Recent Progress in Stimuli-Responsive Antimicrobial Electrospun Nanofibers
Abstract
:1. Introduction
2. Design of Smart Electrospun Nanofibers with Antimicrobial Properties
3. Stimuli-Responsive Electrospun Nanofibers
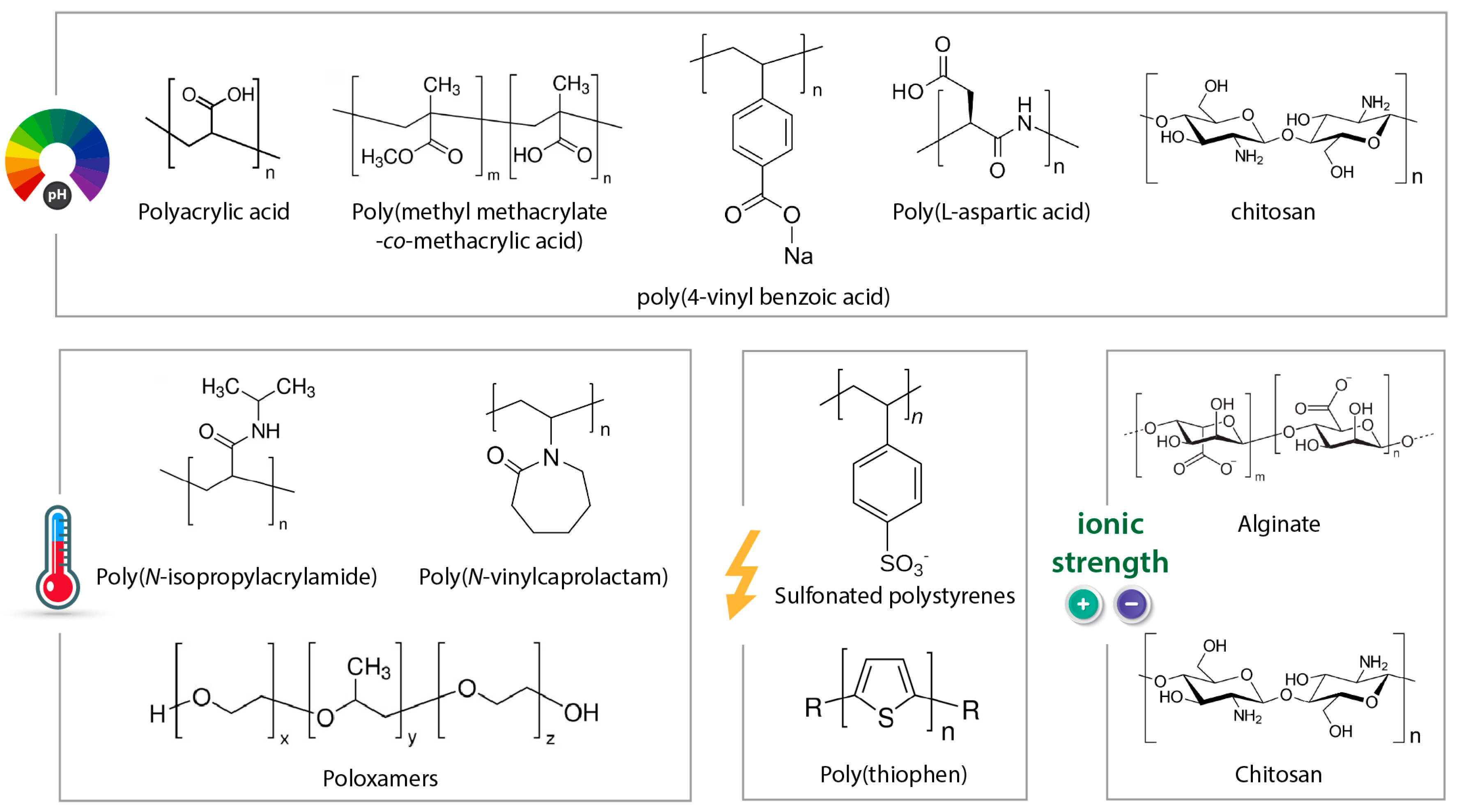
3.1. pH-Responsive Fibers
3.2. Thermo-Responsive Fibers
3.3. Light-Responsive Fibers
3.4. Other Types of Stimuli

3.5. Stimuli-Responsive Antimicrobial Systems Based on Combined Approaches
4. Final Remarks and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Patel, J.; Harant, A.; Fernandes, G.; Mwamelo, A.J.; Hein, W.; Dekker, D.; Sridhar, D. Measuring the global response to antimicrobial resistance, 2020–2021: A systematic governance analysis of 114 countries. Lancet Infect. Dis. 2023, 3099, S1473–S3099. [Google Scholar] [CrossRef]
- Ikuta, K.S.; Swetschinski, L.R.; Robles Aguilar, G.; Sharara, F.; Mestrovic, T.; Gray, A.P.; Davis Weaver, N.; Wool, E.E.; Han, C.; Gershberg Hayoon, A.; et al. Global mortality associated with 33 bacterial pathogens in 2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2022, 400, 2221–2248. [Google Scholar] [CrossRef]
- Tan, C.; Han, F.; Zhang, S.; Li, P.; Shang, N. Novel bio-based materials and applications in antimicrobial food packaging: Recent advances and future trends. Int. J. Mol. Sci. 2021, 22, 9663. [Google Scholar] [CrossRef] [PubMed]
- Edson, J.A.; Kwon, Y.J. Design, challenge, and promise of stimuli-responsive nanoantibiotics. Nano Converg. 2016, 3, 26. [Google Scholar] [CrossRef] [PubMed]
- Wyszogrodzka, G.; Marszałek, B.; Gil, B.; Dorożyński, P. Metal-organic frameworks: Mechanisms of antibacterial action and potential applications. Drug Discov. Today 2016, 21, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.Y.K.; Nang, S.C.; Chan, H.K.; Li, J. Novel antimicrobial agents for combating antibiotic-resistant bacteria. Adv. Drug Deliv. Rev. 2022, 187, 114378. [Google Scholar] [CrossRef]
- Liguori, K.; Keenum, I.; Davis, B.C.; Calarco, J.; Milligan, E.; Harwood, V.J.; Pruden, A. Antimicrobial Resistance Monitoring of Water Environments: A Framework for Standardized Methods and Quality Control. Environ. Sci. Technol. 2022, 56, 9149–9160. [Google Scholar] [CrossRef]
- Sikder, A.; Chaudhuri, A.; Mondal, S.; Singh, N.D.P. Recent Advances on Stimuli-Responsive Combination Therapy against Multidrug-Resistant Bacteria and Biofilm. ACS Appl. Bio Mater. 2021, 4, 4667–4683. [Google Scholar] [CrossRef]
- Facure, M.H.M.; Schneider, R.; dos Santos, D.M.; Correa, D.S. Impedimetric electronic tongue based on molybdenum disulfide and graphene oxide for monitoring antibiotics in liquid media. Talanta 2020, 217, 121039. [Google Scholar] [CrossRef]
- Wahid, F.; Zhong, C.; Wang, H.S.; Hu, X.H.; Chu, L.Q. Recent advances in antimicrobial hydrogels containing metal ions and metals/metal oxide nanoparticles. Polymers 2017, 9, 636. [Google Scholar] [CrossRef]
- Patel, V.C.; Williams, R. Antimicrobial resistance in chronic liver disease. Hepatol. Int. 2020, 14, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Baquero, F.; Martínez, J.L.; Cantón, R. Antibiotics and antibiotic resistance in water environments. Curr. Opin. Biotechnol. 2008, 19, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, S.; Medina-Lara, A.; Smith, R. Antimicrobial resistance and the COVID-19 pandemic. Bull. World Health Organ. 2022, 100, 295–295A. [Google Scholar] [CrossRef] [PubMed]
- Manesh, A.; Varghese, G.M. Rising antimicrobial resistance: An evolving epidemic in a pandemic. Lancet Microbe 2021, 2, e419–e420. [Google Scholar] [CrossRef] [PubMed]
- Makabenta, J.M.V.; Nabawy, A.; Li, C.-H.; Schmidt-Malan, S.; Patel, R.; Rotello, V.M. Nanomaterial-based therapeutics for antibiotic-resistant bacterial infections. Nat. Rev. Microbiol. 2021, 19, 23–36. [Google Scholar] [CrossRef]
- Pham, S.H.; Choi, Y.; Choi, J. Stimuli-Responsive Nanomaterials for Application in Antitumor Therapy and Drug Delivery. Pharmaceutics 2020, 12, 630. [Google Scholar] [CrossRef]
- Shafranek, R.T.; Millik, S.C.; Smith, P.T.; Lee, C.U.; Boydston, A.J.; Nelson, A. Stimuli-responsive materials in additive manufacturing. Prog. Polym. Sci. 2019, 93, 36–67. [Google Scholar] [CrossRef]
- Roy, D.; Cambre, J.N.; Sumerlin, B.S. Future perspectives and recent advances in stimuli-responsive materials. Prog. Polym. Sci. 2010, 35, 278–301. [Google Scholar] [CrossRef]
- Moulin, E.; Faour, L.; Carmona-Vargas, C.C.; Giuseppone, N. From Molecular Machines to Stimuli-Responsive Materials. Adv. Mater. 2020, 32, e1906036. [Google Scholar] [CrossRef]
- Chan, A.; Orme, R.P.; Fricker, R.A.; Roach, P. Remote and local control of stimuli responsive materials for therapeutic applications. Adv. Drug Deliv. Rev. 2013, 65, 497–514. [Google Scholar] [CrossRef]
- White, E.M.; Yatvin, J.; Grubbs, J.B.; Bilbrey, J.A.; Locklin, J. Advances in smart materials: Stimuli-responsive hydrogel thin films. J. Polym. Sci. Part B Polym. Phys. 2013, 51, 1084–1099. [Google Scholar] [CrossRef]
- Anandhakumar, S.; Gokul, P.; Raichur, A.M. Stimuli-responsive weak polyelectrolyte multilayer films: A thin film platform for self triggered multi-drug delivery. Mater. Sci. Eng. C 2016, 58, 622–628. [Google Scholar] [CrossRef]
- Delcea, M.; Möhwald, H.; Skirtach, A.G. Stimuli-responsive LbL capsules and nanoshells for drug delivery. Adv. Drug Deliv. Rev. 2011, 63, 730–747. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Liu, S.; Deng, W. Dual responsive linalool capsules with high loading ratio for excellent antioxidant and antibacterial efficiency. Colloids Surf. B Biointerfaces 2020, 190, 110978. [Google Scholar] [CrossRef]
- Hu, C.; Zhang, F.; Long, L.; Kong, Q.; Luo, R.; Wang, Y. Dual-responsive injectable hydrogels encapsulating drug-loaded micelles for on-demand antimicrobial activity and accelerated wound healing. J. Control. Release 2020, 324, 204–217. [Google Scholar] [CrossRef] [PubMed]
- Seuring, J.; Agarwal, S. Polymers with upper critical solution temperature in aqueous solution: Unexpected properties from known building blocks. ACS Macro Lett. 2013, 2, 597–600. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Hong, J.; Li, C.; Qiu, Y.; Li, M.; Qin, Z.; Ghiladi, R.A.; Yin, X. Electrospinning membranes with Au@carbon dots: Low toxicity and efficient antibacterial photothermal therapy. Biomater. Adv. 2022, 142, 213155. [Google Scholar] [CrossRef]
