Laboratory Studies about Microplastic Aging and Its Effects on the Adsorption of Chlorpyrifos
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Solutions
2.2. Water Samples Used in the Aging Process Systems
2.3. Procedure
2.3.1. MP Aging Process Laboratory Setup
2.3.2. MP Characterization: FTIR, Raman and Thermogravimetry Analysis
2.3.3. Optimization of the Liquid-Liquid Extraction of Chlorpyrifos from Water-MP System
2.3.4. GC Analysis
2.3.5. Preliminary Batch Adsorption Studies for CPF/Pristine MP
2.3.6. Batch Adsorption Experiments
3. Results and Discussion
3.1. Water-MP Aging Systems
3.1.1. Characterization by Optical Microscopy
3.1.2. Characterization by FTIR Analysis
3.1.3. Characterization by Raman
3.1.4. Characterization by TGA
3.2. Liquid-Liquid Extraction of Chlorpyrifos with Different Solvents
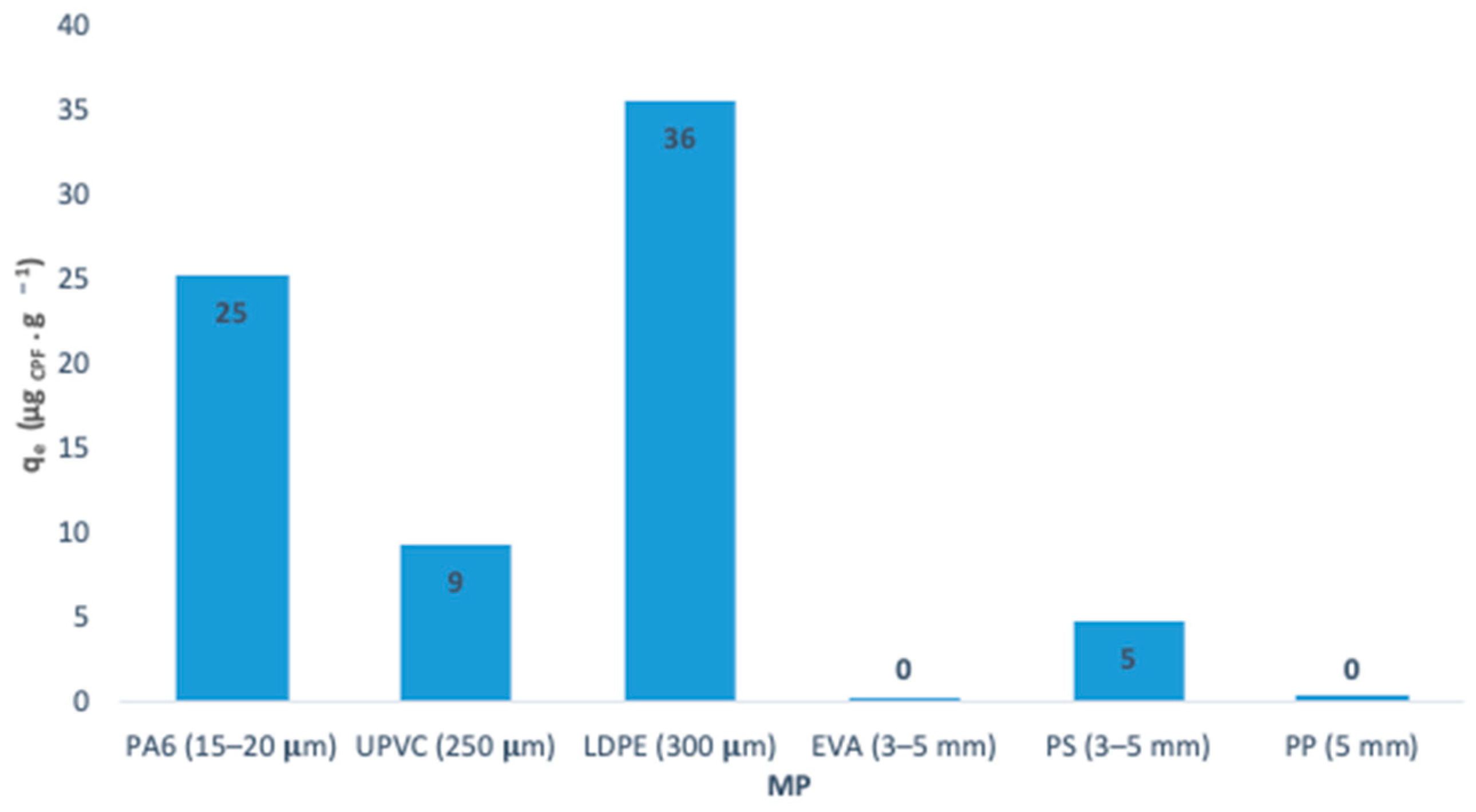
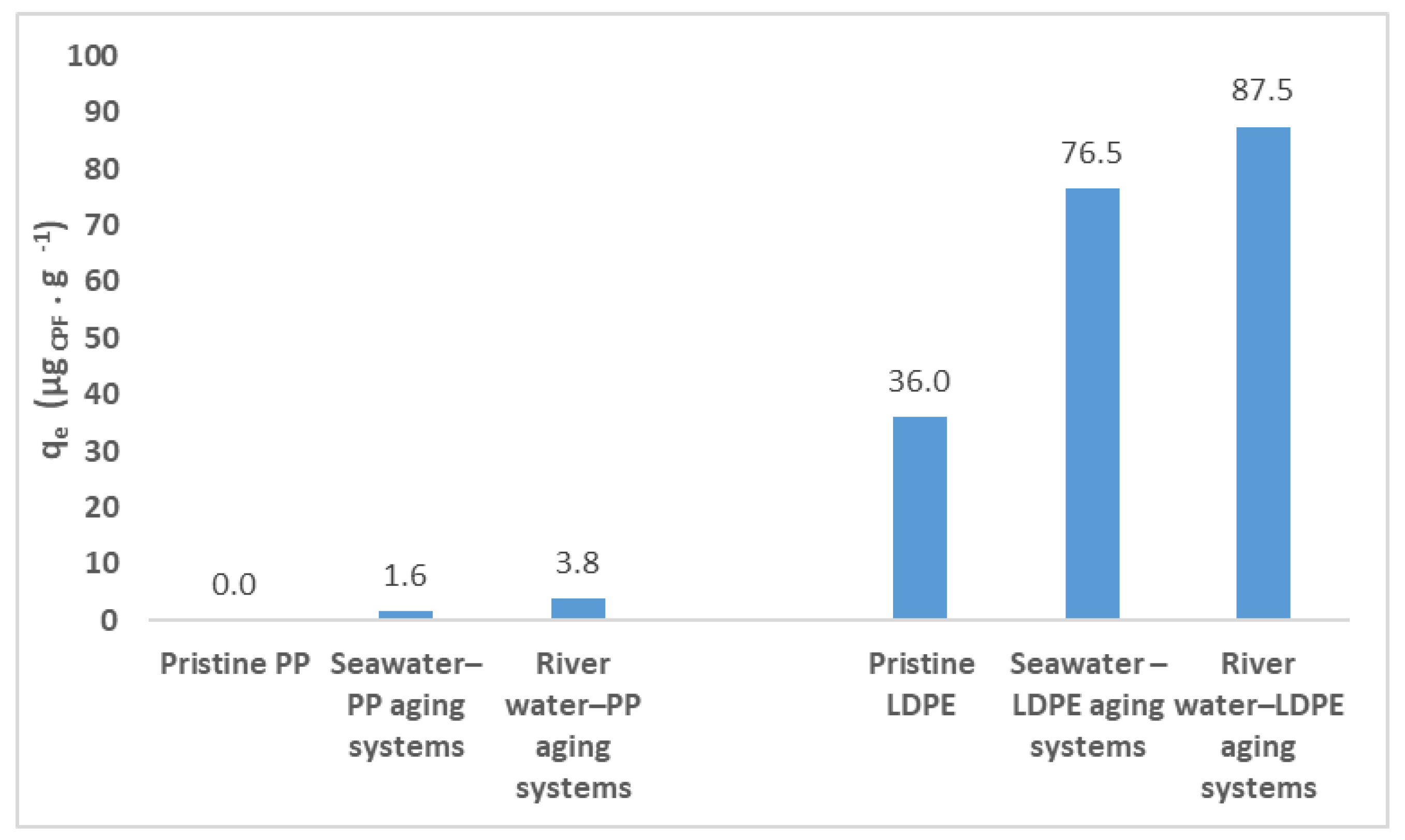
3.3. Batch Adsorption Studies for CPF/Pristine MP Systems
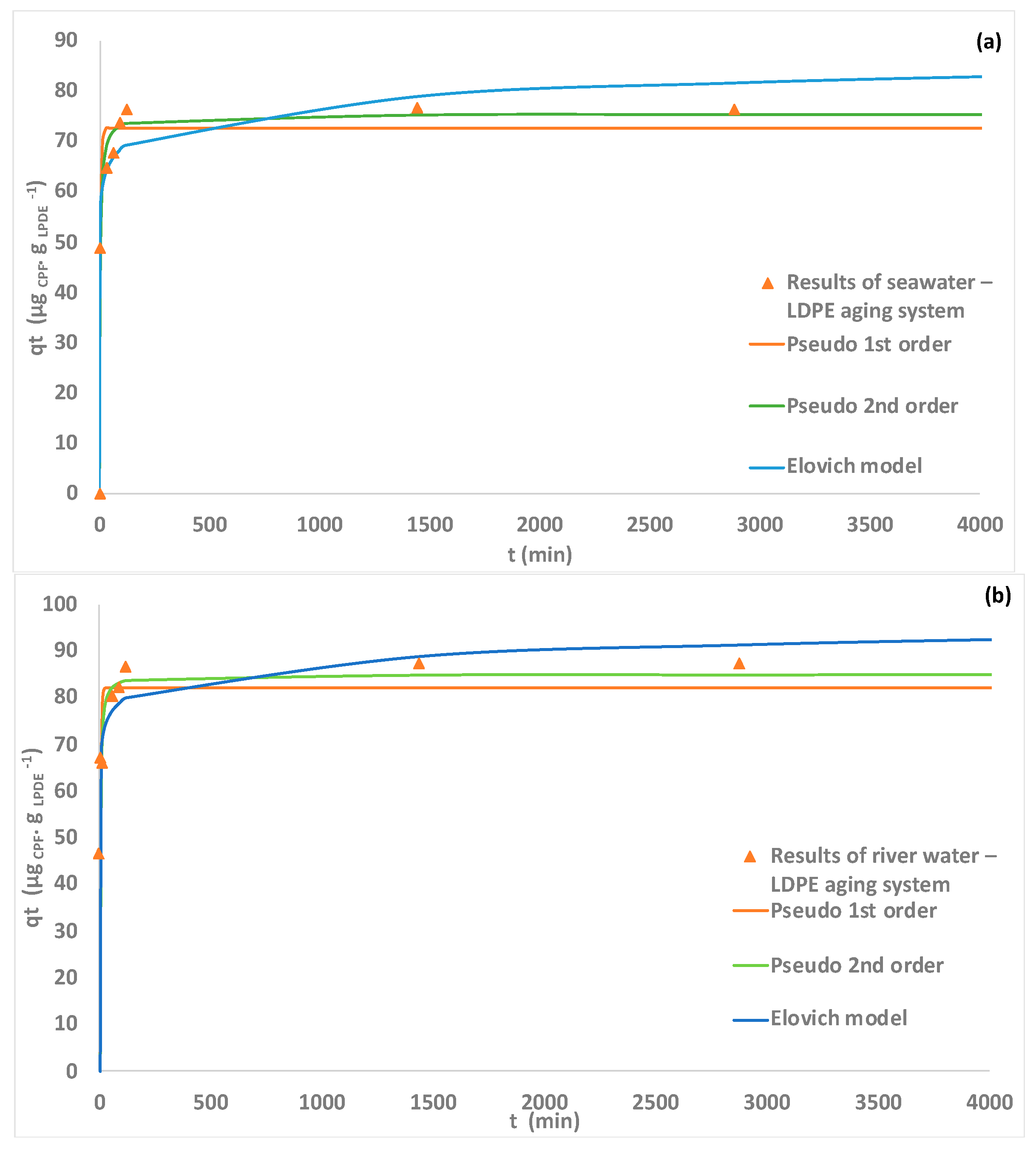
3.4. Adsorption Kinetic Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, J.R.; Zheng, R.; Tang, J.; Sun, H.J.; Wang, J. A mini-review on building insulation materials from perspective of plastic pollution: Current issues and natural fibres as a possible solution. J. Hazard. Mater. 2022, 438, 129449. [Google Scholar] [CrossRef] [PubMed]
- Horton, A.A. Plastic pollution: When do we know enough? J. Hazard. Mater. 2022, 422, 126885. [Google Scholar] [CrossRef] [PubMed]
- Filho, W.L.; Salvia, A.L.; Bonoli, A.; Saari, U.A.; Voronova, V.; Kloga, M.; Kumbhar, S.S.; Olszewski, K.; De Quevedo, D.M.; Barbir, J. An assessment of attitudes towards plastics and bioplastics in Europe. Sci. Total Environ. 2021, 755 Pt 1, 142732. [Google Scholar] [CrossRef]
- Hopewell, J.; Dvorak, R.; Kosior, E. Plastics recycling: Challenges and opportunities. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 2115–2126. [Google Scholar] [CrossRef] [PubMed]
- Phelna, A.; Meissner, K.; Humphrey, J.; Ross, H. Plastic pollution and packaging: Corporate commitments and actions from the food and beverage sector. J. Clean. Prod. 2022, 331, 129827. [Google Scholar] [CrossRef]
- Chen, Y.; Awasthi, A.K.; Wei, F.; Tan, Q.; Li, J. Single-use plastics: Production, usage, disposal, and adverse impacts. Sci. Total Environ. 2021, 752, 141772. [Google Scholar] [CrossRef] [PubMed]
- Ferdous, W.; Manalo, A.; Siddique, R.; Mendis, P.; Zhuge, Y.; Wong, H.S.; Lokuge, W.; Aravinthan, T.; Schubel, P. Recycling of landfill wastes (tyres, plastics and glass) in construction—A review on global waste generation, performance, application and future opportunities. Resour. Conserv. Recycl. 2021, 173, 105745. [Google Scholar] [CrossRef]
- Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T.S. Microplastics as contaminants in the marine environment: A review. Mar. Pollut. Bull. 2011, 62, 2588–2597. [Google Scholar] [CrossRef]
- Hasan Anik, A.; Hossain, S.; Alam, M.; Sultan, M.B.; Hasnine, M.D.T.; Rahman, M.M. Microplastics pollution: A comprehensive review on the sources, fates, effects, and potential remediation. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100530. [Google Scholar] [CrossRef]
