Covalently Functionalized Cellulose Nanoparticles for Simultaneous Enrichment of Pb(II), Cd(II) and Cu(II) Ions
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Chemicals
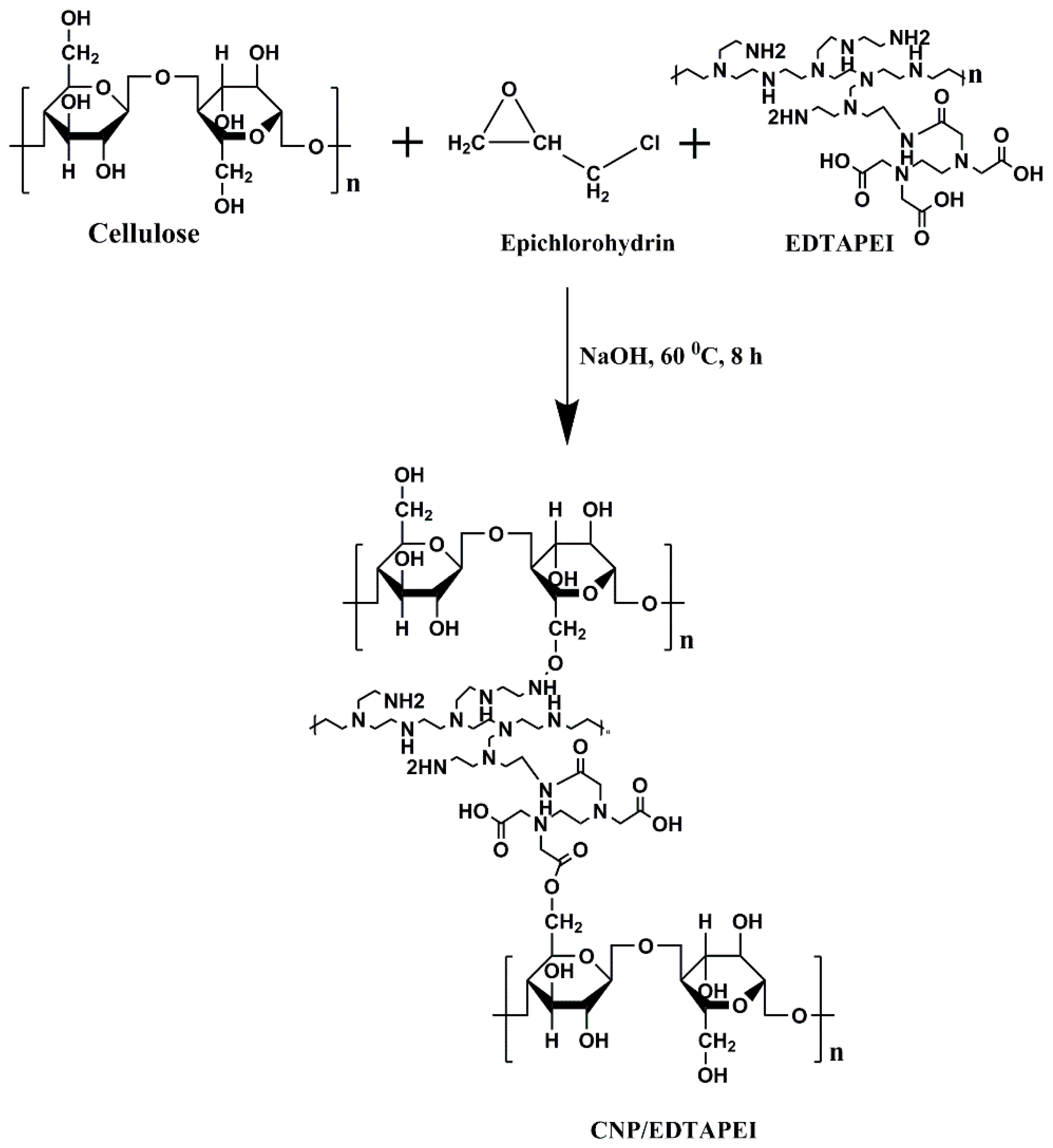
2.2. Synthesis of Nanosorbent
2.2.1. Synthesis of EDTAPEI
2.2.2. Synthesis of CNP/EDTAPEI
2.3. Characterization
2.4. Sample Preparation
2.5. Batch Mode of Operation
2.6. Column Procedure for Simultaneous Sorption of Metal Ions
3. Results and Discussion
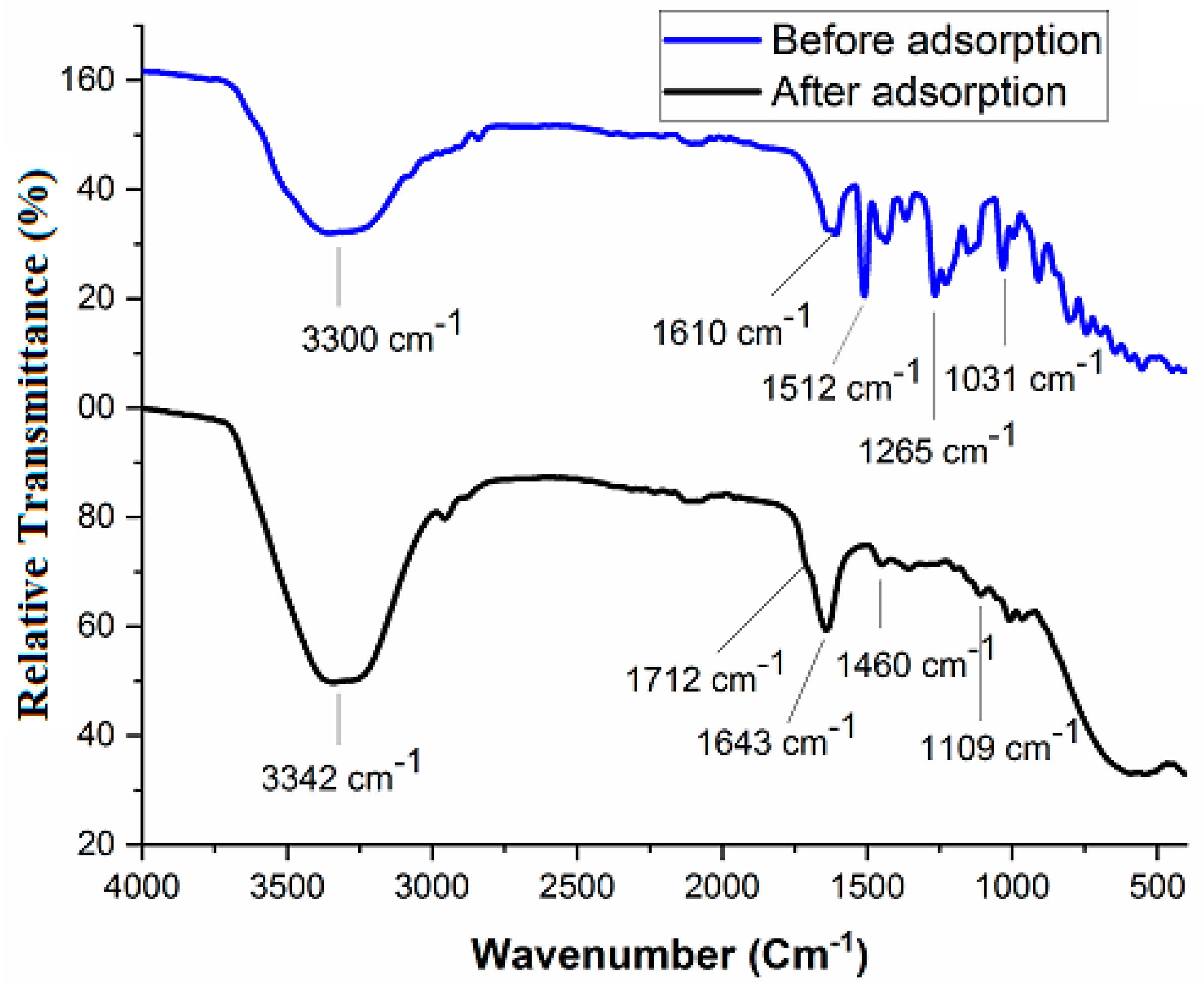
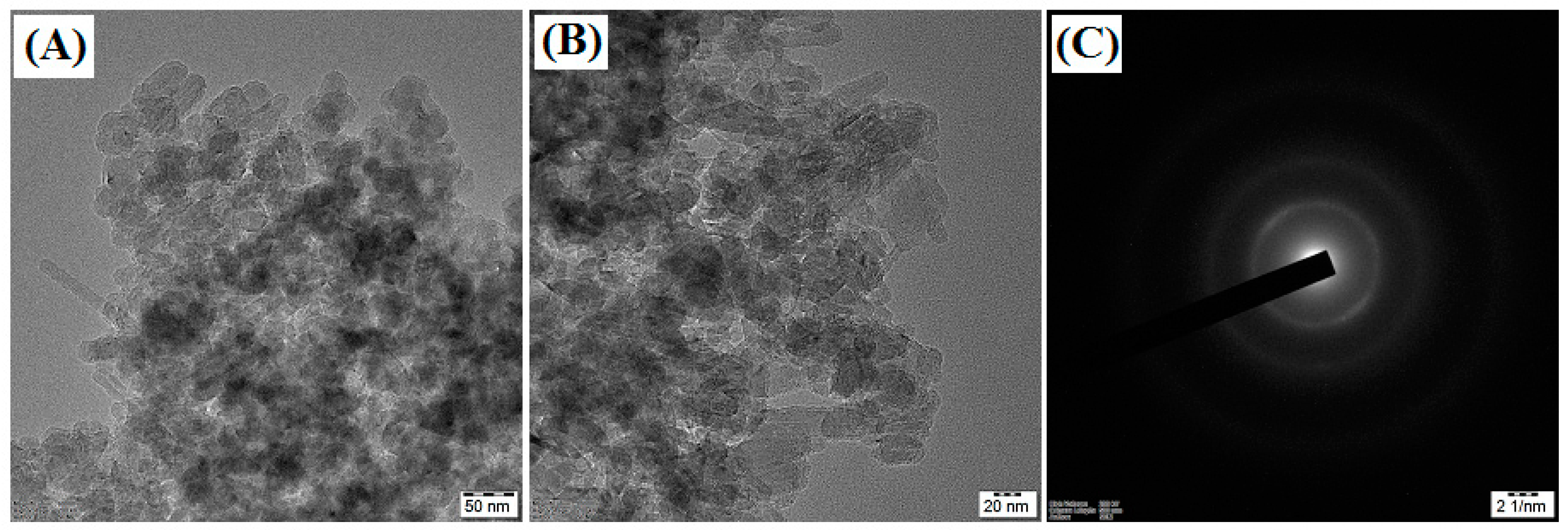
3.1. Characterization
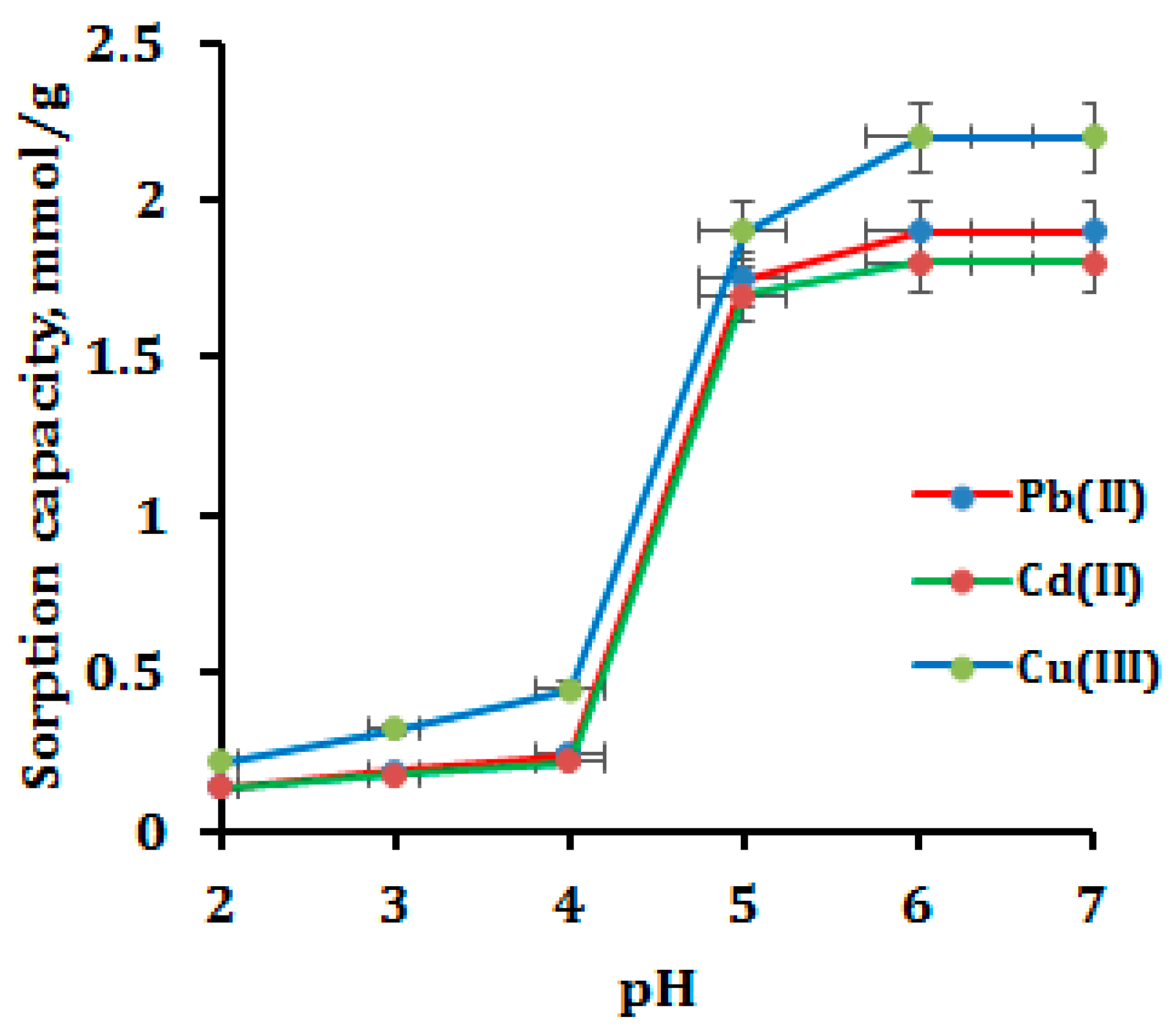
3.2. Optimization of Sample pH
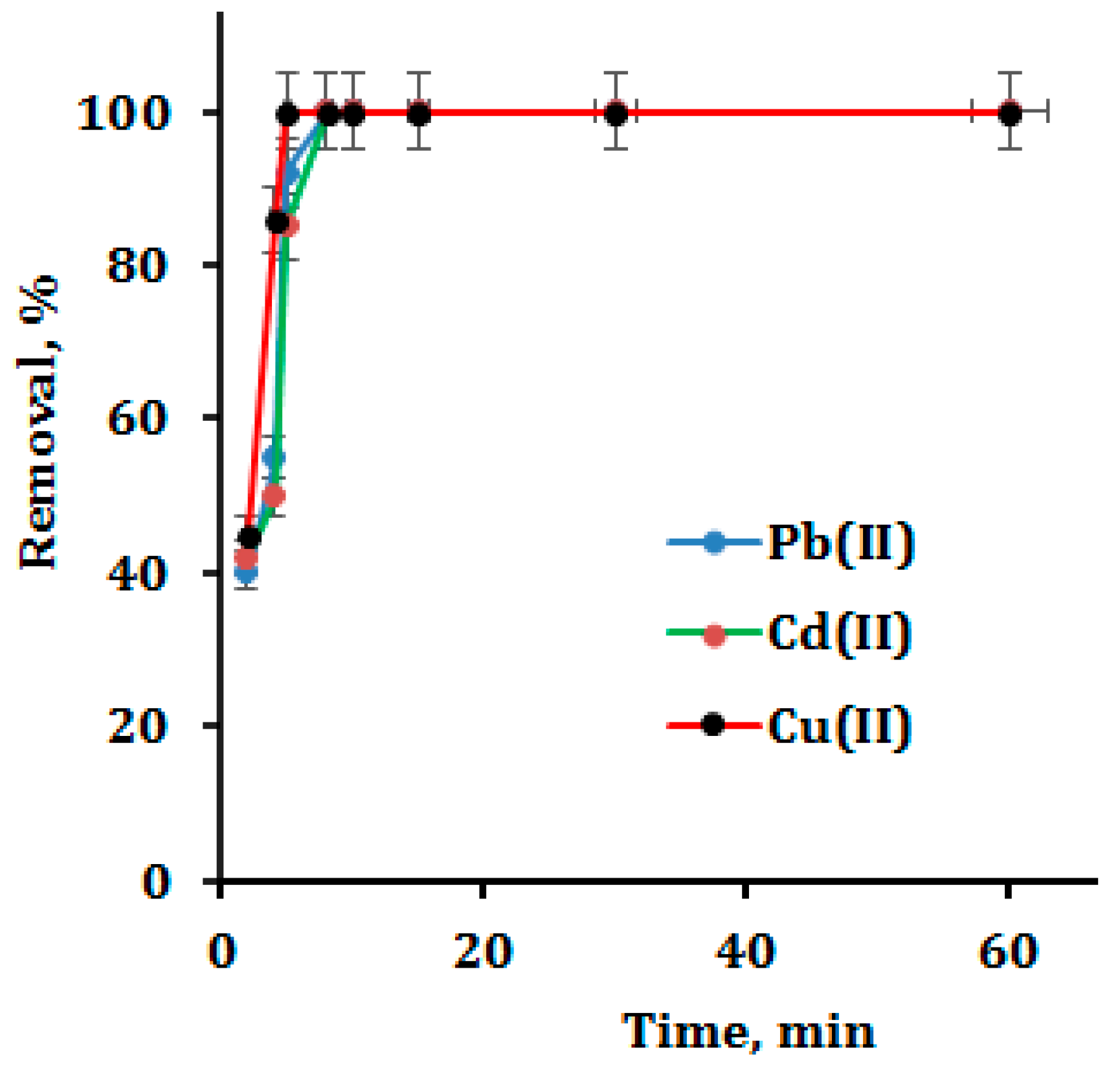
3.3. Optimization of Contact Time
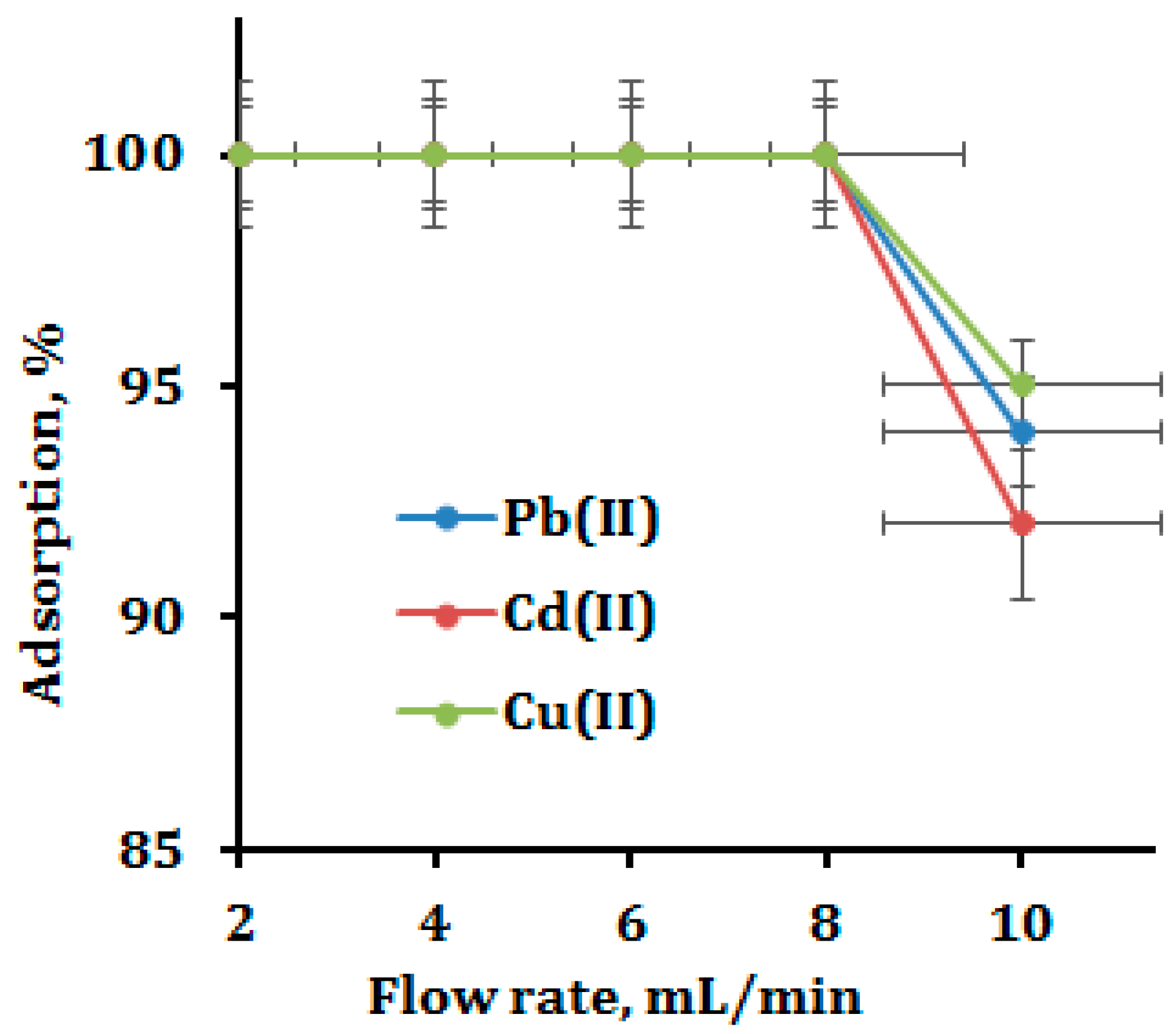
3.4. Effect of Column Flow Rate
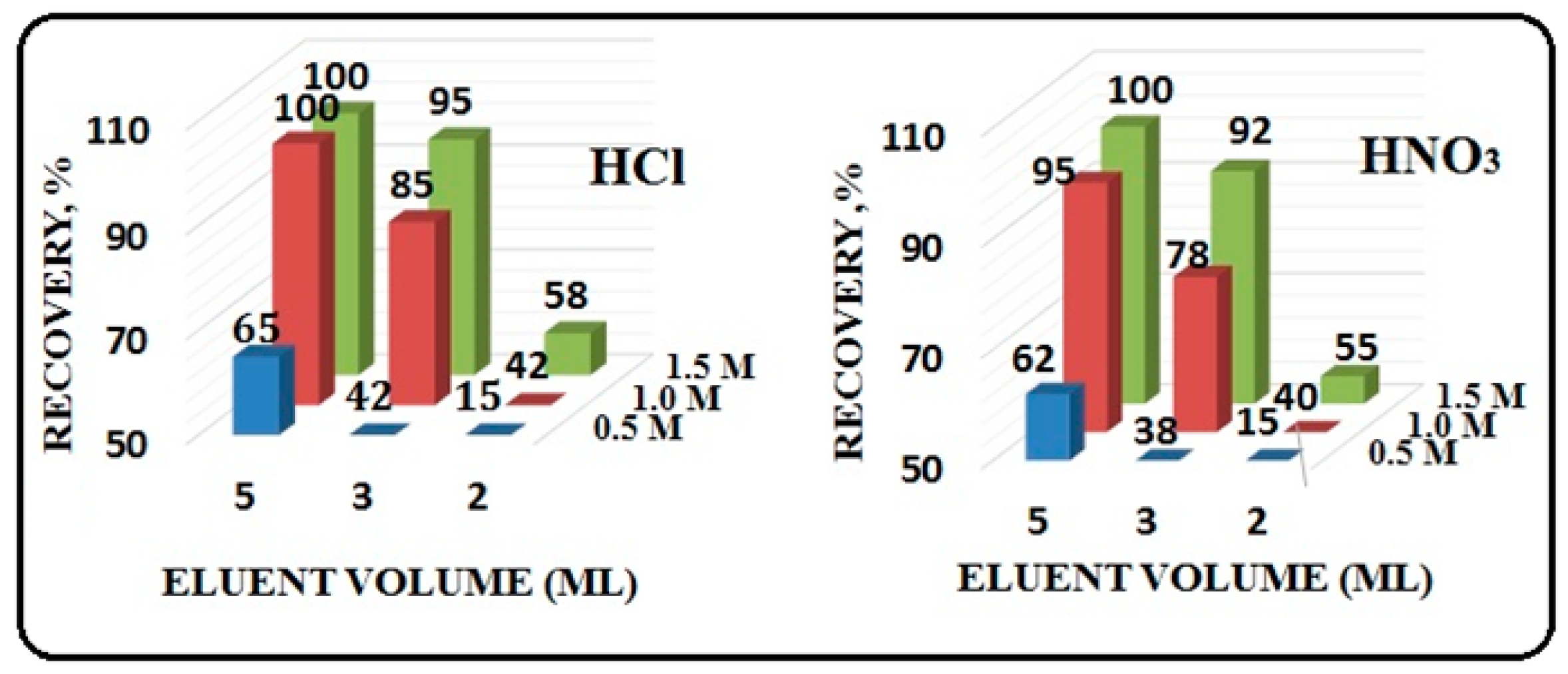
3.5. Type of Eluent
3.6. Effect of Interferents
3.7. Preconcentration Studies
3.8. Analytical Method Validation and Real Sample Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martin, R.B. Metal Ion Toxicity. In Encyclopedia of Inorganic Chemistry; Wiley Online Library: Hoboken, NJ, USA, 2011. [Google Scholar] [CrossRef]
- Martell, A.E. Chemistry of carcinogenic metals. Environ. Health Perspect. 1981, 40, 207–226. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.G.; Nash, S.; Olbert, A.I. A review of water quality index models and their use for assessing surface water quality. Ecol. Indic. 2021, 122, 107218. [Google Scholar] [CrossRef]
- Dabrowiak, J.C. Metal Ion Imbalance in the Body. In Metals in Medicine; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 329–356. [Google Scholar] [CrossRef]
