Conductive Polymers and Their Nanocomposites: Application Features in Biosensors and Biofuel Cells
Abstract
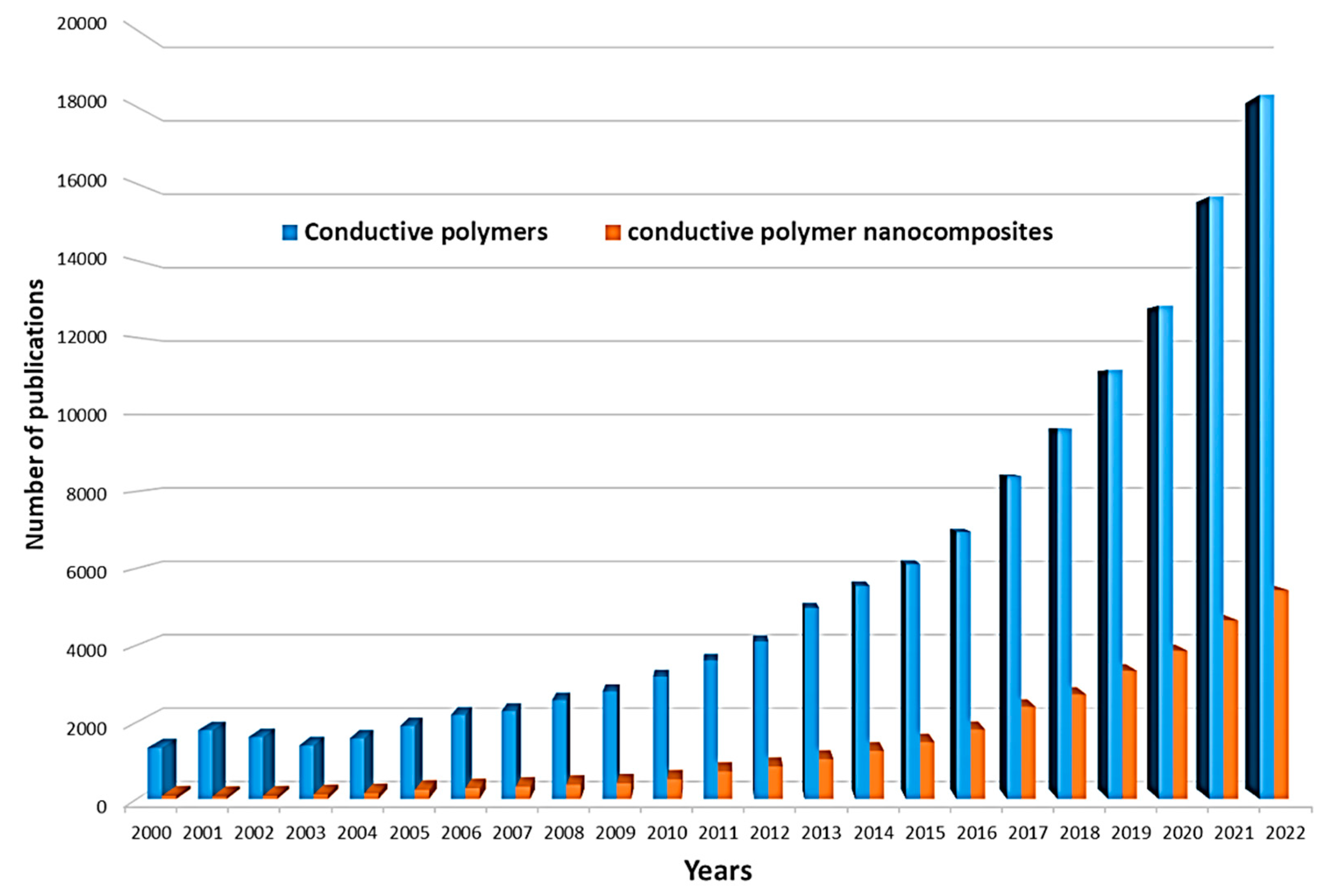
:1. Introduction
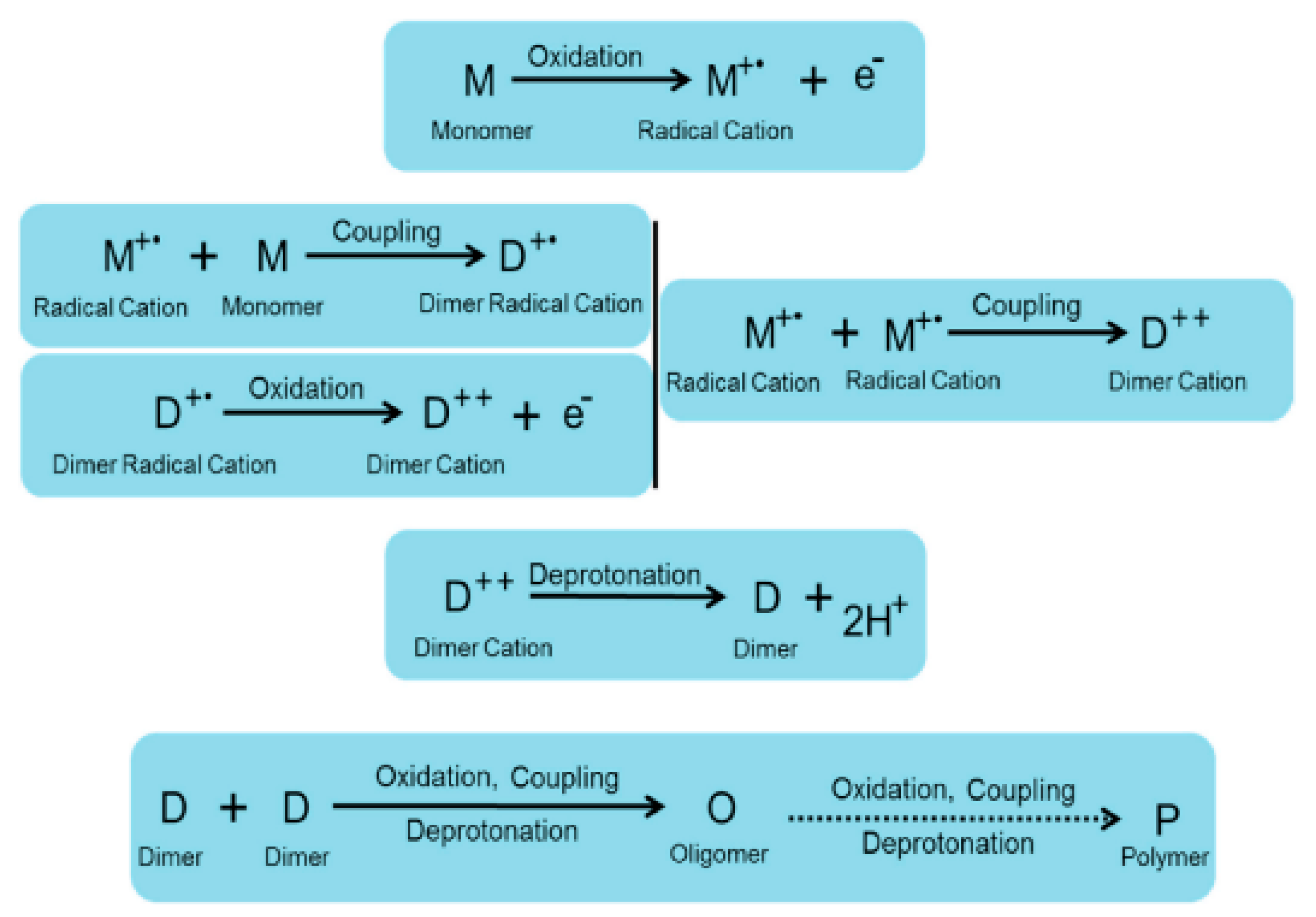
2. Synthesis of Conducting Polymers
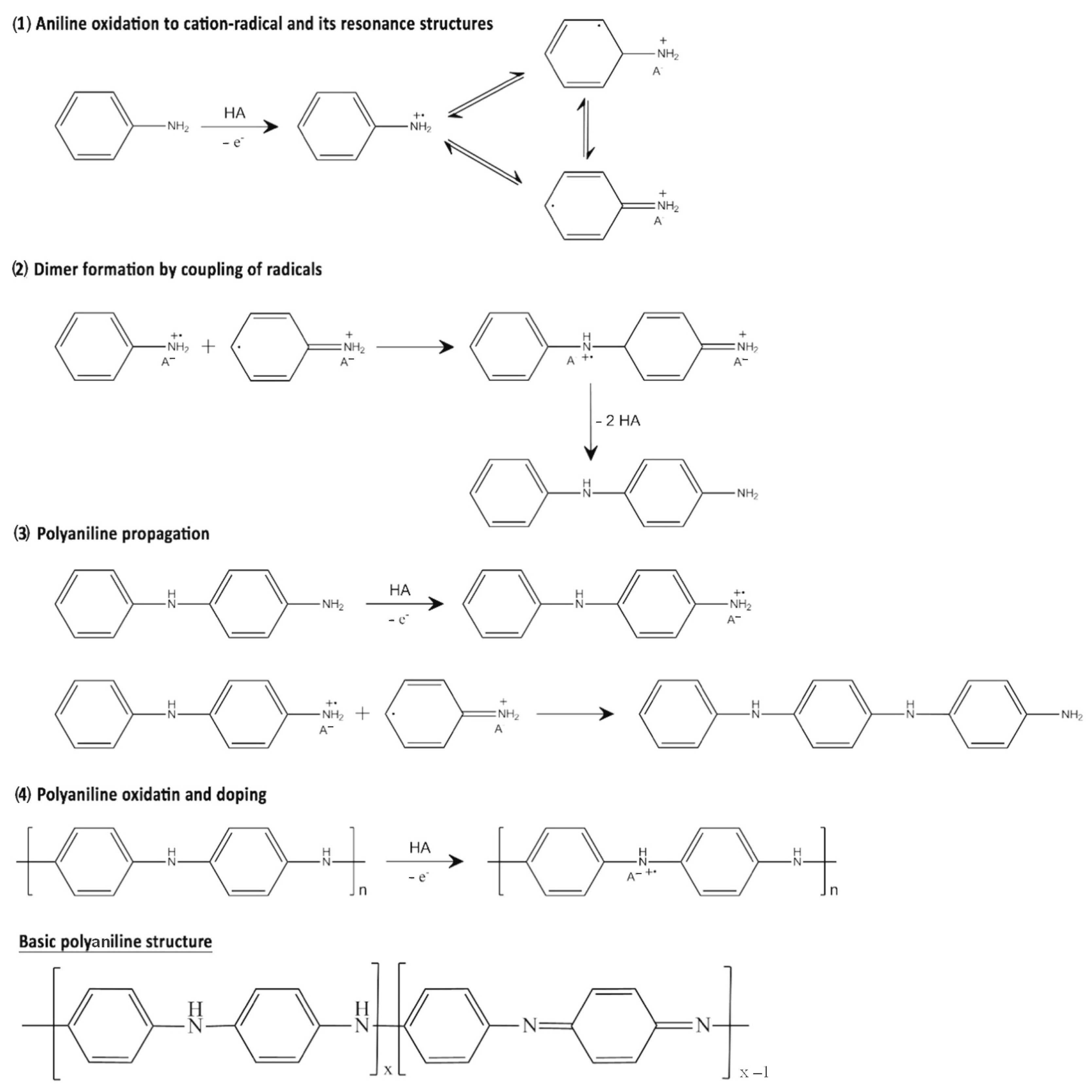
2.1. Chemical Synthesis of Conductive Polymers
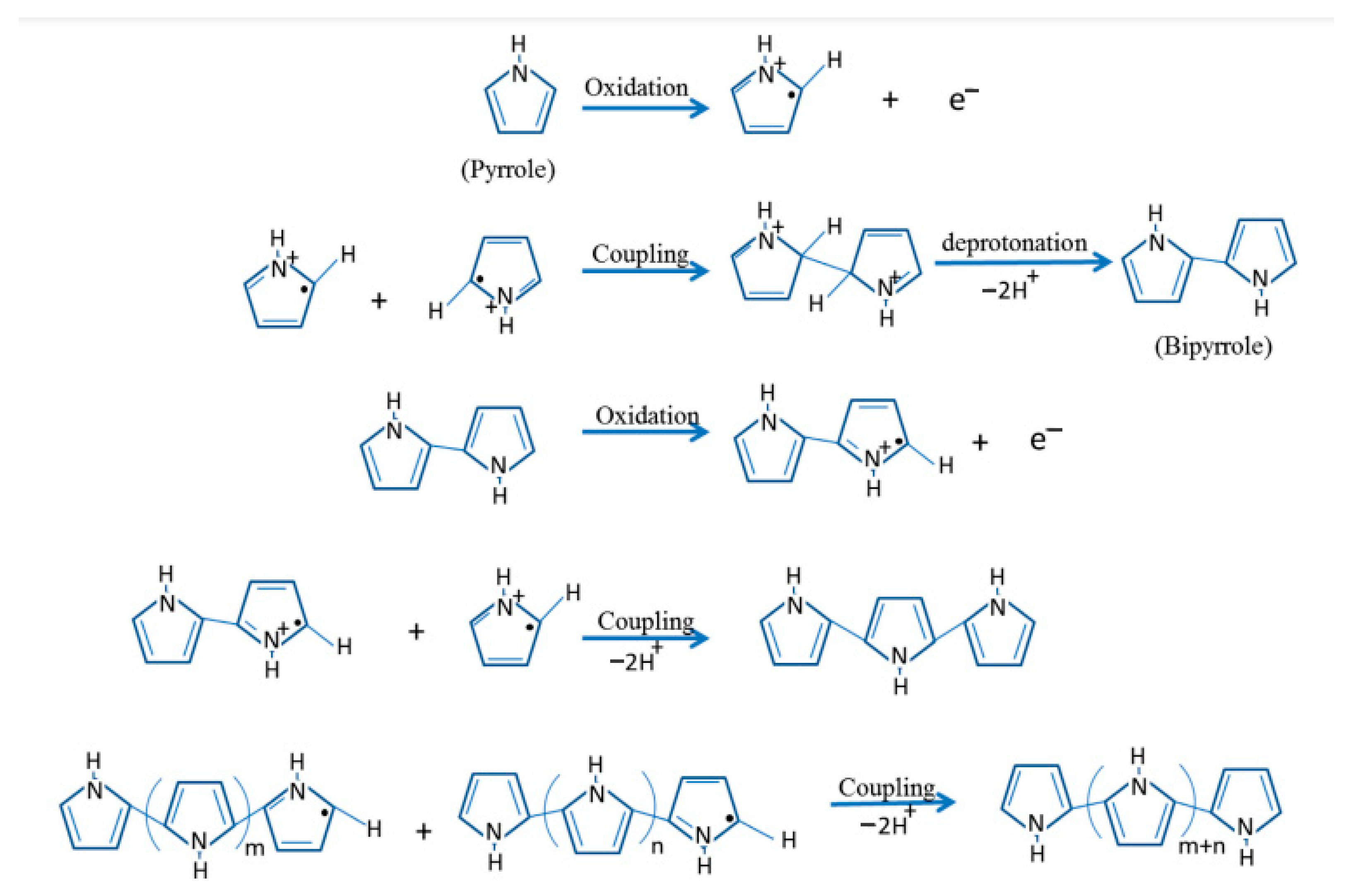
2.2. Electropolymerization of Conductive Polymers
2.3. Photochemical Polymerization of Conductive Polymers
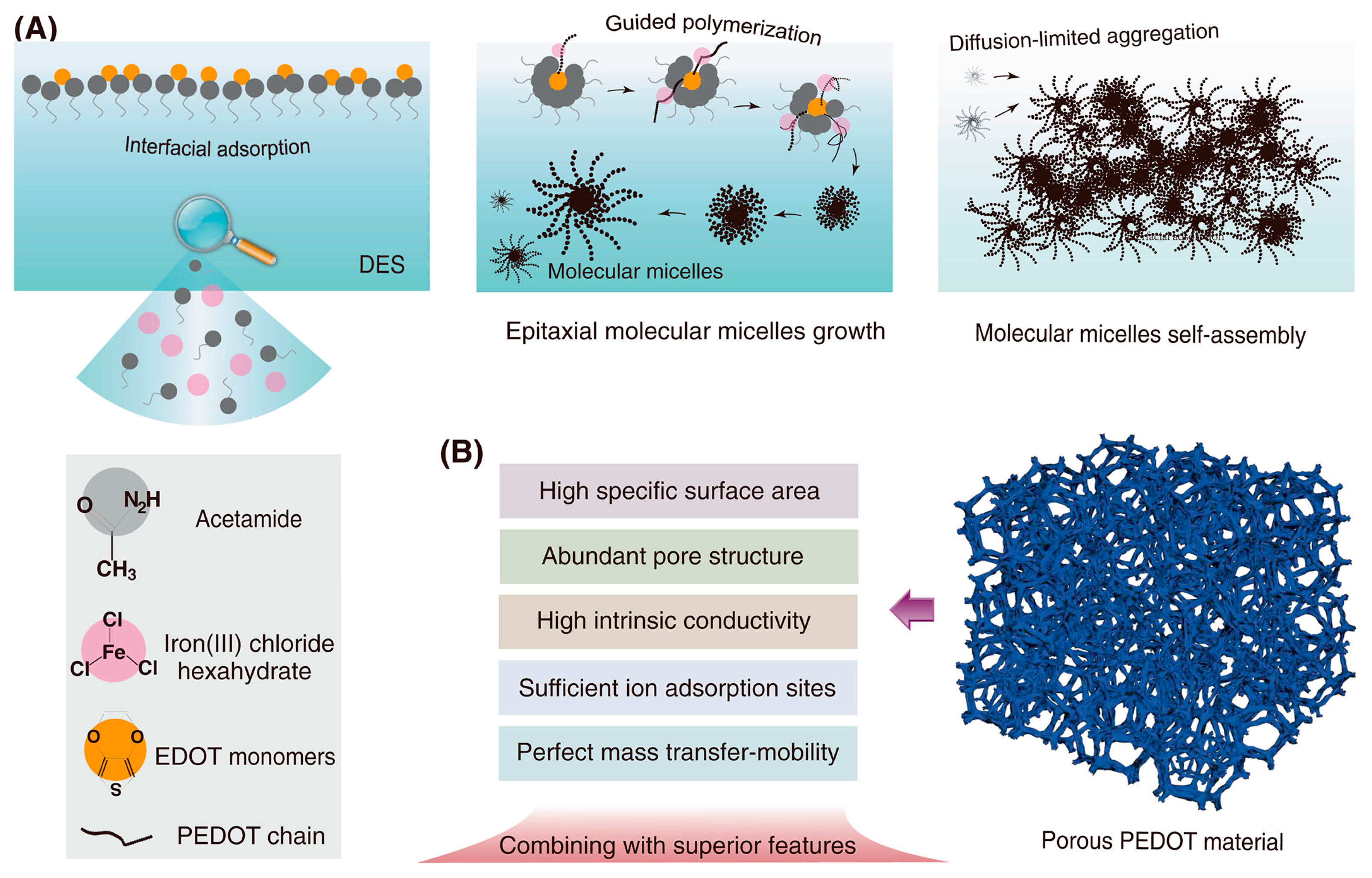
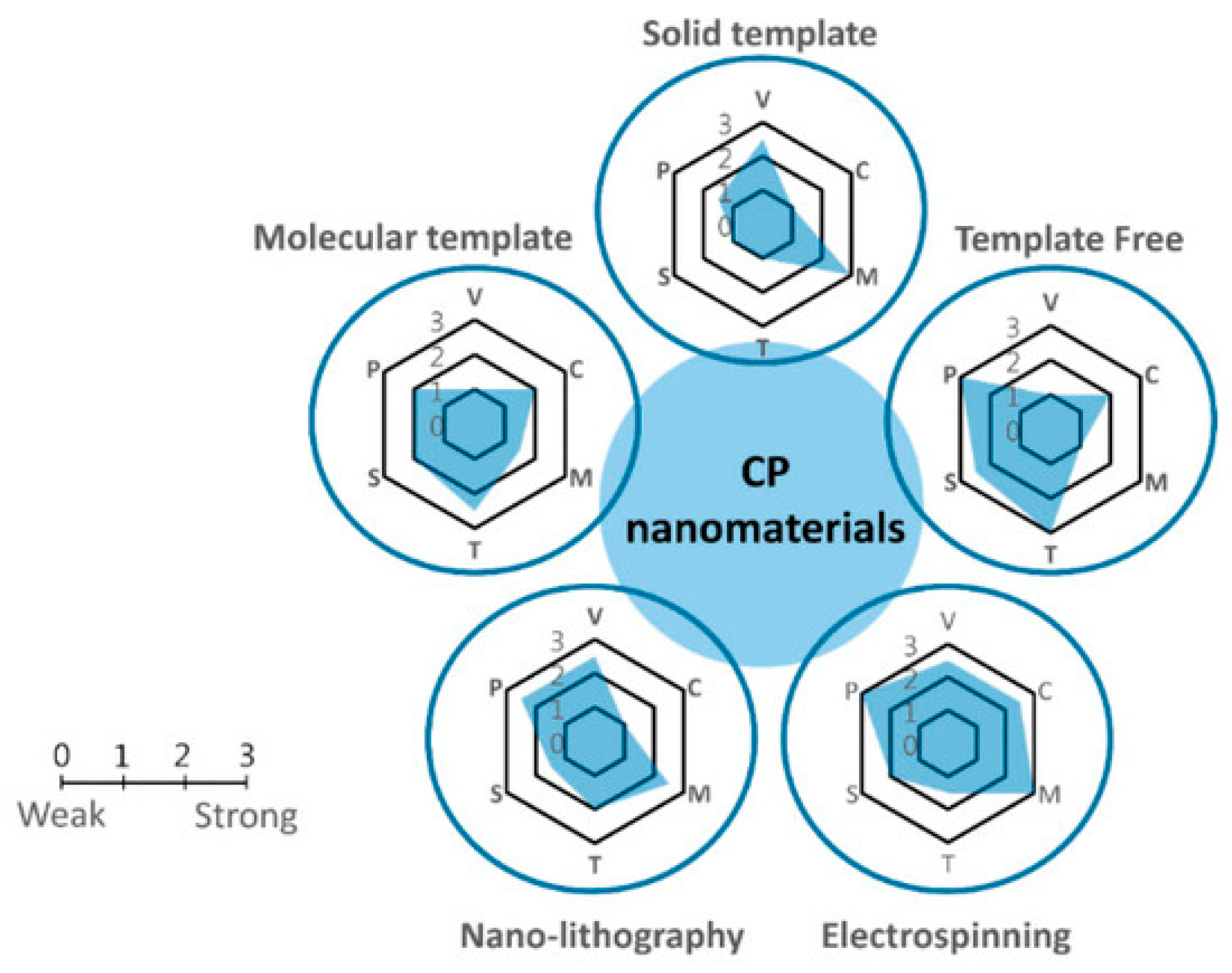
2.4. Synthesis of Conductive Nanocomposites
3. Composite Materials Based on Conductive Polymers
3.1. Composites Based on Carbon Nanomaterials and Conductive Polymers
- Fullerenes are spherical molecules in which carbon atoms are connected to each other through pyramidal hybrid sp2–sp3 orbitals.