- Wang, F.; Wong, C.S.; Chen, D.; Lu, X.; Wang, F.; Zeng, E.Y. Interaction of toxic chemicals with microplastics: A critical review. Water Res. 2018, 139, 208–219. [Google Scholar] [CrossRef]
- Hartmann, N.B.; Huffer, T.; Thompson, R.C.; Hassellov, M.; Verschoor, A.; Daugaard, A.E.; Rist, S.; Karlsson, T.; Brennholt, N.; Cole, M.; et al. Are We Speaking the Same Language? Recommendations for a Definition and Categorization Framework for Plastic Debris. Environ. Sci. Technol. 2019, 53, 1039–1047. [Google Scholar] [PubMed]
- Wang, J.; Liu, X.; Li, Y.; Powell, T.; Wang, X.; Wang, G.; Zhang, P. Microplastics as contaminants in the soil environment: A mini-review. Sci. Total Environ. 2019, 691, 848–857. [Google Scholar] [CrossRef] [PubMed]
- de Souza Machado, A.A.; Kloas, W.; Zarfl, C.; Hempel, S.; Rillig, M.C. Microplastics as an emerging threat to terrestrial ecosystems. Glob. Chang. Biol. 2018, 24, 1405–1416. [Google Scholar] [CrossRef] [PubMed]
- Scherer, C.; Weber, A.; Stock, F.; Vurusic, S.; Egerci, H.; Kochleus, C.; Arendt, N.; Foeldi, C.; Dierkes, G.; Wagner, M.; et al. Comparative assessment of microplastics in water and sediment of a large European river. Sci. Total Environ. 2020, 738, 139866. [Google Scholar] [CrossRef] [PubMed]
- Rakib, M.R.J.; Sarker, A.; Ram, K.; Uddin, M.G.; Walker, T.R.; Chowdhury, T.; Uddin, J.; Khandaker, M.U.; Rahman, M.M.; Idris, A.M. Microplastic Toxicity in Aquatic Organisms and Aquatic Ecosystems: A Review. Water Air Soil. Pollut. 2023, 234, 52. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, C.; Wang, F.; Sun, Q. Behavior and mechanism of atrazine adsorption on pristine and aged microplastics in the aquatic environment: Kinetic and thermodynamic studies. Chemosphere 2022, 292, 133425. [Google Scholar] [CrossRef]
- Fernandez, B.; Campillo, J.A.; Chaves-Pozo, E.; Bellas, J.; Leon, V.M.; Albentosa, M. Comparative role of microplastics and microalgae as vectors for chlorpyrifos bioacumulation and related physiological and immune effects in mussels. Sci. Total Environ. 2022, 807 Pt 3, 150983. [Google Scholar] [CrossRef]
- Yin, R.; Ge, H.; Chen, H.; Du, J.; Sun, Z.; Tan, H.; Wang, S. Sensitive and rapid detection of trace microplastics concentrated through Au-nanoparticle-decorated sponge on the basis of surface-enhanced Raman spectroscopy. Environ. Adv. 2021, 5, 100096. [Google Scholar] [CrossRef]
- Aves, A.R.; Revell, L.E.; Gaw, S.; Ruffell, H.; Schuddeboom, A.; Wotherspoon, N.E.; LaRue, M.; McDonald, A.J. First evidence of microplastics in Antarctic snow. Cryosphere 2022, 16, 2127–2145. [Google Scholar] [CrossRef]
- Castelvetro, V.; Corti, A.; Biale, G.; Ceccarini, A.; Degano, I.; La Nasa, J.; Lomonaco, T.; Manariti, A.; Manco, E.; Modugno, F.; et al. New methodologies for the detection, identification, and quantification of microplastics and their environmental degradation by-products. Environ. Sci. Pollut. Res. Int. 2021, 28, 46764–46780. [Google Scholar] [CrossRef]
- Wu, M.; Yang, C.; Du, C.; Liu, H. Microplastics in waters and soils: Occurrence, analytical methods and ecotoxicological effects. Ecotoxicol. Environ. Saf. 2020, 202, 110910. [Google Scholar] [CrossRef]
- Hurley, R.R.; Nizzetto, L. Fate and occurrence of micro(nano)plastics in soils: Knowledge gaps and possible risks. Curr. Opin. Environ. Sci. Health 2018, 1, 6–11. [Google Scholar] [CrossRef]
