Bioelectrochemical Purification of Biomass Polymer Derived Furfural Wastewater and Its Electric Energy Recovery
Abstract
:1. Introduction
2. Materials and Methods
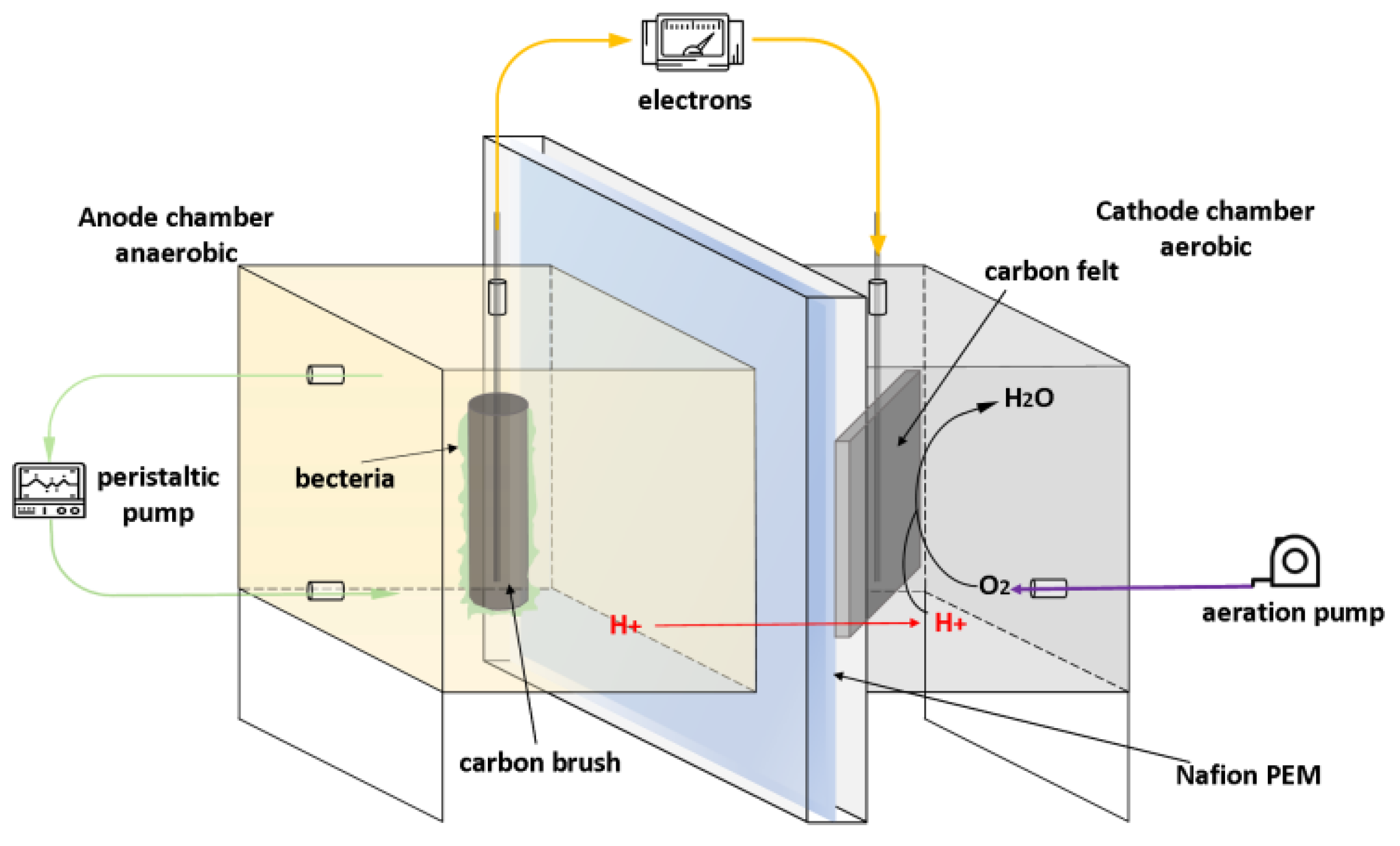
2.1. Reactor Design and Construction
2.2. Inoculation and MFC Operation
2.3. Composition of Biomass Polymer Derived Furfural Wastewater
2.4. Analytical Methods
2.5. Microbial Community Analysis
2.6. Statistical Analysis
3. Results
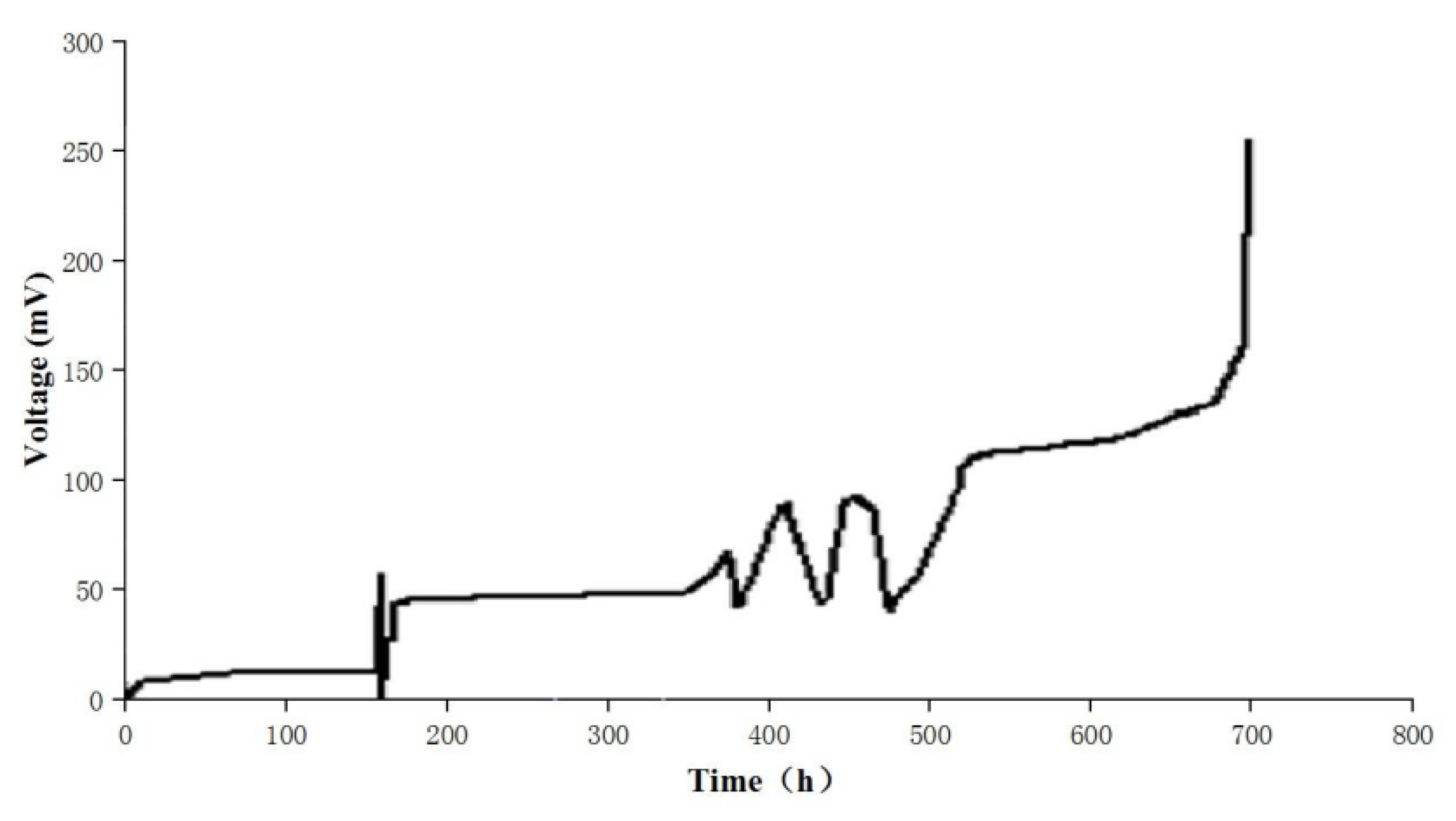
3.1. Start-Up Procedure
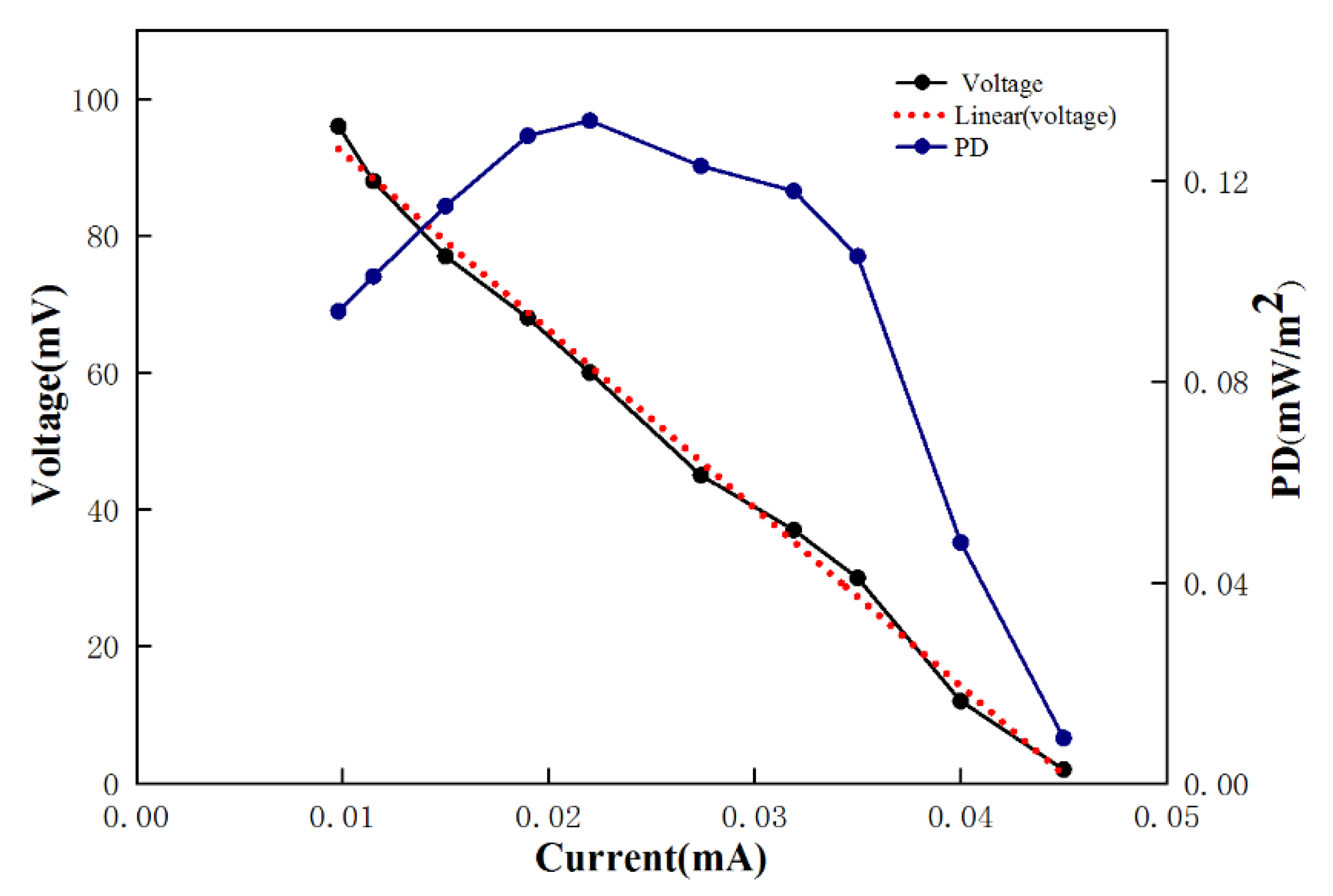
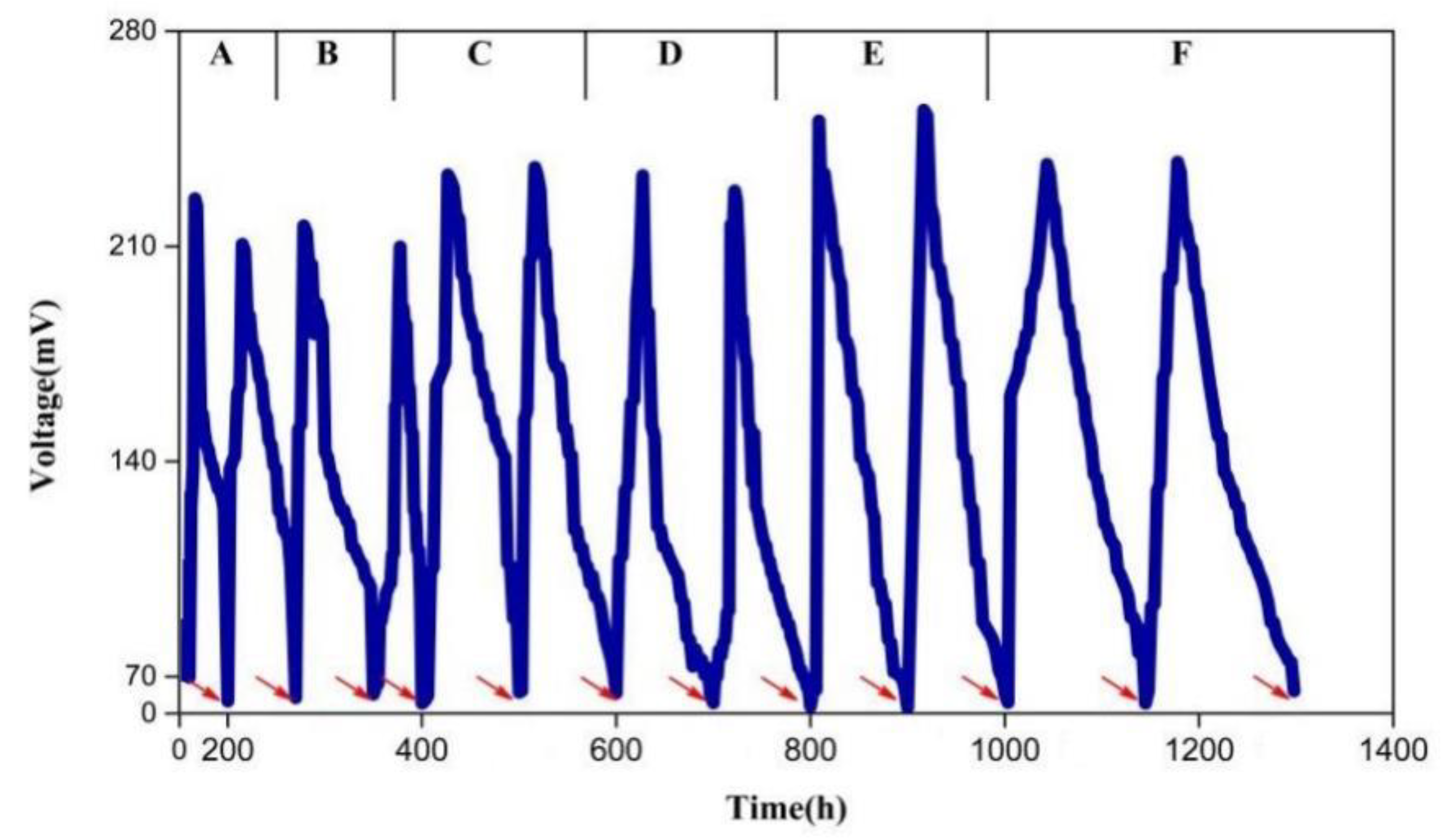
3.2. Electricity Generation
3.3. Biomass-Polymer-Derived Furfural Wastewater Treatment Efficiency
3.3.1. Characterization of Biomass-Polymer-Derived Furfural Wastewater
3.3.2. Standard Curve of Furfural
3.3.3. Removal Performance of Biomass-Polymer-Derived Furfural Wastewater
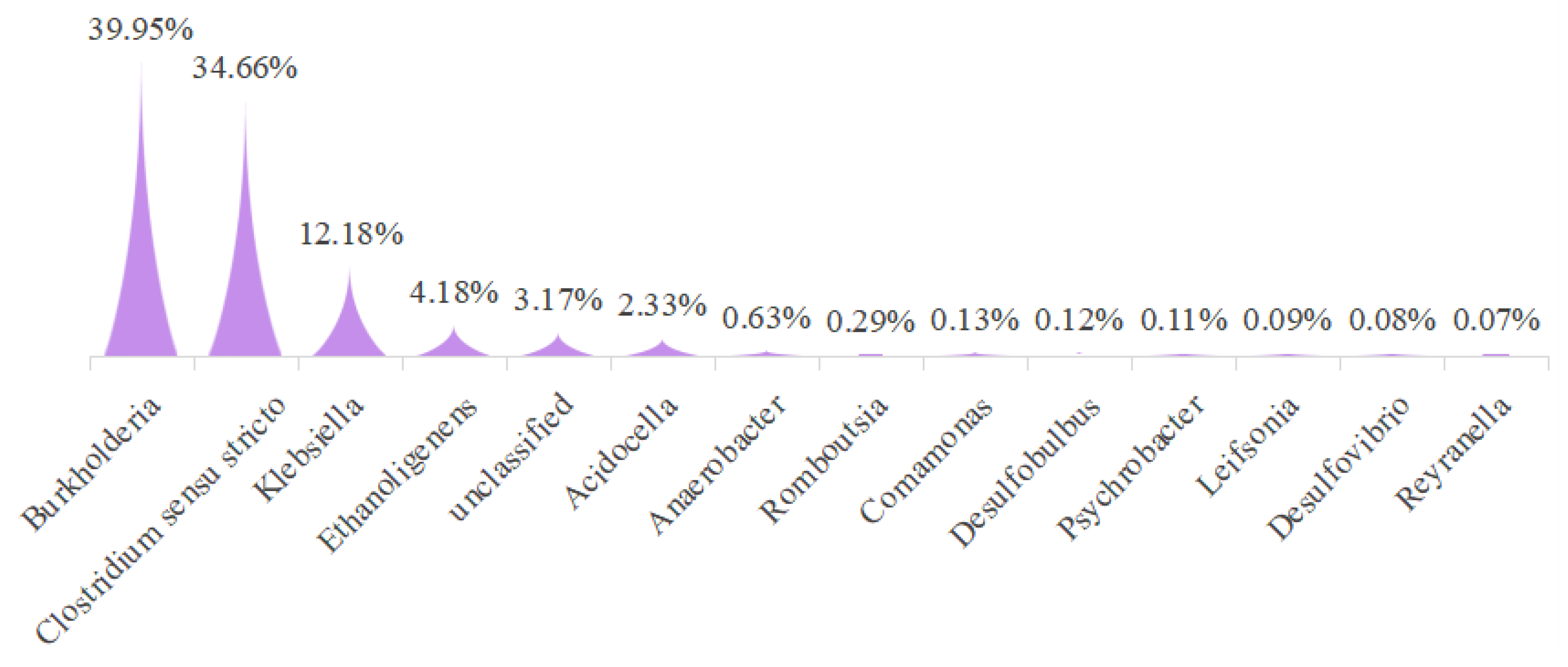
3.4. Microbial Morphology and Community in MFC
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, C.Y.; Wu, H.; Cai, M.Y.; Zhou, Y.T.; Guo, C.Y.; Han, Y.; Zhang, L. Valorization of biomass-derived polymers to functional biochar materials for supercapacitor applications via pyrolysis: Advances and perspectives. Polymers 2023, 15, 2741. [Google Scholar] [CrossRef] [PubMed]
- Anthonia, E.E.; Philip, H.S. An overview of the applications of furfural and its derivatives. Int. J. Adv. Chem. 2015, 3, 42–47. [Google Scholar]
- Xing, Y.; Bu, L.; Sun, D.; Liu, Z.P.; Liu, S.J.; Jiang, J.X. Enhancement of high-solids enzymatic hydrolysis and fermentation of furfural residues by addition of Gleditsia saponin. Fuel 2016, 177, 142–147. [Google Scholar] [CrossRef]
