Non-Covalent Functionalization of Graphene Oxide with POSS to Improve the Mechanical Properties of Epoxy Composites
Abstract
:1. Introduction
2. Experiment
2.1. Raw Materials
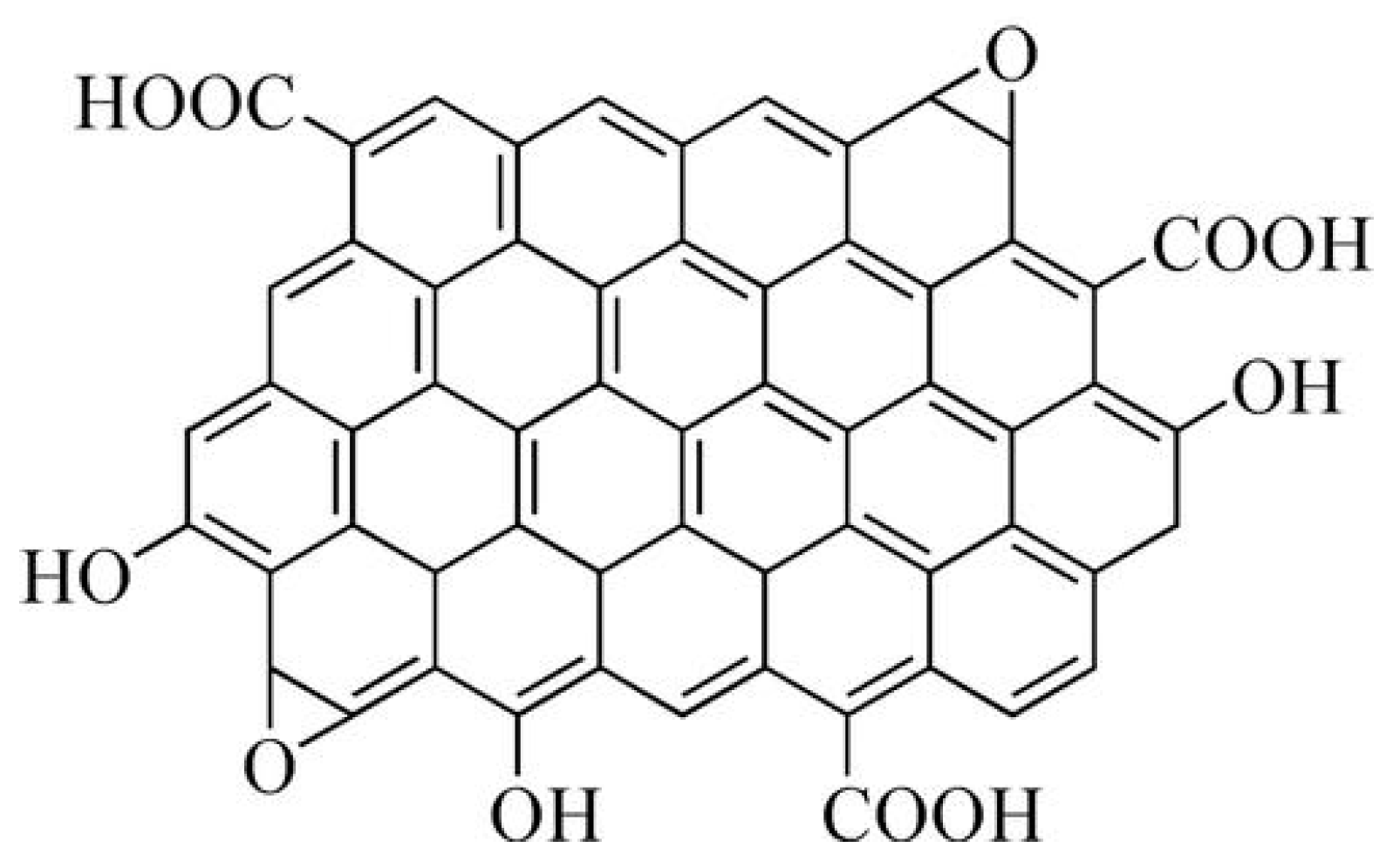
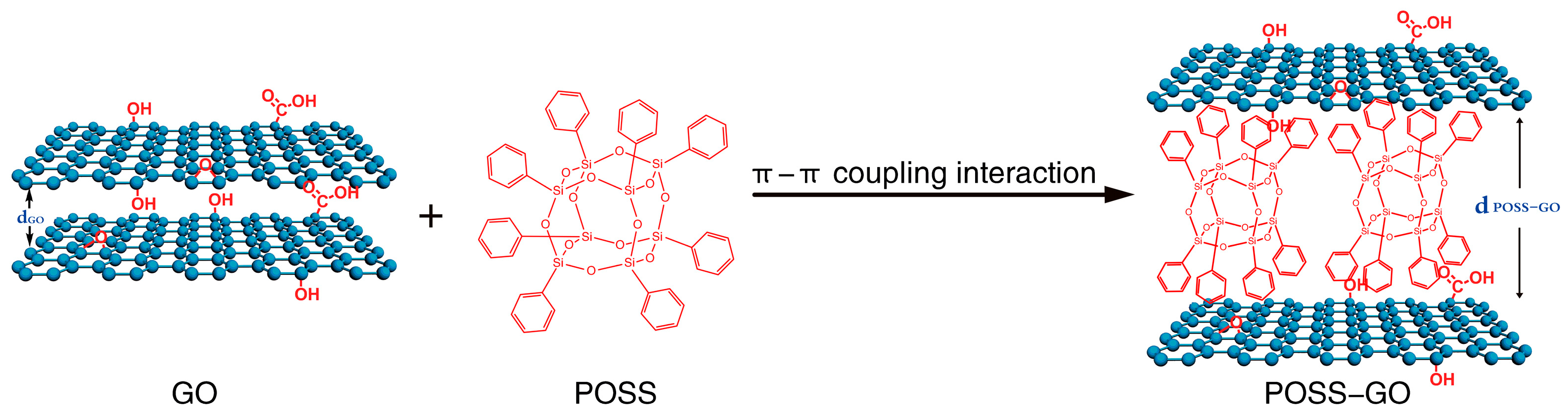
2.2. Preparation and Mechanism of Phenyl POSS-Functionalized GO (POSS-GO)
- (1)
- Firstly, 100 mg of GO was weighed and then dried at 80 °C for 2. Subsequently, it was dissolved in 100 mL of DMAc dispersion at room temperature through mechanical stirring (model XFK JJ-1A, stirring speed: 300 rpm) and an ultrasonic water bath treatment (model JP-010S) for 15 min.
- (2)
- The GO dispersion was transferred to a 500 mL three-necked flask, and then 100 mL of DMAc and 50 mg of POSS were added to it. The solution was mechanically stirred at room temperature (300 rpm) and simultaneously treated with an ultrasonic water bath for 2 h.
- (3)
- The solution was filtered under reduced pressure using a polytetrafluoroethylene (PTFE) filter membrane with a pore size of 0.45 µm, and the filtered material was washed 5 times with anhydrous ethanol. Then, the solvent was placed in a vacuum oven and dried under vacuum at 80 °C for 12 h. Finally, the phenyl POSS non-covalent functionalized GO (abbreviated as POSS-GO) was obtained. The preparation principle is shown in Figure 3.