- Kwon, J.H.; Kim, J.W.; Pham, T.D.; Tarafdar, A.; Hong, S.; Chun, S.H.; Lee, S.H.; Kang, D.Y.; Kim, J.Y.; Kim, S.B.; et al. Microplastics in Food: A Review on Analytical Methods and Challenges. Int. J. Environ. Res. Public. Health 2020, 17, 6710. [Google Scholar] [CrossRef] [PubMed]
- Zurier, H.S.; Goddard, J.M. Biodegradation of microplastics in food and agriculture. Curr. Opin. Food Sci. 2021, 37, 37–44. [Google Scholar] [CrossRef]
- Leslie, H.A.; van Velzen, M.J.M.; Brandsma, S.H.; Vethaak, A.D.; Garcia-Vallejo, J.J.; Lamoree, M.H. Discovery and quantification of plastic particle pollution in human blood. Environ. Int. 2022, 163, 107199. [Google Scholar] [CrossRef] [PubMed]
- Jenner, L.C.; Rotchell, J.M.; Bennett, R.T.; Cowen, M.; Tentzeris, V.; Sadofsky, L.R. Detection of microplastics in human lung tissue using muFTIR spectroscopy. Sci. Total Environ. 2022, 831, 154907. [Google Scholar] [CrossRef]
- Daud, A.; Astuti RD, P.; Basri, K. Detection of Exposure to Microplastics in Humans: A Systematic Review. Open Access Maced. J. Med. Sci. 2021, 9, 275–280. [Google Scholar]
- Ragusa, A.; Svelato, A.; Santacroce, C.; Catalano, P.; Notarstefano, V.; Carnevali, O.; Papa, F.; Rongioletti, M.C.A.; Baiocco, F.; Draghi, S.; et al. Plasticenta: First evidence of microplastics in human placenta. Environ. Int. 2021, 146, 106274. [Google Scholar] [CrossRef]
- Bessa, F.; Barria, P.; Neto, J.M.; Frias, J.; Otero, V.; Sobral, P.; Marques, J.C. Occurrence of microplastics in commercial fish from a natural estuarine environment. Mar. Pollut. Bull. 2018, 128, 575–584. [Google Scholar] [CrossRef]
- Martinho, S.D.; Fernandes, V.C.; Figueiredo, S.A.; Delerue-Matos, C. Study of the Potential Accumulation of the Pesticide Alpha-Endosulfan by Microplastics in Water Systems. Polymers 2022, 14, 3645. [Google Scholar] [CrossRef]
- Selonen, S.; Dolar, A.; Kokalj, A.J.; Sackey, L.N.A.; Skalar, T.; Fernandes, V.C.; Rede, D.; Delerue-Matos, C.; Hurley, R.; Nizzetto, L.; et al. Exploring the impacts of microplastics and associated chemicals in the terrestrial environment—Exposure of soil invertebrates to tire particles. Environ. Res. 2021, 201, 111495. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, X.; Leng, Y.; Wang, J. Defense responses in earthworms (Eisenia fetida) exposed to low-density polyethylene microplastics in soils. Ecotoxicol. Environ. Saf. 2020, 187, 109788. [Google Scholar] [CrossRef] [PubMed]
- de Souza Machado, A.A.; Lau, C.W.; Kloas, W.; Bergmann, J.; Bachelier, J.B.; Faltin, E.; Becker, R.; Görlich, A.S.; Rillig, M.C. Microplastics Can Change Soil Properties and Affect Plant Performance. Environ. Sci. Technol. 2019, 53, 6044–6052. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Xu, S.; Zhou, Q.; Li, H.; Fu, L.; Tang, J.; Wang, Y.; Peng, X.; Xu, Y.; Du, X. A review of microplastics in the aquatic environmental: Distribution, transport, ecotoxicology, and toxicological mechanisms. Environ. Sci. Pollut. Res. Int. 2020, 27, 11494–11505. [Google Scholar] [CrossRef] [PubMed]
