1. Introduction
Implementing biodegradable and renewable natural biopolymers can reduce the harmful effects and environmental pollution caused by non-biodegradable plastics [
1,
2]. Biodegradable polymers can be an alternative to achieving the sustainability of the plastics industry and serve as a possible solution for overcrowded landfills [
3]. The considerable amount of nondegradable polymers used in packaging has led to the emergence of biodegradable plastics, mainly for food packaging and bioplastic industries [
4]. Synthetic polymers applied in food packaging are gradually being replaced by biopolymers, such as polysaccharides (starches [
5], cellulose [
6], pectins [
7], chitosan [
8], sodium alginate [
9]), proteins (casein [
10], gelatin [
11], collagen [
12]), and polymers obtained by microbial production, e.g., polyhydroxyalkanoates (PHA) [
13].
Among these biopolymers, sodium alginate is readily available and has widespread applications. Sodium alginate, mainly extracted from the cell walls and intercellular spaces of marine brown algae, is a linear chain polysaccharide that contains two structural units of 1–4 linked α-L-guluronic acid and β-D-mannuronic acid [
14,
15]. This natural polymer is characterized by film-forming properties, resistance to oxygen permeability, and biocompatibility, making it an excellent component for producing functional packaging materials and in the medical field [
16,
17,
18,
19]. Alginates are used as an emulsifier, stabilizer, thickening agent, and flavor adjuvant. This biopolymer also exhibits properties such as a high capacity to incorporate and release substances, cell affinity, and strong bioadhesion. As a result, it is an ideal polymer for producing hydrogels, scaffolds, tablets, nanoparticles, liposomes, and microparticles [
20]. Nevertheless, alginates display poor mechanical strength and loss of structural integrity, which limits their applications [
20].
The film-forming ability of alginate was used to prepare films functionalized by adding essential oils with various properties. Essential oils are natural aromatic volatile liquids extracted from, i.e., flowers, buds, leaves, bark, roots, fruits, peels, seeds, and resin of plants [
21,
22]. They are secondary metabolites of plants. There are several extraction methods, but steam distillation is the most popular technique for producing essential oils [
23].
Among various essential oils, tansy (
Tanacetum vulgare) essential oil (TO) is extracted from flower baskets or leaves of tansy, and its main components are 1,8-cineole, β -thujone, α-thujone,
cis-chrysanthenol, borneol, myrtenol, camphor,
trans-chrysanthenyl acetate, artemisia ketone, (E)-dihydroxycarvone,
trans-chrysanthenol, bornyl acetate, camphene, sabinene, and carvone [
24,
25,
26,
27,
28,
29,
30,
31,
32]. Tansy is a perennial plant belonging to the Asteraceae family. It grows natively in Europe and Asia in a moderate climate and in North America as introduced species that quickly adapts to new environments [
25,
26,
28,
31]. It grows wildly along roadsides, balks, banks of rivers, and wastelands [
26,
31], achieving 150 cm of height and forming yellow button-like flowers [
28,
33]. Its stems are usually branched from the bottom to the top, and its leaves are similar to fern leaves [
28]. The intraspecific diversity of this plant is enormous. The composition of tansy essential oil varies considerably depending on its geographic origins. Simultaneously, the chemotypes of this oil have been identified according to the first dominant constituent [
28]. A compound of at least 40% determines the chemotype of the plant [
34]. Tansy essential oil has interesting biological features, including anthelmintic and antibacterial properties [
26,
35]. Moreover, it has important anti-inflammatory, diuretic, and antioxidant properties [
36]. The advantages of tansy for processing bioactive components in foods and medicinal or agricultural usage have been revealed. Tansy products (essential oils and extracts) have been applied in cosmetics, perfumery, photography, and culinary [
33,
37]. As an herbal, tansy has been traditionally used in lotions, dyes, repellents against insects, preservatives, and as a remedy for migraine, neuralgia, rheumatism, and loss of appetite [
34].
To the best of our knowledge, polymer materials loaded with tansy (Tanacetum vulgare) essential oil intended for food packaging have not yet been investigated. This research aimed to prepare sodium alginate-based films with the introduced tansy essential oil and to test their physicochemical, antimicrobial, and antioxidant properties. These films were proposed to be used as food packaging; thus, they should protect food from oxidation and human pathogens to extend its shelf life. Three common human pathogens, Escherichia coli, Staphylococcus aureus, and Pseudomonas aeruginosa, were chosen for the tests.