- Ghosh, S.; Falyouna, O.; Malloum, A.; Othmani, A.; Bornman, C.; Bedair, H.; Onyeaka, H.; Al-Sharify, Z.T.; Oluwaseun Jacob, A.; Miri, T.; et al. A general review on the use of advance oxidation and adsorption processes for the removal of furfural from industrial effluents. Micropor. Mesopor. Mat. 2022, 331, 111638. [Google Scholar] [CrossRef]
- Parseh, I.; Hajizadeh, Y.; Jaafarzadeh, N.; Goudarzi, G.; Shakerinejad, G.; Badeenezhad, A.; Fallahizadeh, S. Removal behavior of gaseous furfural using a biofilter packed with perlite, ripe compost, and oak woodchips. Process Saf. Environ. Protect. 2021, 149, 135–143. [Google Scholar] [CrossRef]
- Moussavi, G.; Leili, M.; Nadafi, K. Investigation of furfural biodegradation in a continuous inflow cyclic biological reactor. Water Sci. Technol. 2016, 73, 292–301. [Google Scholar] [CrossRef]
- Belay, N.; Boopathy, R.; Voskuilen, G. Anaerobic transformation of furfural by Methanococcus deltae DLH. Appl. Environ. Microbiol. 1997, 63, 2092–2094. [Google Scholar] [CrossRef]
- Borghei, S.M.; Hosseini, S.N. Comparison of furfural degradation by different photooxidation methods. Chem. Eng. J. 2008, 139, 482–488. [Google Scholar] [CrossRef]
- Anbia, M.; Mohammadi, N. A nanoporous adsorbent for removal of furfural from aqueous solutions. Desalination 2009, 249, 150–153. [Google Scholar] [CrossRef]
- Singh, S.; Srivastava, V.C.; Mall, I.D. Fixed-bed study for adsorptive removal of furfural by activated carbon. Colloid. Surf. A 2009, 332, 50–56. [Google Scholar] [CrossRef]