- Chen, K.; Li, Y.; Li, Y.; Tan, Y.; Liu, Y.; Pan, W.; Tan, G. Stimuli-responsive electrospun nanofibers for drug delivery, cancer therapy, wound dressing, and tissue engineering. J. Nanobiotechnol. 2023, 21, 1–15. [Google Scholar] [CrossRef]
- Kamsani, N.H.; Haris, M.S.; Pandey, M.; Taher, M.; Rullah, K. Biomedical application of responsive ‘smart’ electrospun nanofibers in drug delivery system: A minireview. Arab. J. Chem. 2021, 14, 103199. [Google Scholar] [CrossRef]
- Nadaf, A.; Gupta, A.; Hasan, N.; Fauziya, N.; Ahmad, S.; Kesharwani, P.; Ahmad, F.J. Recent update on electrospinning and electrospun nanofibers: Current trends and their applications. RSC Adv. 2022, 12, 23808–23828. [Google Scholar] [CrossRef]
- Wu, J.H.; Hu, T.G.; Wang, H.; Zong, M.H.; Wu, H.; Wen, P. Electrospinning of PLA Nanofibers: Recent Advances and Its Potential Application for Food Packaging. J. Agric. Food Chem. 2022, 70, 8207–8221. [Google Scholar] [CrossRef] [PubMed]
- Chinnappan, B.A.; Krishnaswamy, M.; Xu, H.; Hoque, M.E. Electrospinning of Biomedical Nanofibers/Nanomembranes: Effects of Process Parameters. Polymers 2022, 14, 3719. [Google Scholar] [CrossRef] [PubMed]
- Liguori, A.; Pandini, S.; Rinoldi, C.; Zaccheroni, N.; Pierini, F.; Focarete, M.L.; Gualandi, C. Thermoactive Smart Electrospun Nanofibers. Macromol. Rapid Commun. 2022, 43, 2100694. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Matsunaga, Y.T. Thermo-responsive polymers and their application as smart biomaterials. J. Mater. Chem. B 2017, 5, 4307–4321. [Google Scholar] [CrossRef] [PubMed]
- Celebioglu, A.; Saporito, A.F.; Uyar, T. Green Electrospinning of Chitosan/Pectin Nanofibrous Films by the Incorporation of Cyclodextrin/Curcumin Inclusion Complexes: pH-Responsive Release and Hydrogel Features. ACS Sustain. Chem. Eng. 2022, 10, 4758–4769. [Google Scholar] [CrossRef]
- Pan, F.; Amarjargal, A.; Altenried, S.; Liu, M.; Zuber, F.; Zeng, Z.; Rossi, R.M.; Maniura-Weber, K.; Ren, Q. Bioresponsive Hybrid Nanofibers Enable Controlled Drug Delivery through Glass Transition Switching at Physiological Temperature. ACS Appl. Bio. Mater. 2021, 4, 4271–4279. [Google Scholar] [CrossRef]
- Li, Q.; Yin, Y.; Cao, D.; Wang, Y.; Luan, P.; Sun, X.; Liang, W.; Zhu, H. Photocatalytic Rejuvenation Enabled Self-Sanitizing, Reusable, and Biodegradable Masks against COVID-19. ACS Nano 2021, 15, 11992–12005. [Google Scholar] [CrossRef]
- Haider, A.; Haider, S.; Kang, I.-K. A comprehensive review summarizing the effect of electrospinning parameters and potential applications of nanofibers in biomedical and biotechnology. Arab. J. Chem. 2018, 11, 1165–1188. [Google Scholar] [CrossRef]
- Ibrahim, H.M.; Klingner, A. A review on electrospun polymeric nanofibers: Production parameters and potential applications. Polym. Test. 2020, 90, 106647. [Google Scholar] [CrossRef]
- Mercante, L.A.; Scagion, V.P.; Migliorini, F.L.; Mattoso, L.H.C.; Correa, D.S. Electrospinning-based (bio)sensors for food and agricultural applications: A review. TrAC-Trends Anal. Chem. 2017, 91, 91–103. [Google Scholar] [CrossRef]
- Mercante, L.A.; Andre, R.S.; Facure, M.H.M.; Correa, D.S.; Mattoso, L.H.C. Recent progress in conductive electrospun materials for flexible electronics: Energy, sensing, and electromagnetic shielding applications. Chem. Eng. J. 2023, 465, 142847. [Google Scholar] [CrossRef]
- Electrospun, A.; Nanofibers, P. Antibacterial Electrospun Polycaprolactone Nanofibers. Polymers 2022, 14, 746. [Google Scholar]
- Parham, S.; Kharazi, A.Z.; Bakhsheshi-Rad, H.R.; Ghayour, H.; Ismail, A.F.; Nur, H.; Berto, F. Electrospun Nano-fibers for biomedical and tissue engineering applications: A comprehensive review. Materials 2020, 13, 2153. [Google Scholar] [CrossRef] [PubMed]
- Topuz, F.; Kilic, M.E.; Durgun, E.; Szekely, G. Fast-dissolving antibacterial nanofibers of cyclodextrin/antibiotic inclusion complexes for oral drug delivery. J. Colloid Interface Sci. 2021, 585, 184–194. [Google Scholar] [CrossRef]
- Locilento, D.A.; Mercante, L.A.; Andre, R.S.; Mattoso, L.H.C.; Luna, G.L.F.; Brassolatti, P.; Anibal, F.D.F.; Correa, D.S. Biocompatible and Biodegradable Electrospun Nanofibrous Membranes Loaded with Grape Seed Extract for Wound Dressing Application. J. Nanomater. 2019, 2019, 1–11. [Google Scholar] [CrossRef]
- Liu, M.; Duan, X.P.; Li, Y.M.; Yang, D.P.; Long, Y.Z. Electrospun nanofibers for wound healing. Mater. Sci. Eng. C 2017, 76, 1413–1423. [Google Scholar] [CrossRef]
- Parham, S.; Kharazi, A.Z.; Bakhsheshi-Rad, H.R.; Kharaziha, M.; Ismail, A.F.; Sharif, S.; Razzaghi, M.; RamaKrishna, S.; Berto, F. Antimicrobial Synthetic and Natural Polymeric Nanofibers as Wound Dressing: A Review. Adv. Eng. Mater. 2022, 24, 2101460. [Google Scholar] [CrossRef]
- Dehnad, D.; Emadzadeh, B.; Ghorani, B.; Rajabzadeh, G.; Tucker, N.; Jafari, S.M. Bioactive-loaded nanovesicles embedded within electrospun plant protein nanofibers; a double encapsulation technique. Food Hydrocoll. 2023, 141, 108683. [Google Scholar] [CrossRef]
- Adamu, B.F.; Gao, J.; Gebeyehu, E.K.; Beyene, K.A.; Tadesse, M.G.; Liyew, E.Z. Self-Responsive Electrospun Nanofibers Wound Dressings: The Future of Wound Care. Adv. Mater. Sci. Eng. 2022, 2022, 2025170. [Google Scholar] [CrossRef]
- Achilleos, M.; Krasia-Christoforou, T. Thermoresponsive Electrospun Polymer-based (Nano)fibers. Temp. Polym. 2018, 329–355. [Google Scholar] [CrossRef]
- Schoeller, J.; Itel, F.; Wuertz-Kozak, K.; Fortunato, G.; Rossi, R.M. PH-Responsive Electrospun Nanofibers and Their Applications. Polym. Rev. 2022, 62, 351–399. [Google Scholar] [CrossRef]
- Williams, L.; Hatton, F.L.; Willcock, H.; Mele, E. Electrospinning of stimuli-responsive polymers for controlled drug delivery: pH- and temperature-driven release. Biotechnol. Bioeng. 2022, 119, 1177–1188. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Yang, J.; Long, S.; Zhang, G.; Wang, X. Smart and in-situ formation electrospun fibrous membrane for the control of antimicrobial efficacy. Smart Mater. Med. 2021, 2, 87–95. [Google Scholar] [CrossRef]
- Raza, A.; Hayat, U.; Rasheed, T.; Bilal, M.; Iqbal, H.M.N. “Smart” materials-based near-infrared light-responsive drug delivery systems for cancer treatment: A review. J. Mater. Res. Technol. 2019, 8, 1497–1509. [Google Scholar] [CrossRef]
