High-Performance Reversible Furan–Maleimide Resins Based on Furfuryl Glycidyl Ether and Bismaleimides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instruments and Measurements
2.3. Synthesis of Furfuryl Glycidyl Ether (FGE)
 ), 2.80–2.61 (t, 2H,
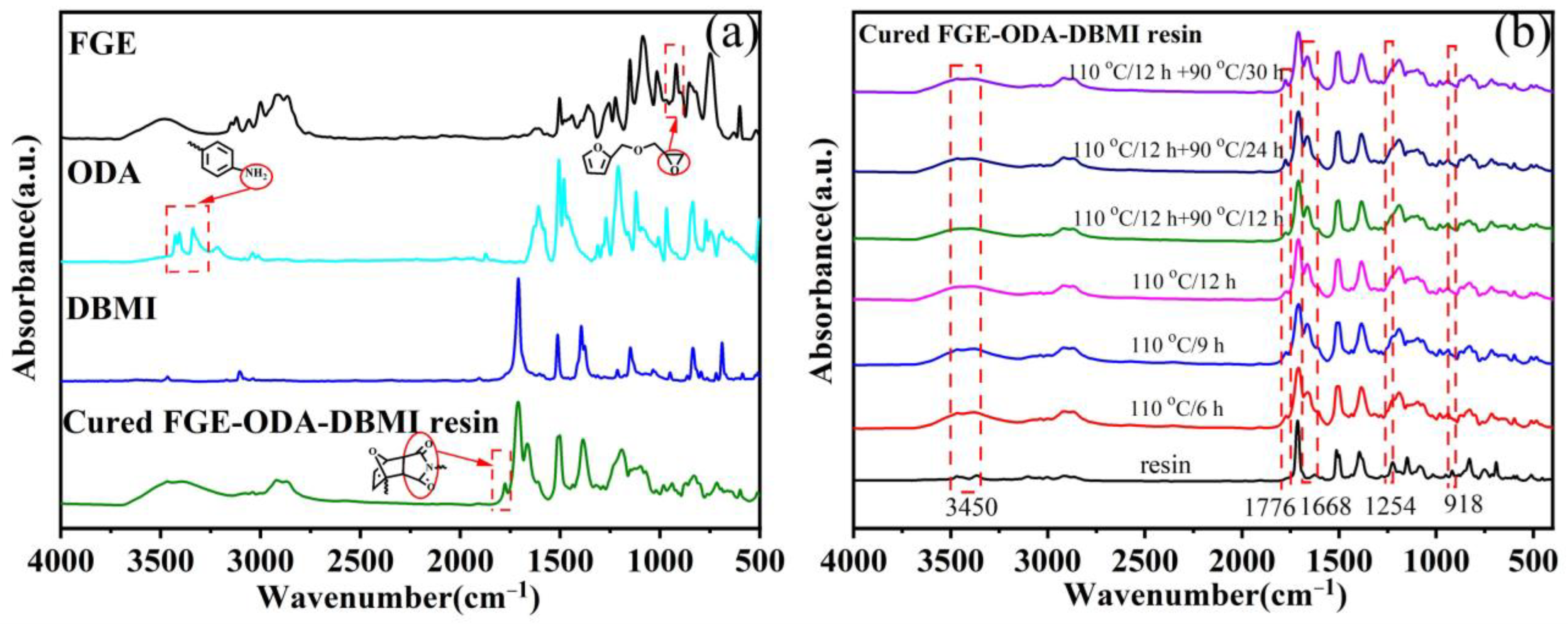
), 2.80–2.61 (t, 2H,  ). FT-IR (KBr, cm−1): 3146, 3125(=C-H, υ), 3062 (-C-H on the oxirane ring, υ), 1504 (-C=C-, υ), 1257, 850 (C-O-C on the oxirane ring, υ), 1217, 1084 (CH2-O-CH2, υ), 1150 (C-O-C on the furan ring, υ), 1008 (C-O-C on the furan ring, δ), 918 (C-O-C on the oxirane ring, δ), and 751 (single-substituted furan ring, δ). EI-MS (m/z): 154 [M]+, 97 [M-
). FT-IR (KBr, cm−1): 3146, 3125(=C-H, υ), 3062 (-C-H on the oxirane ring, υ), 1504 (-C=C-, υ), 1257, 850 (C-O-C on the oxirane ring, υ), 1217, 1084 (CH2-O-CH2, υ), 1150 (C-O-C on the furan ring, υ), 1008 (C-O-C on the furan ring, δ), 918 (C-O-C on the oxirane ring, δ), and 751 (single-substituted furan ring, δ). EI-MS (m/z): 154 [M]+, 97 [M- ]+, 81 [M-
]+, 81 [M- ]+ (Figure S1).