- Leili, M.; Moussavi, G.; Naddafi, K. Removal of furfural from wasterwater using integrated catalytic ozonation and biological approaches. Avicenna J. Environ. Health Eng. 2014, 1, e120. [Google Scholar] [CrossRef]
- Isnail, Z.Z.; Jasim, A.S. Ultrasonic treatment of wastewater contaminated with furfural. IDA J. Desalination Water Reuse 2015, 6, 3–4. [Google Scholar]
- Mustafa, Y.A.; Omran, R.R. Treatment of Furfural Wastewater by (AOPs) Photo-Fenton Method. Eng. J. 2015, 21, 129–141. [Google Scholar] [CrossRef]
- Kay, L.G.; Brouckaert, C.J.; Sindall, R.C. Modelling mesophilic-thermophilic temperature transitions experienced by an aerobic membrane bioreactor treating furfural plant effluent. Water SA 2019, 45, 317–328. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Z.Y.; Noor, R.S.; Cheng, Q.S.; Chu, X.D.; Qu, B.; Zhen, F.; Sun, Y. Furfural wastewater pretreatment of corn stalk for whole slurry anaerobic co-digestion to improve methane production. Sci. Total Environ. 2019, 674, 49–57. [Google Scholar] [CrossRef]
- Yang, Q.; Wu, Z.X.; Liu, L.F.; Zhang, F.X.; Liang, S.N. Treatment of oil wastewater and electricity generation by integrating constructed wetland with microbial fuel cell. Materials 2016, 9, 885. [Google Scholar] [CrossRef]
- Wu, H.; Fu, Y.; Guo, C.Y.; Li, Y.B.; Jiang, N.Z.; Yin, C.R. Electricity generation and removal performance of a microbial fuel cell using sulfonated poly (ether ether ketone) as proton exchange membrane to treat phenol/ acetone wastewater. Bioresour. Technol. 2018, 260, 130–134. [Google Scholar] [CrossRef]
