A Review of Solid-State Proton–Polymer Batteries: Materials and Characterizations
Abstract
:1. Introduction
- An introduction to proton batteries: components, chemistry, and materials.
- The electrochemical characterization of materials: morphologies, structural, ionic conductivity, electrochemical as well as thermal properties, and,
- Performance of PCPE in solid-state batteries: performance characterization, such as open-circuit voltage (OCV), current–voltage (I-V), power density–current density (J-P), discharge, and charge–discharge profile.
2. Components and Mechanism of Proton Battery-Based Polymer Electrolytes
- i.
- Anode (Figure 1a): Oxidation occurs (electrons flow to the external circuit). The anode must be an efficient oxidizing agent, stable in adhesion with electrolyte, and have a useful working voltage, long lifetime, high reversible discharge capacity, and low surface area for safety improvement [22], i.e., combinations of zinc (metal powder), ZnSO4⋅7H2O and graphite powder [23,24].
- ii.
- Cathode (Figure 1b): Reduction occurs (positive terminal of the battery in the discharged mode). i.e., mixtures of lead oxide (PbO2) [25,26], vanadium pentoxide (V2O5) (active cathodic material) [27,28], graphite (provides an adequate electronic conductivity), manganese dioxide (MnO2) [29,30] and small ratio of polymer electrolyte used in the system (to favor electrode/electrolyte interfacial contact and helps in reducing electrode polarization) [31].
- iii.
- Electrolytes (Figure 1c): The medium (ions are transferred between the anode and cathode during charge and discharge). Electrolytes with high ionic-conductance (range between 10−5 to 10−2 S cm−1), high thermal and chemical stability, wide potential window (defined as the range in voltage between the oxidative and reductive decomposition limits of the electrolyte), low reactivity toward other components in the battery and have ionic transference number greater than 0.9 are suitable for battery applications [32], i.e., the use of various type of polymer electrolytes (solid; chitosan [33,34], poly (ethylene oxide) [35,36], liquid; (1,1,2,2-tetrafuoroethyl-2,2,3,3-tetrafuoropropyl ether (TTE) [37] and gel; poly(vinylidene fluoride-co-hexafluoropropylene)-ionic liquid [38]). Table 1 summarizes the ionic conductivities at an ambient temperature of some PCPE systems.
- i.
- At the anode, Zn was oxidized with the release of two electrons and ZnSO4·7H2O is the donor of H+ ions.Zn → Zn2+ + 2e− Eox = 0.76 VZnSO4·7H2O → 7H+ + 7OH− + ZnSO4 Eox = −0.82 V
- ii.
- At the cathode, MnO2 was reduced with the acceptance of electrons.MnO2 + 2e− + 4H+ → Mn2+ + 2H2O Ered = 1.22 V
- iii.
- The overall reaction in the cell isEox + Ered = EcellZn + ZnSO4·7H2O + MnO2 + 2e− + 4H+ → Zn2+ + 7H+ + 7OH− + ZnSO4 + Mn2+ + 2H2O
−(0.76 − 0.82) V + 1.22 V = 1.28 V
