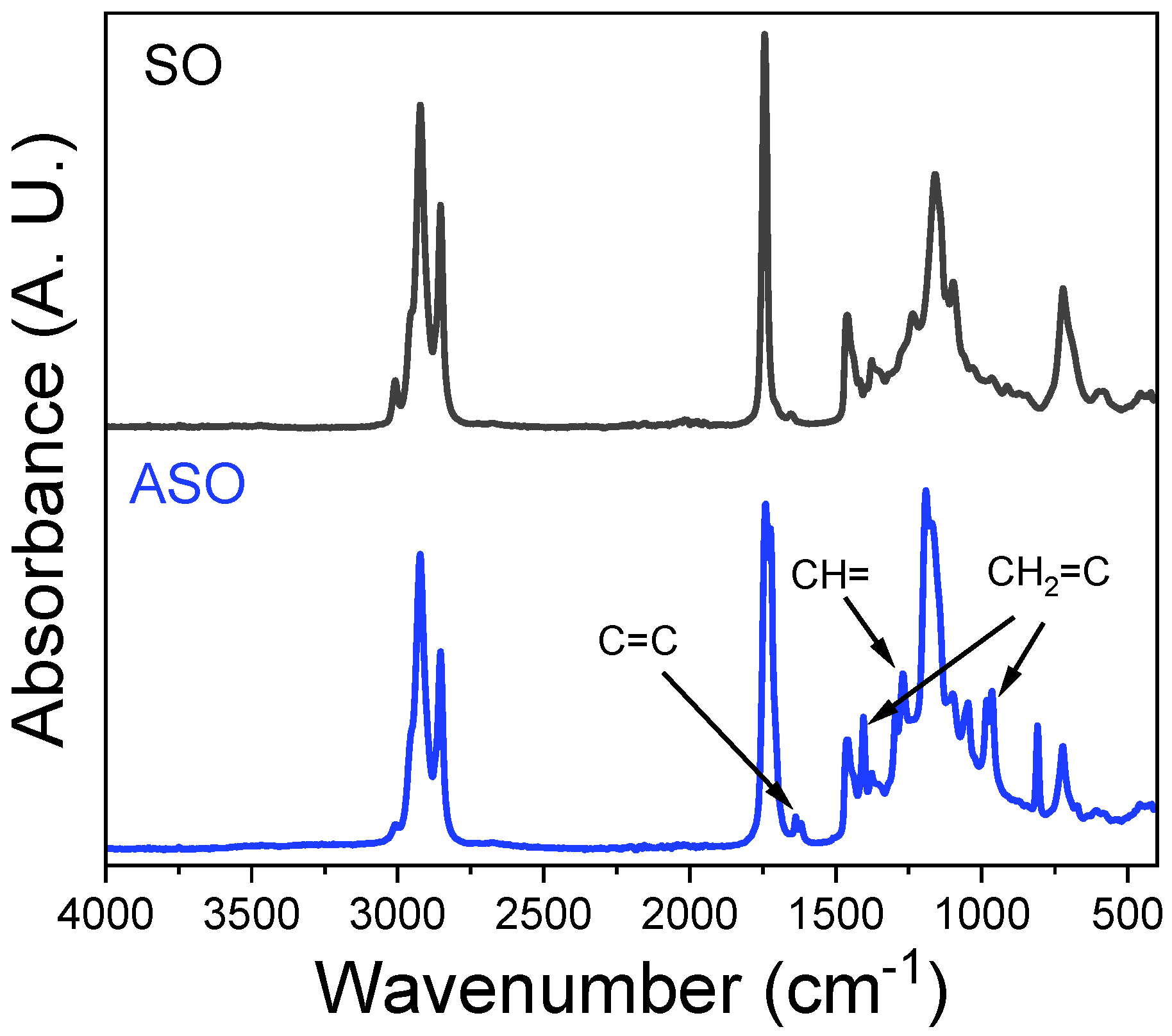
3.2. Synthesis and Characterization of Directly Acrylated Soybean Oil (ASO)
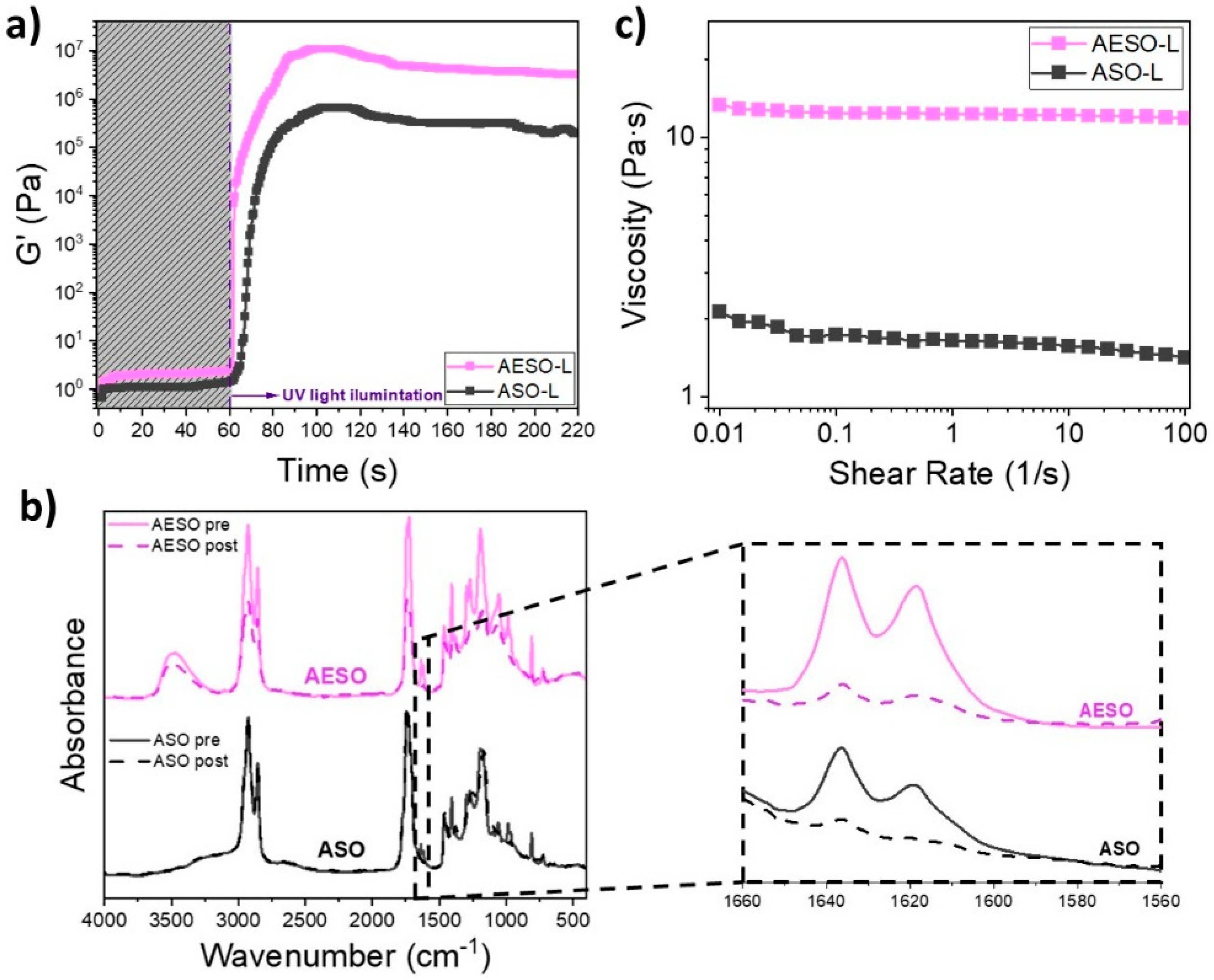
3.3. Characterization of 3D Printable Formulations
3.4. Characterization of Solid Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ghobakhloo, M. Industry 4.0, Digitization, and Opportunities for Sustainability. J. Clean. Prod. 2020, 252, 119869. [Google Scholar] [CrossRef]
- Tofail, S.A.M.; Koumoulos, E.P.; Bandyopadhyay, A.; Bose, S.; O’Donoghue, L.; Charitidis, C. Additive Manufacturing: Scientific and Technological Challenges, Market Uptake and Opportunities. Mater. Today 2018, 21, 22–37. [Google Scholar] [CrossRef]
- Al Rashid, A.; Ahmed, W.; Khalid, M.Y.; Koç, M. Vat Photopolymerization of Polymers and Polymer Composites: Processes and Applications. Addit. Manuf. 2021, 47, 102279. [Google Scholar] [CrossRef]
- Mendes-Felipe, C.; Oliveira, J.; Etxebarria, I.; Vilas-Vilela, J.L.; Lanceros-Mendez, S. State-of-the-Art and Future Challenges of UV Curable Polymer-Based Smart Materials for Printing Technologies. Adv. Mater. Technol. 2019, 4, 1800618. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.A.R.; Razzaq, A.; Yu, Z.; Miller, S. Industry 4.0 and Circular Economy Practices: A New Era Business Strategies for Environmental Sustainability. Bus. Strateg. Environ. 2021, 30, 4001–4014. [Google Scholar] [CrossRef]
- Benson, N.U.; Bassey, D.E.; Palanisami, T. COVID Pollution: Impact of COVID-19 Pandemic on Global Plastic Waste Footprint. Heliyon 2021, 7, e06343. [Google Scholar] [CrossRef] [PubMed]
- Sousa, A.F.; Silvestre, A.J.D. Plastics from Renewable Sources as Green and Sustainable Alternatives. Curr. Opin. Green Sustain. Chem. 2022, 33, 100557. [Google Scholar] [CrossRef]
- Kalita, D.J.; Tarnavchyk, I.; Kalita, H.; Chisholm, B.J.; Webster, D.C. Bio-Based Coating Resins Derived from Cardanol Using Carbocationic Polymerization and Their Evaluation as One-Component Alkyd-Type Coatings. Prog. Org. Coat. 2023, 174, 107252. [Google Scholar] [CrossRef]