- Wu, L.C.; Chen, C.Y.; Lin, T.K.; Su, Y.Y.; Chung, Y.C. Highly efficient removal of victoria blue R and bioelectricity generation from textile wastewater using a novel combined dual microbial fuel cell system. Chemosphere 2020, 258, 127326. [Google Scholar] [CrossRef]
- Pu, K.B.; Li, T.T.; Gao, J.Y.; Chen, Q.Y.; Guo, K.; Zhou, M.; Wang, C.T.; Wang, Y.H. Floating flexible microbial fuel cells for electricity generation and municipal wastewater treatment. Sep. Purif. Technol. 2022, 300, 121915. [Google Scholar] [CrossRef]
- Xin, X.D.; Xie, J.Q.; Li, W.; Lv, S.H.; He, J.G. New insights into microbial fuel cells for saline wastewater treatment: Bioelectrogenesis evaluation, microbial interactions and salinity resource reuse. Process Saf. Environ. 2022, 168, 314–323. [Google Scholar] [CrossRef]
- Zhang, B.; Ning, D.; Yang, Y.; Van Nostrand, J.D.; Zhou, J.; Wen, X. Biodegradability of wastewater determines microbial assembly mechanisms in full-scale wastewater treatment plants. Water Res. 2020, 169, 115276. [Google Scholar] [CrossRef]
- Wei, G.R.; Wei, T.; Li, Z.M.; Wei, C.; Kong, Q.P.; Guan, X.H.; Qiu, G.L.; Hu, Y.; Wei, C.H.; Zhu, S.; et al. BOD/COD ratio as a probing index in the O/H/O process for coking wastewater treatment. Chem. Eng. J. 2023, 466, 143257. [Google Scholar] [CrossRef]
- Wei, C.; Wu, H.; Kong, Q.; Wei, J.; Feng, C.; Qiu, G.; Wei, C.; Li, F. Residual chemical oxygen demand (COD) fractionation in bio-treated coking wastewater integrating solution property characterization. J. Environ. Manag. 2019, 246, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Van Soest, P.J.; Wine, R.H. Use of detergents in the analysis of fibrous feeds. 4. Determination of plant cell-wall constituents. J. Assoc. Off. Anal. Chem. 1967, 50, 50–55. [Google Scholar]
