Alginate–Moroccan Clay, New Bio-Nanocomposite for Removal of H2PO4−, HPO42−, and NO3− Ions from Aqueous Solutions
Abstract
:1. Introduction
2. Materials and Methods
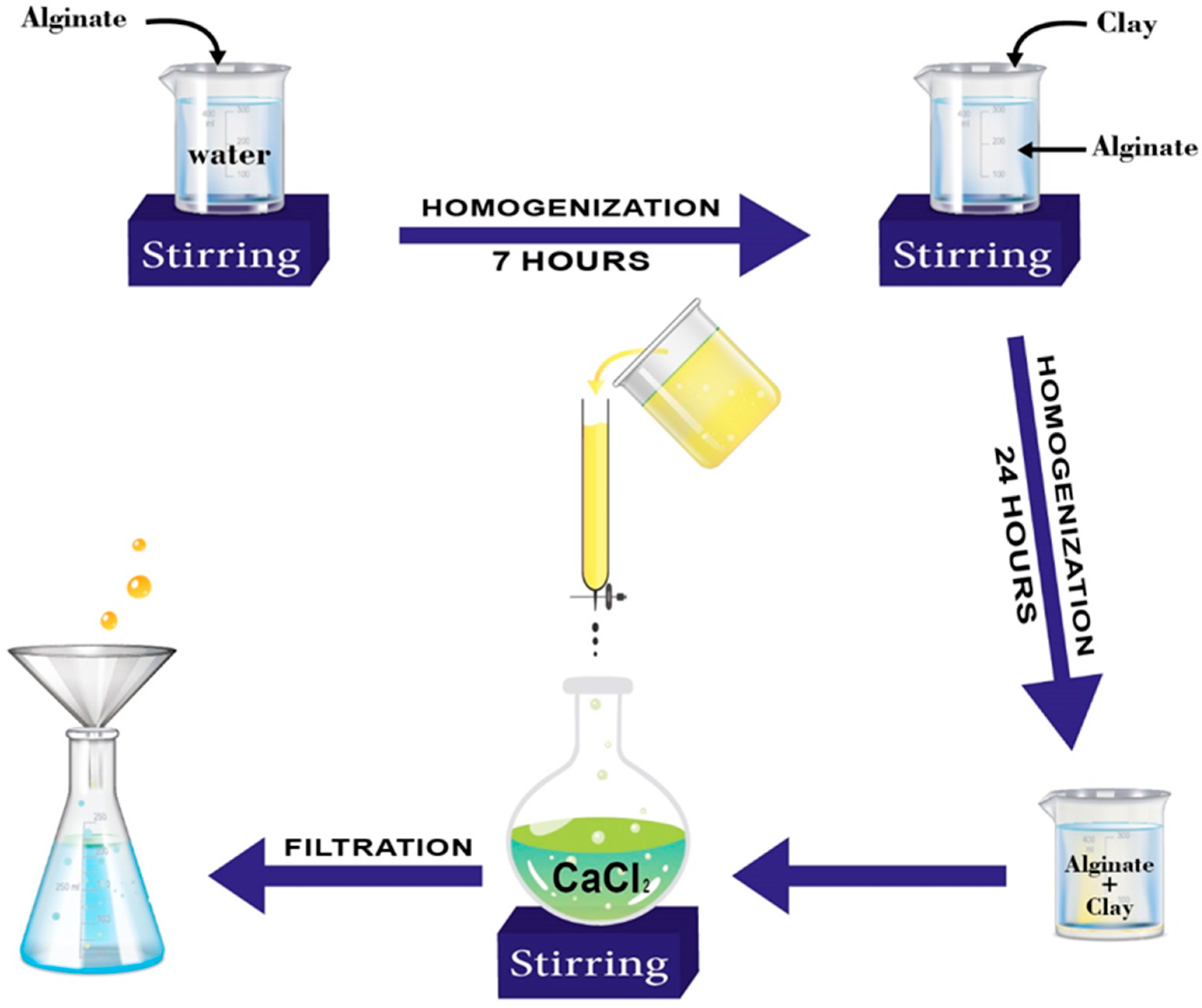
2.1. Adsorbent
2.2. Adsorption Experiments
3. Results and Discussion
3.1. Characterization of Bio-Nanocomposite Beads
3.1.1. XRD Analysis
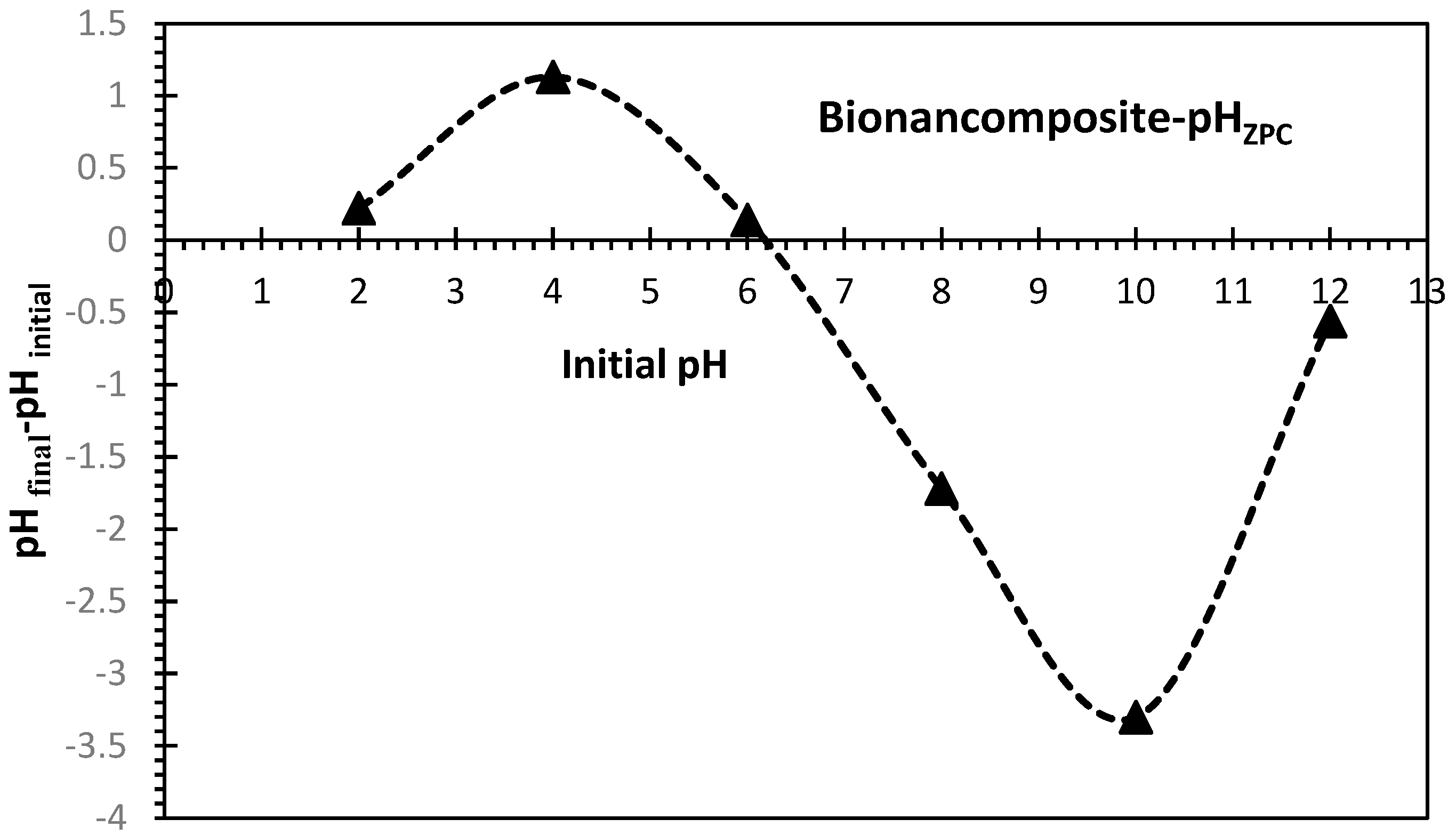
3.1.2. pH of Zero point charge for Bio-Nanocomposite Adsorbent
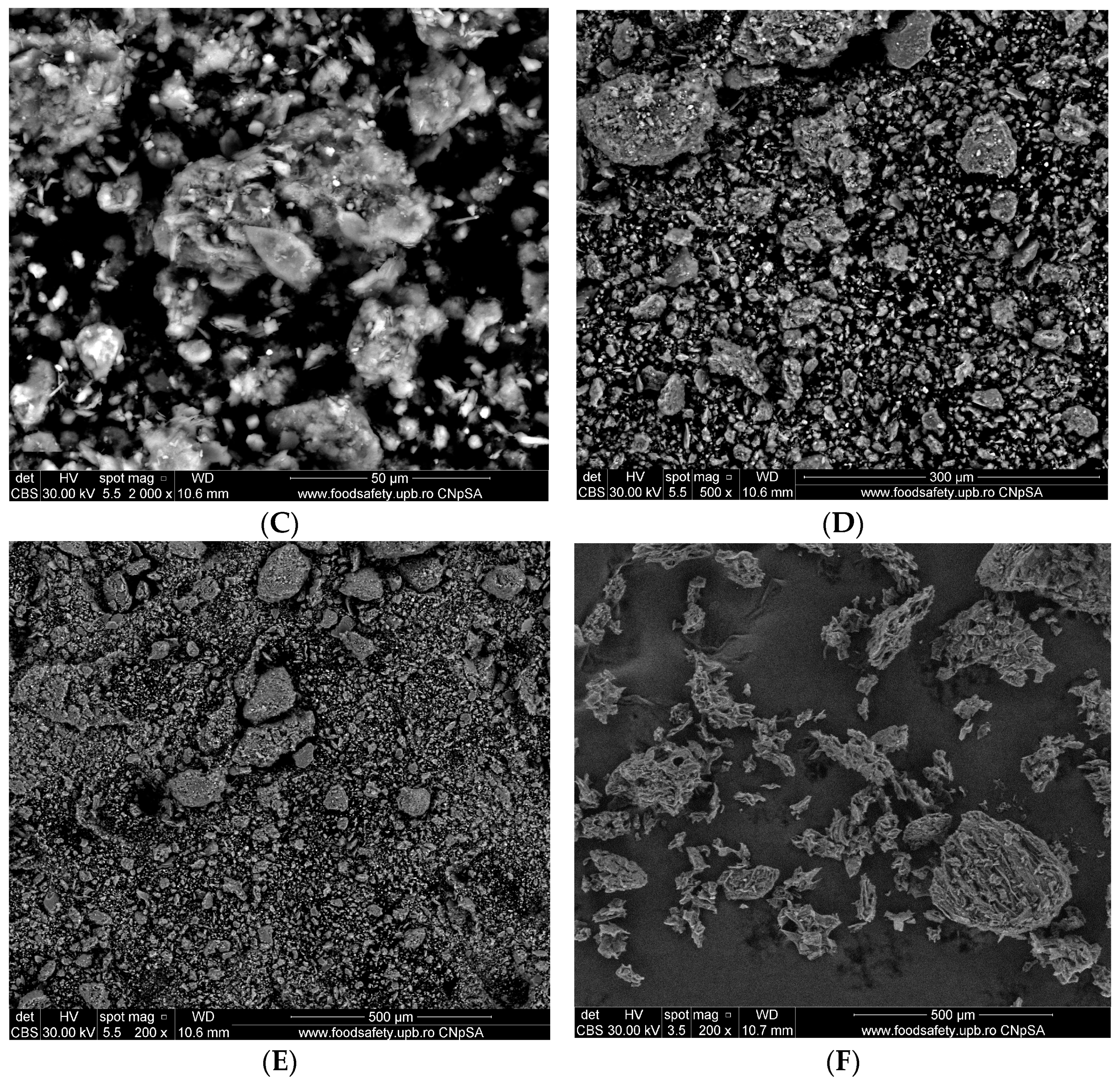
3.1.3. Scanning Electron Microscopy (SEM)
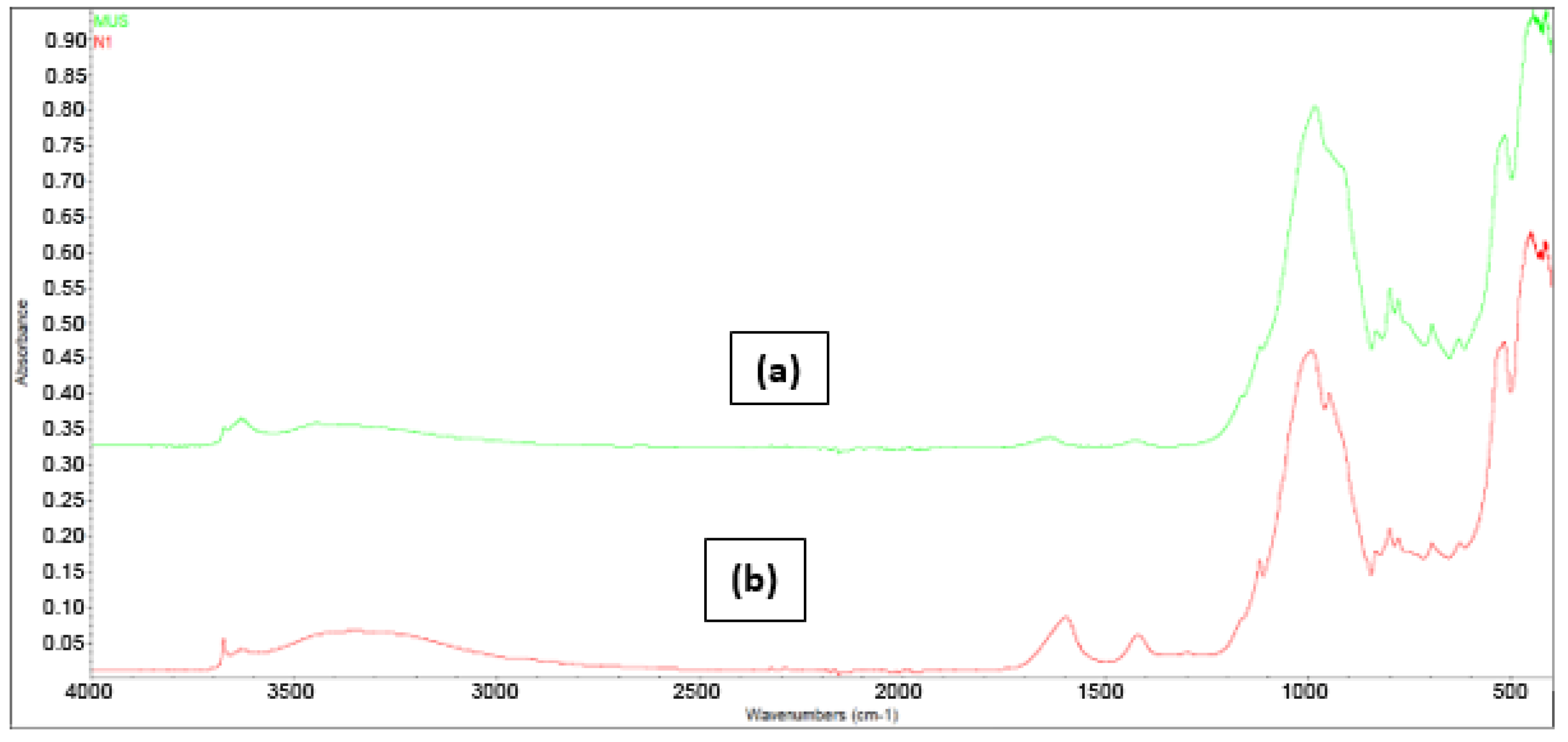
3.1.4. FT-IR Spectroscopy
3.2. Factors Controlling the Adsorption of H2PO4− and HPO42− and NO3− Ions from Aqueous Solution by Alginate–Clay Bio-Nanocomposite
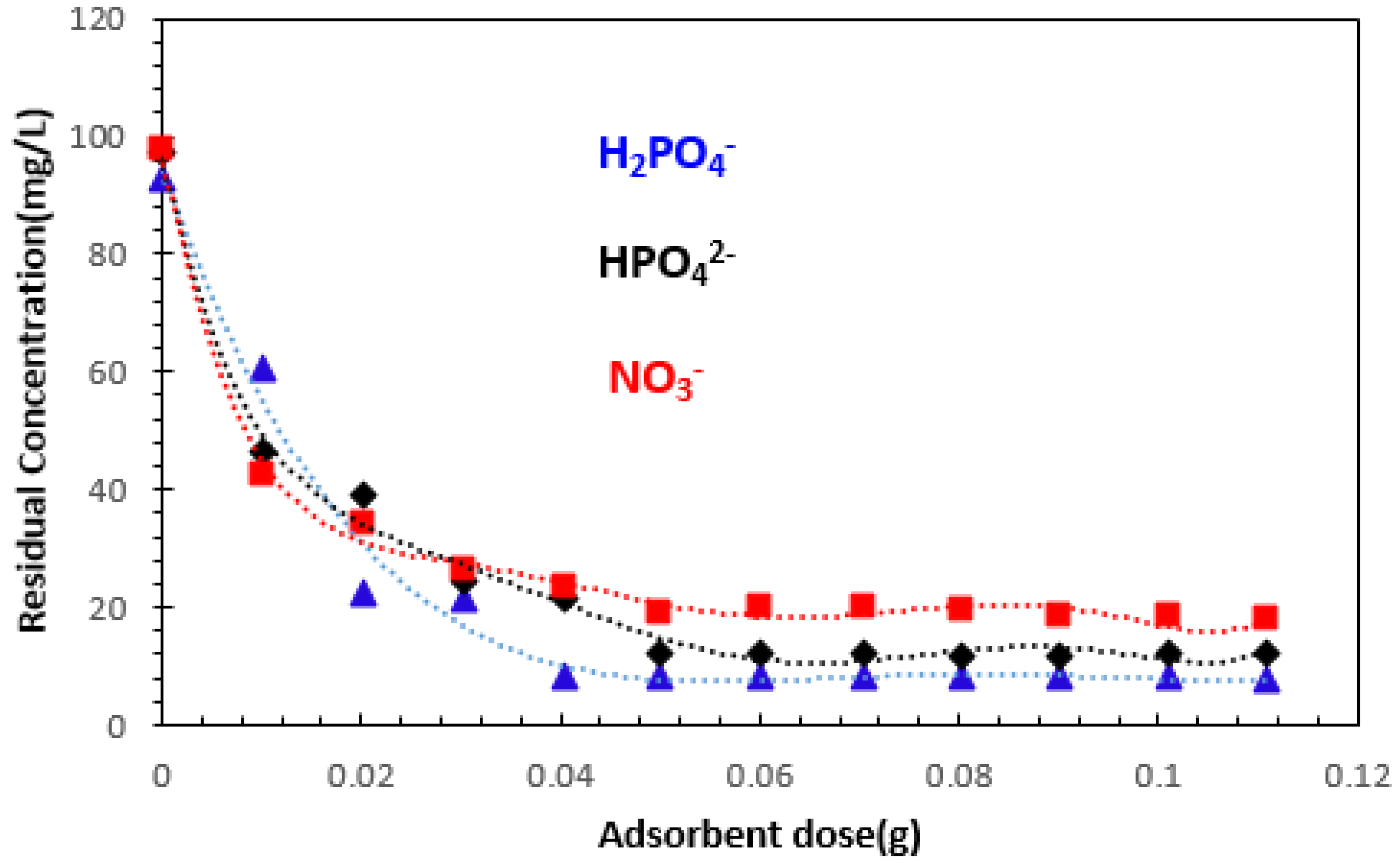
3.2.1. Effect of Adsorbent Dosage
3.2.2. Effect of Contact Time
3.2.3. Effect of Initial Solution pH
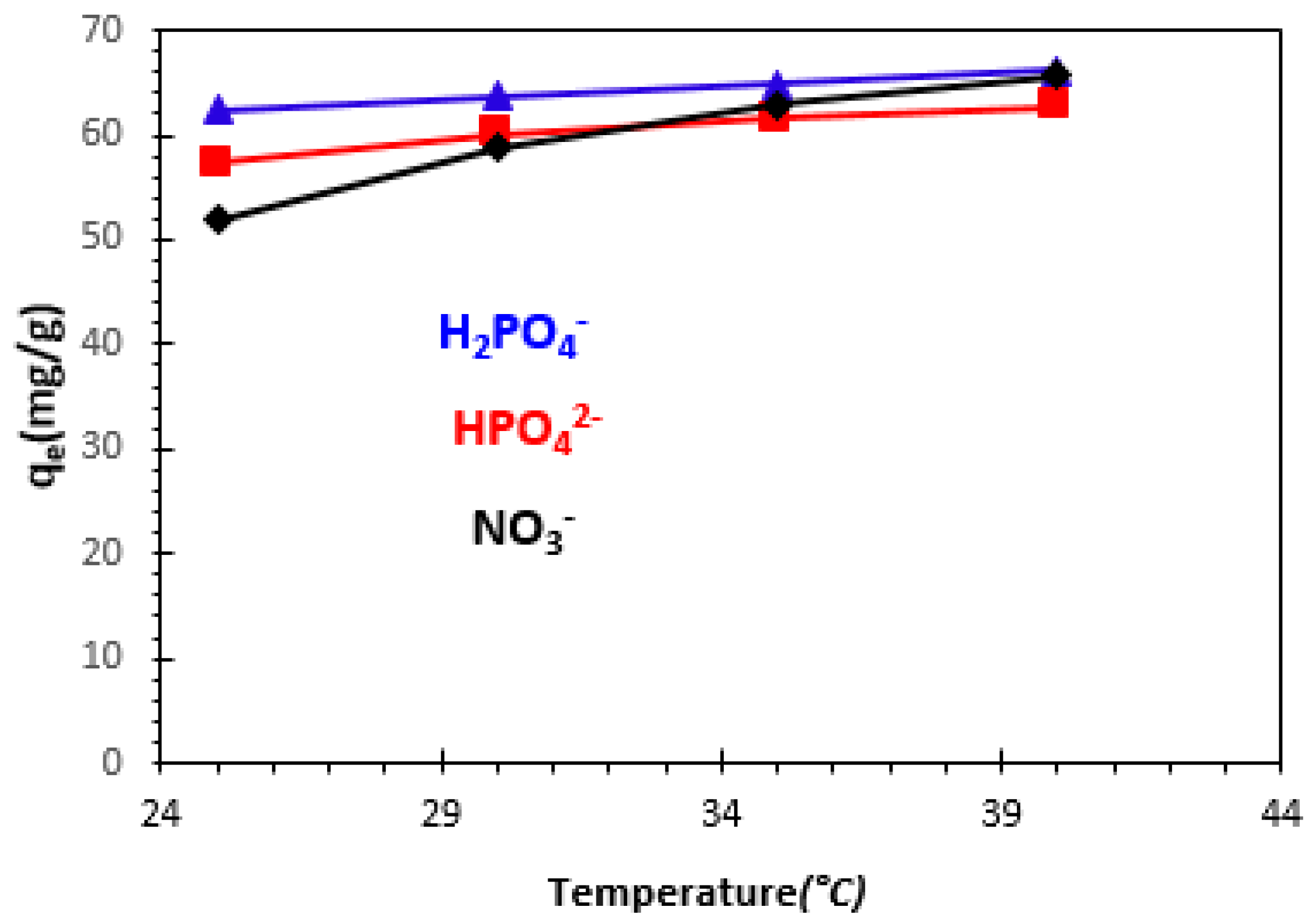
3.2.4. Effect of Temperature
3.3. Adsorption Kinetic Models
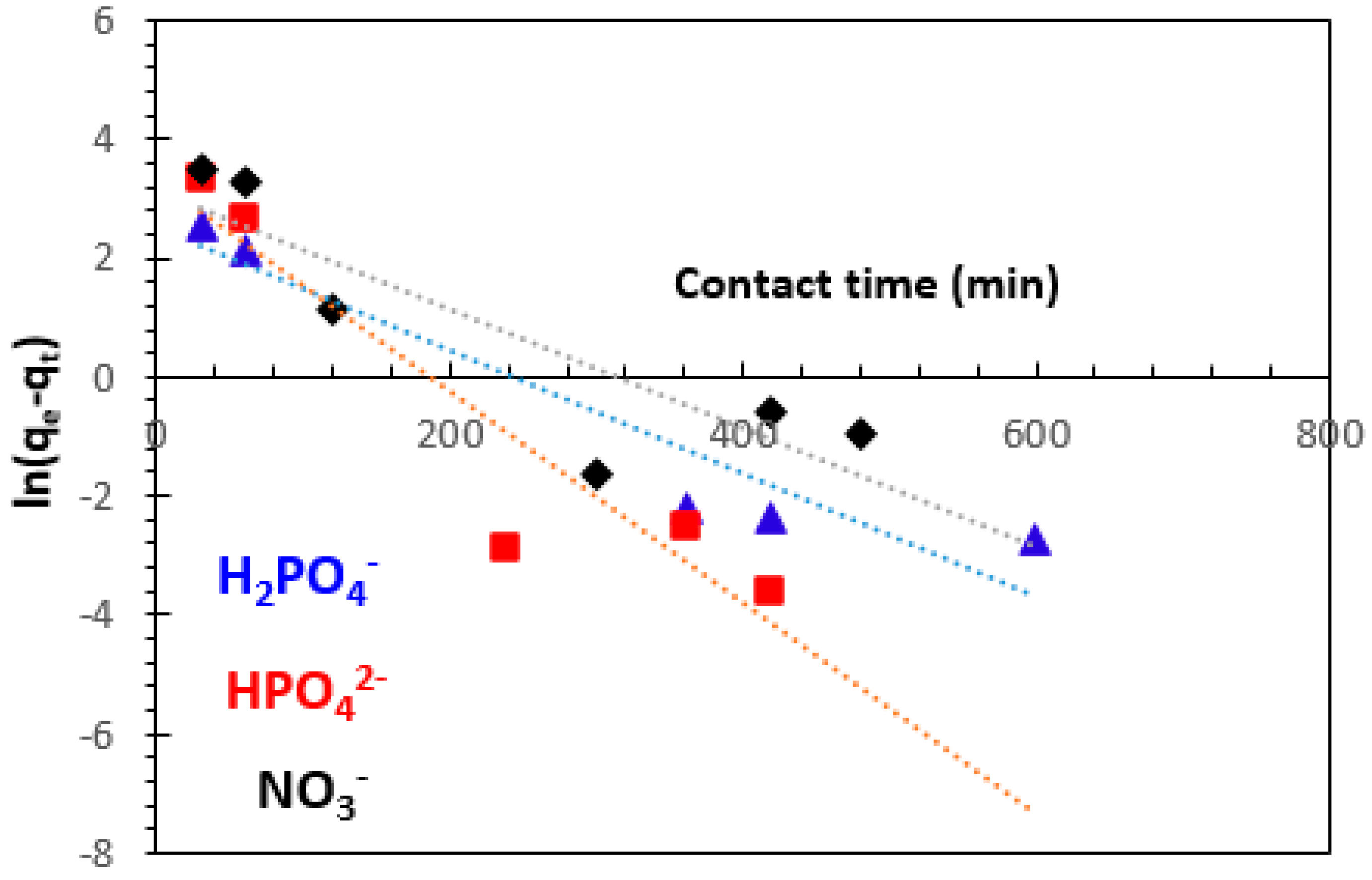
3.3.1. Pseudo-First-Order Kinetics Model
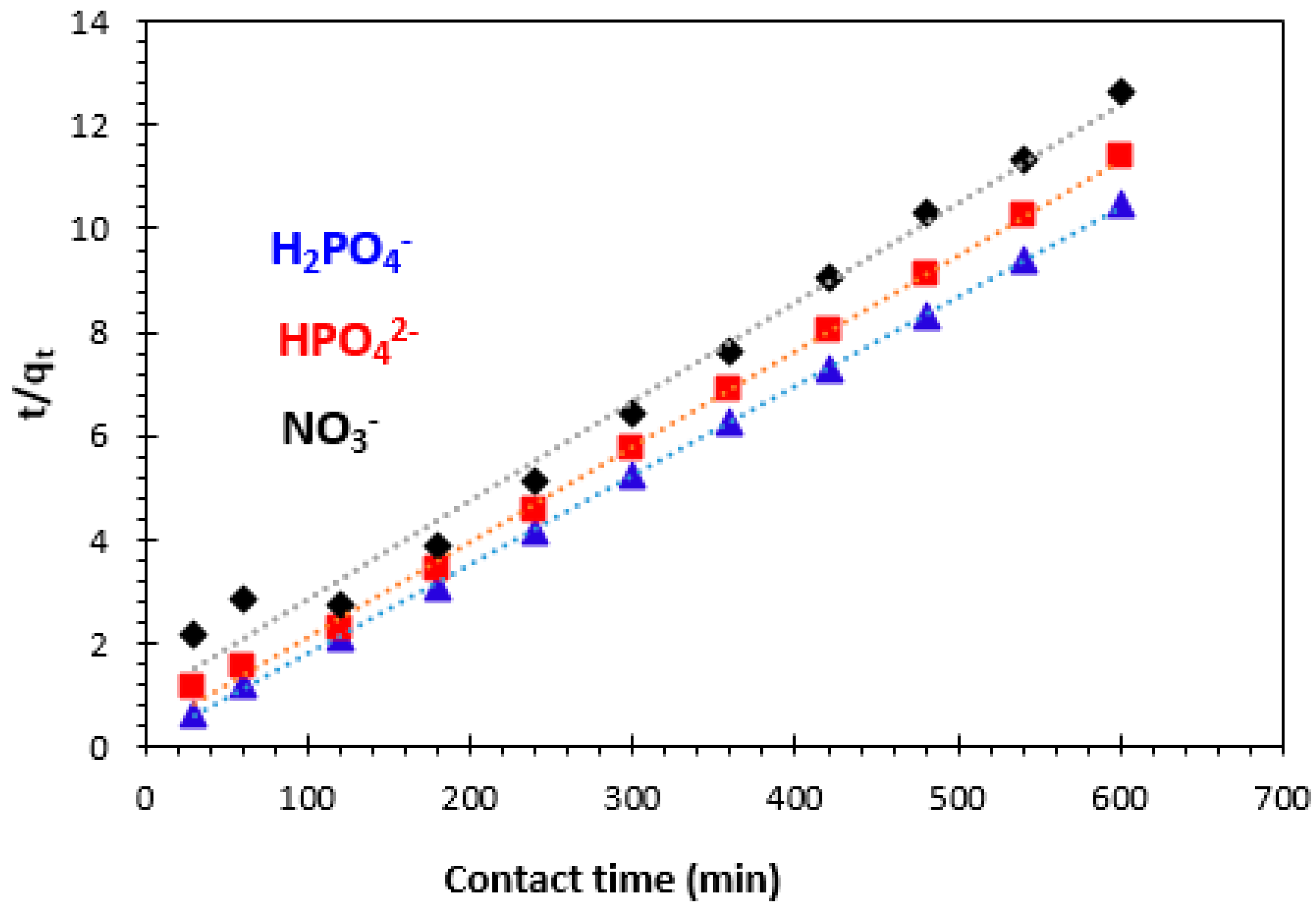
3.3.2. Pseudo-Second-Order Kinetics Model
3.3.3. Elovich Kinetic Model
3.3.4. Intra-Particle Diffusion Kinetics Model
3.4. Isotherm Study
3.4.1. Langmuir Adsorption Isotherm
3.4.2. Freundlich Adsorption Isotherm
3.4.3. Temkin Isotherm
3.4.4. Dubinin–Radushkevich (D–R) Isotherm
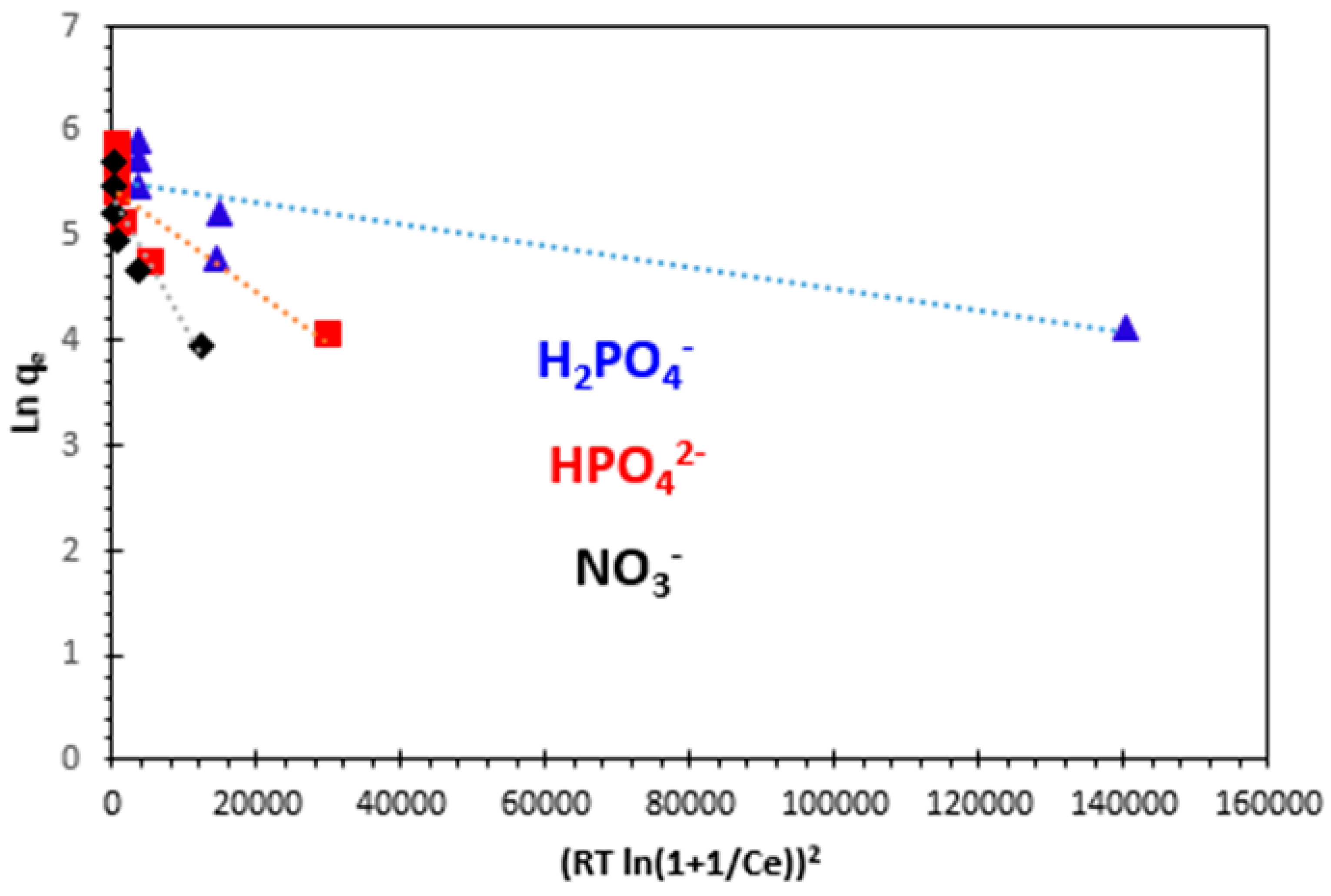
3.5. Thermodynamic Study
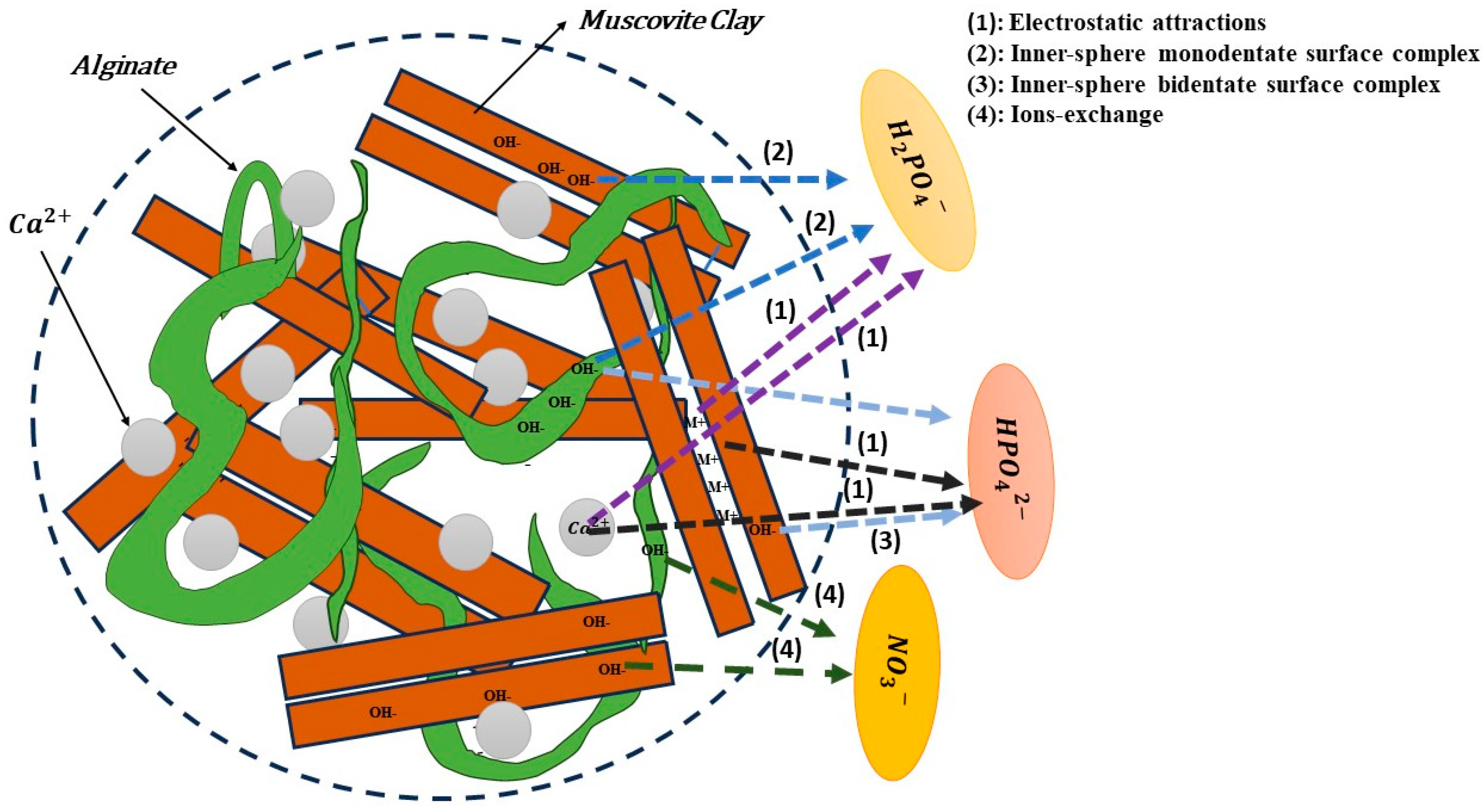
3.6. Adsorption Mechanisms
3.7. Comparison with Published Data
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hosni, K.; Ben Moussa, S.; Ben Amor, M.B. Conditions influencing the removal of phosphate from synthetic wastewater: Influence of the ionic composition. Desalination 2007, 206, 279–285. [Google Scholar] [CrossRef]
- Dévoyé, A.M.; Olayinka, K.; Olukomaiya, O.O.; Iwuchukwu, P.O. The removal of phosphates from laundry wastewater using alum and ferrous sulphate as coagulants. Int. J. Innov. Sci. Res. 2014, 8, 256–260. [Google Scholar]
- Usman, M.O.; Aturagaba, G.; Ntale, M.; Nyakairu, G.W. A review of adsorption techniques for removal of phosphates from wastewater. Water Sci. Technol. 2022, 86, 3113. [Google Scholar] [CrossRef]
- Owodunni, A.A.; Ismail, S.; Kurniawan, S.B.; Ahmad, A.; Imron, M.F.; Abdullah, S.R.S. A review on revolutionary technique for phosphate removal in wastewater using green coagulant. J. Water Process Eng. 2023, 52, 103573. [Google Scholar] [CrossRef]
