Holmium-Containing Metal-Organic Frameworks as Modifiers for PEBA-Based Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Ho-MOFs Preparation
2.3. Ho-MOFs Investigation
2.4. PEBA Investigation
2.5. Dense Membrane Preparation
2.6. Membrane Investigation
3. Results and Discussions
3.1. Ho-MOFs Investigation
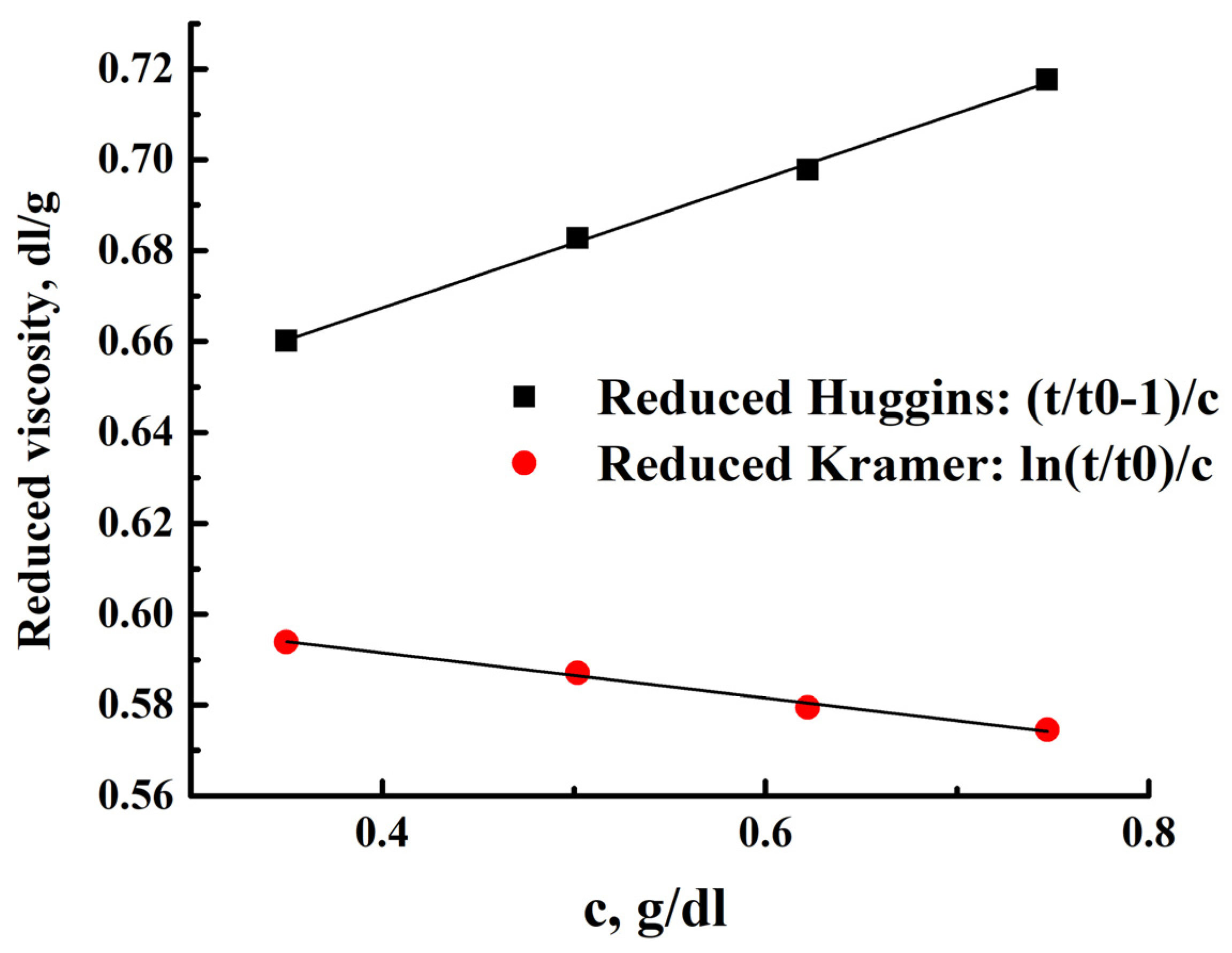
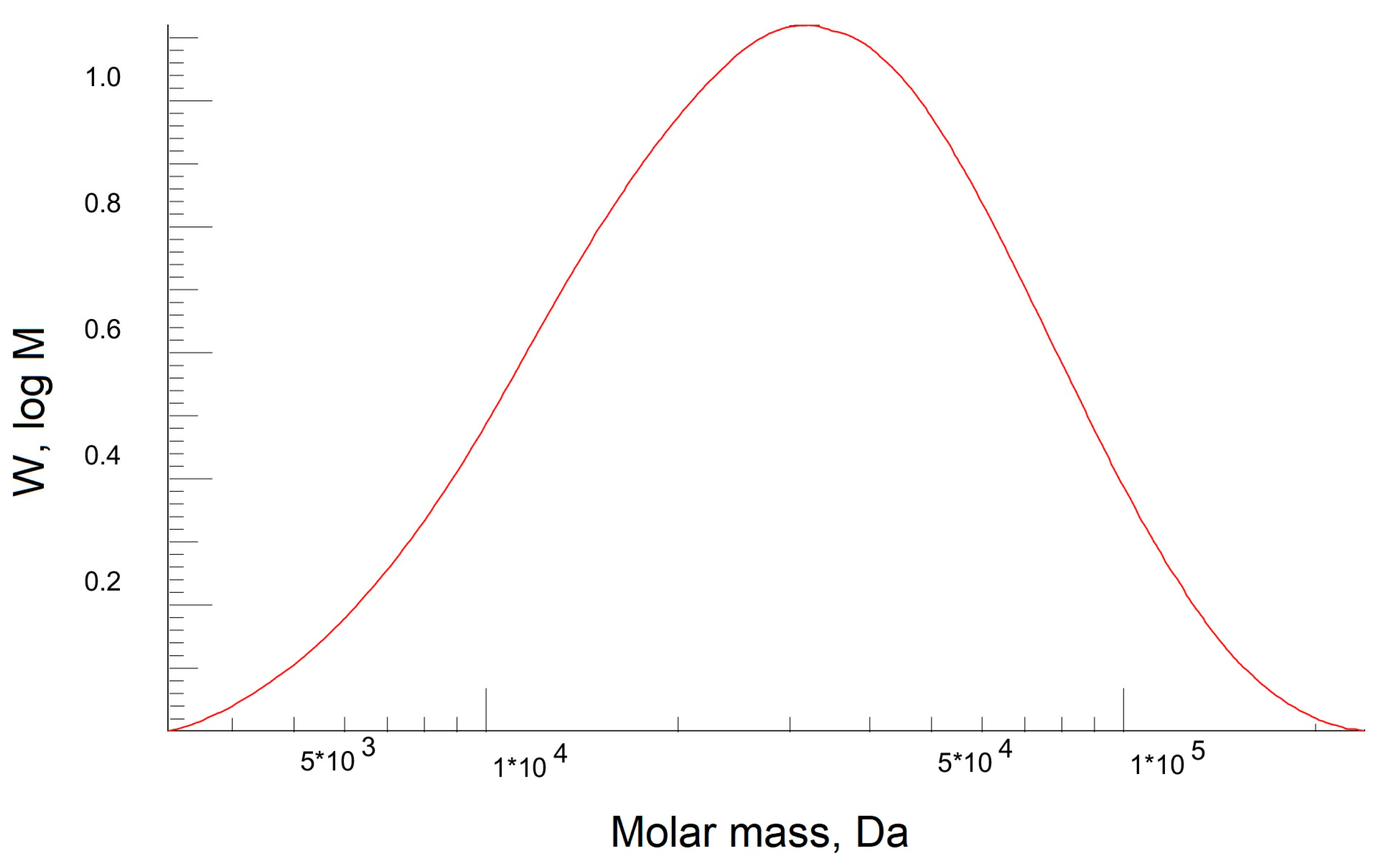
3.2. PEBA Investigation
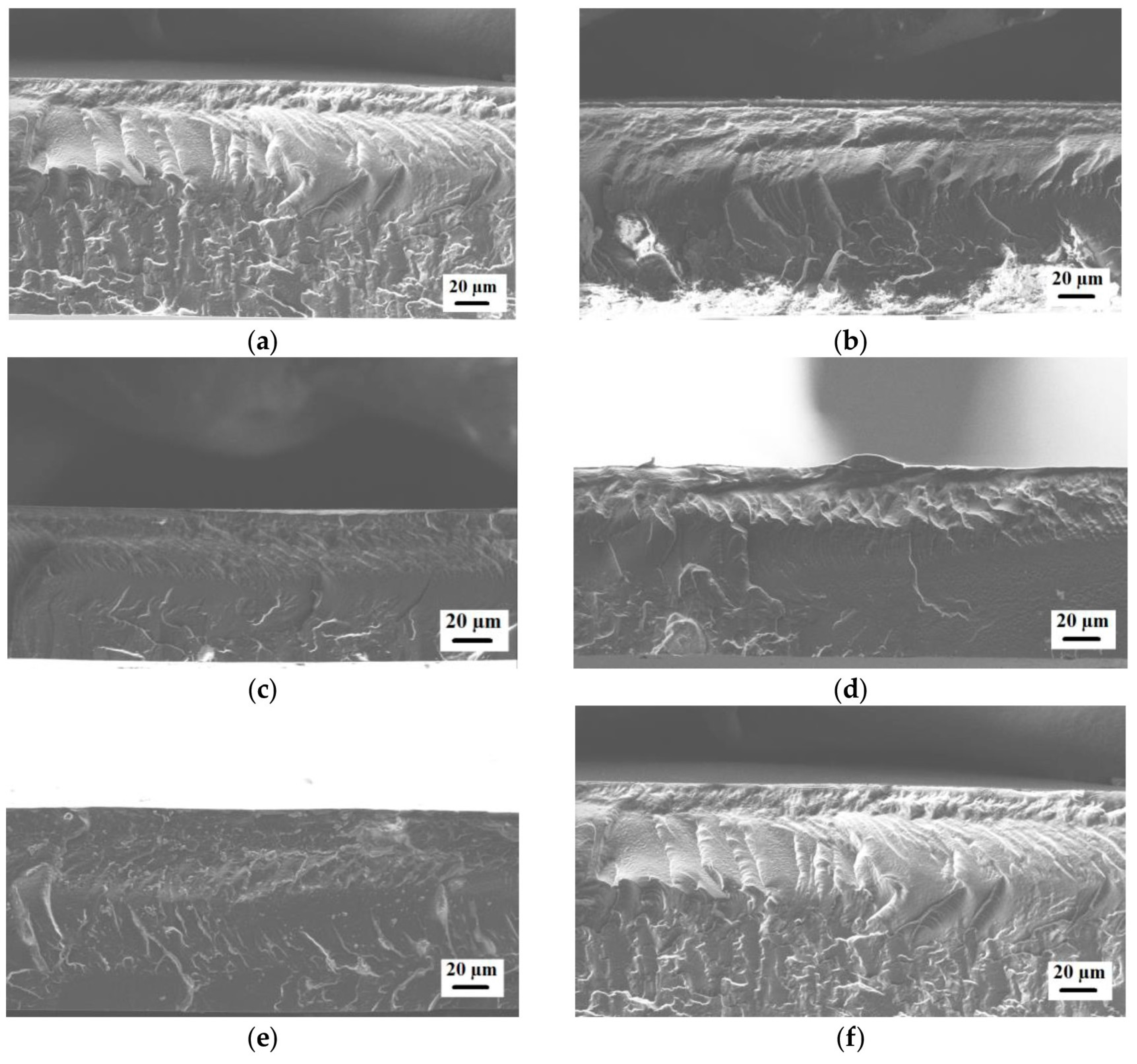
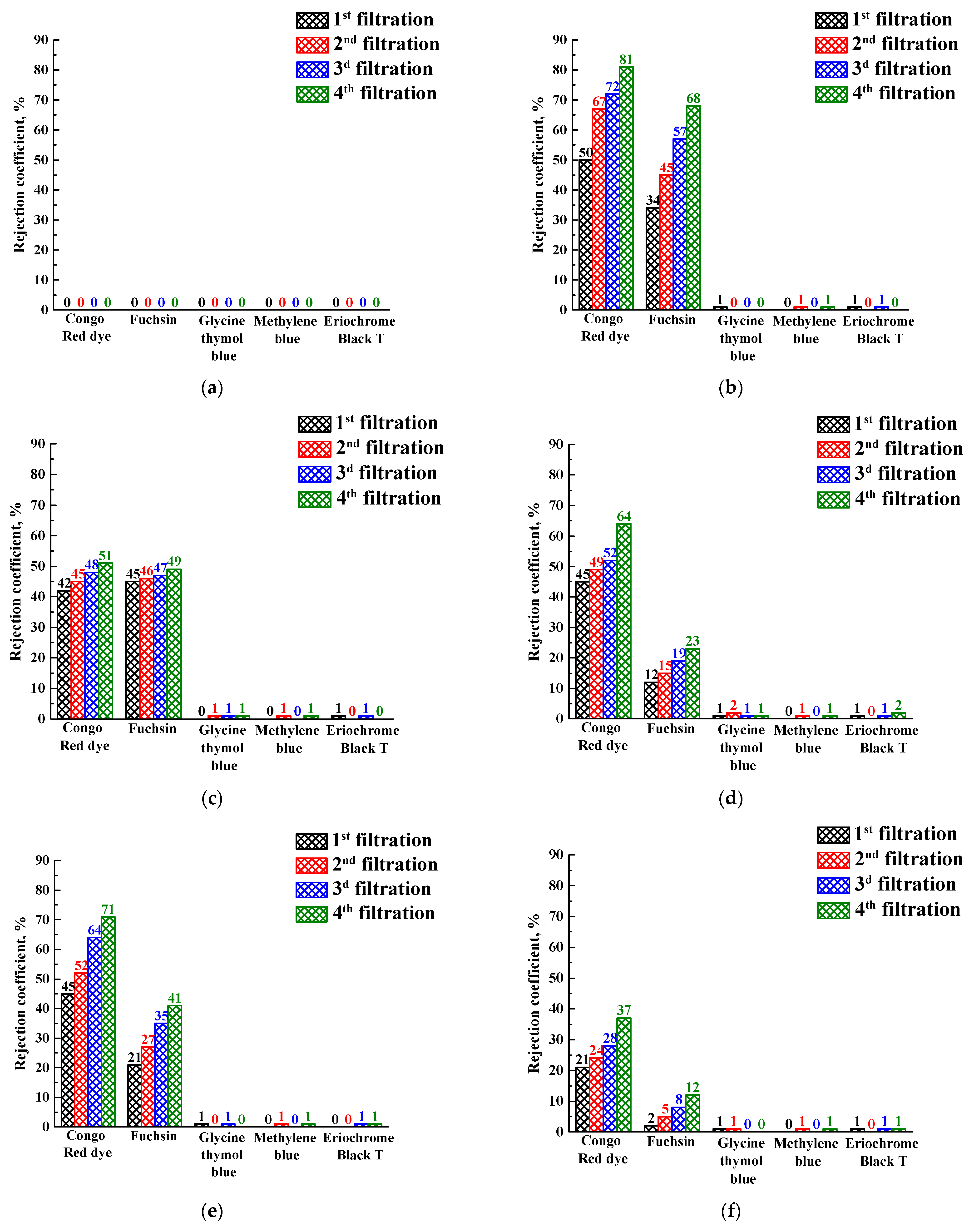
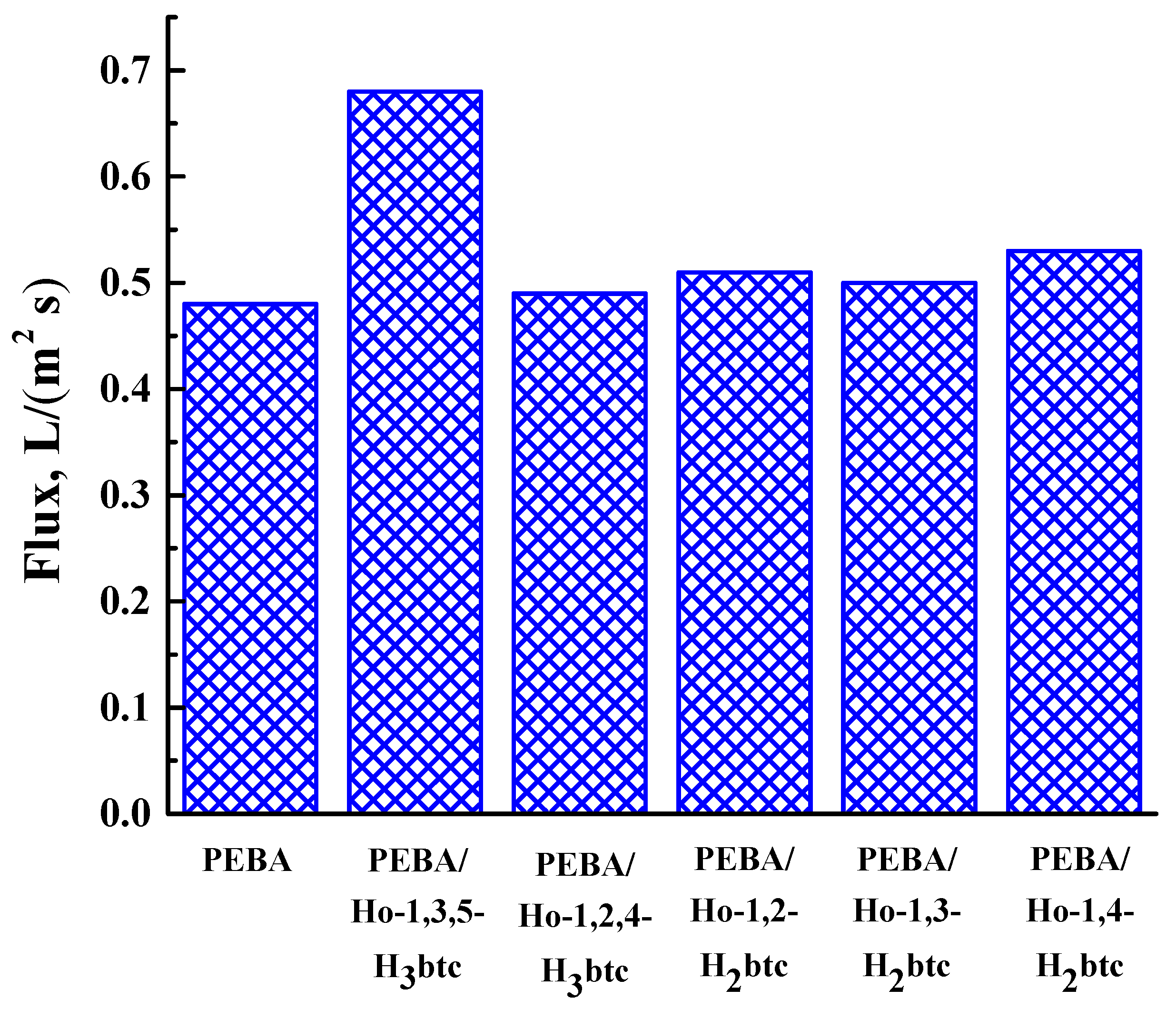
3.3. PEBA and PEBA/Ho-MOFs Membranes Investigation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tobin, G.; Comby, S.; Zhu, N.; Clérac, R.; Gunnlaugsson, T.; Schmitt, W. Towards multifunctional lanthanide-based metal–organic frameworks. Chem. Commun. 2015, 51, 13313–13316. [Google Scholar] [CrossRef] [PubMed]
- Echenique-Errandonea, E.; Mendes, R.F.; Figueira, F.; Choquesillo-Lazarte, D.; Beobide, G.; Cepeda, J.; Ananias, D.; Rodríguez-Diéguez, A.; Almeida Paz, F.A.; Seco, J.M. Multifunctional Lanthanide-Based Metal–Organic Frameworks Derived from 3-Amino-4-hydroxybenzoate: Single-Molecule Magnet Behavior, Luminescent Properties for Thermometry, and CO2 Adsorptive Capacity. Inorg. Chem. 2022, 61, 12977–12990. [Google Scholar] [CrossRef]
- Meng, S.; Li, G.; Wang, P.; He, M.; Sun, X.; Li, Z. Rare earth-based MOFs for photo/electrocatalysis. Mater. Chem. Front. 2023, 7, 806–827. [Google Scholar] [CrossRef]
- Chen, H.; Liu, S.; Lv, H.; Qin, Q.-P.; Zhang, X. Nanoporous {Y2}-Organic Frameworks for Excellent Catalytic Performance on the Cycloaddition Reaction of Epoxides with CO2 and Deacetalization–Knoevenagel Condensation. ACS Appl. Mater. Interfaces 2022, 14, 18589–18599. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Chen, H.; Liu, S.; Lv, H.; Zhang, X.; Li, Q. Highly Robust {Ln4}-Organic Frameworks (Ln = Ho, Yb) for Excellent Catalytic Performance on Cycloaddition Reaction of Epoxides with CO2 and Knoevenagel Condensation. ACS Catal. 2021, 11, 14916–14925. [Google Scholar] [CrossRef]
- Parnicka, P.; Lisowski, W.; Klimczuk, T.; Łuczak, J.; Żak, A.; Zaleska-Medynska, A. Visible-light-driven lanthanide-organic-frameworks modified TiO2 photocatalysts utilizing up-conversion effect. Appl. Catal. B Environ. 2021, 291, 120056. [Google Scholar] [CrossRef]
- Mezenov, Y.A.; Bruyere, S.; Krasilin, A.; Khrapova, E.; Bachinin, S.V.; Alekseevskiy, P.V.; Shipiloskikh, S.; Boulet, P.; Hupont, S.; Nomine, A.; et al. Insights into Solid-To-Solid Transformation of MOF Amorphous Phases. Inorg. Chem. 2022, 61, 13992–14003. [Google Scholar] [CrossRef] [PubMed]
- Vodyashkin, A.A.; Sergorodceva, A.V.; Kezimana, P.; Stanishevskiy, Y.M. Metal-Organic Framework (MOF)—A Universal Material for Biomedicine. Int. J. Mol. Sci. 2023, 24, 7819. [Google Scholar] [CrossRef]
- Pereira, G.A.; Peters, J.A.; Almeida Paz, F.A.; Rocha, J.; Geraldes, C.F.G.C. Evaluation of [Ln(H2cmp)(H2O)] Metal Organic Framework Materials for Potential Application as Magnetic Resonance Imaging Contrast Agents. Inorg. Chem. 2010, 49, 2969–2974. [Google Scholar] [CrossRef]
- Fujita, Y.; Kohaku, K.; Komiyama, N.; Ujiie, K.; Masu, H.; Kojima, T.; Wadati, H.; Kanoh, H.; Kishikawa, K.; Kohri, M. Colorless Magnetic Colloidal Particles Based on an Amorphous Metal-Organic Framework Using Holmium as the Metal Species. ChemNanoMat 2022, 8, e202200078. [Google Scholar] [CrossRef]
- Dalakova, N.V.; Belevtsev, B.I.; Belyaev, E.Y.; Panfilov, A.S.; Bobrysheva, N.P.; Selyutin, A.A. Low-temperature nonlinear effects in the conductivity of lightly doped cuprates La2−xSrxCuO4 in antiferromagnetic state. Low Temp. Phys. 2014, 40, 397–407. [Google Scholar] [CrossRef]
- Belevtsev, B.I.; Dalakova, N.V.; Osmolowsky, M.G.; Beliayev, E.Y.; Selutin, A.A.; Kolesnichenko, Y.A. Percolation effects in the conductivity and magnetoresistance of compacted chromium dioxide powder. Bull. Russ. Acad. Sci. Phys. 2010, 74, 1062–1065. [Google Scholar] [CrossRef]