- Frank, J.A.; Reich, C.I.; Sharma, S.; Weisbaum, J.S.; Wilson, B.A.; Olsen, G.J. Critical evaluation of two primers commonly used for amplification of bacterial 16S rRNA genes. Appl. Environ. Microb. 2008, 74, 2461–2470. [Google Scholar] [CrossRef]
- Chowdhary, P.; Sammi, S.R.; Pandey, R.; Kaithwas, G.; Raj, A.; Singh, J.; Bharagava, R.N. Bacterial degradation of distillery wastewater pollutants and theirmetabolites characterization and its toxicity evaluation by using Caenorhabditis elegans as terrestrial test models. Chemosphere 2020, 261, 127689. [Google Scholar] [CrossRef]
- Portune, K.J.; Pérez, M.C.; Avarez-Hornos, J.; Gabaldón, C. Contribution of bacterial biodiversity on the operational performance of a styrene biotrickling filter. Chemosphere 2020, 247, 125800. [Google Scholar] [CrossRef]
- Chekroud, Z.; Djerrab, L.; Rouabhia, A.; Dems, M.A.; Atailia, I.; Djazy, F.; Smadi, M.A. Valorisation of date fruits by-products forthe production of biopolymer polyhydroxybutyrate (PHB) using the bacterial strain Bacillus paramycoides. Ital. J. Food Sci. 2022, 34, 48–58. [Google Scholar] [CrossRef]
- Saratale, G.D.; Saratale, R.G.; Shahid, M.K.; Zhen, G.; Kumar, G.; Shin, H.S.; Choi, Y.G.; Kim, S.H. A comprehensive overview on electro-active biofilms, role of exo-electrogens and their microbial niches in microbial fuel cells (MFCs). Chemosphere 2017, 178, 534–547. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, G.L.; Zhang, R.D.; Zhang, C.P. Power generation from furfural using the microbial fuel cell. J. Power Sources 2010, 195, 190–194. [Google Scholar] [CrossRef]