- Rashid, M.; Price, N.T.; Pinilla, M.Á.G.; O’Shea, K.E. Effective removal of phosphate from aqueous solution using humic acid coated magnetite nanoparticles. Water Res. 2017, 123, 353–360. [Google Scholar] [CrossRef]
- Almanassra, I.W.; Kochkodan, V.; Mckay, G.; Atieh, M.A.; Al-Ansari, T. Review of phosphate removal from water by carbonaceous sorbents. J. Environ. Manag. 2021, 287, 112245. [Google Scholar] [CrossRef]
- Ownby, M.; Desrosiers, D.A.; Vaneeckhaute, C. Phosphorus removal and recovery from wastewater via hybrid ion exchange nanotechnology: A study on sustainable regeneration chemistries. npj Clean Water 2021, 4, 6. [Google Scholar] [CrossRef]
- Chiban, M.; Soudani, A.; Sinan, F.; Tahrouch, S.; Persin, M. Characterization and application of dried plants to remove heavy metals, nitrate, and phosphate ions from industrial wastewaters. CLEAN Soil Air Water 2011, 39, 376–383. [Google Scholar] [CrossRef]
- Abidar, F.; Morghi, M.; Abali, M.; Sinan, F.; Chiban, M.; Eddaoudi, H.; Zebret, M. Orthophosphate ion adsorption onto raw shrimp shells. Rev. Sci. l’eau 2016, 29, 197–211. [Google Scholar] [CrossRef]
- Öztürk, N.; Bektaş, T.E. Nitrate removal from aqueous solution by adsorption onto various materials. J. Hazard. Mater. 2004, 112, 155–162. [Google Scholar] [CrossRef]
- Barber, W.P.; Stuckey, D.C. Nitrogen removal in a modified anaerobic baffled reactor (ABR): 1, denitrification. Water Res. 2000, 34, 2413–2422. [Google Scholar] [CrossRef]
- Eltaweil, A.S.; Omer, A.M.; El-Aqapa, H.G.; Gaber, N.M.; Attia, N.F.; El-Subruiti, G.M.; Mohy-Eldin, M.S.; El-Monaem, E.M.A. Chitosan based adsorbents for the removal of phosphate and nitrate: A critical review. Carbohydr. Polym. 2021, 274, 118671. [Google Scholar] [CrossRef] [PubMed]
- Aziam, R.; Chiban, M.; Eddaoudi, H.; Soudani, A.; Zerbet, M.; Sinan, F. Kinetic modeling, equilibrium isotherm and thermodynamic studies on a batch adsorption of anionic dye onto eco-friendly dried Carpobrotus edulis plant. Eur. Phys. J. Spec. Top. 2017, 226, 977–992. [Google Scholar] [CrossRef]
- Aziam, R.; Chiban, M.; Eddaoudi, H.E.; Soudani, A.; Zerbet, M.; Sinan, F. Factors controlling the adsorption of acid blue 113 dye from aqueous solution by dried C. edulis plant as natural adsorbent. Arab. J. Geosci. 2016, 9, 659. [Google Scholar] [CrossRef]
- Klein, K.; Mandel, A.; Lilleoja, H.; Salmar, S.; Tenno, T. Assessment of enhanced biological phosphorus removal process inhibition. SN Appl. Sci. 2020, 2, 1489. [Google Scholar] [CrossRef]
- Bektaş, T.E.; Uğurluoğlu, B.K.; Tan, B. Phosphate removal by Ion exchange in batch mode. Water Pract. Technol. 2021, 16, 1343–1354. [Google Scholar] [CrossRef]