- Wu, P.; Xia, L.; Huangfu, M.; Fu, F.; Wang, M.; Wen, B.; Yang, Z.; Wang, J. Lanthanide-Based Metal–Organic Frameworks Containing “V-Shaped” Tetracarboxylate Ligands: Synthesis, Crystal Structures, “Naked-Eye” Luminescent Detection, and Catalytic Properties. Inorg. Chem. 2020, 59, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Zhao, Y.; Wang, J.; Pei, R. Lanthanide-based MOFs: Synthesis approaches and applications in cancer diagnosis and therapy. J. Mater. Chem. B 2022, 10, 9535–9564. [Google Scholar] [CrossRef] [PubMed]
- Vizuet, J.P.; Mortensen, M.L.; Lewis, A.L.; Wunch, M.A.; Firouzi, H.R.; McCandless, G.T.; Balkus, K.J. Fluoro-Bridged Clusters in Rare-Earth Metal–Organic Frameworks. J. Am. Chem. Soc. 2021, 143, 17995–18000. [Google Scholar] [CrossRef]
- Bejan, D.; Bahrin, L.G.; Shova, S.; Marangoci, N.L.; Kökҫam-Demir, Ü.; Lozan, V.; Janiak, C. New Microporous Lanthanide Organic Frameworks. Synthesis, Structure, Luminescence, Sorption, and Catalytic Acylation of 2-Naphthol. Molecules 2020, 25, 3055. [Google Scholar] [CrossRef]
- Vizuet, J.P.; Lewis, A.L.; McCandless, G.T.; Balkus, K.J. Holmium-based metal-organic frameworks using the BDC linker. Polyhedron 2021, 205, 115283. [Google Scholar] [CrossRef]
- Das, A.K.; Vemuri, R.S.; Kutnyakov, I.; McGrail, B.P.; Motkuri, R.K. An Efficient Synthesis Strategy for Metal-Organic Frameworks: Dry-Gel Synthesis of MOF-74 Framework with High Yield and Improved Performance. Sci. Rep. 2016, 6, 28050. [Google Scholar] [CrossRef]
- Leubner, S.; Stäglich, R.; Franke, J.; Jacobsen, J.; Gosch, J.; Siegel, R.; Reinsch, H.; Maurin, G.; Senker, J.; Yot, P.G.; et al. Solvent Impact on the Properties of Benchmark Metal–Organic Frameworks: Acetonitrile-Based Synthesis of CAU-10, Ce-UiO-66, and Al-MIL-53. Chem. Eur. J. 2020, 26, 3877–3883. [Google Scholar] [CrossRef]
- Deegan, M.M.; Antonio, A.M.; Taggart, G.A.; Bloch, E.D. Manipulating solvent and solubility in the synthesis, activation, and modification of permanently porous coordination cages. Coord. Chem. Rev. 2021, 430, 213679. [Google Scholar] [CrossRef]
- Seetharaj, R.; Vandana, P.V.; Arya, P.; Mathew, S. Dependence of solvents, pH, molar ratio and temperature in tuning metal organic framework architecture. Arab. J. Chem. 2019, 12, 295–315. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, J.; Liu, C.; Sang, X.; Peng, L.; Ma, X.; Wu, T.; Han, B.; Yang, G. Solvent determines the formation and properties of metal–organic frameworks. RSC Adv. 2015, 5, 37691–37696. [Google Scholar] [CrossRef]
- Bhindi, M.; Massengo, L.; Hammerton, J.; Derry, M.J.; Worrall, S.D. Structure Control Using Bioderived Solvents in Electrochemical Metal-Organic Framework Synthesis. Appl. Sci. 2023, 13, 720. [Google Scholar] [CrossRef]
- Lozano, L.A.; Iglesias, C.M.; Faroldi, B.M.C.; Ulla, M.A.; Zamaro, J.M. Efficient solvothermal synthesis of highly porous UiO-66 nanocrystals in dimethylformamide-free media. J. Mater. Sci. 2018, 53, 1862–1873. [Google Scholar] [CrossRef]
- Wee, L.H.; Lohe, M.R.; Janssens, N.; Kaskel, S.; Martens, J.A. Fine tuning of the metal–organic framework Cu3(BTC)2 HKUST-1 crystal size in the 100 nm to 5 micron range. J. Mater. Chem. 2012, 22, 13742. [Google Scholar] [CrossRef]
- Chang, J.; Wang, Y.; Chen, L.; Wu, D.; Xu, F.; Bai, Z.; Jiang, K.; Gao, Z. Cobalt nanoparticles embedded nitrogen doped carbon, preparation from alkali deprotonation assisted ZIF-67 and its electrocatalytic performance in oxygen evolution reaction. Int. J. Hydrogen Energy 2020, 45, 12787–12797. [Google Scholar] [CrossRef]
- Wiwasuku, T.; Othong, J.; Boonmak, J.; Ervithayasuporn, V.; Youngme, S. Sonochemical synthesis of microscale Zn(II)-MOF with dual Lewis basic sites for fluorescent turn-on detection of Al3+ and methanol with low detection limits. Dalt. Trans. 2020, 49, 10240–10249. [Google Scholar] [CrossRef]