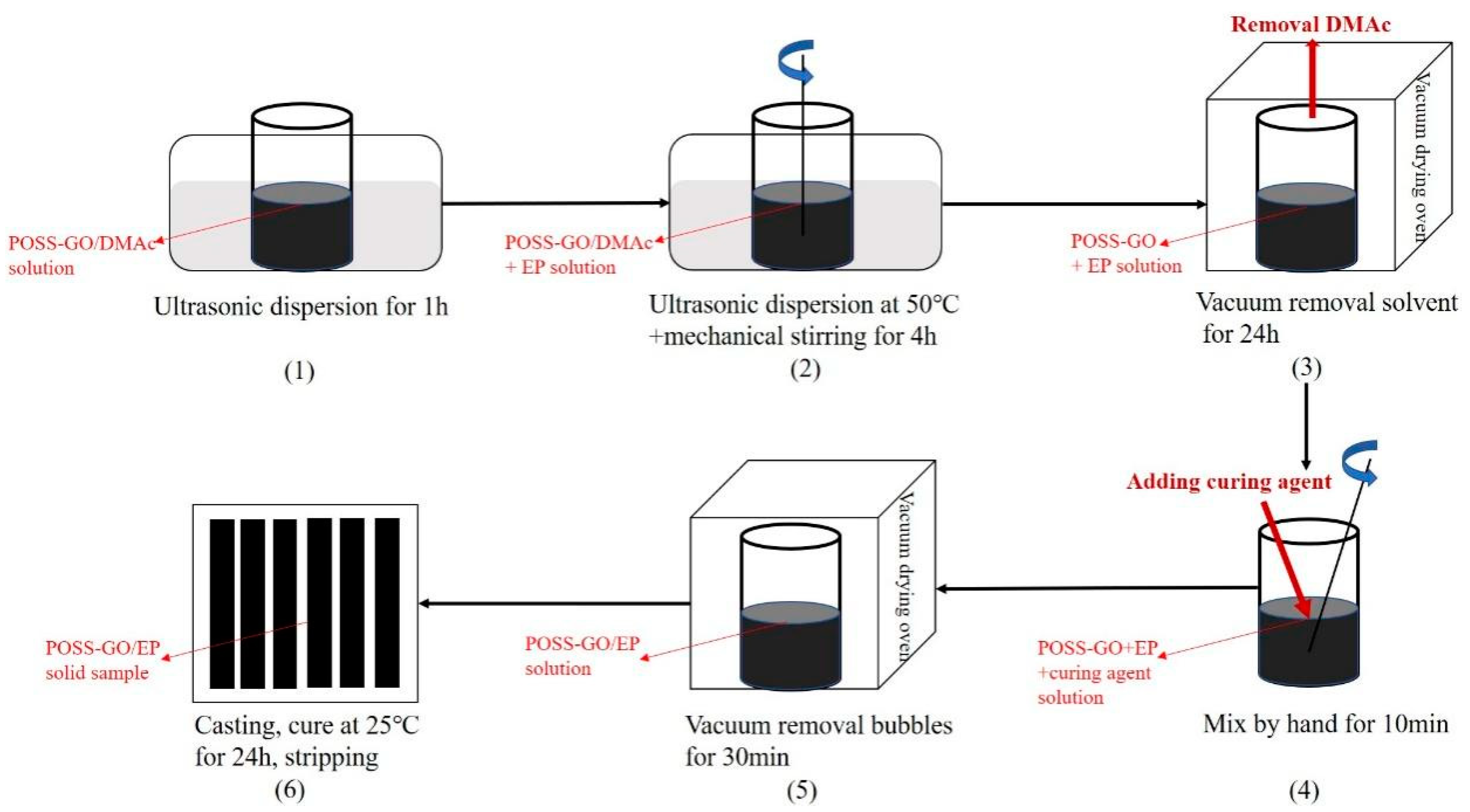
2.3. Preparation of POSS-GO-Reinforced EP (POSS-GO/EP) Composite Material
- (1)
- An appropriate amount of POSS-GO was dispersed in a DMAc solution, which was then sonicated in a water bath for 1 h to obtain the POSS-GO/DMAc dispersion.
- (2)
- The POSS-GO/DMAc dispersion was injected into an EP solution, and the mixture was subjected to an ultrasonic water bath treatment at 50 °C for 4 h. At the same time, a high-speed mechanical mixer was used to stir the mixture at 500 rpm.
- (3)
- The dispersed mixture was placed in a vacuum dryer, and the solvent DMAc removal was realized through a vacuum pump for 24 h.
- (4)
- The mixture with DMAc removed was mixed with the curing agent at a ratio of 100:30 and stirred for 10 min to allow for them to be completely well-mixed.
- (5)
- The fully mixed mixture was placed in a 25 °C constant-temperature vacuum chamber for 30 min to eliminate bubbles.
- (6)
- Finally, the mixture was casted into a PTFE mold, cured at 25 °C for 24 h, and demolded to prepare the POSS-GO-reinforced EP (POSS-GO/EP) composite material.
2.4. Characterization