| Electrolyte System | State | Electrochemical Properties | Ref. | |||
|---|---|---|---|---|---|---|
| Ionic Conductivity σ (S cm−1) | I-TN | Stability (V) | Ea | |||
| Tapioca starch/PEO-NH4NO3 | solid | 2.8 × 10−7 | - | - | - | [40] |
| Sago starch-NH4Br | 6.9 × 10−9 | - | - | 0.07 eV | [41] | |
| Potato starch-NH4I | 2.4 × 10−4 | 0.95 | - | - | [42] | |
| Corn starch/Chitosan NH4I-glycerol | 1.3 × 10−3 | 0.99 | 1.9 | 0.18 eV | [43] | |
| Starch/Chitosan NH4I | 3.0 × 10−4 | - | - | 0.20 eV | [44] | |
| Starch/Chitosan NH4Cl-glycerol | 5.1 × 10−4 | - | - | 0.19 eV | [45] | |
| Starch/Chitosan NH4Br-EC | 1.4 × 10−3 | 0.92 | 0.18 | 0.17 eV | [46] | |
| Rice starch NH4I | 1.4 × 10−4 | - | - | - | [47] | |
| Chitosan acetate—NH4CF3SO3—DMC | ~10−6 | - | - | 0.60 eV | [48] | |
| Chitosan acetate/PEO—NH4NO3 | 1.0 × 10−4 | - | - | - | [49] | |
| Chitosan/PEO—NH4I–I2 | 4.3 × 10−6 | - | - | - | [50] | |
| Cellulose acetate—NH4SCN | 3.3 × 10−3 | 0.99 | - | 0.15 eV | [51] | |
| Chitosan/PEO—NH4NO3—EC | 2.1 × 10−3 | - | 1.75 | 0.18 eV | [52] | |
| MG-30–NH4CF3SO3—EC | ~10−4 | - | - | - | [53] | |
| PEO-NH4ClO4 | ~10−5 | - | - | [54] | ||
| carboxymethyl kappa-carrageenan/CMC-NH4I | 2.41 × 10−3 | - | - | - | [55] | |
| Alginate-(NH4)2SO4 | 3.01 × 10−5 | - | [56] | |||
| Agar-NH4SCN | 1.0 × 10−3 | - | - | - | [57] | |
| Dextran-NH4Br | 1.67 ± 0.36 × 10−6 | 0.92 | 1.62 | - | [58] | |
| Chitosan/PEO–NH4I–I2–[BmIm][I] | gel | 5.5 × 10−4 | - | 2.5 | 0.17 eV | [59] |
| Gelatin-HCL-glycerol | 5.4 × 10−5 | - | - | - | [60] | |
| Gelatin–Acetic acid–glycerol | 8.7 × 10−4 | - | - | - | [60] | |
| MG-49–NH4CF3SO3–PC | 2.9 × 10−2 | - | - | - | [61] | |
| Gellan–H2SO4 | 1.5 × 10−3 | - | - | 0.17 meV | [62] | |
3. Proton-Conducting Polymer-Electrolyte Characterizations and Properties
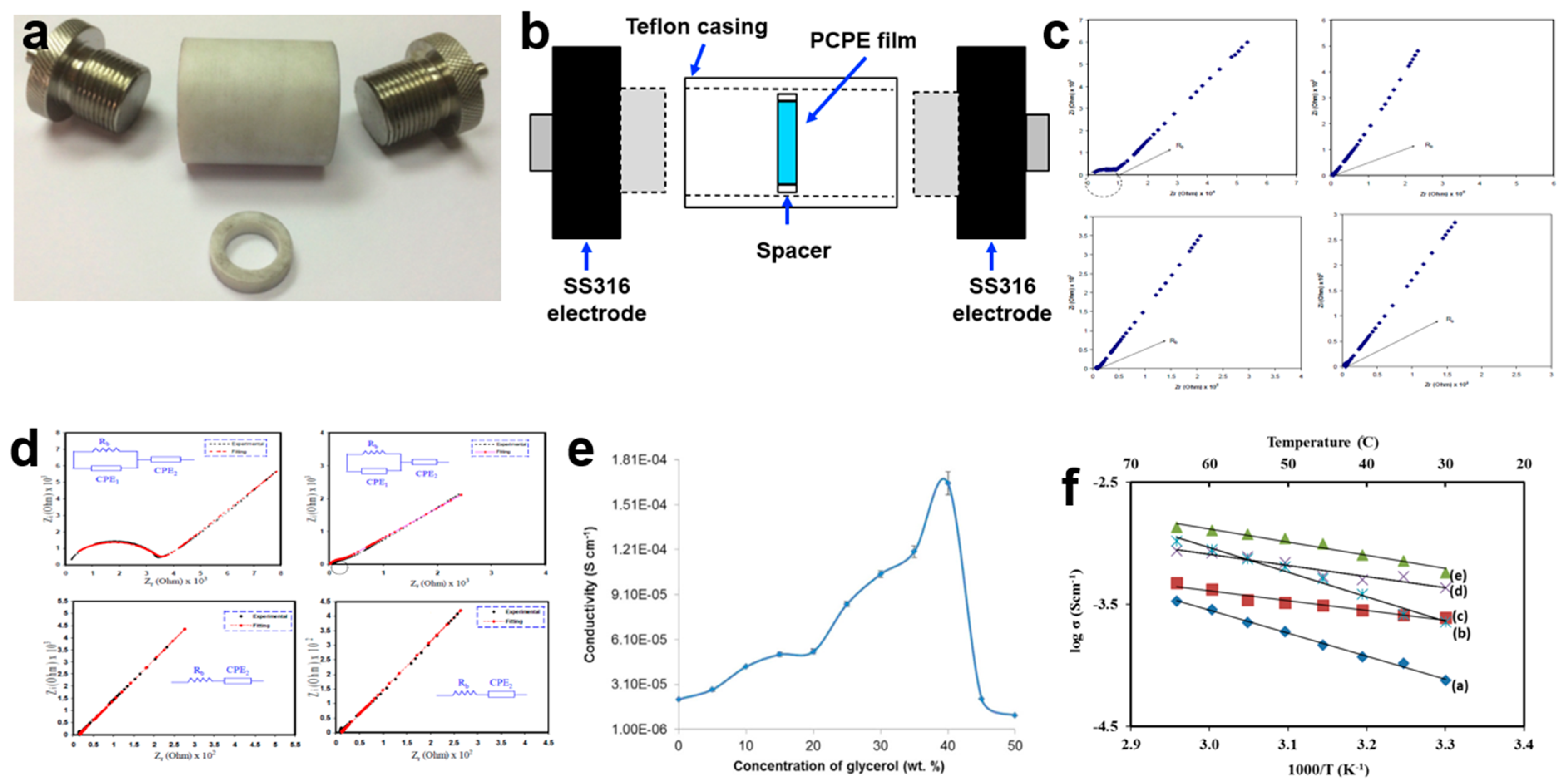
3.1. Ionic Conductivity and Temperature Dependence Analysis
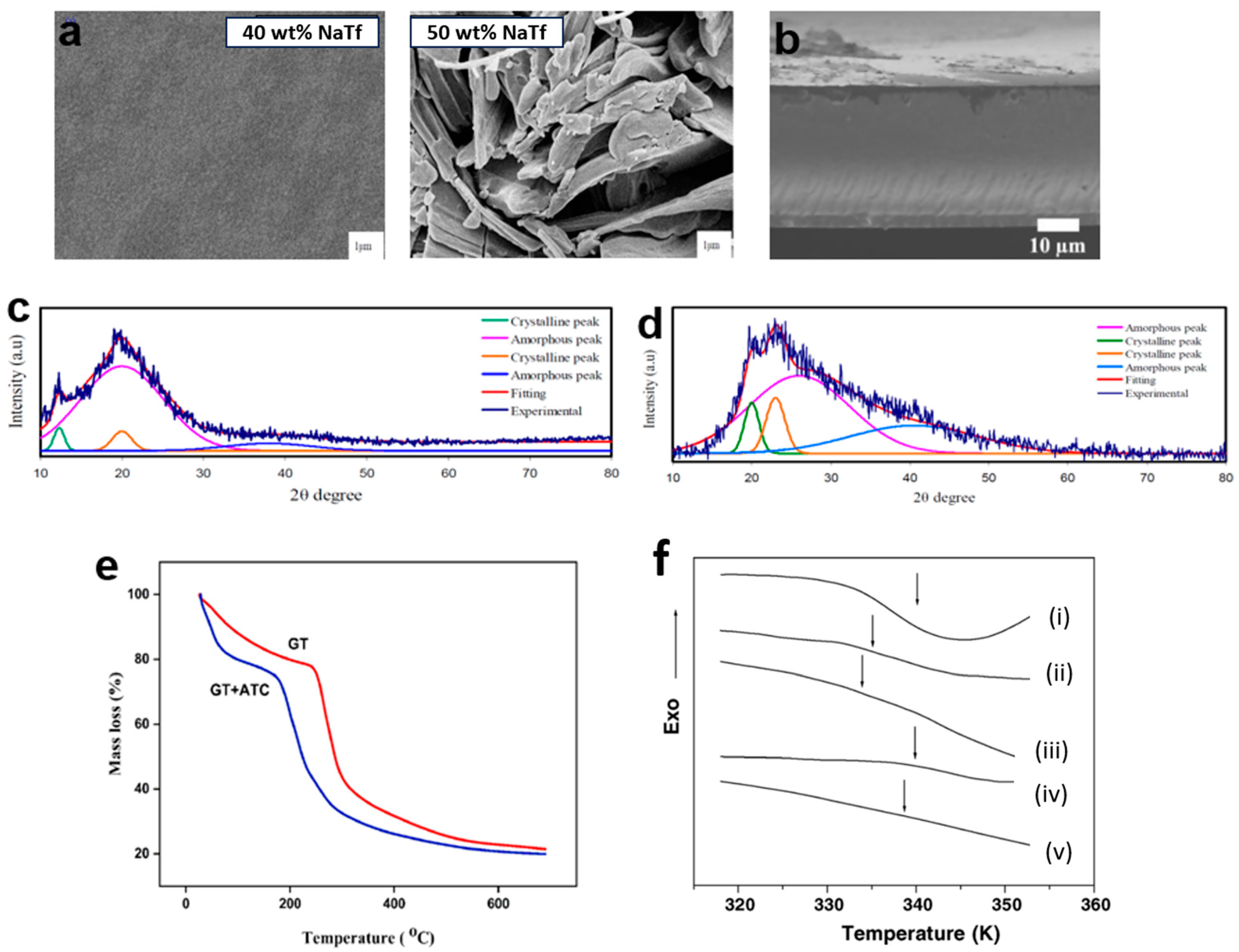
3.2. Morphology Properties
3.3. Structural Characterization
3.4. Thermal Properties
4. Linear Sweep Voltammetry
- i.
- LSV: a voltammetric technique for determining breakdown voltage/decomposition/electrochemical stability of PCPE.
- ii.
- Factors affecting onset current: reaction volume, voltage scan rate, temperature, and the material of the current collector.
- iii.
- The minimum decomposition potential of a PCPE is 1 V (implying that the PCPE is appropriate for use in solid-state polymer batteries).
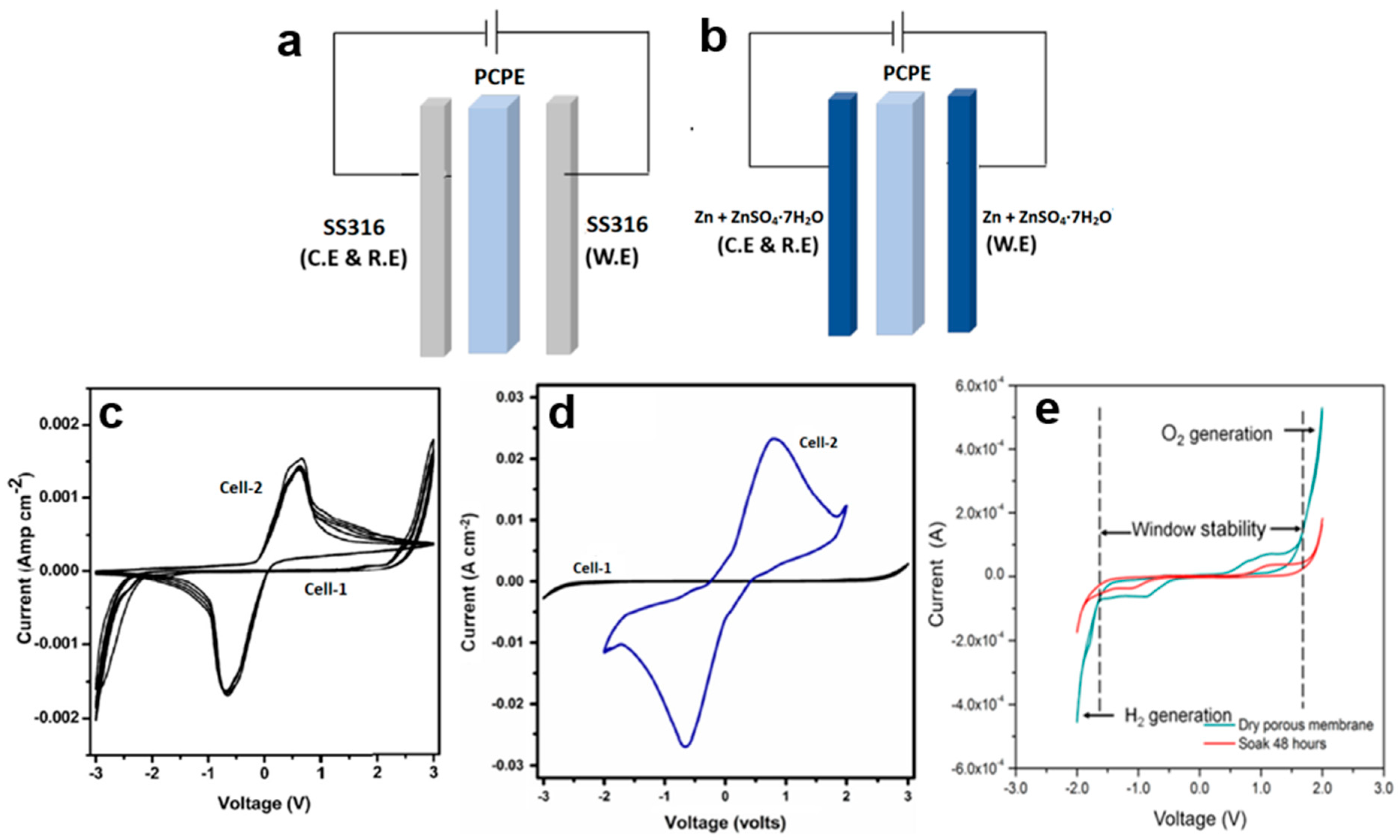
5. Cyclic Voltammetry
- i.
- CV is a valuable technique for confirming protonic conduction in PCPE, complementing complex impedance spectroscopy.
- ii.
- Protonic conduction in PCPE is enhanced through various means, including the addition of ionic salts, plasticizers, ionic liquids, fillers, and other factors.
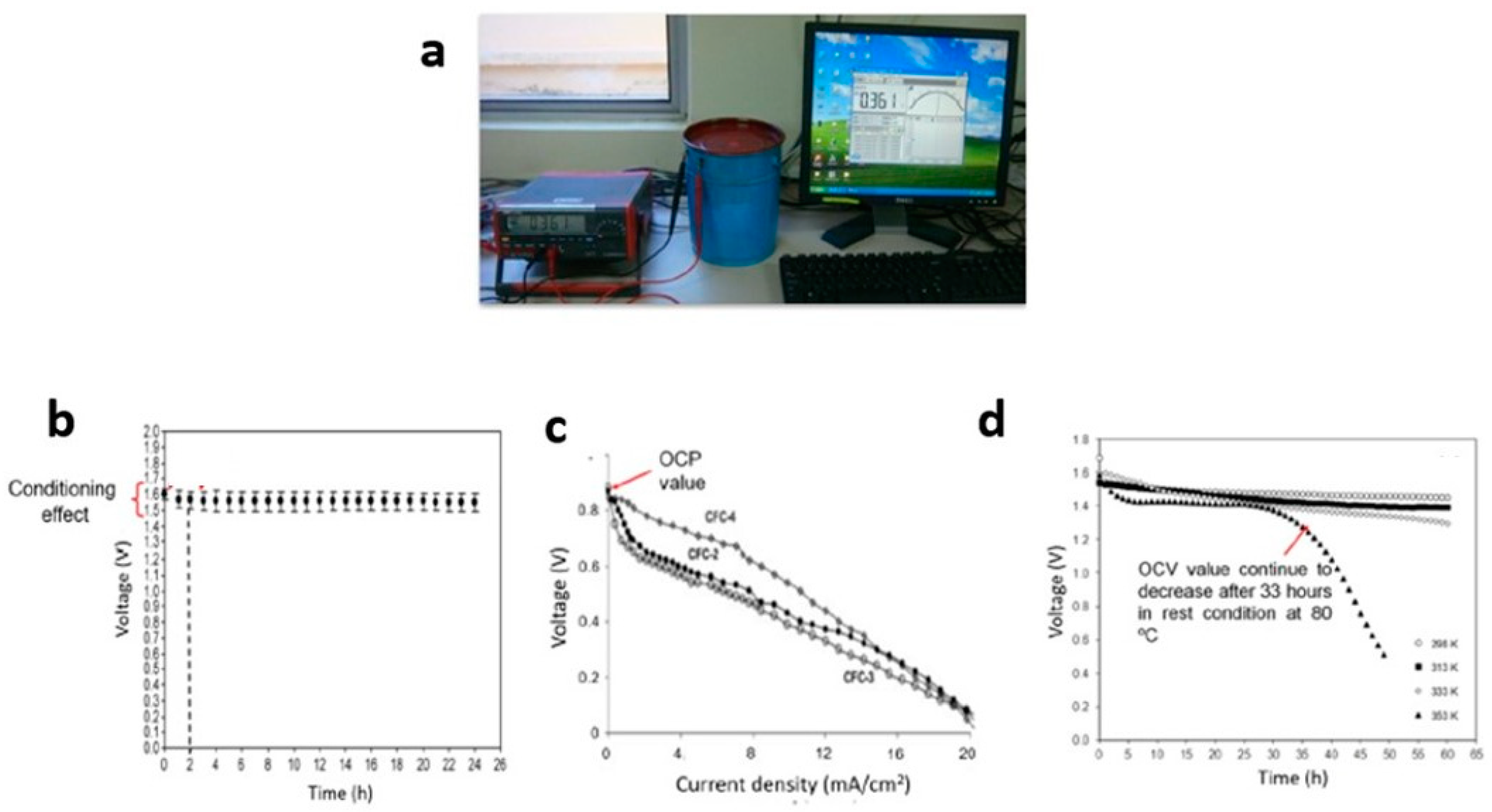
6. Open-Circuit Voltage
- i.