]+ (Figure S1).2.4. Synthesis of N,N′-Hexamethylene-Bismaleimide (HBMI)
 ]+, 82 [M-
]+, 82 [M- ]+ (Figure S2).
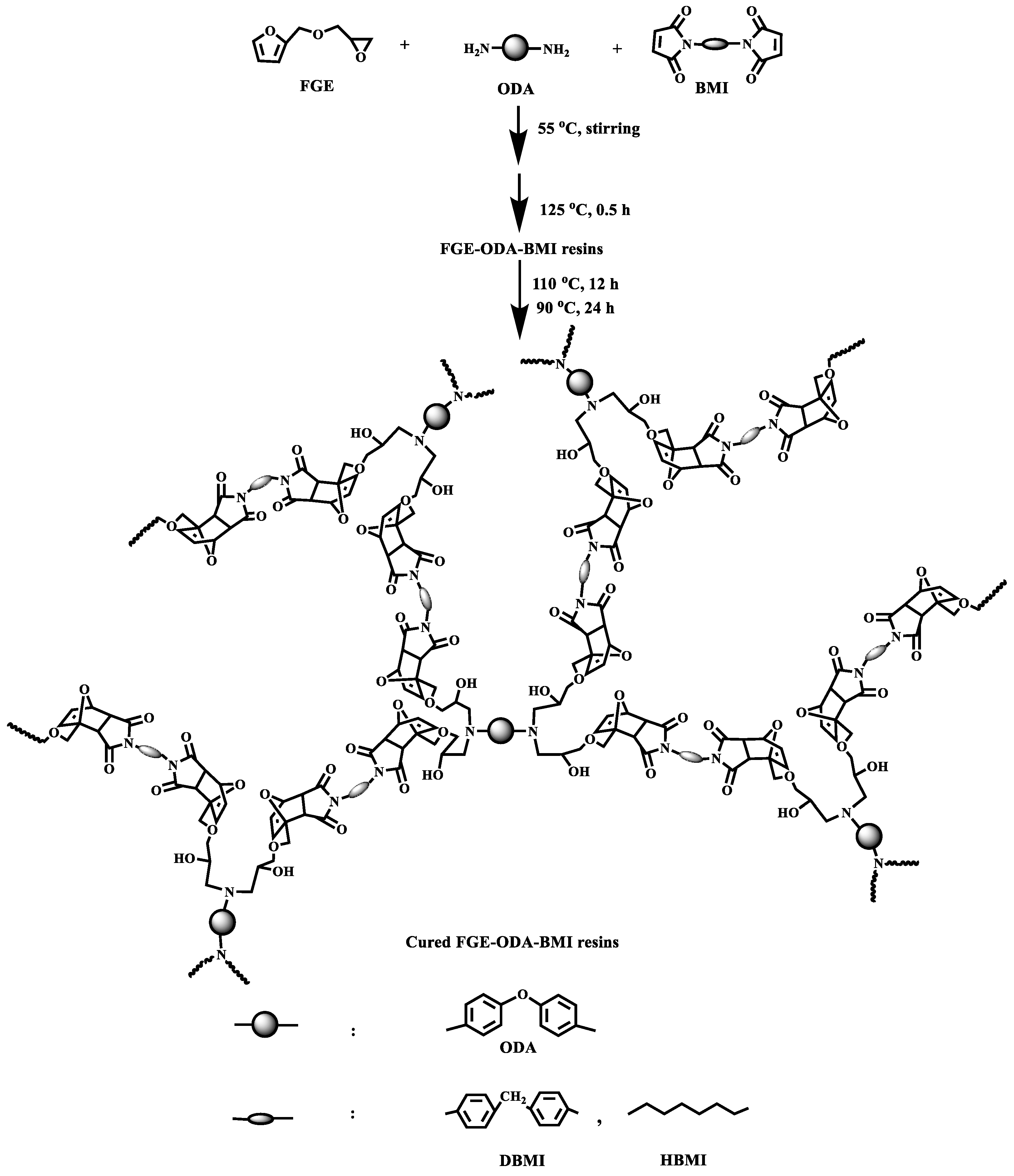
]+ (Figure S2).2.5. Synthesis of Cured FGE-ODA-BMI Resins
3. Results
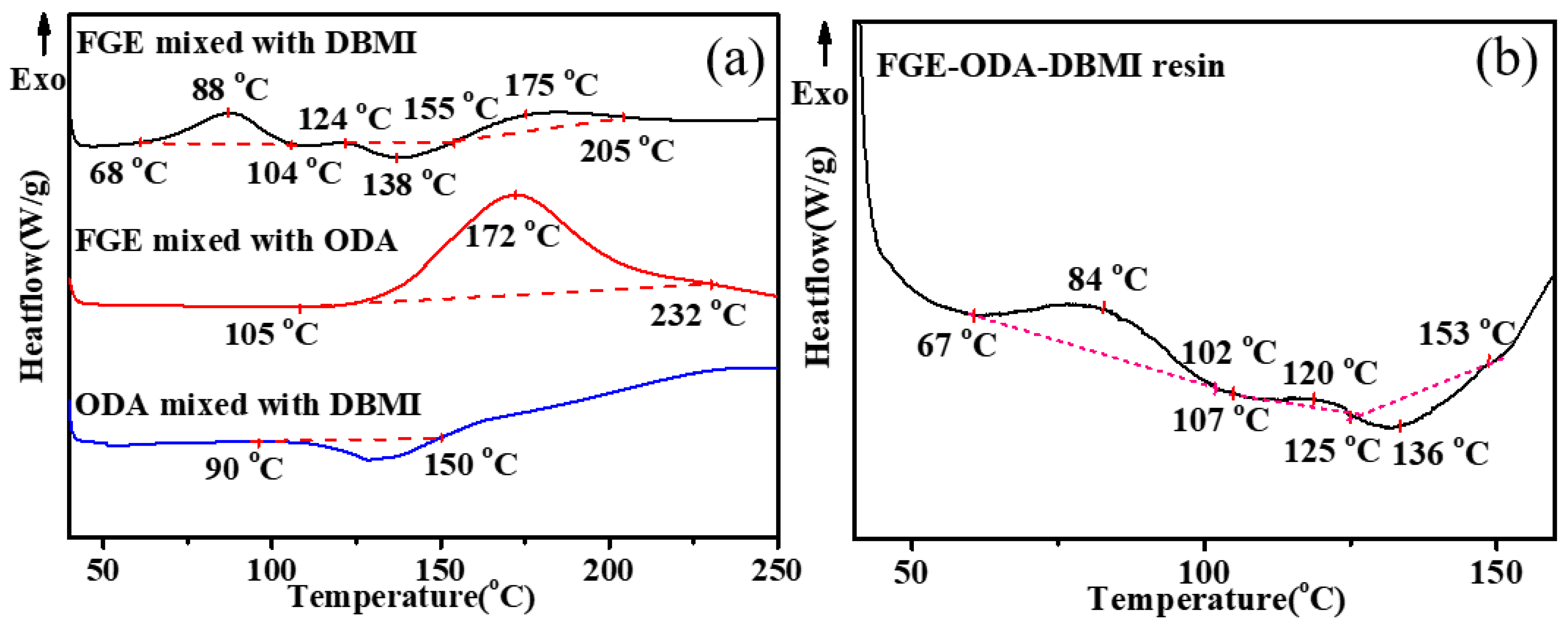
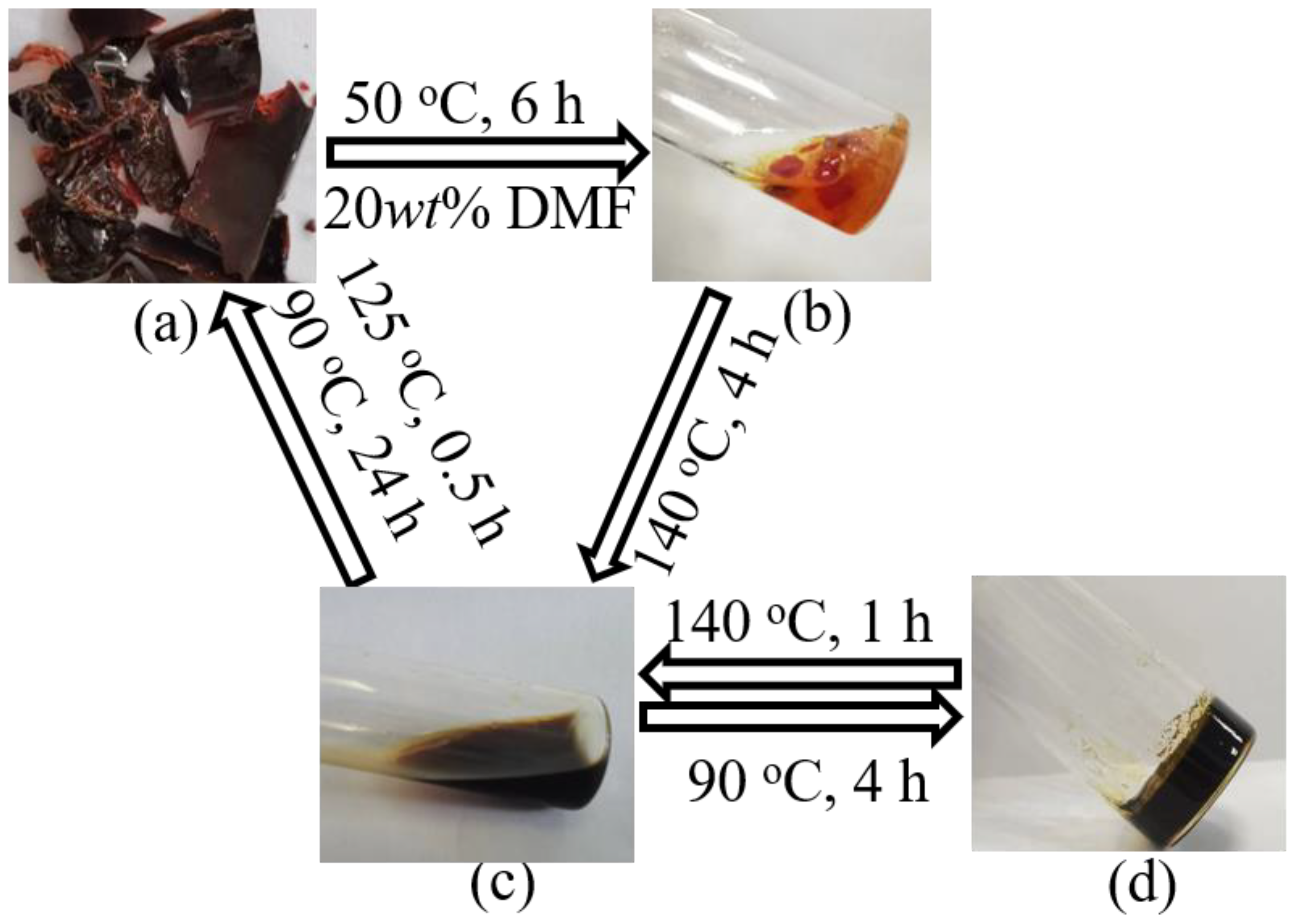
3.1. Curing Procedures and Structural Characterization of FGE-ODA-BMI Resins
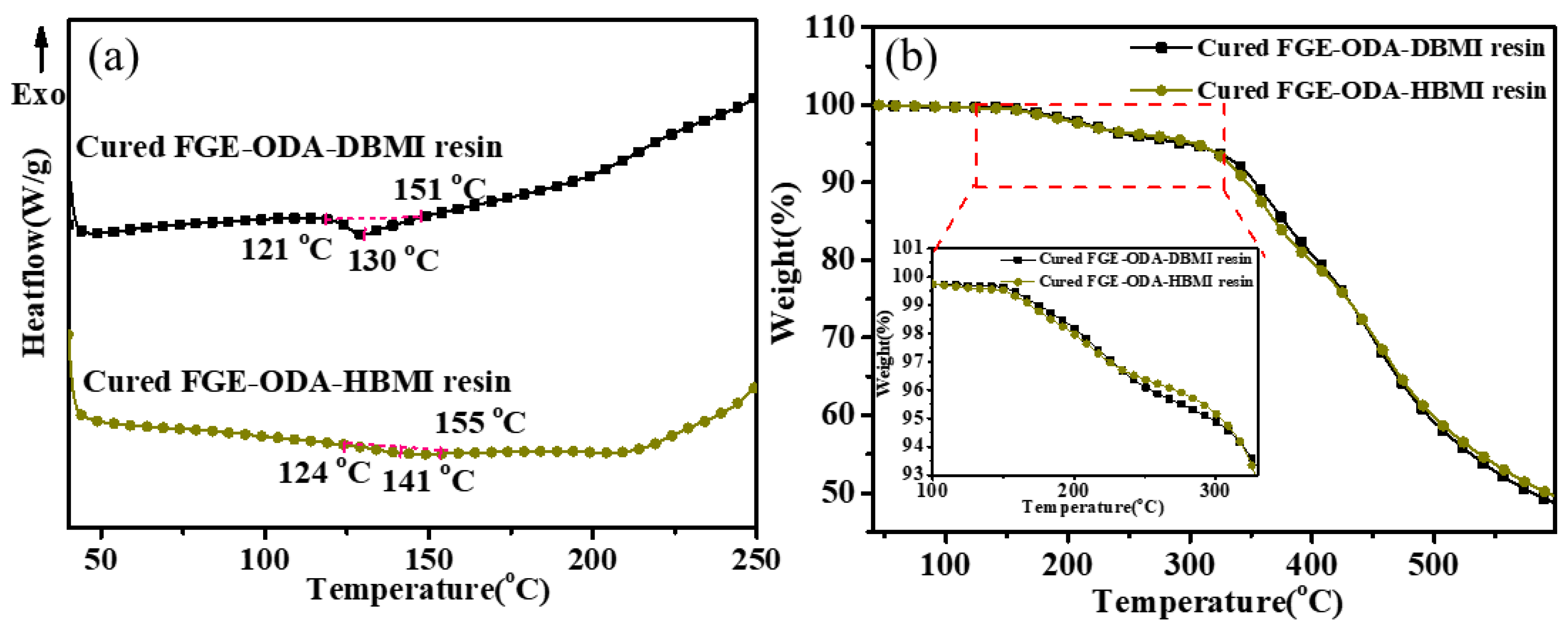
3.2. Thermal Property of the Cured FGE-ODA-BMI Resins
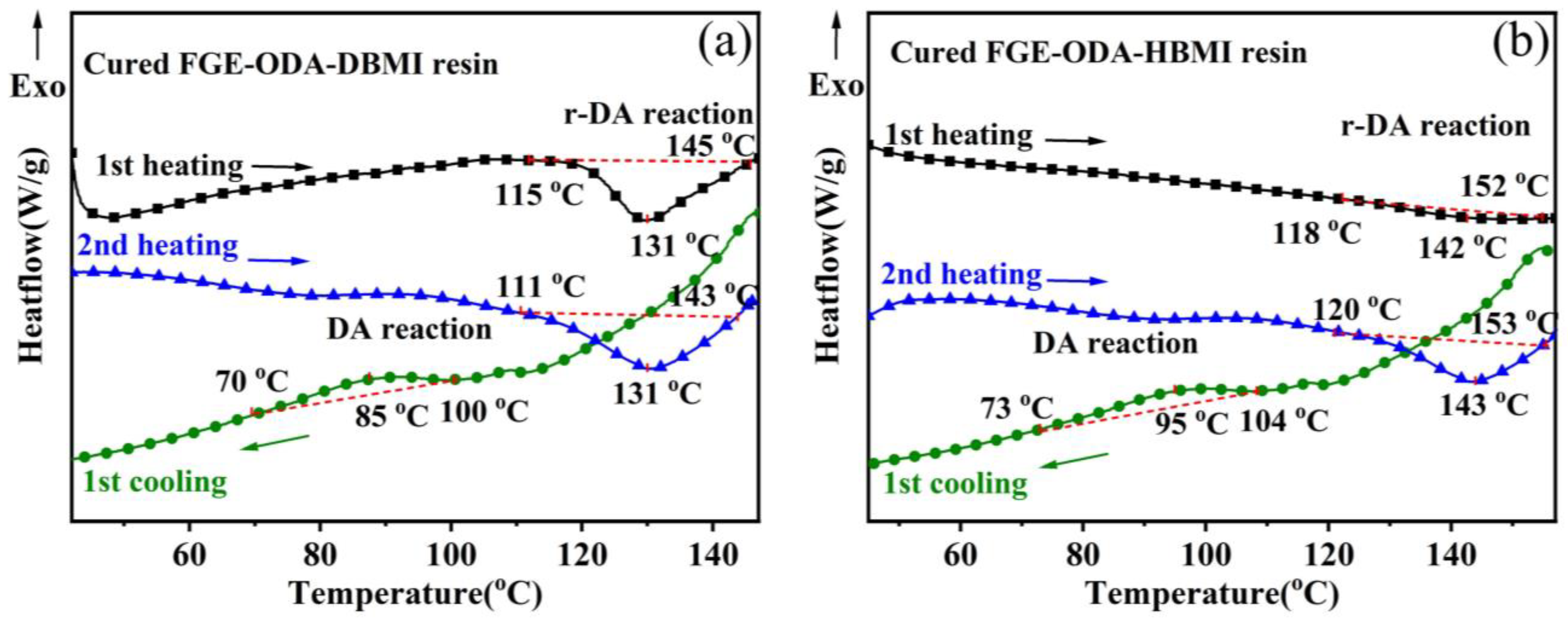
3.3. Reversible Performance of the Cured FGE-ODA-BMI Resins
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Van den Tempel, P.; Picchioni, F.; Bose, R.K. Designing end-of-life recyclable polymers via Diels-Alder chemistry: A review on the kinetics of reversible reactions. Macromol. Rapid. Commun. 2022, 43, 2200023. [Google Scholar] [CrossRef]
- Ma, S.Q.; Webster, D.C. Degradable thermosets based on labile bonds or linkages: A review. Prog. Polym. Sci. 2018, 76, 65–110. [Google Scholar] [CrossRef]
- Zou, W.K.; Dong, J.T.; Luo, Y.W.; Zhao, Q.; Xie, T. Dynamic covalent polymer networks: From old chemistry to modern day innovations. Adv. Mater. 2017, 29, 1606100. [Google Scholar] [CrossRef]
