Fabrication of Phytic Acid/Urea Co-Modified Bamboo Biochar and Its Application as Green Flame Retardant for Polylactic Acid Resins
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials
2.2. Sample Preparation
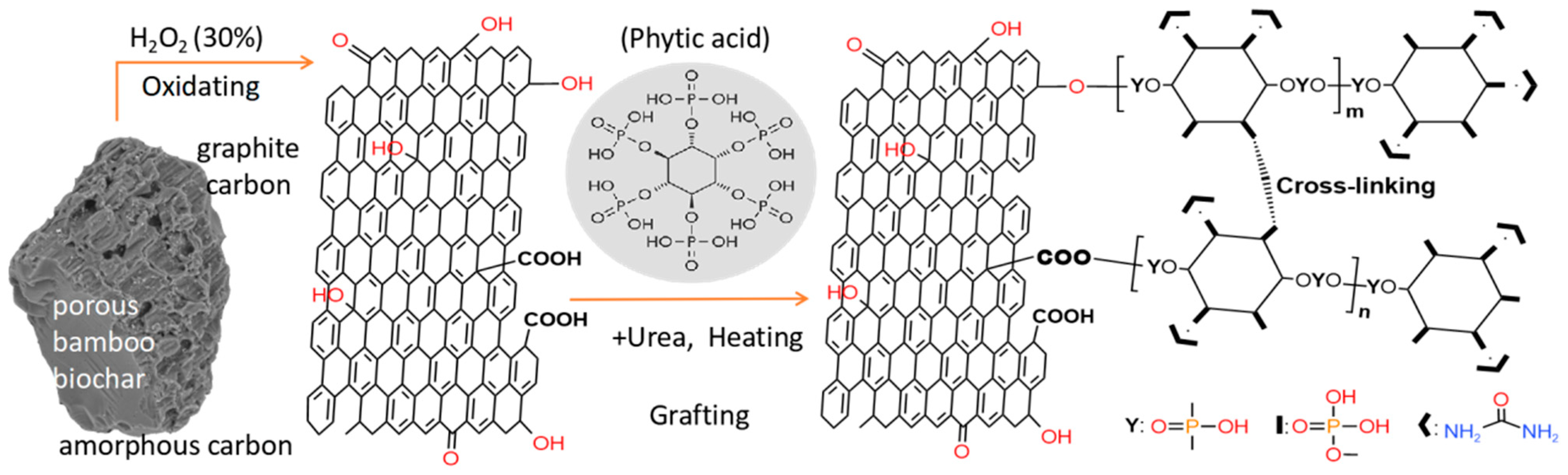
2.2.1. Synthesis of Phytic Acid/Urea Co-Modified BC (mBC)
2.2.2. Preparation of PLA Composites
2.3. Measurements and Characterizations
2.3.1. mBC and Residue Carbon Characterization
2.3.2. Mechanical Properties
2.3.3. Thermal Stability
2.3.4. Combustion Properties
3. Results and Discussion
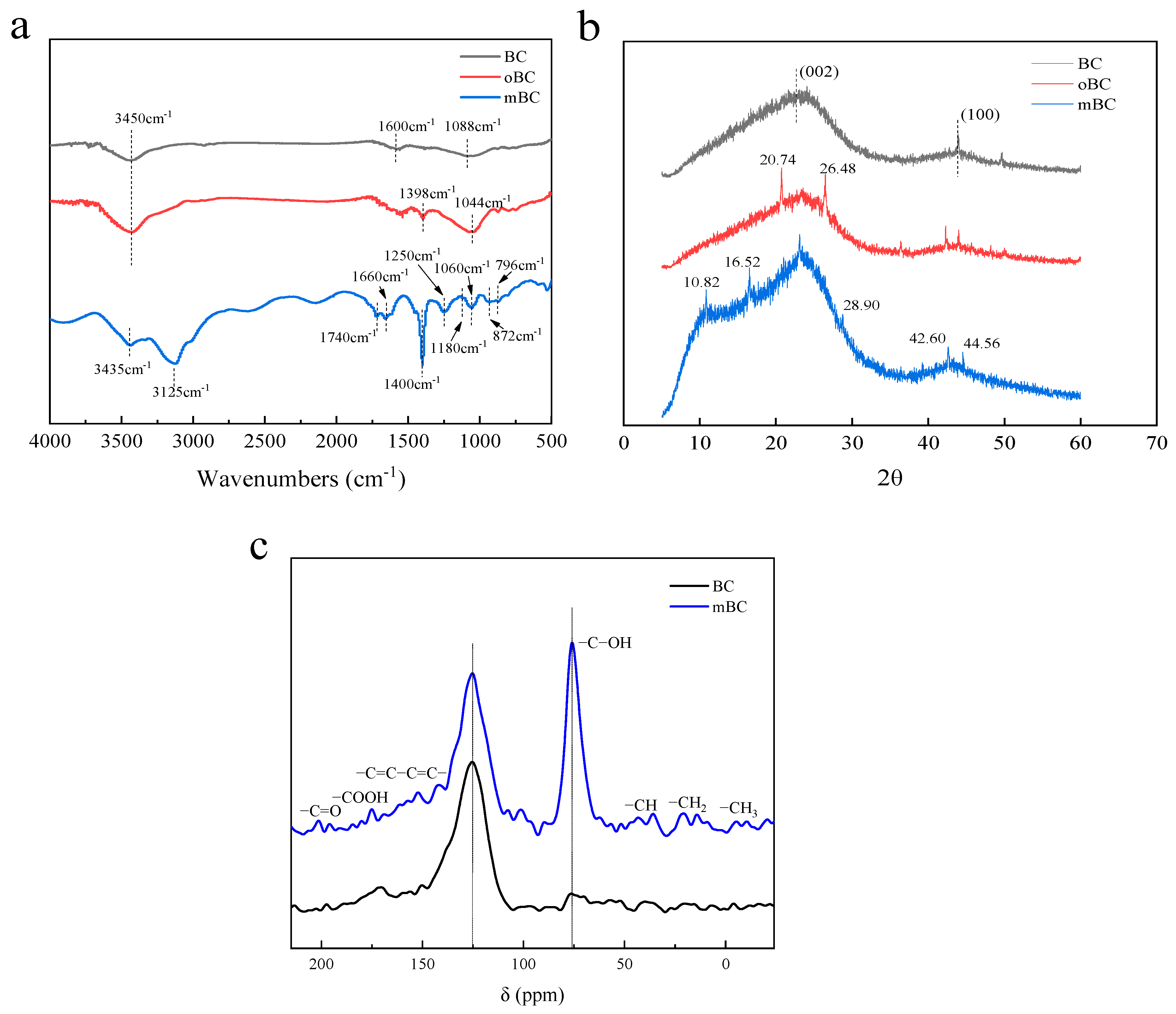
3.1. Characterization of BC, oBC and mBC
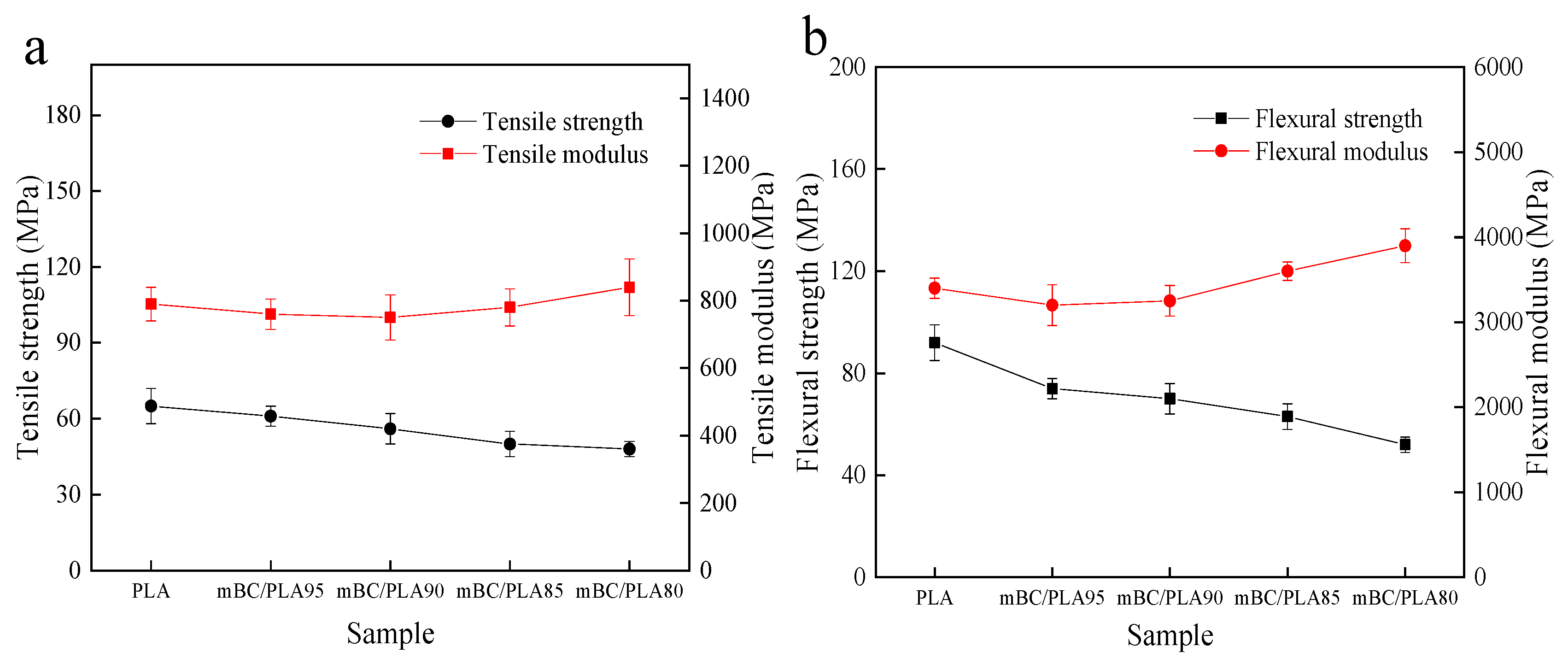
3.2. Mechanical Properties
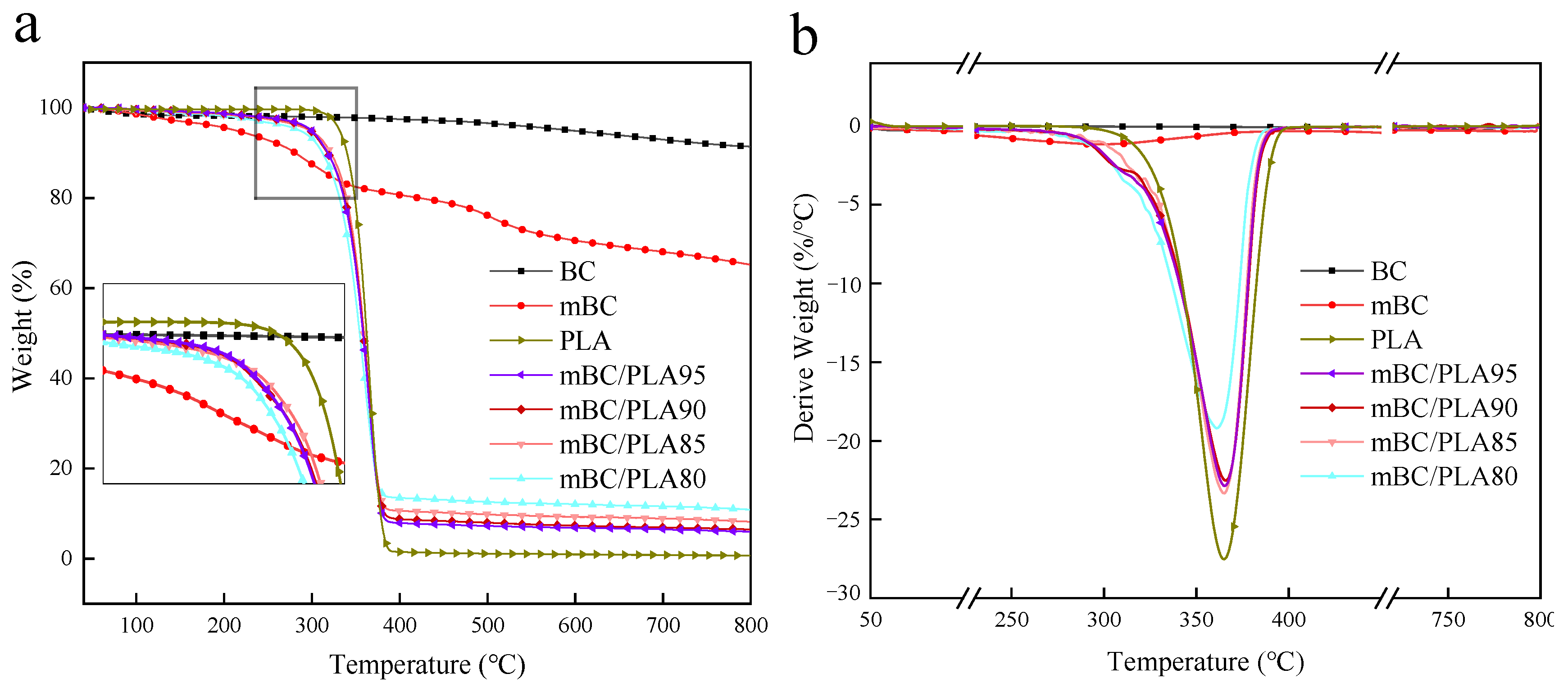
3.3. Thermal Stability
3.4. Combustion Performance
3.4.1. LOI and UL94 Measurements
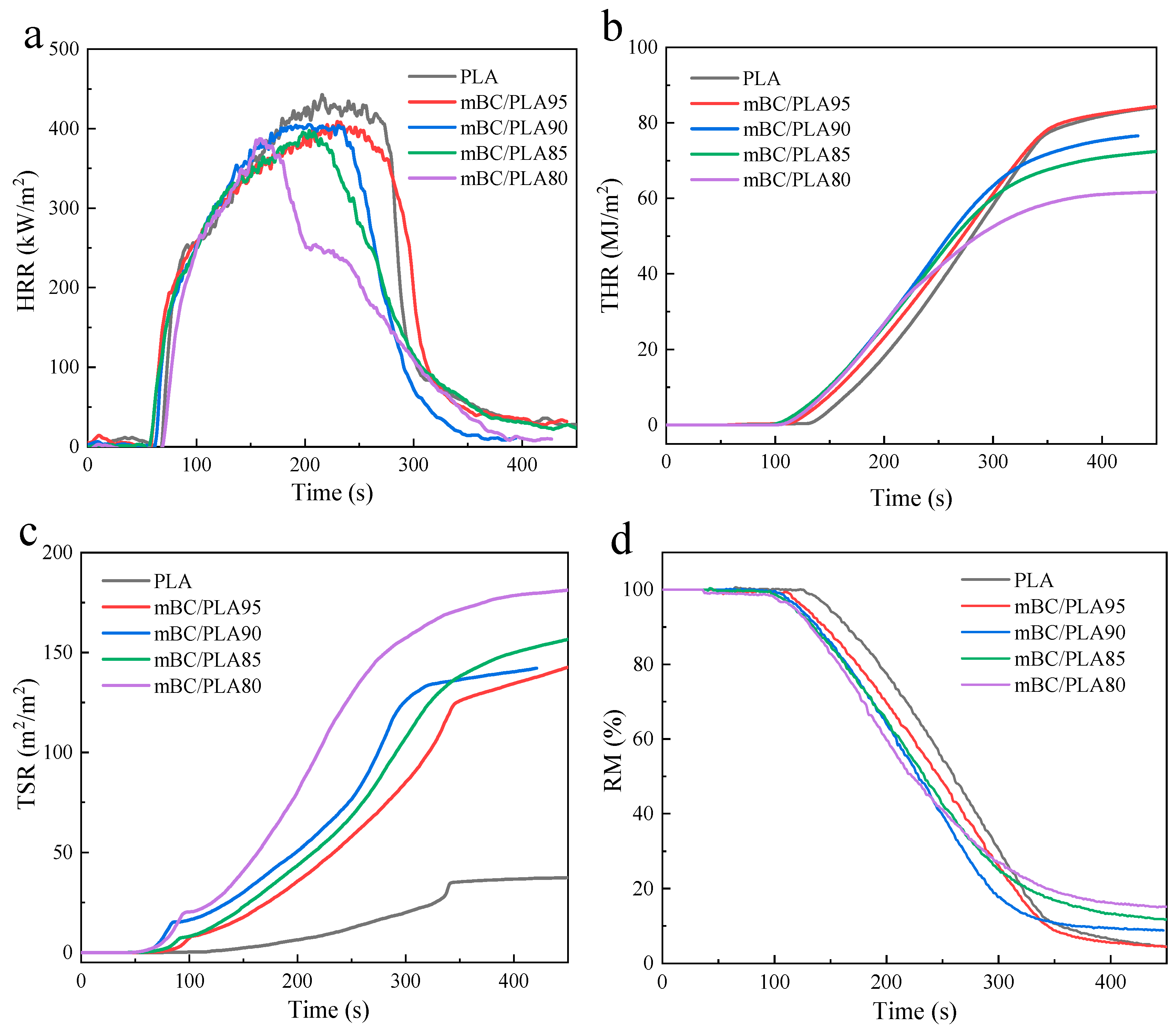
3.4.2. Cone Measurement
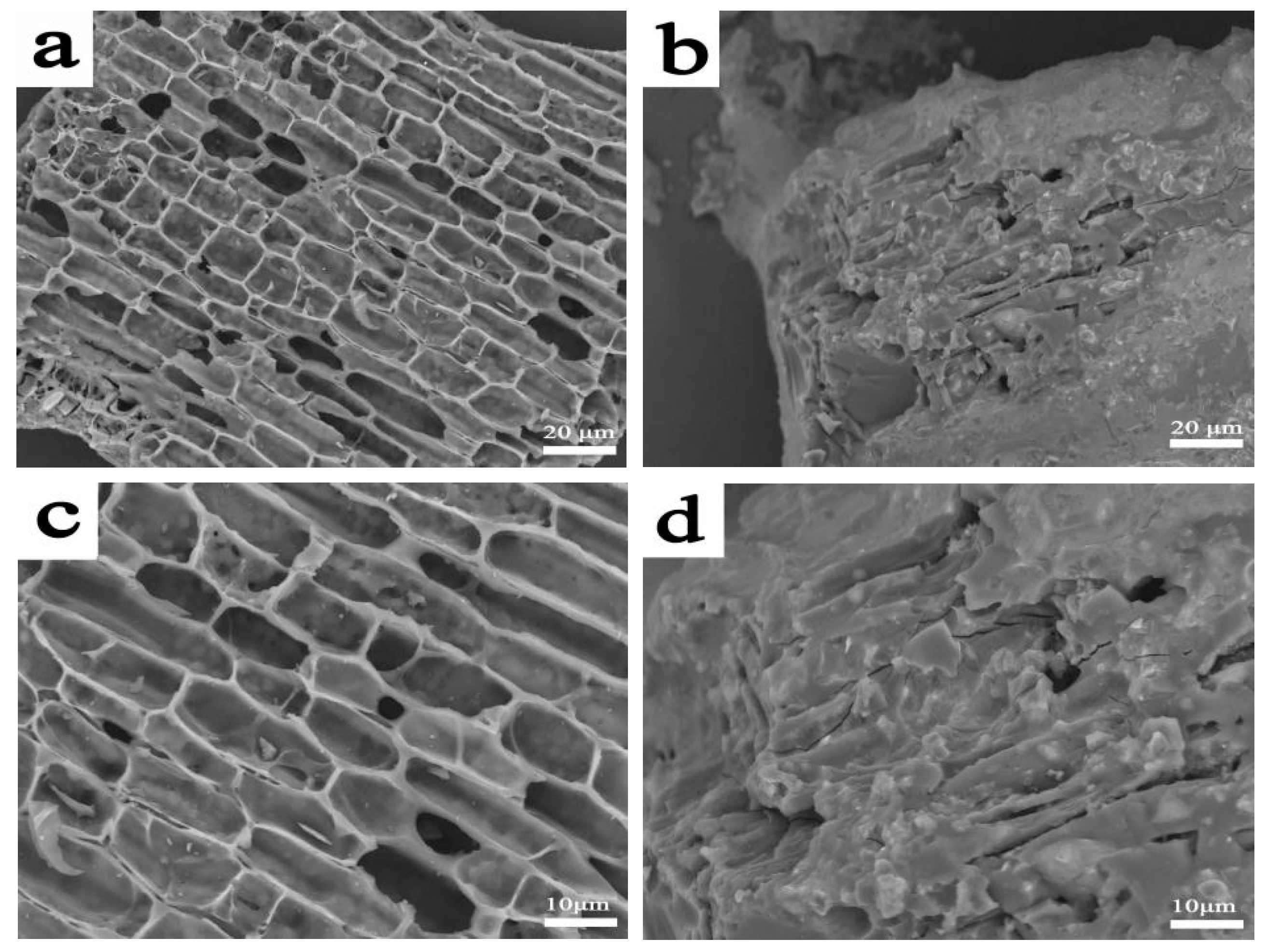
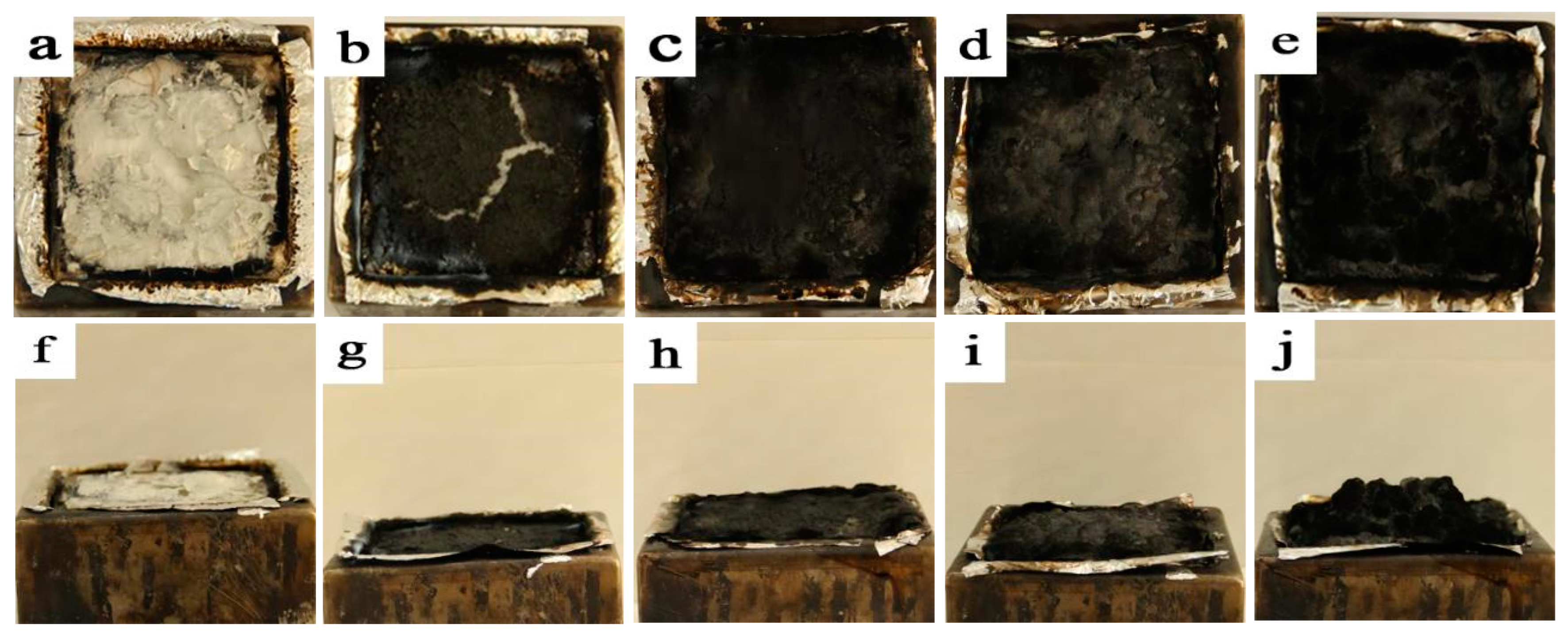
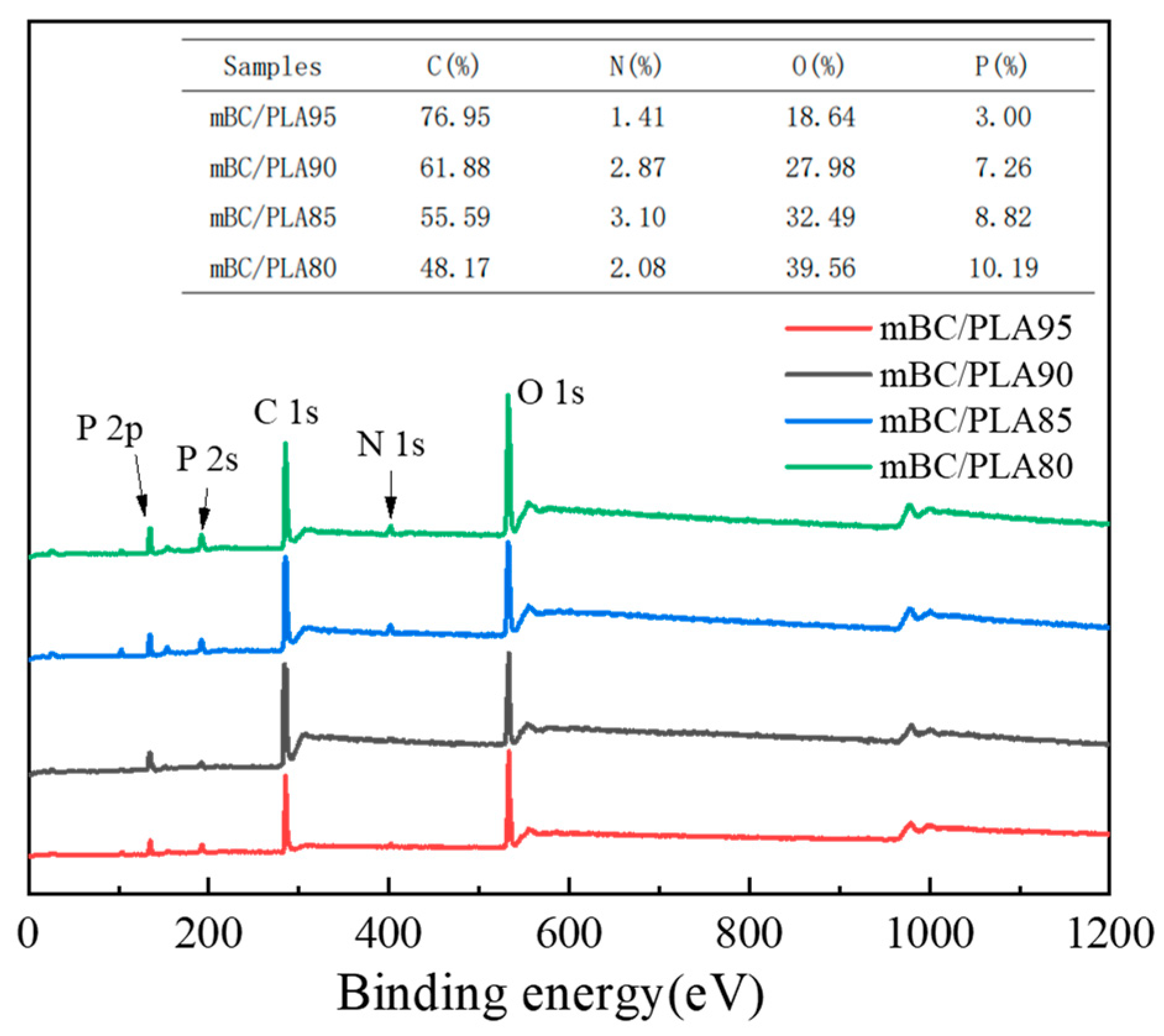
3.5. Residual Carbon Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nawalage, N.S.K.; Bellanthudawa, B.K.A. Synthetic polymers in personal care and cosmetics products (PCCPs) as a source of microplastic (MP) pollution. Mar. Pollut. Bull. 2022, 182, 113927. [Google Scholar] [CrossRef]
- Kazemi, M.; Elham, H.; Fini, E.H. State of the art in the application of functionalized waste polymers in the built environment. Resour. Conserv. Recycl. 2022, 177, 105967. [Google Scholar] [CrossRef]
- Gautam, K.; Vishvakarma, R.; Sharma, R.; Singh, A.; Gaur, V.K.; Varjani, S.; Srivastava, J.K. Production of biopolymers from food waste: Constrains and perspectives. Bioresour. Technol. 2022, 361, 127650. [Google Scholar] [CrossRef]
- Vroman, I.; Tighzert, L. Biodegradable Polymers. Materials 2009, 2, 307–344. [Google Scholar] [CrossRef] [Green Version]
