Enhanced Interfacial Properties of Carbon Fiber/Maleic Anhydride-Grafted Polypropylene Composites via Two-Step Surface Treatment: Electrochemical Oxidation and Silane Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Carbon Fiber Surface Treatment
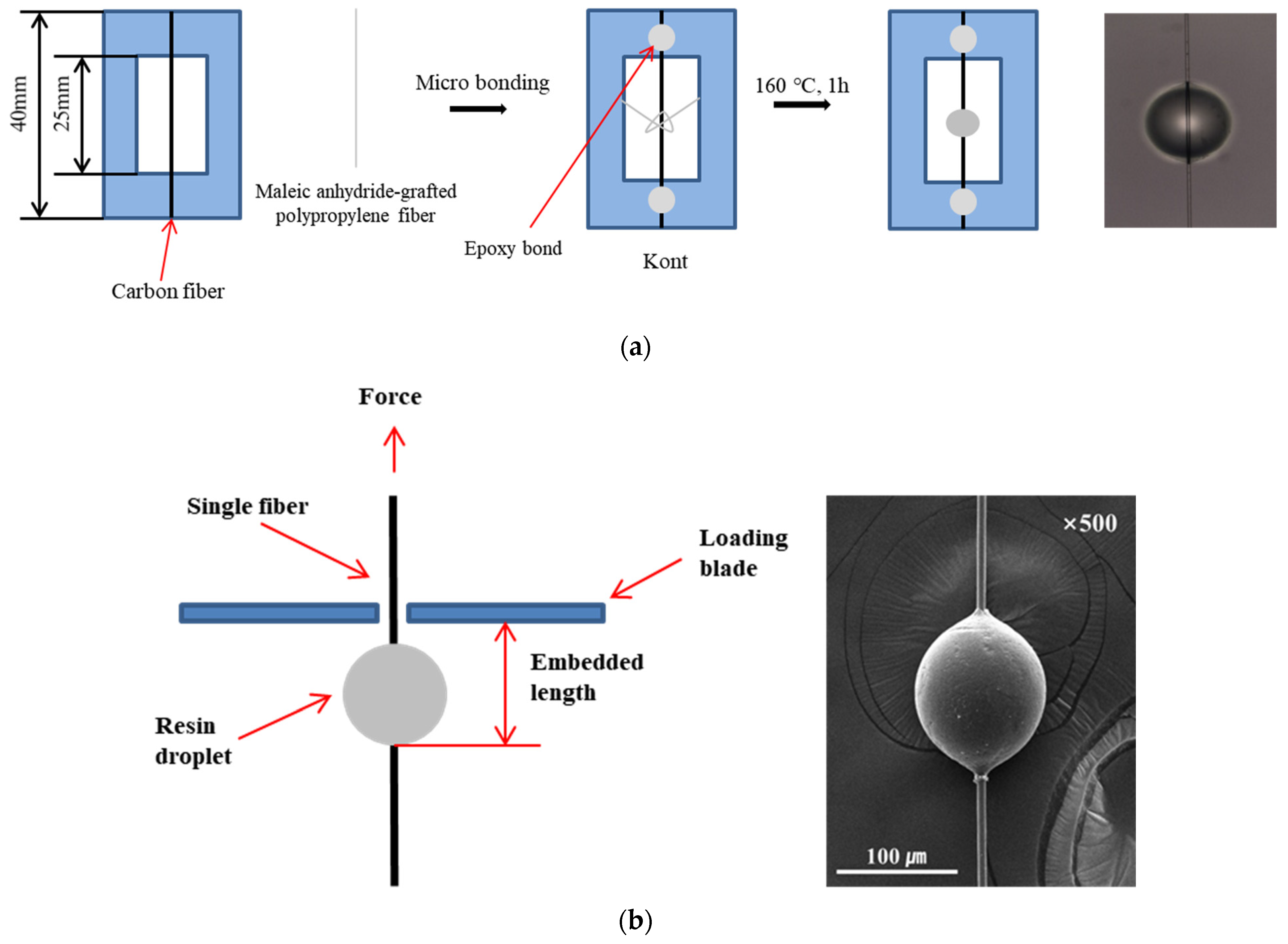
2.3. Single-Carbon-Fiber Microdroplet Test
2.4. Characterization
3. Results
3.1. Surface Morphology and Chemical Structural Analysis
3.2. Mechanical Property Analysis
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, K.-W.; Kim, D.-K.; Kim, B.S.; An, K.-H.; Park, S.-J.; Rhee, K.Y.; Kim, B.-J. Cure behaviors and mechanical properties of carbon fiber-reinforced nylon6/epoxy blended matrix composites. Compos. B Eng. 2023, 112, 15–21. [Google Scholar] [CrossRef]
- Perepelkin, K.E. Oxidized (cyclized) polyacrylonitrile fibers: Oxypan. A review. Fibre Chem. 2003, 35, 409–416. [Google Scholar] [CrossRef]
- Park, S.-J.; Jang, Y.S.; Shim, J.W.; Ryu, S.K. Studies on pore structures and surface functional groups of pitch-based activated carbon fibers. J. Colloid Interface Sci. 2003, 260, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Pan, D. A new cellulose based carbon fiber from a lyocell precursor. Textil Res. J. 2002, 72, 405–410. [Google Scholar]
- Kumar, P.S.; Jayanarayanan, K.; Balachandran, M. High-performance thermoplastic polyaryletherketone/carbon fiber composites: Comparison of plasma, carbon nanotubes/graphene nano-anchoring, surface oxidation techniques for enhanced interface adhesion and properties. Compos. B Eng. 2023, 253, 110560. [Google Scholar] [CrossRef]
- Lin, Y.; Gigliotti, M.; Lafarie-Frenot, M.C.; Bai, J. Effect of carbon nanotubes on the thermoelectric properties of CFRP laminate for aircraft applications. J. Reinf. Plast. Compos. 2015, 34, 173–184. [Google Scholar] [CrossRef]
- Srivastava, S.K.; Singh, I.P. Hybrid epoxy nanocomposites: Lightweight materials for structural applications. Polym. J. 2012, 44, 334–339. [Google Scholar] [CrossRef]
- Sanguesa, J.A.; Torres-Sanz, V.; Garrido, P.; Martinez, F.J.; Marquez-Barja, J.M. A review on electric vehicles: Technologies and challenges. Smart Cities 2021, 4, 372–404. [Google Scholar] [CrossRef]
- Hossain Lipu, M.S.; Hannan, M.A.; Karim, T.F.; Hussain, A.; Saad, M.H.M.; Ayob, A.; Sazal Miah, M.; Indra Mahlia, T.M. Intelligent algorithms and control strategies for battery management system in electric vehicles: Progress, challenges and future outlook. J. Clean. Prod. 2021, 292, 126044. [Google Scholar] [CrossRef]