3. Results and Discussion
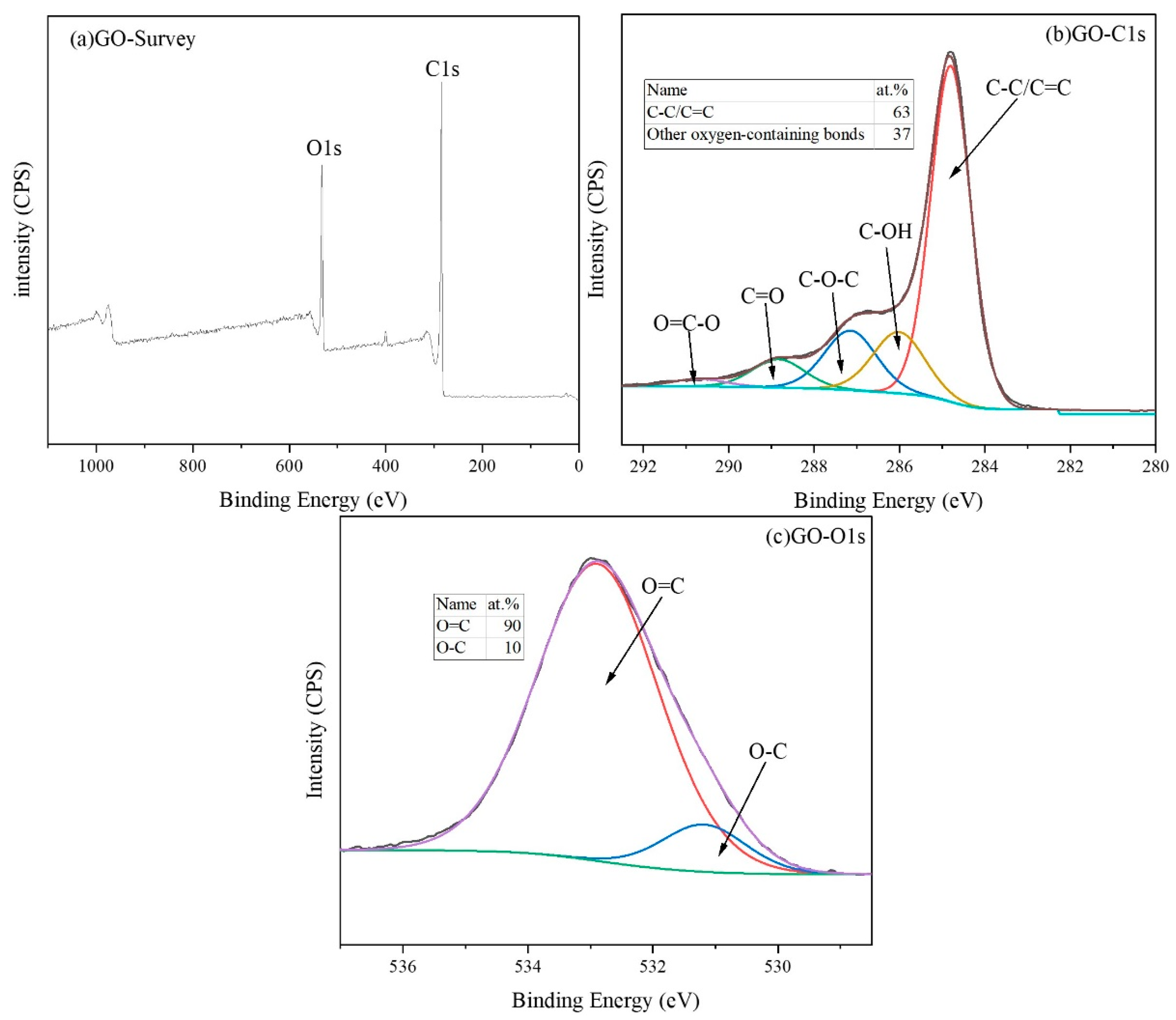
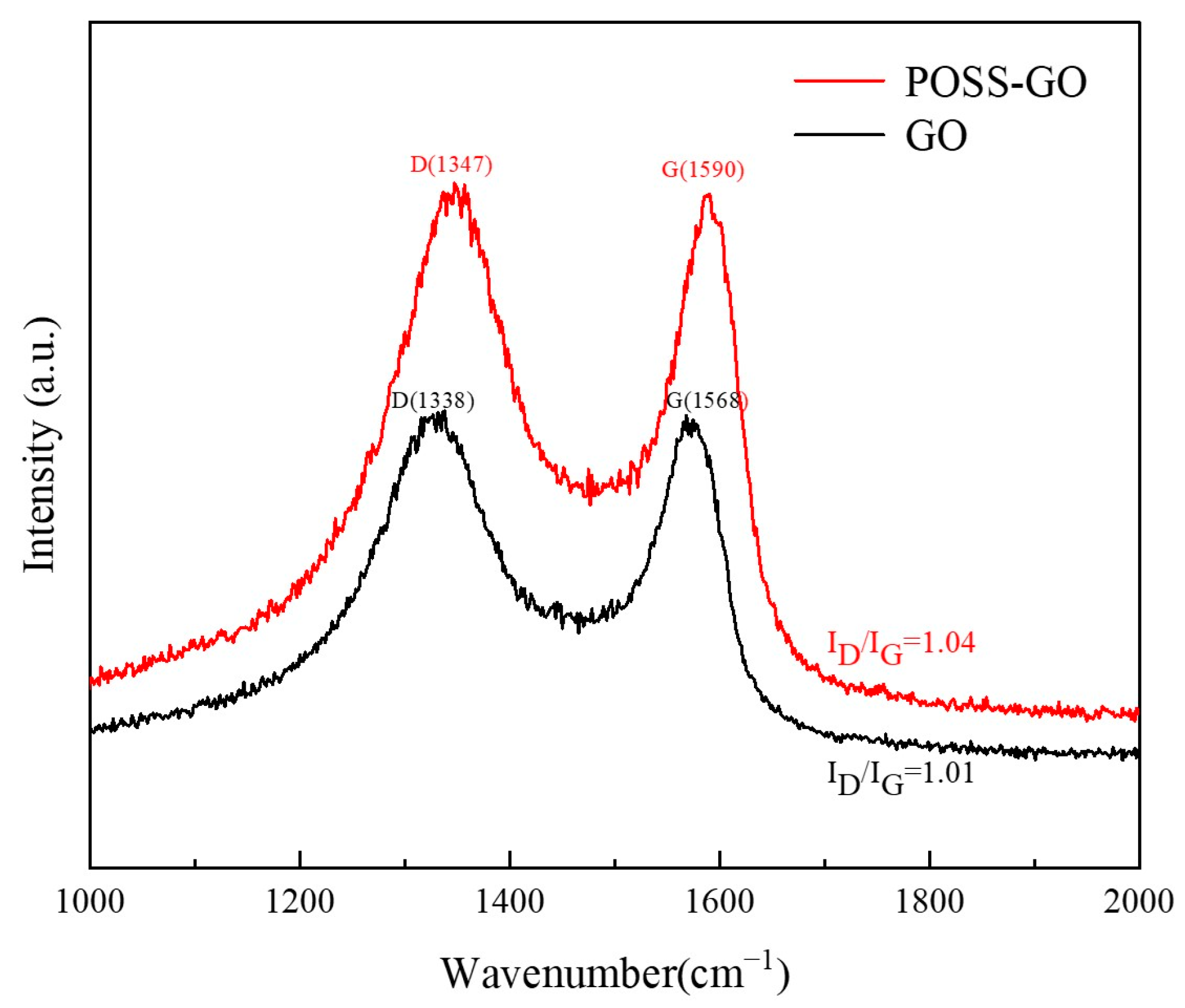
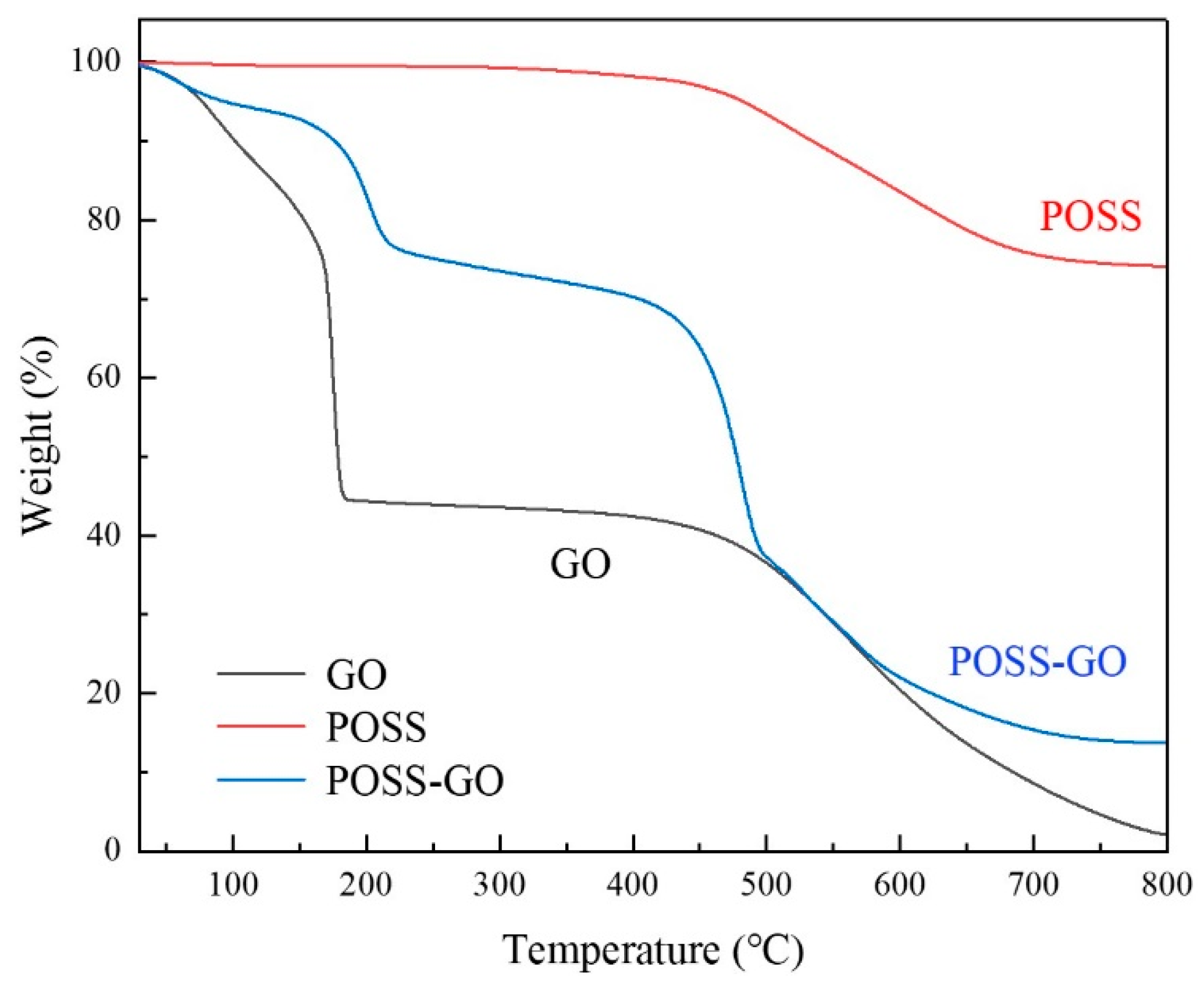
3.1. Characterization of Non-Covalent Functionalized GO
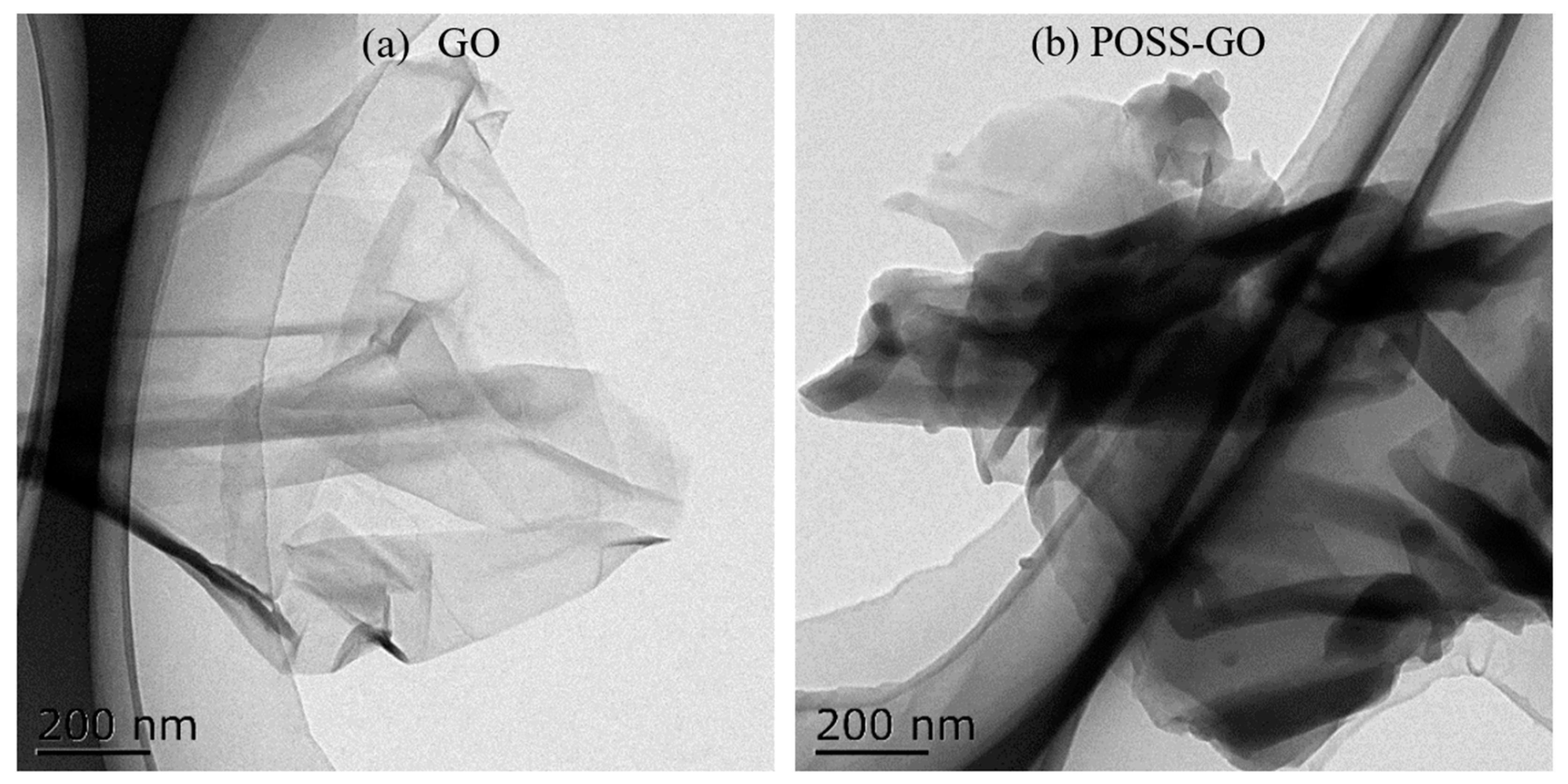
3.2. Morphology and Dispersion Properties of Non-Covalent Functionalized GO
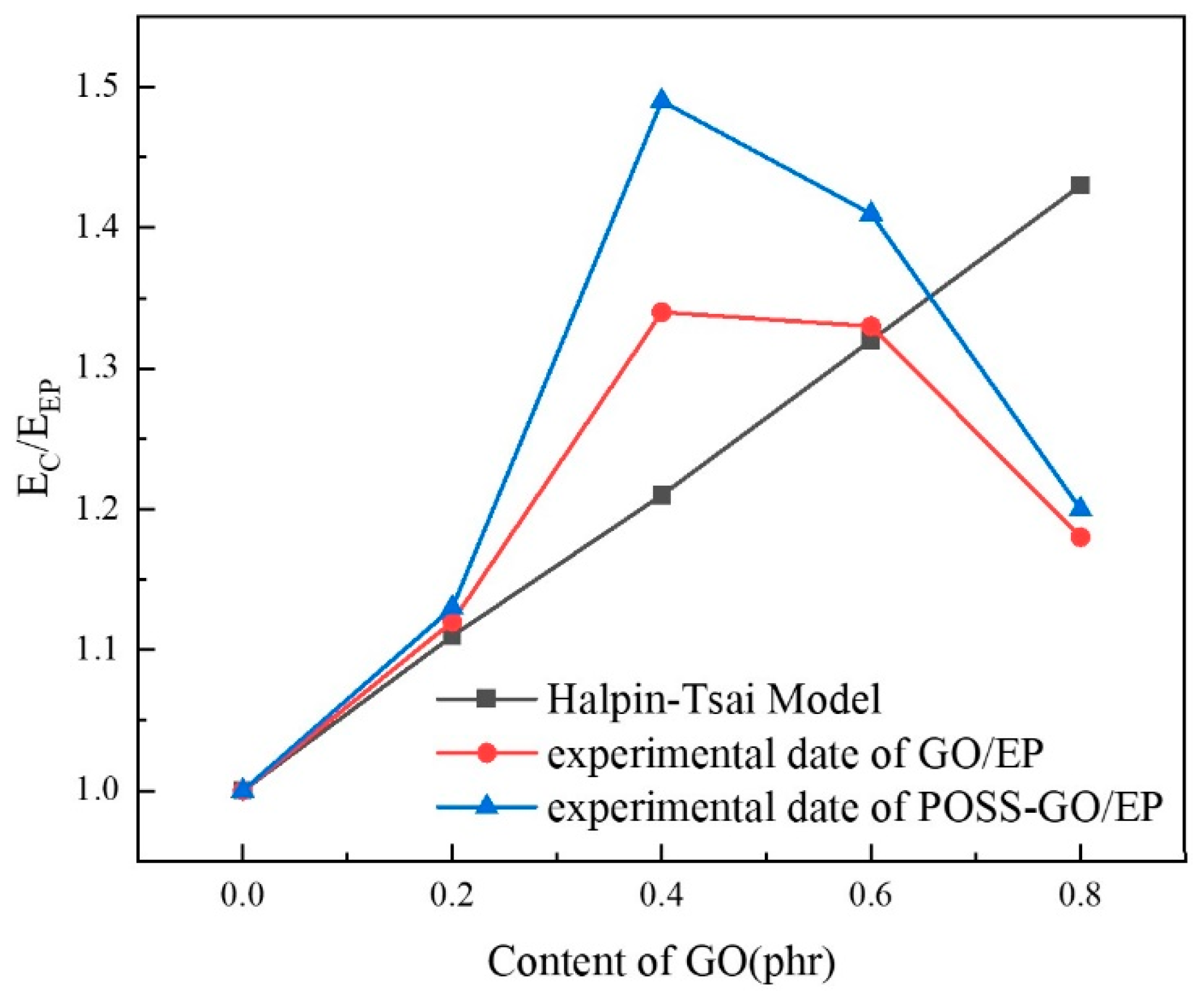
3.3. Mechanical Properties
3.3.1. Microhardness
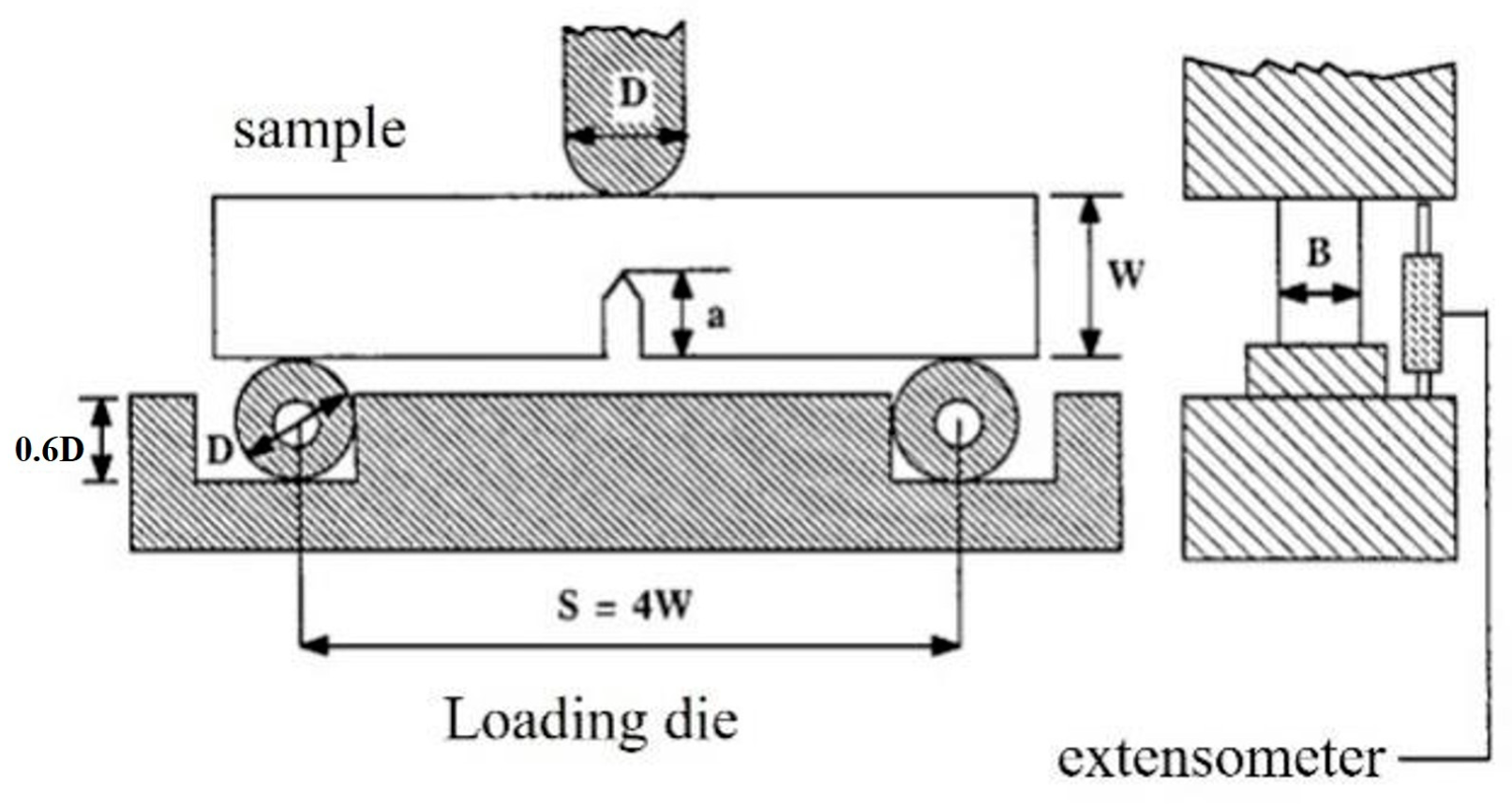
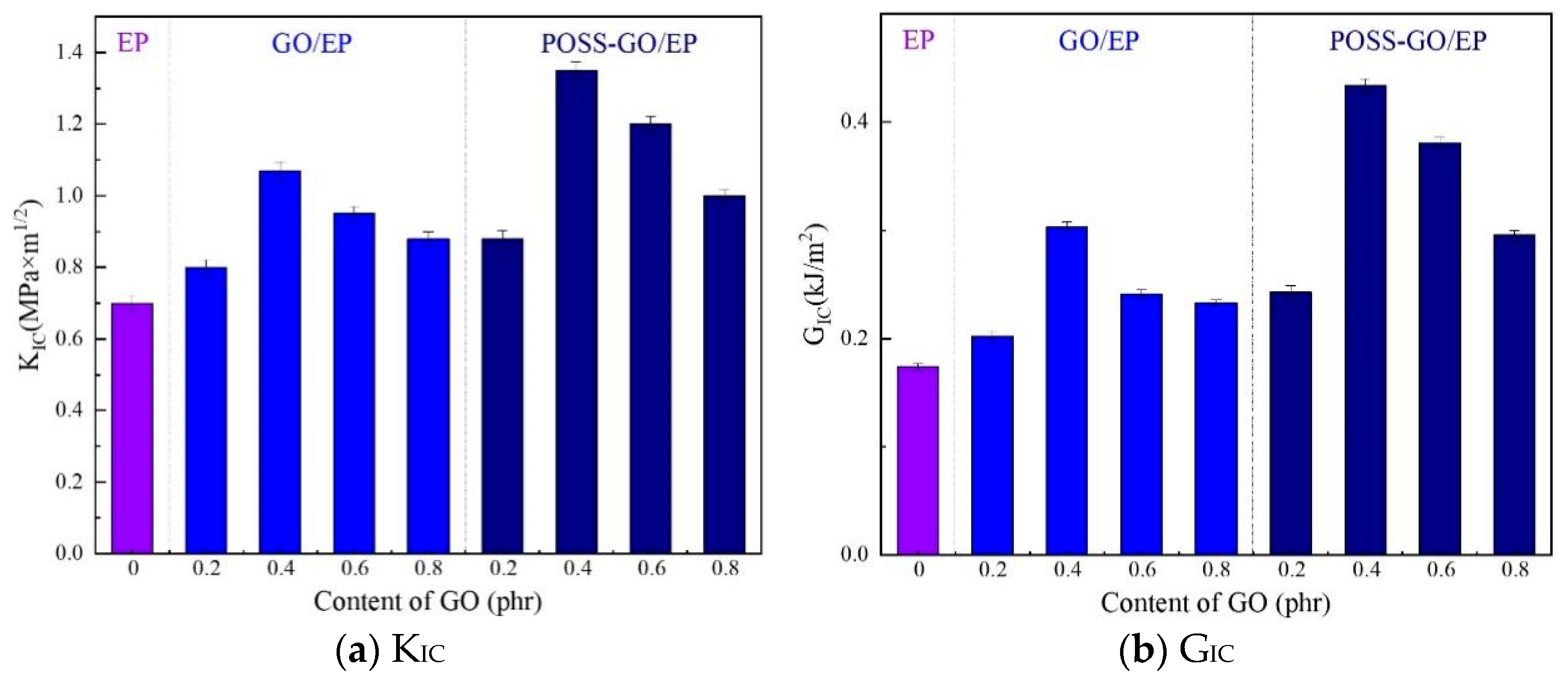
3.3.2. Fracture Toughness
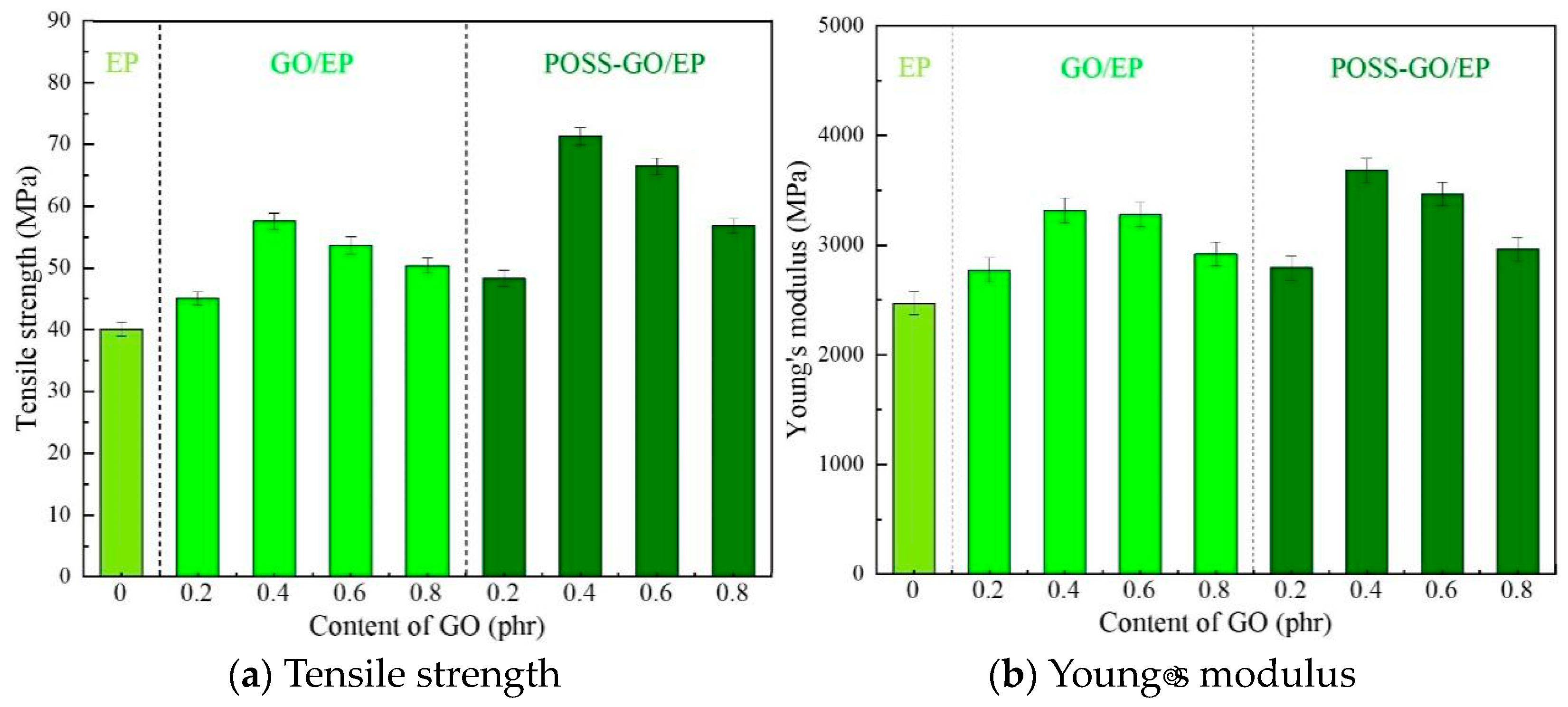
3.3.3. Tensile Properties
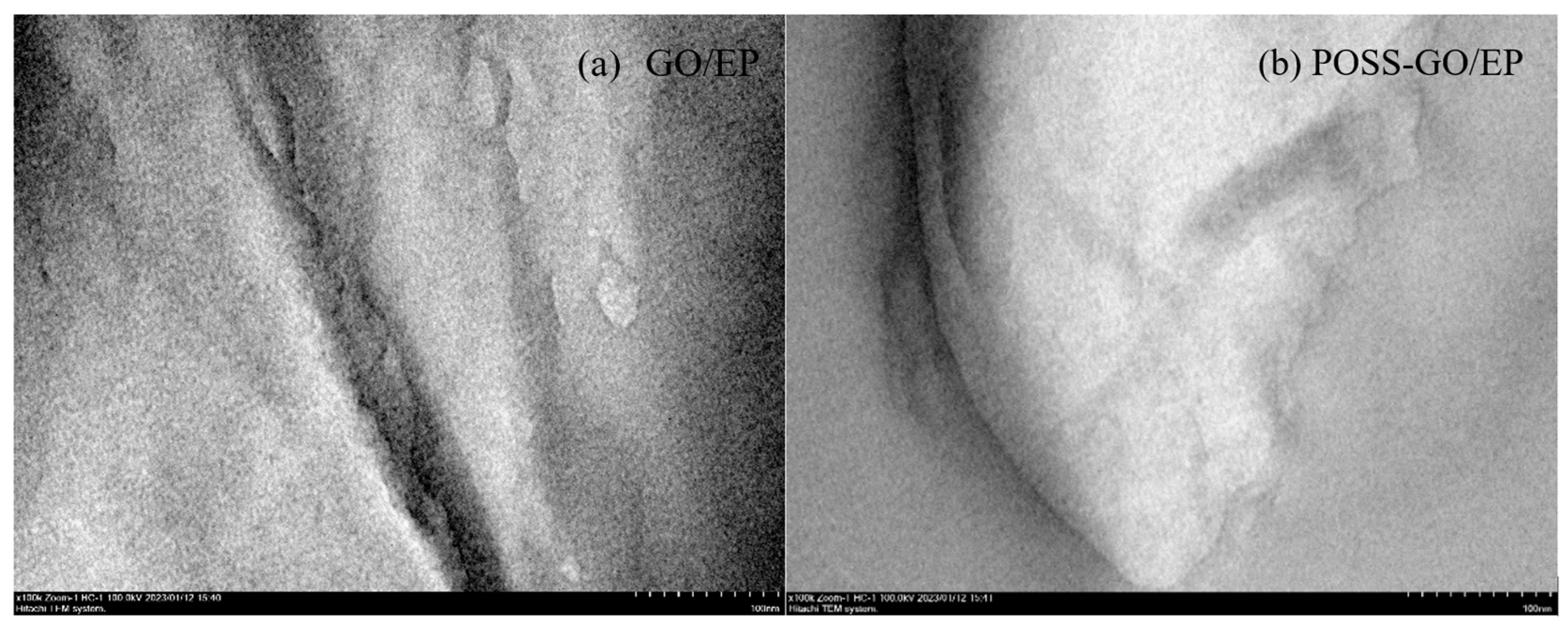
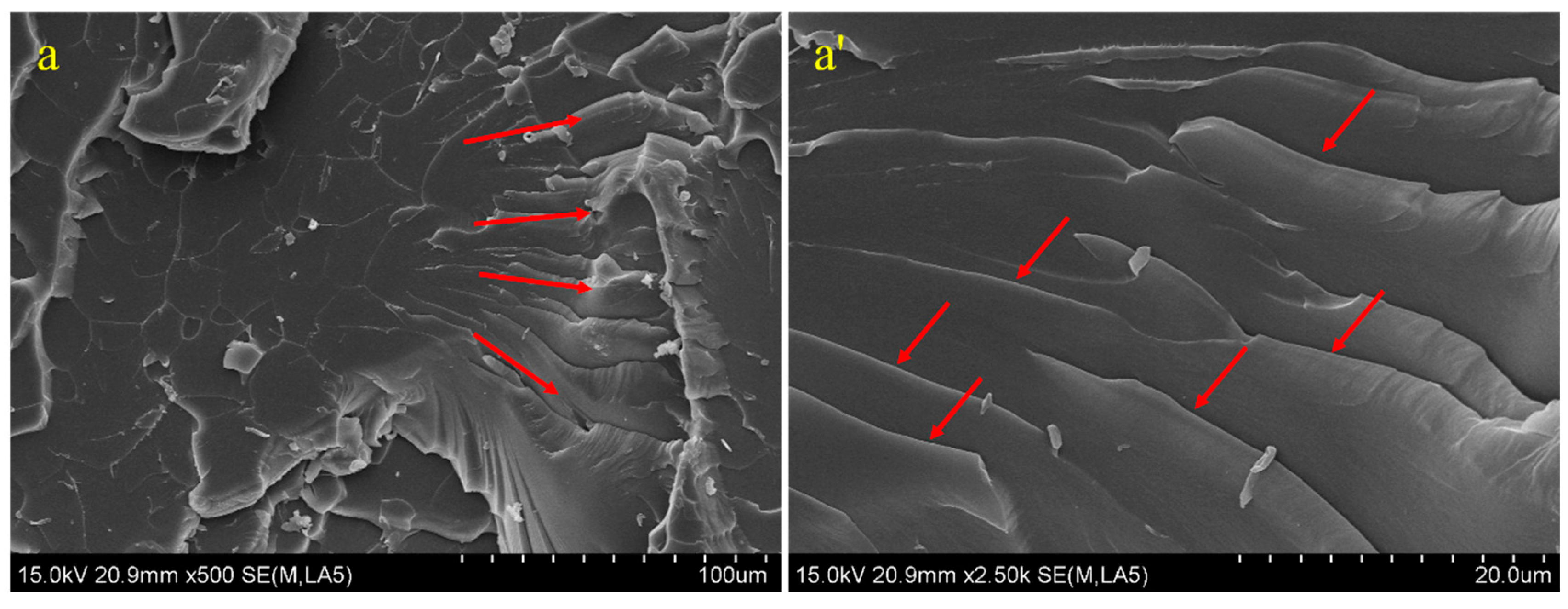
3.4. Tensile Cross-Sectional Morphology and Reinforcement and Toughening Mechanism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yao, J.; Chang, H.C.; Zhang, T.; Niu, Y.F. Synergistic toughening in the interleaved carbon fibre reinforced epoxy composites by thermoplastic resin and nanomaterials. Polym. Test. 2022, 115, 107769. [Google Scholar] [CrossRef]