- Rajaniemi, K.; Hu, T.; Nurmesniemi, E.-T.; Tuomikoski, S.; Lassi, U. Phosphate and ammonium removal from water through electrochemical and chemical precipitation of struvite. Processes 2021, 9, 150. [Google Scholar] [CrossRef]
- Vikrant, K.; Kim, K.-H.; Ok, Y.S.; Tsang, D.C.; Tsang, Y.F.; Giri, B.S.; Singh, R.S. Engineered/designer biochar for the removal of phosphate in water and wastewater. Sci. Total Environ. 2018, 616, 1242–1260. [Google Scholar] [CrossRef]
- Sun, J.; Gao, A.; Wang, X.; Xu, X.; Song, J. Removal of phosphorus from wastewater by different morphological alumina. Molecules 2020, 25, 3092. [Google Scholar] [CrossRef] [PubMed]
- Mekonnen, D.T.; Alemayehu, E.; Lennartz, B. Fixed-bed column technique for the removal of phosphate from water using leftover coal. Materials 2021, 14, 5466. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, E.; Daraei, H.; Ghanbari, R.; Athar, S.D.; Zandsalimi, Y.; Ziaee, A.; Maleki, A.; Yetilmezsoy, K. Synthesis of carboxylated chitosan modified with ferromagnetic nanoparticles for adsorptive removal of fluoride, nitrate, and phosphate anions from aqueous solutions. J. Mol. Liq. 2019, 273, 116–124. [Google Scholar] [CrossRef]
- Barshad, I. Adsorptive and Swelling properties of clay-water system. Clays Clay Miner. 1952, 1, 70–77. [Google Scholar] [CrossRef]
- Amakrane, J.; El Hammouti, K.; Azdimousa, A.; El Halim, M.; El Ouahabi, M.; Lamouri, B.; Fagel, N. Mineralogical and physicochemical characterization of the Jbel Rhassoul clay deposit (Moulouya Plain, Morocco). J. Mater. Environ. Sci. 2018, 9, 2549–2557. [Google Scholar]
- Kevadiya, B.D.; Patel, H.A.; Joshi, G.V.; Abdi, S.H.R.; Bajaj, H.C. Montmorillonite-alginate composites as a drug delivery system: Intercalation and in vitro release of diclofenac sodium. Indian J. Pharm. Sci. 2010, 72, 732–737. [Google Scholar] [CrossRef] [PubMed]
- Ayouch, I.; Barrak, I.; Kassab, Z.; El Achaby, M.; Barhoun, A.; Draoui, K. Improved recovery of cadmium from aqueous medium by alginate composite beads filled by bentonite and phosphate washing sludge. Colloids Surfaces A Physicochem. Eng. Asp. 2020, 604, 125305. [Google Scholar] [CrossRef]
- Zahakifar, F.; Keshtkar, A.R.; Talebi, M. Performance evaluation of sodium alginate/polyvinyl alcohol/polyethylene oxide/ZSM5 zeolite hybrid adsorbent for ion uptake from aqueous solutions: A case study of thorium (IV). J. Radioanal. Nucl. Chem. 2020, 327, 65–72. [Google Scholar] [CrossRef]
- Zia, F.; Salman, M.; Ali, M.; Iqbal, R.; Rasul, A.; Najam-ul-Haq, M.; Asgher, M.; Zia, K.M. Alginate-based bionanocomposites. Bionanocomposites 2020, 173–205. [Google Scholar] [CrossRef]