- Martell, A.E. Complexation of labile metal ions and effect on toxicity. Biol. Trace Elem. Res. 1989, 21, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Rahaman, M.S.; Rahman, M.M.; Mise, N.; Sikder, M.T.; Ichihara, G.; Uddin, M.K.; Kurasaki, M.; Ichihara, S. Environmental arsenic exposure and its contribution to human diseases, toxicity mechanism and management. Environ. Pollut. 2021, 289, 117940. [Google Scholar] [CrossRef]
- Zierold, K.M.; Hagemeyer, A.N.; Sears, C.G. Health symptoms among adults living near a coal-burning power plant. Arch. Environ. Occup. Health 2020, 75, 289–296. [Google Scholar] [CrossRef]
- Zhu, G.; Noman, M.A.; Narale, D.D.; Feng, W.; Pujari, L.; Sun, J. Evaluation of ecosystem health and potential human health hazards in the Hangzhou Bay and Qiantang Estuary region through multiple assessment approaches. Environ. Pollut. 2020, 264, 114791. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, P.; Chen, M.; Dong, Z.; Lu, C. Groundwater quality for potable and irrigation uses and associated health risk in southern part of Gu’an County, North China Plain. Environ. Geochem. Health 2021, 43, 813–835. [Google Scholar] [CrossRef]
- De Souza-Araujo, J.; Hussey, N.E.; Hauser-Davis, R.A.; Rosa, A.H.; de Oliveira Lima, M.; Giarrizzo, T. Human risk assessment of toxic elements (As, Cd, Hg, Pb) in marine fish from the Amazon. Chemosphere 2022, 301, 134575. [Google Scholar] [CrossRef]
- Zheng, S.; Wang, Q.; Yuan, Y.; Sun, W. Human health risk assessment of heavy metals in soil and food crops in the Pearl River Delta urban agglomeration of China. Food Chem. 2020, 316, 126213. [Google Scholar] [CrossRef]
- Zheng, S.; Wang, Q.; Yu, H.; Huang, X.; Li, F. Interactive effects of multiple heavy metal(loid)s on their bioavailability in cocontaminated paddy soils in a large region. Sci. Total Environ. 2020, 708, 135126. [Google Scholar] [CrossRef]