- Graphene is a single layer of graphite that is an atom thick, where the carbon atoms have sp2 hybridization and are arranged in a honeycomb pattern. Graphene derivatives:
- Graphene oxide is functionalized graphene with oxygen-containing functional groups;
- Reduced graphene oxide is treated graphene oxide with reduced oxygen content. The complete reduction of graphene oxide does not lead to the formation of a graphene layer due to residual oxygen-containing functional groups, since not all sp3 bonds return back to the sp2 configuration.
- Single-walled carbon nanotubes (SWCNTs) or graphene nanotubes are graphene planes rolled into a cylinder, and multi-walled carbon nanotubes (MWCNTs) are a set of cylinders with different diameters nested into each other.
- Carbon nanodots or carbon quantum dots are quasi-spherical nanoparticles less than 10 nm in size containing various functional groups (carboxyl, amino groups).
- Graphene nanoribbons are strips of graphene less than 100 nm wide.
3.2. Composites Based on Metal Nanoparticles and Conductive Polymers
4. Biosensors Based on Conductive Polymers
4.1. Enzyme Biosensors
4.2. Microbial Biosensors
4.3. Affinity Biosensors
| Polymer | Polymer Synthesis Method | Composite | Biosensor Formation Time | Determined Compound | Real Samples | Biosensor Type | Detection Method | Main Specifications/Detection Limit/Detection Range | Reference |
|---|---|---|---|---|---|---|---|---|---|
Polypyrrole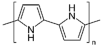 | One-step electrochemical deposition | PPy/polydopamine/(GOx) | 1 h, holding 1 day | Glucose | Human blood serum | Enzymatic (GOX) | CA, CV, EIS | Sensitivity—22.15 A mM−1 cm−2, response time—5–6 s, linear range up to 5.0 mM; LoD—138 µM glucose; stability for 90 days (93.9%). | [144] |
| Electropolymerization | NAD- GDH/poly-TBO (Poly-toluidine blue)/Ppy/SPE | 14 h | Glucose | Synthetic urine | Enzymatic (GDH) | CV, EIS, CA | Linear range—1.0 × 10−3 to 9.0 × 10−3 M; LoD—9.0 × 10−5 M | [145] | |
| One-step electrochemical copolymerization of pyrrole (PPy) and chondroitin sulfate (CS) | CS/PPy nanowires | Approximately 2 h | Acetamiprid (insecticide) | Soil samples | Aptasensor | CC, CA, EIS | The determination time—0.5 s and 2 s; LoD—0.347 pg/mL and 0.065 fg/mL | [146] | |
| Electrochemical polymerization | Polyethylene glycol (PEG)/PPy nanowires | 1 h | MicroRNAs (miRNAs) | serum samples | DNA probes | DPV | Linear range—0.10 pM ∼ 1.0 nM, LoD—0.033 pM; RSD—3.05% | [147] | |
Polyaniline 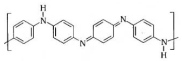 | Electrophoretic deposition | AChE/Ag@CuO/PANI/ITO | 14 h, preliminary procedures for several days | Paraoxon-ethyl | Banana, tomato, and soil | Enzymatic (AChE) | CV, EIS | Linear range—5–100 pM; LoD—11.35 pM; sensitivity—0.5536 μA pM−1 cm−2; RSD—1.74%; After 20 days of storage, the current response remains 71.3% of its initial current | [148] |
| Electropolymerization | Nf/PANI/CuF/Urease | 3 days | Urea | Soil and milk samples | Enzymatic (urease) | CV, DPV | LoD—0.17 µM; linear range—0.5–45.0 µM | [149] | |
| Electropolymerization | Phytic acid/PANI/SCoV2-rS | 1.5 h | Antibodies against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Spike protein | - | recombinant Spike protein (SCoV2-rS) | EIS | LoD—8.00 nM; range to 23.93 nM | [150] | |
| Electropolymerization | Olyaniline titanium oxide (PANI-TiO2)/monoclonal antibodies specific to l-glutamic acid | 14 h | L-glutamic acid | Tomato sauce | Immunosensor (anti-glutamate monoclonal antibodies) | DPV | Sensitivity ~37 mA/nM; detection ranges 1 nM to 500 µM in the electrolyte, 1 µM to 250 µM in tomato sauce | [151] | |
Polythiophene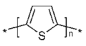 | Electrochemical polymerization | Polythiophene film/graphene oxide (GrO)/GOx | 13 h | Glucose | Commercial fruit juice samples (pear and apricot) | Enzymatic (GOx) | CA | Linear range—0.2–10.0 mM; LoD—0.036 mM; sensitivity—9.4 µA mM−1 cm−2; Response Time—10–20 s; Stability >60 Days | [152] |
| Amperometric depositPon | AuNPs- poly(thiophene-3-boronic acid) (PT3BA)- tyrosinase enzyme | - | Dopamine | human urine sample | Enzymatic (tyrosinase) | DPV, CV | Linear range of detection—5 × 10−8–3 × 10−5 M; LoD—2 × 10−8 M; RSD—3.2%; The lifetime was at least 2 months (89%) | [153] | |
| Electrochemical polymerization | (Poly)thiophenes, namely 2,2′-bithiophene (poly(2,2′-BT))/GOx | 1 day | Glucose | Fruit juices (pear, peach, and apricot) | Enzymatic (GOx) | CA, CV | LoD—30 μM; Linear range of detection—0.09–5.20 mM; response time—120–180 s; stability >15 days | [154] | |
| 4,4′-bis(2-methyl-3-butyn-2-ol)-2,2′-bithiophene (poly(4,4′-bBT))/GOx | LoD—50 μM; Linear range of detection—0.15–5.20 mM; response time 20–50 s; stability >30 days | ||||||||
PEDOT | Electrodeposition | Au-XOR/fMWCNT-PEDOT/GCE | 6 h | Xanthine | Human serum and urine samples; fish and meat samples | Enzymatic (xanthine oxidoreductase (XOR)) | DPV, CV, EIS | LoD—5.45 × 10−2 мкM; Linear range—0.1–10 мкM; response time—4 s, sensitivity—16.075 µA.µM−1cm−2), stability—4 months | [155] |
| Deposition | G. oxydans/PEDOT: PSS/graphene/Nafion | 1 day | Glucose | - | Microbial (G. oxydans) | CV, EIS, CA | Sensitivity—22 μA × mM−1 × cm−2; concentration range 0.02–2 mM; LoD—0.02 mM; Stability >120 Days | [114] | |
| Deposition | Metal-organic framework (MOF), i.e., MIL-53 (Fe) (MIL = Materials of Institut Lavoisier)/PEDOT:PSS/anti-E. coli antibodies | Several days | E. coli | - | Immunosen-sor | DPV, EIS | Concentration range—2.1 × 102 –2.1 × 108 cfu/mL, LoD—4 cfu/mL | [156] | |
Poly(p-phenylene) | Electrodeposition | Poly(p-phenelyne) modified in side chain position with ferrocenyl group (Fc-PPP)/β-alanin/DNA | - | DNA | - | DNA | CV, EIS, | LoD—30 fM; range of detection to 10 pM; electron transfer kinetics with a value of 68 s−1 | [157] |
| Conductive Polymer | Composite on the Electrode Surface | Enzyme | Electrode Material | Determined Compound | Analytical Parameters | Reference |
|---|---|---|---|---|---|---|
| PEI | PEI@AuNP | CDH | Gold disk electrode | Lactose | Electron-transfer (ET) rate (39.6 ± 2.5) s−1; linea range from 1 to 100 μm; response time less than 5 s | [96] |
| PPy | Nafion-GOx-fMWCNTs-PPy | GOx | Pt electrode | Glucose | Sensitivity (54.2 μA mM−1 cm−2) in the linear range up to 4.1 mM, LoD—5 μM, response time within 4 s, | [97] |
| PEDOT | (PAN-MWCNTs)/PEDOT | GOx | Pt disk | Glucose | Sensitivity 92.94 µA/mM cm−2; LoD—2.30 µM; linear range 0.01–1.2 mM | [158] |
| PPy | PAN-MWCNTs)/PPy | GOx | Pt disk | Glucose | Sensitivity 81.72 µA/mM cm−2; LoD—2.38 µM; linear range 0.01–2.0 mM | |
| PEDOT:PSS | GP-PEDOT:PSS | GOx | SPE | Glucose | Sensitivity of 7.23 μA/mM; linear range of 20–900 μM; LoD—0.3 μM; enzyme activity decreases by 30% after 30 days. | [159] |
| PANI | PANI/SnO2-NFs | Catalase | GCE | H2O2 | Linear range 10 to 120 μM; LoD—0.6 μM; stability 92% (35 days) | [160] |
| PPy | PPy-Cl-PPy | GOx | Pt-disc | Glucose | Linear range of 0.5–24 mM and LoD—26.9 μM; Highly stable reponse for more than 2 months; sensitivity 3.5 μA cm−2 mM−1; 1.9% RSD; Rejects interferences from ascorbic acid, glycine, glutamic acid and uric acid | [161] |
| DTP(aryl)aniline | GCE/p DTP(aryl)aniline/ChOx | ChOx | GCE | Cholesterol | Linear range 2.0 μM–23.7 μM; LoD—0.27 μM; sensitivity of 11,246 μA/μM; biosensor lost its 45% of initial activity after 25 days. | [162] |
| PPI dendrimer | GCE/PPI/QDs/ChOx | ChOx | GCE | Cholesterol | Linear Range 0.1–10 mM; LoD—0.075 mM; Sensitivity 111.16 μA mM−1 cm−2. After a month of storage at 4 °C, the biosensor retained 97% of the original response in the same sample. | [163] |
5. BFCs Based on Conductive Polymers
5.1. Enzymatic Anodes
5.2. Microbial Anodes
5.3. Cathodes
| Polymer | Anode Composition | Biocatalyst | Substrate | Real Application | Power | Current Density | Maximum Voltage, mV | Reference |
|---|---|---|---|---|---|---|---|---|
| PEDOT | PEDOT/MWCNT/ GOx | Glucose oxidase (anode)/bilirubin oxidase (cathode) | Glucose | Animal/human body implantation | 236 mW cm−2 | 350 mA cm−2 | 620 | [192] |
| PEDOT | Biochar/NiFe2O4/ PEDOT/bacteria | Pre-acclimated bacteria from an MFC reactor | Glucose | Sustainable green energy generation from wastewater | 1200 mW m−2 | 3324 mA m−2 | 690 | [223] |
| PEDOT | PEDOT/graphene/ nickel | Escherichia coli | Glucose | Generating energy from organic wastes | 0.32 mW cm−2 | 1.7 mA/cm−2 | 210 | [224] |
| PEDOT | Carbon felt/ PEDOT/bacteria | Microbial consortium | Glucose | Sewage wastewater treatment | 2.864 mW m−2 | 3813 mA m−2 | 1470 | [206] |
| PEDOT:PSS | PEDOT:PSS/ sulfonated graphene oxide/ ferritin/GOx | Glucose oxidase | Glucose | Self-powered glucose biosensors | - | 27 ± 2 mA cm−2 | - | [188] |
| PEDOT:PSS |
Carbon veil/ PEDOT:PSS/ sludge | Sludge | Urine | MFC continuously fed with neat human urine | 10.70 µW∙cm−2 | 200 μA∙cm−2 | 705 | [225] |
| PEDOT:PSS | PEDOT:PSS/ graphene/Nafion/G. oxydans | Gluconobacter oxydans |
Synthetic/ municipal wastewater | Treatment of municipal wastewater samples with low pH | 81 mW m−2 | 2.1 mA cm−2 | 550 | [227] |
| PEDOT:PSS | Carbon felt/ PEDOT:PSS/thermally expanded graphite/bacteria | Microbial consortium | Sewage wastewater/ glucose | Sewage wastewater treatment | 68.7 mW m−2 | 969.3 mA m−2 | 540 | [228] |
| PEDOT:PSS | Nickel foam/MgCoO2/PEDOT:PSS/bacteria | Sludge | Wastewater | New materials for wastewater treatment systems | 494 mW m−2 | 900 mA m−2 | [229] | |
| Rectangular polypyrrole | Nickel foam/Nafion/GOx/polyvinylpyrrolidone/polypyrrole | Glucose oxidase/laccase | Glucose | DET anode for glucose fuel cells | 0.350 mW cm−2 | 3.1 mA cm−2 | 1160 | [194] |
| Polypyrrole | Cellulose/ polypyrrole/FDH | Fructose dehydrogenase (anode)/laccase (cathode) | Fructose | Use of hybrid capacitive polymer materials in BFC | 2.1 mW cm−2 | 13 mA cm−2 | 590 | [195] |
| Polypyrrole | Graphite/PPy/yeast | Saccharomyces cerevisiae | Glucose | Evaluation of the possibility of using yeast in BFC | 47.12 mW m−2 | 5.2 mA cm−2 | 390 | [215] |
| Polypyrrole | Stainless steel/PPy/bacteria | Sludge from fruit wastewater treatment | Acetate | Creation of cheap BFC bioanodes | 1190.94 mW m−2 | 1366.4 mA m−2 | 547 | [216] |
| Polypyrrole | Carbon black/PPy/carboxymethyl cellulose/CNTs/bacteria | Electricity-producing microorganisms | Acetate | Environmentally friendly modification of composite anode | 2970 mW m−2 | 5.20 A m−2 | - | [218] |
| Polypyrrole | Carbon felt/PPy/Fe3O4/ bacteria | Electricity-producing bacteria from soil | Molasses wastewate | Degrading molasses wastewater | - | 0.170 A m−2 | - | [219] |
| Polypyrrole | Carbon cloth/PPy/bacteria | - | - | Capacitive bioanode for paper-based microbial fuel cell | 29 µW cm−2 | 299 μA cm−2 | 580 | [220] |
| Sigracell® PV15 | PV15/diethylenetriamine/glutaraldehyde/GOx | Glucose oxidase (anode)/laccase (cathode) | Glucose | Conversion of organic substrates contained in wastewater of oil mills | 2.41 µW cm−2 | 2.8 μA cm−2 | 390 | [200,202] |
| Polyaniline | Polyaniline/ferritin/GOx | Glucose oxidase | Glucose | One-step electrode construction BFC anodes | - | 22.3 ± 2 mA cm−2 | - | [196] |
| Polyaniline | GCE/Au@PANI/GOx | Glucose oxidase | Glucose | High-throughput membrane-less bioenergy devices | 685 µW cm−2 | 12 mA cm−2 | 760 | [197] |