- Wiwasuku, T.; Boonmak, J.; Siriwong, K.; Ervithayasuporn, V.; Youngme, S. Highly sensitive and selective fluorescent sensor based on a multi-responsive ultrastable amino-functionalized Zn(II)-MOF for hazardous chemicals. Sens. Actuators B Chem. 2019, 284, 403–413. [Google Scholar] [CrossRef]
- Ma, K.-B.; Han, S.-B.; Kwon, S.-H.; Kwak, D.-H.; Park, K.-W. High-performance direct ethanol fuel cell using nitrate reduction reaction. Int. J. Hydrogen Energy 2018, 43, 17265–17270. [Google Scholar] [CrossRef]
- Yan, S.; Cheng, K.Y.; Ginige, M.P.; Zheng, G.; Zhou, L.; Kaksonen, A.H. Optimization of nitrate and selenate reduction in an ethanol-fed fluidized bed reactor via redox potential feedback control. J. Hazard. Mater. 2021, 402, 123770. [Google Scholar] [CrossRef]
- Xie, Y.; Ni, S.; Wang, S.; Wu, C.; Huang, K.; Ma, D.; Chen, L.; Liu, R.; Liu, H. Enhanced nitrate removal by alcohol-involved nitrate photolysis-induced advanced reduction process. Chem. Eng. J. 2023, 473, 145244. [Google Scholar] [CrossRef]
- Ladikan, O.; Silyavka, E.; Mitrofanov, A.; Laptenkova, A.; Shilovskikh, V.; Kolonitckii, P.; Ivanov, N.; Remezov, A.; Fedorova, A.; Khripun, V.; et al. Thin Films of Lanthanide Stearates as Modifiers of the Q-Sense Device Sensor for Studying Insulin Adsorption. ACS Omega 2022, 7, 24973–24981. [Google Scholar] [CrossRef] [PubMed]
- Bobrysheva, N.P.; Selyutin, A.A.; Ivanov, N.S.; Sukhodolov, N.G. Magnetic ordering in nanofilms containing 3d elements. Russ. J. Gen. Chem. 2015, 85, 1189–1190. [Google Scholar] [CrossRef]
- Sukhodolov, N.G.; Kel’tsieva, O.A.; Fedorova, A.V.; Selyutin, A.A.; Podol’skaya, E.P. Surface properties of Langmuir–Blodgett films and nanodispersed oxides containing nickel and copper. Russ. J. Gen. Chem. 2015, 85, 1974–1975. [Google Scholar] [CrossRef]
- Lu, Y.; Li, X.; Giovanni, C.; Wang, B. Construction of MOFs-based nanocomposite membranes for emerging organic contaminants abatement in water. Front. Environ. Sci. Eng. 2023, 17, 89. [Google Scholar] [CrossRef]
- Baker, R.W.; Low, B.T. Gas Separation Membrane Materials: A Perspective. Macromolecules 2014, 47, 6999–7013. [Google Scholar] [CrossRef]
- Lively, R.P.; Sholl, D.S. From water to organics in membrane separations. Nat. Mater. 2017, 16, 276–279. [Google Scholar] [CrossRef]
- Dmitrenko, M.; Liamin, V.; Kuzminova, A.; Mazur, A.; Lahderanta, E.; Ermakov, S.; Penkova, A. Novel mixed matrix sodium alginate-fullerenol membranes: Development, characterization, and study in pervaporation dehydration of isopropanol. Polymers 2020, 12, 864. [Google Scholar] [CrossRef]
- Polotskaya, G.A.; Penkova, A.V.; Pientka, Z.; Toikka, A.M. Polymer membranes modified by fullerene C60 for pervaporation of organic mixtures. Desalin. Water Treat. 2010, 14, 83–88. [Google Scholar] [CrossRef]
- Dmitrenko, M.; Kuzminova, A.; Zolotarev, A.; Markelov, D.; Komolkin, A.; Loginova, E.; Plisko, T.; Burts, K.; Bildyukevich, A.; Penkova, A. Modification strategies of polyacrylonitrile ultrafiltration membrane using TiO2 for enhanced antifouling performance in water treatment. Sep. Purif. Technol. 2022, 286, 120500. [Google Scholar] [CrossRef]
- Penkova, A.V.; Kuzminova, A.I.; Dmitrenko, M.E.; Surkova, V.A.; Liamin, V.P.; Markelov, D.A.; Komolkin, A.V.; Poloneeva, D.Y.; Laptenkova, A.V.; Selyutin, A.A.; et al. Novel pervaporation mixed matrix membranes based on polyphenylene isophtalamide modified by metal–organic framework UiO-66(NH2)-EDTA for highly efficient methanol isolation. Sep. Purif. Technol. 2021, 263, 118370. [Google Scholar] [CrossRef]
- Dmitrenko, M.; Kuzminova, A.; Zolotarev, A.; Selyutin, A.; Ermakov, S.; Penkova, A. Nanofiltration Mixed Matrix Membranes from Cellulose Modified with Zn-Based Metal–Organic Frameworks for the Enhanced Water Treatment from Heavy Metal Ions. Polymers 2023, 15, 1341. [Google Scholar] [CrossRef] [PubMed]