- Li, Y.; Lu, C.; Zhu, N.; Chao, J.; Hu, W.; Zhang, Z.; Wang, Y.; Liang, L.; Chen, J.; Xu, D.; et al. Mobilization and methylation of mercury with sulfur addition in paddy soil: Implications for integrated water-sulfur management in controlling Hg accumulation in rice. J. Hazard. Mater. 2022, 430, 128447. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Du, B.; Liu, H.; Cui, H.; Zhang, W.; Fan, X.; Cui, J.; Zhou, J. The bioavailability and contribution of the newly deposited heavy metals (copper and lead) from atmosphere to rice (Oryza sativa L.). J. Hazard. Mater. 2020, 384, 121285. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Zhou, Z.; Rao, M.; Sun, Z. Assessment of heavy metals and arsenic pollution in surface sediments from rivers around a uranium mining area in East China. Environ. Geochem. Health 2020, 42, 1401–1413. [Google Scholar] [CrossRef]
- Zhou, J.; Obrist, D.; Dastoor, A.; Jiskra, M.; Ryjkov, A. Vegetation uptake of mercury and impacts on global cycling. Nat. Rev. Earth Environ. 2021, 2, 269–284. [Google Scholar] [CrossRef]
- Zhen, H.; Jia, L.; Huang, C.; Qiao, Y.; Li, J.; Li, H.; Chen, Q.; Wan, Y. Long-term effects of intensive application of manure on heavy metal pollution risk in protected-field vegetable production. Environ. Pollut. 2020, 263, 114552. [Google Scholar] [CrossRef]
- Zhuang, Z.; Mu, H.Y.; Fu, P.N.; Wan, Y.N.; Yu, Y.; Wang, Q.; Li, H.F. Accumulation of potentially toxic elements in agricultural soil and scenario analysis of cadmium inputs by fertilization: A case study in Quzhou county. J. Environ. Manag. 2020, 269, 110797. [Google Scholar] [CrossRef]
- Zhou, Q.; Yang, N.; Li, Y.; Ren, B.; Ding, X.; Bian, H.; Yao, X. Total concentrations and sources of heavy metal pollution in global river and lake water bodies from 1972 to 2017. Glob. Ecol. Conserv. 2020, 22, e00925. [Google Scholar] [CrossRef]
- Zhang, C.; Xing, H.; Yang, L.; Fei, P.; Liu, H. Development trend and prospect of solid phase extraction technology. Chin. J. Chem. Eng. 2022, 42, 245–255. [Google Scholar] [CrossRef]
