Preparation and Performance of Silicone Rubber Composites Modified by Polyurethane
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Polyurethane Prepolymer
2.3. Preparation of Silicone Rubber Composites
2.4. Characterization
3. Results and Discussion
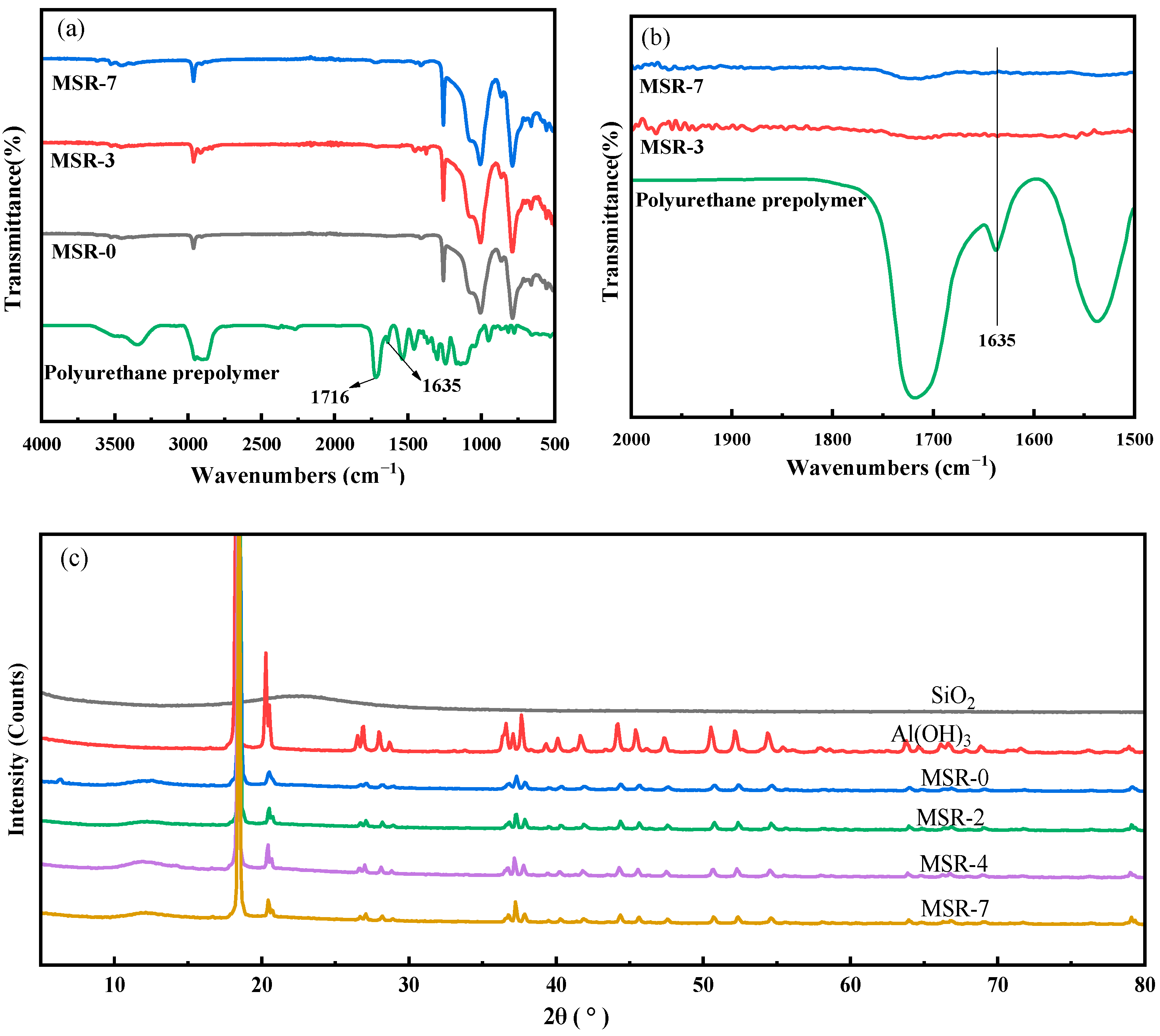
3.1. Structure of Materials
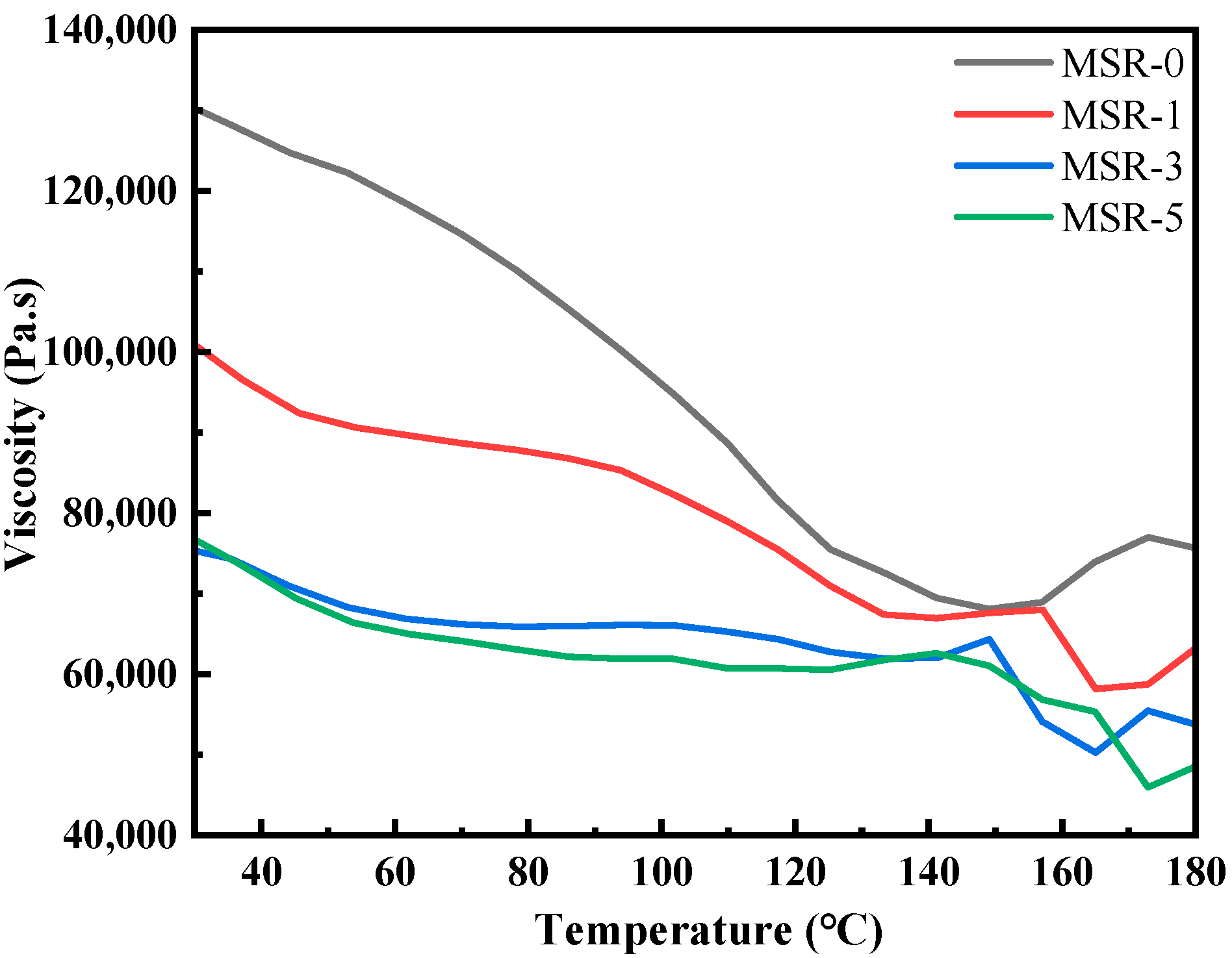
3.2. Viscosity Properties of Materials
3.3. Electrical Insulation Performance
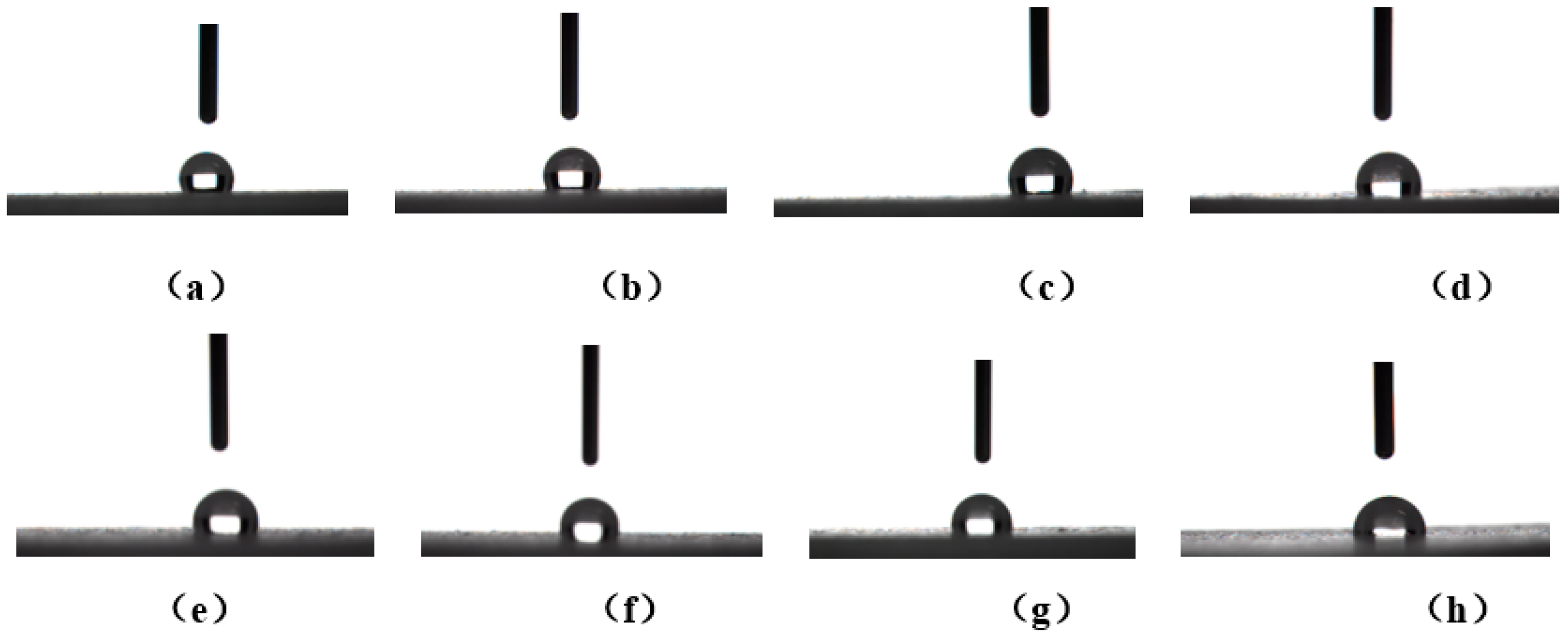
3.4. Hydrophobic Properties of Materials
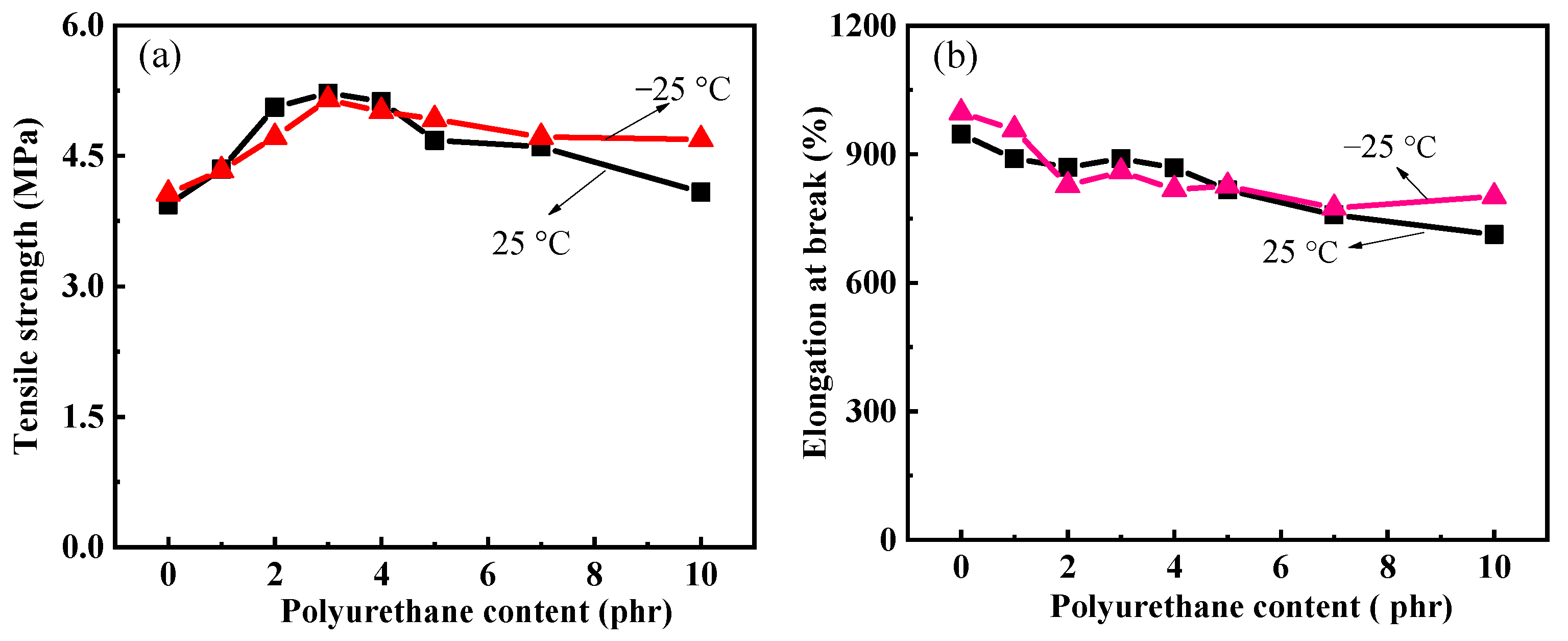
3.5. Mechanical Property
3.6. Thermal Stability
3.7. Flame Retardant Performance
3.8. Morphology of Materials
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Luo, W.H.; Li, L.J.; Luo, B.; Zhang, F.; Wang, T.; Yao, Y. Nanodiamond-filled high-temperature vulcanized silicon rubber composite for high-voltage insulator applications. J. Mater. 2021, 32, 23116–23125. [Google Scholar] [CrossRef]
- Xiao, M.; Du, B.X. Review of high thermal conductivity polymer dielectrics for electrical insulation. High Volt. 2016, 1, 34–42. [Google Scholar] [CrossRef]
- Nelson, J.K.; Fothergill, J.C. Internal charge behaviour of nanocomposites. Nanotechnology 2004, 15, 586–595. [Google Scholar] [CrossRef]