- Martinho, S.D.; Fernandes, V.C.; Figueiredo, S.A.; Delerue-Matos, C. Microplastic Pollution Focused on Sources, Distribution, Contaminant Interactions, Analytical Methods, and Wastewater Removal Strategies: A Review. Int. J. Environ. Res. Public. Health 2022, 19, 5610. [Google Scholar] [CrossRef]
- Rahman, A.; Sarkar, A.; Yadav, O.P.; Achari, G.; Slobodnik, J. Potential human health risks due to environmental exposure to nano- and microplastics and knowledge gaps: A scoping review. Sci. Total Environ. 2021, 757, 143872. [Google Scholar] [CrossRef]
- Prata, J.C.; da Costa, J.P.; Lopes, I.; Duarte, A.C.; Rocha-Santos, T. Environmental exposure to microplastics: An overview on possible human health effects. Sci. Total Environ. 2020, 702, 134455. [Google Scholar] [CrossRef]
- Liu, P.; Lu, K.; Li, J.; Wu, X.; Qian, L.; Wang, M.; Gao, S. Effect of aging on adsorption behavior of polystyrene microplastics for pharmaceuticals: Adsorption mechanism and role of aging intermediates. J. Hazard. Mater. 2020, 384, 121193. [Google Scholar] [CrossRef]
- Huang, Y.; Ding, J.; Zhang, G.; Liu, S.; Zou, H.; Wang, Z.; Zhu, W.; Geng, J. Interactive effects of microplastics and selected pharmaceuticals on red tilapia: Role of microplastic aging. Sci. Total Environ. 2021, 752, 142256. [Google Scholar] [CrossRef]
- Wang, T.; Yu, C.; Chu, Q.; Wang, F.; Lan, T.; Wang, J. Adsorption behavior and mechanism of five pesticides on microplastics from agricultural polyethylene films. Chemosphere 2020, 244, 125491. [Google Scholar] [CrossRef]
- Savino, I.; Campanale, C.; Trotti, P.; Massarelli, C.; Corriero, G.; Uricchio, V.F. Effects and Impacts of Different Oxidative Digestion Treatments on Virgin and Aged Microplastic Particles. Polymers 2022, 14, 1958. [Google Scholar] [CrossRef]
- Rodrigues, J.P.; Duarte, A.C.; Santos-Echeandía, J.; Rocha-Santos, T. Significance of interactions between microplastics and POPs in the marine environment: A critical overview. TrAC Trends Anal. Chem. 2019, 111, 252–260. [Google Scholar] [CrossRef]
- Gao, X.; Hassan, I.; Peng, Y.; Huo, S.; Ling, L. Behaviors and influencing factors of the heavy metals adsorption onto microplastics: A review. J. Clean. Prod. 2021, 319, 128777. [Google Scholar] [CrossRef]
- Hu, J.; Lim, F.Y.; Hu, J. Characteristics and behaviors of microplastics undergoing photoaging and Advanced Oxidation Processes (AOPs) initiated aging. Water Res. 2023, 232, 119628. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Jiang, B.; Guo, J.; Sun, C.; Shi, C.; Huang, S.; Liu, W.; Wu, C.; Zhang, Y. Aging Process of Microplastics in the Aquatic Environments: Aging Pathway, Characteristic Change, Compound Effect, and Environmentally Persistent Free Radicals Formation. Water 2022, 14, 3515. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, J.; Huang, W.; He, Y. Laboratory simulated aging methods, mechanisms and characteristic changes of microplastics: A review. Chemosphere 2023, 315, 137744. [Google Scholar] [CrossRef]
- Brennecke, D.; Duarte, B.; Paiva, F.; Caçador, I.; Canning-Clode, J. Microplastics as vector for heavy metal contamination from the marine environment. Estuarine Coast. Shelf Sci. 2016, 178, 189–195. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Y.; Wangjin, X.; Wang, Y.; Meng, G.; Chen, Y. The adsorption behavior of metals in aqueous solution by microplastics effected by UV radiation. J. Environ. Sci. 2020, 87, 272–280. [Google Scholar] [CrossRef]