- Haseen, U.; Ahmad, H. Preconcentration and Determination of Trace Hg(II) Using a Cellulose Nanofiber Mat Functionalized with MoS2 Nanosheets. Ind. Eng. Chem. Res. 2020, 59, 3198–3204. [Google Scholar] [CrossRef]
- Chunin, N.; Phooplub, K.; Kaewpet, M.; Wattanasin, P.; Kanatharana, P.; Thavarungkul, P.; Thammakhet-Buranachai, C. A novel 3D-printed solid phase microextraction device equipped with silver-polyaniline coated pencil lead for the extraction of phthalate esters in cosmeceutical products. Anal. Chim. Acta 2019, 1091, 30–39. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, Q.; Li, X.; Huang, X. A critical review on chemical analysis of heavy metal complexes in water/wastewater and the mechanism of treatment methods. Chem. Eng. J. 2022, 429, 131688. [Google Scholar] [CrossRef]
- Zhou, Y.; Gao, Y.; Wang, H.; Xia, M.; Yue, Q.; Xue, Z.; Zhu, J.; Yu, J.; Yin, W. Versatile 3D reduced graphene oxide/poly(amino-phosphonic acid) aerogel derived from waste acrylic fibers as an efficient adsorbent for water purification. Sci. Total Environ. 2021, 776, 145973. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Li, P.; Song, X.; Li, J.; Ren, B.; Gao, S.; Cao, R. Recent progress in the removal of mercury ions from water based MOFs materials. Coord. Chem. Rev. 2021, 443, 214034. [Google Scholar] [CrossRef]
- Lin, C.R.; Ivanova, O.S.; Petrov, D.A.; Sokolov, A.E.; Chen, Y.Z.; Gerasimova, M.A.; Zharkov, S.M.; Tseng, Y.T.; Shestakov, N.P.; Edelman, I.S. Amino-Functionalized Fe(3)O(4)@SiO(2) Core-Shell Magnetic Nanoparticles for Dye Adsorption. Nanomaterials 2021, 11, 2371. [Google Scholar] [CrossRef] [PubMed]