- The OCV characterization: (a) voltage versus time, (b) voltage versus current density at different temperatures,
- ii.
- The OCV values of proton batteries based on polymer electrolytes: 1.3–1.7 V,
- iii.
- Techniques to improve OCV value and storage duration: modifications to the polymer electrolyte by the addition of salts, plasticizers, fillers, and by blending with another type of polymer.
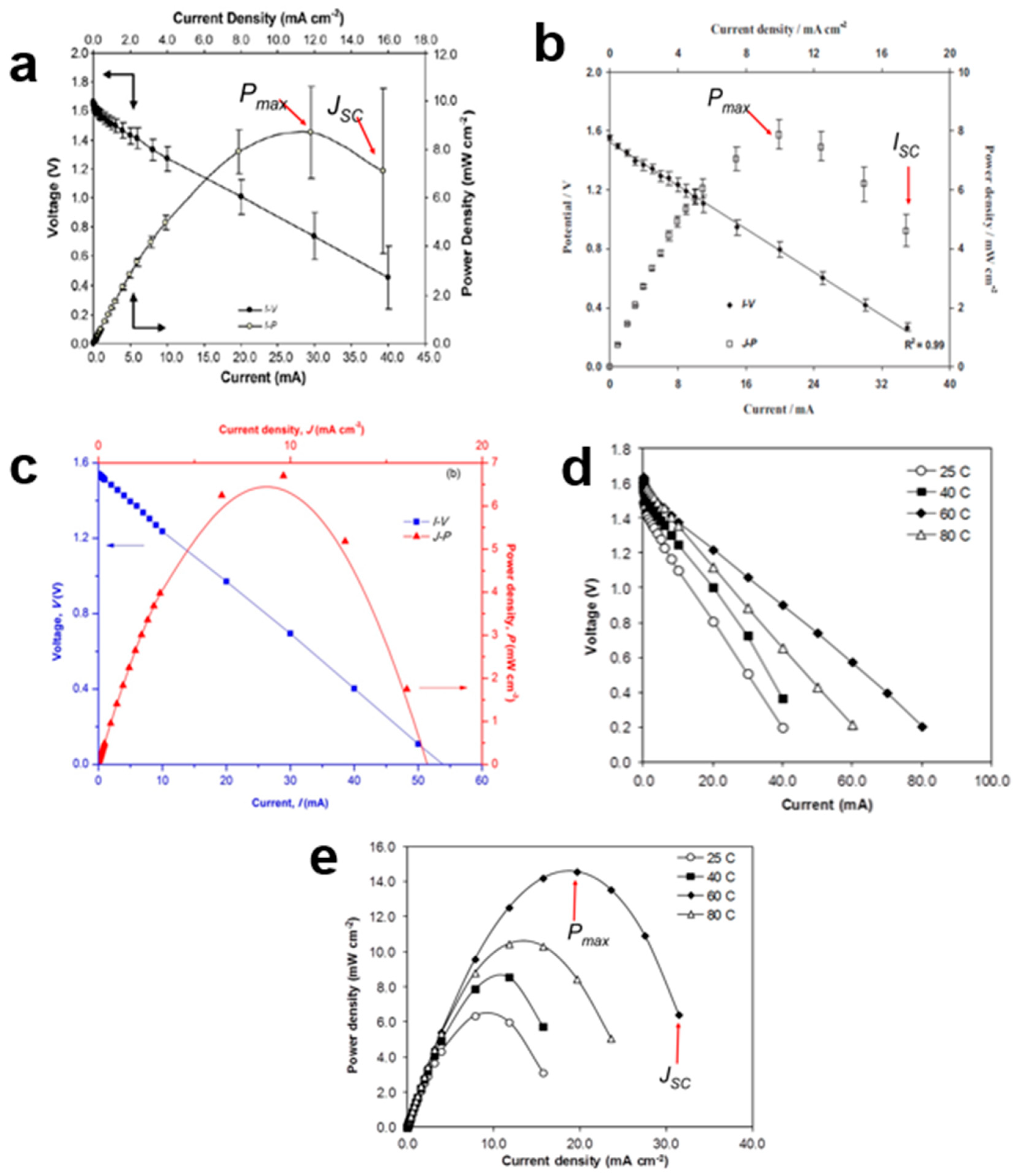
7. Current–Voltage and Power Density–Current Density
- i.
- The I-V plot: small current draws ranging from 5.0 A to 100.0 mA were utilized.
- ii.
- The r-value of the battery: should be as low as possible since a higher r will reduce the Pmax of the battery.
- iii.
- JSC: the lowest value of J in the J-P plot.
- iv.
- Modifications that can be made to reduce the value of r and increase the Pmax: (a) blending a few different types of polymers, (b) using GPE, (c) reducing the size of the battery and (d) varying the type of cathode.
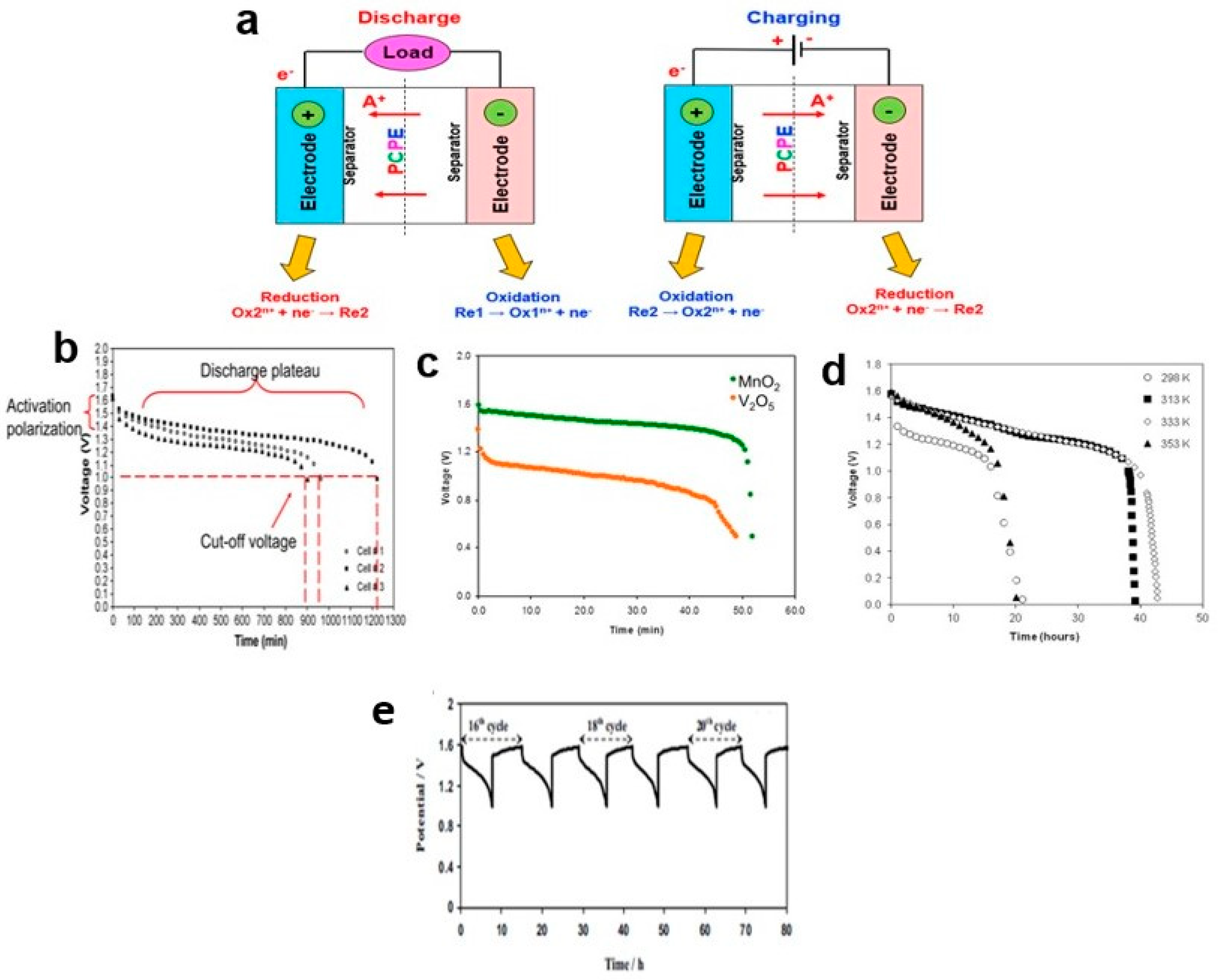
8. Charge–Discharge Profile
8.1. Primary Battery
- i.
- electrode/electrolyte: poor contact,
- ii.
- the anode condition: inability to supply copiously proton in the PCPE,
- iii.
- the cathode condition: undergone structural change during insertion and/or extraction of the proton (developed some interfacial resistance of the cell),
- iv.
- conductivity value: lower than ~10−4 S cm−1.
8.2. Secondary Battery
- i.
- Discharge characterization: specific, small constant currents, i.e., 0.1, 0.5, and 1.0 mA, were typically used.
- ii.
- Variations in the discharge profile graph and discharge capacity values: modification of several parameters, such as blending a few types of polymers, using GPE, and varying the type of cathode.
9. Conclusions and Prospectus/Future/Outlook
- Proton Movement Mechanism: Understanding the movement of protons within polymer electrolytes is essential for optimizing their performance in solid-state batteries.
- Ionic Conductivity: The ionic conductivity of PCPEs is influenced by various factors, including their morphology and structure. Enhancing proton conductivity while maintaining stability is crucial.