- Zhao, J.; Dang, Z.; Muddassir, M.; Raza, S.; Zhong, A.; Wang, X.; Jin, J. A new Cd(II)-based coordination polymer for efficient photocatalytic removal of organic dyes. Molecules 2023, 28, 6848. [Google Scholar] [CrossRef]
- Barrak, I.; Ayouch, I.; Kassab, Z.; El Achaby, M.; Barhoun, A.; Draoui, K. Sodium alginate encapsulated Moroccan clay as eco-friendly and efficient adsorbent for copper ions from aqueous medium. Mater. Today Proc. 2022, 51, 2040–2046. [Google Scholar] [CrossRef]
- Wang, L.; Sun, W.; Hu, Y.-H.; Xu, L.-H. Adsorption mechanism of mixed anionic/cationic collectors in Muscovite—Quartz flotation system. Miner. Eng. 2014, 64, 44–50. [Google Scholar] [CrossRef]
- Aboussabek, A.; Aziam, R.; El Qdhy, S.; Boukarma, L.; Zerbet, M.; Sinan, F.; Chiban, M. Synthesis and characterization of hybrid clay@ Fe3O4 for acid blue113 sequestration using a fixed-bed adsorption column. Int. J. Environ. Sci. Technol. 2023. [Google Scholar] [CrossRef]
- Kausar, A.; Rehman, S.U.; Khalid, F.; Bonilla-Petriciolet, A.; Mendoza-Castillo, D.I.; Bhatti, H.N.; Ibrahim, S.M.; Iqbal, M. Cellulose, clay and sodium alginate composites for the removal of methylene blue dye: Experimental and DFT studies. Int. J. Biol. Macromol. 2022, 209, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Chattopadhyaya, M.C. Adsorption characteristics for the removal of a toxic dye, tartrazine from aqueous solutions by a low cost agricultural by-product. Arab. J. Chem. 2017, 10, S1629–S1638. [Google Scholar] [CrossRef]
- Chang, M.; Ma, X.; Fan, Y.; Dong, X.; Chen, R.; Zhu, B. Adsorption of different valence metal cations on kaolinite: Results from experiments and molecular dynamics simulations. Colloids Surfaces A Physicochem. Eng. Asp. 2023, 656, 130330. [Google Scholar] [CrossRef]
- Belachew, N.; Bekele, G. Synergy of magnetite intercalated bentonite for enhanced adsorption of congo red dye. Silicon 2020, 12, 603–612. [Google Scholar] [CrossRef]
- Bao, T.; Damtie, M.M.; Hosseinzadeh, A.; Frost, R.L.; Yu, Z.M.; Jin, J.; Wu, K. Catalytic degradation of P-chlorophenol by muscovite-supported nano zero valent iron composite: Synthesis, characterization, and mechanism studies. Appl. Clay Sci. 2020, 195, 105735. [Google Scholar] [CrossRef]
- Nouaa, S.; Aziam, R.; Benhiti, R.; Carja, G.; Iaich, S.; Zerbet, M.; Chiban, M. Synthesis of LDH/alginate composite beads as a potential adsorbent for phosphate removal: Kinetic and equilibrium studies. Chem. Pap. 2023, 77, 6689–6705. [Google Scholar] [CrossRef]