- Aparecido, M.; Cristina, K.; Bento, P.; Gaspar, L.; Toledo, D.; Davi, G.; Fernanda, C.; Almeida, B.; De Camargo, F.; Capaldi, G.; et al. Nanotechnological strategies for systemic microbial infections treatment: A review. Int. J. Pharm. 2020, 589, 119780. [Google Scholar] [CrossRef]
- Gyawali, R.; Ibrahim, S.A. Natural products as antimicrobial agents. Food Control 2014, 46, 412–429. [Google Scholar] [CrossRef]
- Macedo, J.; Sanfelice, R.; Mercante, L.; Santos, D.; Habitzreuter, F.; Campana-Filho, S.; Pavinatto, A. Atividade antimicrobiana de quitosanas e seus derivados: Influência das características estruturais. Quim. Nova 2022, 45, 690–704. [Google Scholar] [CrossRef]
- Ślosarczyk, A.; Klapiszewska, I.; Skowrońska, D.; Janczarek, M.; Jesionowski, T.; Klapiszewski, Ł. A comprehensive review of building materials modified with metal and metal oxide nanoparticles against microbial multiplication and growth. Chem. Eng. J. 2023, 466, 143276. [Google Scholar] [CrossRef]
- Nikolova, M.P.; Chavali, M.S. Metal oxide nanoparticles as biomedical materials. Biomimetics 2020, 5, 27. [Google Scholar] [CrossRef]
- Xu, X.; Wang, S.; Wu, H.; Liu, Y.; Xu, F.; Zhao, J. A multimodal antimicrobial platform based on MXene for treatment of wound infection. Colloids Surf. B Biointerfaces 2021, 207, 111979. [Google Scholar] [CrossRef]
- Mayerberger, E.A.; Street, R.M.; McDaniel, R.M.; Barsoum, M.W.; Schauer, C.L. Antibacterial properties of electrospun Ti3C2Tz (MXene)/chitosan nanofibers. RSC Adv. 2018, 8, 35386–35394. [Google Scholar] [CrossRef] [PubMed]
- Rafiq, M.; Rather, S.; Wani, T.U.; Rather, A.H.; Khan, R.S.; Khan, A.E.; Hamid, I.; Khan, H.A.; Alhomida, A.S.; Sheikh, F.A. Recent progress in MXenes incorporated into electrospun nanofibers for biomedical application: Study focusing from 2017 to 2022. Chin. Chem. Lett. 2023, 34, 108463. [Google Scholar] [CrossRef]
- Saatchi, A.; Arani, A.R.; Moghanian, A.; Mozafari, M. Cerium-doped bioactive glass-loaded chitosan/polyethylene oxide nanofiber with elevated antibacterial properties as a potential wound dressing. Ceram. Int. 2021, 47, 9447–9461. [Google Scholar] [CrossRef]
- Deliormanlı, A.M. Electrospun cerium and gallium-containing silicate based 13-93 bioactive glass fibers for biomedical applications. Ceram. Int. 2016, 42, 897–906. [Google Scholar] [CrossRef]
- Goh, Y.; Akram, M.; Alshemary, A.; Hussain, R. Antibacterial polylactic acid/chitosan nanofibers decorated with bioactive glass. Appl. Surf. Sci. 2016, 387, 1–7. [Google Scholar] [CrossRef]
- Yildiz, A.; Vatansever Bayramol, D.; Atav, R.; Ağirgan, A.Ö.; Aydin Kurç, M.; Ergünay, U.; Mayer, C.; Hadimani, R.L. Synthesis and characterization of Fe3O4@Cs@Ag nanocomposite and its use in the production of magnetic and antibacterial nanofibrous membranes. Appl. Surf. Sci. 2020, 521, 146332. [Google Scholar] [CrossRef]
- Cai, N.; Li, C.; Han, C.; Luo, X.; Shen, L.; Xue, Y.; Yu, F. Tailoring mechanical and antibacterial properties of chitosan/gelatin nanofiber membranes with Fe3O4 nanoparticles for potential wound dressing application. Appl. Surf. Sci. 2016, 369, 492–500. [Google Scholar] [CrossRef]
- Kandel, R.; Jang, S.R.; Ghimire, U.; Shrestha, S.; Shrestha, B.K.; Park, C.H.; Kim, C.S. Engineered nanostructure fibrous cell-laden biointerfaces integrating Fe3O4/SrO2-fMWCNTs induce osteogenesis and anti-bacterial effect. J. Ind. Eng. Chem. 2023, 120, 216–230. [Google Scholar] [CrossRef]
- Li, R.; Chen, T.; Pan, X. Metal–Organic-Framework-Based Materials for Antimicrobial Applications. ACS Nano 2021, 15, 3808–3848. [Google Scholar] [CrossRef]
- Quirós, J.; Boltes, K.; Aguado, S.; de Villoria, R.G.; Vilatela, J.J.; Rosal, R. Antimicrobial metal–organic frameworks incorporated into electrospun fibers. Chem. Eng. J. 2015, 262, 189–197. [Google Scholar] [CrossRef]
- Yan, L.; Gopal, A.; Kashif, S.; Hazelton, P.; Lan, M.; Zhang, W.; Chen, X. Metal organic frameworks for antibacterial applications. Chem. Eng. J. 2022, 435, 134975. [Google Scholar] [CrossRef]
- Wang, S.; Yan, F.; Ren, P.; Li, Y.; Wu, Q.; Fang, X.; Chen, F.; Wang, C. Incorporation of metal-organic frameworks into electrospun chitosan/poly (vinyl alcohol) nanofibrous membrane with enhanced antibacterial activity for wound dressing application. Int. J. Biol. Macromol. 2020, 158, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Mallakpour, S.; Azadi, E.; Hussain, C.M. The latest strategies in the fight against the COVID-19 pandemic: The role of metal and metal oxide nanoparticles. New J. Chem. 2021, 45, 6167–6179. [Google Scholar] [CrossRef]
- Zare, M.; Dziemidowicz, K.; Williams, G.R.; Ramakrishna, S. Encapsulation of pharmaceutical and nutraceutical active ingredients using electrospinning processes. Nanomaterials 2021, 11, 1968. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, W.; Wang, W.; Xiao, Y.; Chen, Y.; Wang, X. Antibacterial Electrospun Nanofibrous Materials for Wound Healing. Adv. Fiber Mater. 2023, 5, 107–129. [Google Scholar] [CrossRef]
- Khorshidi, S.; Karkhaneh, A. On-demand release of ciprofloxacin from a smart nanofiber depot with acoustic stimulus. J. Biosci. 2018, 43, 959–967. [Google Scholar] [CrossRef]
- Kai, D.; Liow, S.S.; Loh, X.J. Biodegradable polymers for electrospinning: Towards biomedical applications. Mater. Sci. Eng. C 2015, 45, 659–670. [Google Scholar] [CrossRef]
- Zhang, C.; Feng, F.; Zhang, H. Emulsion electrospinning: Fundamentals, food applications and prospects. Trends Food Sci. Technol. 2018, 80, 175–186. [Google Scholar] [CrossRef]
- Yu, D.G.; Li, X.Y.; Wang, X.; Yang, J.H.; Bligh, S.W.A.; Williams, G.R. Nanofibers Fabricated Using Triaxial Electrospinning as Zero Order Drug Delivery Systems. ACS Appl. Mater. Interfaces 2015, 7, 18891–18897. [Google Scholar] [CrossRef]
- Yarin, A.L. Coaxial electrospinning and emulsion electrospinning of core-shell fibers. Polym. Adv. Technol. 2011, 22, 310–317. [Google Scholar] [CrossRef]
- Wang, H.; Huang, Q.; Chang, H.; Xiao, J.; Cheng, Y. Stimuli-responsive dendrimers in drug delivery. Biomater. Sci. 2016, 4, 375–390. [Google Scholar] [CrossRef] [PubMed]
- Quek, J.Y.; Uroro, E.; Goswami, N.; Vasilev, K. Design principles for bacteria-responsive antimicrobial nanomaterials. Mater. Today Chem. 2022, 23, 100606. [Google Scholar] [CrossRef]