- Kuzminova, A.; Dmitrenko, M.; Zolotarev, A.; Markelov, D.; Komolkin, A.; Dubovenko, R.; Selyutin, A.; Wu, J.; Su, R.; Penkova, A. Novel Mixed Matrix Membranes Based on Poly(vinylidene fluoride): Development, Characterization, Modeling. Polymers 2023, 15, 1222. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Huang, J.; Li, Z.; Han, Z.; Fan, J. Review of Synthesis and Separation Application of Metal-Organic Framework-Based Mixed-Matrix Membranes. Polymers 2023, 15, 1950. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Navarro, J.A.; Hernandez-Garcia, F.; Alvarez Romero, G.A. Novel applications of metal-organic frameworks (MOFs) as redox-active materials for elaboration of carbon-based electrodes with electroanalytical uses. Coord. Chem. Rev. 2020, 412, 213263. [Google Scholar] [CrossRef]
- Kuzminova, A.; Dmitrenko, M.; Zolotarev, A.; Korniak, A.; Poloneeva, D.; Selyutin, A.; Emeline, A.; Yushkin, A.; Foster, A.; Budd, P.; et al. Novel Mixed Matrix Membranes Based on Polymer of Intrinsic Microporosity PIM-1 Modified with Metal—Organic Frameworks for Removal of Heavy Metal Ions and Food Dyes by Nanofiltration. Membrane 2022, 12, 1–24. [Google Scholar] [CrossRef]
- Shah Buddin, M.M.H.; Ahmad, A.L. A review on metal-organic frameworks as filler in mixed matrix membrane: Recent strategies to surpass upper bound for CO2 separation. J. CO2 Util. 2021, 51, 101616. [Google Scholar] [CrossRef]
- Duan, Y.; Li, L.; Shen, Z.; Cheng, J.; He, K. Engineering Metal-Organic-Framework (MOF)-Based Membranes for Gas and Liquid Separation. Membranes 2023, 13, 480. [Google Scholar] [CrossRef]
- Farahani, S.K.; Hosseini, S.M. A highly promoted nanofiltration membrane by incorporating of aminated Zr-based MOF for efficient salts and dyes removal with excellent antifouling properties. Chem. Eng. Res. Des. 2022, 188, 764–778. [Google Scholar] [CrossRef]
- Fang, X.; Wei, S.; Liu, S.; Li, R.; Zhang, Z.; Liu, Y.; Zhang, X.; Lou, M.; Chen, G.; Li, F. Metal-Coordinated Nanofiltration Membranes Constructed on Metal Ions Blended Support toward Enhanced Dye/Salt Separation and Antifouling Performances. Membranes 2022, 12, 340. [Google Scholar] [CrossRef]
- Shu, L.; Xie, L.-H.; Meng, Y.; Liu, T.; Zhao, C.; Li, J.-R. A thin and high loading two-dimensional MOF nanosheet based mixed-matrix membrane for high permeance nanofiltration. J. Memb. Sci. 2020, 603, 118049. [Google Scholar] [CrossRef]
- Meng, Y.; Shu, L.; Liu, L.; Wu, Y.; Xie, L.-H.; Zhao, M.-J.; Li, J.-R. A high-flux mixed matrix nanofiltration membrane with highly water-dispersible MOF crystallites as filler. J. Memb. Sci. 2019, 591, 117360. [Google Scholar] [CrossRef]
- Konyukhova, E.V.; Buzin, A.I.; Godovsky, Y.K. Melting of polyether block amide (Pebax): The effect of stretching. Thermochim. Acta 2002, 391, 271–277. [Google Scholar] [CrossRef]
- Yave, W.; Car, A.; Peinemann, K.-V. Nanostructured membrane material designed for carbon dioxide separation. J. Memb. Sci. 2010, 350, 124–129. [Google Scholar] [CrossRef]
- Vasileiou, A.N.; Theodorakopoulos, G.V.; Karousos, D.S.; Bouroushian, M.; Sapalidis, A.A.; Favvas, E.P. Nanocarbon-Based Mixed Matrix Pebax-1657 Flat Sheet Membranes for CO2/CH4 Separation. Membranes 2023, 13, 470. [Google Scholar] [CrossRef]
- Nguyen, Q.T.; Sublet, J.; Langevin, D.; Chappey, C.; Marais, S.; Valleton, J.-M.; Poncin–Epaillard, F. CO2 permeation with Pebax -based membranes for global warming reduction. In Membrane Gas Separation; John Wiley & Sons: Hoboken, NJ, USA, 2010; pp. 255–277. [Google Scholar]
- Nobakht, D.; Abedini, R. A new ternary Pebax®1657/maltitol/ZIF-8 mixed matrix membrane for efficient CO2 separation. Process Saf. Environ. Prot. 2023, 170, 709–719. [Google Scholar] [CrossRef]
- Du, X.; Feng, S.; Luo, J.; Zhuang, Y.; Song, W.; Li, X.; Wan, Y. Pebax mixed matrix membrane with bimetallic CeZr-MOFs to enhance CO2 separation. Sep. Purif. Technol. 2023, 322, 124251. [Google Scholar] [CrossRef]
- Azizi, N.; Jazebizadeh, M.H.; Azizi, F.; Jahanmahin, O.; Parsamehr, P.S.; Arzani, M. Enhancing CO2 permeation features of PEBAX-based membrane via incorporating MgO nanoparticles in its polymeric matrix. Mater. Today Commun. 2023, 34, 105460. [Google Scholar] [CrossRef]