- Hamad, K.; Kaseem, M.; Ayyoob, M.; Joo, J.; Deri, F. Polylactic acid blends: The future of green, light and tough. Prog. Polym. Sci. 2018, 85, 83–127. [Google Scholar] [CrossRef]
- Kukkonen, J.; Ervasti, T.; Laitinen, R. Production and characterization of glibenclamide incorporated PLA filaments for 3D printing by fused deposition modeling. J. Drug Deliv. Sci. Technol. 2022, 77, 103843. [Google Scholar] [CrossRef]
- Pisani, S.; Dorati, R.; Conti, B.; Modena, T.; Bruni, G.; Genta, I. Design of copolymer PLA-PCL electrospun matrix for biomedical applications. React. Funct. Polym. 2018, 124, 77–89. [Google Scholar] [CrossRef]
- Yusoff, N.H.; Pal, K.; Narayanan, T.; Souza, F. Recent trends on bioplastics synthesis and characterizations: Polylactic acid (PLA) incorporated with tapioca starch for packaging applications. J. Mol. Struct. 2021, 1232, 129954. [Google Scholar] [CrossRef]
- Lee, C.; Liu, C. The influence of forced-air cooling on a 3D printed PLA part manufactured by fused filament fabrication. Addit. Manuf. 2019, 25, 196–203. [Google Scholar] [CrossRef]
- Varsavas, S.D.; Kaynak, C. Weathering degradation performance of PLA and its glass fiber reinforced composite. Mater. Today Commun. 2018, 15, 344–353. [Google Scholar] [CrossRef]
- Hassouna, F.; Raquez, J.; Addiego, F.; Toniazzo, V.; Dubois, F.; Ruch, D. New development on plasticized poly(lactide): Chemical grafting of citrate on PLA by reactive extrusion. Eur. Polym. J. 2012, 48, 404–415. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, Z.; Li, Y.; Lin, H.; Li, Y.; Pan, M.; Zhong, G.; Li, Z. Tuning wettability and mechanical property of polylactide composite films with in-situ nanofibrils of poly(butylene adipate-co-terephthalate). Compos. Commun. 2020, 22, 100515. [Google Scholar] [CrossRef]
- Mohammadi, M.; Heuzey, M.; Carreau, P.J.; Taguet, A. Interfacial localization of CNCs in PLA/PBAT blends and its effect on rheological, thermal, and mechanical properties. Polymer 2021, 233, 124229. [Google Scholar] [CrossRef]