- Electrochemical Properties: LSV and CV techniques are used to determine the operational voltage limits of proton–polymer batteries.
- Thermal Analysis: Thermal stability and degradation characteristics of PCPEs at specific temperature ranges play a critical role in determining the operating temperature range of PCPE batteries.
- Performance: Although PCPE batteries may have lower performance compared to other electrochemical devices, they are still beneficial for small electronic devices.
- Polymer Host Modification: Various methods, such as the use of additives, block copolymers, fabrication techniques, and materials, can improve the performance of proton–polymer batteries.
- Low ionic conductivity: PCPEs often exhibit lower ionic conductivity compared to traditional liquid electrolytes. Enhancing proton mobility within the polymer matrix and optimizing proton transport pathways are ongoing challenges.
- Chemical stability: PCPEs are prone to chemical degradation, especially under high-temperature and voltage conditions. Developing PCPE materials with improved chemical stability is crucial.
- Electrode–electrolyte interface compatibility: Ensuring a compatible interface between PCPE electrolytes and electrode materials is critical for efficient charge transfer and overall battery performance.
- Mechanical stability and flexibility: PCPEs must withstand volume changes and mechanical stresses during charge/discharge cycles while remaining mechanically stable and flexible.
- Scalability and cost: Developing scalable and cost-effective processes for PCPE synthesis and fabrication is essential for commercial viability.
- The compilation of all the characterizations into one precise report as a main reference will be beneficial to future researchers as most of the previous studies preferred to focus on a specific type of characterization.
- Method for preparing the PCPE: varying the physical form of the electrolyte and the materials that are blended with PCPE, i.e., the addition of perovskite proton conductors, can improve the characterization of the electrolyte.
- Combination of theory and modeling: gain a better knowledge of proton-conducting mechanisms, and the comparison of simulated and real proton–polymer batteries can serve as a benchmark for future research breakthroughs in this field.
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, Y.; Xiao, S.; Li, M.; Chang, Z.; Wang, F.; Gao, J.; Wu, Y. Natural macromolecule based carboxymethyl cellulose as a gel polymer electrolyte with adjustable porosity for lithium ion batteries. J. Power Sources 2015, 288, 368–375. [Google Scholar] [CrossRef]
- Rani, M.S.A.; Mohammad, M.; Sua’it, M.S.; Ahmad, A.; Mohamed, N.S. Novel approach for the utilization of ionic liquid-based cellulose derivative biosourced polymer electrolytes in safe sodium-ion batteries. Polym. Bull. 2021, 78, 5355–5377. [Google Scholar] [CrossRef]
- Hasan, M.M.; Islam, D.; Rashid, T.U. Biopolymer-Based Electrolytes for Dye-Sensitized Solar Cells: A Critical Review. Energy Fuels 2020, 34, 15634–15671. [Google Scholar] [CrossRef]
- Rani, M.S.A.; Abdullah, N.A.; Sainorudin, M.H.; Mohammad, M.; Ibrahim, S. The development of poly(ethylene oxide) reinforced with a nanocellulose-based nanocomposite polymer electrolyte in dye-sensitized solar cells. Mater. Adv. 2021, 2, 5465–5470. [Google Scholar] [CrossRef]
- Rudhziah, S.; Rani, M.S.A.; Subban, R.H.Y.; Ahmad, A.; Mohamed, N.S.; Huzaifah, M.R.M. Hybrid carboxymethyl kappa-carrageenan/carboxymethyl cellulose- based biopolymer electrolytes for dye-sensitized solar cell application. Int. J. Electrochem. Sci. 2022, 17, 220143. [Google Scholar] [CrossRef]
- Latif, F.A.; Zailani, N.A.M.; Al Shukaili, Z.S.M.; Zamri, S.F.M.; Kasim, N.A.M.; Rani, M.S.A.; Norrrahim, M.N.F. Review of poly (methyl methacrylate) based polymer electrolytes in solid-state supercapacitors. Int. J. Electrochem. Sci. 2022, 17, 22013. [Google Scholar] [CrossRef]
- Rani, M.S.A.; Isa, N.S.; Nurazzi, N.M.; Norrrahim, M.N.F. Nanocomposite polymer electrolyte-based poly (ethylene oxide) reinforced with nanocellulose from oil palm empty fruit bunch: Electrical and electrochemical insights. In Synthetic and Natural Nanofillers in Polymer Composites; Woodhead Publishing: Sawston, UK, 2023; pp. 173–185. [Google Scholar]
- Kamarudin, K.H.; Rani, M.S.A.; Isa, M.I.N. Ionic conductivity and conduction mechanism of biodegradable dual polysaccharides blend electrolytes. Am.-Eurasian J. Sustain. Agric. 2015, 9, 8–14. [Google Scholar]
- Rani, M.S.A.; Ahamd, A.; Mohamed, N.S. A comprehensive investigation on electrical characterization and ionic transport properties of cellulose derivative from kenaf fibre-based biopolymer electrolytes. Polym. Bull. 2018, 75, 5061–5074. [Google Scholar] [CrossRef]
- Jeżowski, P.; Kowalczewski, P.Ł. Starch as a Green Binder for the Formulation of Conducting Glue in Supercapacitors. Polymers 2019, 11, 1648. [Google Scholar] [CrossRef]
- Ali, A.M.M.; Subban, R.H.Y.; Bahron, H.; Yahya, M.; Kamisan, A. Investigation on modified natural rubber gel polymer electrolytes for lithium polymer battery. J. Power Sources 2013, 244, 636–640. [Google Scholar] [CrossRef]
- Yaksic, A.; Tilton, J.E. Using the cumulative availability curve to assess the threat of mineral depletion: The case of lithium. Resour. Policy 2009, 34, 185–194. [Google Scholar] [CrossRef]
- Rani, M.S.A.; Sainorudin, M.H.; Asim, N.; Mohammad, M. Formation of biopolymer electrolyte by interaction between carboxymethyl cellulose and NaCH3COO and its Na+ ion transport properties. Int. J. Electrochem. Sci. 2020, 15, 11833–11844. [Google Scholar] [CrossRef]
- Ellis, B.L.; Nazar, L.F. Sodium and sodium-ion energy storage batteries. Curr. Opin. Solid State Mater. Sci. 2012, 16, 168–177. [Google Scholar] [CrossRef]
- Polu, A.R.; Rhee, H.-W. Nanocomposite solid polymer electrolytes based on poly (ethylene oxide)/POSS-PEG (n = 13.3) hybrid nanoparticles for lithium ion batteries. J. Ind. Eng. Chem. 2015, 31, 323–329. [Google Scholar] [CrossRef]
- Egbue, O.; Long, S.; Kim, S.D. Resource availability and implications for the development of plug-in electric vehicles. Sustainability 2022, 14, 1665. [Google Scholar] [CrossRef]
- Rani, M.S.A.; Isa, N.S.; Nurazzi, N.M.; Norrrahim, M.N.F. Effect of SiO2 ceramic filler on carboxymethyl cellulose from palm oil empty fruit bunch-based nanocomposite biopolymer electrolyte. In Synthetic and Natural Nanofillers in Polymer Composites; Woodhead Publishing: Sawston, UK, 2023; pp. 127–139. [Google Scholar]
- Dzulkurnain, N.A.; Rani, M.S.A.; Ahmad, A.; Mohamed, N.S. Effect of lithium salt on physicochemical properties of P(MMA-co-EMA) based copolymer electrolytes for dye-sensitized solar cell application. Ionics 2018, 24, 269–276. [Google Scholar] [CrossRef]
- Rani, M.S.A.; Dzulkurnain, N.A.; Ahmad, A.; Mohamed, N.S. Conductivity and dielectric behavior studies of carboxymethyl cellulose from kenaf bast fiber incorporated with ammonium acetate-BMATFSI biopolymer electrolytes. Int. J. Polym. Anal. Charact. 2015, 20, 250–260. [Google Scholar] [CrossRef]
- Fan, W.; Li, N.-W.; Zhang, X.; Zhao, S.; Cao, R.; Yin, Y.; Xing, Y.; Wang, J.; Guo, Y.-G.; Li, C. A Dual-Salt Gel Polymer Electrolyte with 3D Cross-Linked Polymer Network for Dendrite-Free Lithium Metal Batteries. Adv. Sci. 2018, 5, 1800559. [Google Scholar] [CrossRef]
- Al Munsur, A.Z.; Goo, B.-H.; Kim, Y.; Kwon, O.J.; Paek, S.Y.; Lee, S.Y.; Kim, H.-J.; Kim, T.-H. Nafion-Based Proton-Exchange Membranes Built on Cross-Linked Semi-Interpenetrating Polymer Networks between Poly(acrylic acid) and Poly(vinyl alcohol). ACS Appl. Mater. Interfaces 2021, 13, 28188–28200. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, B.; Mallakpour, S.; Taki, M. Application of ionic liquids as an electrolyte additive on the electrochemical behavior of lead acid battery. J. Power Sources 2009, 187, 605–612. [Google Scholar] [CrossRef]