- Cao, H.; Gou, M.; Wang, C.; Guo, R. Constructing solubility-diffusion domain in pebax by hybrid-phase MOFs for efficient separation of carbon dioxide and methane. Microporous Mesoporous Mater. 2022, 346, 112328. [Google Scholar] [CrossRef]
- Maleh, M.S.; Raisi, A. In-situ growth of ZIF-8 nanoparticles in Pebax-2533 for facile preparation of high CO2-selective mixed matrix membranes. Colloids Surf. A Physicochem. Eng. Asp. 2023, 659, 130747. [Google Scholar] [CrossRef]
- Liu, J.; Pan, Y.; Xu, J.; Wang, Z.; Zhu, H.; Liu, G.; Zhong, J.; Jin, W. Introducing amphipathic copolymer into intermediate layer to fabricate ultra-thin Pebax composite membrane for efficient CO2 capture. J. Memb. Sci. 2023, 667, 121183. [Google Scholar] [CrossRef]
- Hasan, M.R.; Zhao, H.; Steunou, N.; Serre, C.; Malankowska, M.; Téllez, C.; Coronas, J. Optimization of MIL-178(Fe) and Pebax® 3533 loading in mixed matrix membranes for CO2 capture. Int. J. Greenh. Gas Control 2022, 121, 103791. [Google Scholar] [CrossRef]
- Suhaimi, N.H.; Jusoh, N.; Rashidi, S.S.; Ch’ng, C.W.M.; Sambudi, N.S. Ethylene Recovery via Pebax-Based Composite Membrane: Numerical Optimization. Sustainability 2023, 15, 1856. [Google Scholar] [CrossRef]
- Berned-Samatán, V.; Téllez, C.; Coronas, J. Double-Layered Pebax® 3533/ZIF-8 Membranes with Single-Walled Carbon Nanotube Buckypapers as Support for Gas Separation. Membranes 2023, 13, 71. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.-C.; Wichidit, T.; Narkkun, T.; Tung, K.-L.; Faungnawakij, K.; Klaysom, C. Aminosilane-Functionalized Zeolite Y in Pebax Mixed Matrix Hollow Fiber Membranes for CO2/CH4 Separation. Polymers 2022, 15, 102. [Google Scholar] [CrossRef]
- Abi, Y.; Li, W.; Chang, Z. PEBAX 3533/PAA/CNC Composite Fiber Membranes as the Humidifier Membrane for Proton Exchange Membrane Fuel Cells. Ind. Eng. Chem. Res. 2022, 61, 1375–1385. [Google Scholar] [CrossRef]
- Liu, J.; Fan, S.; Li, C.; Qing, H.; Xiao, Z. Sandwich Structure Membrane with Enhanced Anti-Swelling Property and Mechanical Strength for Bioethanol Separation by Pervaporation. Ind. Eng. Chem. Res. 2023, 62, 5262–5273. [Google Scholar] [CrossRef]
- Xue, Y.X.; Dai, F.F.; Yang, Q.; Chen, J.H.; Lin, Q.J.; Fang, L.J.; Lin, W.W. Fabrication of PEBA/HZIF-8 Pervaporation Membranes for High Efficiency Phenol Recovery. ACS Omega 2022, 7, 23467–23478. [Google Scholar] [CrossRef]
- Han, Z.; Zhao, Y.; Jiang, H.; Sheng, A.; Li, H.; Jia, H.; Yun, Z.; Wei, Z.; Wang, H. (3-Aminopropyl) Triethoxysilane-Modified ZIF-90 Nanoparticle/Polydimethylsiloxane Mixed Matrix Membranes for Ethanol Recovery via Pervaporation. ACS Appl. Nano Mater. 2022, 5, 183–194. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, Z.; Song, J.; Song, J.; Pan, F.; Zhang, P.; Cao, X. Elevated Pervaporative Desulfurization Performance of Pebax-Ag + @MOFs Hybrid Membranes by Integrating Multiple Transport Mechanisms. Ind. Eng. Chem. Res. 2019, 58, 16911–16921. [Google Scholar] [CrossRef]
- Zuo, J.; Shi, G.M.; Wei, S.; Chung, T.-S. The Development of Novel Nexar Block Copolymer/Ultem Composite Membranes for C2–C4 Alcohols Dehydration via Pervaporation. ACS Appl. Mater. Interfaces 2014, 6, 13874–13883. [Google Scholar] [CrossRef] [PubMed]
- Castro-Muñoz, R.; De La Iglesia, Ó.; Fila, V.; Téllez, C.; Coronas, J. Pervaporation-assisted esterification reactions by means of mixed matrix membranes. Ind. Eng. Chem. Res. 2018, 57, 15998–16011. [Google Scholar] [CrossRef]
- Soloukipour, S.; Saljoughi, E.; Mousavi, S.M.; Pourafshari Chenar, M. PEBA/PVDF blend pervaporation membranes: Preparation and performance. Polym. Adv. Technol. 2017, 28, 113–123. [Google Scholar] [CrossRef]
- Sforça, M.L.; Yoshida, I.V.P.; Borges, C.P.; Nunes, S.P. Hybrid membranes based on SiO2/polyether-b-polyamide: Morphology and applications. J. Appl. Polym. Sci. 2001, 82, 178–185. [Google Scholar] [CrossRef]