- Mahmud, M.S.; Buys, Y.F.; Anuar, H.; Sopyan, I. Miscibility, Morphology and Mechanical Properties of Compatibilized Polylactic Acid/Thermoplastic Polyurethane Blends. Mater. Today Proc. 2019, 17, 778–786. [Google Scholar] [CrossRef]
- Sharma, S.; Majumdar, A.; Butola, B.S. Tailoring the biodegradability of polylactic acid (PLA) based films and ramie- PLA green composites by using selective additives. Int. J. Biol. Macromol. 2021, 181, 1092–1103. [Google Scholar] [CrossRef]
- Ma, X.; Wu, N.; Liu, P.; Cui, H. Fabrication of highly efficient phenylphosphorylated chitosan bio-based flame retardants for flammable PLA biomaterial. Carbohydr. Polym. 2022, 287, 119317. [Google Scholar] [CrossRef]
- Zhang, M.; Ding, X.; Zhan, Y.; Wang, Y.; Wang, X. Improving the flame retardancy of poly(lactic acid) using an efficient ternary hybrid flame retardant by dual modification of graphene oxide with phenylphosphinic acid and nano MOFs. J. Hazard. Mater. 2020, 384, 121260. [Google Scholar] [CrossRef]
- Wang, X.; Sun, J.; Liu, X.; Jiang, S.; Wang, J.; Li, H.; Bourbigot, S.; Gu, X.; Zhang, S. An effective flame retardant containing hypophosphorous acid for poly (lactic acid): Fire performance, thermal stability and mechanical properties. Polym. Test. 2019, 78, 105940. [Google Scholar] [CrossRef]
- Xue, Y.; Ma, Z.; Xu, X.; Shen, M.; Huang, G.; Bourbigot, S.; Liu, X.; Song, P. Mechanically robust and flame-retardant polylactide composites based on molecularly-engineered polyphosphoramides. Compos. Part A Appl. Sci. Manuf. 2021, 144, 106317. [Google Scholar] [CrossRef]
- Liao, F.; Ju, Y.; Dai, X.; Cao, Y.; Li, J.; Wang, X. A novel efficient polymeric flame retardant for poly (lactic acid) (PLA): Synthesis and its effects on flame retardancy and crystallization of PLA. Polym. Degrad. Stab. 2015, 120, 251–261. [Google Scholar] [CrossRef]