- Chowaniec, A.; Sadowski, Ł.; Żak, A. The chemical and microstructural analysis of the adhesive properties of epoxy resin coatings modified using waste glass powder. Appl. Surf. Sci. 2020, 504, 144373. [Google Scholar] [CrossRef]
- Jiang, T.; Guan, H.L.; Chen, X.R.; Zou, H.L. Review of Electrical Properties of Epoxy Resin Nanocomposites. Zhejiang Electr. Power 2019, 38, 65–71. (In Chinese) [Google Scholar]
- Li, L.L.; Li, Q.; Xiao, W.G.; Chai, P.J. Research progress in graphene/epoxy resin nanocomposites. Compos. Mater. Sci. Eng. 2021, 5, 110–119. (In Chinese) [Google Scholar]
- Palraj, S.; Selvaraj, M. Corrosion and wear resistance behavior of nano-silica epoxy composite coatings. Prog. Org. Coat. 2015, 81, 132–139. [Google Scholar] [CrossRef]
- Peng, J.; Qie, J.J.; Zhu, J.J.; Lun, W.C.; Deng, S.J.; Luo, P. Research progress in toughening modification of epoxy resin. New Chem. Mater. 2022, 50, 221–224. (In Chinese) [Google Scholar]
- Amirbek, B.; Anton, M.; Andrey, S.; Lyazzat, T.; Marzhan, A.; Ainagul, A.; Raigul, O.; Marina, L. The Influence of Pristine and Aminoacetic Acid-Treated Aluminum Nitride on the Structure, Curing Processes, and Properties of Epoxy Nanocomposites. J. Compos. Sci. 2023, 7, 482. [Google Scholar]