- Zhu, Y. Influence of corona discharge on hydrophobicity of silicone rubber used for outdoor insulation. Polym. Test. 2019, 74, 14–20. [Google Scholar] [CrossRef]
- Hackam, R. Outdoor HV composite polymeric insulators. IEEE Trans. Electr. Insul. 1999, 6, 557–585. [Google Scholar] [CrossRef]
- Gorur, R.S.; De, L.O.; EI-Kishky, A.; Chowdhary, H.; Mukherjee, M.; Sundaram, H.; Burnham, J.T. Sudden flashover of nonceramic insulators in artificial contamination tests. IEEE Trans. Dielectr. Electr. Insul. 1997, 4, 79–87. [Google Scholar] [CrossRef]
- Boinovich, L.; Emelyanenko, A.M.; Pashinin, A.S. Analysis of long-term durability of superhydrophobic properties under continuous contact with water. ACS Appl. Mater. Interfaces 2010, 2, 1754–1758. [Google Scholar] [CrossRef]
- Sunanda, C.; Dinesh, M.N.; Vasudev, N. Performance evalution of Silicon Rubber insulating material with MgO and ZnO nanofillers. In Proceedings of the 2016 IEEE International Conference on High Voltage Engineering and Application, Chongqing, China, 19–22 September 2016. [Google Scholar]