- Gugliuzza, A. Poly(ether-block-amide) Copolymers Synthesis, Properties and Applications. In Handbook of Engineering and Specialty Thermoplastics; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; pp. 111–140. [Google Scholar]
- Mendoza-Mendoza, E.; España-Sánchez, B.L.; de Jesús Montes-Luna, A.; Castruita-de León, G. Effect of poly(ether block amide)-graphene/ZnO membranes in mixed gas separation performance. J. Appl. Polym. Sci. 2023, 140, e53453. [Google Scholar] [CrossRef]
- Amirkhani, F.; Harami, H.R.; Asghari, M. CO2/CH4 mixed gas separation using poly(ether-b-amide)-ZnO nanocomposite membranes: Experimental and molecular dynamics study. Polym. Test. 2020, 86, 106464. [Google Scholar] [CrossRef]
- Mousavi, S.R.; Asghari, M.; Mahmoodi, N.M. Chitosan-wrapped multiwalled carbon nanotube as filler within PEBA thin film nanocomposite (TFN) membrane to improve dye removal. Carbohydr. Polym. 2020, 237, 116128. [Google Scholar] [CrossRef]
- Yousef, S.; Sarwar, Z.; Šereika, J.; Striūgas, N.; Krugly, E.; Danilovas, P.P.; Martuzevicius, D. A New Industrial Technology for Mass Production of Graphene/PEBA Membranes for CO2/CH4 Selectivity with High Dispersion, Thermal and Mechanical Performance. Polymers 2020, 12, 831. [Google Scholar] [CrossRef]
- Amirkhani, F.; Mosadegh, M.; Asghari, M.; Parnian, M.J. The beneficial impacts of functional groups of CNT on structure and gas separation properties of PEBA mixed matrix membranes. Polym. Test. 2020, 82, 106285. [Google Scholar] [CrossRef]
- Pazirofteh, M.; Abdolmajidi, M.; Samipoorgiri, M.; Dehghani, M.; Mohammadi, A.H. Separation and transport specification of a novel PEBA-1074/PEG-400/TiO2 nanocomposite membrane for light gas separation: Molecular simulation study. J. Mol. Liq. 2019, 291, 111268. [Google Scholar] [CrossRef]
- Pan, F.; Wang, M.; Ding, H.; Song, Y.; Li, W.; Wu, H.; Jiang, Z.; Wang, B.; Cao, X. Embedding Ag + @COFs within Pebax membrane to confer mass transport channels and facilitated transport sites for elevated desulfurization performance. J. Memb. Sci. 2018, 552, 1–12. [Google Scholar] [CrossRef]
- Pakizeh, M.; Rouhani, R.; Pourafshari Chenar, M. A new route for ZIF-8 synthesis and its application in MMM preparation for toluene removal from water using PV process. Chem. Eng. Res. Des. 2023, 190, 730–744. [Google Scholar] [CrossRef]
- Cao, X.; Wang, K.; Feng, X. Incorporating ZIF-71 into poly(ether-block-amide) (PEBA) to form mixed matrix membranes for enhanced separation of aromatic compounds from aqueous solutions by pervaporation. Sep. Purif. Technol. 2022, 300, 121924. [Google Scholar] [CrossRef]
- Erfani, A.; Asghari, M. Comparison of micro- and nano-sized CuBTC particles on the CO2/CH4 separation performance of PEBA mixed matrix membranes. J. Chem. Technol. Biotechnol. 2020, 95, 2951–2963. [Google Scholar] [CrossRef]
- Sun, J.; Li, Q.; Chen, G.; Duan, J.; Liu, G.; Jin, W. MOF-801 incorporated PEBA mixed-matrix composite membranes for CO2 capture. Sep. Purif. Technol. 2019, 217, 229–239. [Google Scholar] [CrossRef]
- Armarego, W.L.F.; Chai, C.L.L. Purification of Laboratory Chemicals; Elsevier: Amsterdam, The Netherlands, 2009; ISBN 9781856175678. [Google Scholar]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Harkins, W.D.; Jura, G. Surfaces of Solids. XII. An Absolute Method for the Determination of the Area of a Finely Divided Crystalline Solid. J. Am. Chem. Soc. 1944, 66, 1362–1366. [Google Scholar] [CrossRef]
- Zimm, B.H. Apparatus and Methods for Measurement and Interpretation of the Angular Variation of Light Scattering; Preliminary Results on Polystyrene Solutions. J. Chem. Phys. 1948, 16, 1099–1116. [Google Scholar] [CrossRef]
- Wu, H. Correlations between the Rayleigh ratio and the wavelength for toluene and benzene. Chem. Phys. 2010, 367, 44–47. [Google Scholar] [CrossRef]