- Amamoto, Y.; Kamada, J.; Otsuka, H.; Takahara, A.; Matyjaszewski, K. Repeatable photoinduced self-healing of covalently cross-linked polymers through reshuffling of trithiocarbonate units. Angew. Chem. Int. Ed. 2011, 123, 1698–1701. [Google Scholar] [CrossRef]
- Yoon, J.A.; Kamada, J.; Koynov, K.; Mohin, J.; Nicolaÿ, R.; Zhang, Y.; Balazs, A.C.; Kowalewski, T.; Matyjaszewski, K. Self-healing polymer films based on thiol-disulfide exchange reactions and self-healing kinetics measured using atomic force microscopy. Macromolecules 2011, 45, 142–149. [Google Scholar] [CrossRef]
- Montarnal, D.; Capelot, M.; Tournihac, F.; Leibler, L. Silica-like malleable materials from permanent organic networks. Science 2011, 334, 965–968. [Google Scholar] [CrossRef] [PubMed]
- Demongeot, A.; Mougnier, J.; Okada, S.; Soulié-Ziakovic, C.; Tournilhac, F. Coordination and catalysis of Zn2+ in epoxy-based vitrimers. Polym. Chem. 2016, 7, 4486–4493. [Google Scholar] [CrossRef]
- Denissen, W.; Rivero, G.; Nicolaÿ, R.; Leibler, L.; Winne, J.M.; Du Prez, F.E. Vinylogous urethane vitrimers. Adv. Funct. Mater. 2015, 25, 2451–2457. [Google Scholar] [CrossRef]
- Taynton, P.; Yu, K.; Shoemaker, R.K.; Jin, Y.H.; Qi, H.J.; Zhang, W. Heat- or water-driven malleability in a highly recyclable covalent network polymer. Adv. Mater. 2014, 26, 3938–3942. [Google Scholar] [CrossRef]
- Lei, X.F.; Jin, Y.H.; Sun, H.L.; Zhang, W. Rehealable imide-imine hybrid polymers with full recyclability. J. Mater. Chem. A 2017, 5, 21140–21145. [Google Scholar] [CrossRef]