- Gao, S.H.; Gao, L.H.; Zhou, K.S. Super-hydrophobicity and oleophobicity of silicone rubber modified by CF4 radio frequency plasma. Appl. Surf. Sci. 2011, 257, 4945–4950. [Google Scholar] [CrossRef]
- Akbar, M.; Ullah, R.; Alam, S. Aging of silicone rubber-based composite insulators under multi-stressed conditions: An overview. Mater. Res. Express. 2019, 6, 102003. [Google Scholar] [CrossRef]
- Liu, X.H.; Shen, H.K.; Cao, C.; Song, S.H.; Zhou, Z.F.; Xun, W.B. Preparation and properties of room temperature vulcanized thermal-conductive silicone rubber with high electrical insulation. IEEE Electr. Insul. Mag. 2017, 50, 6–9. [Google Scholar]
- Li, W.; Gedde, U.W.; Hillborg, H. Structure and electrical properties of silicone rubber filled with thermally reduced graphene oxide. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 1156–1163. [Google Scholar] [CrossRef]
- Liu, C.; Xu, Y.; Bi, D.; Luo, B.; Zhang, F.; Wang, T.; Yao, Y.; Lu, S.; Xu, W. The effects of aluminum-nitride nano-fillers on the mechanical, electrical, and thermal properties of high temperature vulcanized silicon rubber for high-voltage outdoor insulator applications. Materials 2019, 12, 3562. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Li, X.F.; Zhang, D.H.; Wang, H.S.; Chen, Y.; Chen, Y.F. Comparison of ATH and SiO2 fillers filled silicone rubber composites for HTV insulators. Compos. Sci. Technol. 2018, 155, 137–143. [Google Scholar] [CrossRef]