- Meenu, P.; Sreelekshmy, B.R.; Basheer, R.; Sadasivan, S.M.; Vijayakumari Ramakrishnan, R.M.; Shibli, S.M.A. Development of a high-performance mediatorless microbial fuel cell comprising a catalytic steel anode. ACS Appl. Bio. Mater. 2018, 1, 1124–1133. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Yu, Y.L.; Li, H.N.; Yan, Z.Y.; Li, Z.; Liu, G.H.; Zhang, Z.H.; Feng, Y.J. Enhancing carbon and nitrogen removals by a novel tubular bio-electrochemical system with functional biocathode coupling with oxygen-producing submerged plants. Chem. Eng. J. 2020, 402, 125400. [Google Scholar] [CrossRef]
- Liu, T.X.; Li, X.M.; Zhang, W.; Hu, M.; Li, F.B. Fe(III) oxides accelerate microbial nitrate reduction and electricity generation by Klebsiella pneumoniae L17. J. Colloid Interf. Sci. 2014, 423, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.F.; Liu, Y.Y.; Yang, Y.W.; Tang, M.Z.; Wang, R.J.; Jiang, L.T.; Tian, Y.P.; Hu, H.W.; Zhang, X.; Wei, Y.S. Bacterial community structure and gene function prediction in response to long-term running of dual graphene modified bioelectrode bioelectrochemical systems. Bioresour. Technol. 2020, 309, 123398. [Google Scholar] [CrossRef] [PubMed]
- Rabaey, K.; Read, S.T.; Clauwaert, P.; Frguia, S.; Bond, P.L.; Blackall, L.L.; Keller, J. Cathodic oxygen reduction catalyzed by bacteria in microbial fuel cells. ISME J. 2008, 2, 519–527. [Google Scholar] [CrossRef]