- Fertier, L.; Koleilat, H.; Stemmelen, M.; Giani, O.; Joly-Duhamel, C.; Lapinte, V.; Robin, J.J. The Use of Renewable Feedstock in UV-Curable Materials-A New Age for Polymers and Green Chemistry. Prog. Polym. Sci. 2013, 38, 932–962. [Google Scholar] [CrossRef]
- Zhang, C.; Garrison, T.F.; Madbouly, S.A.; Kessler, M.R. Recent Advances in Vegetable Oil-Based Polymers and Their Composites. Prog. Polym. Sci. 2017, 71, 91–143. [Google Scholar] [CrossRef]
- Mendes-Felipe, C.; Costa, P.; Roppolo, I.; Sangermano, M.; Lanceros-Mendez, S. Bio-Based Piezo- and Thermoresistive Photocurable Sensing Materials from Acrylated Epoxidized Soybean Oil. Macromol. Mater. Eng. 2022, 307, 2100934. [Google Scholar] [CrossRef]
- Voet, V.S.D.; Guit, J.; Loos, K. Sustainable Photopolymers in 3D Printing: A Review on Biobased, Biodegradable, and Recyclable Alternatives. Macromol. Rapid Commun. 2021, 42, 2000475. [Google Scholar] [CrossRef]
- Gan, Y.; Jiang, X. Photo-Cured Materials from Vegetable Oils. In Green Materials from Plant Oils; Royal Society of Chemistry: London, UK, 2014. [Google Scholar]
- Malburet, S.; Di Mauro, C.; Noè, C.; Mija, A.; Sangermano, M.; Graillot, A. Sustainable Access to Fully Biobased Epoxidized Vegetable Oil Thermoset Materials Prepared by Thermal or UV-Cationic Processes. RSC Adv. 2020, 10, 41954–41966. [Google Scholar] [CrossRef]
- Noè, C.; Hakkarainen, M.; Sangermano, M. Cationic UV-Curing of Epoxidized Biobased Resins. Polymers 2020, 13, 89. [Google Scholar] [CrossRef]
- Danov, S.M.; Kazantsev, O.A.; Esipovich, A.L.; Belousov, A.S.; Rogozhin, A.E.; Kanakov, E.A. Recent Advances in the Field of Selective Epoxidation of Vegetable Oils and Their Derivatives: A Review and Perspective. Catal. Sci. Technol. 2017, 7, 3659–3675. [Google Scholar] [CrossRef]
- Khot, S.N.; Lascala, J.J.; Can, E.; Morye, S.S.; Williams, G.I.; Palmese, G.R.; Kusefoglu, S.H.; Wool, R.P. Development and Application of Triglyceride-Based Polymers and Composites. J. Appl. Polym. Sci. 2001, 82, 703–723. [Google Scholar] [CrossRef]
- Ho, Y.H.; Parthiban, A.; Thian, M.C.; Ban, Z.H.; Siwayanan, P. Acrylated Biopolymers Derived via Epoxidation and Subsequent Acrylation of Vegetable Oils. Int. J. Polym. Sci. 2022, 2022, 6210128. [Google Scholar] [CrossRef]