- Khan, H.; Amin, M.; Ahmad, A. Erosion/tracking resistance investigation of micro/nano-SiO2 filled RTV-SiR composites for outdoor high voltage insulations. In Proceedings of the International Bhurban Conference on Applied Sciences & Technology, Islamabad, Pakistan, 10–14 January 2017. [Google Scholar]
- Chen, X.; Wang, J.Q.; Zhang, C.; Yang, W.; Lin, J.; Bian, X.M.; He, S.J. Performance of silicone rubber composites using boron nitride to replace alumina tri-ydrate. High Volt. 2021, 6, 480–486. [Google Scholar] [CrossRef]
- Zhang, X.; Yi, J.; Yin, Y.; Song, Y.H.; Xiong, C.X. Thermal conductivity and electrical insulation properties of h-BN@PDA/silicone rubber composites. Diam. Relat. Mater. 2021, 117, 108485. [Google Scholar] [CrossRef]
- Khanum, K.K.; Jayaram, S.H. Improved Thermal Properties and Erosion Resistance of Silicone Composites with Hexagonal Boron Nitride. IEEE Trans. Ind. Appl. 2022, 58, 6583–6590. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Zeng, X.G.; Lai, X.J.; Zhou, Q.; Huang, X.Y. Suppression effect and mechanism of amine-containing MQ silicone resin on the tracking and erosion resistance of silicone rubber. ACS Omega 2017, 2, 5111–5121. [Google Scholar] [CrossRef]