- Marcos-Hernández, M.; Arrieta, R.A.; Ventura, K.; Hernández, J.; Powell, C.D.; Atkinson, A.J.; Markovski, J.S.; Gardea-Torresdey, J.; Hristovski, K.D.; Westerhoff, P.; et al. Superparamagnetic nanoadsorbents for the removal of trace As(III) in drinking water. Environ. Adv. 2021, 4, 100046. [Google Scholar] [CrossRef]
- Sun, Y.; Wu, Y.; Fu, Y.; Yang, C.; Jiang, J.; Yan, G.; Hu, J. Rapid and high selective removal of Hg(II) ions using tannic acid cross-linking cellulose/polyethyleneimine functionalized magnetic composite. Int. J. Biol. Macromol. 2021, 182, 1120–1129. [Google Scholar] [CrossRef]
- Topuz, B.; Batmaz, F.; Külköylüoğlu, O.; Çapraz, Ç. First usage of ostracod species (Herpetocypris brevicaudata) carapace as a biosorbent with XAD-4 resin to determine Co(II), Cu(II) and Mn(II) trace metal ions. Microchem. J. 2021, 167, 106335. [Google Scholar] [CrossRef]
- Pietrucci, F.; Boero, M.; Andreoni, W. How natural materials remove heavy metals from water: Mechanistic insights from molecular dynamics simulations. Chem. Sci. 2021, 12, 2979–2985. [Google Scholar] [CrossRef]
- Ricardo Teixeira Tarley, C.; Antonio Cajamarca Suquila, F.; Casarin, J.; Celso Goncalves Junior, A.; Gava Segatelli, M. Development of selective preconcentration/clean-up method for imidazolinone herbicides determination in natural water and rice samples by HPLC-PAD using an imazethapyr imprinted poly(vinylimidazole-TRIM). Food Chem. 2021, 334, 127345. [Google Scholar] [CrossRef]
- Lima, I.S.; Lazarin, A.M.; Airoldi, C. Favorable chitosan/cellulose film combinations for copper removal from aqueous solutions. Int. J. Biol. Macromol. 2005, 36, 79–83. [Google Scholar] [CrossRef]