- Maheshwari, T.; Tamilarasan, K.; Selvasekarapandian, S.; Chitra, R.; Kiruthika, S. Investigation of blend biopolymer electrolytes based on Dextran-PVA with ammonium thiocyanate. J. Solid State Electrochem. 2021, 25, 755–765. [Google Scholar] [CrossRef]
- Moni, P. Solid polymer electrolyte based on tragacanth gum-ammonium thiocyanate. J. Solid State Electrochem. 2021, 25, 2371–2383. [Google Scholar] [CrossRef]
- Moniha, V.; Alagar, M.; Selvasekarapandian, S.; Sundaresan, B.; Boopathi, G. Conductive bio-polymer electrolyte iota-carrageenan with ammonium nitrate for application in electrochemical devices. J. Non-Cryst. Solids 2018, 481, 424–434. [Google Scholar] [CrossRef]
- Hemalatha, R.; Alagar, M.; Rameshbabu, P.; Parvathi, A.A.; Devamani, R.H.P. Development of medicinal plant (Centella Asiatica—Gotu Kola) based proton conducting polymer electrolytes for electrochemical device applications. Mater. Today Proc. 2021, 81, 330–335. [Google Scholar] [CrossRef]
- Unnisa, C.N.; Chitra, S.; Selvasekarapandian, S.; Monisha, S.; Devi, G.N.; Moniha, V.; Hema, M. Development of poly(glycerol suberate) polyester (PGS)–PVA blend polymer electrolytes with NH4SCN and its application. Ionics 2018, 24, 1979–1993. [Google Scholar] [CrossRef]
- Monisha, S.; Mathavan, T.; Selvasekarapandian, S.; Benial, A.M.F.; Aristatil, G.; Mani, N.; Premalatha, M.; Pandi, D.V. Investigation of bio polymer electrolyte based on cellulose acetate-ammonium nitrate for potential use in electrochemical devices. Carbohydr. Polym. 2017, 157, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Ambika, C.; Karuppasamy, K.; Vikraman, D.; Lee, J.Y.; Regu, T.; Raj, T.A.B.; Prasanna, K.; Kim, H.-S. Effect of dimethyl carbonate (DMC) on the electrochemical and cycling properties of solid polymer electrolytes (PVP-MSA) and its application for proton batteries. Solid State Ion. 2018, 321, 106–114. [Google Scholar] [CrossRef]
- Isa, M.I.N.; Sohaimy, M.I.H.; Ahmad, N.H. Carboxymethyl cellulose plasticized polymer application as bio-material in solid-state hydrogen ionic cell. Int. J. Hydrogen Energy 2021, 46, 8030–8039. [Google Scholar] [CrossRef]
- Samsudin, A.S.; Lai, H.M.; Isa, M.I.N. Biopolymer Materials Based Carboxymethyl Cellulose as a Proton Conducting Biopolymer Electrolyte for Application in Rechargeable Proton Battery. Electrochim. Acta 2014, 129, 1–13. [Google Scholar] [CrossRef]
- MacCallum, J.R.; Vincent, C.A. Polymer Electrolyte Reviews; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1989; Volume 2. [Google Scholar]
- Aziz, S.B.; Brza, M.A.; Hamsan, H.M.; Kadir, M.F.Z.; Abdulwahid, R.T. Electrochemical characteristics of solid state double-layer capacitor constructed from proton conducting chitosan-based polymer blend electrolytes. Polym. Bull. 2021, 78, 3149–3167. [Google Scholar] [CrossRef]
- Rani, M.S.A.; Mohamed, N.S.; Isa, M.I.N. Characterization of Proton Conducting Carboxymethyl Cellulose/Chitosan Dual-Blend Based Biopolymer Electrolytes. Mater. Sci. Forum 2016, 846, 539–544. [Google Scholar] [CrossRef]
- Rani, M.S.A.; Mohamed, N.S.; Isa, M.I.N. Investigation of the ionic conduction mechanism in carboxymethyl cellulose/chitosan biopolymer blend electrolyte impregnated with ammonium nitrate. Int. J. Polym. Anal. Charact. 2015, 20, 491–503. [Google Scholar] [CrossRef]
- Yao, Y.; Cao, Y.; Li, G.; Liu, C.; Jiang, Z.; Pan, F.; Sun, J. Enhanced Electrochemical Performance of Poly(ethylene oxide) Composite Polymer Electrolyte via Incorporating Lithiated Covalent Organic Framework. Trans. Tianjin Univ. 2021, 28, 67–72. [Google Scholar] [CrossRef]
- Azimi, N.; Xue, Z.; Bloom, I.; Gordin, M.L.; Wang, D.; Daniel, T.; Takoudis, C.; Zhang, Z. Understanding the Effect of a Fluorinated Ether on the Performance of Lithium–Sulfur Batteries. ACS Appl. Mater. Interfaces 2015, 7, 9169–9177. [Google Scholar] [CrossRef]
- Ravi, M.; Kim, S.; Ran, F.; Kim, D.S.; Lee, Y.M.; Ryou, M.-H. Hybrid gel polymer electrolyte based on 1-methyl-1-Propylpyrrolidinium Bis(Trifluoromethanesulfonyl) imide for flexible and shape-variant lithium secondary batteries. J. Membr. Sci. 2021, 621, 119018. [Google Scholar] [CrossRef]
- Hamsan, M.H.; Kadir, M.F.Z.; Aziz, M.F.; Shukur, M.F. Branched Glucan from Leuconostoc Mesenteroides as the channel for ionic migration in the fabrication of protonic (H+) battery. Int. J. Hydrogen Energy 2022, 47, 38690–38702. [Google Scholar] [CrossRef]
- Ramly, K.; Isa, M.I.N.; Khiar, A.S.A. Conductivity and dielectric behaviour studies of starch/PEO+ x wt-% NH4NO3 polymer electrolyte. Mater. Res. Innov. 2011, 15 (Suppl. S2), s82–s85. [Google Scholar] [CrossRef]
- Samsudin, A.S.; Aziz, M.I.A.; Isa, M.I.N. Natural Polymer Electrolyte System Based on Sago: Structural and Transport Behavior Characteristics. Int. J. Polym. Anal. Charact. 2012, 17, 600–607. [Google Scholar] [CrossRef]
- Kumar, M.; Tiwari, T.; Srivastava, N. Electrical transport behaviour of bio-polymer electrolyte system: Potato starch+ammonium iodide. Carbohydr. Polym. 2012, 88, 54–60. [Google Scholar] [CrossRef]
- Yusof, Y.M.; Majid, N.A.; Kasmani, R.M.; Illias, H.A.; Kadir, M.F.Z. The Effect of Plasticization on Conductivity and Other Properties of Starch/Chitosan Blend Biopolymer Electrolyte Incorporated with Ammonium Iodide. Mol. Cryst. Liq. Cryst. 2014, 603, 73–88. [Google Scholar] [CrossRef]
- Yusof, Y.M.; Shukur, M.F.; Illias, H.A.; Kadir, M.F.Z. Conductivity and electrical properties of corn starch–chitosan blend biopolymer electrolyte incorporated with ammonium iodide. Phys. Scr. 2014, 89, 035701. [Google Scholar] [CrossRef]
- Shukur, M.F.; Ithnin, R.; Kadir, M.F.Z. Electrical properties of proton conducting solid biopolymer electrolytes based on starch–chitosan blend. Ionics 2014, 20, 977–999. [Google Scholar] [CrossRef]
- Shukur, M.F.; Ithnin, R.; Kadir, M.F.Z. Protonic Transport Analysis of Starch-Chitosan Blend Based Electrolytes and Application in Electrochemical Device. Mol. Cryst. Liq. Cryst. 2014, 603, 52–65. [Google Scholar] [CrossRef]
- Khanmirzaei, M.H.; Ramesh, S.; Ramesh, K. Effect of different iodide salts on ionic conductivity and structural and thermal behavior of rice-starch-based polymer electrolytes for dye-sensitized solar cell application. Ionics 2015, 21, 2383–2391. [Google Scholar] [CrossRef]
- Khiar, A.; Majid, S.; Idris, N.; Hassan, M.; Puteh, R.; Arof, A. Ionic Hopping Transport in Chitosan-Based Polymer Electrolytes. Mater. Sci. Forum 2006, 517, 237–241. [Google Scholar] [CrossRef]
- Kadir, M.F.Z.; Aspanut, Z.; Yahya, R.; Arof, A.K. Chitosan–PEO proton conducting polymer electrolyte membrane doped with NH4NO3. Mater. Res. Innov. 2011, 15 (Suppl. S2), s164–s167. [Google Scholar] [CrossRef]
- Mohamad, S.A.; Ali, M.H.; Yahya, R.; Ibrahim, Z.A.; Arof, A.K. Photovoltaic activity in a ZnSe/PEO–chitosan blend electrolyte junction. Ionics 2007, 13, 235–240. [Google Scholar] [CrossRef]
- Monisha, S.; Selvasekarapandian, S.; Mathavan, T.; Benial, A.M.F.; Manoharan, S.; Karthikeyan, S. Preparation and characterization of biopolymer electrolyte based on cellulose acetate for potential applications in energy storage devices. J. Mater. Sci. Mater. Electron. 2016, 27, 9314–9324. [Google Scholar] [CrossRef]
- Shukur, M.; Ithnin, R.; Illias, H.; Kadir, M. Proton conducting polymer electrolyte based on plasticized chitosan–PEO blend and application in electrochemical devices. Opt. Mater. 2013, 35, 1834–1841. [Google Scholar] [CrossRef]
- Zaki, N.H.M.; Mahmud, Z.S.; Adam, N.I.; Ahmad, A.H.; Ali, A.M.M.; Yahya, M.Z.A. Characterization of plasticized grafted natural rubber-30% poly (methyl methacrylate) (MG30) based polymer electrolytes. In Proceedings of the IEEE Symposium on Business, Engineering and Industrial Applications, Bandung, Indonesia, 23–26 September 2012; pp. 705–708. [Google Scholar] [CrossRef]