- Pradeep, M.A.; Vasudev, N.; Reddy, P.V.; Khastgir, D. Effect of ATH content on electrical and aging properties of EVA and silicone rubber blends for high voltage insulator compound. J. Appl. Polym. Sci. 2007, 104, 3505–3516. [Google Scholar] [CrossRef]
- Li, C.N.; Cao, X.W.; Tong, Y.Z.; Yang, A.T.; Gao, D.L.; Ru, Y.; He, G.J. Hybrid filler with nanoparticles grown in situ on the surface for the modification of thermal conductive and insulating silicone rubber. ACS Appl. Polym. Mater. 2022, 4, 7152–7161. [Google Scholar] [CrossRef]
- Tang, W.Y.; Liao, X.; Shi, S.Z.; Wang, B.; Lv, C.F.; Zou, F.F.; Li, G.X. Significantly enhanced porosity of silicone rubber nanocomposite foams via cross-linking structure regulation and heterogeneous nucleation by CNTs for promising ultralow-k dielectrics. Ind. Eng. Chem. Res. 2022, 61, 14251–14259. [Google Scholar] [CrossRef]
- GB/T18474-2001; Pipes and Fittings Made of Crosslinked polyethylene(PE-X)—Estimation of the Degree of Cross Linking by Determination of the Gel Content. Chinese Technical Standard: Beijing, China, 2001.