- Li, Z.; Khajepour, A.; Song, J. A comprehensive review of the key technologies for pure electric vehicles. Materials 2019, 182, 824–839. [Google Scholar] [CrossRef]
- Kim, D.-K.; Han, W.; Kim, K.-W.; Kim, B.-J. Electromagnetic interference shielding effectiveness of direct-grown-carbon nanotubes/carbon and glass fiber-reinforced epoxy matrix composites. Materials 2023, 15, 2604–2616. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-W.; Kim, D.-K.; Han, W.; Kim, B.-J. Comparison of the characteristics of recycled carbon fibers/polymer composites by different recycling techniques. Molecules 2022, 27, 5663–5670. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Song, J.H. Improvement in the mechanical properties of carbon and aramid composites by fiber surface modification using polydopamine. Compos. B Eng. 2019, 160, 31–36. [Google Scholar] [CrossRef]
- Wong, K.H.; Syed Mohammed, D.; Pickering, S.J.; Brooks, R. Effect of coupling agents on reinforcing potential of recycled carbon fibre for polypropylene composite. Compos. Sci. Technol. 2012, 72, 835–844. [Google Scholar] [CrossRef]
- Wang, J.; Anthony, D.B.; Fuentes, C.A.; De Luca, H.G.; Zhang, D.; Bismarck, A.; Van Vuure, A.W.; Shaffer, M.S.P.; Seveno, D. Wettability of carbon nanotube-grafted carbon fibers and their interfacial properties in polypropylene thermoplastic composite. Compos. Part A Appl. Sci. Manuf. 2022, 159, 106993. [Google Scholar] [CrossRef]
- Wen, Z.; Xu, C.; Qian, X.; Zhang, Y.; Wang, X.; Song, S.; Dai, M.; Zhang, C. A two-step carbon fiber surface treatment and its effect on the interfacial properties of CF/EP composites: The electrochemical oxidation followed by grafting of silane coupling agent. Appl. Surf. Sci. 2019, 486, 546–554. [Google Scholar] [CrossRef]
- Lin, S.P.; Han, J.L.; Yeh, J.T.; Chang, F.C.; Hsieh, K.H. Composites of UHMWPE fiber reinforced PU/epoxy grafted interpenetrating polymer networks. Eur. Polym. J. 2007, 43, 996–1008. [Google Scholar] [CrossRef]
- Cao, X.; Li, J.L. Enhanced interfacial property of carbon fiber reinforced epoxy composite based on carbon fiber treated by supercritical water/nitrate system. J. Compos. Mater. 2021, 55, 3719–3727. [Google Scholar] [CrossRef]
- Yenier, Z.; Altay, L.; Sarikanat, M. Effect of surface modification of carbon fibers on properties of carbon/epoxy composites. Emerg. Mater. Res. 2020, 9, 110–118. [Google Scholar] [CrossRef]