- Li, B.; Yin, H. Superior adsorption property of a novel green biosorbent yttrium/alginate gel beads for dyes from aqueous solution. J. Polym. Environ. 2020, 28, 2137–2148. [Google Scholar] [CrossRef]
- Ullah, A.; Zahoor, M.; Din, W.U.; Muhammad, M.; Khan, F.A.; Sohail, A.; Ullah, R.; Ali, E.A.; Murthy, H.C.A. Removal of methylene blue from aqueous solution using black tea wastes: Used as efficient adsorbent. Adsorpt. Sci. Technol. 2022, 2022, 5713077. [Google Scholar] [CrossRef]
- Hameed, K.S.; Muthirulan, P.; Sundaram, M.M. Adsorption of chromotrope dye onto activated carbons obtained from the seeds of various plants: Equilibrium and kinetics studies. Arab. J. Chem. 2017, 10, S2225–S2233. [Google Scholar] [CrossRef]
- Amrhar, O.; Berisha, A.; El Gana, L.; Nassali, H.; Elyoubi, M.S. Removal of methylene blue dye by adsorption onto Natural Muscovite Clay: Experimental, theoretical and computational investigation. Int. J. Environ. Anal. Chem. 2023, 103, 2419–2444. [Google Scholar] [CrossRef]
- Stefan, D.S.; Meghea, I. Mechanism of simultaneous removal of Ca2+, Ni2+, Pb2+ and Al3+ ions from aqueous solutions using Purolite® S930 ion exchange resin. Comptes Rendus Chim. 2014, 17, 496–502. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, X.; He, Y.; Chen, Y.; Zhou, J.; Luo, X. Adsorption of phosphorus from slaughterhouse wastewater by carboxymethyl konjac glucomannan loaded with lanthanum. Int. J. Biol. Macromol. 2018, 119, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Trinh, V.T.; Pham, T.T.H.; Van, H.T.; Trinh, M.V.; Thang, P.Q.; Vu, X.H.; Nguyen, V.Q.; Dang, T.T. Phosphorus removal from aqueous solution by adsorption using silver nanoparticles: Batch experiment. J. Hazard. Toxic Radioact. Waste 2020, 24, 04020038. [Google Scholar] [CrossRef]
- Chiban, M.; Zerbet, M.; Sinan, F. Low-cost materials for phosphate removal from aqueous solutions. In Phosphates: Sources, Properties and Applications; Nova Science Publishers Inc.: New York, NY, USA, 2012; pp. 1–43. [Google Scholar]
- Mandal, S.; Calderon, J.; Marpu, S.B.; Omary, M.A.; Shi, S.Q. Mesoporous activated carbon as a green adsorbent for the removal of heavy metals and Congo red: Characterization, adsorption kinetics, and isotherm studies. J. Contam. Hydrol. 2021, 243, 103869. [Google Scholar] [CrossRef]
- Morghi, M.; Abidar, F.; Soudani, A.; Zerbet, M.; Chiban, M.; Kabli, H.; Sinan, F. Removal of nitrate ions from aqueous solution using chitin as natural adsorbent. Int. J. Res. Environ. Stud. 2015, 2, 8–20. [Google Scholar]