- Gorur, R.S. Aging in silicone rubber used for outdoor Insulation. IEEE Trans. Power Deliv. 1992, 7, 525–531. [Google Scholar] [CrossRef]
- Kim, S.H. Suppession mechanism of leakage current on RTV coated porcelain and silicone rubber insulators. IEEE Trans. Power Deliv. 1991, 6, 1549–1556. [Google Scholar] [CrossRef]
- Wu, P.X.; Zhang, L.C. Polymer Blending Modification, 1st ed.; China Light Industry Press: Beijing, China, 1996; p. 251. [Google Scholar]
- Su, Z.T.; Qian, H.H.; Mi, Z.A. Low temperature characteristics of SKTFT-25 copolymer fluorosilicone rubber. Rubber Ind. 2004, 51, 281–283. [Google Scholar]
- Tang, Z.H.; Xie, Z.J.; Qu, L.L. Effect of phenyl content on properties of methyl vinyl phenyl silicone rubber. Rubber Ind. 2007, 54, 610–612. [Google Scholar]
- Xiao, J.B.; Gao, H.Q.; Liu, W. Study on the properties of fluororubber/silicone rubber/fluorosilicone rubber blend. Rubber Ind. 2016, 63, 394–397. [Google Scholar]
- Xiao, Q.; Pi, M.L.; Zhang, L.X.; Zhang, Y.Y.; Fu, S.Y. Mechanical properties of silicone rubber composed of diverse vinyl content silicone gums blending. Mater. Des. 2010, 31, 4083–4087. [Google Scholar]