| Polyaniline | Nafion/PANI1600@CNTs/GOx | Glucose oxidase (anode)/laccase (cathode) | Glucose | DET anode/cathode for glucose fuel cells | 1.12 mW cm−2 | 6.2 mA cm−2 | 780 | [198] |
| Polyaniline | Nickel foam/graphene oxide/PANI/GOx | Glucose oxidase | Glucose | Flow-through electrodes for glucose-based enzymatic microfuel cells | 118 µW cm−2 | - | - | [199] |
| Polyaniline | Chitosan@reduced graphene oxide/polyaniline/ferritin/GOx | Glucose oxidase | Glucose | Glucose-based EFCs | - | 3.5 mA·cm−2 | - | [201] |
| Polyaniline | Bacterial cellulose/polyaniline/TiO2/S. xiamenensis | Shewanella xiamenensis | Glucose | Low-cost compact microbial fuel cells | 40.66 W m−3 | 116.72 A m−3 | 790 | [208] |
| Polyaniline | Carbon cloth/rGO/polyanliline/bacteria | Microbial consortium | Domestic wastewater | MFC for wastewater recovery | 306 mW m2 | 1050 mA m−2 | 381 | [210] |
| Polyaniline | Graphite/PANI/bacteria | Activated sludge | Potato powder/soybean powder | Purification of biodegradable organic compounds in wastewater | 256.4 mW cm−2 | 324.2 mA cm−2 | - | [211] |
| Polyaniline | Carbon paper/PANI/TiO2/graphene/Nafion/S. oneidensis | Shewanella oneidensis | Trypton | Bifunctional catalyst to improve the performance of both the anode and cathode of MFCs | 79.3 mW m2 | 135 mA m−2 | 650 | [235] |
| Polydopamine | Activated carbon/PDA/bacteria | Microbial consortium | Acetate | Superhydrophilic surface for microbial anodes | 803 mW m−2 | 4 A m−2 | 540 | [212] |
| Polydopamine | Carbon felt/PDA/S. xiamenensis | Shewanella xiamenensis | Lactate | Possibility of immobilization of redox-active PDA on the surface of individual cells | 452.8 mW m−2 | 142.7 μA cm−2 | 750 | [214] |
| Polymer | Cathode Composition | Biocatalyst | Substrate | Real Application | Power | Current Density | Maximum Voltage, mV | Reference |
|---|---|---|---|---|---|---|---|---|
| PVA | Carbon cloth/PVA/WRF | White rot fungi | Copper-containing solution | Removing Cu2+ from the wastewater | 41.3 mW m−2 | 260 mA m−2 | 710 mV | [171] |
| PVA | CNTs/PVA/sorbitol/laccase | Laccase | ABTS/O2 | Printable laccase-based biocathode for fuel cell applications | 11 µW∙cm−2 | 50 mA cm−2 | 1100 | [252] |
| Polyindole | Polyindole/iron phthalocyanine/CNTs | - | Domestic wastewater/acetate | Low-cost composite with high ORR | 799 mW m−2 | 3480 mA m−2 | 695 | [236] |
| Polypyrrole | Carbon cloth/PPy | Shewanella putrefaciens (in anode) | Glucose | Iron removal from wastewater | 190 mW m−2 | 1.27 A m−2 | 690 | [237] |
| Polypyrrole | Carbon paper/Ni–NiO/PPy–rGO | Mixed bacterial culture from a previously used MFC | - | COD removal | 678.79 mW m−2 | 2134.56 mA m−2 | 610 | [238] |
| Polypyrrole | Stainless steel/PPy/poly(methylene blue)/C. vulgaris | Chlorella vulgaris (cathode)/g Saccharomyces cerevisiae (anode) | Carbon dioxide | A photosynthetic biocathodic half-cell | 7 mW m2 | 65 mA m−2 | 370 | [239] |
| Poly(pyrrole-2-carboxylic acid) | Graphite rod/PPCA/Prussian blue/GOx | Glucose oxidase | Glucose | Biocathode for glucose-powered single-enzyme biofuel cell | - | 31.68 μA cm−2 | 430 | [257] |
| Polyaniline | Carbon felt/PANI/sludge | Aerobic sludge | NaHCO3 | Utilization of nitrates | 199 mW m2 | 1420 mA m−2 | 482 | [181] |
| Polyaniline | Graphite/PANI/tourmaline/bacteria | Sludge | NaHCO3 | Mineral-based biocathode | 266 mW m−2 | 1220 mA m−2 | - | [246] |
| Polyaniline | PANI/laccase | Laccase | ABTS/O2 | Biocathode for azo dye decolorization | 38 mW m−2 | 175 mA m−2 | - | [249] |
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Pal, T.; Banerjee, S.; Manna, P.K.; Kar, K.K. Characteristics of Conducting Polymers BT. In Handbook of Nanocomposite Supercapacitor Materials I: Characteristics; Kar, K.K., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 247–268. ISBN 978-3-030-43009-2. [Google Scholar]
- Shirakawa, H.; Louis, E.J.; MacDiarmid, A.G.; Chiang, C.K.; Heeger, A.J. Synthesis of electrically conducting organic polymers: Halogen derivatives of polyacetylene,(CH) x. J. Chem. Soc. Chem. Commun. 1977, 16, 578–580. [Google Scholar] [CrossRef]
- Kanazawa, K.K.; Diaz, A.F.; Geiss, R.H.; Gill, W.D.; Kwak, J.F.; Logan, J.A.; Rabolt, J.F.; Street, G.B. ‘Organic metals’: Polypyrrole, a stable synthetic ‘metallic’polymer. J. Chem. Soc. Chem. Commun. 1979, 19, 854–855. [Google Scholar] [CrossRef]
- Namsheer, K.; Rout, C.S. Conducting polymers: A comprehensive review on recent advances in synthesis, properties and applications. RSC Adv. 2021, 11, 5659–5697. [Google Scholar] [CrossRef]
- Park, Y.; Jung, J.; Chang, M. Research progress on conducting polymer-based biomedical applications. Appl. Sci. 2019, 9, 1070. [Google Scholar] [CrossRef]
- Nair, S.S.; Mishra, S.K.; Kumar, D. Recent progress in conductive polymeric materials for biomedical applications. Polym. Adv. Technol. 2019, 30, 2932–2953. [Google Scholar] [CrossRef]
- Guo, B.; Ma, P.X. Conducting Polymers for Tissue Engineering. Biomacromolecules 2018, 19, 1764–1782. [Google Scholar] [CrossRef]
- Dong, R.; Ma, P.X.; Guo, B. Conductive biomaterials for muscle tissue engineering. Biomaterials 2020, 229, 119584. [Google Scholar] [CrossRef]
- Krukiewicz, K.; Bednarczyk-Cwynar, B.; Turczyn, R.; Zak, J.K. EQCM verification of the concept of drug immobilization and release from conducting polymer matrix. Electrochim. Acta 2016, 212, 694–700. [Google Scholar] [CrossRef]
- Boehler, C.; Oberueber, F.; Asplund, M. Tuning drug delivery from conducting polymer films for accurately controlled release of charged molecules. J. Control. Release 2019, 304, 173–180. [Google Scholar] [CrossRef]
- Lakard, S.; Morrand-Villeneuve, N.; Lesniewska, E.; Lakard, B.; Michel, G.; Herlem, G.; Gharbi, T.; Fahys, B. Synthesis of polymer materials for use as cell culture substrates. Electrochim. Acta 2007, 53, 1114–1126. [Google Scholar] [CrossRef]
- Sardana, S.; Gupta, A.; Singh, K.; Maan, A.S.; Ohlan, A. Conducting polymer hydrogel based electrode materials for supercapacitor applications. J. Energy Storage 2022, 45, 103510. [Google Scholar] [CrossRef]
- El Rhazi, M.; Majid, S.; Elbasri, M.; Salih, F.E.; Oularbi, L.; Lafdi, K. Recent progress in nanocomposites based on conducting polymer: Application as electrochemical sensors. Int. Nano Lett. 2018, 8, 79–99. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, Y.; Guo, X.; Yu, G. Conductive polymers for stretchable supercapacitors. Nano Res. 2019, 12, 1978–1987. [Google Scholar] [CrossRef]
- Agobi, A.U.; Louis, H.; Magu, T.O.; Dass, P.M. A review on conducting polymers-based composites for energy storage application. J. Chem. Rev 2019, 1, 19–34. [Google Scholar]
- Li, J.; Levitt, A.; Kurra, N.; Juan, K.; Noriega, N.; Xiao, X.; Wang, X.; Wang, H.; Alshareef, H.N.; Gogotsi, Y. MXene-conducting polymer electrochromic microsupercapacitors. Energy Storage Mater. 2019, 20, 455–461. [Google Scholar] [CrossRef]
- Liu, S.; Pan, T.J.; Wang, R.F.; Yue, Y.; Shen, J. Anti-corrosion and conductivity of the electrodeposited graphene/polypyrrole composite coating for metallic bipolar plates. Prog. Org. Coat. 2019, 136, 105237. [Google Scholar] [CrossRef]
- Humpolíček, P.; Kašpárková, V.; Pacherník, J.; Stejskal, J.; Bober, P.; Capáková, Z.; Radaszkiewicz, K.A.; Junkar, I.; Lehocký, M. The biocompatibility of polyaniline and polypyrrole: A comparative study of their cytotoxicity, embryotoxicity and impurity profile. Mater. Sci. Eng. C 2018, 91, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Ramanavicius, S.; Ramanavicius, A. Charge transfer and biocompatibility aspects in conducting polymer-based enzymatic biosensors and biofuel cells. Nanomaterials 2021, 11, 371. [Google Scholar] [CrossRef]
- He, H.; Zhang, L.; Guan, X.; Cheng, H.; Liu, X.; Yu, S.; Wei, J.; Ouyang, J. Biocompatible conductive polymers with high conductivity and high stretchability. ACS Appl. Mater. Interfaces 2019, 11, 26185–26193. [Google Scholar] [CrossRef]
- Timonov, A.M.; Vasilyeva, S.V. Electronic conductivity of polymer compounds. Soros Educ. J. 2000, 6, 33–39. [Google Scholar]
- Perchikov, R.N.; Provotorova, D.V.; Kharkova, A.S.; Arlyapov, V.A.; Medvedeva, A.S.; Machulin, A.V.; Filonov, A.E.; Reshetilov, A.N. Bioanalytical System for Determining the Phenol Index Based on Pseudomonas putida BS394 (pBS216) Bacteria Immobilized in a Redox-Active Biocompatible Composite Polymer “Bovine Serum Albumin–Ferrocene–Carbon Nanotubes”. Polymers 2022, 14, 5366. [Google Scholar] [CrossRef] [PubMed]
- Karyakin, A.A.; Karyakina, E.E.; Schmidt, H. Electropolymerized Azines: A New Group of Electroactive Polymers. Electroanalysis 1999, 11, 149–155. [Google Scholar] [CrossRef]
- Dalkiran, B.; Brett, C.M.A. Polyphenazine and polytriphenylmethane redox polymer/nanomaterial–based electrochemical sensors and biosensors: A review. Microchim. Acta 2021, 188, 178. [Google Scholar] [CrossRef]
- Kuznetsova, L.S.; Arlyapov, V.A.; Kamanina, O.A.; Lantsova, E.A.; Tarasov, S.E.; Reshetilov, A.N. Development of Nanocomposite Materials Based on Conductive Polymers for Using in Glucose Biosensor. Polymers 2022, 14, 1543. [Google Scholar] [CrossRef] [PubMed]
- Medvedeva, A.S.; Dyakova, E.I.; Kuznetsova, L.S.; Mironov, V.G.; Gurkin, G.K.; Rogova, T.V.; Kharkova, A.S.; Melnikov, P.V.; Naumova, A.O.; Butusov, D.N. A Two-Mediator System Based on a Nanocomposite of Redox-Active Polymer Poly (thionine) and SWCNT as an Effective Electron Carrier for Eukaryotic Microorganisms in Biosensor Analyzers. Polymers 2023, 15, 3335. [Google Scholar] [CrossRef]
- Xing, S. Nanomaterials of conducting polymers and its application in energy conversion and storage. In Advanced Nanomaterials for Electrochemical-Based Energy Conversion and Storage; Elsevier: Amsterdam, The Netherlands, 2020; pp. 325–354. ISBN 9780128145586. [Google Scholar]
- Choudhary, R.B.; Ansari, S.; Majumder, M. Recent advances on redox active composites of metal-organic framework and conducting polymers as pseudocapacitor electrode material. Renew. Sustain. Energy Rev. 2021, 145, 110854. [Google Scholar] [CrossRef]
- Nezakati, T.; Seifalian, A.; Tan, A.; Seifalian, A.M. Conductive Polymers: Opportunities and Challenges in Biomedical Applications. Chem. Rev. 2018, 118, 6766–6843. [Google Scholar] [CrossRef]
- Sumdani, M.G.; Islam, M.R.; Yahaya, A.N.A.; Safie, S.I. Recent advancements in synthesis, properties, and applications of conductive polymers for electrochemical energy storage devices: A review. Polym. Eng. Sci. 2022, 62, 269–303. [Google Scholar] [CrossRef]
- Guo, X.; Facchetti, A. The journey of conducting polymers from discovery to application. Nat. Mater. 2020, 19, 922–928. [Google Scholar] [CrossRef]
- Zhang, L.; Du, W.; Nautiyal, A.; Liu, Z.; Zhang, X. Recent progress on nanostructured conducting polymers and composites: Synthesis, application and future aspects. Sci. China Mater. 2018, 61, 303–352. [Google Scholar] [CrossRef]
- Zhou, W.; Xu, J. Progress in Conjugated Polyindoles: Synthesis, Polymerization Mechanisms, Properties, and Applications. Polym. Rev. 2017, 57, 248–275. [Google Scholar] [CrossRef]
- Nguyen, D.N.; Yoon, H. Recent Advances in Nanostructured Conducting Polymers: From Synthesis to Practical Applications. Polymers 2016, 8, 118. [Google Scholar] [CrossRef] [PubMed]
- Malev, V.V.; Kondratiev, V.V.; Timonov, A.M. Polymer-modified electrodes. Nestor-History SPb 2012, 344. [Google Scholar]