- Fleige, E.; Quadir, M.A.; Haag, R. Stimuli-responsive polymeric nanocarriers for the controlled transport of active compounds: Concepts and applications. Adv. Drug Deliv. Rev. 2012, 64, 866–884. [Google Scholar] [CrossRef] [PubMed]
- Mounesan, M.; Akbari, S.; Brycki, B.E. Extended-release essential oils from poly(acrylonitrile) electrospun mats with dendritic materials. Ind. Crops Prod. 2021, 160, 113094. [Google Scholar] [CrossRef]
- Yoon, J.; Yang, H.S.; Lee, B.S.; Yu, W.R. Recent Progress in Coaxial Electrospinning: New Parameters, Various Structures, and Wide Applications. Adv. Mater. 2018, 30, e1704765. [Google Scholar] [CrossRef]
- Lu, Y.; Huang, J.; Yu, G.; Cardenas, R.; Wei, S.; Wujcik, E.K.; Guo, Z. Coaxial electrospun fibers: Applications in drug delivery and tissue engineering. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016, 8, 654–677. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, L.; Zhu, K. Coaxial electrospinning for encapsulation and controlled release of fragile water-soluble bioactive agents. J. Control. Release 2014, 193, 296–303. [Google Scholar] [CrossRef]
- Mickova, A.; Buzgo, M.; Benada, O.; Rampichova, M.; Fisar, Z.; Filova, E.; Tesarova, M.; Lukas, D.; Amler, E. Core/shell nanofibers with embedded liposomes as a drug delivery system. Biomacromolecules 2012, 13, 952–962. [Google Scholar] [CrossRef]
- Li, D.; Yue, G.; Li, S.; Liu, J.; Li, H.; Gao, Y.; Liu, J.; Hou, L.; Liu, X.; Cui, Z.; et al. Fabrication and Applications of Multi-Fluidic Electrospinning Multi-Structure Hollow and Core–Shell Nanofibers. Engineering 2022, 13, 116–127. [Google Scholar] [CrossRef]
- Ji, W.; Sun, Y.; Yang, F.; Van Den Beucken, J.J.J.P.; Fan, M.; Chen, Z.; Jansen, J.A. Bioactive electrospun scaffolds delivering growth factors and genes for tissue engineering applications. Pharm. Res. 2011, 28, 1259–1272. [Google Scholar] [CrossRef]
- Yu, D.G.; Li, J.J.; Zhang, M.; Williams, G.R. High-quality Janus nanofibers prepared using three-fluid electrospinning. Chem. Commun. 2017, 53, 4542–4545. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, K.; Yu, D.G.; Yang, Y.; Bligh, S.W.A.; Williams, G.R. Electrospun Janus nanofibers loaded with a drug and inorganic nanoparticles as an effective antibacterial wound dressing. Mater. Sci. Eng. C 2020, 111, 110805. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lv, R.; Chen, L.; Sun, R.; Zhang, Y.; Sheng, R.; Du, T.; Li, Y.; Qi, Y. A Multifunctional Janus Electrospun Nanofiber Dressing with Biofluid Draining, Monitoring, and Antibacterial Properties for Wound Healing. ACS Appl. Mater. Interfaces 2022, 14, 12984–13000. [Google Scholar] [CrossRef]
- Wang, Z.; Li, J.; Qiao, Y.; Liu, X.; Zheng, Y.; Li, Z.; Shen, J.; Zhang, Y.; Zhu, S.; Jiang, H.; et al. Rapid Ferroelectric-Photoexcited Bacteria-Killing of Bi4Ti3O12/Ti3C2Tx Nanofiber Membranes. Adv. Fiber Mater. 2023, 5, 484–496. [Google Scholar] [CrossRef]
- Ziai, Y.; Petronella, F.; Rinoldi, C.; Nakielski, P.; Zakrzewska, A.; Kowalewski, T.A.; Augustyniak, W.; Li, X.; Calogero, A.; Sabała, I.; et al. Chameleon-inspired multifunctional plasmonic nanoplatforms for biosensing applications. NPG Asia Mater. 2022, 14, 18. [Google Scholar] [CrossRef]
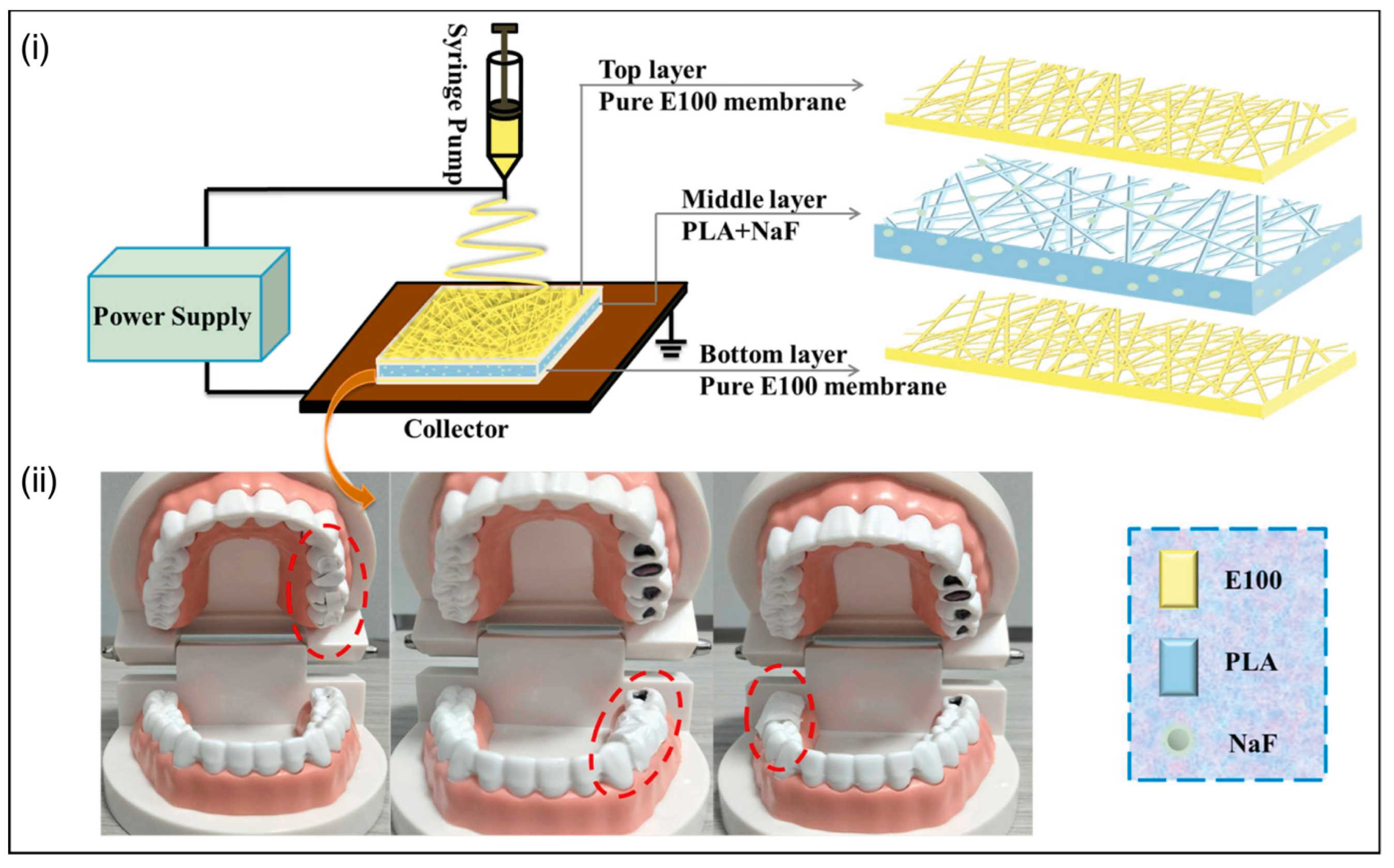
- Lang, Y.; Wang, B.; Chang, M.-W.; Sun, R.; Zhang, L. Sandwich-structured electrospun pH-responsive dental pastes for anti-caries. Colloids Surf. A Physicochem. Eng. Asp. 2023, 668, 131399. [Google Scholar] [CrossRef]
- Cao, H.; Chai, S.; Tan, Z.; Wu, H.; Mao, X.; Wei, L.; Zhou, F.; Sun, R.; Liu, C. Recent Advances in Physical Sensors Based on Electrospinning Technology. ACS Mater. Lett. 2023, 5, 1627–1648. [Google Scholar] [CrossRef]
- Wang, T.; Ke, H.; Chen, S.; Wang, J.; Yang, W.; Cao, X.; Liu, J.; Wei, Q.; Ghiladi, R.A.; Wang, Q. Porous protoporphyrin IX-embedded cellulose diacetate electrospun microfibers in antimicrobial photodynamic inactivation. Mater. Sci. Eng. C 2021, 118, 111502. [Google Scholar] [CrossRef]
- Yang, D.; Faraz, F.; Wang, J.; Radacsi, N. Combination of 3D Printing and Electrospinning Techniques for Biofabrication. Adv. Mater. Technol. 2022, 7, 2101309. [Google Scholar] [CrossRef]
- Chen, T.; Bakhshi, H.; Liu, L.; Ji, J.; Agarwal, S. Combining 3D Printing with Electrospinning for Rapid Response and Enhanced Designability of Hydrogel Actuators. Adv. Funct. Mater. 2018, 28, 1800514. [Google Scholar] [CrossRef]
- Ghosh, A.; Orasugh, J.T.; Ray, S.S.; Chattopadhyay, D. Integration of 3D Printing–Coelectrospinning: Concept Shifting in Biomedical Applications. ACS Omega 2023, 8, 28002–28025. [Google Scholar] [CrossRef] [PubMed]
- De Sio, L.; Ding, B.; Focsan, M.; Kogermann, K.; Pascoal-Faria, P.; Petronela, F.; Mitchell, G.; Zussman, E.; Pierini, F. Personalized Reusable Face Masks with Smart Nano-Assisted Destruction of Pathogens for COVID-19: A Visionary Road. Chem.-A Eur. J. 2021, 27, 6112–6130. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, D.M.; De Annunzio, S.R.; Carmello, J.C.; Pavarina, A.C.; Fontana, C.R.; Correa, D.S. Combining Coaxial Electrospinning and 3D Printing: Design of Biodegradable Bilayered Membranes with Dual Drug Delivery Capability for Periodontitis Treatment. ACS Appl. Bio Mater. 2022, 5, 146–159. [Google Scholar] [CrossRef]
- Boda, S.K.; Fischer, N.G.; Ye, Z.; Aparicio, C. Dual Oral Tissue Adhesive Nanofiber Membranes for pH-Responsive Delivery of Antimicrobial Peptides. Biomacromolecules 2020, 21, 4945–4961. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Calderon, L.; Yus, C.; Landa, G.; Mendoza, G.; Arruebo, M.; Irusta, S. Pharmacokinetic control on the release of antimicrobial drugs from pH-responsive electrospun wound dressings. Int. J. Pharm. 2022, 624, 122003. [Google Scholar] [CrossRef]
- Liao, X.; Xiang, Z.; Lei, Y.; Zhu, Z.; Guo, J.; Lin, S.; Shang, J. Facile fabrication of pH-controlled drug release mat via engineering 3D reversible gel-like nanofibers. Polymer 2022, 252, 124925. [Google Scholar] [CrossRef]
- Arafat, M.T.; Mahmud, M.M.; Wong, S.Y.; Li, X. PVA/PAA based electrospun nanofibers with pH-responsive color change using bromothymol blue and on-demand ciprofloxacin release properties. J. Drug Deliv. Sci. Technol. 2021, 61, 102297. [Google Scholar] [CrossRef]