- Zheng, H.; Zhang, W.; Li, B.; Zhu, J.; Wang, C.; Song, G.; Wu, G.; Yang, X.; Huang, Y.; Ma, L. Recent advances of interphases in carbon fiber-reinforced polymer composites: A review. Compos. B Eng. 2022, 233, 109639. [Google Scholar] [CrossRef]
- Huang, C.; Chen, G.; Wang, Q.; Wang, Z.; Yu, Q.; Liu, X. Research progress of carbon fiber surface modification technology. Eng. Plast. Appl. 2022, 50, 170–174. [Google Scholar]
- Hu, J.Q.; Li, F.; Wang, B.; Zhang, H.Q.; Ji, C.M.; Wang, S.X.; Zhou, Z.G.; Compos, B.E. A two-step combination strategy for significantly enhancing the interfacial adhesion of CF/PPS composites: The liquid-phase oxidation followed by grafting of silane coupling agent. Compos. B Eng. 2020, 191, 107966. [Google Scholar]
- Kim, D.-K.; An, K.-H.; Bang, Y.H.; Kwac, L.-K.; Oh, S.-Y.; Kim, B.-J. Effects of electrochemical oxidation of carbon fibers on interfacial shear strength using a micro-bond method. Carbon Lett. 2016, 19, 32–39. [Google Scholar] [CrossRef]
- Kim, D.K.; Han, W.; Kim, B.; Kim, B.; An, K. A study on EMI shielding enhancement behaviors of Ni-plated CFs-reinforced polymer matrix composites by post heat treatment. Appl. Surf. Sci. 2017, 415, 55–60. [Google Scholar] [CrossRef]
- Wang, S.; Chen, Z.H.; Ma, W.J.; Ma, Q.S. Influence of heat treatment on physical–chemical properties of PAN-based carbon fibers. Ceram. Int. 2006, 32, 291–295. [Google Scholar] [CrossRef]
- Kim, K.-W.; Jeong, J.S.; An, K.H.; Kim, B.J. A study on the microstructural changes and mechanical behaviors of carbon fibers induced by optimized electrochemical etching. Compos. B Eng. 2019, 165, 764–771. [Google Scholar] [CrossRef]
- Kim, D.-K.; Kang, S.H.; Han, W.; Kim, K.W.; Kim, B.J. Facile method to enhance the mechanical interfacial strength between carbon fibers and polyamide 6 using modified silane coupling agents. Carbon Lett. 2022, 32, 1463–1472. [Google Scholar] [CrossRef]
- Liao, M.; Yang, Y.; Hamada, H. Mechanical performance of glass woven fabric composite: Effect of different surface treatment agents. Compos. B Eng. 2016, 86, 17–26. [Google Scholar] [CrossRef]
- Xie, Y.; Hill, C.A.S.; Xiao, Z.; Militz, H.; Mai, C. Silane coupling agents used for natural fiber/polymer composites: A review. Compos. Part A Appl. Sci. Manuf. 2010, 41, 806–819. [Google Scholar] [CrossRef]
- Downey, M.A.; Drzal, L.T. Toughening of carbon fiber-reinforced epoxy polymer composites utilizing fiber surface treatment and sizing. Compos. Part A Appl. Sci. Manuf. 2016, 90, 687–698. [Google Scholar] [CrossRef]