- Chen, X.; Chen, C.E.; Guo, X.; Sweetman, A.J. Sorption and desorption of bisphenols on commercial plastics and the effect of UV aging. Chemosphere 2023, 310, 136867. [Google Scholar] [CrossRef]
- Zhou, B.; Wang, J.; Zhang, H.; Shi, H.; Fei, Y.; Huang, S.; Tong, Y.; Wen, D.; Luo, Y.; Barcelo, D. Microplastics in agricultural soils on the coastal plain of Hangzhou Bay, east China: Multiple sources other than plastic mulching film. J. Hazard. Mater. 2020, 388, 121814. [Google Scholar] [CrossRef]
- Lan, T.; Wang, T.; Cao, F.; Yu, C.; Chu, Q.; Wang, F. A comparative study on the adsorption behavior of pesticides by pristine and aged microplastics from agricultural polyethylene soil films. Ecotoxicol. Environ. Saf. 2021, 209, 111781. [Google Scholar] [CrossRef] [PubMed]
- Ccanccapa, A.; Masiá, A.; Navarro-Ortega, A.; Picó, Y.; Barceló, D. Pesticides in the Ebro River basin: Occurrence and risk assessment. Environ. Pollut. 2016, 211, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Dolar, A.; Selonen, S.; van Gestel, C.A.M.; Perc, V.; Drobne, D.; Kokalj, A.J. Microplastics, chlorpyrifos and their mixtures modulate immune processes in the terrestrial crustacean Porcellio scaber. Sci. Total Environ. 2021, 772, 144900. [Google Scholar] [CrossRef] [PubMed]
- Bellas, J.; Gil, I. Polyethylene microplastics increase the toxicity of chlorpyrifos to the marine copepod Acartia tonsa. Environ. Pollut. 2020, 260, 114059. [Google Scholar] [CrossRef] [PubMed]
- Rial, D.; León, V.M.; Bellas, J. Integrative assessment of coastal marine pollution in the Bay of Santander and the Upper Galician Rias. J. Sea Res. 2017, 130, 239–247. [Google Scholar] [CrossRef]
- Leon, V.M.; Garcia, I.; Gonzalez, E.; Samper, R.; Fernandez-Gonzalez, V.; Muniategui-Lorenzo, S. Potential transfer of organic pollutants from littoral plastics debris to the marine environment. Environ. Pollut. 2018, 236, 442–453. [Google Scholar] [CrossRef]
- Fernandes, V.C.; Freitas, M.; Pacheco, J.P.G.; Oliveira, J.M.; Domingues, V.F.; Delerue-Matos, C. Magnetic dispersive micro solid-phase extraction and gas chromatography determination of organophosphorus pesticides in strawberries. J. Chromatogr. A 2018, 1566, 1–12. [Google Scholar] [CrossRef]
- Ho, Y.S. Citation review of Lagergren kinetic rate equation on adsorption reactions. Scientometrics 2004, 59, 171–177. [Google Scholar]
- Lagergeren, S. About theory of so-called adsorption of soluble substances. Kongl Vetensk. Acad. Handl. 1898, 24, 1–39. [Google Scholar]
- Low, M.J.D. Kinetics of chemisorption of gases on solids. Chem. Rev. 1960, 60, 267–312. [Google Scholar] [CrossRef]
- Liu, G.; Zhu, Z.; Yang, Y.; Sun, Y.; Yu, F.; Ma, J. Sorption behavior and mechanism of hydrophilic organic chemicals to virgin and aged microplastics in freshwater and seawater. Environ. Pollut. 2019, 246, 26–33. [Google Scholar] [CrossRef] [PubMed]