- Elemile, O.O.; Akpor, B.O.; Ibitogbe, E.M.; Afolabi, Y.T.; Ajani, D.O. Adsorption isotherm and kinetics for the removal of nitrate from wastewater using chicken feather fiber. Cogent Eng. 2022, 9, 2043227. [Google Scholar] [CrossRef]
- Abidar, F.; Morghi, M.; Ait Ichou, A.; Soudani, A.; Chiban, M.; Sinan, F.; Zerbet, M. Removal of orthophosphate ions from aqueous solution using chitin as natural adsorbent. Desalination Water Treat. 2016, 57, 14739–14749. [Google Scholar] [CrossRef]
- Akram, M.; Xu, X.; Gao, B.; Wang, S.; Khan, R.; Yue, Q.; Duan, P.; Dan, H.; Pan, J. Highly efficient removal of phosphate from aqueous media by pomegranate peel co-doping with ferric chloride and lanthanum hydroxide nanoparticles. J. Clean. Prod. 2021, 292, 125311. [Google Scholar] [CrossRef]
- Sabzehmeidani, M.M.; Mahnaee, S.; Ghaedi, M.; Heidari, H.; Roy, V.A.L. Carbon based materials: A review of adsorbents for inorganic and organic compounds. Mater. Adv. 2021, 2, 598–627. [Google Scholar] [CrossRef]
- Photiou, P.; Koutsokeras, L.; Constantinides, G.; Koutinas, M.; Vyrides, I. Phosphate removal from synthetic and real wastewater using thermally treated seagrass residues of Posidonia oceanica. J. Clean. Prod. 2021, 278, 123294. [Google Scholar] [CrossRef]
- Wang, A.; Zhou, K.; Liu, X.; Liu, F.; Zhang, C.; Chen, Q. Granular tri-metal oxide adsorbent for fluoride uptake: Adsorption kinetic and equilibrium studies. J. Colloid Interface Sci. 2017, 505, 947–955. [Google Scholar] [CrossRef]
- Wu, C.; Li, L.; Zhou, H.; Ai, J.; Zhang, H.; Tao, J.; Wang, D.; Zhang, W. Effects of chemical modification on physicochemical properties and adsorption behavior of sludge-based activated carbon. J. Environ. Sci. 2021, 100, 340–352. [Google Scholar] [CrossRef]
- Kalam, S.; Abu-Khamsin, S.A.; Kamal, M.S.; Patil, S. Surfactant adsorption isotherms: A review. ACS Omega 2021, 6, 32342–32348. [Google Scholar] [CrossRef]
- Ahmadou, F.; Abahdou, F.Z.; Slimani, R.; El Hajjaji, S. Methylen blue removal by adsorption on Moringa oleifera pods powder and Moringa oleifera kernels powder: Kinetic, isotherm and thermodynamic studies. Moroc. J. Chem. 2023, 11, 11-1. [Google Scholar]
- Elsherif, K.M.; El-Dali, A.; Ewlad-Ahmed, A.M.; Treban, A.A.; Alqadhi, H.; Alkarewi, S. Kinetics and isotherms studies of safranin adsorption onto two surfaces prepared from orange peels. Moroc. J. Chem. 2022, 10, 10-4. [Google Scholar]
- Nguyen, V.T.; Nguyen, T.A.; Tran, T.H.; Le, T.N.; Nguyen, N.H. Batch and column adsorption of reactive dye by eggshell powder–chitosan gel core-shell material. Moroc. J. Chem. 2021, 9, 9-1. [Google Scholar]