- Fan, Y.; Bai, W.; Mu, P.; Su, Y.; Zhu, Z.; Sun, H.; Liang, W.; Li, A. Conductively monolithic polypyrrole 3-D porous architecture with micron-sized channels as superior salt-resistant solar steam generators. Sol. Energy Mater. Sol. Cells 2020, 206, 110347. [Google Scholar] [CrossRef]
- Nie, S.; Li, Z.; Yao, Y.; Jin, Y. Progress in Synthesis of Conductive Polymer Poly(3,4-Ethylenedioxythiophene). Front. Chem. 2021, 9, 803509. [Google Scholar] [CrossRef]
- Fadel, M.; Fadeel, D.A.; Ibrahim, M.; Hathout, R.M.; El-Kholy, A.I. One-step synthesis of polypyrrole-coated gold nanoparticles for use as a photothermally active nano-system. Int. J. Nanomed. 2020, 15, 2605–2615. [Google Scholar] [CrossRef]
- Jangid, N.K.; Jadoun, S.; Kaur, N. A review on high-throughput synthesis, deposition of thin films and properties of polyaniline. Eur. Polym. J. 2020, 125, 109485. [Google Scholar]
- Pang, A.L.; Arsad, A.; Ahmadipour, M. Synthesis and factor affecting on the conductivity of polypyrrole: A short review. Polym. Adv. Technol. 2021, 32, 1428–1454. [Google Scholar] [CrossRef]
- Majeed, A.H.; Mohammed, L.A.; Hammoodi, O.G.; Sehgal, S.; Alheety, M.A.; Saxena, K.K.; Dadoosh, S.A.; Mohammed, I.K.; Jasim, M.M.; Salmaan, N.U. A Review on Polyaniline: Synthesis, Properties, Nanocomposites, and Electrochemical Applications. Int. J. Polym. Sci. 2022, 2022, 9047554. [Google Scholar] [CrossRef]
- Walde, P.; Kashima, K.; Ćirić-Marjanović, G. Synthesizing Polyaniline With Laccase/O2 as Catalyst. Front. Bioeng. Biotechnol. 2019, 7, 165. [Google Scholar] [CrossRef]
- Song, Y.; Fan, J.B.; Wang, S. Recent progress in interfacial polymerization. Mater. Chem. Front. 2017, 1, 1028–1040. [Google Scholar] [CrossRef]
- Cheng, W.; Liu, Y.; Tong, Z.; Zhu, Y.; Cao, K.; Chen, W.; Zhao, D.; Yu, H. Micro-interfacial polymerization of porous PEDOT for printable electronic devices. EcoMat 2023, 5, e12288. [Google Scholar] [CrossRef]
- Thao, V.D.; Giang, B.L.; Thu, T.V. Free-standing polypyrrole/polyaniline composite film fabricated by interfacial polymerization at the vapor/liquid interface for enhanced hexavalent chromium adsorption. RSC Adv. 2019, 9, 5445–5452. [Google Scholar] [CrossRef]
- Konwar, G.; Sarma, S.C.; Mahanta, D.; Peter, S.C. Polyaniline hybrid nanofibers via green interfacial polymerization for all-solid-state symmetric supercapacitors. ACS Omega 2020, 5, 14494–14501. [Google Scholar] [CrossRef]
- Saheeda, P.; Jayaleksmi, S. Liquid/liquid interfacial polymerization as an effective synthesis approach for polypyrrole/MWCNTs nanocomposite with impressive nonlinear optical properties. Opt. Mater. 2020, 104, 109940. [Google Scholar] [CrossRef]
- Dey Sadhu, S.; Meena, P.L.; Kumar, J.; Gupta, J.; Choudhary, S.; Gupta, A. Preparation and characterization of polyaniline-and polythiophene-based copolymer and its nanocomposite suitable for electro-optical devices. Polym. Compos. 2020, 41, 4619–4630. [Google Scholar] [CrossRef]
- Inagaki, C.S.; Oliveira, M.M.; Bergamini, M.F.; Marcolino-Junior, L.H.; Zarbin, A.J.G. Facile synthesis and dopamine sensing application of three component nanocomposite thin films based on polythiophene, gold nanoparticles and carbon nanotubes. J. Electroanal. Chem. 2019, 840, 208–217. [Google Scholar] [CrossRef]
- Korent, A.; Žagar Soderžnik, K.; Šturm, S.; Žužek Rožman, K. A Correlative Study of Polyaniline Electropolymerization and its Electrochromic Behavior. J. Electrochem. Soc. 2020, 167, 106504. [Google Scholar] [CrossRef]
- Ashassi-Sorkhabi, H.; Kazempour, A. Incorporation of organic/inorganic materials into polypyrrole matrix to reinforce its anticorrosive properties for the protection of steel alloys: A review. J. Mol. Liq. 2020, 309, 113085. [Google Scholar] [CrossRef]
- Chakraborty, R.; Seesala, V.S.; Manna, J.S.; Saha, P.; Dhara, S. Synthesis, characterization and cytocompatibility assessment of hydroxyapatite-polypyrrole composite coating synthesized through pulsed reverse electrochemical deposition. Mater. Sci. Eng. C 2019, 94, 597–607. [Google Scholar] [CrossRef]
- Hamdi-Mohammadabad, P.; Tohidi, T.; Talebzadeh, R.; Mohammad-Rezaei, R.; Rahmatallahpur, S. Preparation and characterization of gamma irradiated ZnO/PANI hybrid films. J. Radioanal. Nucl. Chem. 2021, 330, 785–796. [Google Scholar] [CrossRef]
- Gizdavic-Nikolaidis, M.R.; Stanisavljev, D.R.; Easteal, A.J.; Zujovic, Z.D. A rapid and facile synthesis of nanofibrillar polyaniline using microwave radiation. Macromol. Rapid Commun. 2010, 31, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Sury, S.V.J.; Ulianas, A.; Aini, S. Synthesis of conducting polyaniline with photopolymerization method and characterization. In Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2021; Volume 1788, p. 12004. [Google Scholar]
- Arlyapov, V.A.; Yudina, N.Y.; Asulyan, L.D.; Kamanina, O.A.; Alferov, S.V.; Shumsky, A.N.; Machulin, A.V.; Alferov, V.A.; Reshetilov, A.N. Registration of BOD using Paracoccus yeei bacteria isolated from activated sludge. 3 Biotech 2020, 10, 207. [Google Scholar] [CrossRef] [PubMed]
- Krishnaswamy, S.; Ragupathi, V.; Raman, S.; Panigrahi, P.; Nagarajan, G.S. Optical properties of P-type polypyrrole thin film synthesized by pulse laser deposition technique: Hole transport layer in electroluminescence devices. Optik 2019, 194, 163034. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Saeb, M.R. Synthetic route of polyaniline (IV): Irradiation path. In Fundamentals and Emerging Applications of Polyaniline; Elsevier: Amsterdam, The Netherlands, 2019; pp. 91–103. [Google Scholar]
- Idumah, C.I.; Ezeani, E.O.; Nwuzor, I.C. A review: Advancements in conductive polymers nanocomposites. Polym. Technol. Mater. 2021, 60, 756–783. [Google Scholar] [CrossRef]
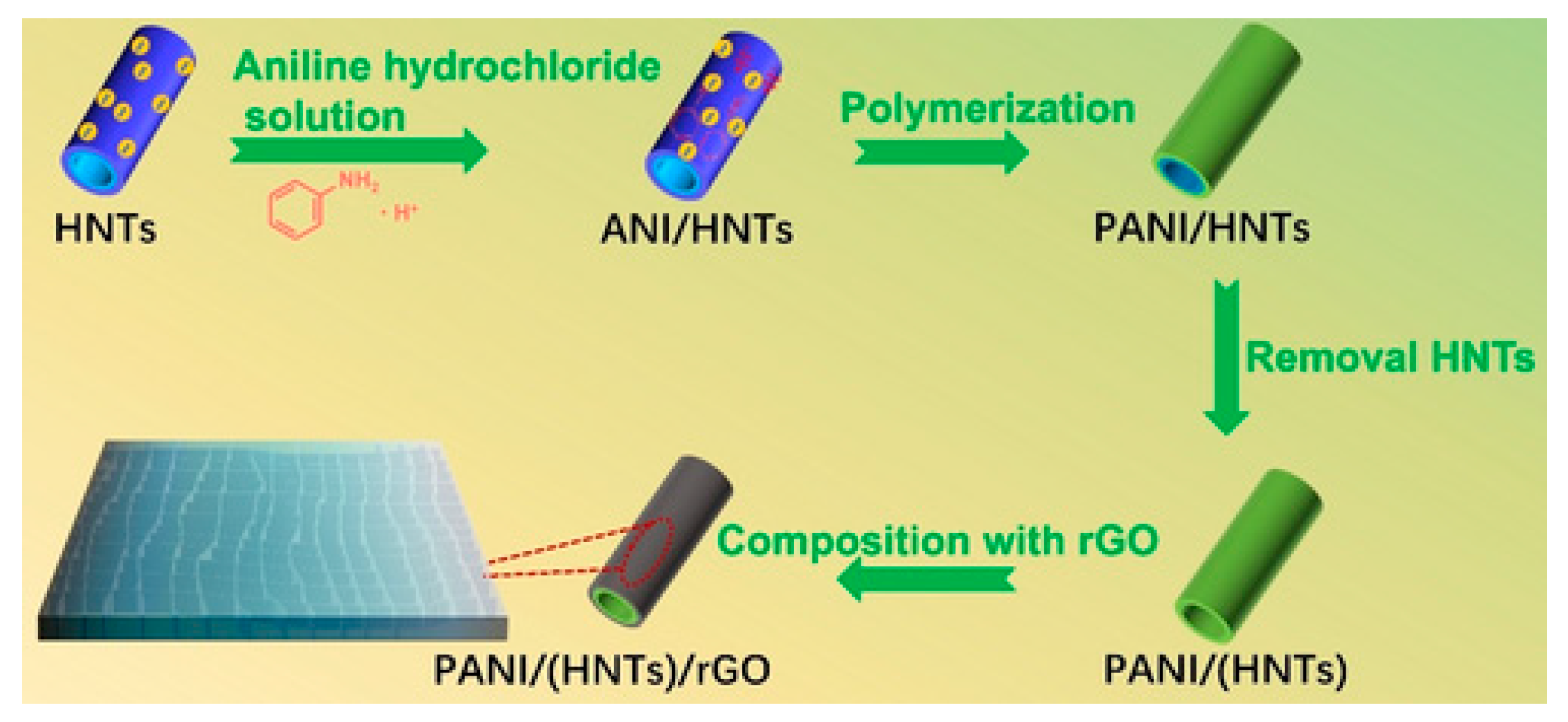
- Wang, X.; Fan, P.; Wang, S.; Liu, H.; Liao, L. Nanotubular Polyaniline/Reduced Graphene Oxide Composite Synthesized from a Natural Halloysite Template for Application as a High Performance Supercapacitor Electrode. ChemistrySelect 2022, 7, e202104402. [Google Scholar] [CrossRef]
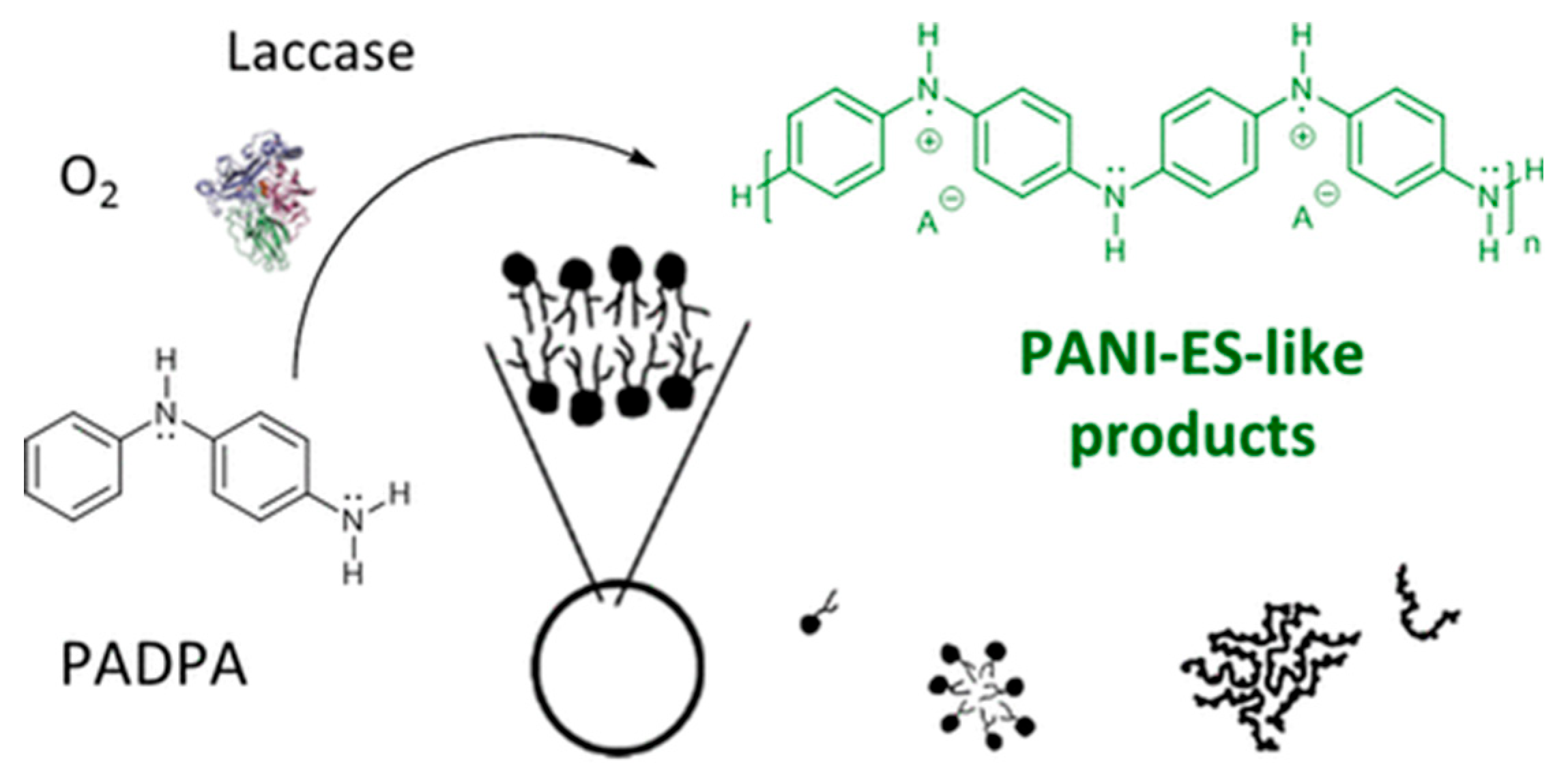
- Kashima, K.; Fujisaki, T.; Serrano-Luginbühl, S.; Kissner, R.; Janošević Ležaić, A.; Bajuk-Bogdanović, D.; Ćirić-Marjanović, G.; Busato, S.; Ishikawa, T.; Walde, P. Effect of Template Type on the Trametes versicolor Laccase-Catalyzed Oligomerization of the Aniline Dimer p-Aminodiphenylamine (PADPA). ACS Omega 2019, 4, 2931–2947. [Google Scholar] [CrossRef]
- Wang, X.X.; Yu, G.F.; Zhang, J.; Yu, M.; Ramakrishna, S.; Long, Y.Z. Conductive polymer ultrafine fibers via electrospinning: Preparation, physical properties and applications. Prog. Mater. Sci. 2021, 115, 100704. [Google Scholar] [CrossRef]
- Maharjan, B.; Kaliannagounder, V.K.; Jang, S.R.; Awasthi, G.P.; Bhattarai, D.P.; Choukrani, G.; Park, C.H.; Kim, C.S. In-situ polymerized polypyrrole nanoparticles immobilized poly(ε-caprolactone) electrospun conductive scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2020, 114, 111056. [Google Scholar] [CrossRef]
- Ramanaviciene, A.; Plikusiene, I. Polymers in Sensor and Biosensor Design. Polymers 2021, 13, 917. [Google Scholar] [CrossRef]
- Xiao, X.; Yan, X.; Magner, E.; Ulstrup, J. Polymer coating for improved redox-polymer-mediated enzyme electrodes: A mini-review. Electrochem. Commun. 2021, 124, 106931. [Google Scholar] [CrossRef]
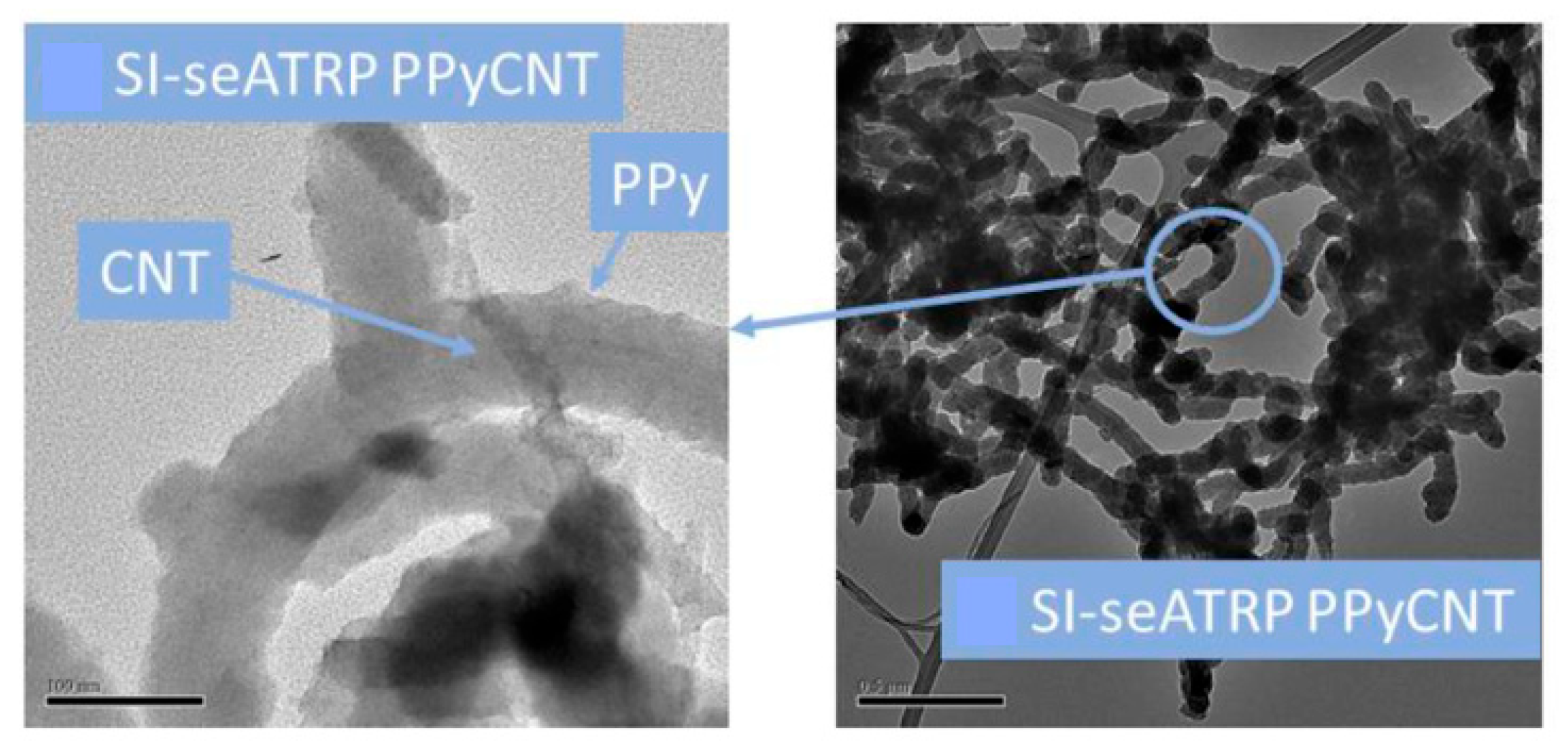
- O’Brien, C.; Ignaszak, A. Polymer-grafted-carbon assembled via an electrochemically-aided atom transfer radical polymerization: Towards improved energy storage electrode. Electrochem. Commun. 2022, 135, 107198. [Google Scholar] [CrossRef]
- Yan, W.; Li, J.; Zhang, G.; Wang, L.; Ho, D. A synergistic self-assembled 3D PEDOT: PSS/graphene composite sponge for stretchable microsupercapacitors. J. Mater. Chem. A 2020, 8, 554–564. [Google Scholar] [CrossRef]
- Wilczewska, P.; Breczko, J.; Bobrowska, D.M.; Wysocka-Żołopa, M.; Goclon, J.; Basa, A.; Winkler, K. Enhancement of polypyrrole electrochemical performance with graphene quantum dots in polypyrrole nanoparticle/graphene quantum dot composites. J. Electroanal. Chem. 2022, 923, 116767. [Google Scholar] [CrossRef]
- Bedioui, F.; Devynck, J.; Bied-Charreton, C. Immobilization of metalloporphyrins in electropolymerized films: Design and applications. Acc. Chem. Res. 1995, 28, 30–36. [Google Scholar] [CrossRef]
- You, H.; Mu, Z.; Zhao, M.; Zhou, J.; Yuan, Y.; Bai, L. Functional fullerene-molybdenum disulfide fabricated electrochemical DNA biosensor for Sul1 detection using enzyme-assisted target recycling and a new signal marker for cascade amplification. Sensors Actuators B Chem. 2020, 305, 127483. [Google Scholar] [CrossRef]
- Zhao, Z.; Das, S.; Zharnikov, M. Poly (ethylene glycol)–Fullerene Composite Films and Free-Standing Nanosheets for Flexible Electronic Devices and Sensors. ACS Appl. Nano Mater. 2023, 6, 2151–2161. [Google Scholar] [CrossRef]
- Rajagopalan, M.; Oh, I.-K.; Palanivel, J.; Sabarathinam, S.; Cifuentes-Faura, J. Fabrication and characterizations of electro-mechanical actuators based on fullerene-reinforced biocompatible polymer. Sensors Actuators A Phys. 2022, 339, 113510. [Google Scholar] [CrossRef]
- Zhang, Y.; Xia, Z.; Gui, G.; Zhao, G.; Meng, L. An Electrochemical Benzylpenicillin Biosensor Based on ß-Lactamase and Fullerene Supported by A Bilayer Lipid Membrane. Int. J. Electrochem. Sci. 2020, 15, 12007–12014. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, X.; Chen, Y.; Yu, P.; Wang, C.; Ma, Y. Enhanced capacitance and rate capability of graphene/polypyrrole composite as electrode material for supercapacitors. J. Power Sources 2011, 196, 5990–5996. [Google Scholar] [CrossRef]
- Bose, S.; Kuila, T.; Uddin, M.E.; Kim, N.H.; Lau, A.K.T.; Lee, J.H. In-situ synthesis and characterization of electrically conductive polypyrrole/graphene nanocomposites. Polymer 2010, 51, 5921–5928. [Google Scholar] [CrossRef]
- Chaughtai, Z.; Hashmi, M.A.; Yar, M.; Ayub, K. Electronic structure of polypyrrole composited with a low percentage of graphene nanofiller. Phys. Chem. Chem. Phys. 2021, 23, 8557–8570. [Google Scholar] [CrossRef] [PubMed]
- Troncoso, O.P.; Torres, F.G. Bacterial cellulose—Graphene based nanocomposites. Int. J. Mol. Sci. 2020, 21, 6532. [Google Scholar] [CrossRef] [PubMed]
- Hiemenz, P.C.; Rajagopalan, R. Principles of Colloid and Surface Chemistry, 3rd ed.; Marcel Dekker: New York, NY, USA, 1997; pp. 105–114. [Google Scholar]
- Suryanarayana, C.; Froes, F.H. The structure and mechanical properties of metallic nanocrystals. Metall. Trans. A 1992, 23, 1071–1081. [Google Scholar] [CrossRef]
- Osman, M.A.; Rupp, J.E.P.; Suter, U.W. Effect of non-ionic surfactants on the exfoliation and properties of polyethylene-layered silicate nanocomposites. Polymer 2005, 46, 8202–8209. [Google Scholar] [CrossRef]
- Chandra, A.; Turng, L.-S.; Gopalan, P.; Rowell, R.M.; Gong, S. Study of utilizing thin polymer surface coating on the nanoparticles for melt compounding of polycarbonate/alumina nanocomposites and their optical properties. Compos. Sci. Technol. 2008, 68, 768–776. [Google Scholar] [CrossRef]
- Martinazzo, J.; Brezolin, A.N.; Paschoalin, R.T.; Soares, A.C.; Steffens, J.; Steffens, C. Correction: Sexual pheromone detection using PANI· Ag nanohybrid and PANI/PSS nanocomposite nanosensors. Anal. Methods 2021, 13, 4528. [Google Scholar] [CrossRef]