- Tripathi, N.; Misra, M.; Mohanty, A.K. Durable Polylactic Acid (PLA)-Based Sustainable Engineered Blends and Biocomposites: Recent Developments, Challenges, and Opportunities. ACS Eng. Au 2021, 1, 7–38. [Google Scholar] [CrossRef]
- Wang, M.; Huang, Z.; Liu, G.; Kang, F. Adsorption of dimethyl sulfide from aqueous solution by a cost-effective bamboo charcoal. J. Hazard. Mater. 2011, 190, 1009–1015. [Google Scholar] [CrossRef] [PubMed]
- You, Z.; Li, D. Highly filled bamboo charcoal powder reinforced ultra-high molecular weight polyethylene. Mater. Lett. 2014, 122, 121–124. [Google Scholar] [CrossRef]
- Ho, M.; Lau, K.; Wang, H.; Hui, D. Improvement on the properties of polylactic acid (PLA) using bamboo charcoal particles. Compos. Part B Eng. 2015, 81, 14–25. [Google Scholar] [CrossRef]
- Zou, D.; Zheng, X.; Ye, Y.; Yan, D.; Xu, H.; Si, S.; Li, X. Effect of different amounts of bamboo charcoal on properties of biodegradable bamboo charcoal/polylactic acid composites. Macromolecules 2022, 216, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Sheng, K.; Zhang, S.; Qian, S.; Lopez, C. High-toughness PLA/Bamboo cellulose nanowhiskers bionanocomposite strengthened with silylated ultrafine bamboo-char. Compos. Part B Eng. 2019, 165, 174–182. [Google Scholar] [CrossRef]
- Kumar, R.; Gunjal, J.; Chauhan, S. Effect of carbonization temperature on properties of natural fiber and charcoal filled hybrid polymer composite. Compos. Part B Eng. 2021, 217, 108846. [Google Scholar] [CrossRef]
- Li, S.; Li, X.; Chen, C.; Wang, H.; Deng, Q.; Gong, M.; Li, D. Development of electrically conductive nano bamboo charcoal/ultra-high molecular weight polyethylene composites with a segregated network. Compos. Sci. Technol. 2016, 132, 31–37. [Google Scholar] [CrossRef]