- He, H.; Cheng, M.; Liang, Y.; Zhu, H.; Sun, Y.; Dong, D.; Wang, S. Intelligent Cellulose Nanofibers with Excellent Biocompatibility Enable Sustained Antibacterial and Drug Release via a pH-Responsive Mechanism. J. Agric. Food Chem. 2020, 68, 3518–3527. [Google Scholar] [CrossRef]
- Xu, J.; Liu, L.; Yu, J.; Zou, Y.; Wang, Z.; Fan, Y. DDA (degree of deacetylation) and pH-dependent antibacterial properties of chitin nanofibers against Escherichia coli. Cellulose 2019, 26, 2279–2290. [Google Scholar] [CrossRef]
- Huang, C.; Soenen, S.J.; van Gulck, E.; Vanham, G.; Rejman, J.; Van Calenbergh, S.; Vervaet, C.; Coenye, T.; Verstraelen, H.; Temmerman, M.; et al. Electrospun cellulose acetate phthalate fibers for semen induced anti-HIV vaginal drug delivery. Biomaterials 2012, 33, 962–969. [Google Scholar] [CrossRef]
- Hosseini-Alvand, E.; Khorasani, M.T. Fabrication of electrospun nanofibrous thermoresponsive semi-interpenetrating poly(N-isopropylacrylamide)/polyvinyl alcohol networks containing ZnO nanoparticle mats: Characterization and antibacterial and cytocompatibility evaluation. J. Mater. Chem. B 2022, 11, 890–904. [Google Scholar] [CrossRef] [PubMed]
- Elashnikov, R.; Slepička, P.; Rimpelova, S.; Ulbrich, P.; Švorčík, V.; Lyutakov, O. Temperature-responsive PLLA/PNIPAM nanofibers for switchable release. Mater. Sci. Eng. C 2017, 72, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Elsherbiny, D.A.; Abdelgawad, A.M.; Shaheen, T.I.; Abdelwahed, N.A.M.; Jockenhoevel, S.; Ghazanfari, S. Thermoresponsive nanofibers loaded with antimicrobial α-aminophosphonate-o/w emulsion supported by cellulose nanocrystals for smart wound care patches. Int. J. Biol. Macromol. 2023, 233, 123655. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Tan, P.; Luo, H.; Lan, J.; Shi, Y.; Zhang, Y.; Fan, H.; Tan, L. Study on the release behaviors of berberine hydrochloride based on sandwich nanostructure and shape memory effect. Mater. Sci. Eng. C 2020, 109, 110541. [Google Scholar] [CrossRef] [PubMed]
- Preis, E.; Anders, T.; Širc, J.; Hobzova, R.; Cocarta, A.I.; Bakowsky, U.; Jedelská, J. Biocompatible indocyanine green loaded PLA nanofibers for in situ antimicrobial photodynamic therapy. Mater. Sci. Eng. C 2020, 115, 111068. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wen, H.; Li, H.; Yan, Z.C.; Li, Y.; Wang, L.; Wang, D.; Tang, B.Z. AIEgen-loaded nanofibrous membrane as photodynamic/photothermal antimicrobial surface for sunlight-triggered bioprotection. Biomaterials 2021, 276, 121007. [Google Scholar] [CrossRef]
- Ademola Bode-Aluko, C.; Pereao, O.; Kyaw, H.H.; Al-Naamani, L.; Al-Abri, M.Z.; Tay Zar Myint, M.; Rossouw, A.; Fatoba, O.; Petrik, L.; Dobretsov, S. Photocatalytic and antifouling properties of electrospun TiO2 polyacrylonitrile composite nanofibers under visible light. Mater. Sci. Eng. B 2021, 264, 114913. [Google Scholar] [CrossRef]
- Ballesteros, C.A.S.; Correa, D.S.; Zucolotto, V. Polycaprolactone nanofiber mats decorated with photoresponsive nanogels and silver nanoparticles: Slow release for antibacterial control. Mater. Sci. Eng. C 2020, 107, 110334. [Google Scholar] [CrossRef]
- Elashnikov, R.; Lyutakov, O.; Ulbrich, P.; Svorcik, V. Light-activated polymethylmethacrylate nanofibers with antibacterial activity. Mater. Sci. Eng. C 2016, 64, 229–235. [Google Scholar] [CrossRef]
- Nie, X.; Wu, S.; Mensah, A.; Lu, K.; Wei, Q. Carbon quantum dots embedded electrospun nanofibers for efficient antibacterial photodynamic inactivation. Mater. Sci. Eng. C 2020, 108, 110377. [Google Scholar] [CrossRef]
- Patil, T.V.; Deb Dutta, S.; Patel, D.K.; Ganguly, K.; Lim, K.T. Electrospinning near infra-red light-responsive unzipped CNT/PDA nanofibrous membrane for enhanced antibacterial effect and rapid drug release. Appl. Surf. Sci. 2023, 612, 155949. [Google Scholar] [CrossRef]
- Shen, H.; Zhou, Z.; Wang, H.; Chen, J.; Zhang, M.; Han, M.; Shen, Y.; Shuai, D. Photosensitized Electrospun Nanofibrous Filters for Capturing and Killing Airborne Coronaviruses under Visible Light Irradiation. Environ. Sci. Technol. 2022, 56, 4295–4304. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, D.; Cheng, K.; Li, W.; Yu, Q.; Wang, L. Photothermal-responsive fiber dressing with enhanced antibacterial activity and cell manipulation towards promoting wound-healing. J. Colloid Interface Sci. 2022, 623, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Croitoru, A.-M.; Karaçelebi, Y.; Saatcioglu, E.; Altan, E.; Ulag, S.; Aydoğan, H.K.; Sahin, A.; Motelica, L.; Oprea, O.; Tihauan, B.-M.; et al. Electrically Triggered Drug Delivery from Novel Electrospun Poly(Lactic Acid)/Graphene Oxide/Quercetin Fibrous Scaffolds for Wound Dressing Applications. Pharmaceutics 2021, 13, 957. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, R.; Sun, X.; An, H.; Min, T.; Zhu, Z.; Wen, Y. Leaf-stomata-inspired packaging nanofibers with humidity-triggered thymol release based on thymol/EVOH coaxial electrospinning. Food Res. Int. 2022, 162, 112093. [Google Scholar] [CrossRef]
- Shi, R.; Ye, J.; Li, W.; Zhang, J.; Li, J.; Wu, C.; Xue, J.; Zhang, L. Infection-responsive electrospun nanofiber mat for antibacterial guided tissue regeneration membrane. Mater. Sci. Eng. C 2019, 100, 523–534. [Google Scholar] [CrossRef]
- Aytac, Z.; Xu, J.; Raman Pillai, S.K.; Eitzer, B.D.; Xu, T.; Vaze, N.; Ng, K.W.; White, J.C.; Chan-Park, M.B.; Luo, Y.; et al. Enzyme- and Relative Humidity-Responsive Antimicrobial Fibers for Active Food Packaging. ACS Appl. Mater. Interfaces 2021, 13, 50298–50308. [Google Scholar] [CrossRef]
- Zafar, S.; Arshad, M.S.; Rana, S.J.; Patel, M.; Yousef, B.; Ahmad, Z. Engineering of clarithromycin loaded stimulus responsive dissolving microneedle patches for the treatment of biofilms. Int. J. Pharm. 2023, 640, 123003. [Google Scholar] [CrossRef]
- Elashnikov, R.; Rimpelová, S.; Lyutakov, O.; Pavlíčková, V.S.; Khrystonko, O.; Kolská, Z.; Švorčík, V. Ciprofloxacin-Loaded Poly(N-isopropylacrylamide- co-acrylamide)/Polycaprolactone Nanofibers as Dual Thermo- and pH-Responsive Antibacterial Materials. ACS Appl. Bio Mater. 2022, 5, 1700–1709. [Google Scholar] [CrossRef]