- Asad, H.; Mohammad, I.; Iftikhar, A.; Nasir, M.; Shaukat, S.; Hassan, J. Thermal and mechanical properties of carbon nanotube/epoxy nanocomposites reinforced with pristine and functionalized multiwalled carbon nanotubes. Polym. Compos. 2015, 36, 1891–1898. [Google Scholar]
- Hesam, A.; Hamed, K.; Esmaeil, T. Reinforcing effects of aminosilane-functionalized graphene on the tribological and mechanical behaviors of epoxy nanocomposites. Appl. Polym. Sci. 2019, 136, 47410. [Google Scholar]
- Rao, C.N.R.; Biswas, K.; Subrahmanyam, K.S.; Govindaraj, A. Graphene, the new nanocarbon. J. Mater. Chem. 2009, 19, 2457–2469. [Google Scholar] [CrossRef]
- Yang, S.Y.; Lin, W.N. Synergetic effects of graphene platelets and carbon nanotubes on the mechanical and thermal properties of epoxy composites. Carbon. 2011, 49, 793–803. [Google Scholar] [CrossRef]
- Li, Z.; Wang, R. Control of the functionality of graphene oxide for its application in epoxy nanocomposites. Polymer 2013, 54, 6437–6446. [Google Scholar] [CrossRef]
- Wan, Y.J.; Gong, L.X.; Tang, L.C.; Wu, L.B.; Jiang, J.X. Mechanical properties of epoxy composites filled with silane-functionalized graphene oxide. Compos. Part A Appl. S 2014, 64, 79–89. [Google Scholar] [CrossRef]