- Peng, Z.H.; Li, Q.F.; Zhou, Q.S. Kinetic Studies on dehydration kinetics of aluminum hydroxide. Light Metals 2010, 16–18. [Google Scholar] [CrossRef]
- Redaoui, D.; Sahnoune, F.; Heraiz, M. Mechanism and kinetic parameters of the thermal decomposition of gibbsite Al(OH)3 by thermogravimetric analysis. Acta Phys. Pol. 2017, 131, 562–565. [Google Scholar] [CrossRef]
- Zhu, B.Q.; Fang, B.X.; Li, X.C. Dehydration reactions and kinetic parameters of gibbsite. Ceram. Int. 2010, 36, 2493–2498. [Google Scholar] [CrossRef]



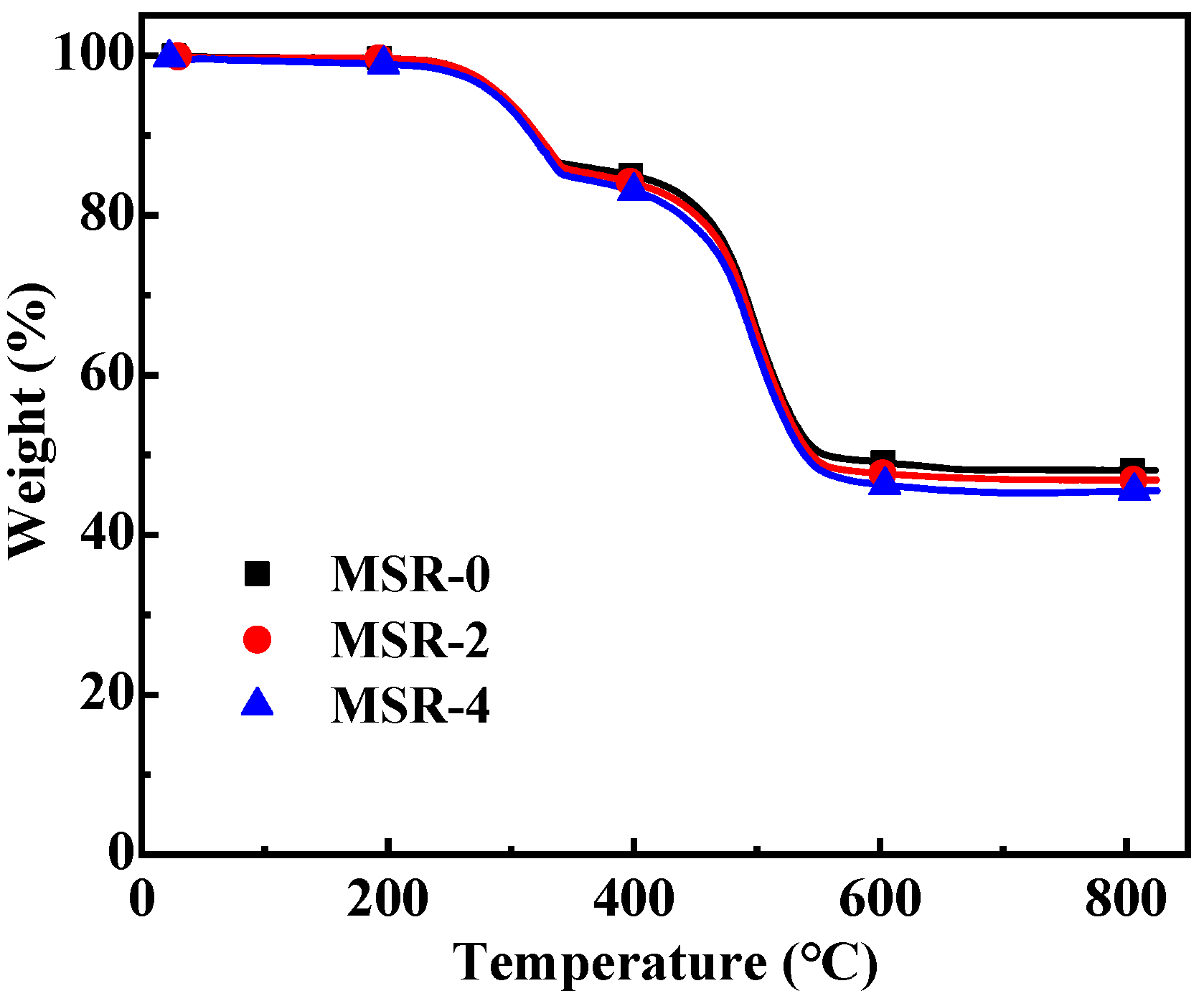
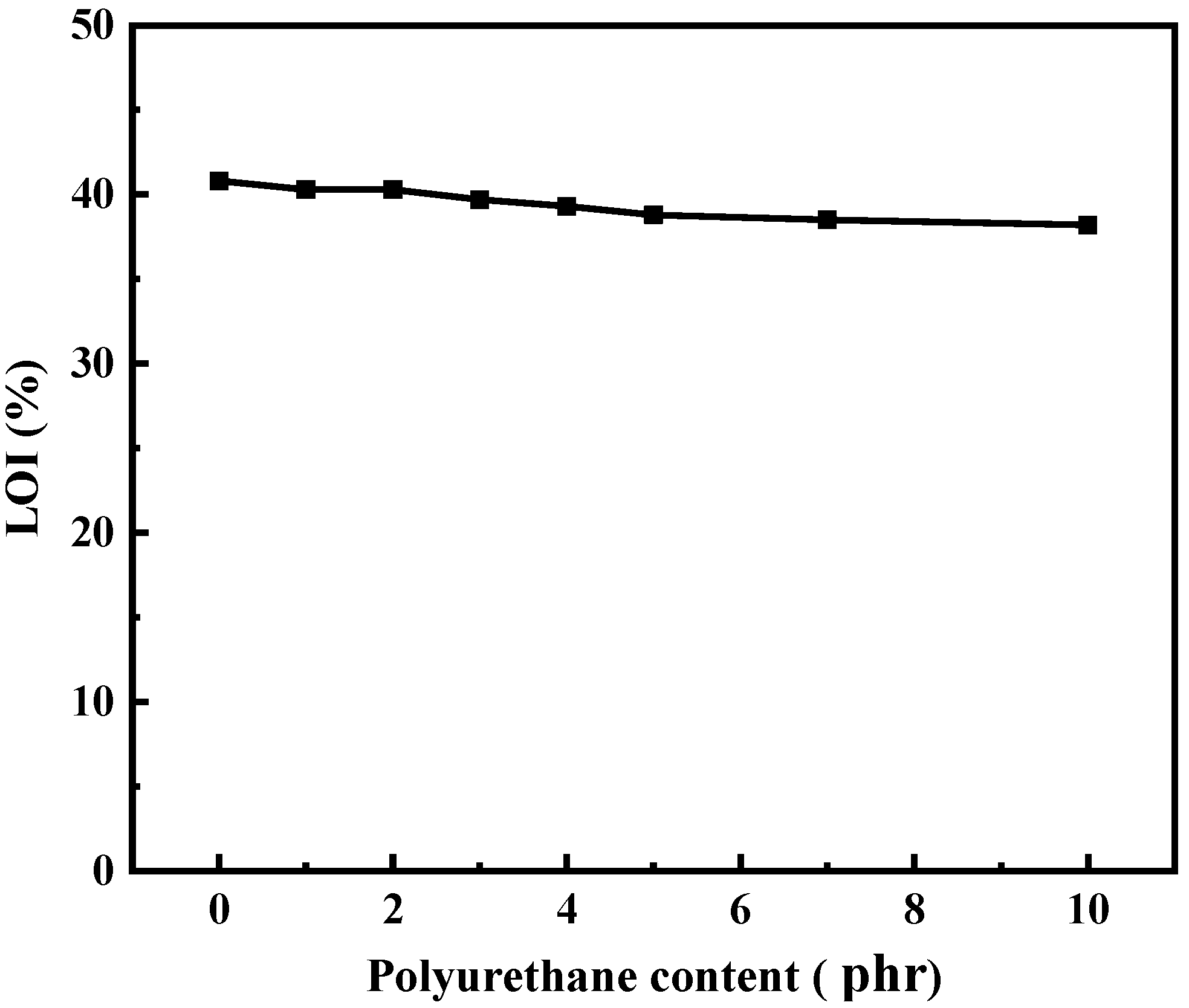

| Composites | MSR-0 | MSR-1 | MSR-2 | MSR-3 | MSR-4 | MSR-5 | MSR-7 | MSR-10 |
|---|---|---|---|---|---|---|---|---|
| Weight loss rate (%) | 0 | 0.001 | 0.001 | 0 | 0.001 | 0.001 | 0 | 0.001 |
| Composites | The First-Step Thermal Decomposition | The Second-Step Thermal Decomposition | Total Weight Loss Rate (%) | Residual Rate (%) | ||
|---|---|---|---|---|---|---|
| Temperature Range (°C) | Weight Loss Rate (%) | Temperature Range (°C) | Weight Loss Rate (%) | |||