- Abdalkarim, S.Y.H.; Yu, H.; Wang, C.; Chen, Y.; Zou, Z.; Han, L.; Yao, J.; Tam, K.C. Thermo and light-responsive phase change nanofibers with high energy storage efficiency for energy storage and thermally regulated on–off drug release devices. Chem. Eng. J. 2019, 375, 121979. [Google Scholar] [CrossRef]
- Gorji, M.; Zarbaf, D.; Mazinani, S.; Noushabadi, A.S.; Cella, M.A.; Sadeghianmaryan, A.; Ahmadi, A. Multi-responsive on-demand drug delivery PMMA-co-PDEAEMA platform based on CO2, electric potential, and pH switchable nanofibrous membranes. J. Biomater. Sci. Polym. Ed. 2023, 34, 351–371. [Google Scholar] [CrossRef] [PubMed]
- Abdella, S.; Abid, F.; Youssef, S.H.; Kim, S.; Afinjuomo, F.; Malinga, C.; Song, Y.; Garg, S. pH and its applications in targeted drug delivery. Drug Discov. Today 2023, 28, 103414. [Google Scholar] [CrossRef]
- Lambers, H.; Piessens, S.; Bloem, A.; Pronk, H.; Finkel, P. Natural skin surface pH is on average below 5, which is beneficial for its resident flora. Int. J. Cosmet. Sci. 2006, 28, 359–370. [Google Scholar] [CrossRef]
- Percival, S.L.; McCarty, S.; Hunt, J.A.; Woods, E.J. The effects of pH on wound healing, biofilms, and antimicrobial efficacy. Wound Repair Regen. 2014, 22, 174–186. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, S.; Zhang, F.; Yu, J.; Zhang, X.; Yang, G.; Liu, X. pH-sensitive biomaterials for drug delivery. Molecules 2020, 25, 5649. [Google Scholar] [CrossRef] [PubMed]
- Demirci, S.; Celebioglu, A.; Aytac, Z.; Uyar, T. PH-responsive nanofibers with controlled drug release properties. Polym. Chem. 2014, 5, 2050–2056. [Google Scholar] [CrossRef]
- Chanmugam, A.; Langemo, D.; Thomason, K.; Haan, J.; Altenburger, E.A.; Tippett, A.; Henderson, L.; Zortman, T.A. Relative Temperature Maximum in Wound Infection and Inflammation as Compared with a Control Subject Using Long-Wave Infrared Thermography. Adv. Ski. Wound Care 2017, 30, 406–414. [Google Scholar] [CrossRef]
- Sponchioni, M.; Capasso Palmiero, U.; Moscatelli, D. Thermo-responsive polymers: Applications of smart materials in drug delivery and tissue engineering. Mater. Sci. Eng. C 2019, 102, 589–605. [Google Scholar] [CrossRef]
- Zhu, Y.; Batchelor, R.; Lowe, A.B.; Roth, P.J. Design of Thermoresponsive Polymers with Aqueous LCST, UCST, or Both: Modification of a Reactive Poly(2-vinyl-4,4-dimethylazlactone) Scaffold. Macromolecules 2016, 49, 672–680. [Google Scholar] [CrossRef]
- Zhao, C.; Ma, Z.; Zhu, X.X. Rational design of thermoresponsive polymers in aqueous solutions: A thermodynamics map. Prog. Polym. Sci. 2019, 90, 269–291. [Google Scholar] [CrossRef]
- Gu, S.Y.; Wang, Z.M.; Li, J.B.; Ren, J. Switchable wettability of thermo-responsive biocompatible nanofibrous films created by electrospinninga. Macromol. Mater. Eng. 2010, 295, 32–36. [Google Scholar] [CrossRef]
- Chen, M.; Dong, M.; Havelund, R.; Regina, V.R.; Meyer, R.L.; Besenbacher, F.; Kingshott, P. Thermo-responsive core-sheath electrospun nanofibers from poly (N-isopropylacrylamide)/polycaprolactone blends. Chem. Mater. 2010, 22, 4214–4221. [Google Scholar] [CrossRef]
- Song, F.; Wang, X.L.; Wang, Y.Z. Fabrication of novel thermo-responsive electrospun nanofibrous mats and their application in bioseparation. Eur. Polym. J. 2011, 47, 1885–1892. [Google Scholar] [CrossRef]
- Tzeng, P.; Kuo, C.C.; Lin, S.T.; Chiu, Y.C.; Chen, W.C. New thermoresponsive luminescent electrospun nanofibers prepared from poly[2,7-(9,9-dihexylfluorene)]-block-poly(Nisopropylacrylamide)/ PMMA blends. Macromol. Chem. Phys. 2010, 211, 1408–1416. [Google Scholar] [CrossRef]
- Vanangamudi, A.; Dumée, L.F.; Des Ligneris, E.; Duke, M.; Yang, X. Thermo-responsive nanofibrous composite membranes for efficient self-cleaning of protein foulants. J. Membr. Sci. 2019, 574, 309–317. [Google Scholar] [CrossRef]
- González, E.; Frey, M.W. Synthesis, characterization and electrospinning of poly(vinyl caprolactam-co-hydroxymethyl acrylamide) to create stimuli-responsive nanofibers. Polymer 2017, 108, 154–162. [Google Scholar] [CrossRef]
- Li, G.; Fei, G.; Xia, H.; Han, J.; Zhao, Y. Spatial and temporal control of shape memory polymers and simultaneous drug release using high intensity focused ultrasound. J. Mater. Chem. 2012, 22, 7692–7696. [Google Scholar] [CrossRef]
- Chang, D.; Ma, Y.; Xu, X.; Xie, J.; Ju, S. Stimuli-Responsive Polymeric Nanoplatforms for Cancer Therapy. Front. Bioeng. Biotechnol. 2021, 9, 707319. [Google Scholar] [CrossRef]
- Municoy, S.; Álvarez Echazú, M.I.; Antezana, P.E.; Galdopórpora, J.M.; Olivetti, C.; Mebert, A.M.; Foglia, M.L.; Tuttolomondo, M.V.; Alvarez, G.S.; Hardy, J.G.; et al. Stimuli-responsive materials for tissue engineering and drug delivery. Int. J. Mol. Sci. 2020, 21, 4724. [Google Scholar] [CrossRef]
- Maliszewska, I.; Czapka, T. Electrospun Polymer Nanofibers with Antimicrobial Activity. Polymers 2022, 14, 1661. [Google Scholar] [CrossRef]
- Shi, J.; Li, J.; Wang, Y.; Zhang, C.Y. TiO2-based nanosystem for cancer therapy and antimicrobial treatment: A review. Chem. Eng. J. 2022, 431, 133714. [Google Scholar] [CrossRef]
- Delaney, L.J.; Isguven, S.; Eisenbrey, J.R.; Hickok, N.J.; Forsberg, F. Making waves: How ultrasound-targeted drug delivery is changing pharmaceutical approaches. Mater. Adv. 2022, 3, 3023–3040. [Google Scholar] [CrossRef] [PubMed]
- Roovers, S.; Segers, T.; Lajoinie, G.; Deprez, J.; Versluis, M.; De Smedt, S.C.; Lentacker, I. The Role of Ultrasound-Driven Microbubble Dynamics in Drug Delivery: From Microbubble Fundamentals to Clinical Translation. Langmuir 2019, 35, 10173–10191. [Google Scholar] [CrossRef]
- Jamburidze, A.; Huerre, A.; Baresch, D.; Poulichet, V.; De Corato, M.; Garbin, V. Nanoparticle-Coated Microbubbles for Combined Ultrasound Imaging and Drug Delivery. Langmuir 2019, 35, 10087–10096. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Chen, J.; Wu, W.; Liu, Y.; Li, H.; Xu, Z.; Zhu, Y. Flexible and breathable all-nanofiber iontronic pressure sensors with ultraviolet shielding and antibacterial performances for wearable electronics. Nano Energy 2022, 95, 107022. [Google Scholar] [CrossRef]
- Chao, M.; Di, P.; Yuan, Y.; Xu, Y.; Zhang, L.; Wan, P. Flexible breathable photothermal-therapy epidermic sensor with MXene for ultrasensitive wearable human-machine interaction. Nano Energy 2023, 108, 108201. [Google Scholar] [CrossRef]