- Liu, D.; Li, B.; Li, G.; Wang, L.; Yang, X. Tagged and enhanced interface of carbon fiber/epoxy by doping sizing agent with upconversion luminescent nanoparticles. Mater. Lett. 2017, 196, 37–41. [Google Scholar] [CrossRef]
- Jiang, B.; Zhang, T.; Zhao, L.; Huang, Y. Interfacially reinforced carbon fiber composites by grafting modified methylsilicone resin. Compos. Sci. Technol. 2017, 140, 39–45. [Google Scholar] [CrossRef]
- Park, S.; Jin, J. Effect of silane coupling agent on interphase and performance of glass fibers/unsaturated polyester composites. J. Colloid Interface Sci. 2001, 242, 174–179. [Google Scholar] [CrossRef]
- Sae-Oui, P.; Sirisinha, C.; Thepsuwan, U.; Hatthapanit, K. Roles of silane coupling agents on properties of silica-filled polychloroprene. Eur. Polym. J. 2006, 42, 479–486. [Google Scholar] [CrossRef]
- Dai, Z.; Shi, F.; Zhang, B.; Li, M.; Zhang, Z. Effect of sizing on carbon fiber surface properties and fibers/epoxy interfacial adhesion. Appl. Surf. Sci. 2011, 257, 6980–6985. [Google Scholar] [CrossRef]
- Lee, M.-S.; Park, M.R.; Kim, H.Y.; Park, S.-J. Effects of Microporosity and Surface Chemistry on Separation Performances of N-Containing Pitch-Based Activated Carbons for CO2/N2 Binary Mixture. Sci. Rep. 2016, 6, 23224. [Google Scholar] [CrossRef]







| Sample Name | Treatment Conditions |
|---|---|
| AS-CF | Unsized carbon fiber |
| EO-CF | Electrochemical-oxidation-treated carbon fiber |
| EOS1-CF | Silane-treated carbon fiber at a concentration of 1 wt.% |
| EOS2-CF | Silane-treated carbon fiber at a concentration of 2 wt.% |
| EOS3-CF | Silane-treated carbon fiber at a concentration of 3 wt.% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.-K.; Han, W.; Kim, K.-W.; Kim, B.-J. Enhanced Interfacial Properties of Carbon Fiber/Maleic Anhydride-Grafted Polypropylene Composites via Two-Step Surface Treatment: Electrochemical Oxidation and Silane Treatment. Polymers 2023, 15, 3784. https://doi.org/10.3390/polym15183784
Kim D-K, Han W, Kim K-W, Kim B-J. Enhanced Interfacial Properties of Carbon Fiber/Maleic Anhydride-Grafted Polypropylene Composites via Two-Step Surface Treatment: Electrochemical Oxidation and Silane Treatment. Polymers. 2023; 15(18):3784. https://doi.org/10.3390/polym15183784
Chicago/Turabian StyleKim, Dong-Kyu, Woong Han, Kwan-Woo Kim, and Byung-Joo Kim. 2023. "Enhanced Interfacial Properties of Carbon Fiber/Maleic Anhydride-Grafted Polypropylene Composites via Two-Step Surface Treatment: Electrochemical Oxidation and Silane Treatment" Polymers 15, no. 18: 3784. https://doi.org/10.3390/polym15183784