- Gunasekar, V.; Ponnusami, V. Kinetics, equilibrium, and thermodynamic studies on adsorption of methylene blue by carbonized plant leaf powder. J. Chem. 2012, 2013, 415280. [Google Scholar] [CrossRef]
- Farch, S.; Yahoum, M.M.; Toumi, S.; Tahraoui, H.; Lefnaoui, S.; Kebir, M.; Zamouche, M.; Amrane, A.; Zhang, J.; Hadadi, A.; et al. Application of Walnut Shell Biowaste as an Inexpensive Adsorbent for Methylene Blue Dye: Isotherms, Kinetics, Thermodynamics, and Modeling. Separations 2023, 10, 60. [Google Scholar] [CrossRef]
- Kończak, M.; Huber, M. Application of the engineered sewage sludge-derived biochar to minimize water eutrophication by removal of ammonium and phosphate ions from water. J. Clean. Prod. 2022, 331, 129994. [Google Scholar] [CrossRef]
- Benhiti, R.; Ait Ichou, A.; Zaghloul, A.; Aziam, R.; Carja, G.; Zerbet, M.; Sinan, F.; Chiban, M. Synthesis, characterization, and comparative study of MgAl-LDHs prepared by standard coprecipitation and urea hydrolysis methods for phosphate removal. Environ. Sci. Pollut. Res. 2020, 27, 45767–45774. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, X.; Wang, J. A critical review of various adsorbents for selective removal of nitrate from water: Structure, performance and mechanism. Chemosphere 2022, 291, 132728. [Google Scholar] [CrossRef] [PubMed]
- Arai, Y.; Sparks, D. ATR–FTIR spectroscopic investigation on phosphate adsorption mechanisms at the ferrihydrite–Water interface. J. Colloid Interface Sci. 2001, 241, 317–326. [Google Scholar] [CrossRef]
- Chiban, M.; Soudani, A.; Sinan, F.; Persin, M. Single, binary and multi-component adsorption of some anions and heavy metals on environmentally friendly Carpobrotus edulis plant. Colloids Surfaces B Biointerfaces 2022, 82, 267–276. [Google Scholar] [CrossRef] [PubMed]







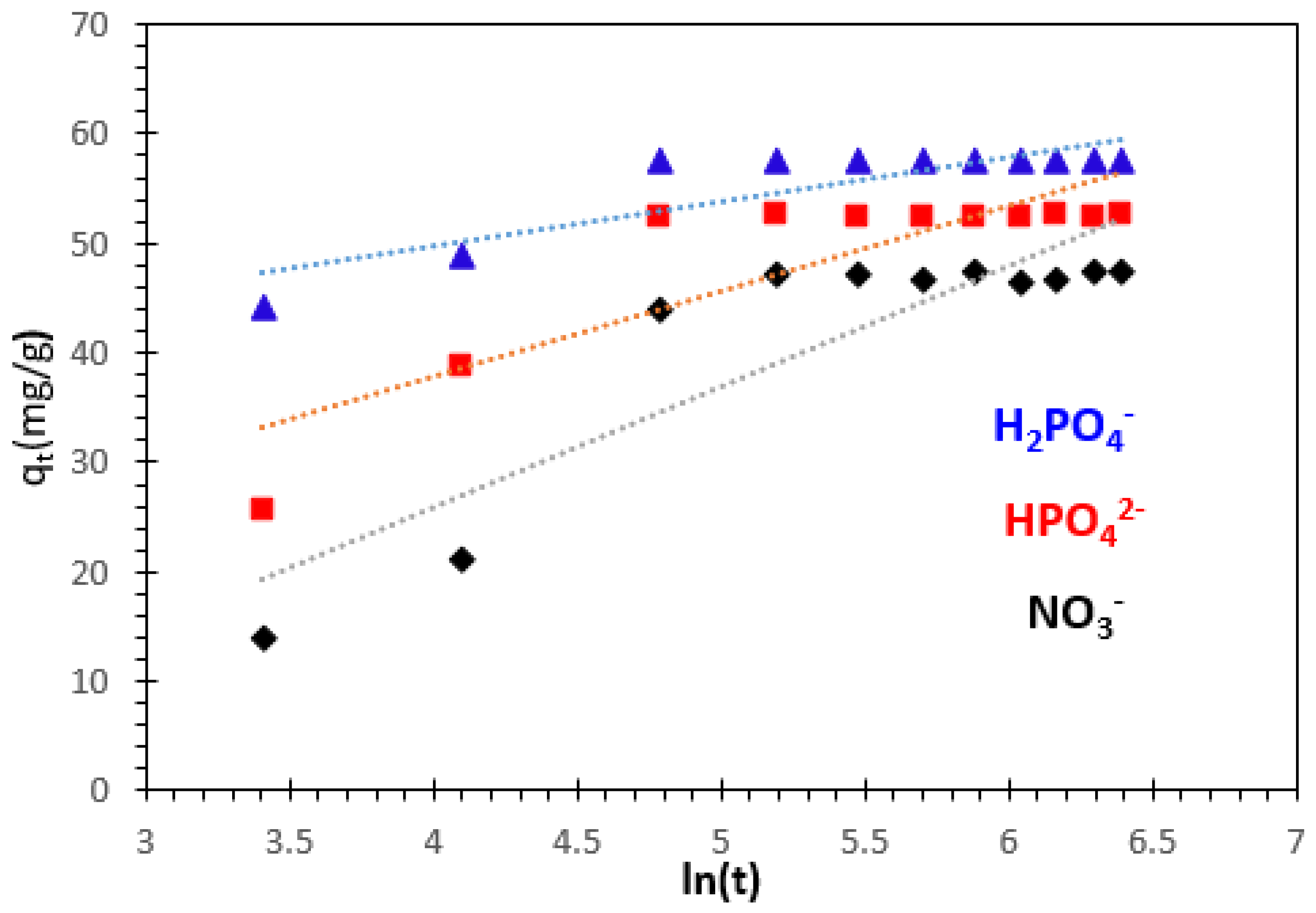
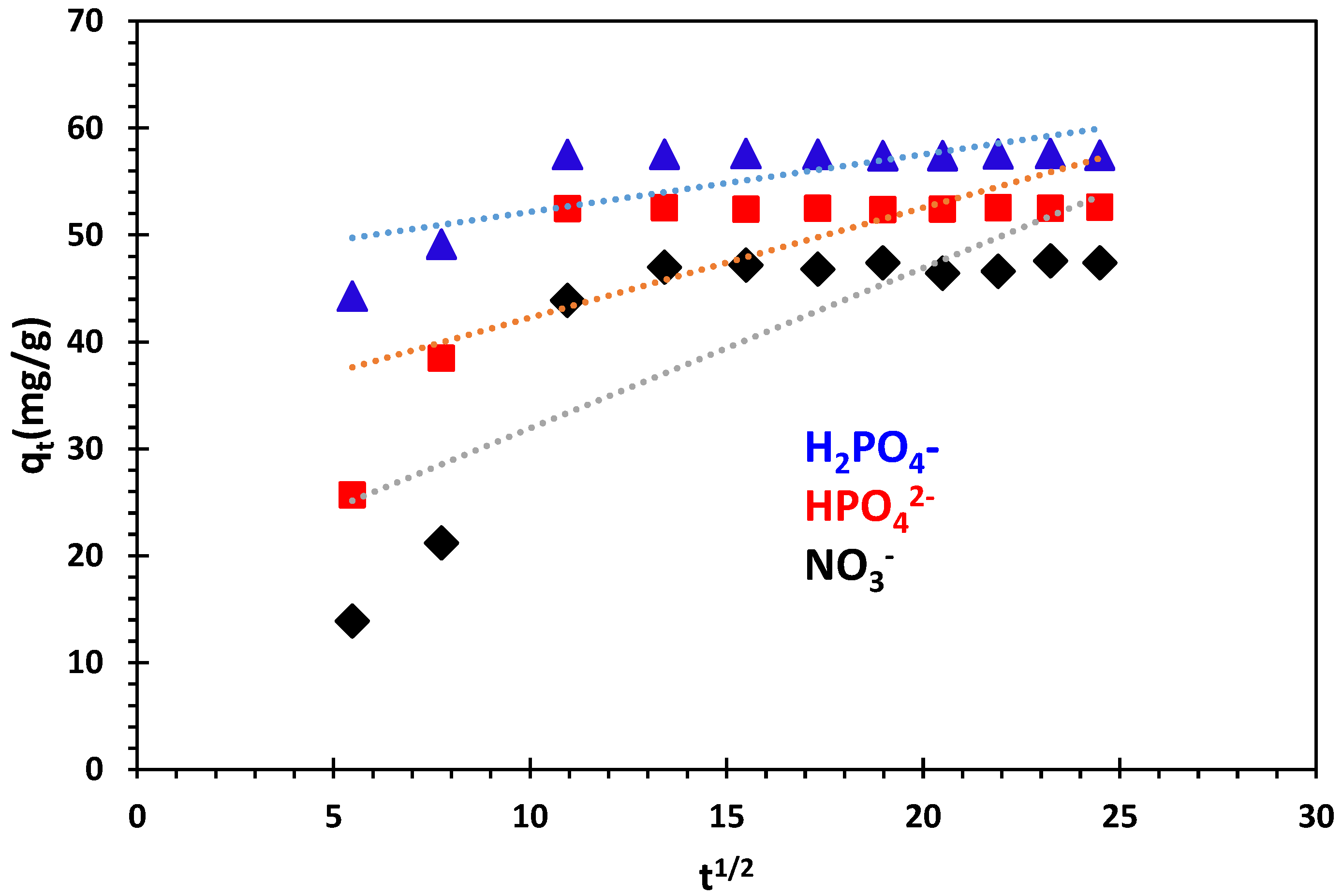

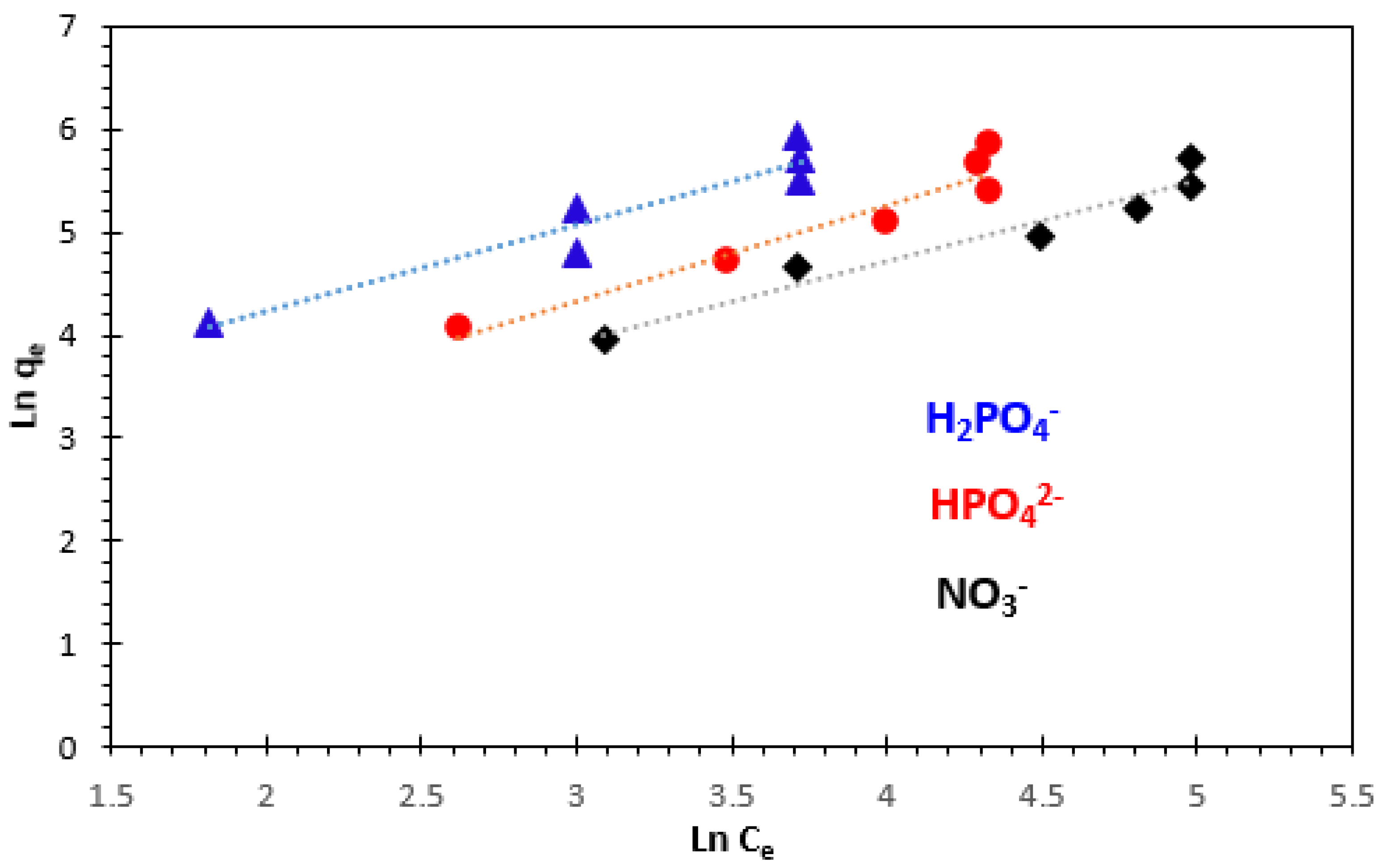
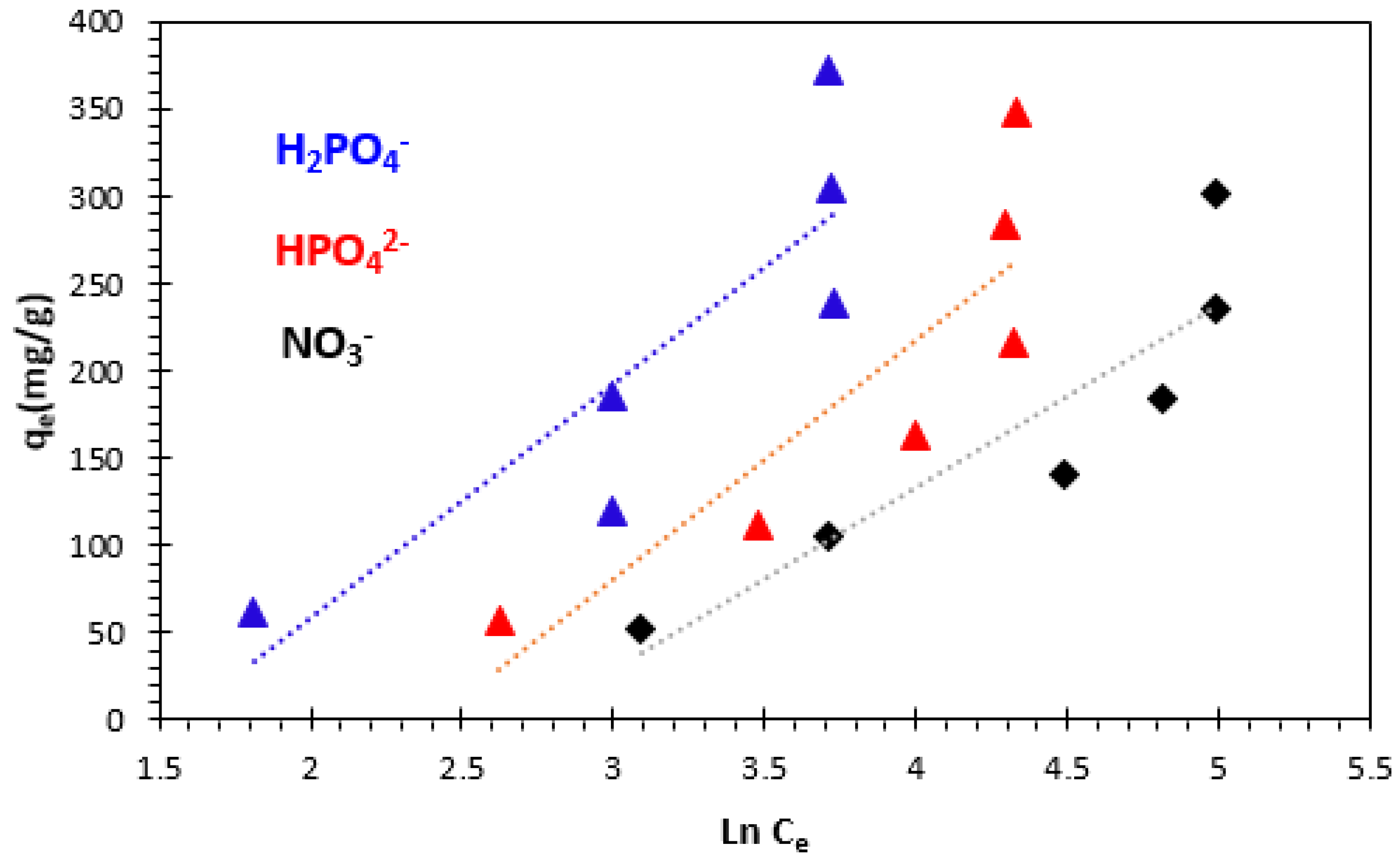
| Kinetics Model | Parameters | Phosphate Ions | Nitrate Ions | |
|---|---|---|---|---|
| H2PO4− | HPO42− | NO3− | ||
| Pseudo-first-order model | qe,exp (mg.g−1) | 62.37 | 57.30 | 51.85 |
| K1 (min−1) | 0.0104 | 0.0178 | 0.0101 | |
| qe,cal (mg.g−1) | 6.853 | 9.079 | 8.584 | |
| R2 | 0.916 | 0.888 | 0.771 | |
| Pseudo-second-order model | qe,exp (mg.g−1) | 62.37 | 57.30 | 51.85 |
| K2 (g.mg−1min−1) | 0.0030 | 0.0011 | 3.7 × 10−4 | |
| qe,the (mg.g−1) | 58.139 | 54.64 | 52.63 | |
| R2 | 0.999 | 0.998 | 0.986 | |
| Elovich model | qe,exp (mg.g−1) | 62.37 | 57.30 | 51.85 |
| α | 91.46 | 18.05 | 50.06 | |
| β | 0.246 | 0.128 | 0.090 | |