- Wang, P.; Liu, J.; Li, Y.; Li, G.; Yu, W.; Zhang, Y.; Meng, C.; Guo, S. Recent Advances in Wearable Tactile Sensors Based on Electrospun Nanofiber Platform. Adv. Sens. Res. 2023, 2, 2200047. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, L.; Xia, C.; Gan, L. High performance flexible and antibacterial strain sensor based on silver-carbon nanotubes coated cellulose/polyurethane nanofibrous membrane: Cellulose as reinforcing polymer blend and polydopamine as compatibilizer. Int. J. Biol. Macromol. 2022, 223, 184–192. [Google Scholar] [CrossRef]
- Zhang, W.; Lin, L.; Zhang, L.; Choi, Y.; Cho, Y.; Chen, T.; Gao, J.; Yao, H.; Piao, Y. An In Situ Self-Assembly Dual Conductive Shell Nanofiber Strain Sensor with Superior Sensitivity and Antibacterial Property. Adv. Mater. Interfaces 2022, 9, 2101107. [Google Scholar] [CrossRef]
- Peng, X.; Dong, K.; Ye, C.; Jiang, Y.; Zhai, S.; Cheng, R.; Liu, D.; Gao, X.; Wang, J.; Wang, Z.L. A breathable, biodegradable, antibacterial, and self-powered electronic skin based on all-nanofiber triboelectric nanogenerators. Sci. Adv. 2020, 6, eaba9624. [Google Scholar] [CrossRef]
- Li, M.; Zou, X.; Ding, Y.; Wang, W.; Cheng, Z.; Wang, D.; Wang, Z.; Shao, Y.; Bai, J. Multifunctional sensors for respiration monitoring and antibacterial activity based on piezoelectric PVDF/BZT-0.5BCT nanoparticle composite nanofibers. Smart Mater. Struct. 2022, 31, 125002. [Google Scholar] [CrossRef]
- Ye, J.; Zhang, X.; Xie, W.; Gong, M.; Liao, M.; Meng, Q.; Xue, J.; Shi, R.; Zhang, L. An Enzyme-Responsive Prodrug with Inflammation-Triggered Therapeutic Drug Release Characteristics. Macromol. Biosci. 2020, 20, e2000116. [Google Scholar] [CrossRef]
- Woeppel, K.M.; Zheng, X.S.; Schulte, Z.M.; Rosi, N.L.; Cui, X.T. Nanoparticle Doped PEDOT for Enhanced Electrode Coatings and Drug Delivery. Adv. Healthc. Mater. 2019, 8, e1900622. [Google Scholar] [CrossRef] [PubMed]
- Kleber, C.; Lienkamp, K.; Rühe, J.; Asplund, M. Electrochemically Controlled Drug Release from a Conducting Polymer Hydrogel (PDMAAp/PEDOT) for Local Therapy and Bioelectronics. Adv. Healthc. Mater. 2019, 8, e1801488. [Google Scholar] [CrossRef] [PubMed]
- Svirskis, D.; Travas-Sejdic, J.; Rodgers, A.; Garg, S. Electrochemically controlled drug delivery based on intrinsically conducting polymers. J. Control. Release 2010, 146, 6–15. [Google Scholar] [CrossRef]
- Sengupta, A.; Das, S.; Dasgupta, S.; Sengupta, P.; Datta, P. Flexible Nanogenerator from Electrospun PVDF–Polycarbazole Nanofiber Membranes for Human Motion Energy-Harvesting Device Applications. ACS Biomater. Sci. Eng. 2021, 7, 1673–1685. [Google Scholar] [CrossRef]
- Druvari, D.; Kyriakopoulou, F.; Lainioti, G.C.; Vlamis-Gardikas, A.; Kallitsis, J.K. Humidity-Responsive Antimicrobial Membranes Based on Cross-Linked Copolymers Functionalized with Ionic Liquid Moieties. ACS Appl. Mater. Interfaces 2023, 15, 11193–11207. [Google Scholar] [CrossRef]
- Shaghaleh, H.; Hamoud, Y.A.; Xu, X.; Liu, H.; Wang, S.; Sheteiwy, M.; Dong, F.; Guo, L.; Qian, Y.; Li, P.; et al. Thermo-/pH-responsive preservative delivery based on TEMPO cellulose nanofiber/cationic copolymer hydrogel film in fruit packaging. Int. J. Biol. Macromol. 2021, 183, 1911–1924. [Google Scholar] [CrossRef]
- Chen, G.; Chen, G.; Pan, L.; Chen, D. Electrospun flexible PVDF/GO piezoelectric pressure sensor for human joint monitoring. Diam. Relat. Mater. 2022, 129, 109358. [Google Scholar] [CrossRef]
- Fu, G.; Shi, Q.; Liang, Y.; He, Y.; Xue, R.; He, S.; Chen, Y. Fluorescent markable multi-mode pressure sensors achieved by sandwich-structured electrospun P(VDF-HFP) nanocomposite films. Polymer 2022, 254, 125087. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, X.; Li, X.; Wang, H.; Sang, X.; Zhu, G.; Yeung, Y. Enhanced piezoelectric performance of PVDF/BiCl3/ZnO nanofiber-based piezoelectric nanogenerator. Eur. Polym. J. 2022, 166, 110956. [Google Scholar] [CrossRef]
- Veeramuthu, L.; Cho, C.; Venkatesan, M.; Kumar, G.R.; Hsu, H.; Zhuo, B.; Kau, L.; Chung, M.; Lee, W.; Kuo, C. Muscle fibers inspired electrospun nanostructures reinforced conductive fibers for smart wearable optoelectronics and energy generators. Nano Energy 2022, 101, 107592. [Google Scholar] [CrossRef]
- Sam, S.; Joseph, B.; Thomas, S. Exploring the antimicrobial features of biomaterials for biomedical applications. Results Eng. 2023, 17, 100979. [Google Scholar] [CrossRef]
- Caracciolo, P.C.; Abraham, G.A.; Battaglia, E.S.; Bongiovanni Abel, S. Recent Progress and Trends in the Development of Electrospun and 3D Printed Polymeric-Based Materials to Overcome Antimicrobial Resistance (AMR). Pharmaceutics 2023, 15, 1964. [Google Scholar] [CrossRef]

| Stimuli | Nanofibers Matrix | Antimicrobial Agent | Microorganism | Observations | Ref. |
|---|---|---|---|---|---|
| pH | Chi/Pect/HPγCD | Curcumin (polyphenol) | - | Nanofibers exhibited a pH-responsive release profile of curcumin in pH 5.4 and 7.4. | [35] |
| Chi/oxidized pectin | D-GL13K or IDR-1018 (peptides) | S. gordonii, S. mutans | Oxidized pectin aid in the pH-controlled delivery of cationic peptides. | [104] | |
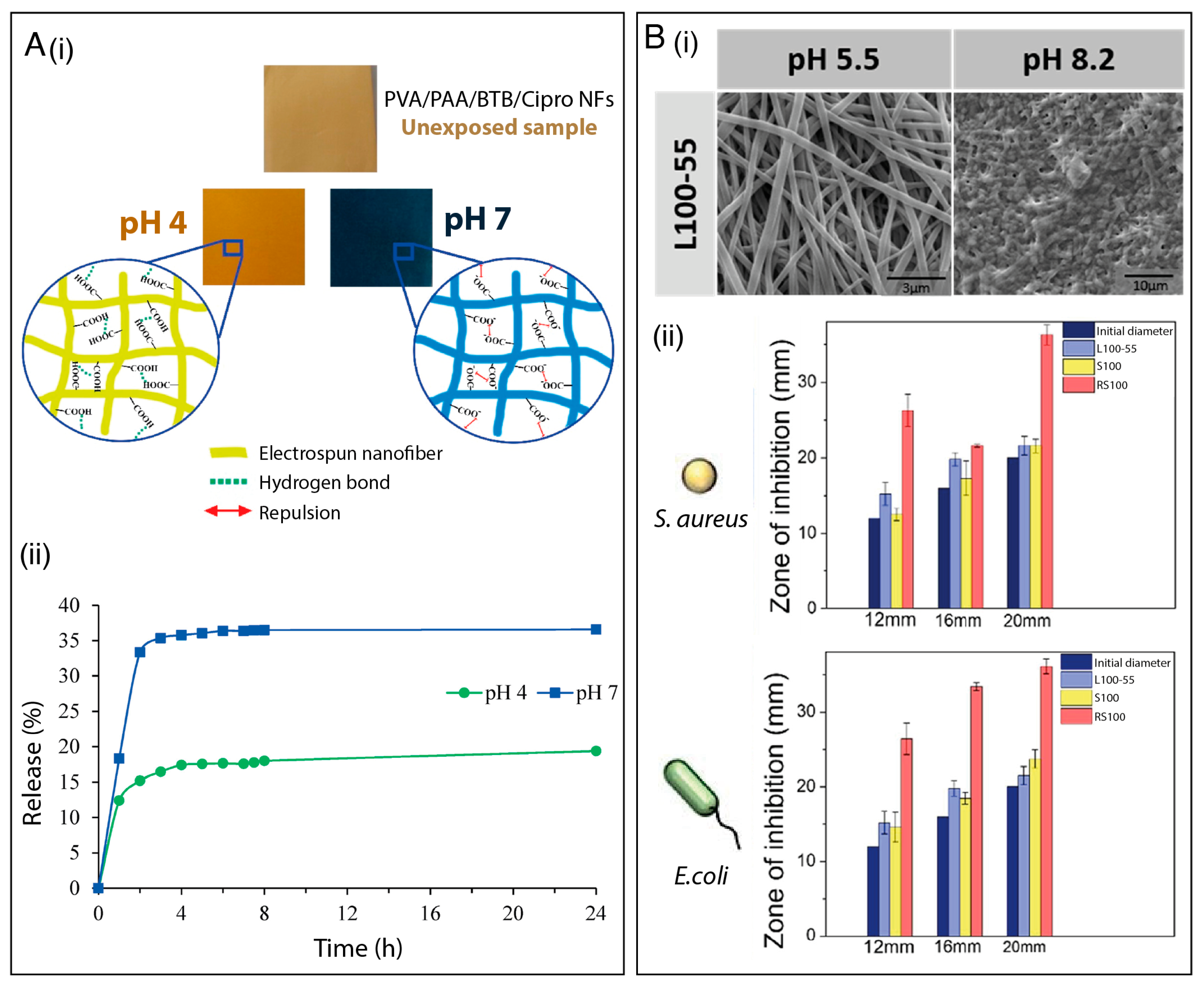
| Eudragit® L100-55 or Eudragit® S100 or Eudragit® RS100 | Thymol (monoterpene) Rifampicin (antibiotic) | E. coli, S. aureus | Varying the polymeric matrix of nanofibrous membranes enabled different antimicrobial release kinetics. | [105] | |
| PDMA | Amoxicillin (antibiotic) | E. coli, S. aureus | The different release profiles observed upon different pH was due to the reversible protonation and deprotonation of side-chain tertiary amine groups in PDMA. | [106] | |
| PVA/PAA/BTB | Ciprofloxacin (antibiotic) | E. coli, S. aureus | The ionization of –COOH groups of PAA at higher pH (>7) causes repulsion among -COO- enhancing the drug release. | [107] | |
| CNF-PEI | - | E. coli, L. monocytogenes | The protonation and deprotonation of NH2 groups and the competition of intermolecular hydrogen bonds in CNF-PEI as a function of pH led to a reversible change of the wettability and antibacterial properties. | [108] | |
| PD-ChNFs | - | E. coli | The antibacterial effect is caused by the cationic amino group content that was affected by degree of deacetylation and pH. | [109] | |
| CAP | Reverse transcriptase inhibitors TMC 125 or tenofovir disoproxil fumarate (Viread) | BAL virus | CAP fibers rapidly dissolve at pH 7.4, releasing the encapsulated drugs. | [110] | |