- Pratap, R.; Singh, A.; Singh, R.C.; Singh, M.K.; Alheety, M.A.; Aldbea, F.W.; Samsudin, A.S.; Singh, P.K. Dynamics of Proton Transport in Polyethylene Oxide (PEO) with Ammonium Perchlorate (NH4ClO4). Macromol. Symp. 2023, 407, 2100450. [Google Scholar] [CrossRef]
- Rani, M.S.A.; Rudhziah, S.; Ahmad, A.; Mohamed, N.S. Effects of different iodide salts on the electrical and electrochemical properties of hybrid biopolymer electrolytes for dye-sensitized solar cells application. Polym. Bull. 2022, 79, 9813–9832. [Google Scholar] [CrossRef]
- Khan, N.M.; Norman, N.M.; Samsudin, A.S. Studies on the effect of H+ carrier toward ionic conduction properties in alginate-ammonium sulfate complexes–based polymer electrolytes system. High Perform. Polym. 2022, 34, 637–644. [Google Scholar] [CrossRef]
- Selvalakshmi, S.; Vijaya, N.; Selvasekarapandian, S.; Premalatha, M. Biopolymer agar-agar doped with NH4SCN as solid polymer electrolyte for electrochemical cell application. J. Appl. Polym. Sci. 2017, 134, 44702. [Google Scholar] [CrossRef]
- Hamsan, M.H.; Shukur, M.F.; Aziz, S.B.; Yusof, Y.M.; Kadir, M.F.Z. Influence of NH4Br as an ionic source on the structural/electrical properties of dextran-based biopolymer electrolytes and EDLC application. Bull. Mater. Sci. 2020, 43, 1–7. [Google Scholar] [CrossRef]
- Buraidah, M.H.; Teo, L.P.; Yusuf, S.N.F.; Noor, M.M.; Kufian, M.Z.; Careem, M.A.; Majid, S.R.; Taha, R.M.; Arof, A.K. TiO2/chitosan-NH4I (+I2)-BMII-based dye-sensitized solar cells with anthocyanin dyes extracted from black rice and red cabbage. Int. J. Photoenergy 2011, 2011, 273683. [Google Scholar] [CrossRef]
- Pawlicka, A.; Firmino, A.; Vieira, D.; Sentanin, F.; Grote, J.G.; Kajzar, F. Gelatin- and DNA-based ionic conducting membranes for electrochromic devices. In Optical Materials in Defence Systems Technology VI; SPIE: Bellingham, WA, USA, 2009. [Google Scholar] [CrossRef]
- Kamisan, A.S.; Kudin, T.I.T.; Ali, A.M.M.; Yahya, M.Z.A. Polymer gel electrolytes based on 49% methyl-grafted natural rubber. Sains Malays 2011, 40, 49–54. [Google Scholar]
- Sudhakar, Y.N.; Kumar, S.; Bhat, D.K. Effect of acid dopants in biodegradable gel polymer electrolyte and the performance in an electrochemical double layer capacitor. Phys. Scr. 2015, 90, 95702. [Google Scholar] [CrossRef]
- Rani, M.S.A.; Isa, N.S.; Sainorudin, M.H.; Abdullah, N.A.; Mohammad, M.; Asim, N.; Razali, H.; Ibrahim, M. Magnesium Ion-Conducting Biopolymer Electrolytes Based on Carboxymethyl Cellulose Derived from Palm Oil Empty Fruit Bunch Fibre. Int. J. Electrochem. Sci. 2021, 16, 210354. [Google Scholar] [CrossRef]
- Albu, A.-M.; Maior, I.; Nicolae, C.A.; Bocăneală, F.L. Novel PVA proton conducting membranes doped with polyaniline generated by in-situ polymerization. Electrochim. Acta 2016, 211, 911–917. [Google Scholar] [CrossRef]
- Khalil, R.; Sheha, E.; Hanafy, T.; Al-Hartomy, O. Synthesis and characterization of poly(vinyl alcohol)-acid salt polymer electrolytes. Mater. Express 2014, 4, 483–490. [Google Scholar] [CrossRef]
- Correia, D.M.; Costa, C.M.; Nunes-Pereira, J.; Silva, M.M.; Botelho, G.; Ribelles, J.L.G.; Lanceros-Mendez, S. Physicochemical properties of poly (vinylidene fluoride-trifluoroethylene)/poly (ethylene oxide) blend membranes for lithium ion battery applications: Influence of poly (ethylene oxide) molecular weight. Solid State Ion. 2014, 268, 54–67. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, H.; Girard, G.M.A.; Yunis, R.; MacFarlane, D.R.; Mecerreyes, D.; Bhattacharyya, A.J.; Howlett, P.C.; Forsyth, M. Preparation and characterization of gel polymer electrolytes using poly(ionic liquids) and high lithium salt concentration ionic liquids. J. Mater. Chem. A 2017, 5, 23844–23852. [Google Scholar] [CrossRef]
- Gümüşoğlu, T.; Arı, G.A.; Deligöz, H. Investigation of salt addition and acid treatment effects on the transport properties of ionically cross-linked polyelectrolyte complex membranes based on chitosan and polyacrylic acid. J. Membr. Sci. 2011, 376, 25–34. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Wu, H.; Xiao, L.; Jiang, Z. Fabrication and performances of solid superacid embedded chitosan hybrid membranes for direct methanol fuel cell. J. Power Sources 2010, 195, 2526–2533. [Google Scholar] [CrossRef]
- Pawlicka, A.; Mattos, R.; Tambelli, C.; Silva, I.; Magon, C.; Donoso, J. Magnetic resonance study of chitosan bio-membranes with proton conductivity properties. J. Membr. Sci. 2013, 429, 190–196. [Google Scholar] [CrossRef]
- Cheng, S.-W.; Tsai, C.-H.; Wu, S.-H.; Liu, C.-K.; Cheng, Y.-N.; Lee, R.-Y. Effects of reduction process on the electrochemical and microstructural properties for electrolyte-supported SOFC. Int. J. Hydrogen Energy 2015, 40, 1534–1540. [Google Scholar] [CrossRef]
- Aggas, J.R.; Harrell, W.; Lutkenhaus, J.; Guiseppi-Elie, A. Metal–polymer interface influences apparent electrical properties of nano-structured polyaniline films. Nanoscale 2018, 10, 672–682. [Google Scholar] [CrossRef] [PubMed]
- Mobarak, N.M.; Ahmad, A.; Abdullah, M.P.; Ramli, N.; Rahman, M.Y.A. Conductivity enhancement via chemical modification of chitosan based green polymer electrolyte. Electrochim. Acta 2013, 92, 161–167. [Google Scholar] [CrossRef]
- Liao, G.-M.; Yang, C.-C.; Hu, C.-C.; Pai, Y.-L.; Lue, S.J. Novel quaternized polyvinyl alcohol/quaternized chitosan nano-composite as an effective hydroxide-conducting electrolyte. J. Membr. Sci. 2015, 485, 17–29. [Google Scholar] [CrossRef]
- Chen, X.; Xie, Y.; Ling, Y.; Zhao, J.; Xu, Y.; Tong, Y.; Li, S.; Wang, Y. Ionic liquid crystal induced morphological control of solid composite polymer electrolyte for lithium-ion batteries. Mater. Des. 2020, 192, 108760. [Google Scholar] [CrossRef]
- Fadzallah, I.; Majid, S.; Careem, M.; Arof, A. A study on ionic interactions in chitosan–oxalic acid polymer electrolyte membranes. J. Membr. Sci. 2014, 463, 65–72. [Google Scholar] [CrossRef]
- Yusof, Y.M.; Illias, H.A.; Kadir, M.F.Z. Incorporation of NH4Br in PVA-chitosan blend-based polymer electrolyte and its effect on the conductivity and other electrical properties. Ionics 2014, 20, 1235–1245. [Google Scholar] [CrossRef]
- Shukur, M.F.; Kadir, M.F.Z. Hydrogen ion conducting starch-chitosan blend based electrolyte for application in electrochemical devices. Electrochim. Acta 2015, 158, 152–165. [Google Scholar] [CrossRef]
- Pandi, D.V.; Selvasekarapandian, S.; Bhuvaneswari, R.; Premalatha, M.; Monisha, S.; Arunkumar, D.; Junichi, K. Development and characterization of proton conducting polymer electrolyte based on PVA, amino acid glycine and NH4SCN. Solid State Ion. 2016, 298, 15–22. [Google Scholar] [CrossRef]
- Naachiyar, R.M.; Ragam, M.; Selvasekarapandian, S.; Krishna, M.V.; Buvaneshwari, P. Development of biopolymer electrolyte membrane using Gellan gum biopolymer incorporated with NH4SCN for electro-chemical application. Ionics 2021, 27, 3415–3429. [Google Scholar] [CrossRef]
- Aziz, S.B.; Hadi, J.M.; Dannoun, E.M.A.; Abdulwahid, R.T.; Saeed, S.R.; Marf, A.S.; Karim, W.O.; Kadir, M.F. The Study of Plasticized Amorphous Biopolymer Blend Electrolytes Based on Polyvinyl Alcohol (PVA): Chitosan with High Ion Conductivity for Energy Storage Electrical Double-Layer Capacitors (EDLC) Device Application. Polymers 2020, 12, 1938. [Google Scholar] [CrossRef] [PubMed]
- Brza, M.A.; Aziz, S.B.; Anuar, H.; Dannoun, E.M.A.; Ali, F.; Abdulwahid, R.T.; Al-Zangana, S.; Kadir, M.F. The Study of EDLC Device with High Electrochemical Performance Fabricated from Proton Ion Conducting PVA-Based Polymer Composite Electrolytes Plasticized with Glycerol. Polymers 2020, 12, 1896. [Google Scholar] [CrossRef] [PubMed]
- Chai, M.N.; Isa, M.I.N. Novel Proton Conducting Solid Bio-polymer Electrolytes Based on Carboxymethyl Cellulose Doped with Oleic Acid and Plasticized with Glycerol. Sci. Rep. 2016, 6, 27328. [Google Scholar] [CrossRef]