- Zheng, P.W.; McCarthy, T.J. A surprise from 1954: Siloxane equilibration is a simple, robust, and obvious polymer self-healing mechanism. J. Am. Chem. Soc. 2012, 134, 2024–2027. [Google Scholar] [CrossRef] [PubMed]
- Obadia, M.; Mudraboyina, B.P.; Serghei, A.; Montarnal, D.; Drockenmuller, E. Reprocessing and recycling of highly cross-linked ion-conducting networks through transalkylation exchanges of C-N bonds. J. Am. Chem. Soc. 2015, 137, 6078–6083. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.X.; Tournilhac, F.; Leibler, K.; Guan, Z.B. Making insoluble polymer networks malleable via olefin metathesis. J. Am. Chem. Soc. 2012, 134, 8424–8427. [Google Scholar] [CrossRef] [PubMed]
- Goodman, S.H.; Dodiuk, H. Handbook of Thermoset Plastics; William Andrew: San Diego, CA, USA, 2014. [Google Scholar]
- Liu, Y.L.; Chuo, T.W. Self-healing polymers based on thermally reversible Diels-Alder chemistry. Polym. Chem. 2013, 4, 2194–2205. [Google Scholar] [CrossRef]
- Min, Y.; Huang, S.; Wang, Y.; Zhang, Z.; Du, B.; Zhang, X.; Fan, Z. Sonochemical transformation of epoxy-amine thermoset into soluble and reusable polymers. Macromolecules 2015, 48, 316–322. [Google Scholar] [CrossRef]