- Qiu, B.B.; Shi, J.C.; Hu, W.; Gao, J.; Li, S.T.; Chu, H.Q. Construction of hydrothermal liquefaction system for efficient production of biomass-derived furfural: Solvents, catalysts and mechanisms. Fuel 2023, 354, 129278. [Google Scholar] [CrossRef]
- Wu, J.Y.; Lay, C.H.; Chia, S.R.; Show, P.L.; Hsieh, P.H.; Chen, C.C. Economic potential of bioremediation using immobilized microalgae-based microbial fuel cells. Clean Technol. Envir. 2021, 23, 2251–2264. [Google Scholar] [CrossRef]
- Silva-Palaciosa, F.; Salvador-Salinasa, A.; Quezada-Alvarezb, M.A.; Rodriguez-Yupanquia, M.; Segundoc, R.F.; Rennyc, N.N.; Cabanillas-Chirinos, L. Bioelectricity generation through Microbial Fuel Cells using Serratia fonticola bacteria and Rhodotorula glutinis yeast. Energy Rep. 2023, 9, 295–301. [Google Scholar] [CrossRef]
- Mukimin, A.; Vistanty, H. Low carbon development based on microbial fuel cells as electrical generation and wastewater treatment unit. Renew. Energ. Focus 2023, 44, 132–138. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, X.; Lin, J.; Han, S.; Lei, L. Azo dye treatment with simultaneous electricity production in an anaerobic-aerobic sequential reactor and microbial fuel cell coupled system. Bioresour. Technol. 2010, 101, 4440–4445. [Google Scholar] [CrossRef]
- Wang, K.; Li, W.; Gong, X.; Li, X.; Liu, W.; He, C.; Wang, Z.; Nguyen, Q.; Chen, C.; Wang, J. International Biodeterioration & Biodegradation Biological pretreatment of tannery wastewater using a full-scale hydrolysis acidi fi cation system. Int. Biodeterior. Biodegrad. 2014, 95, 41–45. [Google Scholar]