- You, H.; Huang, B.; Cao, C.; Liu, X.; Sun, X.; Xiao, L.; Qiu, J.; Luo, Y.; Qian, Q.; Chen, Q. Adsorption-desorption behavior of methylene blue onto aged polyethylene microplastics in aqueous environments. Mar. Pollut. Bull. 2021, 167, 112287. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Wang, J.; Peng, J.; Wu, Z.; Tan, X. Observation of the degradation of three types of plastic pellets exposed to UV irradiation in three different environments. Sci. Total Environ. 2018, 628–629, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wang, Q.; Zhang, C.; Zhang, J.; Dong, Z.; Xu, Q. Aging simulation of thin-film plastics in different environments to examine the formation of microplastic. Water Res. 2021, 202, 117462. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Wu, X.; Huang, H.; Wang, H.; Shi, Y.; Gao, S. Simulation of natural aging property of microplastics in Yangtze River water samples via a rooftop exposure protocol. Sci. Total Environ. 2021, 785, 147265. [Google Scholar] [CrossRef]
- Meng, J.; Xu, B.; Liu, F.; Li, W.; Sy, N.; Zhou, X.; Yan, B. Effects of chemical and natural ageing on the release of potentially toxic metal additives in commercial PVC microplastics. Chemosphere 2021, 283, 131274. [Google Scholar] [CrossRef]
- Woo, H.; Seo, K.; Choi, Y.; Kim, J.; Tanaka, M.; Lee, K.; Choi, J. Methods of Analyzing Microsized Plastics in the Environment. Appl. Sci. 2021, 11, 10640. [Google Scholar] [CrossRef]
- Andrade, J.; Fernández-González, V.; López-Mahía, P.; Muniategui, S. A low-cost system to simulate environmental microplastic weathering. Mar. Pollut. Bull. 2019, 149, 10640. [Google Scholar] [CrossRef]
- Gardette, M.; Perthue, A.; Gardette, J.-L.; Janecska, T.; Földes, E.; Pukánszky, B.; Therias, S. Photo- and thermal-oxidation of polyethylene: Comparison of mechanisms and influence of unsaturation content. Polym. Degrad. Stab. 2013, 98, 2383–2390. [Google Scholar] [CrossRef]
- Gao, L.; Fu, D.; Zhao, J.; Wu, W.; Wang, Z.; Su, Y.; Peng, L. Microplastics aged in various environmental media exhibited strong sorption to heavy metals in seawater. Mar. Pollut. Bull. 2021, 169, 112480. [Google Scholar] [CrossRef]
- Sun, Y.; Yuan, J.; Zhou, T.; Zhao, Y.; Yu, F.; Ma, J. Laboratory simulation of microplastics weathering and its adsorption behaviors in an aqueous environment: A systematic review. Environ. Pollut. 2020, 265 Pt B, 114864. [Google Scholar] [CrossRef]
- Sorasan, C.; Edo, C.; Gonzalez-Pleiter, M.; Fernandez-Pinas, F.; Leganes, F.; Rodriguez, A.; Rosal, R. Ageing and fragmentation of marine microplastics. Sci. Total Environ. 2022, 827, 154438. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Shi, Y.; Wu, X.; Wang, H.; Huang, H.; Guo, X.; Gao, S. Review of the artificially-accelerated aging technology and ecological risk of microplastics. Sci. Total Environ. 2021, 768, 144969. [Google Scholar] [CrossRef] [PubMed]
- Kappler, A.; Fischer, D.; Oberbeckmann, S.; Schernewski, G.; Labrenz, M.; Eichhorn, K.J.; Voit, B. Analysis of environmental microplastics by vibrational microspectroscopy: FTIR, Raman or both? Anal. Bioanal. Chem. 2016, 408, 8377–8391. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Liu, P.; Wang, H.; Huang, H.; Shi, Y.; Yang, C.; Gao, S. Photo aging of polypropylene microplastics in estuary water and coastal seawater: Important role of chlorine ion. Water Res. 2021, 202, 117396. [Google Scholar] [CrossRef] [PubMed]