- Gandini, A.; Lacerda, T.M. Furan polymers: State of the art and perspectives. Macromol. Mater. Eng. 2022, 307, 2100902. [Google Scholar] [CrossRef]
- Yang, T.C.; Yeh, C.H. Morphology and mechanical properties of 3D printed wood fiber/polylactic acid composite parts using fused deposition modeling (FDM): The effects of printing speed. Polymers 2020, 12, 1334. [Google Scholar] [CrossRef]
- Navarro-Baena, I.; Sessini, V.; Dominici, F.; Torre, L.; Kenny, J.M.; Peponi, L. Design of biodegradable blends based on PLA and PCL: From morphological, thermal and mechanical studies to shape memory behavior. Polym. Degrad. Stab. 2016, 132, 97–108. [Google Scholar] [CrossRef]
- Pauloehrl, T.; Delaittre, G.; Winkler, V.; Welle, A.; Bruns, M.; Börner, H.G.; Greiner, A.M.; Bastmeyer, M.; Barner-Kowollik, C. Adding spatial control to click chemistry: Phototriggered Diels-Alder surface (bio)functionalization at ambient temperature. Angew. Chem. Int. Ed. 2012, 51, 1071–1074. [Google Scholar] [CrossRef]
- Fan, M.J.; Liu, J.L.; Li, X.Y.; Zhang, J.Y.; Cheng, J. Recyclable Diels-Alder furan/maleimide polymer networks with shape memory effect. Ind. Eng. Chem. Res. 2014, 53, 16156–16163. [Google Scholar] [CrossRef]
- Yasuda, K.; Takahashi, Y.; Sugane, K.; Shibata, M. Self-healing high-performance thermosets utilizing furan/maleimide Diels-Alder, epoxy/amine nucleophilic ring-opening, and maleimide/amine michael reactions. Polym. Bull. 2021, 79, 8455–8469. [Google Scholar] [CrossRef]