- Li, J.H.; Wang, R.H.; Wang, T.Y.; Gu, S.Q.; Lyu, Z.P.; Chen, T. Preparation and properties of graphene/cyanate ester-epoxy composites. Acta Mater. Compos. Sin. 2014, 5, 34–39. [Google Scholar]
- Ding, J.; Huang, Y.; Sun, X.; Wu, H.W.; Wang, Y.L. Synthesis, mechanical properties and thermal stability of the functional reduced graphene oxide /bisphenol A cyanate ester nanocomposites. J. Mater. Sci. Mater. Electron. 2016, 27, 3462–3473. [Google Scholar] [CrossRef]
- Georgakilas, V.; Otyepka, M. Functionalization of Graphene: Covalent and Non-Covalent Approaches, Derivatives and Applications. Chem. Rev. 2012, 11, 6156–6214. [Google Scholar] [CrossRef] [PubMed]
- Baney, R.H.; Itoh, M.; Sakakibara, A.; Suzuki, T. Silsesquioxanes. Chem. Rev. 1995, 95, 1409–1430. [Google Scholar] [CrossRef]
- Kuo, S.W.; Chang, F.C. POSS related polymer nanocomposites. Prog. Polym. Sci. 2011, 36, 1649–1696. [Google Scholar] [CrossRef]