- Zhao, J.; Huang, P.; Jin, W. Electrochemical sensor based on TiO2/polyvinyl alcohol nanocomposite for detection of ciprofloxacin in rainwater. Int. J. Electrochem. Sci. 2021, 16, 2. [Google Scholar] [CrossRef]
- Mahalakshmi, S.; Sridevi, V. In situ electrodeposited gold nanoparticles on polyaniline-modified electrode surface for the detection of dopamine in presence of ascorbic acid and uric acid. Electrocatalysis 2021, 12, 415–435. [Google Scholar] [CrossRef]
- German, N.; Ramanaviciene, A.; Ramanavicius, A. Dispersed conducting polymer nanocomposites with glucose oxidase and gold nanoparticles for the design of enzymatic glucose biosensors. Polymers 2021, 13, 2173. [Google Scholar] [CrossRef]
- Phasuksom, K.; Sirivat, A. Chronoampermetric detection of enzymatic glucose sensor based on doped polyindole/MWCNT composites modified onto screen-printed carbon electrode as portable sensing device for diabetes. RSC Adv. 2022, 12, 28505–28518. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, Y.; So, K.; Xia, H.-Q.; Kano, K. Orientation-Oriented Adsorption and Immobilization of Redox Enzymes for Electrochemical Communication with Electrodes; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Xue, H.; Shen, Z.; Li, Y. Polyaniline–polyisoprene composite film based glucose biosensor with high permselectivity. Synth. Met. 2001, 124, 345–349. [Google Scholar] [CrossRef]
- Bulut, U.; Sayin, V.O.; Cevher, S.C.; Cirpan, A.; Soylemez, S. Benzodithiophene bearing conjugated polymer-based surface anchoring for sensitive electrochemical glucose detection. Express Polym. Lett. 2022, 16, 1012–1021. [Google Scholar] [CrossRef]
- Neupane, S.; Bhusal, S.; Subedi, V.; Nakarmi, K.B.; Gupta, D.K.; Yadav, R.J.; Yadav, A.P. Preparation of an amperometric glucose biosensor on polyaniline-coated graphite. J. Sensors 2021, 2021, 8832748. [Google Scholar] [CrossRef]
- Shen, F.; Arshi, S.; Magner, E.; Ulstrup, J.; Xiao, X. One-step electrochemical approach of enzyme immobilization for bioelectrochemical applications. Synth. Met. 2022, 291, 117205. [Google Scholar] [CrossRef]
- Ramanavicius, A.; Rekertaitė, A.I.; Valiūnas, R.; Valiūnienė, A. Single-step procedure for the modification of graphite electrode by composite layer based on polypyrrole, Prussian blue and glucose oxidase. Sensors Actuators B Chem. 2017, 240, 220–223. [Google Scholar] [CrossRef]
- Pramanik, K.; Sarkar, P.; Bhattacharyay, D.; Majumdar, P. One step electrode fabrication for direct electron transfer cholesterol biosensor based on composite of polypyrrole, green reduced graphene oxide and cholesterol oxidase. Electroanalysis 2018, 30, 2719–2730. [Google Scholar] [CrossRef]
- Ramírez-Sánchez, K.; Alvarado-Hidalgo, F.; Zamora-Sequeira, R.; Sáenz-Arce, G.; Rojas-Carrillo, O.; Avedaño-Soto, E.; Ruepert, C.; Mena-Torres, F.; Starbird-Pérez, R. Biosensor based on the directly enzyme immobilization into a gold nanotriangles/conductive polymer biocompatible coat for electrochemical detection of Chlorpyrifos in water. Med. Devices Sensors 2019, 2, e10047. [Google Scholar] [CrossRef]
- Mazurenko, I.; Hitaishi, V.P.; Lojou, E. Recent advances in surface chemistry of electrodes to promote direct enzymatic bioelectrocatalysis. Curr. Opin. Electrochem. 2020, 19, 113–121. [Google Scholar] [CrossRef]
- Tavahodi, M.; Ortiz, R.; Schulz, C.; Ekhtiari, A.; Ludwig, R.; Haghighi, B.; Gorton, L. Direct electron transfer of cellobiose dehydrogenase on positively charged polyethyleneimine gold nanoparticles. Chempluschem 2017, 82, 546–552. [Google Scholar] [CrossRef]
- Shrestha, B.K.; Ahmad, R.; Shrestha, S.; Park, C.H.; Kim, C.S. Globular Shaped Polypyrrole Doped Well-Dispersed Functionalized Multiwall Carbon Nanotubes/Nafion Composite for Enzymatic Glucose Biosensor Application. Sci. Rep. 2017, 7, 16191. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.; Sima, M.; Stewart, R.J.; Minteer, S.D. Direct bioelectrocatalysis by redox enzymes immobilized in electrostatically condensed oppositely charged polyelectrolyte electrode coatings. Analyst 2020, 145, 1250–1257. [Google Scholar] [CrossRef] [PubMed]
- Kitova, A.; Tarasov, S.; Plekhanova, Y.; Bykov, A.; Reshetilov, A. Direct bioelectrocatalytic oxidation of glucose by Gluconobacter oxydans membrane fractions in PEDOT: PSS/TEG-modified biosensors. Biosensors 2021, 11, 144. [Google Scholar] [CrossRef]
- Hickey, D.P.; Lim, K.; Cai, R.; Patterson, A.R.; Yuan, M.; Sahin, S.; Abdellaoui, S.; Minteer, S.D. Pyrene hydrogel for promoting direct bioelectrochemistry: ATP-independent electroenzymatic reduction of N 2. Chem. Sci. 2018, 9, 5172–5177. [Google Scholar] [CrossRef] [PubMed]
- Clark, L.C., Jr.; Lyons, C. Electrode systems for continuous monitoring in cardiovascular surgery. Ann. N. Y. Acad. Sci. 1962, 102, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Ohara, T.J.; Rajagopalan, R.; Heller, A. “Wired” enzyme electrodes for amperometric determination of glucose or lactate in the presence of interfering substances. Anal. Chem. 1994, 66, 2451–2457. [Google Scholar] [CrossRef]
- Kumar, A.; Gupta, G.H.; Singh, G.; More, N.; Keerthana, M.; Sharma, A.; Jawade, D.; Balu, A.; Kapusetti, G. Ultrahigh sensitive graphene oxide/conducting polymer composite based biosensor for cholesterol and bilirubin detection. Biosens. Bioelectron. X 2023, 13, 100290. [Google Scholar] [CrossRef]
- Dong, S.; Zhang, J.; Huang, G.; Wei, W.; Huang, T. Conducting microporous organic polymer with–OH functional groups: Special structure and multi-functional integrated property for organophosphorus biosensor. Chem. Eng. J. 2021, 405, 126682. [Google Scholar] [CrossRef]
- Loos, K. Polymer Synthesis by Enzymatic Catalysis. In Macromolecular Engineering: Polymer Synthesis by Enzymatic Catalysis; Wiley: Hoboken, NJ, USA, 2022; pp. 1–75. [Google Scholar]
- German, N.; Popov, A.; Ramanaviciene, A.; Ramanavicius, A. Enzymatic formation of polyaniline, polypyrrole, and polythiophene nanoparticles with embedded glucose oxidase. Nanomaterials 2019, 9, 806. [Google Scholar] [CrossRef]
- German, N.; Popov, A.; Ramanaviciene, A.; Ramanavicius, A. Formation and electrochemical characterisation of enzyme-assisted formation of polypyrrole and polyaniline nanocomposites with embedded glucose oxidase and gold nanoparticles. J. Electrochem. Soc. 2020, 167, 165501. [Google Scholar] [CrossRef]
- Apetrei, R.-M.; Cârâc, G.; Bahrim, G.; Camurlu, P. Utilization of enzyme extract self-encapsulated within polypyrrole in sensitive detection of catechol. Enzym. Microb. Technol. 2019, 128, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Li, C.M.; Zang, J.; Zhou, Q.; Gan, Y.; Bao, H.; Guo, J.; Lee, V.S.; Moochhala, S.M. Biocatalytic generation of ppy-enzyme-CNT nanocomposite: From network assembly to film growth. J. Phys. Chem. C 2007, 111, 2025–2031. [Google Scholar] [CrossRef]
- Kurbanalieva, S.; Arlyapov, V.; Kharkova, A.; Perchikov, R.; Kamanina, O.; Melnikov, P.; Popova, N.; Machulin, A.; Tarasov, S.; Saverina, E.; et al. Electroactive Biofilms of Activated Sludge Microorganisms on a Nanostructured Surface as the Basis for a Highly Sensitive Biochemical Oxygen Demand Biosensor. Sensors 2022, 22, 6049. [Google Scholar] [CrossRef]
- Andryukov, B.G.; Romashko, R.V.; Efimov, T.A.; Lyapun, I.N.; Bynina, M.P. Mechanisms of adhesive–cohesive interaction of bacteria in the formation of biofilm. Mol. Genet. Microbiol. Virol. 2020, 35, 195–201. [Google Scholar] [CrossRef]
- Qi, X.; Wang, S.; Li, T.; Wang, X.; Jiang, Y.; Zhou, Y.; Zhou, X.; Huang, X.; Liang, P. An electroactive biofilm-based biosensor for water safety: Pollutants detection and early-warning. Biosens. Bioelectron. 2021, 173, 112822. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Wang, S.; Jiang, Y.; Liu, P.; Hao, W.; Han, J.; Zhou, Y.; Huang, X.; Liang, P. Additional polypyrrole as conductive medium in artificial electrochemically active biofilm (EAB) to increase the sensitivity of EAB based biosensor in water quality early-warning. Biosens. Bioelectron. 2021, 190, 113453. [Google Scholar] [CrossRef]
- Plekhanova, Y.; Tarasov, S.; Reshetilov, A. Use of PEDOT: PSS/Graphene/Nafion composite in biosensors based on acetic acid bacteria. Biosensors 2021, 11, 332. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Gao, G.; Xia, S. Development of a mediator-type bioelectrochemical sensor based on polypyrrole immobilized ferricyanide and microorganisms for biochemical oxygen demand fast detection. Int. J. Electrochem. Sci. 2015, 10, 9695–9705. [Google Scholar] [CrossRef]
- Ionescu, R.E.; Abu-Rabeah, K.; Cosnier, S.; Durrieu, C.; Chovelon, J.; Marks, R.S. Amperometric algal Chlorella vulgaris cell biosensors based on alginate and polypyrrole-alginate gels. Electroanal. Int. J. Devoted Fundam. Pract. Asp. Electroanal. 2006, 18, 1041–1046. [Google Scholar]
- Jha, S.K.; Kanungo, M.; Nath, A.; D’Souza, S.F. Entrapment of live microbial cells in electropolymerized polyaniline and their use as urea biosensor. Biosens. Bioelectron. 2009, 24, 2637–2642. [Google Scholar] [CrossRef]
- Yousif, N.M.; Gomaa, O.M. Screen-printed biosensor based on electro-polymerization of bio-composite for nitrate detection in aqueous media. Environ. Technol. 2023, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Apetrei, R.-M.; Carac, G.; Ramanaviciene, A.; Bahrim, G.; Tanase, C.; Ramanavicius, A. Cell-assisted synthesis of conducting polymer–polypyrrole–for the improvement of electric charge transfer through fungal cell wall. Colloids Surf. B Biointerfaces 2019, 175, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Ramanavicius, A.; Andriukonis, E.; Stirke, A.; Mikoliunaite, L.; Balevicius, Z.; Ramanaviciene, A. Synthesis of polypyrrole within the cell wall of yeast by redox-cycling of [Fe(CN)6]3−/[Fe(CN)6]4−. Enzym. Microb. Technol. 2016, 83, 40–47. [Google Scholar] [CrossRef]
- Apetrei, R.-M.; Cârâc, G.; Bahrim, G.; Camurlu, P. Sensitivity enhancement for microbial biosensors through cell Self-Coating with polypyrrole. Int. J. Polym. Mater. Polym. Biomater. 2018, 68, 1058–1067. [Google Scholar] [CrossRef]
- Arlyapov, V.A.; Kharkova, A.S.; Kurbanaliyeva, S.K.; Kuznetsova, L.S.; Machulin, A.V.; Tarasov, S.E.; Melnikov, P.V.; Ponamoreva, O.N.; Alferov, V.A.; Reshetilov, A.N. Use of biocompatible redox-active polymers based on carbon nanotubes and modified organic matrices for development of a highly sensitive BOD biosensor. Enzym. Microb. Technol. 2021, 143, 109706. [Google Scholar] [CrossRef]
- Hu, J.; Li, Y.; Gao, G.; Xia, S. A mediated BOD biosensor based on immobilized B. subtilis on three-dimensional porous graphene-polypyrrole composite. Sensors 2017, 17, 2594. [Google Scholar] [CrossRef]
- Zhou, T.; Han, H.; Liu, P.; Xiong, J.; Tian, F.; Li, X. Microbial fuels cell-based biosensor for toxicity detection: A review. Sensors 2017, 17, 2230. [Google Scholar] [CrossRef]
- Lakard, B. Electrochemical Biosensors Based on Conducting Polymers: A Review. Appl. Sci. 2020, 10, 6614. [Google Scholar] [CrossRef]
- Aynalem, B.; Muleta, D. Microbial Biosensors as Pesticide Detector: An Overview. J. Sensors 2021, 2021, 5538857. [Google Scholar] [CrossRef]
- Ma, Z.; Meliana, C.; Munawaroh, H.S.H.; Karaman, C.; Karimi-Maleh, H.; Low, S.S.; Show, P.L. Recent advances in the analytical strategies of microbial biosensor for detection of pollutants. Chemosphere 2022, 306, 135515. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Wan, N.; Fu, T.; Wang, L.; Li, C.; Qie, Z.; Zhu, A. A current sensing biosensor for BOD rapid measurement. Archaea 2020, 2020, 8894925. [Google Scholar] [CrossRef] [PubMed]