- Wang, D.; Cheng, W.; Yue, Y.; Xuan, L.; Ni, X.; Han, G. Electrospun Cellulose Nanocrystals/Chitosan/Polyvinyl Alcohol Nanofibrous Films and their Exploration to Metal Ions Adsorption. Polymers 2018, 10, 1046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coates, J.P. The interpretation of infrared spectra: Published reference sources. Appl. Spectrosc. Rev. 1996, 31, 179–192. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts. 3rd ed By George Socrates (The University of West London, Middlesex, U.K.). J. Wiley and Sons: Chichester. 2001. xviii + 348 pp. $185.00. ISBN: 0-471-85298-8. J. Am. Chem. Soc. 2002, 124, 1830. [Google Scholar] [CrossRef]
- He, H.; Martell, A.E.; Motekaitis, R.J.; Reibenspies, J.H. Interactions between divalent metal ions and an octacoordinate macrocyclic ligand. Inorg. Chem. 2000, 39, 1586–1592. [Google Scholar] [CrossRef] [PubMed]
- Martell, A.E. Chelation: Stability and selectivity. Ann. N. Y. Acad. Sci. 1960, 88, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Pearson, R.G. Hard and soft acids and bases, HSAB, part 1: Fundamental principles. J. Chem. Educ. 1968, 45, 581. [Google Scholar] [CrossRef]
- Pearson, R.G. Hard and soft acids and bases, HSAB, part II: Underlying theories. J. Chem. Educ. 1968, 45, 643. [Google Scholar] [CrossRef]
- Tyson, C.A.; Martell, A.E. Kinetics and mechanism of the metal chelate catalyzed oxidation of pyrocatechols. J. Am. Chem. Soc. 1972, 94, 939–945. [Google Scholar] [CrossRef]
- Lenz, G.R.; Martell, A.E. Metal Complexes of Carnosine. Biochemistry 1964, 3, 750–753. [Google Scholar] [CrossRef]
- Long, G.L.; Winefordner, J.D. Limit of Detection A Closer Look at the IUPAC Definition. Anal. Chem. 1983, 55, 712A–724A. [Google Scholar] [CrossRef]
- Jin, X.; Xiang, Z.; Liu, Q.; Chen, Y.; Lu, F. Polyethyleneimine-bacterial cellulose bioadsorbent for effective removal of copper and lead ions from aqueous solution. Bioresour. Technol. 2017, 244, 844–849. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, H.; Alharbi, W.; BinSharfan, I.I.; Khan, R.A.; Alsalme, A. Aminophosphonic Acid Functionalized Cellulose Nanofibers for Efficient Extraction of Trace Metal Ions. Polymers 2020, 12, 2370. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Liu, Z.; Zhang, J.; Jiao, C.; Ding, L.; Yang, S. Synthesis and Adsorption Properties of Novel Bacterial Cellulose/Graphene Oxide/Attapulgite Materials for Cu and Pb Ions in Aqueous Solutions. Materials 2020, 13, 3703. [Google Scholar] [CrossRef] [PubMed]
- Daochalermwong, A.; Chanka, N.; Songsrirote, K.; Dittanet, P.; Niamnuy, C.; Seubsai, A. Removal of Heavy Metal Ions Using Modified Celluloses Prepared from Pineapple Leaf Fiber. ACS Omega 2020, 5, 5285–5296. [Google Scholar] [CrossRef]
| Co-Existing Ions | Salt Added | Amount Added (×103 µg L−1) | Recovery % (RSD) | ||
|---|---|---|---|---|---|
| Pb(II) | Cd(II) | Cu(II) | |||
| Na+ | NaCl | 580 | 99.4 (4.15) | 97.9 (4.75) | 99.9 (3.52) |
| K+ | KCl | 540 | 99.5 (4.65) | 98.7 (4.90) | 99.6 (4.15) |
| Ca2+ | CaCl2 | 75 | 99.6 (4.28) | 98.5 (3.15) | 99.4 (3.83) |
| Mg2+ | MgCl2 | 120 | 98.9 (3.34) | 98.5 (4.16) | 99.3 (3.94) |
| Cl− | NaCl | 980 | 99.3 (4.16) | 98.6 (4.12) | 98.6 (3.56) |
| Br− | NaBr | 820 | 98.7 (3.14) | 98.8 (4.12) | 99.6 (2.44) |
| CO32− | Na2CO3 | 340 | 97.9 (4.56) | 97.5 (4.15) | 98.3 (3.39) |
| SO42− | Na2SO4 | 220 | 97.6 (4.15) | 98.5 (3.32) | 97.5 (2.34) |
| NO3− | NaNO3 | 300 | 99.5 (2.05) | 97.3 (3.65) | 99.5 (4.05) |
| CH3COO− | CH3COONa | 320 | 98.9 (2.96) | 98.5 (3.05) | 97.2 (3.96) |
| C6H5O73− | Na3C6H5O7 | 260 | 98.8 (4.15) | 98.5 (3.42) | 98.5 (3.34) |
| Humic acid | - | 20 | 97.2 (3.54) | 97.8 (3.24) | 97.6 (3.64) |
| Fulvic acid | - | 20 | 98.5 (3.05) | 97.6 (4.12) | 97.5 (4.05) |
| Fe(II) | FeCl2 | 1.2 | 97.7 (4.21) | 97.0 (3.98) | 98.6 (3.88) |
| Fe(III) | FeCl3 | 1.5 | 99.0 (2.88) | 98.7 (4.76) | 98.2 (3.56) |
| As(III) | AsCl3 | 1.5 | 99.4 (3.76) | 99.7 (4.03) | 98.9 (3.84) |