- Jiang, D.W.; Xing, L.X.; Liu, L.; Yan, X.R.; Guo, J.; Zhang, X.; Zhang, Q.B.; Wu, Z.J.; Zhao, F.; Huang, Y.G.; et al. Interfacially reinforced unsaturated polyester composites by chemically grafting different functional POSS onto carbon fibers. J. Mater. Chem. A 2014, 2, 18293. [Google Scholar] [CrossRef]
- Zhao, F.; Huang, Y.D. Improved interfacial properties of carbon fiber/epoxy composites through grafting polyhedral oligomeric silsesquioxane on carbon fiber surface. Mater. Lett. 2010, 64, 2742–2744. [Google Scholar] [CrossRef]
- Liu, K.; Lu, S.R.; Li, S.R.; Huang, B.; Wei, C. Mechanical and thermal properties of POSS-g-GO reinforced epoxy composites. Iran. Polym. J. 2012, 21, 497–503. [Google Scholar] [CrossRef]
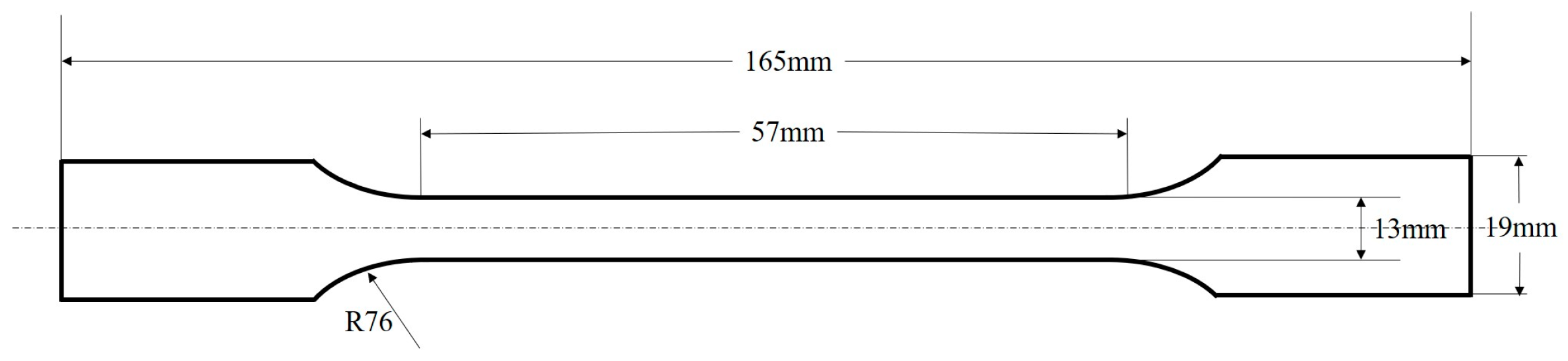
- ASTM D5045-99; ASTM International. Standard Test Methods for Plane-Strain Fracture Toughness and Strain Energy Release Rate of Plastic Materials. ASTM International: West Conshohocken, PA, USA, 1999.
- Kinloch, A.J. Adhesion and Adhesives: Science and Technology; Chapman & Hall: London, UK, 1987; pp. 209–237. [Google Scholar]
- ASTM D638-2010; ASTM International. Standard Test Method for Tensile Properties of Plastics. ASTM International: West Conshohocken, PA, USA, 2010.