| Temperature | PNIPAM/PVA | ZnO | S. aureus | Nanofibers exhibited a thermo-controllable ZnO release profile upon temperature variation between 28 and 32 °C. | [111] |
| Eudragit® RS 100/PMMA | Octenidine dihydrochloride (antiseptic) | S. aureus, P. aeruginosa | The thermal switch can be turned on at 37 °C and off at 25 °C, conferring a controlled release of the antiseptic. | [36] | |
| PLLA/PNIPAM | Crystal violet (non-toxic dye) | E. coli, S. epidermidis | Switchable wettability and controlled release were achieved by changing the environmental temperature across the LCST of PNIPAM. | [112] | |
| PBS | Aminophosphonates derivatives | E. coli, S. aureus, C. albicans, K. pneumonia, B. subtilis | Nanofibers exhibited biocidal activity against all tested microorganisms at 39 °C (temperature of an infected wound). | [113] | |
| SMPU | Berberine hydrochloride (alkaloid) | E. coli, S. aureus | The alkaloid could be released in a controlled manner owing to the thermo-sensitive shape memory effect of the polymer. | [114] | |
| Light | PLA | Indocyanine green (non-toxic dye) | S. saprophyticus, E. coli, S. aureus | Upon laser irradiation at 810 nm for 30 min, the bacterial viability was significantly reduced. | [115] |
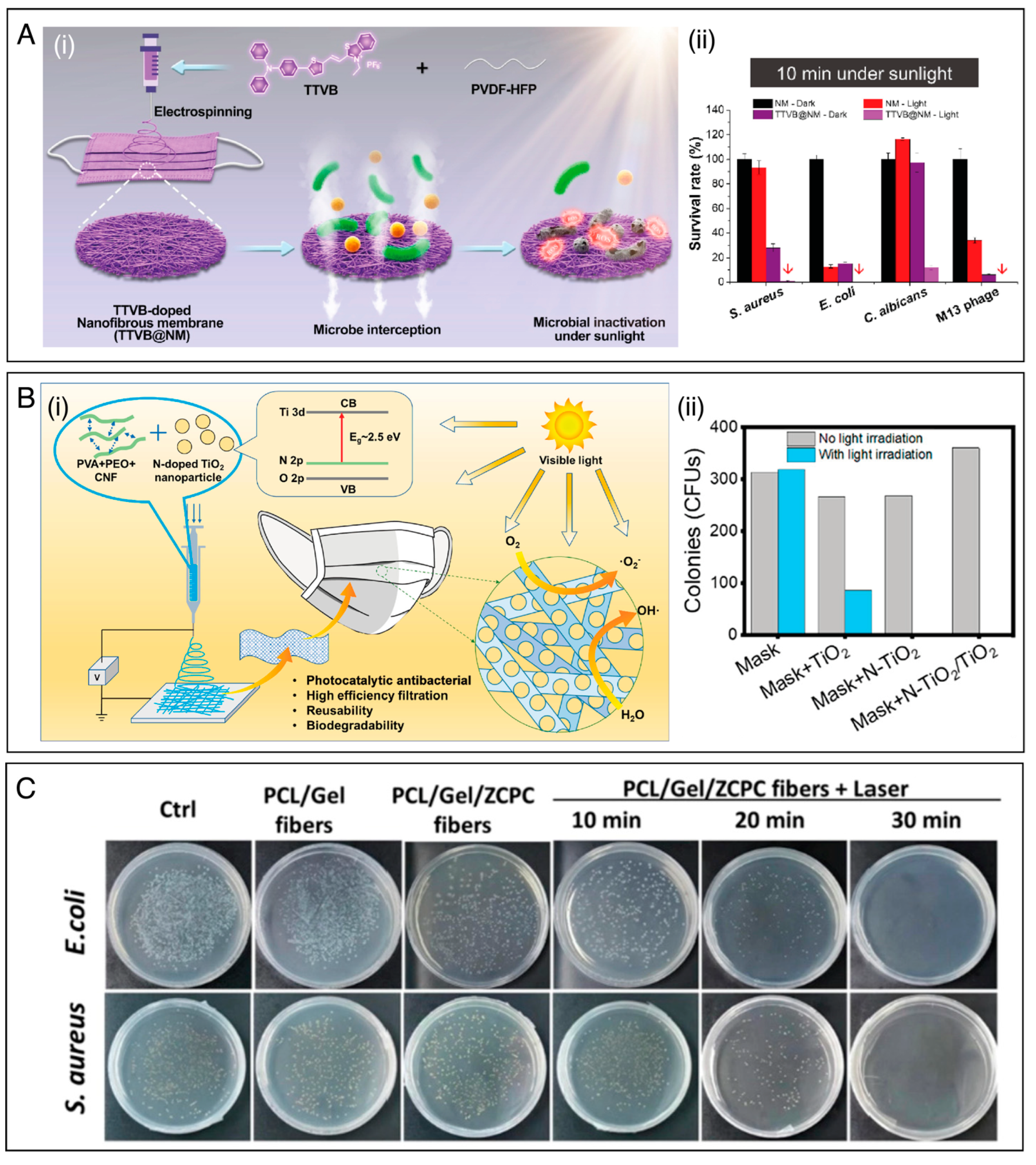
| PVDF-HFP | AIEgens | S. aureus, E. coli, S. cerevisiae, M13 bacteriophage | Enhanced antimicrobial performance against pathogens was achieved under sunlight irradiation for 5–10 min. | [116] | |
| PAN | TiO2 | E. coli, Bacillus sp. | At light conditions, PAN-TiO2 inhibited 3-fold the growth of bacteria compared to PAN after 24 h. | [117] | |
| PVA/PEO/CNF | N-TiO2/TiO2 | E. coli, S. aureus | Antibacterial mask reached 100% bacteria disinfection under 0.1 sun simulation or natural sunlight for only 10 min. | [37] | |
| PCL | AgNPs | E. coli, S. aureus | Nanofibrous mats functionalized with photoresponsive nanogels released AgNPs when irradiated by light at 405 nm. | [118] | |
| PMMA/TPP | AgNPs | S. epidermidis, E. faecalis | Combining TPP and AgNPs enabled light-triggered tuning of AgNPs release from nanofibers. | [119] | |
| PAN | CQDs | E. coli, S. aureus, P. aeruginosa, B. subtilis | 1O2 generated from the CQDs were responsible for the pathogen inactivation. | [120] | |
| PCL | uCNT@PDA | E. coli, B. subtilis | The temperature increase triggered by NIR exposure was enough to kill bacteria and destroy bacterial biofilms. | [121] | |
| PVDF | Rose bengal | Murine hepatitis virus A59 (MHV-A59) | The membranes rapidly inactivated 97.1% of MHV-A59 in virus-laden droplets after 15 min irradiation of simulated reading light. | [122] | |
| PVA | - | E. coli, S. aureus | The temperature increase (up to 50 °C) upon NIR irradiation induced by the Au@carbon dots could effectively eradicate bacteria at the wound site. | [27] | |
| PCL/gelatin | Ciprofloxacin (antibiotic) and Zn2+ | E. coli, S. aureus | The composite fiber membrane with NIR-induced hyperthermia and Zn2+ release exhibited bacteriostatic properties | [123] | |
| Ultrasound | PEO | Ciprofloxacin (antibiotic) | E. coli, S. aureus | Ultrasound stimulus of 15 W/cm2 increased the antibiotic release more than three times. | [77] |
| Electrical | PLA/GO | Quercetin (flavonoid) | E. coli, S. aureus, C. albicans | 10 s of electric stimulation at 10 and 50 Hz ensured the complete delivery of the quercetin. | [124] |
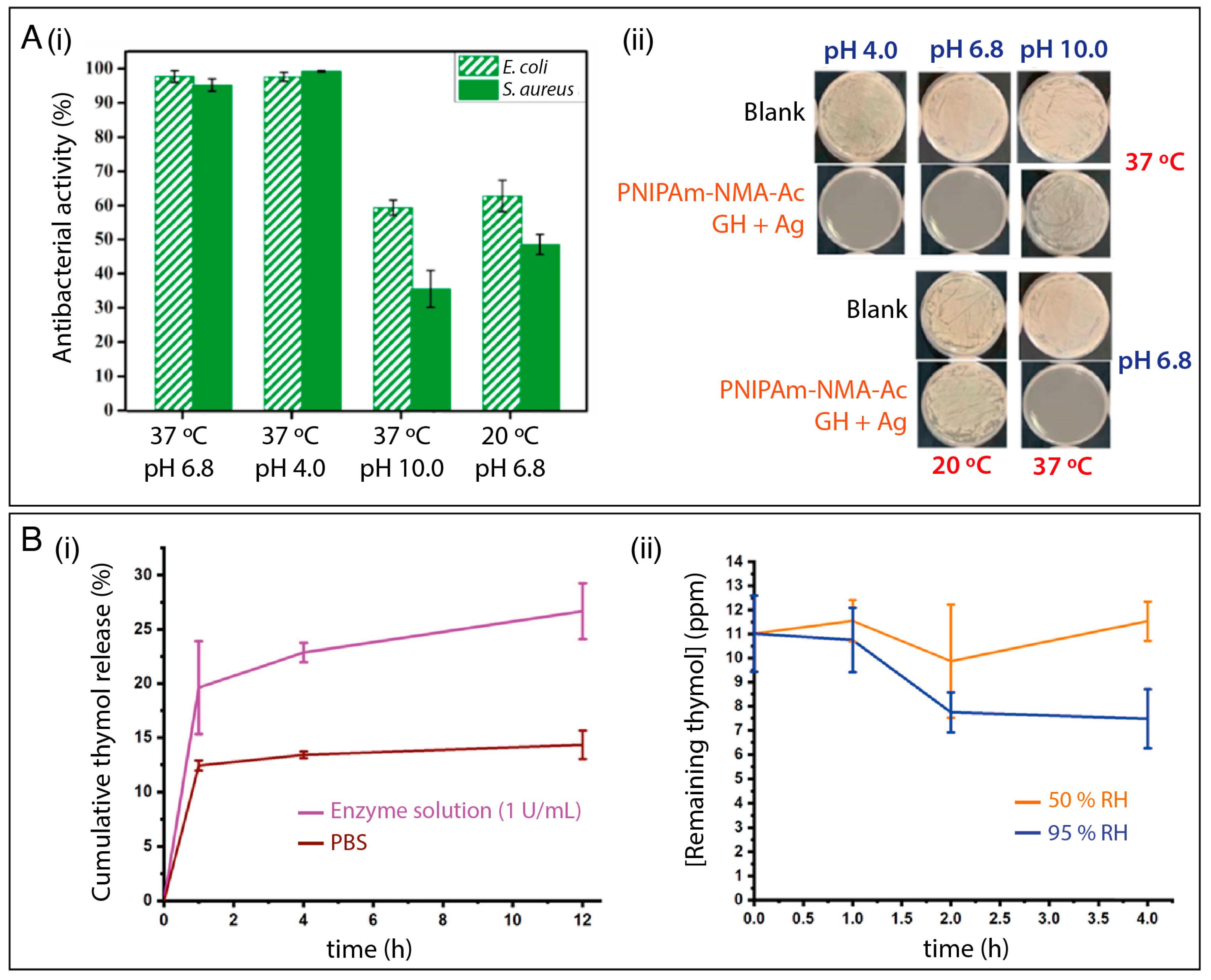
| Humidity | EVOH | Thymol (monoterpene) | E. coli, S. aureus | Nanofibers released more thymol at 90% RH than at 30% RH. | [125] |
| Enzyme | PCL | Metronidazole (antibiotic) | H. pylori | As the concentration of cholesterol esterase (CE) (an enzyme secreted by macrophagocytes that accumulates at the site of infection) increased, a higher amount of the antibiotic was released from the nanofiber mat. | [126] |
| Enzyme and humidity | Starch/zein/CNC | AIs (thyme oil, citric acid, nisin) and CD-ICs of AIs | E. coli, L. innocua, A. fumigatus | Fibers could release free AIs when triggered by microorganism-exudated enzymes and AIs from CD-IC in response to high relative humidity (95% RH). | [127] |
| Enzyme and pH | Eudragit S-100 | Clarithromycin (antibiotic) | E. coli, S. aureus, S. enterica | The nanofibers released more antibiotic in response to a pathologic (enzyme hyaluronate lyase) and physiologic (pH 7.4) stimulus. | [128] |
| Temperature and pH | PNIPAm-co-Aam/PCL | Ciprofloxacin (antibiotic) | S. epidermidis, E. coli | LCSTs of the PCL/PNIPAm-co-AAm nanofibers were 32 °C for pH 4 and 37.5 °C for pH 7.4, leading to different ciprofloxacin release kinetics. | [129] |
| Temperature and light | PEG/PHBV/f-CNC-ZnO | Tetracycline hydrochloride (antibiotic) | - | Beyond the PEG melting point, the encapsulated antibiotic was readily released from composite nanofibers. | [130] |
| CO2, pH, and electrical | PMMA-co-PDEAEMA | Curcumin (polyphenol) | E. coli, S. aureus | Increasing voltage from 2 to 8 V, bubbling CO2 gas and lowering the pH from 7.4 to 5 lead to an enhanced curcumin release. | [131] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mercante, L.A.; Teodoro, K.B.R.; dos Santos, D.M.; dos Santos, F.V.; Ballesteros, C.A.S.; Ju, T.; Williams, G.R.; Correa, D.S. Recent Progress in Stimuli-Responsive Antimicrobial Electrospun Nanofibers. Polymers 2023, 15, 4299. https://doi.org/10.3390/polym15214299
Mercante LA, Teodoro KBR, dos Santos DM, dos Santos FV, Ballesteros CAS, Ju T, Williams GR, Correa DS. Recent Progress in Stimuli-Responsive Antimicrobial Electrospun Nanofibers. Polymers. 2023; 15(21):4299. https://doi.org/10.3390/polym15214299
Chicago/Turabian StyleMercante, Luiza A., Kelcilene B. R. Teodoro, Danilo M. dos Santos, Francisco V. dos Santos, Camilo A. S. Ballesteros, Tian Ju, Gareth R. Williams, and Daniel S. Correa. 2023. "Recent Progress in Stimuli-Responsive Antimicrobial Electrospun Nanofibers" Polymers 15, no. 21: 4299. https://doi.org/10.3390/polym15214299
APA StyleMercante, L. A., Teodoro, K. B. R., dos Santos, D. M., dos Santos, F. V., Ballesteros, C. A. S., Ju, T., Williams, G. R., & Correa, D. S. (2023). Recent Progress in Stimuli-Responsive Antimicrobial Electrospun Nanofibers. Polymers, 15(21), 4299. https://doi.org/10.3390/polym15214299