- Zhao, M.; Hu, L.; Dai, L.; Wang, Z.; He, J.; Wang, Z.; Chen, J.; Hrynsphan, D.; Tatsiana, S. Bamboo charcoal powder-based polyurethane as packing material in biotrickling filter for simultaneous removal of n-hexane and dichloromethane. Bioresour. Technol. 2022, 345, 126427. [Google Scholar] [CrossRef]
- Narakaew, S.; Thungprasert, S.; Janprommin, S.; Chaisena, A. Silver-nanowire/bamboo-charcoal composite percolation network on nylon sheet for improved PM2.5 capture efficiency. Appl. Surf. Sci. 2022, 596, 153666. [Google Scholar] [CrossRef]
- Gezahegn, S.; Garcia, C.; Lai, R.; Zhou, X.; Tjong, J.; Thomas, S.C.; Huang, F.; Jaffer, S.; Yang, W.; Sain, M. Benign species-tuned biomass carbonization to nano-layered graphite for EMI filtering and greener energy storage functions. Renew. Energy 2021, 164, 1039–1051. [Google Scholar] [CrossRef]
- Cui, X.; Wu, Q.; Sun, J.; Gu, X.; Li, H.; Zhang, S. Preparation of 4-formylphenylboronic modified chitosan and its effects on the flame retardancy of poly(lactic acid). Polym. Degrad. Stab. 2022, 202, 110037. [Google Scholar] [CrossRef]
- Shi, X.; Peng, X.; Zhu, J.; Lin, G.; Kuang, T. Synthesis of DOPO-HQ-functionalized graphene oxide as a novel and efficient flame retardant and its application on polylactic acid: Thermal property, flame retardancy, and mechanical performance. J. Colloid Interface Sci. 2018, 524, 267–278. [Google Scholar] [CrossRef]
- Suparanon, T.; Phetwarotai, W. Fire-extinguishing characteristics and flame retardant mechanism of polylactide foams: Influence of tricresyl phosphate combined with natural flame retardant. Int. J. Biol. Macromol. 2020, 158, 1090–1101. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Xiang, H.; Zhou, J.; Zhu, M. Enhanced flame-retardant performance of poly (lactic acid) (PLA) composite by using intrinsically phosphorus-containing PLA. Prog. Nat. Sci. Mater. Int. 2018, 28, 590–597. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, L.; Semple, K.; Zhang, M.; Zhang, W.; Dai, C. Development of Biodegradable Flame-Retardant Bamboo Charcoal Composites, Part I: Thermal and Elemental Analyses. Polymers 2020, 12, 2217. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, L.; Semple, K.; Zhang, M.; Zhang, W.; Dai, C. Development of Biodegradable Flame-Retardant Bamboo Charcoal Composites, Part II: Thermal Degradation, Gas Phase, and Elemental Analyses. Polymers 2020, 12, 2238. [Google Scholar] [CrossRef]
- Xu, J.; Fei, J.; Tang, T.; Ma, M.; Shi, Y.; He, H.; Chen, S.; Wang, X. Achieving high flame retardancy, crystallization and biodegradability PLA based on 1 wt% addition of novel fully bio-based flame retardant. Polymer 2022, 258, 125263. [Google Scholar] [CrossRef]
- Fang, Q.; Zhan, Y.; Chen, X.; Wu, R.; Zhang, W.; Wang, Y.; Wu, X.; He, Y.; Zhou, J.; Yuan, B. A bio-based intumescent flame retardant with biomolecules functionalized ammonium polyphosphate enables polylactic acid with excellent flame retardancy. Eur. Polym. J. 2022, 177, 111479. [Google Scholar] [CrossRef]
- Zhang, L.; Chai, W.; Li, W.; Semple, K.; Yin, N.; Zhang, W.; Dai, C. Intumescent-Grafted Bamboo Charcoal: A Natural Nontoxic Fire-Retardant Filler for Polylactic Acid (PLA) Composites. ACS Omega 2021, 6, 26990–27006. [Google Scholar] [CrossRef]
- Kong, F.; He, Q.; Peng, W.; Nie, S.; Dong, X.; Yang, J. Eco-friendly flame retardant poly(lactic acid) composites based on banana peel powders and phytic acid: Flame retardancy and thermal property. J. Polym. Res. 2020, 27, 204. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Q.; Cheng, B.; Ren, Y.; Zhang, Y.; Ding, C. Durable flame retardant cellulosic fibers modified with novel, facile and efficient phytic acid-based finishing agent. Cellulose 2018, 25, 799–811. [Google Scholar] [CrossRef]
- Bansal, K.; Swarup, S.; Quadir, M. Halogen free organic coatings for flame retarding applications using phytic acid conjugated UV-curable resin. Prog. Org. Coat. 2022, 172, 107093. [Google Scholar] [CrossRef]