- Rani, M.S.A.; Rudhziah, S.; Ahmad, A.; Mohamed, N.S. Biopolymer Electrolyte Based on Derivatives of Cellulose from Kenaf Bast Fiber. Polymers 2014, 6, 2371–2385. [Google Scholar] [CrossRef]
- Verma, M.L.; Minakshi, M.; Singh, N.K. Synthesis and Characterization of Solid Polymer Electrolyte based on Activated Carbon for Solid State Capacitor. Electrochim. Acta 2014, 137, 497–503. [Google Scholar] [CrossRef]
- Ghani, N.A.A.; Othaman, R.; Ahmad, A.; Anuar, F.H.; Hassan, N.H. Impact of purification on iota carrageenan as solid polymer electrolyte. Arab. J. Chem. 2019, 12, 370–376. [Google Scholar] [CrossRef]
- Marf, A.S.; Abdullah, R.M.; Aziz, S.B. Structural, Morphological, Electrical and Electrochemical Properties of PVA: CS-Based Proton-Conducting Polymer Blend Electrolytes. Membranes 2020, 10, 71. [Google Scholar] [CrossRef]
- Kadir, M.F.Z.; Majid, S.R.; Arof, A.K. Plasticized chitosan–PVA blend polymer electrolyte based proton battery. Electrochim. Acta 2010, 55, 1475–1482. [Google Scholar] [CrossRef]
- Rani, M.S.A.; Ahmad, A.; Mohamed, N.S. Influence of nano-sized fumed silica on physicochemical and electrochemical properties of cellulose derivatives-ionic liquid biopolymer electrolytes. Ionics 2018, 24, 807–814. [Google Scholar] [CrossRef]
- Yang, H.; Liu, Y.; Kong, L.; Kang, L.; Ran, F. Biopolymer-based carboxylated chitosan hydrogel film crosslinked by HCl as gel polymer electrolyte for all-solid-sate supercapacitors. J. Power Sources 2019, 426, 47–54. [Google Scholar] [CrossRef]
- Raut, P.; Li, S.; Chen, Y.-M.; Zhu, Y.; Jana, S.C. Strong and Flexible Composite Solid Polymer Electrolyte Membranes for Li-Ion Batteries. ACS Omega 2019, 4, 18203–18209. [Google Scholar] [CrossRef] [PubMed]
- Rani, M.S.A.; Hassan, N.H.; Ahmad, A.; Kaddami, H.; Mohamed, N.S. Investigation of biosourced carboxymethyl cellulose-ionic liquid polymer electrolytes for potential application in electrochemical devices. Ionics 2016, 22, 1855–1864. [Google Scholar] [CrossRef]
- Aziz, S.B.; Abdullah, O.G.; Rasheed, M.A.; Ahmed, H.M. Effect of High Salt Concentration (HSC) on Structural, Morphological, and Electrical Characteristics of Chitosan Based Solid Polymer Electrolytes. Polymers 2017, 9, 187. [Google Scholar] [CrossRef] [PubMed]
- Brza, M.A.; Aziz, S.B.; Nofal, M.M.; Saeed, S.R.; Al-Zangana, S.; Karim, W.O.; Hussen, S.A.; Abdulwahid, R.T.; Kadir, M.F.Z. Drawbacks of Low Lattice Energy Ammonium Salts for Ion-Conducting Polymer Electrolyte Preparation: Structural, Morphological and Electrical Characteristics of CS:PEO:NH4BF4-Based Polymer Blend Electrolytes. Polymers 2020, 12, 1885. [Google Scholar] [CrossRef]
- Hirankumar, G.; Selvasekarapandian, S.; Kuwata, N.; Kawamura, J.; Hattori, T. Thermal, electrical and optical studies on the poly (vinyl alcohol) based polymer electrolytes. J. Power Sources 2005, 144, 262–267. [Google Scholar] [CrossRef]
- Song, K.; Wu, L.-F.; Liu, D.; Li, L.; Song, J.; Wang, Z. Revealing the detailed structure in flow-induced crystallization of semicrystalline polymers. Phys. Chem. Chem. Phys. 2020, 22, 25206–25214. [Google Scholar] [CrossRef]
- Hassan, F.; Woo, H.J.; Aziz, N.A.; Kufian, M.Z.; Majid, S.R. Synthesis of Al2TiO5 and its effect on the properties of chitosan–NH4SCN polymer electrolytes. Ionics 2013, 19, 483–489. [Google Scholar] [CrossRef]
- Maheshwari, T.; Tamilarasan, K.; Selvasekarapandian, S.; Chitra, R.; Muthukrishnan, M. Synthesis and characterization of Dextran, poly (vinyl alcohol) blend biopolymer electrolytes with NH4NO3, for electrochemical applications. Int. J. Green Energy 2022, 19, 314–330. [Google Scholar] [CrossRef]
- Hemalatha, R.; Alagar, M.; Selvasekarapandian, S.; Sundaresan, B.; Moniha, V. Studies of proton conducting polymer electrolyte based on PVA, amino acid proline and NH4SCN. J. Sci. Adv. Mater. Devices 2019, 4, 101–110. [Google Scholar] [CrossRef]
- Rudhziah, S.; Rani, M.S.A.; Ahmad, A.; Mohamed, N.S.; Kaddami, H. Potential of blend of kappa-carrageenan and cellulose derivatives for green polymer electrolyte application. Ind. Crops Prod. 2015, 72, 133–141. [Google Scholar] [CrossRef]
- Altaf, F.; Gill, R.; Batool, R.; Drexler, M.; Alamgir, F.; Abbas, G.; Jacob, K. Proton conductivity and methanol permeability study of polymer electrolyte membranes with range of functionalized clay content for fuel cell application. Eur. Polym. J. 2019, 110, 155–167. [Google Scholar] [CrossRef]
- Hande, V.R.; Rath, S.K.; Rao, S.; Patri, M. Cross-linked sulfonated poly (ether ether ketone) (SPEEK)/reactive organoclay nanocomposite proton exchange membranes (PEM). J. Membr. Sci. 2011, 372, 40–48. [Google Scholar] [CrossRef]
- Shaari, N.; Kamarudin, S.K.; Basri, S.; Shyuan, L.K.; Masdar, M.S.; Nordin, D. Enhanced mechanical flexibility and performance of sodium alginate polymer electrolyte bio-membrane for application in direct methanol fuel cell. J. Appl. Polym. Sci. 2018, 135, 46666. [Google Scholar] [CrossRef]
- Sivadevi, S.; Selvasekarapandian, S.; Karthikeyan, S.; Sanjeeviraja, C.; Nithya, H.; Iwai, Y.; Kawamura, J. Proton-conducting polymer electrolyte based on PVA-PAN blend doped with ammonium thiocyanate. Ionics 2015, 21, 1017–1029. [Google Scholar] [CrossRef]
- Saeed, M.A.M.; Abdullah, O.G. Effect of High Ammonium Salt Concentration and Temperature on the Structure, Morphology, and Ionic Conductivity of Proton-Conductor Solid Polymer Electrolytes Based PVA. Membranes 2020, 10, 262. [Google Scholar] [CrossRef] [PubMed]
- Ketabi, S.; Lian, K. Effect of SiO2 on conductivity and structural properties of PEO–EMIHSO4 polymer electrolyte and enabled solid electrochemical capacitors. Electrochim. Acta 2013, 103, 174–178. [Google Scholar] [CrossRef]
- Venkatesan, P.N.; Dharmalingam, S. Development of cation exchange resin-polymer electrolyte membranes for microbial fuel cell application. J. Mater. Sci. 2015, 50, 6302–6312. [Google Scholar] [CrossRef]
- Aziz, S.B.; Brza, M.A.; Brevik, I.; Hafiz, M.H.; Asnawi, A.S.; Yusof, Y.M.; Abdulwahid, R.T.; Kadir, M.F.Z. Blending and characteristics of electrochemical double-layer capacitor device assembled from plasticized proton ion conducting chitosan:dextran:NH4PF6 polymer electrolytes. Polymers 2020, 12, 2103. [Google Scholar] [CrossRef] [PubMed]
- Liew, C.-W.; Arifin, K.; Kawamura, J.; Iwai, Y.; Ramesh, S.; Arof, A.K. Electrical and structural studies of ionic liquid-based poly(vinyl alcohol) proton conductors. J. Non-Cryst. Solids 2015, 425, 163–172. [Google Scholar] [CrossRef]
- Alves, R.; Donoso, J.; Magon, C.; Silva, I.; Pawlicka, A.; Silva, M. Solid polymer electrolytes based on chitosan and europium triflate. J. Non-Cryst. Solids 2016, 432, 307–312. [Google Scholar] [CrossRef]
- Choi, K.-M.; Lim, S.-W.; Choi, M.-C.; Han, D.-H.; Ha, C.-S. Properties of poly(ethylene glycol)-grafted poly(lactic acid) plasticized with poly(ethylene glycol). Macromol. Res. 2014, 22, 1312–1319. [Google Scholar] [CrossRef]
- Yang, J.-M.; Wang, S.-A. Preparation of graphene-based poly (vinyl alcohol)/chitosan nanocomposites membrane for alkaline solid electrolytes membrane. J. Membr. Sci. 2015, 477, 49–57. [Google Scholar] [CrossRef]
- Choudhury, N.A.; Ma, J.; Sahai, Y. High performance and eco-friendly chitosan hydrogel membrane electrolytes for direct borohydride fuel cells. J. Power Sources 2012, 210, 358–365. [Google Scholar] [CrossRef]
- Aziz, N.A.; Majid, S.R.; Arof, A.K. Synthesis and characterizations of phthaloyl chitosan-based polymer electrolytes. J. Non-Cryst. Solids 2012, 358, 1581–1590. [Google Scholar] [CrossRef]
- Kam, W.; Liew, C.W.; Lim, J.Y.; Ramesh, S. Electrical, structural, and thermal studies of antimony trioxide-doped poly (acrylic acid)-based composite polymer electrolytes. Ionics 2014, 20, 665–674. [Google Scholar] [CrossRef]