- Arlyapov, V.A.; Plekhanova, Y.V.; Kamanina, O.A.; Nakamura, H.; Reshetilov, A.N. Microbial Biosensors for Rapid Determination of Biochemical Oxygen Demand: Approaches, Tendencies and Development Prospects. Biosensors 2022, 12, 842. [Google Scholar] [CrossRef] [PubMed]
- Ramanavicius, S.; Jagminas, A.; Ramanavicius, A. Advances in molecularly imprinted polymers based affinity sensors. Polymers 2021, 13, 974. [Google Scholar] [CrossRef] [PubMed]
- Abdelwahab, A.A.; Won, M.; Shim, Y. Direct electrochemistry of cholesterol oxidase immobilized on a conducting polymer: Application for a cholesterol biosensor. Electroanal. Int. J. Devoted Fundam. Pract. Asp. Electroanal. 2010, 22, 21–25. [Google Scholar] [CrossRef]
- Gholami, M.D.; O’mullane, A.P.; Sonar, P.; Ayoko, G.A.; Izake, E.L. Antibody coated conductive polymer for the electrochemical immunosensing of Human Cardiac Troponin I in blood plasma. Anal. Chim. Acta 2021, 1185, 339082. [Google Scholar] [CrossRef]
- Nasrin, F.; Khoris, I.M.; Chowdhury, A.D.; Boonyakida, J.; Park, E.Y. Impedimetric biosensor of Norovirus with low variance using simple bioconjugation on conductive polymer-Au nanocomposite. Sensors Actuators B Chem. 2022, 369, 132390. [Google Scholar] [CrossRef]
- Gao, S.; Guisán, J.M.; Rocha-Martin, J. Oriented immobilization of antibodies onto sensing platforms-A critical review. Anal. Chim. Acta 2022, 1189, 338907. [Google Scholar] [CrossRef]
- Zhou, Y.; Lü, H.; Zhang, D.; Xu, K.; Hui, N.; Wang, J. Electrochemical biosensors based on conducting polymer composite and PAMAM dendrimer for the ultrasensitive detection of acetamiprid in vegetables. Microchem. J. 2023, 185, 108284. [Google Scholar] [CrossRef]
- Plekhanova, Y.V.; Rai, M.; Reshetilov, A.N. Nanomaterials in bioelectrochemical devices: On applications enhancing their positive effect. 3 Biotech 2022, 12, 231. [Google Scholar] [CrossRef]
- Mohammadpour-Haratbar, A.; Zare, Y.; Rhee, K.Y. Electrochemical biosensors based on polymer nanocomposites for detecting breast cancer: Recent progress and future prospects. Adv. Colloid Interface Sci. 2022, 309, 102795. [Google Scholar] [CrossRef]
- Moon, J.-M.; Thapliyal, N.; Hussain, K.K.; Goyal, R.N.; Shim, Y.-B. Conducting polymer-based electrochemical biosensors for neurotransmitters: A review. Biosens. Bioelectron. 2018, 102, 540–552. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.; Akhtar, M.H.; Benboudiaf, A.; Park, D.-S.; Shim, Y.-B. A Sensor for Serotonin and Dopamine Detection in Cancer Cells Line Based on the Conducting Polymer−Pd Complex Composite. Electroanalysis 2020, 32, 520–527. [Google Scholar] [CrossRef]
- Taylor, I.M.; Robbins, E.M.; Catt, K.A.; Cody, P.A.; Happe, C.L.; Cui, X.T. Enhanced dopamine detection sensitivity by PEDOT/graphene oxide coating on in vivo carbon fiber electrodes. Biosens. Bioelectron. 2017, 89, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Yazdanparast, S.; Benvidi, A.; Banaei, M.; Nikukar, H.; Tezerjani, M.D.; Azimzadeh, M. Dual-aptamer based electrochemical sandwich biosensor for MCF-7 human breast cancer cells using silver nanoparticle labels and a poly (glutamic acid)/MWNT nanocomposite. Microchim. Acta 2018, 185, 405. [Google Scholar] [CrossRef]
- Shafiei, F.; Saberi, R.S.; Mehrgardi, M.A. A label-free electrochemical aptasensor for breast cancer cell detection based on a reduced graphene oxide-chitosan-gold nanoparticle composite. Bioelectrochemistry 2021, 140, 107807. [Google Scholar] [CrossRef]
- Yang, T.; Gao, Y.; Liu, Z.; Xu, J.; Lu, L.; Yu, Y. Three-dimensional gold nanoparticles/prussian blue-poly (3, 4-ethylenedioxythiophene) nanocomposite as novel redox matrix for label-free electrochemical immunoassay of carcinoembryonic antigen. Sensors Actuators B Chem. 2017, 239, 76–84. [Google Scholar] [CrossRef]
- Zhang, D.; Lang, X.; Hui, N.; Wang, J. Dual-Mode electrochemical biosensors based on Chondroitin sulfate functionalized polypyrrole nanowires for ultrafast and ultratrace detection of acetamiprid pesticide. Microchem. J. 2022, 179, 107530. [Google Scholar] [CrossRef]
- Lee, M.; Kim, S.; Jang, M.; Park, H.S.; Lee, J.Y. One-Pot electrochemical fabrication of high performance amperometric enzymatic biosensors using polypyrrole and polydopamine. J. Ind. Eng. Chem. 2021, 97, 316–325. [Google Scholar] [CrossRef]
- Wang, J.; Hui, N. Electrochemical functionalization of polypyrrole nanowires for the development of ultrasensitive biosensors for detecting microRNA. Sensors Actuators B Chem. 2019, 281, 478–485. [Google Scholar] [CrossRef]
- Pinyou, P.; Blay, V.; Monkrathok, J.; Janphuang, P.; Chansaenpak, K.; Pansalee, J.; Lisnund, S. A facile method for generating polypyrrole microcapsules and their application in electrochemical sensing. Microchim. Acta 2022, 189, 410. [Google Scholar] [CrossRef]
- Paneru, S.; Kumar, D. Ag-doped-CuO nanoparticles supported polyaniline (PANI) based novel electrochemical sensor for sensitive detection of paraoxon-ethyl in three real samples. Sensors Actuators B Chem. 2023, 379, 133270. [Google Scholar] [CrossRef]
- Sanko, V.; Şenocak, A.; Tümay, S.O.; Demirbas, E. A novel comparative study for electrochemical urea biosensor design: Effect of different ferrite nanoparticles (MFe2O4, M: Cu, Co, Ni, Zn) in urease immobilized composite system. Bioelectrochemistry 2023, 149, 108324. [Google Scholar] [CrossRef] [PubMed]
- Drobysh, M.; Ramanavicius, A.; Baradoke, A. Polyaniline-based electrochemical immunosensor for the determination of antibodies against SARS-CoV-2 spike protein. Sci. Total Environ. 2023, 862, 160700. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Dutta, H.S.; Dhayal, M. Langmuir-Blodgett monolayer of electrochemically synthesized PANI-TiO2 nanocomposites for MSG biosensor. Appl. Surf. Sci. Adv. 2022, 10, 100264. [Google Scholar] [CrossRef]
- Pilo, M.I.; Baluta, S.; Loria, A.C.; Sanna, G.; Spano, N. Poly (Thiophene)/Graphene Oxide-Modified Electrodes for Amperometric Glucose Biosensing. Nanomaterials 2022, 12, 2840. [Google Scholar] [CrossRef]
- Sethuraman, V.; Sridhar, T.M.; Sasikumar, R. Development of an electrochemical biosensor for determination of dopamine by gold modified poly (thiophene-3-boronic acid)-polyphenol oxidase modified electrode. Mater. Lett. 2021, 302, 130387. [Google Scholar] [CrossRef]
- Pilo, M.; Farre, R.; Lachowicz, J.I.; Masolo, E.; Panzanelli, A.; Sanna, G.; Senes, N.; Sobral, A.; Spano, N. Design of amperometric biosensors for the detection of glucose prepared by immobilization of glucose oxidase on conducting (poly) thiophene films. J. Anal. Methods Chem. 2018, 2018, 1849439. [Google Scholar] [CrossRef]
- Sen, S.; Sarkar, P. An interference-free new xanthine biosensor based on immobilized enzyme-nanogold conjugate on carbon nanotube doped poly (3, 4-Ethylenedioxythiophene) composite film. Int. J. Biol. Macromol. 2022, 199, 275–286. [Google Scholar] [CrossRef]
- Gupta, A.; Sharma, A.L.; Deep, A. Sensitive impedimetric detection of E. coli with metal-organic framework (MIL-53)/polymer (PEDOT) composite modified screen-printed electrodes. J. Environ. Chem. Eng. 2021, 9, 104925. [Google Scholar] [CrossRef]
- Bizid, S.; Mlika, R.; Haj Said, A.; Chemli, M.; Korri Youssoufi, H. Investigations of poly(p-phenylene) modified with ferrocene and their application in electrochemical DNA sensing. Sensors Actuators B Chem. 2016, 226, 370–380. [Google Scholar] [CrossRef]
- Çetin, M.Z.; Guven, N.; Apetrei, R.-M.; Camurlu, P. Highly sensitive detection of glucose via glucose oxidase immobilization onto conducting polymer-coated composite polyacrylonitrile nanofibers. Enzym. Microb. Technol. 2023, 164, 110178. [Google Scholar] [CrossRef]
- Wisitsoraat, A.; Pakapongpan, S.; Sriprachuabwong, C.; Phokharatkul, D.; Sritongkham, P.; Lomas, T.; Tuantranont, A. Graphene–PEDOT: PSS on screen printed carbon electrode for enzymatic biosensing. J. Electroanal. Chem. 2013, 704, 208–213. [Google Scholar] [CrossRef]
- Alim, S.; Vejayan, J.; Kafi, A.K.M. Direct Electrochemistry of Catalase Immobilized at Polymerized-SnO. Biomed. J. 2018, 2, 6. [Google Scholar]
- Ayenimo, J.G.; Adeloju, S.B. Amperometric detection of glucose in fruit juices with polypyrrole-based biosensor with an integrated permselective layer for exclusion of interferences. Food Chem. 2017, 229, 127–135. [Google Scholar] [CrossRef]
- Cevik, E.; Cerit, A.; Gazel, N.; Yildiz, H.B. Construction of an amperometric cholesterol biosensor based on DTP (aryl) aniline conducting polymer bound cholesterol oxidase. Electroanalysis 2018, 30, 2445–2453. [Google Scholar] [CrossRef]
- Mokwebo, K.V.; Oluwafemi, O.S.; Arotiba, O.A. An electrochemical cholesterol biosensor based on a CdTe/CdSe/ZnSe quantum dots—Poly (propylene imine) dendrimer nanocomposite immobilisation layer. Sensors 2018, 18, 3368. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, I.V.; Pasquini, L.; Narducci, R.; Sgreccia, E.; Di Vona, M.L.; Knauth, P. A short overview of biological fuel cells. Membranes 2022, 12, 427. [Google Scholar] [CrossRef] [PubMed]
- Nasar, A.; Perveen, R. Applications of enzymatic biofuel cells in bioelectronic devices–A review. Int. J. Hydrogen Energy 2019, 44, 15287–15312. [Google Scholar] [CrossRef]
- Verma, M.; Mishra, V. Recent trends in upgrading the performance of yeast as electrode biocatalyst in microbial fuel cells. Chemosphere 2021, 284, 131383. [Google Scholar] [CrossRef] [PubMed]
- Fadzli, F.S.; Bhawani, S.A.; Adam Mohammad, R.E. Microbial fuel cell: Recent developments in organic substrate use and bacterial electrode interaction. J. Chem. 2021, 2021, 4570388. [Google Scholar] [CrossRef]
- Ren, J.; Li, N.; Du, M.; Zhang, Y.; Hao, C.; Hu, R. Study on the effect of synergy effect between the mixed cultures on the power generation of microbial fuel cells. Bioengineered 2021, 12, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Mu, H.; Liu, W.; Zhang, R.; Guo, J.; Xian, M.; Liu, H. Electricigens in the anode of microbial fuel cells: Pure cultures versus mixed communities. Microb. Cell Fact. 2019, 18, 39. [Google Scholar] [CrossRef] [PubMed]
- Mani, P.; VT, F.; Bowman, K.; TS, C.; Keshavarz, T.; Kyazze, G. Development of an electroactive aerobic biocathode for microbial fuel cell applications. Environ. Microbiol. Rep. 2020, 12, 607–612. [Google Scholar] [CrossRef]
- Lin, C.-W.; Lai, C.-Y.; Liu, S.-H.; Chen, Y.-R.; Alfanti, L.K. Enhancing bioelectricity generation and removal of copper in microbial fuel cells with a laccase-catalyzed biocathode. J. Clean. Prod. 2021, 298, 126726. [Google Scholar] [CrossRef]
- Castro, C.J.; Taha, K.; Kenney, I.; Yeh, D.H. The Role of Carbon to Nitrogen Ratio on the Performance of Denitrifying Biocathodes for Decentralized Wastewater Treatment. Water 2022, 14, 3076. [Google Scholar] [CrossRef]
- Tofighi, A.; Rahimnejad, M. Synthetic polymer-based membranes for microbial fuel cells. In Synthetic Polymeric Membranes for Advanced Water Treatment, Gas Separation, and Energy Sustainability; Elsevier: Amsterdam, The Netherlands, 2020; pp. 309–335. [Google Scholar]
- Solomon, J.; Dharmalingam, S. Modified polymer electrolyte membrane for microbial fuel cell: Performance analysis, investigation on anti-biofouling effect, and microbial community analysis on biofouled membrane. J. Power Sources 2023, 580, 233452. [Google Scholar] [CrossRef]