- Briede, S.; Barkane, A.; Jurinovs, M.; Thakur, V.K.; Gaidukovs, S. Acrylation of Biomass: A Review of Synthesis Process: Know-How and Future Application Directions. Curr. Opin. Green Sustain. Chem. 2022, 35, 100626. [Google Scholar] [CrossRef]
- Zhang, P.; Xin, J.; Zhang, J. Effects of Catalyst Type and Reaction Parameters on One-Step Acrylation of Soybean Oil. ACS Sustain. Chem. Eng. 2014, 2, 181–187. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, J. One-Step Acrylation of Soybean Oil (SO) for the Preparation of SO-Based Macromonomers. Green Chem. 2013, 15, 641. [Google Scholar] [CrossRef]
- Briede, S.; Jurinovs, M.; Nechausov, S.; Platnieks, O.; Gaidukovs, S. State-of-the-Art UV-Assisted 3D Printing via a Rapid Syringe-Extrusion Approach for Photoactive Vegetable Oil Acrylates Produced in One-Step Synthesis. Mol. Syst. Des. Eng. 2022, 7, 1434–1448. [Google Scholar] [CrossRef]
- UNE-EN-ISO 660; Animal and Vegetable Fats and Oils—Determination of Acid Value and Acidity. Asociación Española de Normalización, UNE: Madrid, Spain, 2021.
- McCutcheon, J.W. Wijs Iodine Method. Ind. Eng. Chem. Anal. Ed. 1940, 12, 465. [Google Scholar] [CrossRef]
- Bukowska, A.; Bukowski, W. Reactivity of Some Carboxylic Acids in Reactions with Some Epoxides in the Presence Chromium (III) Ethanoate. Org. Process Res. Dev. 2002, 6, 234–237. [Google Scholar] [CrossRef]
- Baghban, S.A.; Ebrahimi, M.; Khorasani, M. A Facile Method to Synthesis of a Highly Acrylated Epoxidized Soybean Oil with Low Viscosity: Combined Experimental and Computational Approach. Polym. Test. 2022, 115, 107727. [Google Scholar] [CrossRef]
- Li, Y.; Sun, X.S. Synthesis and Characterization of Acrylic Polyols and Polymers from Soybean Oils for Pressure-Sensitive Adhesives. RSC Adv. 2015, 5, 44009–44017. [Google Scholar] [CrossRef]
- Fu, L.; Yang, L.; Dai, C.; Zhao, C.; Ma, L. Thermal and Mechanical Properties of Acrylated Expoxidized-Soybean Oil-Based Thermosets. J. Appl. Polym. Sci. 2010, 117, 2220–2225. [Google Scholar] [CrossRef]
- Lebedevaite, M.; Talacka, V.; Ostrauskaite, J. High Biorenewable Content Acrylate Photocurable Resins for DLP 3D Printing. J. Appl. Polym. Sci. 2021, 138, 50233. [Google Scholar] [CrossRef]