- Nath, A.K.; Talukdar, R. Ionic liquid-based novel polymer electrolytes: Electrical and thermal properties. Int. J. Polym. Anal. Charact. 2020, 25, 597–603. [Google Scholar] [CrossRef]
- Premalatha, M.; Mathavan, T.; Selvasekarapandian, S.; Monisha, S.; Pandi, D.V.; Selvalakshmi, S. Investigations on proton conducting biopolymer membranes based on tamarind seed polysaccharide incorporated with ammonium thiocyanate. J. Non-Cryst. Solids 2016, 453, 131–140. [Google Scholar] [CrossRef]
- Yusuf, S.N.F.; Kufian, M.Z.; Teo, L. Preparation and electrical characterization of polymer electrolytes: A review. Mater. Today Proc. 2019, 17, 446–458. [Google Scholar] [CrossRef]
- Karthikeyan, S.; Selvasekarapandian, S.; Premalatha, M.; Monisha, S.; Boopathi, G.; Aristatil, G.; Arun, A.; Madeswaran, S. Proton-conducting I-Carrageenan-based biopolymer electrolyte for fuel cell application. Ionics 2017, 23, 2775–2780. [Google Scholar] [CrossRef]
- Noor, N.A.M.; Isa, M.I.N. Investigation on transport and thermal studies of solid polymer electrolyte based on carboxymethyl cellulose doped ammonium thiocyanate for potential application in electrochemical devices. Int. J. Hydrogen Energy 2019, 44, 8298–8306. [Google Scholar] [CrossRef]
- Deivanayagam, R.; Shahbazian-Yassar, R. Electrochemical Methods and Protocols for Characterization of Ceramic and Polymer Electrolytes for Rechargeable Batteries. Batter. Supercaps 2021, 4, 596–606. [Google Scholar] [CrossRef]
- Gupta, H.; Shalu; Balo, L.; Singh, V.K.; Singh, S.K.; Tripathi, A.K.; Verma, Y.L.; Singh, R.K. Effect of temperature on electrochemical performance of ionic liquid based polymer electrolyte with Li/LiFePO4 electrodes. Solid State Ion. 2017, 309, 192–199. [Google Scholar] [CrossRef]
- Aziz, S.B.; Hamsan, M.H.; Karim, W.O.; Marif, A.S.; Abdulwahid, R.T.; Kadir, M.F.Z.; Brza, M.A. Study of impedance and solid-state double-layer capacitor behavior of proton (H+)-conducting polymer blend electrolyte-based CS:PS polymers. Ionics 2020, 26, 4635–4649. [Google Scholar] [CrossRef]
- Arof, A.K.; Shuhaimi, N.E.A.; Alias, N.A.; Kufian, M.Z.; Majid, S.R. Application of chitosan/iota-carrageenan polymer electrolytes in electrical double layer capacitor (EDLC). J. Solid State Electrochem. 2010, 14, 2145–2152. [Google Scholar] [CrossRef]
- Mishra, K.; Hashmi, S.A.; Rai, D.K. Studies on a proton battery using gel polymer electrolyte. High Perform. Polym. 2014, 26, 672–676. [Google Scholar] [CrossRef]
- Aziz, S.B.; Asnawi, A.S.F.M.; Kadir, M.F.Z.; Alshehri, S.M.; Ahamad, T.; Yusof, Y.M.; Hadi, J.M. Structural, Electrical and Electrochemical Properties of Glycerolized Biopolymers Based on Chitosan (CS): Methylcellulose (MC) for Energy Storage Application. Polymers 2021, 13, 1183. [Google Scholar] [CrossRef]
- Mabbott, G.A. An introduction to cyclic voltammetry. J. Chem. Educ. 1983, 60, 697. [Google Scholar] [CrossRef]
- Mishra, K.; Hashmi, S.A.; Rai, D.K. Protic ionic liquid-based gel polymer electrolyte: Structural and ion transport studies and its application in proton battery. J. Solid State Electrochem. 2014, 18, 2255–2266. [Google Scholar] [CrossRef]
- Hamsan, M.H.; Aziz, S.B.; Shukur, M.F.; Kadir, M.F.Z. Protonic cell performance employing electrolytes based on plasticized methylcellulose-potato starch-NH4NO3. Ionics 2019, 25, 559–572. [Google Scholar] [CrossRef]
- Yusof, Y.M.; Illias, H.A.; Shukur, M.F.; Kadir, M.F.Z. Characterization of starch-chitosan blend-based electrolyte doped with ammonium iodide for application in proton batteries. Ionics 2017, 23, 681–697. [Google Scholar] [CrossRef]
- Pundir, S.; Mishra, K.; Rai, D. Ion transport studies in nanocomposite polymer electrolyte membrane of PVA–[C4C1Im][HSO4]–SiO2. J. Solid State Electrochem. 2018, 22, 1801–1815. [Google Scholar] [CrossRef]
- Mishra, K.; Pundir, S.S.; Rai, D.K. Effect of polysorbate plasticizer on the structural and ion conduction properties of PEO–NH4PF6 solid polymer electrolyte. Ionics 2017, 23, 105–112. [Google Scholar] [CrossRef]
- Pundir, S.; Mishra, K.; Rai, D. Structural, thermal and electrochemical studies of PVA/PVP—NH4SCN—[C2C1Im][SCN] polymer electrolyte system. J. Mater. Sci. Mater. Electron. 2021, 32, 1476–1490. [Google Scholar] [CrossRef]
- Pundir, S.; Mishra, K.; Rai, D. Poly(vinyl)alcohol/1-butyl-3-methylimidazolium hydrogen sulfate solid polymer electrolyte: Structural and electrical studies. Solid State Ion. 2015, 275, 86–91. [Google Scholar] [CrossRef]
- Majid, S.R.; Arof, A.K. Conductivity studies and performance of chitosan based polymer electrolytes in H2/air fuel cell. Polym. Adv. Technol. 2009, 20, 524–528. [Google Scholar] [CrossRef]
- Ummartyotin, S.; Pechyen, C. Strategies for development and implementation of bio-based materials as effective renewable resources of energy: A comprehensive review on adsorbent technology. Renew. Sustain. Energy Rev. 2016, 62, 654–664. [Google Scholar] [CrossRef]
- Jothi, M.A.; Vanitha, D.; Bahadur, S.A.; Nallamuthu, N. Proton conducting polymer electrolyte based on cornstarch, PVP, and NH4Br for energy storage applications. Ionics 2021, 27, 225–237. [Google Scholar] [CrossRef]
- Linden, D.; Reddy, T.B. (Eds.) Handbook of Batteries; McGraw-Hill: New York, NY, USA, 2002. [Google Scholar]
- Aurbach, D.; Markovsky, B.; Salitra, G.; Markevich, E.; Talyossef, Y.; Koltypin, M.; Nazar, L.; Ellis, B.; Kovacheva, D. Review on electrode–electrolyte solution interactions, related to cathode materials for Li-ion batteries. J. Power Sources 2007, 165, 491–499. [Google Scholar] [CrossRef]
- Selvakumaran, D.; Pan, A.; Liang, S.; Cao, G. A review on recent developments and challenges of cathode materials for rechargeable aqueous Zn-ion batteries. J. Mater. Chem. A 2019, 7, 18209–18236. [Google Scholar] [CrossRef]
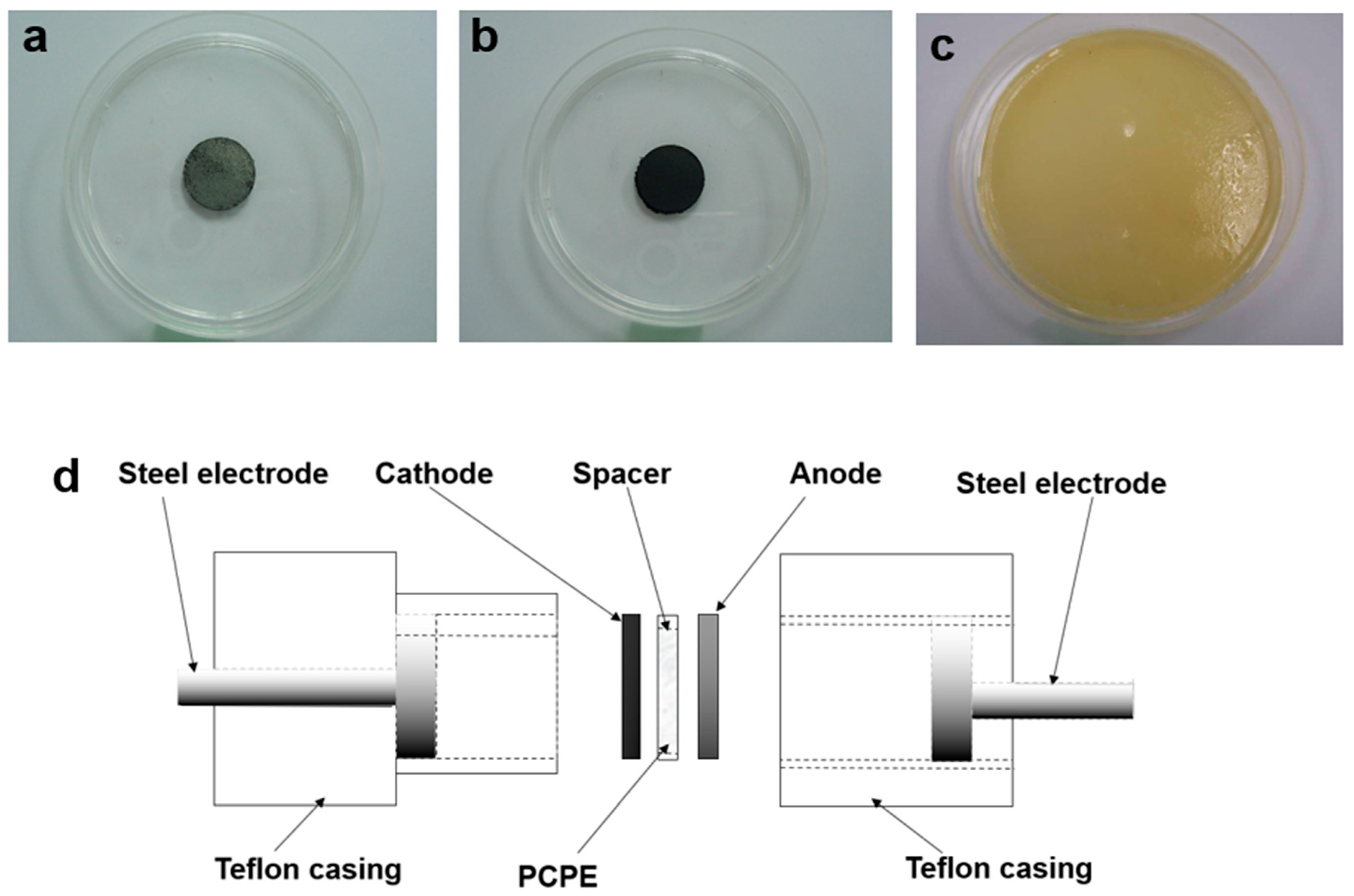
| Characterization | Materials | Scan Rate (mVs−1) | Range (V) | Highlights | Ref. |
|---|---|---|---|---|---|
| LSV |
| 1 | 0–5 |
| [98] |
| 10 | 0–3 |
| [126] | |
| 10 | 0–2.5 |
| [82] | |
| CV | Cell 1: SS|poly(vinylidenefluoride-co-hexafluoropropylene) + poly(vinylpyrrolidone) + 1-butyl-3-methylimidazolium hydrogen sulfate, immobilized|SS and Cell 2: Zn + ZnSO4·7H2O|poly(vinylidenefluoride-co-hexafluoropropylene) + poly(vinylpyrrolidone) + 1-butyl-3-methylimidazolium hydrogen sulfate, immobilized|Zn + ZnSO4·7H2O | 5 |
| [128] | |
| Cell A: SS|methylcellulose/potato starch ammonium nitrate|SS Cell B: Zn + ZnSO4·7H2O|methylcellulose/potato starch ammonium nitrate|Zn + ZnSO4·7H2O |
| [129] | |||
| OCV—Initial voltage of primary/secondary battery |
|
| |||
| [80] | ||||
| |||||
|
| [28] | |||
| Discharge Profile—Primary battery |
|
| [25] | ||
|
| [79] | |||
|
| [137] | |||
|
| [99] | |||
| Charge–Discharge Profile—Secondary battery |
|
| [31] | ||
|
| [30] | |||
|
| [128] | |||
|
| [125] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rani, M.S.A.; Norrrahim, M.N.F.; Knight, V.F.; Nurazzi, N.M.; Abdan, K.; Lee, S.H. A Review of Solid-State Proton–Polymer Batteries: Materials and Characterizations. Polymers 2023, 15, 4032. https://doi.org/10.3390/polym15194032
Rani MSA, Norrrahim MNF, Knight VF, Nurazzi NM, Abdan K, Lee SH. A Review of Solid-State Proton–Polymer Batteries: Materials and Characterizations. Polymers. 2023; 15(19):4032. https://doi.org/10.3390/polym15194032
Chicago/Turabian StyleRani, M. S. A., M. N. F. Norrrahim, V. F. Knight, N. M. Nurazzi, K. Abdan, and S. H. Lee. 2023. "A Review of Solid-State Proton–Polymer Batteries: Materials and Characterizations" Polymers 15, no. 19: 4032. https://doi.org/10.3390/polym15194032
APA StyleRani, M. S. A., Norrrahim, M. N. F., Knight, V. F., Nurazzi, N. M., Abdan, K., & Lee, S. H. (2023). A Review of Solid-State Proton–Polymer Batteries: Materials and Characterizations. Polymers, 15(19), 4032. https://doi.org/10.3390/polym15194032