- Rytelewska, S.; Dąbrowska, A. The Raman Spectroscopy Approach to Different Freshwater Microplastics and Quantitative Characterization of Polyethylene Aged in the Environment. Microplastics 2022, 1, 263–282. [Google Scholar] [CrossRef]
- Hiejima, Y.; Kida, T.; Takeda, K.; Igarashi, T.; Nitta, K.-H. Microscopic structural changes during photodegradation of low-density polyethylene detected by Raman spectroscopy. Polym. Degrade. Stab. 2018, 150, 67–72. [Google Scholar] [CrossRef]
- Marica, I.; Aluas, M.; Pinzaru, S.C. Raman technology application for plastic waste management aligned with FAIR principle to support the forthcoming plastic and environment initiatives. Waste Manag. 2022, 144, 479–489. [Google Scholar] [CrossRef]
- Imhof, H.K.; Laforsch, C.; Wiesheu, A.C.; Schmid, J.; Anger, P.M.; Niessner, R.; Ivleva, N.P. Pigments and plastic in limnetic ecosystems: A qualitative and quantitative study on microparticles of different size classes. Water Res. 2016, 98, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Dalai, S.; Wenxiu, C. Radiation effects on LDPE/EVA blends. J. Appl. Polym. Sci. 2002, 86, 1296–1302. [Google Scholar] [CrossRef]
- Zhao, M.; Yi, D.; Camino, G.; Frache, A.; Yang, R. Interdigitated crystalline MMT–MCA in polyamide 6. RSC Adv. 2017, 7, 861–869. [Google Scholar] [CrossRef]
- Miandad, R.; Nizami, A.S.; Rehan, M.; Barakat, M.A.; Khan, M.I.; Mustafa, A.; Ismail, I.M.I.; Murphy, J.D. Influence of temperature and reaction time on the conversion of polystyrene waste to pyrolysis liquid oil. Waste Manag. 2016, 58, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Armenta, J.L.; Salazar-Cruz, B.A.; Espindola-Flores, A.C.; Villarreal-Lucio, D.S.; De León-Almazán, C.M.; Estrada-Martinez, J. Thermal and Thermomechanical Characterization of Polypropylene-Seed Shell Particles Composites. Appl. Sci. 2022, 12, 8336. [Google Scholar] [CrossRef]
- Ainali, N.M.; Bikiaris, D.N.; Lambropoulou, D.A. Aging effects on low- and high-density polyethylene, polypropylene and polystyrene under UV irradiation: An insight into decomposition mechanism by Py-GC/MS for microplastic analysis. J. Anal. Appl. Pyrolysis 2021, 158, 105207. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, M.; Sha, W.; Wang, Y.; Hao, H.; Dou, Y.; Li, Y. Sorption Behavior and Mechanisms of Organic Contaminants to Nano and Microplastics. Molecules 2020, 25, 1827. [Google Scholar] [CrossRef]
- Concha-Grana, E.; Moscoso-Perez, C.M.; Lopez-Mahia, P.; Muniategui-Lorenzo, S. Adsorption of pesticides and personal care products on pristine and weathered microplastics in the marine environment. Comparison between bio-based and conventional plastics. Sci. Total Environ. 2022, 848, 157703. [Google Scholar] [CrossRef]
- Garrido, S.; Linares, M.; Campillo, J.A.; Albentosa, M. Effect of microplastics on the toxicity of chlorpyrifos to the microalgae Isochrysis galbana, clone t-ISO. Ecotoxicol. Environ. Saf. 2019, 173, 103–109. [Google Scholar] [CrossRef]
- Ziccardi, L.M.; Edgington, A.; Hentz, K.; Kulacki, K.J.; Driscoll, S.K. Microplastics as vectors for bioaccumulation of hydrophobic organic chemicals in the marine environment: A state-of-the-science review. Environ. Toxicol. Chem. 2016, 35, 1667–1676. [Google Scholar] [CrossRef]