| MSR-0 | 274.6–357.9 | 13.87 | 462.4–592.8 | 37.66 | 51.53 | 48.47 |
| MSR-2 | 275.6–359.3 | 14.68 | 462.2–595.7 | 38.59 | 53.27 | 46.73 |
| MSR-4 | 278.1–356.8 | 15.49 | 459.8–605.6 | 39.25 | 54.74 | 45.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, L.; Li, Y.; Fu, W.; Zhou, L.; Fang, S. Preparation and Performance of Silicone Rubber Composites Modified by Polyurethane. Polymers 2023, 15, 3920. https://doi.org/10.3390/polym15193920
Gao L, Li Y, Fu W, Zhou L, Fang S. Preparation and Performance of Silicone Rubber Composites Modified by Polyurethane. Polymers. 2023; 15(19):3920. https://doi.org/10.3390/polym15193920
Chicago/Turabian StyleGao, Lijun, Ying Li, Wensheng Fu, Liming Zhou, and Shaoming Fang. 2023. "Preparation and Performance of Silicone Rubber Composites Modified by Polyurethane" Polymers 15, no. 19: 3920. https://doi.org/10.3390/polym15193920
APA StyleGao, L., Li, Y., Fu, W., Zhou, L., & Fang, S. (2023). Preparation and Performance of Silicone Rubber Composites Modified by Polyurethane. Polymers, 15(19), 3920. https://doi.org/10.3390/polym15193920