- Daud, S.M.; Kim, B.H.; Ghasemi, M.; Daud, W.R.W. Separators used in microbial electrochemical technologies: Current status and future prospects. Bioresour. Technol. 2015, 195, 170–179. [Google Scholar] [CrossRef]
- Parekh, A. Recent developments of proton exchange membranes for PEMFC: A review. Front. Energy Res. 2022, 10, 956132. [Google Scholar] [CrossRef]
- Borja-Maldonado, F.; Zavala, M.Á.L. Contribution of configurations, electrode and membrane materials, electron transfer mechanisms, and cost of components on the current and future development of microbial fuel cells. Heliyon 2022, 8, e09849. [Google Scholar] [CrossRef]
- Szakács, S.; Koók, L.; Nemestóthy, N.; Bélafi-Bakó, K.; Bakonyi, P. Studying microbial fuel cells equipped with heterogeneous ion exchange membranes: Electrochemical performance and microbial community assessment of anodic and membrane-surface biofilms. Bioresour. Technol. 2022, 360, 127628. [Google Scholar] [CrossRef]
- Banerjee, A.; Calay, R.K.; Eregno, F.E. Role and important properties of a membrane with its recent advancement in a microbial fuel cell. Energies 2022, 15, 444. [Google Scholar] [CrossRef]
- Kumar, V.; Mondal, S.; Nandy, A.; Kundu, P.P. Analysis of polybenzimidazole and polyvinylpyrrolidone blend membranes as separating barrier in single chambered microbial fuel cells. Biochem. Eng. J. 2016, 111, 34–42. [Google Scholar] [CrossRef]
- Li, C.; Ding, L.; Cui, H.; Zhang, L.; Xu, K.; Ren, H. Application of conductive polymers in biocathode of microbial fuel cells and microbial community. Bioresour. Technol. 2012, 116, 459–465. [Google Scholar] [CrossRef]
- Xiao, X.; Xia, H.; Wu, R.; Bai, L.; Yan, L.; Magner, E.; Cosnier, S.; Lojou, E.; Zhu, Z.; Liu, A. Tackling the challenges of enzymatic (bio) fuel cells. Chem. Rev. 2019, 119, 9509–9558. [Google Scholar] [CrossRef]
- Wang, L.; Wu, X.; Su, B.S.Q.; Song, R.; Zhang, J.-R.; Zhu, J.-J. Enzymatic biofuel cell: Opportunities and intrinsic challenges in futuristic applications. Adv. Energy Sustain. Res. 2021, 2, 2100031. [Google Scholar] [CrossRef]
- Ul Haque, S.; Yasir, M.; Cosnier, S. Recent advancements in the field of flexible/wearable enzyme fuel cells. Biosens. Bioelectron. 2022, 214, 114545. [Google Scholar] [CrossRef] [PubMed]
- Boehler, C.; Aqrawe, Z.; Asplund, M. Applications of PEDOT in bioelectronic medicine. Bioelectron. Med. 2019, 2, 89–99. [Google Scholar] [CrossRef]
- Gangopadhyay, R.; Das, B.; Molla, M.R. How does PEDOT combine with PSS? Insights from structural studies. RSC Adv. 2014, 4, 43912–43920. [Google Scholar] [CrossRef]
- Sun, K.; Zhang, S.; Li, P.; Xia, Y.; Zhang, X.; Du, D.; Isikgor, F.H.; Ouyang, J. Review on application of PEDOTs and PEDOT: PSS in energy conversion and storage devices. J. Mater. Sci. Mater. Electron. 2015, 26, 4438–4462. [Google Scholar] [CrossRef]
- Ahamed, M.I.; Asiri, A.M.; AlAmry, K.A. Biocompatible mediated bioanode prepared by using poly (3, 4-ethylene dioxythiophene) poly (styrene sulfonate)(PEDOT: PSS) and sulfonated graphene oxide integrated enzyme for biofuel cells applications. Mater. Sci. Energy Technol. 2018, 1, 63–69. [Google Scholar]
- Abd-Wahab, F.; Abdul Guthoos, H.F.; Wan Salim, W.W.A. Solid-state rGO-PEDOT: PSS transducing material for cost-effective enzymatic sensing. Biosensors 2019, 9, 36. [Google Scholar] [CrossRef]
- Cho, E.; Mohammadifar, M.; Choi, S. A self-powered sensor patch for glucose monitoring in sweat. In Proceedings of the 2017 IEEE 30th International Conference on Micro Electro Mechanical Systems (MEMS), Las Vegas, NV, USA, 22–26 January 2017; pp. 366–369. [Google Scholar]
- Antipova, C.G.; Parunova, Y.M.; Vishnevskaya, M.V.; Krasheninnikov, S.V.; Lukanina, K.I.; Grigoriev, T.E.; Chvalun, S.N.; Gotovtsev, P.M. Biomechanical behaviour of PEDOT: PSS-based hydrogels as an electrode for stent integrated enzyme biofuel cells. Heliyon 2022, 8, e09218. [Google Scholar] [CrossRef]
- Kwon, C.H.; Park, Y.B.; Lee, J.A.; Choi, Y.-B.; Kim, H.-H.; Lima, M.D.; Baughman, R.H.; Kim, S.J. Mediator-free carbon nanotube yarn biofuel cell. RSC Adv. 2016, 6, 48346–48350. [Google Scholar] [CrossRef]
- Sintayehu, Y.D.; Ananda Murthy, H.C. Polypyrrole based biofunctional composite layer for bioelectrocatalytic device system. Adv. Mater. Lett. 2019, 10, 524–532. [Google Scholar] [CrossRef]
- Kang, Z.; Zhang, Y.-H.P.J.; Zhu, Z. A shriveled rectangular carbon tube with the concave surface for high-performance enzymatic glucose/O2 biofuel cells. Biosens. Bioelectron. 2019, 132, 76–83. [Google Scholar] [CrossRef]
- Kizling, M.; Stolarczyk, K.; Tammela, P.; Wang, Z.; Nyholm, L.; Golimowski, J.; Bilewicz, R. Bioelectrodes based on pseudocapacitive cellulose/polypyrrole composite improve performance of biofuel cell. Bioelectrochemistry 2016, 112, 184–190. [Google Scholar] [CrossRef] [PubMed]
- ul Haque, S.; Nasar, A.; Asiri, A.M. Fabrication and characterization of electrochemically prepared bioanode (polyaniline/ferritin/glucose oxidase) for biofuel cell application. Chem. Phys. Lett. 2018, 692, 277–284. [Google Scholar] [CrossRef]
- Mishra, P.; Lakshmi, G.; Mishra, S.; Avasthi, D.K.; Swart, H.C.; Turner, A.P.F.; Mishra, Y.K.; Tiwari, A. Electrocatalytic biofuel cell based on highly efficient metal-polymer nano-architectured bioelectrodes. Nano Energy 2017, 39, 601–607. [Google Scholar] [CrossRef]
- Kang, Z.; Jiao, K.; Cheng, J.; Peng, R.; Jiao, S.; Hu, Z. A novel three-dimensional carbonized PANI1600@ CNTs network for enhanced enzymatic biofuel cell. Biosens. Bioelectron. 2018, 101, 60–65. [Google Scholar] [CrossRef]
- Kumar, R.; Bhuvana, T.; Mishra, G.; Sharma, A. A polyaniline wrapped aminated graphene composite on nickel foam as three-dimensional electrodes for enzymatic microfuel cells. RSC Adv. 2016, 6, 73496–73505. [Google Scholar] [CrossRef]
- Pelosi, D.; Barelli, L.; Montegiove, N.; Calzoni, E.; Cesaretti, A.; Di Michele, A.; Emiliani, C.; Gammaitoni, L. Immobilizing Enzymes on a Commercial Polymer: Performance Analysis of a GOx-Laccase Based Enzymatic Biofuel Cell Assembly. Energies 2022, 15, 2182. [Google Scholar] [CrossRef]
- Haque, S.; Nasar, A.; Inamuddin; Rahman, M.M. Applications of chitosan (CHI)-reduced graphene oxide (rGO)-polyaniline (PAni) conducting composite electrode for energy generation in glucose biofuel cell. Sci. Rep. 2020, 10, 10428. [Google Scholar] [CrossRef] [PubMed]
- Montegiove, N.; Calzoni, E.; Pelosi, D.; Gammaitoni, L.; Barelli, L.; Emiliani, C.; Di Michele, A.; Cesaretti, A. Optimizing Covalent Immobilization of Glucose Oxidase and Laccase on PV15 Fluoropolymer-Based Bioelectrodes. J. Funct. Biomater. 2022, 13, 270. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Das, S.; Mosquera, M.E.G. Conducting polymer-based nanohybrids for fuel cell application. Polymers 2020, 12, 2993. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ren, K.; Zhu, Y.; Huang, J.; Liu, S. A Review of Recent Advances in Microbial Fuel Cells: Preparation, Operation, and Application. BioTech 2022, 11, 44. [Google Scholar] [CrossRef]
- Mahmoud, R.H.; Gomaa, O.M.; Hassan, R.Y.A. Bio-electrochemical frameworks governing microbial fuel cell performance: Technical bottlenecks and proposed solutions. RSC Adv. 2022, 12, 5749–5764. [Google Scholar] [CrossRef]
- Mishra, P.; Malla, M.A.; Gupta, S.K.; Mishra, P.; Malla, M.A.; Gupta, S.K. Poly (3,4-ethylenedioxythiophene)-Modified Graphite Felt and Carbon Cloth Anodes for Use in Microbial Fuel Cells. ChemistrySelect 2022, 7, e202103920. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, Y.; Han, X.; Shan, Y.; Li, F.; Shi, L. Biofilm biology and engineering of Geobacter and Shewanella spp. for energy applications. Front. Bioeng. Biotechnol. 2021, 9, 786416. [Google Scholar] [CrossRef]
- Truong, D.H.; Dam, M.S.; Bujna, E.; Rezessy-Szabo, J.; Farkas, C.; Vi, V.N.H.; Csernus, O.; Nguyen, V.D.; Gathergood, N.; Friedrich, L. In situ fabrication of electrically conducting bacterial cellulose-polyaniline-titanium-dioxide composites with the immobilization of Shewanella xiamenensis and its application as bioanode in microbial fuel cell. Fuel 2021, 285, 119259. [Google Scholar] [CrossRef]
- Sharma, S.C.D.; Li, J.; Hu, A.; Chang, C.-C.; Yu, C.-P. Integration of pre-colonized and mediator immobilized mixed culture for the improvement of electricity production of microbial fuel cells. Environ. Technol. Innov. 2021, 22, 101514. [Google Scholar] [CrossRef]
- Lin, X.-Q.; Li, Z.-L.; Liang, B.; Nan, J.; Wang, A.-J. Identification of biofilm formation and exoelectrogenic population structure and function with graphene/polyanliline modified anode in microbial fuel cell. Chemosphere 2019, 219, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Hemdan, B.A.; El-Taweel, G.E.; Naha, S.; Goswami, P. Bacterial community structure of electrogenic biofilm developed on modified graphite anode in microbial fuel cell. Sci. Rep. 2023, 13, 1255. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.; An, J.; Li, J.; Zhou, L.; Li, N.; Wang, X. Polydopamine as a new modification material to accelerate startup and promote anode performance in microbial fuel cells. J. Power Sources 2017, 343, 477–482. [Google Scholar] [CrossRef]
- Mukherjee, P.; Mishra, N.S.; Saravanan, P. Polydopamine modified silk fibroin 3-D anode for enhanced microbial fuel cell operation. Sustain. Energy Technol. Assess. 2022, 49, 101696. [Google Scholar] [CrossRef]
- Liu, S.-R.; Cai, L.-F.; Wang, L.-Y.; Yi, X.-F.; Peng, Y.-J.; He, N.; Wu, X.; Wang, Y.-P. Polydopamine coating on individual cells for enhanced extracellular electron transfer. Chem. Commun. 2019, 55, 10535–10538. [Google Scholar] [CrossRef] [PubMed]
- Zinovicius, A.; Rozene, J.; Merkelis, T.; Bruzaite, I.; Ramanavicius, A.; Morkvenaite-Vilkonciene, I. Evaluation of a yeast–polypyrrole biocomposite used in microbial fuel cells. Sensors 2022, 22, 327. [Google Scholar] [CrossRef]
- Pu, K.-B.; Ma, Q.; Cai, W.-F.; Chen, Q.-Y.; Wang, Y.-H.; Li, F.-J. Polypyrrole modified stainless steel as high performance anode of microbial fuel cell. Biochem. Eng. J. 2018, 132, 255–261. [Google Scholar] [CrossRef]
- Gniadek, M.; Wichowska, A.; Antos-Bielska, M.; Orlowski, P.; Krzyzowska, M.; Donten, M. Synthesis and characterization of polypyrrole and its composites coatings on flexible surface and its antibacterial properties. Synth. Met. 2020, 266, 116430. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, L.; An, L. Electricity generation and storage in microbial fuel cells with porous polypyrrole-base composite modified carbon brush anodes. Renew. Energy 2020, 162, 2220–2226. [Google Scholar] [CrossRef]
- Fan, L.; Xi, Y. Effect of polypyrrole-Fe3O4 composite modified anode and its electrodeposition time on the performance of microbial fuel cells. Energies 2021, 14, 2461. [Google Scholar] [CrossRef]
- Fang, Y.; Yuan, B.; Jiang, Y.; Song, R.-B.; Zhang, J.-R.; Zhu, J.-J. Layer-by-layer construction of in situ formed polypyrrole and bacterial cells as capacitive bioanodes for paper-based microbial fuel cells. J. Mater. Chem. A 2022, 10, 4915–4925. [Google Scholar] [CrossRef]