- Cosola, A.; Conti, R.; Grützmacher, H.; Sangermano, M.; Roppolo, I.; Pirri, C.F.; Chiappone, A. Multiacrylated Cyclodextrin: A Bio-Derived Photocurable Macromer for VAT 3D Printing. Macromol. Mater. Eng. 2020, 305, 2000350. [Google Scholar] [CrossRef]
- Anseth, K.S.; Wang, C.M.; Bowman, C.N. Reaction Behaviour and Kinetic Constants for Photopolymerizations of Multi(Meth)Acrylate Monomers. Polymer 1994, 35, 3243–3250. [Google Scholar] [CrossRef]
- Barkane, A.; Platnieks, O.; Jurinovs, M.; Kasetaite, S.; Ostrauskaite, J.; Gaidukovs, S.; Habibi, Y. UV-Light Curing of 3D Printing Inks from Vegetable Oils for Stereolithography. Polymers 2021, 13, 1195. [Google Scholar] [CrossRef]
- Arslan, A.; Steiger, W.; Roose, P.; Van den Bergen, H.; Gruber, P.; Zerobin, E.; Gantner, F.; Guillaume, O.; Ovsianikov, A.; Van Vlierberghe, S.; et al. Polymer Architecture as Key to Unprecedented High-Resolution 3D-Printing Performance: The Case of Biodegradable Hexa-Functional Telechelic Urethane-Based Poly-ε-Caprolactone. Mater. Today 2021, 44, 25–39. [Google Scholar] [CrossRef]
- Weng, Z.; Zhou, Y.; Lin, W.; Senthil, T.; Wu, L. Structure-Property Relationship of Nano Enhanced Stereolithography Resin for Desktop SLA 3D Printer. Compos. Part A Appl. Sci. Manuf. 2016, 88, 234–242. [Google Scholar] [CrossRef]
- Rengasamy, S.; Mannari, V. Development of Soy-Based UV-Curable Acrylate Oligomers and Study of Their Film Properties. Prog. Org. Coat. 2013, 76, 78–85. [Google Scholar] [CrossRef]
- Kuhnt, T.; Morgan, F.L.C.; Baker, M.B.; Moroni, L. An Efficient and Easily Adjustable Heating Stage for Digital Light Processing Set-Ups. Addit. Manuf. 2021, 46, 102102. [Google Scholar] [CrossRef]
- Chen, Z.; Li, J.; Liu, C.; Liu, Y.; Zhu, J.; Lao, C. Preparation of High Solid Loading and Low Viscosity Ceramic Slurries for Photopolymerization-Based 3D Printing. Ceram. Int. 2019, 45, 11549–11557. [Google Scholar] [CrossRef]