| R2 | 0.746 | 0.737 | 0.794 | |
| Intra-particle diffusion | KInt (mg.g−1min−1/2) | 0.537 | 1.027 | 1.488 |
| CI | 46.78 | 31.99 | 16.94 | |
| R2 | 0.568 | 0.554 | 0.630 | |
| Ions | Orthophosphates | Nitrates | |
|---|---|---|---|
| H2PO4− | HPO42− | NO3− | |
| RL | 0.495 | 0.261 | 0.270 |
| Model | Parameters | Orthophosphate Ions | Nitrate Ions | |
|---|---|---|---|---|
| H2PO4− | HPO42− | NO3− | ||
| Langmuir | qL (mg/g) | 625 | 909.09 | 588.23 |
| KL (L/mg) | 0.017 | 0.0047 | 0.0045 | |
| R2 | 0.940 | 0.975 | 0.970 | |
| Freundlich | 1/n | 0.835 | 0.933 | 0.782 |
| KF (mg·g−1) | 13.06 | 4.63 | 4.89 | |
| R2 | 0.899 | 0.925 | 0.937 | |
| Temkin | KT (L.mg–1) | 0.210 | 0.090 | 0.065 |
| bT (J.mol–1) | 18.509 | 15.92 | 23.73 | |
| R2 | 0.757 | 0.743 | 0.813 | |
| D–R | KD (mol2J–1) | 10−5 | 5 × 10−5 | 0.0001 |
| qm (mg·g−1) | 249.011 | 235.003 | 209.013 | |
| E (J.mol−1) | 223.60 | 44.72 | 70.71 | |
| R2 | 0.717 | 0.747 | 0.821 | |
| Ion | ΔG° (kJ/mol) | ΔH° (kJ/mol) | ΔS° (J/K/mol) | |||
|---|---|---|---|---|---|---|
| 25 °C | 30 °C | 35 °C | 40 °C | |||
| H2PO4− | −17.089 | −18.436 | −20.140 | −25.038 | 134.603 | 506.40 |
| HPO42− | −14.85 | −16.167 | −17.178 | −18.231 | 51.692 | 223.45 |
| NO3− | −12.359 | −14.138 | −16.938 | −21.291 | 161.32 | 581.45 |
| Adsorbent | Anions | qm (mg/g) | Reference |
|---|---|---|---|
| Raw shrimp shells | HPO42− | 92 | [9] |
| H2PO4− | 133 | [9] | |
| Chitin | NO3− | 200 | [47] |
| H2PO4− | 336 | [49] | |
| HPO42− | 110 | [49] | |
| C. edulis plant | H2PO4− | 26.1 | [65] |
| NO3− | 141.1 | [65] | |
| LDH/alginate composite | Phosphate | 400 | [27] |
| Alginate–Moroccan clay bio-nanocomposite | H2PO4− | 625 | This study |
| HPO42− | 909.09 | ||
| NO3− | 588.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aziam, R.; Stefan, D.S.; Aboussabek, A.; Chiban, M.; Croitoru, A.-M. Alginate–Moroccan Clay, New Bio-Nanocomposite for Removal of H2PO4−, HPO42−, and NO3− Ions from Aqueous Solutions. Polymers 2023, 15, 4666. https://doi.org/10.3390/polym15244666
Aziam R, Stefan DS, Aboussabek A, Chiban M, Croitoru A-M. Alginate–Moroccan Clay, New Bio-Nanocomposite for Removal of H2PO4−, HPO42−, and NO3− Ions from Aqueous Solutions. Polymers. 2023; 15(24):4666. https://doi.org/10.3390/polym15244666
Chicago/Turabian StyleAziam, Rachid, Daniela Simina Stefan, Abdelali Aboussabek, Mohamed Chiban, and Alexa-Maria Croitoru. 2023. "Alginate–Moroccan Clay, New Bio-Nanocomposite for Removal of H2PO4−, HPO42−, and NO3− Ions from Aqueous Solutions" Polymers 15, no. 24: 4666. https://doi.org/10.3390/polym15244666