- Pankratova, G.; Bollella, P.; Pankratov, D.; Gorton, L. Supercapacitive biofuel cells. Curr. Opin. Biotechnol. 2022, 73, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Sirajudeen, A.A.O.; Annuar, M.S.M. Polymeric nanocomposition for innovative functional enhancement of electrodes and proton exchange membrane in microbial fuel cell. J. Serbian Chem. Soc. 2021, 86, 1–23. [Google Scholar] [CrossRef]
- Senthilkumar, N.; Pannipara, M.; Al-Sehemi, A.G. PEDOT/NiFe2O4 nanocomposites on biochar as a free-standing anode for high-performance and durable microbial fuel cells. New J. Chem. 2019, 43, 7743–7750. [Google Scholar] [CrossRef]
- Hernández, L.A.; Riveros, G.; González, D.M.; Gacitua, M.; del Valle, M.A. PEDOT/graphene/nickel-nanoparticles composites as electrodes for microbial fuel cells. J. Mater. Sci. Mater. Electron. 2019, 30, 12001–12011. [Google Scholar] [CrossRef]
- Salar-García, M.J.; Montilla, F.; Quijada, C.; Morallon, E.; Ieropoulos, I. Improving the power performance of urine-fed microbial fuel cells using PEDOT-PSS modified anodes. Appl. Energy 2020, 278, 115528. [Google Scholar] [CrossRef]
- Smith, R.E.; Totti, S.; Velliou, E.; Campagnolo, P.; Hingley-Wilson, S.M.; Ward, N.I.; Varcoe, J.R.; Crean, C. Development of a novel highly conductive and flexible cotton yarn for wearable pH sensor technology. Sensors Actuators B Chem. 2019, 287, 338–345. [Google Scholar] [CrossRef]
- Tarasov, S.; Plekhanova, Y.; Kashin, V.; Gotovtsev, P.; Signore, M.A.; Francioso, L.; Kolesov, V.; Reshetilov, A. Gluconobacter Oxydans-Based MFC with PEDOT: PSS/Graphene/Nafion Bioanode for Wastewater Treatment. Biosensors 2022, 12, 699. [Google Scholar] [CrossRef]
- Rajendran, J.; Shetty, B.H.; Ganapathy, D.; Murugan, P.; Atchudan, R.; Umapathy, D.; Khosla, A.; Sundramoorthy, A.K. Thermally expanded graphite incorporated with PEDOT: PSS based anode for microbial fuel cells with high bioelectricity production. J. Electrochem. Soc. 2022, 169, 17515. [Google Scholar] [CrossRef]
- Shetty, B.H.; Sundramoorthy, A.K.; Annamalai, J.; Murugan, P.; Atchudan, R.; Arya, S.; Alothman, A.A.; Ouladsmane, M. Fabrication of High-Performance MgCoO2/PEDOT: PSS@ Nickel Foam Anode for Bioelectricity Generation by Microbial Fuel Cells. J. Nanomater. 2022, 2022, 6358852. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, J.; Zhang, Y.; Guo, Q.; Hu, T.; Xiao, H.; Lu, W.; Jia, J. Progress on anodic modification materials and future development directions in microbial fuel cells. J. Power Sources 2023, 556, 232486. [Google Scholar] [CrossRef]
- Park, S.-G.; Rhee, C.; Jadhav, D.A.; Eisa, T.; Al-Mayyahi, R.B.; Shin, S.G.; Abdelkareem, M.A.; Chae, K.-J. Tailoring a highly conductive and super-hydrophilic electrode for biocatalytic performance of microbial electrolysis cells. Sci. Total Environ. 2023, 856, 159105. [Google Scholar] [CrossRef] [PubMed]
- Anjum, A.; Mazari, S.A.; Hashmi, Z.; Jatoi, A.S.; Abro, R. A review of role of cathodes in the performance of microbial fuel cells. J. Electroanal. Chem. 2021, 899, 115673. [Google Scholar] [CrossRef]
- Sugioka, M.; Yoshida, N.; Yamane, T.; Kakihana, Y.; Higa, M.; Matsumura, T.; Sakoda, M.; Iida, K. Long-term evaluation of an air-cathode microbial fuel cell with an anion exchange membrane in a 226L wastewater treatment reactor. Environ. Res. 2022, 205, 112416. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; He, W.; Liu, J.; Wang, X.; Qu, Y.; Ren, N. A horizontal plug flow and stackable pilot microbial fuel cell for municipal wastewater treatment. Bioresour. Technol. 2014, 156, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Han, T.H.; Parveen, N.; Shim, J.H.; Nguyen, A.T.N.; Mahato, N.; Cho, M.H. Ternary composite of polyaniline graphene and TiO2 as a bifunctional catalyst to enhance the performance of both the bioanode and cathode of a microbial fuel cell. Ind. Eng. Chem. Res. 2018, 57, 6705–6713. [Google Scholar] [CrossRef]
- Nguyen, M.-T.; Mecheri, B.; Iannaci, A.; D’Epifanio, A.; Licoccia, S. Iron/polyindole-based electrocatalysts to enhance oxygen reduction in microbial fuel cells. Electrochim. Acta 2016, 190, 388–395. [Google Scholar] [CrossRef]
- Sumisha, A.; Haribabu, K. Nanostructured polypyrrole as cathode catalyst for Fe (III) removal in single chamber microbial fuel cell. Biotechnol. Bioprocess Eng. 2020, 25, 78–85. [Google Scholar] [CrossRef]
- Pattanayak, P.; Papiya, F.; Pramanik, N.; Kundu, P.P. Deposition of Ni–NiO nanoparticles on the reduced graphene oxide filled polypyrrole: Evaluation as cathode catalyst in microbial fuel cells. Sustain. Energy Fuels 2019, 3, 1808–1826. [Google Scholar] [CrossRef]
- Mukherjee, P.; Sharma, U.; Saravanan, P. PEDOT modified MIL-53 (Al) as high throughput cathode catalyst for durable and economical power generation in microbial fuel cell. Int. J. Energy Res. 2022, 46, 23326–23340. [Google Scholar] [CrossRef]
- Simeon, I.M.; Herkendell, K.; Pant, D.; Freitag, R. Electrochemical evaluation of different polymer binders for the production of carbon-modified stainless-steel electrodes for sustainable power generation using a soil microbial fuel cell. Chem. Eng. J. Adv. 2022, 10, 100246. [Google Scholar] [CrossRef]
- Milner, E.M.; Popescu, D.; Curtis, T.; Head, I.M.; Scott, K.; Eileen, H.Y. Microbial fuel cells with highly active aerobic biocathodes. J. Power Sources 2016, 324, 8–16. [Google Scholar] [CrossRef]
- Koltysheva, D.; Shchurska, K.; Kuzminskyi, Y. Microalgae and cyanobacteria as biological agents of biocathodes in biofuel cells. Biotechnol. J. Biotechnol. Comput. Biol. Bionanotechnol. 2021, 102, 437–444. [Google Scholar] [CrossRef]
- Ramanaiah, S.V.; Cordas, C.M.; Matias, S.C.; Reddy, M.V.; Leitao, J.H.; Fonseca, L.P. Bioelectricity generation using long-term operated biocathode: RFLP based microbial diversity analysis. Biotechnol. Rep. 2021, 32, e00693. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Angenent, L.T. Application of bacterial biocathodes in microbial fuel cells. Electroanal. Int. J. Devoted Fundam. Pract. Asp. Electroanal. 2006, 18, 2009–2015. [Google Scholar] [CrossRef]
- Godwin, J.M.; Evitts, R.W.; Kennell, G.F. Microbial fuel cell with a polypyrrole/poly (methylene blue) composite electrode. Rep. Electrochem. 2012, 2, 3–11. [Google Scholar]
- Zhang, H.; Zhang, R.; Zhang, G.; Yang, F.; Gao, F. Modified graphite electrode by polyaniline/tourmaline improves the performance of bio-cathode microbial fuel cell. Int. J. Hydrogen Energy 2014, 39, 11250–11257. [Google Scholar] [CrossRef]
- Le Goff, A.; Holzinger, M.; Cosnier, S. Recent progress in oxygen-reducing laccase biocathodes for enzymatic biofuel cells. Cell. Mol. Life Sci. 2015, 72, 941–952. [Google Scholar] [CrossRef]
- El Ichi-Ribault, S.; Zebda, A.; Laaroussi, A.; Reverdy-Bruas, N.; Chaussy, D.; Belgacem, M.N.; Suherman, A.L.; Cinquin, P.; Martin, D.K. Laccase-based biocathodes: Comparison of chitosan and Nafion. Anal. Chim. Acta 2016, 937, 43–52. [Google Scholar] [CrossRef]
- Mani, P.; Fidal, V.T.; Keshavarz, T.; Chandra, T.S.; Kyazze, G. Laccase immobilization strategies for application as a cathode catalyst in microbial fuel cells for azo dye decolourization. Front. Microbiol. 2021, 11, 620075. [Google Scholar] [CrossRef]
- Mazur, M.; Krywko-Cendrowska, A.; Krysiński, P.; Rogalski, J. Encapsulation of laccase in a conducting polymer matrix: A simple route towards polypyrrole microcontainers. Synth. Met. 2009, 159, 1731–1738. [Google Scholar] [CrossRef]
- Wu, G.; Gao, Y.; Zhao, D.; Ling, P.; Gao, F. Methanol/oxygen enzymatic biofuel cell using laccase and NAD+-dependent dehydrogenase cascades as biocatalysts on carbon nanodots electrodes. ACS Appl. Mater. Interfaces 2017, 9, 40978–40986. [Google Scholar] [CrossRef] [PubMed]
- Smolander, M.; Boer, H.; Valkiainen, M.; Roozeman, R.; Bergelin, M.; Eriksson, J.-E.; Zhang, X.-C.; Koivula, A.; Viikari, L. Development of a printable laccase-based biocathode for fuel cell applications. Enzym. Microb. Technol. 2008, 43, 93–102. [Google Scholar] [CrossRef]
- Martinez-Ortiz, J.; Flores, R.; Vazquez-Duhalt, R. Molecular design of laccase cathode for direct electron transfer in a biofuel cell. Biosens. Bioelectron. 2011, 26, 2626–2631. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Huang, X.; Wang, T. Construction and direct electrochemistry of orientation controlled laccase electrode. Biochem. Biophys. Res. Commun. 2014, 446, 201–205. [Google Scholar] [CrossRef]
- Shrier, A.; Giroud, F.; Rasmussen, M.; Minteer, S.D. Operational stability assays for bioelectrodes for biofuel cells: Effect of immobilization matrix on laccase biocathode stability. J. Electrochem. Soc. 2014, 161, H244. [Google Scholar] [CrossRef]
- Wang, X.; Sjöberg-Eerola, P.; Immonen, K.; Bobacka, J.; Bergelin, M. Immobilization of Trametes hirsuta laccase into poly (3,4-ethylenedioxythiophene) and polyaniline polymer-matrices. J. Power Sources 2011, 196, 4957–4964. [Google Scholar] [CrossRef]
- Kausaite-Minkstimiene, A.; Kaminskas, A.; Popov, A.; Ramanavicius, A.; Ramanaviciene, A. Development of a new biocathode for a single enzyme biofuel cell fuelled by glucose. Sci. Rep. 2021, 11, 18568. [Google Scholar] [CrossRef]
- Kaur, G.; Kaur, A.; Kaur, H. Review on nanomaterials/conducting polymer based nanocomposites for the development of biosensors and electrochemical sensors. Polym. Technol. Mater. 2021, 60, 504–521. [Google Scholar] [CrossRef]
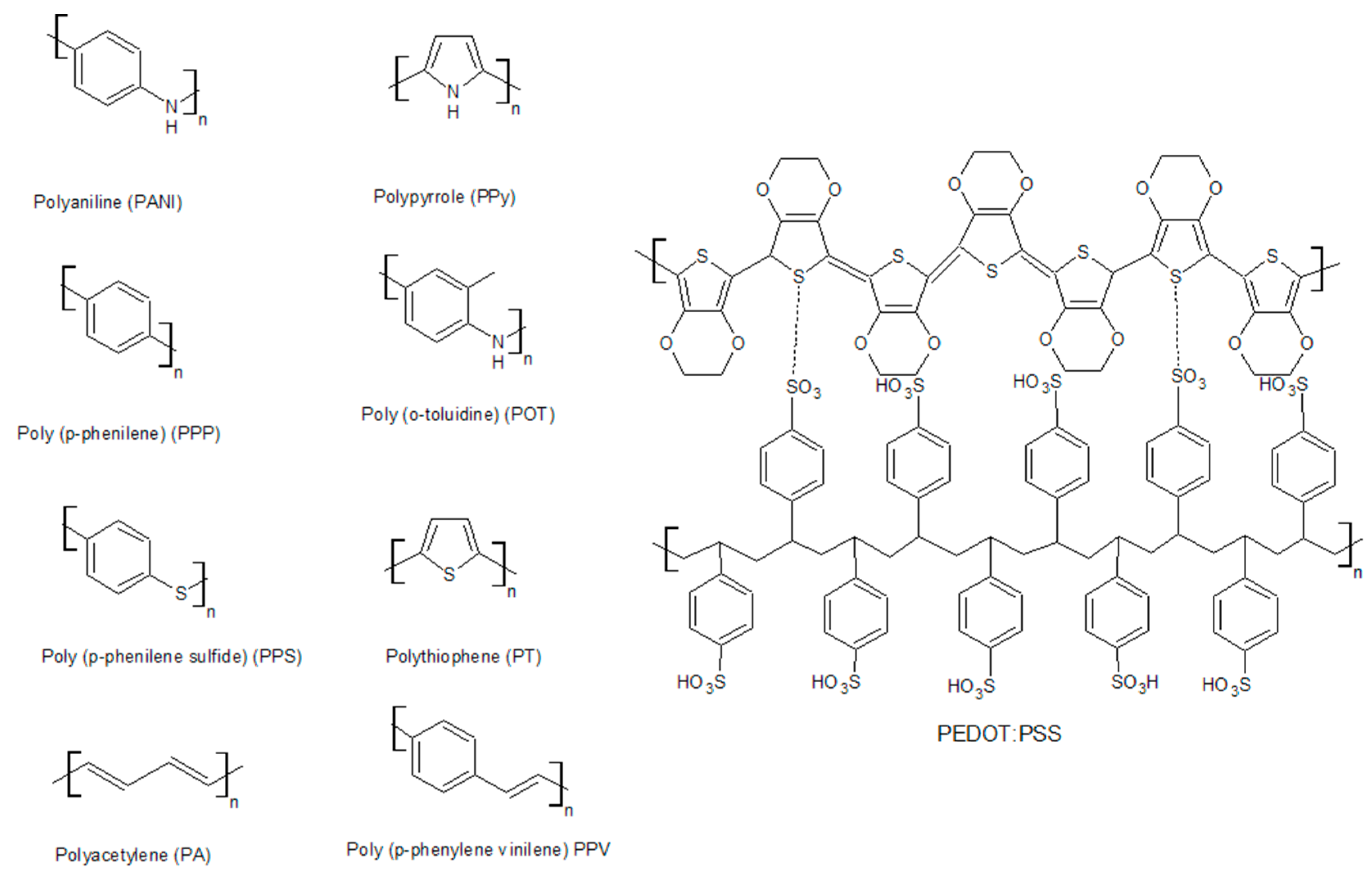




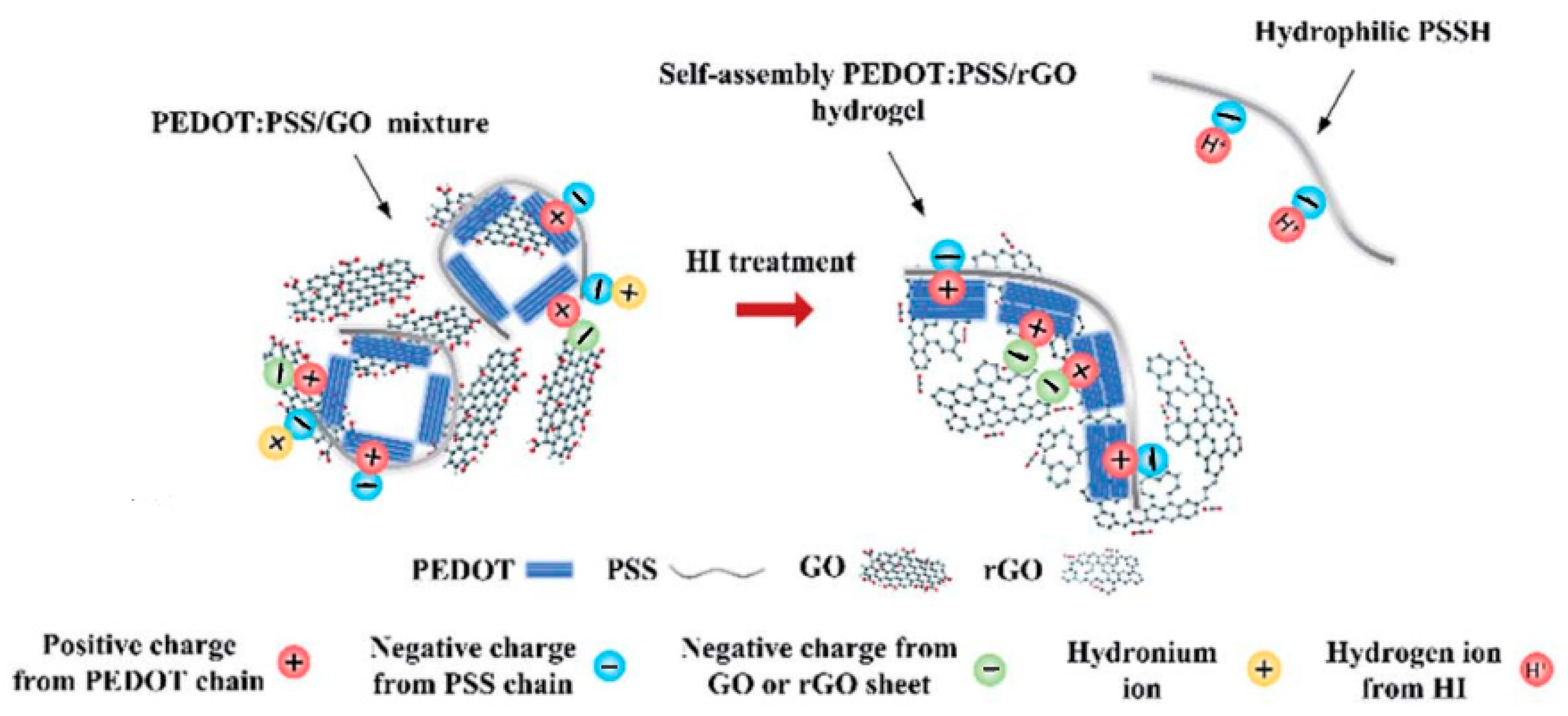
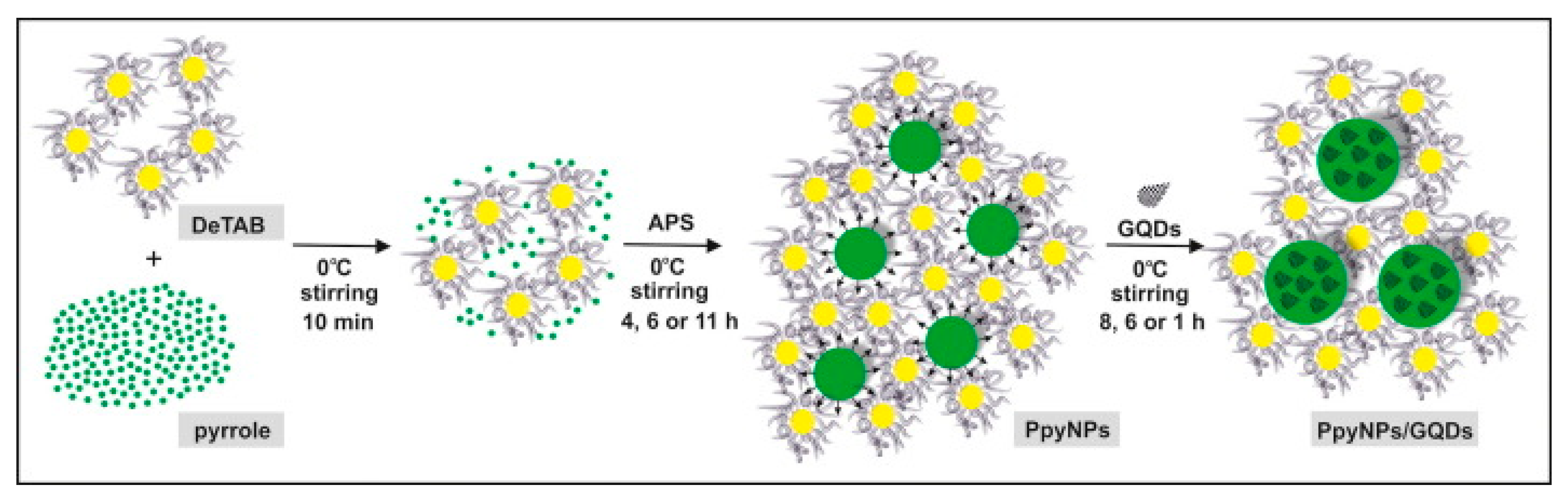
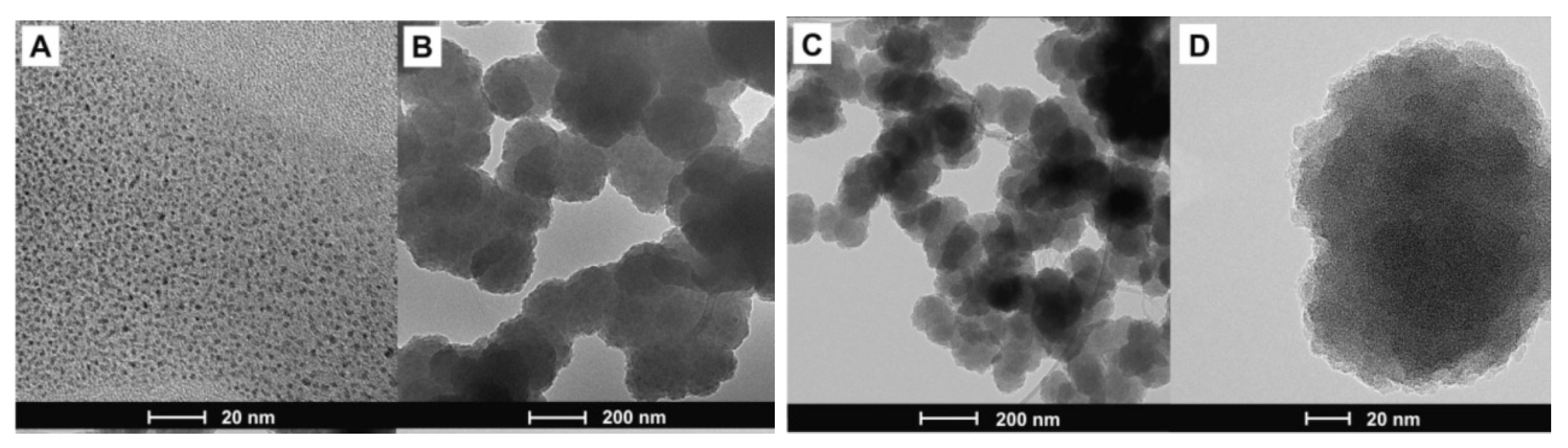
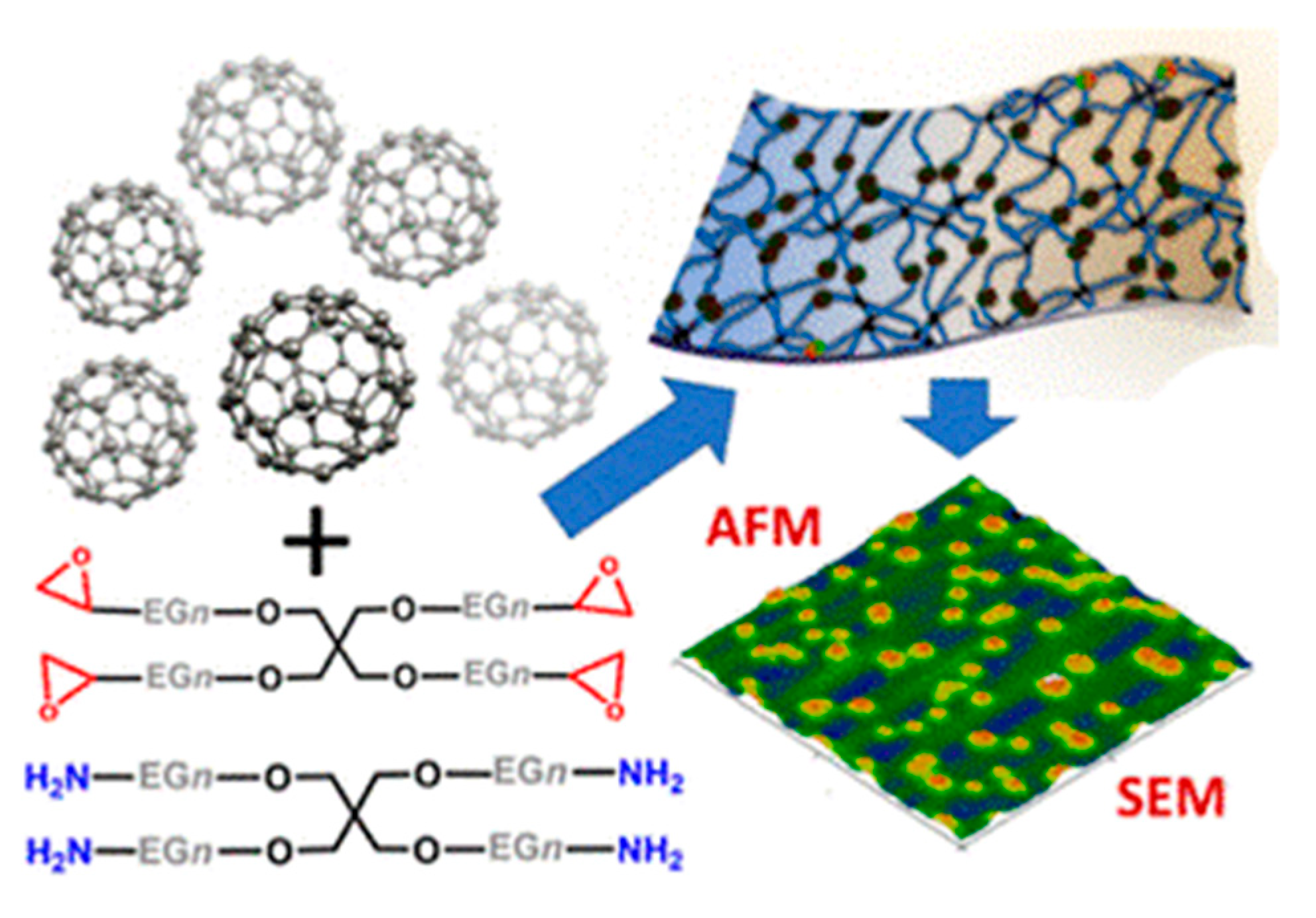

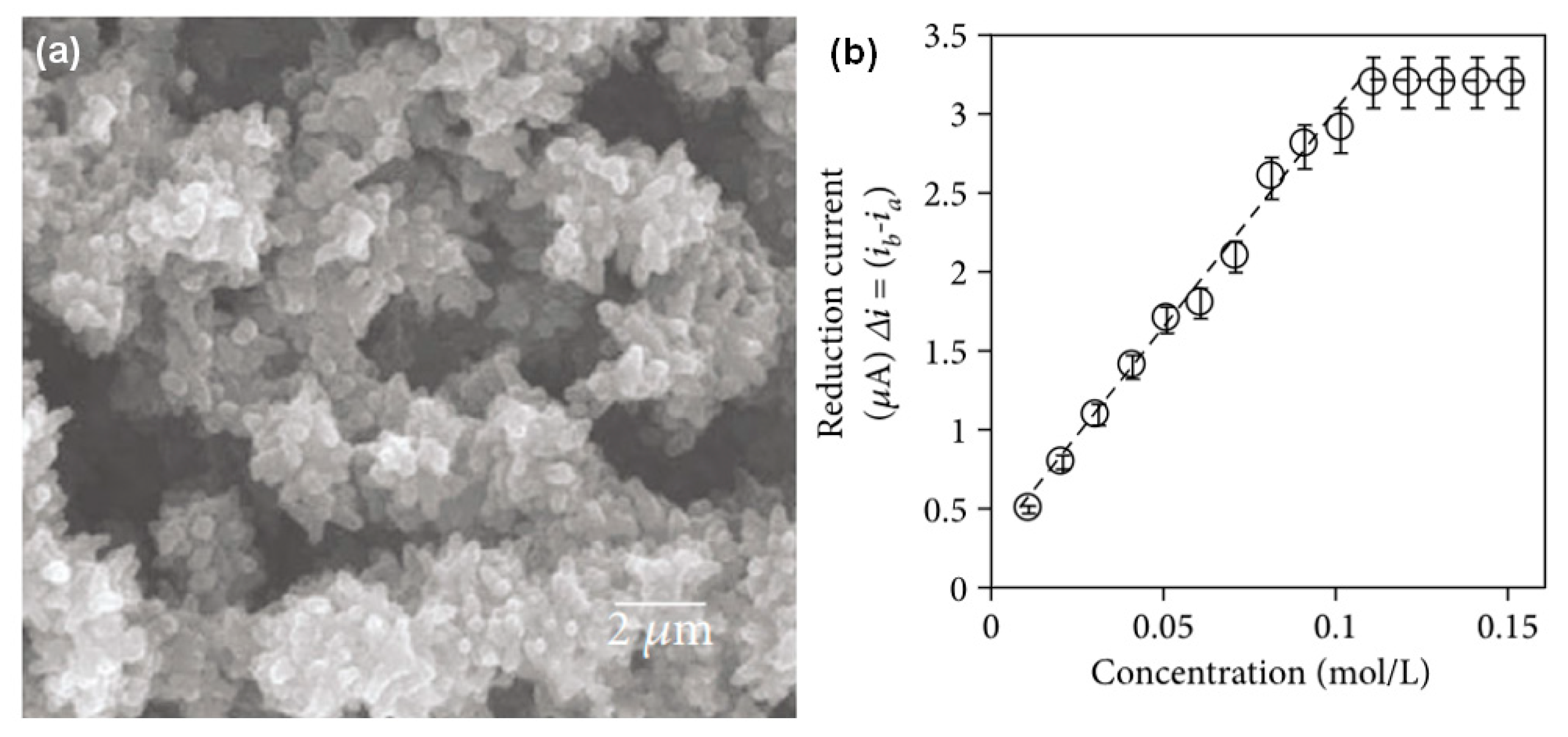

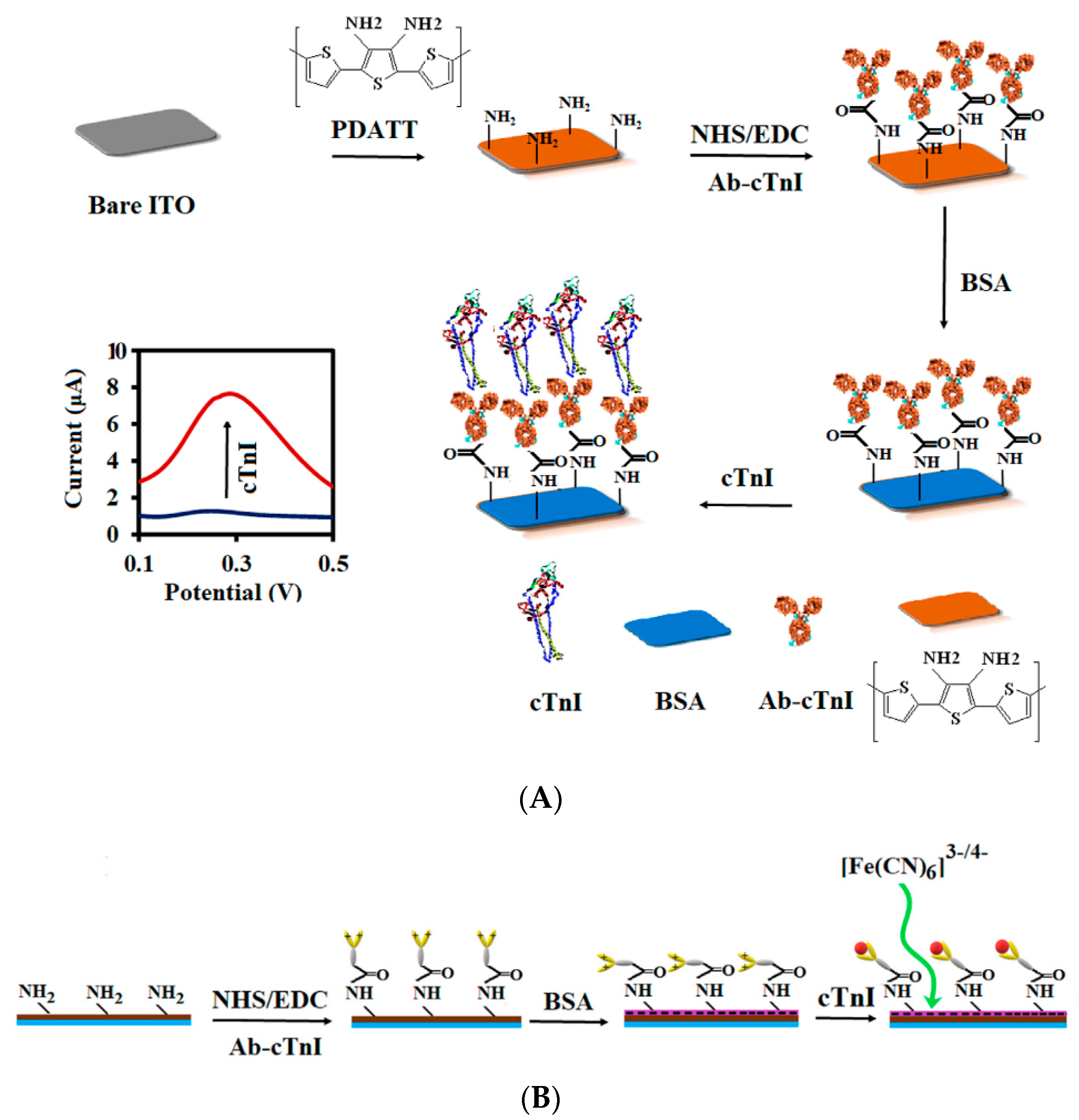
| Conductive Polymer | First Synthesized | Molecular Weight of the Monomer (g mol−1) | Conductivity Type | Specific Capacitance (Fg−1) | Conductivity (S cm−1) |
|---|---|---|---|---|---|
| Polyacetylene | 1977 | 26 | n, p | 241 | 103–1.7 × 105 |
| Polypyrrole | 1979 | 67 | P | 530 | 102–7.5 × 103 |
| Polyparaphenylene | 1979 | 78 | n, p | - | 102–103 |
| Polyparavinylene | 1979 | 28 | P | - | 3–5 × 103 |
| Poly(3,4-ethylenedioxythiophene) | 1980 | 142 | n, p | 92 | 300 |
| Polyaniline | 1980 | 93 | n, p | 240 | 30–200 |
| Polythiophene | 1981 | 84 | p | 485 | 10–103 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuznetsova, L.S.; Arlyapov, V.A.; Plekhanova, Y.V.; Tarasov, S.E.; Kharkova, A.S.; Saverina, E.A.; Reshetilov, A.N. Conductive Polymers and Their Nanocomposites: Application Features in Biosensors and Biofuel Cells. Polymers 2023, 15, 3783. https://doi.org/10.3390/polym15183783
Kuznetsova LS, Arlyapov VA, Plekhanova YV, Tarasov SE, Kharkova AS, Saverina EA, Reshetilov AN. Conductive Polymers and Their Nanocomposites: Application Features in Biosensors and Biofuel Cells. Polymers. 2023; 15(18):3783. https://doi.org/10.3390/polym15183783
Chicago/Turabian StyleKuznetsova, Lyubov S., Vyacheslav A. Arlyapov, Yulia V. Plekhanova, Sergei E. Tarasov, Anna S. Kharkova, Evgeniya A. Saverina, and Anatoly N. Reshetilov. 2023. "Conductive Polymers and Their Nanocomposites: Application Features in Biosensors and Biofuel Cells" Polymers 15, no. 18: 3783. https://doi.org/10.3390/polym15183783
APA StyleKuznetsova, L. S., Arlyapov, V. A., Plekhanova, Y. V., Tarasov, S. E., Kharkova, A. S., Saverina, E. A., & Reshetilov, A. N. (2023). Conductive Polymers and Their Nanocomposites: Application Features in Biosensors and Biofuel Cells. Polymers, 15(18), 3783. https://doi.org/10.3390/polym15183783