- Noè, C.; Iannucci, L.; Malburet, S.; Graillot, A.; Sangermano, M.; Grassini, S. New UV-Curable Anticorrosion Coatings from Vegetable Oils. Macromol. Mater. Eng. 2021, 306, 2100029. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, X.; Liu, R.; Liu, X.; Liu, J. Highly Functional Bio-Based Acrylates with a Hard Core and Soft Arms: From Synthesis to Enhancement of an Acrylated Epoxidized Soybean Oil-Based UV-Curable Coating. Prog. Org. Coat. 2019, 134, 342–348. [Google Scholar] [CrossRef]
- Sahoo, S.K.; Khandelwal, V.; Manik, G. Synthesis and Characterization of Low Viscous and Highly Acrylated Epoxidized Methyl Ester Based Green Adhesives Derived from Linseed Oil. Int. J. Adhes. Adhes. 2019, 89, 174–177. [Google Scholar] [CrossRef]
- Landel, R.F.; Nielsen, L.E. Mechanical Properties of Polymers and Composites; CRC Press: Boca Raton, FL, USA, 1993. [Google Scholar]
- Bandzierz, K.; Reuvekamp, L.; Dryzek, J.; Dierkes, W.; Blume, A.; Bielinski, D. Influence of Network Structure on Glass Transition Temperature of Elastomers. Materials 2016, 9, 607. [Google Scholar] [CrossRef] [Green Version]
- Wu, N.; Wu, B.; Xu, Y.; Tang, X. Relationships of the Degree of C=C Double Bond Conversion with the Dielectric Properties for SiO2/1,2-PB/SBS/EPDM Composites Cured by Organic Peroxide. ChemistrySelect 2022, 7, e202104078. [Google Scholar] [CrossRef]
- Sahoo, S.K.; Mohanty, S.; Nayak, S.K. Study of Thermal Stability and Thermo-Mechanical Behavior of Functionalized Soybean Oil Modified Toughened Epoxy/Organo Clay Nanocomposite. Prog. Org. Coat. 2015, 88, 263–271. [Google Scholar] [CrossRef]
- Chen, D.; Li, J.; Yuan, Y.; Gao, C.; Cui, Y.; Li, S.; Liu, X.; Wang, H.; Peng, C.; Wu, Z. A Review of the Polymer for Cryogenic Application: Methods, Mechanisms and Perspectives. Polymers 2021, 13, 320. [Google Scholar] [CrossRef]
- Su, Y.; Lin, H.; Zhang, S.; Yang, Z.; Yuan, T. One-Step Synthesis of Novel Renewable Vegetable Oil-Based Acrylate Prepolymers and Their Application in UV-Curable Coatings. Polymers 2020, 12, 1165. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Yuan, T.; Yang, Z.; Man, L.; Hu, Y.; Yang, Z. UV/Thermal Dual Curing of Tung Oil-Based Polymers Induced by Cationic Photoinitiator. Prog. Org. Coat. 2019, 126, 8–17. [Google Scholar] [CrossRef]