- Shabani, P.; Shokrieh, M.M.; Zibaei, I. Effect of the conversion degree and multiple healing on the healing efficiency of a thermally reversible self-healing polymer. Polym. Adv. Technol. 2019, 30, 2906–2917. [Google Scholar] [CrossRef]
- Abdollahi, H.; Salimi, A.; Barikani, M.; Samadi, A.; Rad, S.; Zanjanijam, A.R. Systematic investigation of mechanical properties and fracture toughness of epoxy networks: Role of the polyetheramine structural parameters. J. Appl. Polym. Sci. 2018, 136, 47121. [Google Scholar] [CrossRef]
- Ma, M.M.; Wang, X.Y.; Yu, Z.E.; Wan, L.Q.; Huang, F.R. High impact polytriazole resins for advanced composites. Des. Monomers Polym. 2020, 23, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Dhanaraju, G.; Golla, S.; Ben, B.S.; Vikram, K.A. Interfacial and matrix healing of thermally reversible bismaleimide infused Graphene Nano Platelets reinforced polymer nanocomposite through Diels-Alder bonding. Mater. Today Commun. 2022, 31, 103753. [Google Scholar]
- Polgar, L.M.; Cerpentier, R.R.J.; Vermeij, G.H.; Picchioni, F.; van Duin, M. Influence of the chemical structure of cross-linking agents on properties of thermally reversible networks. Pure Appl. Chem. 2016, 88, 1103–1116. [Google Scholar] [CrossRef]