2. Materials and Methods
2.1. Materials
Sodium alginate (ALG) was acquired from Büchi Labortechnik AG (Flawil, Switzerland), glycerol (G) and methanol (pure for analysis) were bought from Avantor Performance Materials Poland S.A. (Gliwice, Poland), tansy essential oil (TO) from Tanacetum vulgare was purchased from Herbapol w Krakowie SA (Cracow, Poland), a surfactant TWEEN 80 was bought from Greenaction (Kielce, Poland), and 2,2-diphenyl-1-picrylhydrazyl (DPPH, 95%) and 6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid (Trolox, 97%) were supplied by Sigma–Aldrich (Poznań, Poland).
2.2. Determination of Sodium Alginate Molecular Weight
Sodium alginate molecular weight was determined using the viscometric method. The biopolymer was dissolved in 0.1 M NaCl aqueous solution and placed in a Ubbelohde viscometer (type 532 10, K constant 0.01 mm2/s) immersed in water bath CT72/P (Si Analytics, Mainz, Germany) at 25 °C. The flow time of sodium alginate solutions of different concentrations was measured automatically with a Viscoclock Plus (Si Analytics, Mainz, Germany) device.
The limiting viscosity number, [η], was estimated from Huggins and Kraemer equations equaling 993 [cm
3/g]. Next, the limiting viscosity number was taken to calculate the viscosity-average molecular weight based on the Mark-Houwink-Sakurada equation,
. The viscosity-average molecular weight was 55,800 for K = 0.0178 cm
3/g and a = 1 [
38].
2.3. Preparation of Solutions and Films
Sodium alginate was dissolved in distilled water to form a 2% (w/v) aqueous alginate solution. To this solution, glycerol in the amount of 2.5% (w/v) was added. Separately, tansy essential oil was mixed with the surfactant with a volume ratio of 2:1. Subsequently, various amounts of this mixture were added to 30 cm3 portions of sodium alginate solution with glycerol. These solution portions were poured into Petri dishes to evaporate the solvent. 1%; 1.33%; 1.67%; or 2% (w/v) of tansy essential oil was added to the biopolymer solution. After water evaporation, the biopolymeric films enriched in tansy essential oil were obtained, and the content of the essential oil in these films with respect to the weight of the biopolymer was as follows: 50%, 66%, 83%, and 100%, respectively.
The film thickness was measured with a digital gauge (Sylvac GC-050, Yverdon, Switzerland) with an accuracy and resolution of 0.001 mm. The thickness values are the averages of several measurements for each sample.
2.4. Viscosity Measurements
The viscosity studies were conducted using a DV1 viscometer (Brookfield Ametek, Middleboro, MA, USA) viscometer equipped with spindle no. 5. The tests were conducted in solutions; therefore, 2% aqueous solutions of sodium alginate with glycerol and tansy essential oil of different concentrations were prepared in 100 mL beakers, to which the spindle was immersed in the determined position. The rotation speed was in the range of 1.5 to 60 rpm. The temperature of the environment where the measurements were conducted was 20 °C. The tests were performed three times for each solution.
2.5. ATR-FTIR Spectroscopy
Infrared spectra of the samples were collected with a Nicolet iS5 (Thermo Fisher Scientific, Waltham, MA, USA) spectrophotometer with ID7 ATR equipment containing ZnSe crystal, whose angle of incidence was 45°. The apparatus operating parameters were applied: 4 cm−1 resolution, 32 scans, and the range of wavenumbers 4000–550 cm−1.
2.6. The Uniaxial Tensile Tests
Tensile tests were conducted with a mechanical testing machine EZ-Test SX Texture Analyzer (Shimadzu, Kyoto, Japan). Paddle-shaped samples were cut out of the films, and then these samples were stretched until rupture with a stretching speed of 10 mm/min. The device registered a stress-strain curve from which quantities such as Young’s modulus (E), stress (σ) and strain (ε) at break were calculated. Five tests for each film type were conducted, and the average values were calculated. Trapezium X software version 1.4.5 (Shimadzu, Kyoto, Japan) was used to calculate the mentioned quantities.
2.7. Moisture Content
Moisture content (Mc, %) in the films was tested gravimetrically. The samples were dried to a constant weight in an oven at 105 °C. The obtained values were the averages of three repetitions. The following Formula (1) was used for the calculation of moisture content:
where W
0 is the sample weight before drying, and W
d the sample weight after drying.
2.8. UV-VIS Spectroscopy
The UV-VIS spectra of the solutions were performed using a UV-1601 PC spectrophotometer (Shimadzu, Kyoto, Japan).
2.9. Scanning Electron Microscopy
The images of the studied samples were performed using a scanning electron microscope (SEM), a model 1430 VP, 2001 (LEO Electron Microscopy Ltd., Cambridge, UK), with an acceleration voltage of 10 kV. Before observation, the samples were covered with about a 10 nm layer of gold and palladium. Images of the surfaces and the fractures of the samples were obtained. To get the fracture, a sample was immersed in liquid nitrogen and then mechanically broken.