- Noè, C.; Cosola, A.; Tonda-Turo, C.; Sesana, R.; Delprete, C.; Chiappone, A.; Hakkarainen, M.; Sangermano, M. DLP-Printable Fully Biobased Soybean Oil Composites. Polymer 2022, 247, 124779. [Google Scholar] [CrossRef]
- Gastaldi, M.; Roppolo, I.; Chiappone, A.; Garino, C.; Fin, A.; Manachino, M.; Sirianni, P.; Viscardi, G.; Scaltrito, L.; Zanetti, M.; et al. Thermochromic Photoluminescent 3D Printed Polymeric Devices Based on Copper-Iodide Clusters. Addit. Manuf. 2022, 49, 102504. [Google Scholar] [CrossRef]



| Property | AESO | ASO |
|---|---|---|
| tgel (s) | 1.3 ± 0.2 | 7.0 ± 1.4 |
| ΔG′/Δt (kPa/s) | 72 ± 6 | 8.6 ± 0.2 |
| Conversion (%) | 79 ± 3 | 93 ± 2 |
| (Pa·s) | 11.8 ± 0.6 | 1.66 ± 0.04 |
| E′R (MPa) | 40 | 2.6 |
| Tg (°C) | 48 | −6 |
| νc (mmol·cm–3) | 2.58 | 0.26 |
| ∆V(%) | 5.0 ± 0.2 | 4.3 ± 0.4 |
| E (MPa) | 1.433 ± 0.370 | 0.085 ± 0.007 |
| σb (MPa) | 3.46 ± 0.25 | 0.43 ± 0.07 |
| εb (%) | 3.44 ± 0.46 | 11.04 ± 1.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendes-Felipe, C.; Isusi, I.; Gómez-Jiménez-Aberasturi, O.; Prieto-Fernandez, S.; Ruiz-Rubio, L.; Sangermano, M.; Vilas-Vilela, J.L. One-Step Method for Direct Acrylation of Vegetable Oils: A Biobased Material for 3D Printing. Polymers 2023, 15, 3136. https://doi.org/10.3390/polym15143136
Mendes-Felipe C, Isusi I, Gómez-Jiménez-Aberasturi O, Prieto-Fernandez S, Ruiz-Rubio L, Sangermano M, Vilas-Vilela JL. One-Step Method for Direct Acrylation of Vegetable Oils: A Biobased Material for 3D Printing. Polymers. 2023; 15(14):3136. https://doi.org/10.3390/polym15143136
Chicago/Turabian StyleMendes-Felipe, Cristian, Igor Isusi, Olga Gómez-Jiménez-Aberasturi, Soraya Prieto-Fernandez, Leire Ruiz-Rubio, Marco Sangermano, and José Luis Vilas-Vilela. 2023. "One-Step Method for Direct Acrylation of Vegetable Oils: A Biobased Material for 3D Printing" Polymers 15, no. 14: 3136. https://doi.org/10.3390/polym15143136
APA StyleMendes-Felipe, C., Isusi, I., Gómez-Jiménez-Aberasturi, O., Prieto-Fernandez, S., Ruiz-Rubio, L., Sangermano, M., & Vilas-Vilela, J. L. (2023). One-Step Method for Direct Acrylation of Vegetable Oils: A Biobased Material for 3D Printing. Polymers, 15(14), 3136. https://doi.org/10.3390/polym15143136