- Rodríguez-Seijo, A.; Santos, B.; da Silva, E.F.; Cachada, A.; Pereira, R. Low-density polyethylene microplastics as a source and carriers of agrochemicals to soil and earthworms. Environ. Chem. 2019, 16, 1667–1676. [Google Scholar] [CrossRef]
- Karapanagioti, H.K.; Werner, D. Sorption of Hydrophobic Organic Compounds to Plastics in the Marine Environment: Sorption and Desorption Kinetics. In Hazardous Chemicals Associated with Plastics in the Marine Environment; Spinger: Berlin/Heidelberg, Germany, 2018; pp. 205–219. [Google Scholar]
- Liu, X.; Xu, J.; Zhao, Y.; Shi, H.; Huang, C.H. Hydrophobic sorption behaviors of 17beta-Estradiol on environmental microplastics. Chemosphere 2019, 226, 726–735. [Google Scholar] [CrossRef]
- Pascall, M.A.; Zabik, M.E.; Zabik, M.J.; Hernandez, R.J. Uptake of Polychlorinated Biphenyls (PCBs) from an Aqueous Medium by Polyethylene, Polyvinyl Chloride, and Polystyrene Films. J. Agric. Food Chem. 2005, 53, 164–169. [Google Scholar] [CrossRef] [PubMed]
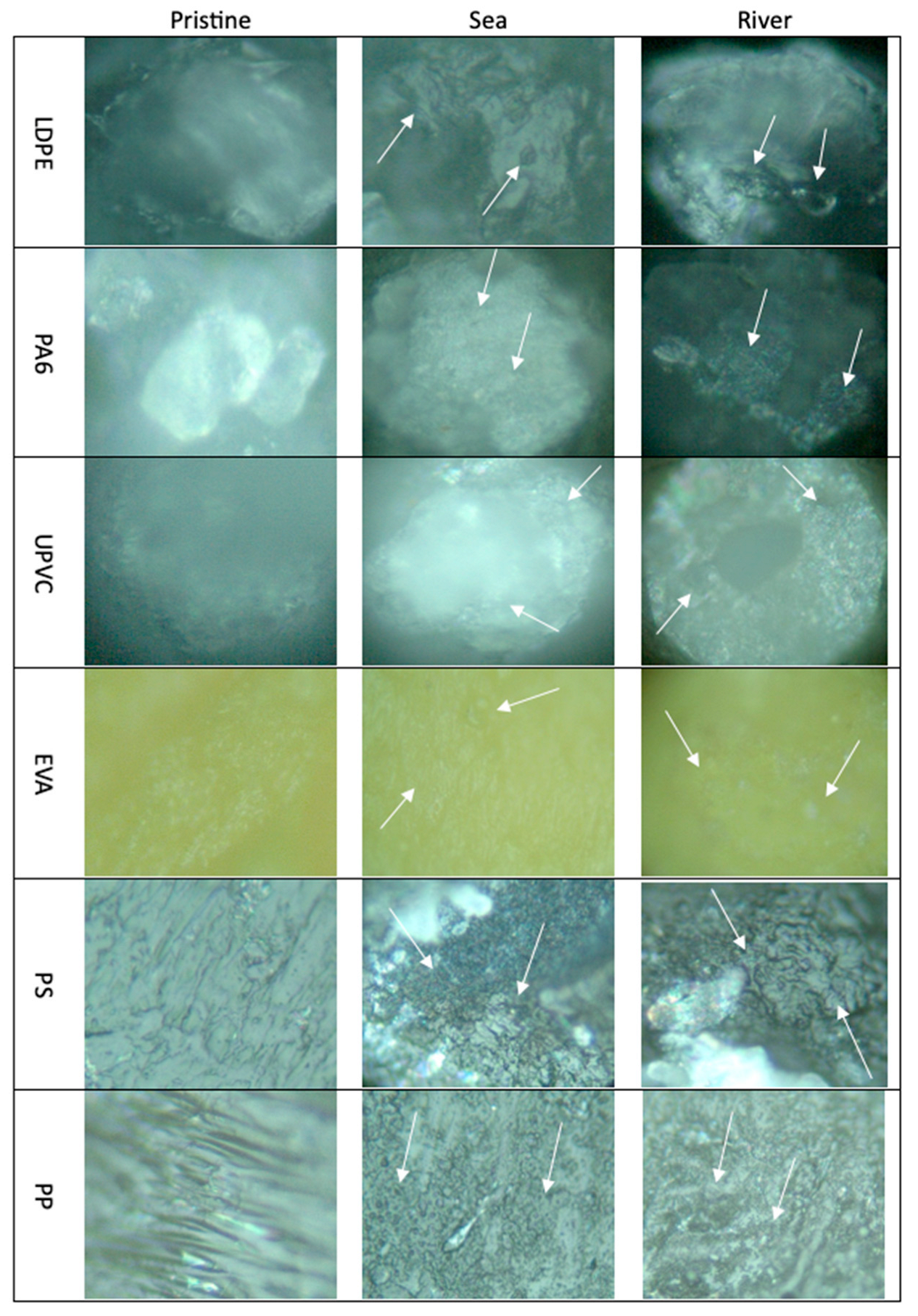
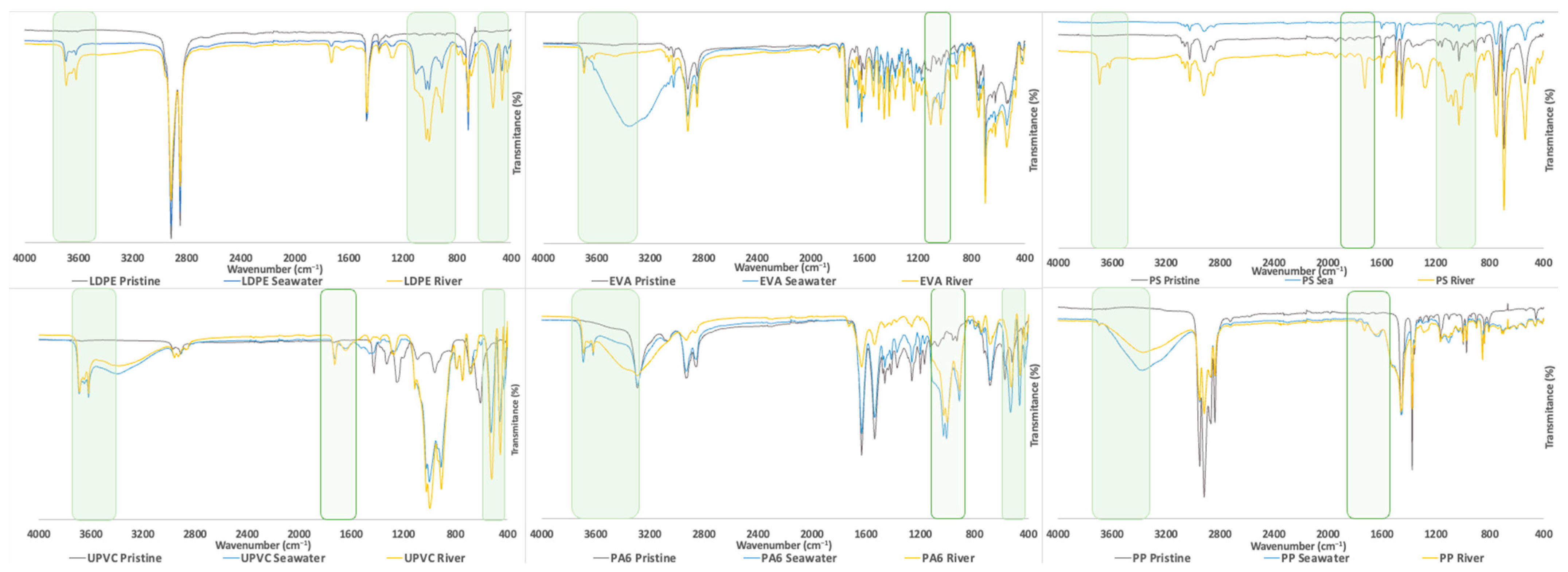
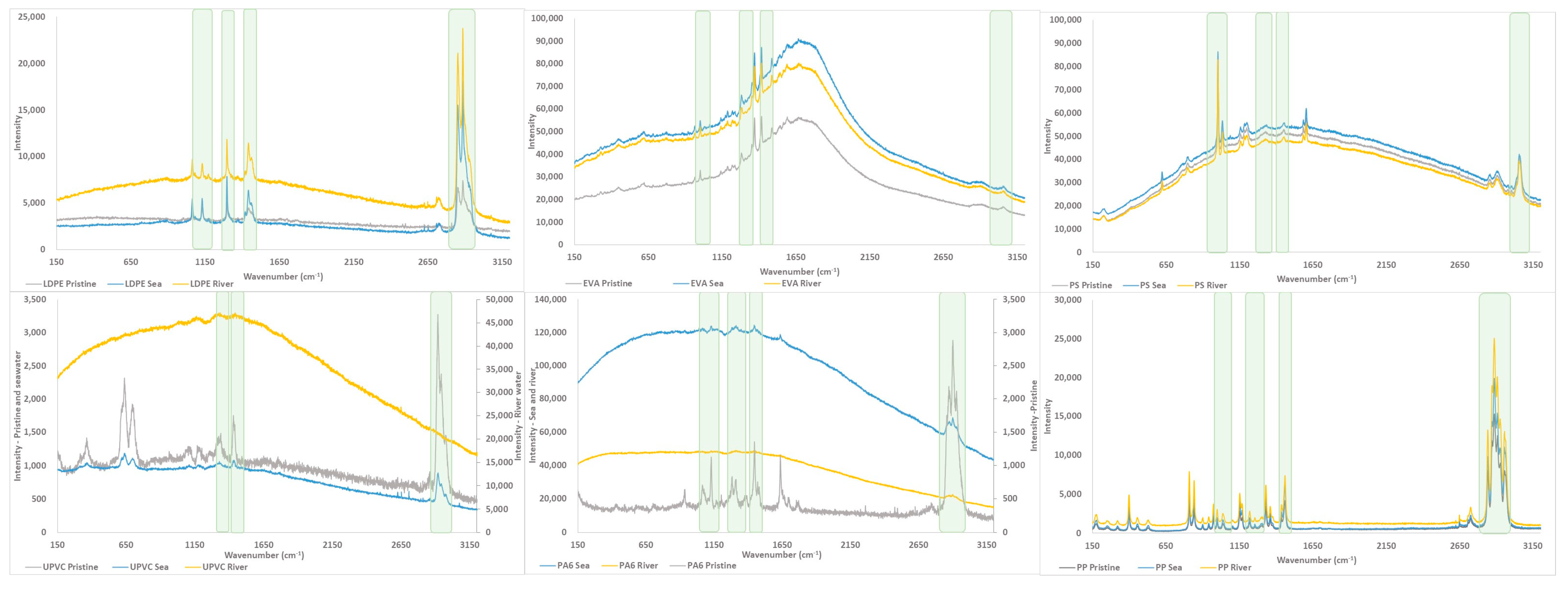
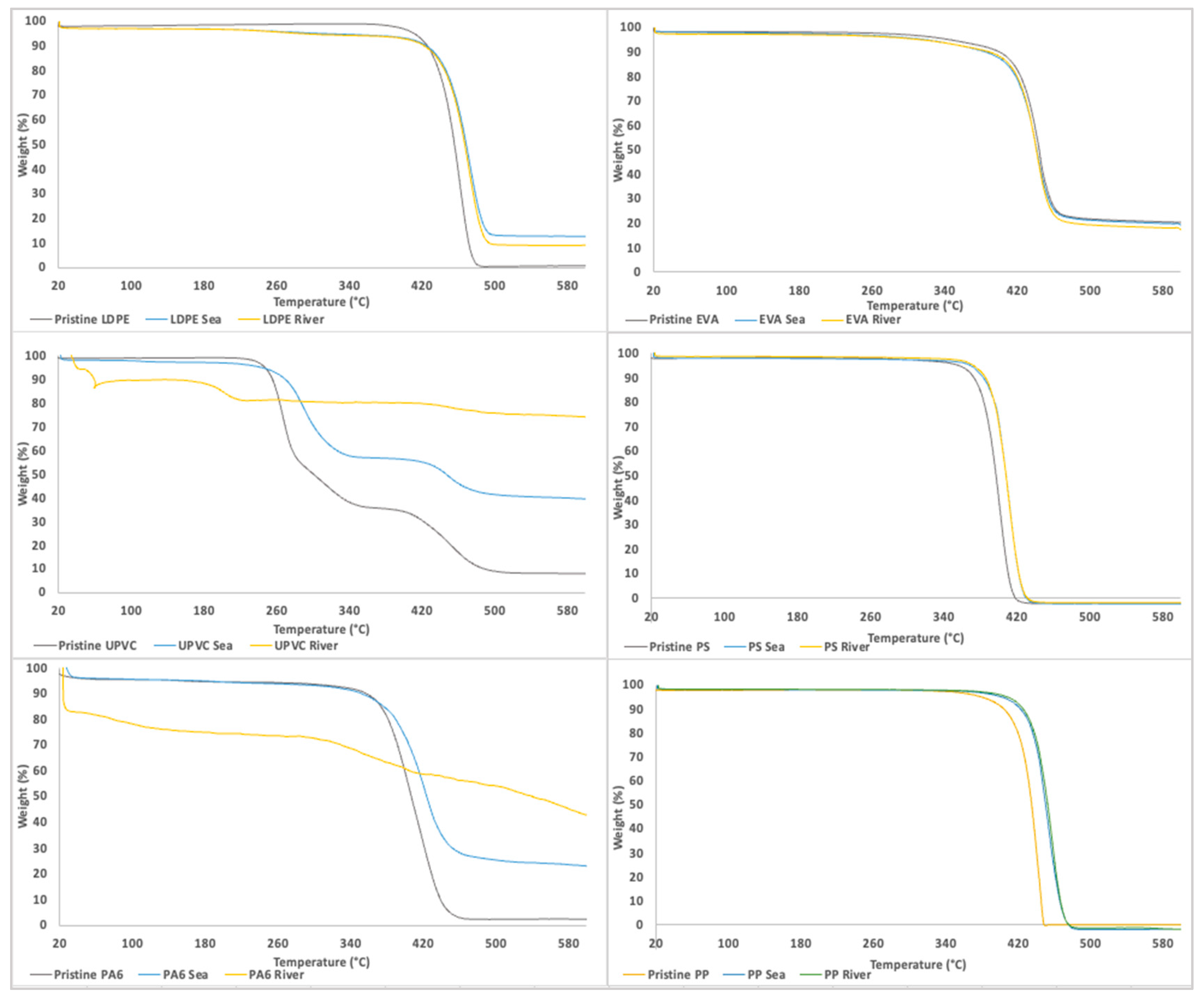

| Organic Solvent | C0 (μg·L−1) | Recovery Ratio (%) | RSD (%) |
|---|---|---|---|
| n-hexane | 150 | 99 | 5 |
| Ethyl acetate | 91 | 12 | |
| Dichloromethane | 38 | 8 |
| Models | Parameters | Seawater–LDPE Aging System | River Water–LDPE Aging System |
|---|---|---|---|
| Pseudo-1st order | k1 (min−1) | 0.22 ± 0.04 | 0.31 ± 0.09 |
| qe (µg CPF. g LPDE -1) | 72.70 ± 1.89 | 82.15 ± 3.06 | |
| R2 | 0.973 | 0.947 | |
| Residual sum of squares | 128.94 | 324.57 | |
| Pseudo-2nd order | k2 (g LPDE . µg CPF −1 . min−1) | 0.0044 ± 7.78 × 10−4 | 0.006 ± 2.12 × 10−3 |
| qe (µg CPF. g LPDE-1) | 75.39 ± 1.29 | 85.03 ± 2.36 | |
| R2 | 0.991 | 0.975 | |
| Residual sum of squares | 44.82 | 148.98 | |
| Elovich | α (µg . g LPDE −1 . min−1) | 1.83 × 106 ± 8.27 × 106 | 1.79 × 108 ± 8.96 × 108 |
| Β (µg . g LPDE −1) | 0.258 ± 6.88 × 10−2 | 0.286 ± 6.58 × 10−2 | |
| R2 | 0.962 | 0.979 | |
| Residual sum of squares | 183.24 | 127.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinho, S.D.; Fernandes, V.C.; Figueiredo, S.A.; Vilarinho, R.; Moreira, J.A.; Delerue-Matos, C. Laboratory Studies about Microplastic Aging and Its Effects on the Adsorption of Chlorpyrifos. Polymers 2023, 15, 3468. https://doi.org/10.3390/polym15163468
Martinho SD, Fernandes VC, Figueiredo SA, Vilarinho R, Moreira JA, Delerue-Matos C. Laboratory Studies about Microplastic Aging and Its Effects on the Adsorption of Chlorpyrifos. Polymers. 2023; 15(16):3468. https://doi.org/10.3390/polym15163468
Chicago/Turabian StyleMartinho, Sílvia D., Vírgínia Cruz Fernandes, Sónia A. Figueiredo, Rui Vilarinho, J. Agostinho Moreira, and Cristina Delerue-Matos. 2023. "Laboratory Studies about Microplastic Aging and Its Effects on the Adsorption of Chlorpyrifos" Polymers 15, no. 16: 3468. https://doi.org/10.3390/polym15163468
APA StyleMartinho, S. D., Fernandes, V. C., Figueiredo, S. A., Vilarinho, R., Moreira, J. A., & Delerue-Matos, C. (2023). Laboratory Studies about Microplastic Aging and Its Effects on the Adsorption of Chlorpyrifos. Polymers, 15(16), 3468. https://doi.org/10.3390/polym15163468