2.10. Thermal Analysis
The thermal stability of the prepared samples was tested on a thermoanalyzer SDT 2960 Simultaneous DSC/TGA analyzer (TA Instruments, New Castle, DE, USA) in a nitrogen atmosphere with a heating rate of 10 °C/min to 600 °C. Thermogravimetric (TG) and derivative thermogravimetric (DTG) curves were used to determine the characteristic parameters such as Tonset (°C), the temperature of the beginning of the process; Tmax (°C), the temperature at which rate of the process was the maximum (maximum on DTG curve); Δm (%), the weight loss during the process; and a residue (%) at 600 °C after the degradation of the sample.
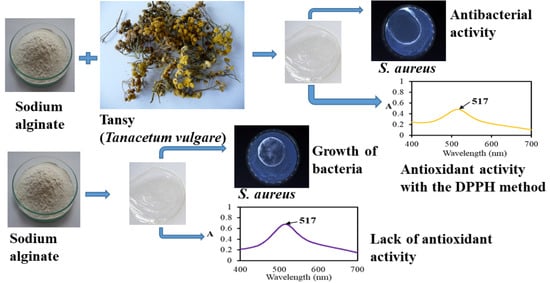
2.11. Antioxidant Capacity
The antioxidant capacity (AC) of the films was tested using DPPH (2,2-diphenyl-1-picrylhydrazyl) radical and applying the QUENCHER method [
39], which is based on the direct contact of a solid film with DPPH solution. The tested film was insoluble in the radical solution, and the reactions occurred at the sample’s surface. To determine the AC of the prepared films, 0.1 g of the film was ground in a mill and placed in a test tube to which 6 cm
3 of 60.86 µmol/dm
3 DPPH solution in methanol was added. Then, the test tube was put to a shaker (Thermoshaker, VWR International, LLC, Gdańsk, Poland) at 650 rpm for 15 min. Afterward, the test tube was kept in the dark place for 15 min, and then the UV-VIS spectrum of supernatant was registered at 517 nm using a UV-VIS spectrophotometer. The analysis was conducted in triplicate. The percentage of scavenging of the DPPH radical was calculated according to Formula (2):
where A
0 is the absorbance of DPPH solution, and A
s the absorbance of DPPH solution after contacting the studied film.
A calibration curve based on Trolox solutions showing a linear dependence of the percentage of DPPH scavenging (% DPPH) on Trolox concentration was prepared. The AC of the tested film was expressed in μmol of Trolox equivalents per 100 g of the studied film [
40].
2.12. Antibacterial Assay
The prepared films were examined in terms of antibacterial activity, which was carried out based on the standard ISO 20645: 2006 [
41]. A disk diffusion method was applied for this assessment. The following bacteria strains were used in these tests:
Escherichia coli (ATCC 8739),
Staphylococcus aureus (ATCC 6538P), and
Pseudomonas aeruginosa (ATCC 13388) (Microbiologics
®, St. Cloud, MN, USA). The agar medium was inoculated with the bacterial culture at a concentration of 1.5 × 10
8 CFU/mL (0.5 McFarland). The agar medium (AM, Oxoid Company, Napean, ON, Canada) containing the components [g/L] tryptone peptone—15, phyton peptone—5, sodium chloride—5, and agar-agar—15 was poured onto Petri dishes to form a gel. The tested and the control sample had a circle shape with a 25 ± 5 mm diameter, and they were put in the inoculated agar medium. The samples studied were incubated at 37 ± 1 °C for 20 h. After the end of the incubation time, the films were taken off and the presence or absence of zones inhibiting the growth of microorganisms was determined by ISO standard [
41]. The analyses were repeated four times.
To check the antibacterial properties of the films quantitatively, the analyses were performed using the ISO 22196:2011 standard [
42].
Specified amounts of bacterial cells (10
6) were placed on sodium alginate film—a control sample—and the biopolymeric films with tansy essential oil. After 0 h for the reference sample and 24 h for the reference and tested samples, the bacteria were retrieved from the films’ surfaces and placed in a neutralizing solution. The number of cultured cells was then determined by placing them in a plate count agar (PCA; Oxoid Company, Nepean, ON, Canada) medium, which is used to determine the total bacterial growth of a sample. The incubation of microorganisms on plates containing the medium was carried out at 35 °C for 48 h. Antibacterial activity (R) was determined according to the standard [
42].
2.13. Statistical Analysis
One-way ANOVA with Tukey’s post hoc analysis (p < 0.05) was performed to compare the results statistically. Different letters (a–e) within the same column indicate significant differences between the compared values.