- Daraei, P.; Madaeni, S.S.; Ghaemi, N.; Khadivi, M.A.; Rajabi, L.; Derakhshan, A.A.; Seyedpour, F. PAA grafting onto new acrylate-alumoxane/PES mixed matrix nano-enhanced membrane: Preparation, characterization and performance in dye removal. Chem. Eng. J. 2013, 221, 111–123. [Google Scholar] [CrossRef]
- Singha, D.K.; Majee, P.; Mondal, S.K.; Mahata, P. Detection of pesticide using the large stokes shift of luminescence of a mixed lanthanide co-doped metal–organic framework. Polyhedron 2019, 158, 277–282. [Google Scholar] [CrossRef]
- Fröhlich, D.; Pantatosaki, E.; Kolokathis, P.D.; Markey, K.; Reinsch, H.; Baumgartner, M.; van der Veen, M.A.; De Vos, D.E.; Stock, N.; Papadopoulos, G.K.; et al. Water adsorption behaviour of CAU-10-H: A thorough investigation of its structure–property relationships. J. Mater. Chem. A 2016, 4, 11859–11869. [Google Scholar] [CrossRef]
- Almáši, M.; Zeleňák, V.; Kuchár, J.; Bourrelly, S.; Llewellyn, P.L. New members of MOF-76 family containing Ho(III) and Tm(III) ions: Characterization, stability and gas adsorption properties. Colloids Surf. A Physicochem. Eng. Asp. 2016, 496, 114–124. [Google Scholar] [CrossRef]
- Kuzminova, A.; Dmitrenko, M.; Zolotarev, A.; Myznikov, D.; Selyutin, A.; Su, R.; Penkova, A. Pervaporation Polyvinyl Alcohol Membranes Modified with Zr-Based Metal Organic Frameworks for Isopropanol Dehydration. Membranes 2022, 12, 908. [Google Scholar] [CrossRef] [PubMed]
- Ambroz, F.; Macdonald, T.J.; Martis, V.; Parkin, I.P. Evaluation of the BET Theory for the Characterization of Meso and Microporous MOFs. Small Methods 2018, 2, 1800173. [Google Scholar] [CrossRef]
- Walton, K.S.; Snurr, R.Q. Applicability of the BET Method for Determining Surface Areas of Microporous Metal−Organic Frameworks. J. Am. Chem. Soc. 2007, 129, 8552–8556. [Google Scholar] [CrossRef]
- Batonneau-Gener, I.; Sachse, A. Determination of the Exact Microporous Volume and BET Surface Area in Hierarchical ZSM-5. J. Phys. Chem. C 2019, 123, 4235–4242. [Google Scholar] [CrossRef]
- Koutsianos, A.; Pallach, R.; Frentzel-Beyme, L.; Das, C.; Paulus, M.; Sternemann, C.; Henke, S. Breathing porous liquids based on responsive metal-organic framework particles. Nat. Commun. 2023, 14, 4200. [Google Scholar] [CrossRef]
- Wang, L.; Li, T.; Dong, X.; Pang, M.; Xiao, S.; Zhang, W. Thiophene-based MOFs for iodine capture: Effect of pore structures and interaction mechanism. Chem. Eng. J. 2021, 425, 130578. [Google Scholar] [CrossRef]
- Vizuet, J.P.; Lewis, A.L.; McCandless, G.T.; Balkus, K.J. Characterization of a Holmium 4,4′-Biphenyldicarboxylate Metal-Organic Framework and Its Potential as a Holmium Carrier System. J. Nanosci. Nanotechnol. 2020, 20, 3019–3024. [Google Scholar] [CrossRef] [PubMed]
- Dar, F.A.; Want, B. Structure, optical transition analysis and magnetic study of lanthanide based metal-organic framework: Holmium bi-tartrate trihydrate. Phys. Scr. 2023, 98, 095943. [Google Scholar] [CrossRef]
- Liu, S.; Liu, G.; Shen, J.; Jin, W. Fabrication of MOFs/PEBA mixed matrix membranes and their application in bio-butanol production. Sep. Purif. Technol. 2014, 133, 40–47. [Google Scholar] [CrossRef]
- Liu, Q.; Li, Y.; Li, Q.; Liu, G.; Liu, G.; Jin, W. Mixed-matrix hollow fiber composite membranes comprising of PEBA and MOF for pervaporation separation of ethanol/water mixtures. Sep. Purif. Technol. 2019, 214, 2–10. [Google Scholar] [CrossRef]
- Zhang, A.-S.; Li, S.-H.; Ahmad, A.; Mao, H.; Xu, L.-H.; Zhao, Z.-P. Coordinate covalent grafted ILs-modified MIL-101/PEBA membrane for pervaporation: Adsorption simulation and separation characteristics. J. Memb. Sci. 2021, 619, 118807. [Google Scholar] [CrossRef]
- Mao, H.; Li, S.-H.; Xu, L.-H.; Wang, S.; Liu, W.-M.; Lv, M.-Y.; Lv, J.; Zhao, Z.-P. Zeolitic imidazolate frameworks in mixed matrix membranes for boosting phenol/water separation: Crystal evolution and preferential orientation. J. Memb. Sci. 2021, 638, 119611. [Google Scholar] [CrossRef]