- Lorero, I.; Rodríguez, A.; Campo, M.; Prolongo, S.G. Thermally remendable, weldable, and recyclable epoxy network crosslinked with reversible Diels-alder bonds. Polymer 2022, 259, 125334. [Google Scholar] [CrossRef]
- Li, J.; Zhang, G.; Deng, L.; Jiang, K.; Zhao, S.; Gao, Y.; Sun, R.; Wong, C. Thermally reversible self-healing novolac epoxy resins based on Diels-Alder chemistry. J. Appl. Polym. Sci. 2015, 132, 42167. [Google Scholar] [CrossRef]
- Zolghadr, M.; Shakeri, A.; Zohuriaan-Mehr, M.J.; Salimi, A. Self-healing semi-IPN materials from epoxy resin by solvent-free furan-maleimide Diels-Alder polymerization. J. Appl. Polym. Sci. 2019, 136, 48015. [Google Scholar] [CrossRef]
- Hopewell, J.L.; Hill, D.J.T.; Pomery, P.J. Electron spin resonance study of the homopolymerization of aromatic bismaleimides. Polymer 1998, 39, 5601–5607. [Google Scholar] [CrossRef]
- Settle, A.E.; Berstis, L.; Rorrer, N.A.; Roman-Leshkóv, Y.; Beckham, G.T.; Richards, R.M.; Vardon, D.R. Heterogeneous Diels-Alder catalysis for biomass-derived aromatic compounds. Green Chem. 2017, 19, 3468–3492. [Google Scholar] [CrossRef]
- Canary, S.A.; Stevens, P.M. Thermally reversible crosslinking of polystyrene via the furan-maleimide Diels-Alder reaction. J. Polym. Sci. Part A Polym. Chem. 1992, 30, 1755–1760. [Google Scholar] [CrossRef]