- Kim, J.Y.; Kim, H.; Kim, T.Y.; Yu, S.; Suk, J.W.; Jeong, T.; Song, S.J.; Bae, M.J.; Han, I.; Jung, D.G.; et al. A chlorinated barium titanate-filled polymer composite with a high dielectric constant and its application to electroluminescent devices. J. Mater. Chem. C 2013, 1, 5078–5083. [Google Scholar] [CrossRef]
- McNally, T.; Potschke, P.; Halley, P.; Murphy, M.; Martin, D.J.; Bell, S.E.; Brennan, G.P.; Bein, D.; Lemoine, P.; Quinn, J.P. Polyethylene multiwalled carbon nanotube composites. Polymer 2005, 46, 8222–8232. [Google Scholar] [CrossRef]
- Wang, S.; Chia, P.J.; Chua, L.L.; Zhao, L.H.; Png, R.Q.; Sivaramakrishnan, S.; Zhou, M.; Goh, R.G.; Friend, R.H.; Wee, A.T.; et al. Band-like Transport in Surface-Functionalized Highly Solution-Processable Graphene Nanosheets. Adv. Mater. 2008, 20, 3440–3446. [Google Scholar] [CrossRef]
- Zhang, M.M.; Yan, H.X.; Yang, X.W.; Liu, C. Effect of functionalized graphene oxide with a hyperbranched cyclotriphosphazene polymer on mechanical and thermal properties of cyanate ester composites. RSC Adv. 2014, 4, 45930–45938. [Google Scholar] [CrossRef]
- Teng, C.C.; Ma, C.C.M.; Lu, C.H.; Yang, S.Y.; Lee, S.H.; Hsiao, M.C.; Yen, M.Y.; Chiou, K.C.; Lee, T.M. Thermal conductivity and structure of non-covalent functionalized graphene/epoxy composites. Carbon. 2011, 49, 5107–5116. [Google Scholar] [CrossRef]
- Kiran, J.U.; Roners, J.P.; Mathew, S. XPS and thermal studies of silver doped SiO2 matrices for plasmonic applications. Mater. Today Proc. 2020, 33, 1263–1267. [Google Scholar] [CrossRef]
- Subramaniam, K.; Das, A.; Heinrich, G. Development of conducting polychloroprene rubber using imidazolium based ionic liquid modified multi-walled carbon nanotubes. Compos. Sci. Technol. 2011, 71, 1441–1449. [Google Scholar] [CrossRef]
- Fang, M.; Wang, K.; Lu, H.; Yang, Y.; Nutt, S. Covalent polymer functionalization of graphene nanosheets and mechanical properties of composites. J. Mater. Chem. 2009, 19, 7098–7105. [Google Scholar] [CrossRef]
- Li, Y.Z.; Guan, Y.Q.; Liu, Y.; Yin, J.B.; Zhao, X.P. Highly stable nanofluid based on polyhedral oligomeric silsesquioxane-decorated graphene oxide nanosheets and its enhanced electro-responsive behavior. Nanotechnology 2016, 27, 195702–195713. [Google Scholar] [CrossRef]
- He, K.; Chen, N.F.; Wang, C.J.; Wei, L.S.; Chen, J.K. Method for Determining Crystal Grain Size by X-Ray Diffraction. Cryst. Res. Technol. 2018, 53, 1700157. [Google Scholar] [CrossRef]
- Zhang, D.; Ma, Q.; Fan, H.; Yang, H.; Liu, S. Millimeter-long multilayer graphene nanoribbons prepared by wet chemical processing. Carbon 2014, 71, 120–126. [Google Scholar] [CrossRef]
- Shen, S.L.; Bi, S.S.; Hu, F.F.; Tan, Y.N.; Ma, L.J. Dispersion stability of SiO2/H2O nanofluid based on dynamic light scattering method. J. Eng. Thermophys. 2016, 37, 33–36. (In Chinese) [Google Scholar]
- Liu, H.; Liu, J.X.; Sun, Y.S.; Deng, J.H.; Chen, J.J.; Gao, C.Y. Study on influencing factors of dispersion of nano-silica under dynamic light scattering. Inorg. Chem. Ind. 2022, 54, 74–79. (In Chinese) [Google Scholar]
- Cordes, D.B.; Lickiss, P.D.; Rataboul, F. Recent Developments in the Chemistry of Cubic Polyhedral Oligosilsesquioxanes. Chem. Rev. 2010, 110, 2081–2173. [Google Scholar] [CrossRef]
- Phillips, S.H.; Haddad, T.S.; Tomczak, S.J. Developments in nanoscience: Polyhedral oligomeric silsesquioxane (POSS)-polymers. Curr. Opin. Solid. St. M. 2004, 8, 21–29. [Google Scholar] [CrossRef]
- Wang, L.L.; Tan, Y.F.; Wang, X.L.; Xu, T.; Xiao, C.F.; Qi, Z.H. Mechanical and Fracture Properties of Hyperbranched Polymer Covalent Functionalized Multiwalled Carbon Nanotube-Reinforced Epoxy Composites. Chem. Phys. Lett. 2018, 706, 31–39. [Google Scholar] [CrossRef]
- Ahmed, S.; Jones, F.R. A review of particulate reinforcement theories for polymer composites. J. Mater. Sci. 1990, 25, 4933–4942. [Google Scholar] [CrossRef]
- Jancar, J.; Dianselmo, A.; Dibenedetto, A. The yield strength of particulate reinforced thermoplastic composites. Polym. Eng. Sci. 1992, 32, 1394–1399. [Google Scholar] [CrossRef]
- Rong, M.Z.; Zhang, M.Q.; Zheng, Y.X.; Zeng, H.M.; Friedrich, K. Improvement of tensile properties of nano-SiO2/PP composites in relation to percolation mechanism. Polymer 2001, 42, 3301–3304. [Google Scholar] [CrossRef]
- Mallick, P.K. Fiber-Reinforced Composites: Materials, Manufacturing, and Design; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Thostenson, E.T.; Chou, T.W. On the elastic properties of carbon nanotube-based composites: Modelling and characterization. J. Phys. D Appl. Phys. 2003, 5, 573–582. [Google Scholar] [CrossRef]
- Xiao, C.F.; Tan, Y.F.; Yang, X.P.; Xu, T.; Wang, L.L.; Qi, Z.H. Mechanical properties and strengthening mechanism of epoxy resin reinforced with nano-SiO2, particles and multi-walled carbon nanotubes. Chem. Phys. Lett. 2018, 695, 34–43. [Google Scholar] [CrossRef]
- Xu, T.; Tian, J.; An, L.Z.; Jiao, Y.M.; Yin, Q.; Tan, Y.F. Study on the Construction of Dopamine/Poly(ethyleneimine)/Aminoated Carbon Nanotube Multilayer Films on Aramid Fiber Surfaces to Improve the Mechanical Properties of Aramid Fibers/Epoxy Composites. ACS Omega 2022, 7, 35610–35625. [Google Scholar] [CrossRef]
- Tian, J.; Tan, Y.F.; Wang, X.L.; Gao, L.; Zhang, Z.W.; Tang, B.H. Investigation on mechanical properties and reinforced mechanisms of hyperbranched polyesters functionalized nano-silica modified epoxy composites. Mater. Res. Express 2019, 6, 085347. [Google Scholar] [CrossRef]










| Series | EP Matrix (phr) | GO (phr) | POSS-GO (phr) |
|---|---|---|---|
| Neat epoxy | 100 | - | - |
| GO/EP composites | 99.8 | 0.2 | - |
| 99.6 | 0.4 | - | |
| 99.4 | 0.6 | - | |
| 99.2 | 0.8 | - | |
| POSS-GO/EP composites | 99.8 | - | 0.2 |
| 99.6 | - | 0.4 | |
| 99.4 | - | 0.6 | |
| 99.2 | - | 0.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, T.; Jiao, Y.; Su, Z.; Yin, Q.; An, L.; Tan, Y. Non-Covalent Functionalization of Graphene Oxide with POSS to Improve the Mechanical Properties of Epoxy Composites. Polymers 2023, 15, 4726. https://doi.org/10.3390/polym15244726
Xu T, Jiao Y, Su Z, Yin Q, An L, Tan Y. Non-Covalent Functionalization of Graphene Oxide with POSS to Improve the Mechanical Properties of Epoxy Composites. Polymers. 2023; 15(24):4726. https://doi.org/10.3390/polym15244726
Chicago/Turabian StyleXu, Ting, Yumin Jiao, Zhenglian Su, Qin Yin, Lizhou An, and Yefa Tan. 2023. "Non-Covalent Functionalization of Graphene Oxide with POSS to Improve the Mechanical Properties of Epoxy Composites" Polymers 15, no. 24: 4726. https://doi.org/10.3390/polym15244726
APA StyleXu, T., Jiao, Y., Su, Z., Yin, Q., An, L., & Tan, Y. (2023). Non-Covalent Functionalization of Graphene Oxide with POSS to Improve the Mechanical Properties of Epoxy Composites. Polymers, 15(24), 4726. https://doi.org/10.3390/polym15244726