- Fang, L.J.; Chen, J.H.; Yang, Q.; Lin, W.W.; Lin, Q.J.; He, Y.S.; Zhuo, Y.Z. S-ZIF-8/PEBA/ZIF-8 pervaporation membrane with in situ growing of ZIF-8 active layer on the surface owing outstanding phenol enrichment performance. J. Taiwan Inst. Chem. Eng. 2022, 134, 104356. [Google Scholar] [CrossRef]
- Liu, L.; Li, Y.; Xu, M.; Wang, C. 2D Co-UMOFNs filled PEBA composite membranes for pervaporation of phenol solution. Sep. Purif. Technol. 2022, 285, 120414. [Google Scholar] [CrossRef]
- Mao, H.; Li, S.-H.; Zhang, A.-S.; Xu, L.-H.; Lu, H.-X.; Lv, J.; Zhao, Z.-P. Furfural separation from aqueous solution by pervaporation membrane mixed with metal organic framework MIL-53(Al) synthesized via high efficiency solvent-controlled microwave. Sep. Purif. Technol. 2021, 272, 118813. [Google Scholar] [CrossRef]
- Ansari, A.A.; Ganaie, A.B.; Iftikhar, K. Synthesis and 4f 4f absorption studies of tris(acetylacetonato)praseodymium(III) and holmium(III) complexes with imidazole and pyrazole in non-aqueous solvents. Structure elucidation by Sparkle/PM7. J. Mol. Struct. 2019, 1198, 126826. [Google Scholar] [CrossRef]
- Liu, Y.; Ho, L.T.A.; Huang, G.; Chen, Y.; Ungur, L.; Liu, J.; Tong, M. Magnetization Dynamics on Isotope-Isomorphic Holmium Single-Molecule Magnets. Angew. Chemie Int. Ed. 2021, 60, 27282–27287. [Google Scholar] [CrossRef] [PubMed]
- Iftikhar, K.; Ahmad, N. f-f spectral studies of holmium(III) complexes. Aust. J. Chem. 1983, 36, 695. [Google Scholar] [CrossRef]
- Hua, X.-H.; Xue, J.-H.; Yang, L.-M.; Xu, Y.-Z.; Wu, J.-G. (Butane-1,2,3,4-tetraol-κ3O1,O2,O3)(ethanol-κO)tris(nitrato-κ2O,O′)holmium(III). Acta Crystallogr. Sect. E Struct. Rep. Online 2013, 69, m162–m163. [Google Scholar] [CrossRef] [PubMed]
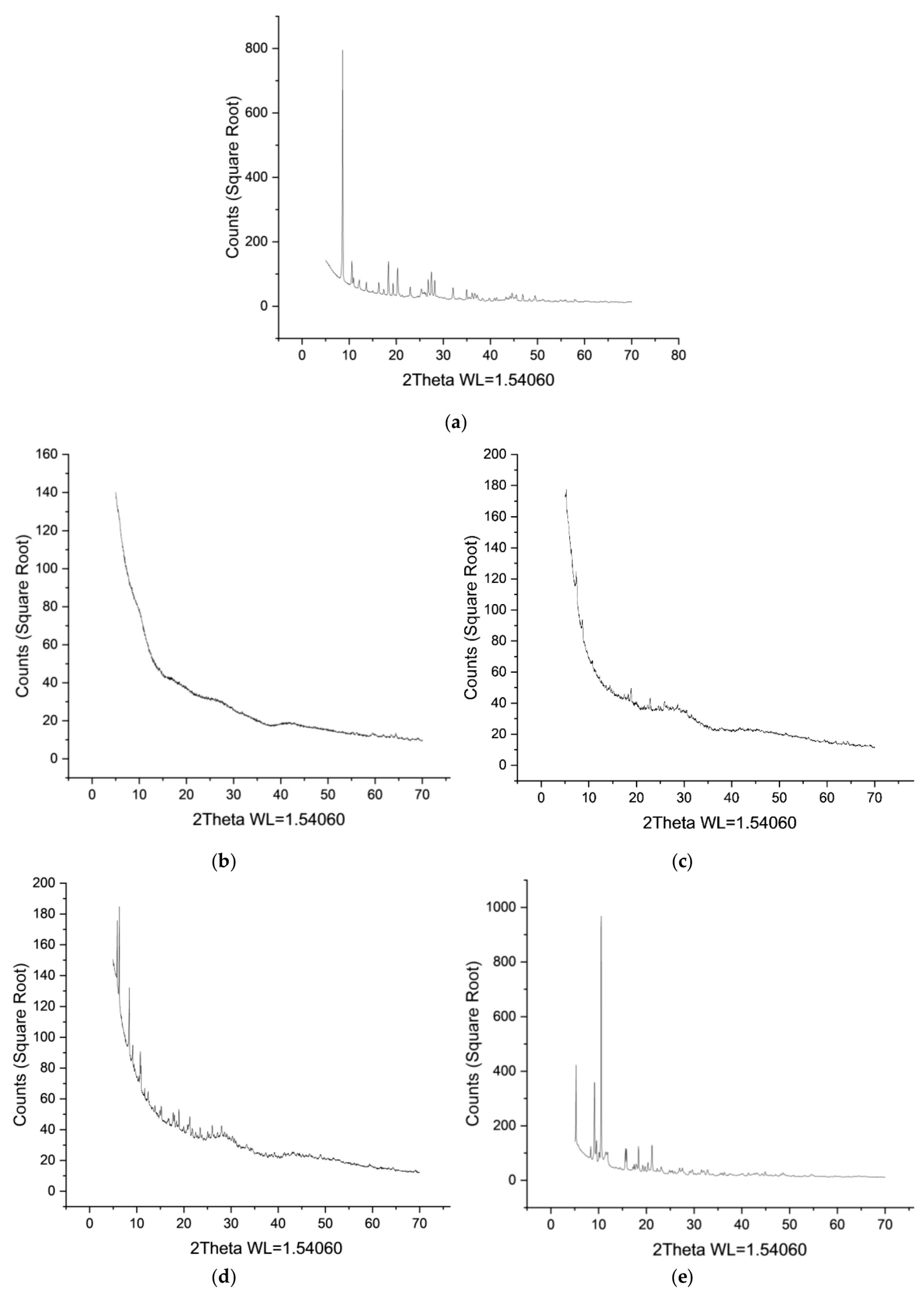






| Ho-MOF | Specific Surface Area, m2/g |
|---|---|
| Ho-1,3,5-H3btc | 10 |
| Ho-1,2,4-H3btc | 1 |
| Ho-1,2-H2bdc | 241 |
| Ho-1,3-H2bdc | 10 |
| Ho-1,4-H2bdc | 1 |
| T, °C | [η], dL/g | ||
|---|---|---|---|
| Huggins | Kramer | Average | |
| 10 | 0.615 | 0.617 | 0.616 |
| 20 | 0.611 | 0.613 | 0.612 |
| 25 | 0.610 | 0.612 | 0.611 |
| 30 | 0.610 | 0.610 | 0.610 |
| 40 | 0.606 | 0.607 | 0.607 |
| 50 | 0.604 | 0.603 | 0.604 |
| T, °C | Huggins | Kramer |
|---|---|---|
| 10 | 0.411 | −0.119 |
| 20 | 0.392 | −0.129 |
| 25 | 0.384 | −0.134 |
| 30 | 0.365 | −0.143 |
| 40 | 0.362 | −0.145 |
| 50 | 0.346 | −0.153 |
| Membrane | Ra, nm | Rq, nm | Contact Angle of Water, ° |
|---|---|---|---|
| PEBA | 9.4 ± 0.5 | 11.9 ± 0.6 | 78 ± 1 |
| PEBA/Ho-1,3,5-H3btc | 1.3 ± 0.1 | 1.7 ± 0.1 | 85 ± 1 |
| PEBA/Ho-1,2,4-H3btc | 2.4 ± 0.1 | 3.0 ± 0.1 | 86 ± 1 |
| PEBA/Ho-1,2-H2bdc | 2.0 ± 0.1 | 2.6 ± 0.1 | 86 ± 1 |
| PEBA/Ho-1,3-H2bdc | 3.9 ± 0.2 | 5.0 ± 0.2 | 87 ± 1 |
| PEBA/Ho-1,4-H2bdc | 4.8 ± 0.2 | 6.1 ± 0.2 | 86 ± 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuzminova, A.; Dmitrenko, M.; Salomatin, K.; Vezo, O.; Kirichenko, S.; Egorov, S.; Bezrukova, M.; Karyakina, A.; Eremin, A.; Popova, E.; et al. Holmium-Containing Metal-Organic Frameworks as Modifiers for PEBA-Based Membranes. Polymers 2023, 15, 3834. https://doi.org/10.3390/polym15183834
Kuzminova A, Dmitrenko M, Salomatin K, Vezo O, Kirichenko S, Egorov S, Bezrukova M, Karyakina A, Eremin A, Popova E, et al. Holmium-Containing Metal-Organic Frameworks as Modifiers for PEBA-Based Membranes. Polymers. 2023; 15(18):3834. https://doi.org/10.3390/polym15183834
Chicago/Turabian StyleKuzminova, Anna, Mariia Dmitrenko, Kirill Salomatin, Olga Vezo, Sergey Kirichenko, Semyon Egorov, Marina Bezrukova, Anna Karyakina, Alexey Eremin, Ekaterina Popova, and et al. 2023. "Holmium-Containing Metal-Organic Frameworks as Modifiers for PEBA-Based Membranes" Polymers 15, no. 18: 3834. https://doi.org/10.3390/polym15183834
APA StyleKuzminova, A., Dmitrenko, M., Salomatin, K., Vezo, O., Kirichenko, S., Egorov, S., Bezrukova, M., Karyakina, A., Eremin, A., Popova, E., Penkova, A., & Selyutin, A. (2023). Holmium-Containing Metal-Organic Frameworks as Modifiers for PEBA-Based Membranes. Polymers, 15(18), 3834. https://doi.org/10.3390/polym15183834