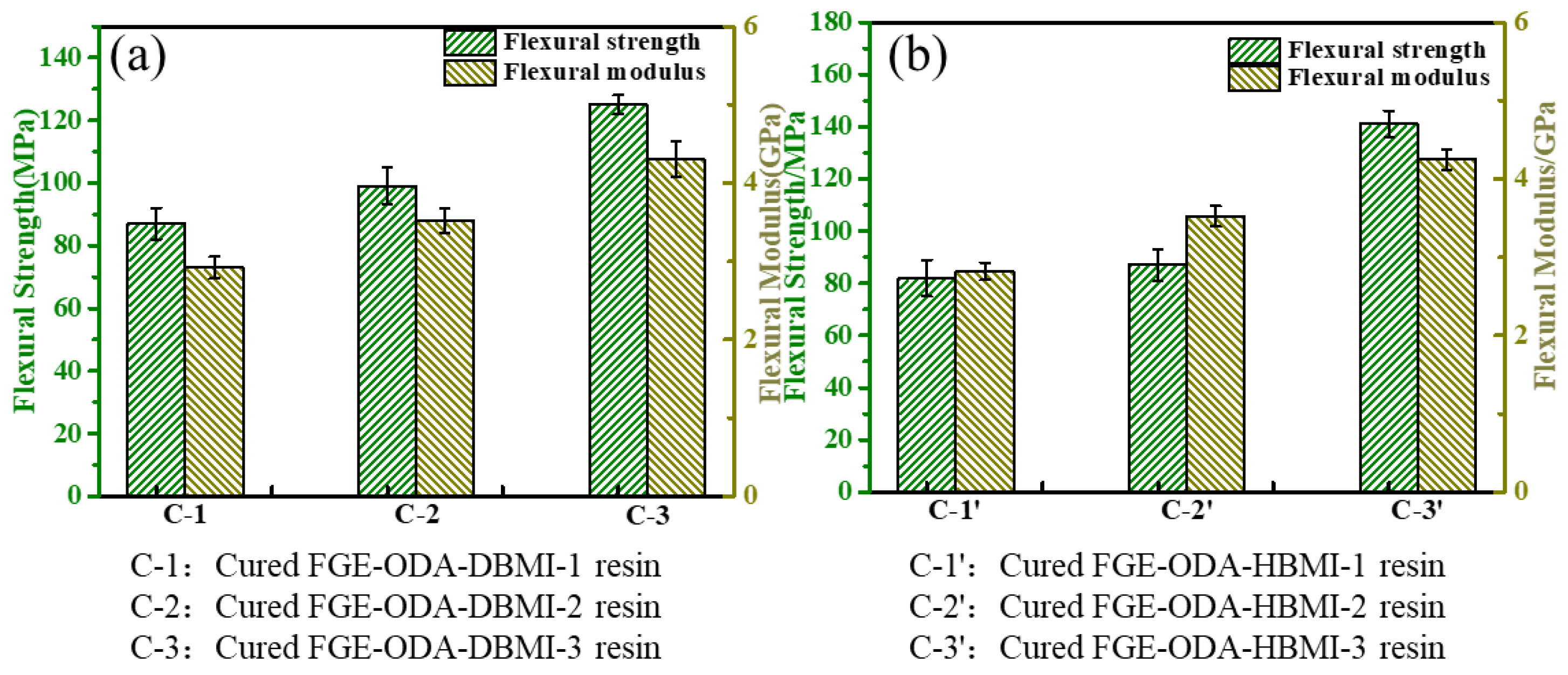

| Sample | Cured FGE-ODA-DBMI Resin | Cured FGE-ODA-HBMI Resin | ||||
|---|---|---|---|---|---|---|
| Flexural Strength (MPa) | Flexural Modulus (GPa) | Retention Rate (%) | Flexural Strength (MPa) | Flexural Modulus (GPa) | Retention Rate (%) | |
| R-0 | 125 ± 3 | 4.30 ± 0.23 | - | 141 ± 5 | 4.25 ± 0.13 | - |
| R-1 | 109 ± 7 | 4.08 ± 0.15 | 87.2 | 111 ± 9 | 3.95 ± 0.17 | 78.7 |
| R-2 | 98 ± 8 | 3.82 ± 0.23 | 78.4 | 102 ± 5 | 3.87 ± 0.21 | 72.3 |
| R-3 | 83 ± 6 | 3.69 ± 0.19 | 66.4 | 86 ± 3 | 3.68 ± 0.16 | 61.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Li, J.; Zhang, J.; Liu, S.; Wan, L.; Liu, Z.; Huang, F. High-Performance Reversible Furan–Maleimide Resins Based on Furfuryl Glycidyl Ether and Bismaleimides. Polymers 2023, 15, 3470. https://doi.org/10.3390/polym15163470
Wang J, Li J, Zhang J, Liu S, Wan L, Liu Z, Huang F. High-Performance Reversible Furan–Maleimide Resins Based on Furfuryl Glycidyl Ether and Bismaleimides. Polymers. 2023; 15(16):3470. https://doi.org/10.3390/polym15163470
Chicago/Turabian StyleWang, Jiawen, Jixian Li, Jun Zhang, Shuyue Liu, Liqiang Wan, Zuozhen Liu, and Farong Huang. 2023. "High-Performance Reversible Furan–Maleimide Resins Based on Furfuryl Glycidyl Ether and Bismaleimides" Polymers 15, no. 16: 3470. https://doi.org/10.3390/polym15163470