- Yu, S.; Xia, Z.; Kiratitanavit, W.; Kulkarni, S.; Kumar, J.; Mosurkal, R.; Nagarajan, R. Facile microwave assisted flame retardant treatment for cotton fabric using a biobased industrial byproduct: Phytic acid. Cellulose 2021, 28, 10655–10674. [Google Scholar] [CrossRef]
- Yan, W.; Xu, S.; Ding, C.; Liu, Z.; Hu, Y.; Fan, Q.; Li, J.; Tian, X.; Zeng, H. Novel bio-derived phytic acid and melamine interlayered/surface dual modified layered double hydroxide by one-pot method and its highly efficient flame retardant performance for polypropylene. Appl. Clay Sci. 2022, 228, 106620. [Google Scholar] [CrossRef]


| Sample | Ingredients (wt%) | ||
|---|---|---|---|
| PLA | mBC | Lubricant | |
| Virgin PLA | 100 | 0 | 0.5–1 |
| mBC/PLA95 | 95 | 5 | 0.5–1 |
| mBC/PLA90 | 90 | 10 | 0.5–1 |
| mBC/PLA85 | 85 | 15 | 0.5–1 |
| mBC/PLA80 | 80 | 20 | 0.5–1 |
| Sample | Tensile Strength (MPa) | Tensile Modulus (MPa) | Flexural Strength (MPa) | Flexural Modulus (GPa) |
|---|---|---|---|---|
| Virgin PLA | 65.25 ± 7.21 | 790.65 ± 50.27 | 92.52 ± 7.21 | 3.40 ± 0.12 |
| mBC/PLA95 | 61.54 ± 4.52 | 761.54 ± 45.11 | 74.13 ± 4.12 | 3.20 ± 0.24 |
| mBC/PLA90 | 56.22 ± 6.10 | 750.84 ± 67.89 | 70.20 ± 6.32 | 3.25 ± 0.18 |
| mBC/PLA85 | 50.35 ± 7.21 | 779.61 ± 55.74 | 63.19 ± 5.25 | 3.61 ± 0.11 |
| mBC/PLA80 | 48.01 ± 3.02 | 840.66 ± 84.75 | 52.45 ± 3.02 | 3.90 ± 0.20 |
| Sample | T5% (°C) | Tmax (°C) | Carbon Residue Rate (wt%) | ||
|---|---|---|---|---|---|
| 400 (°C) | 600 (°C) | 800 (°C) | |||
| BC | 593 | 365 | 97.46 | 94.87 | 91.40 |
| mBC | 207 | 298 | 80.65 | 70.35 | 65.21 |
| PLA | 331 | 366 | 2.28 | 1.63 | 1.35 |
| mBC/PLA95 | 299 | 337 | 7.84 | 6.82 | 5.98 |
| mBC/PLA90 | 291 | 337 | 8.70 | 7.30 | 6.47 |
| mBC/PLA85 | 296 | 338 | 10.60 | 9.25 | 8.22 |
| mBC/PLA80 | 280 | 329 | 13.42 | 12.06 | 10.88 |
| Samples | LOI (%) | UL-94 Test | |
|---|---|---|---|
| Dripping | Rating | ||