- Wang, X.; Yue, X.; Guo, Q. Production of electricity during wastewater treatment using fluidized-bed microbial fuel cells. Chem. Eng. Technol. 2014, 37, 703–708. [Google Scholar] [CrossRef]


| Parameter (g/L) | Furfural Wastewater | After Treatment by MFC |
|---|---|---|
| COD | 16.31 ± 0.01 | 1.79 ± 0.06 |
| BOD | 5.07 ± 0.01 | 3.73 ± 0.12 |
| TN | 1.61 ± 0.01 | 0.51 ± 0.05 |
| TP | (31.90 ± 0.01) × 10−3 | (0.16 ± 0.01) × 10−3 |
| DO | (0.12 ± 0.01) × 10−3 | (1.86 ± 0.03) × 10−3 |
| pH | 2.15 ± 0.02 | 6.10 ± 0.07 |
| T, °C | 55.00 ± 0.01 | 30.00 ± 2.2 |
| H2SO4 | 8.27 ± 0.01 | 0.82 ± 0.17 |
| CH3COOH | 10.38 ± 0.14 | 1.60 ± 0.08 |
| hemicellulose | 29.73 ± 0.68 | 21.99 ± 0.50 |
| cellulose | 34.90 ± 0.46 | 30.71 ± 0.41 |
| lignin | 8.15 ± 0.22 | 7.50 ± 0.20 |
| furfural | 6.89 ± 0.11 | 0.32 ± 0.01 |
| BOD/COD | 0.31 ± 0.01 | 0.48 ± 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, H.; Quan, Y.; Yin, Z.; Yin, C.; Fu, Y. Bioelectrochemical Purification of Biomass Polymer Derived Furfural Wastewater and Its Electric Energy Recovery. Polymers 2023, 15, 3422. https://doi.org/10.3390/polym15163422
Tian H, Quan Y, Yin Z, Yin C, Fu Y. Bioelectrochemical Purification of Biomass Polymer Derived Furfural Wastewater and Its Electric Energy Recovery. Polymers. 2023; 15(16):3422. https://doi.org/10.3390/polym15163422
Chicago/Turabian StyleTian, Hailing, Yue Quan, Zhenhao Yin, Chengri Yin, and Yu Fu. 2023. "Bioelectrochemical Purification of Biomass Polymer Derived Furfural Wastewater and Its Electric Energy Recovery" Polymers 15, no. 16: 3422. https://doi.org/10.3390/polym15163422