| Virgin PLA | 20.3 | Yes | NR |
| BC/PLA90 * | 21.2 | Yes | NR |
| BC/PLA80 * | 21.8 | Yes | NR |
| BC-m/PLA90 * | 28.0 | Yes | V-2 |
| BC-m/PLA80 * | 29.2 | No | V-0 |
| mBC/PLA95 | 24.7 | Yes | V-2 |
| mBC/PLA90 | 28.3 | No | V-0 |
| mBC/PLA85 | 29.9 | No | V-0 |
| mBC/PLA80 | 31.2 | No | V-0 |
| Sample | TTI (s) | THR (MJ/m2) | pHRR (kW/m2) | pMLR (g/s) | TSR (m2/m2) | RM (%) |
|---|---|---|---|---|---|---|
| Virgin PLA | 63 | 86.09 | 447.26 | 0.72 | 36.84 | 2.98 |
| mBC/PLA95 | 51 | 85.61 | 411.77 | 0.59 | 149.40 | 3.77 |
| mBC/PLA90 | 43 | 76.58 | 425.96 | 0.76 | 142.19 | 8.82 |
| mBC/PLA85 | 40 | 74.16 | 402.52 | 0.58 | 165.64 | 10.23 |
| mBC/PLA80 | 38 | 61.77 | 389.56 | 0.56 | 181.56 | 14.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, J.; Wang, E.; Sun, Y.; Yin, N.; Tian, S.; Ying, W.; Li, W.; Zhang, W. Fabrication of Phytic Acid/Urea Co-Modified Bamboo Biochar and Its Application as Green Flame Retardant for Polylactic Acid Resins. Polymers 2023, 15, 360. https://doi.org/10.3390/polym15020360
Zhong J, Wang E, Sun Y, Yin N, Tian S, Ying W, Li W, Zhang W. Fabrication of Phytic Acid/Urea Co-Modified Bamboo Biochar and Its Application as Green Flame Retardant for Polylactic Acid Resins. Polymers. 2023; 15(2):360. https://doi.org/10.3390/polym15020360
Chicago/Turabian StyleZhong, Jinhuan, Enfu Wang, Yi Sun, Ningning Yin, Shuo Tian, Weijun Ying, Wenzhu Li, and Wenbiao Zhang. 2023. "Fabrication of Phytic Acid/Urea Co-Modified Bamboo Biochar and Its Application as Green Flame Retardant for Polylactic Acid Resins" Polymers 15, no. 2: 360. https://doi.org/10.3390/polym15020360
APA StyleZhong, J., Wang, E., Sun, Y., Yin, N., Tian, S., Ying, W., Li, W., & Zhang, W. (2023). Fabrication of Phytic Acid/Urea Co-Modified Bamboo Biochar and Its Application as Green Flame Retardant for Polylactic Acid Resins. Polymers, 15(2), 360. https://doi.org/10.3390/polym15020360