| As(V) | AsOCl | 2.0 | 99.0 (4.42) | 98.7 (3.49) | 98.7 (4.72) |
| Hg(II) | HgCl2 | 1.8 | 97.8 (4.33) | 98.5 (2.98) | 98.0 (4.06) |
| Sample Volume (mL) | Analyte Concentration (µg L−1) | Preconcentration Limit | Preconcentration Factor | ||||
|---|---|---|---|---|---|---|---|
| Cu(II) | Pb(II) | Cd(II) | Cu(II) | Pb(II) | Cd(II) | ||
| 1000 | 1.0 | 1.0 | 1.0 | 1.0 | 200 | 200 | 200 |
| 1200 | 0.83 | 0.83 | 0.83 | 0.83 | 240 | 240 | 240 |
| 1400 | 0.71 | 0.71 | 0.71 | 0.71 | 280 | 280 | 280 |
| 1500 | 0.66 | - | - | - | - | - | - |
| Metal Ion | Regression Equation | R2 |
|---|---|---|
| Cu(II) | A = 19.4708 XCu + 3.0125 | 0.9999 |
| Pb(II) | A = 109.6104 XPb + 4.1675 | 0.9997 |
| Cd(II) | A = 9.8504 XCd + 4.1525 | 0.9996 |
| Samples | Certified Values (µg g−1) | Values Found by Proposed Method (µg g−1) a | Value of t-Test b |
|---|---|---|---|
| NIES 10C | Cd: 1.82; Cu: 4.1 | Cd: 1.84 ± 0.008; Cu: 4.2 ± 0.041 | 1.150; 1.242 |
| SRM 1572b | Pb: 13.3; Cu: 16.5 | Pb: 12.8 ± 0.082; Cu: 16.2 ± 0.057 | 1.126; 1.312 |
| Samples | Amount Added (µg) | Metal Ion Found (µg L−1) ± Standard Deviation a (% Recovery of Added Amount; RSD) | ||
|---|---|---|---|---|
| Cu(II) | Pb(II) | Cd(II) | ||
| Industrial effluent | 0 | 18.5 ± 0.52 | 7.30 ± 0.85 | 3.57 ± 0.56 |
| 5 | 23.4 ± 0.63 (98; 3.35) | 12.30 ± 0.61 (100; 4.19) | 8.56 ± 0.89 (99.8; 3.87) | |
| Groundwater | 0 | 10.5 ± 0.41 | 2.8 ± 0.02 | 2.4 ± 0.09 |
| 5 | 15.4 ± 0.22 (98; 1.58) | 7.8 ± 0.37 (100; 1.68) | 7.42 ± 0.31 (100.4; 2.14) | |
| Tap water | 0 | 3.7 ± 0.27 | 2.2 ± 0.08 | 3.1 ± 0.31 |
| 5 | 8.7 ± 0.16 (100; 2.03) | 7.24 ± 0.12 (100.8; 1.34) | 8.12 ± 0.40 (100.4; 1.56) | |
| River water | 0 | nd b | 1.1 ± 0.02 | nd b |
| 5 | 4.99 ± 0.15 (99.8; 2.87) | 6.08 ± 0.16 (99.6; 4.05) | 5.06 ± 0.38 (101.2; 2.53) | |
| Adsorbent | Adsorption Capacity (mg g−1) | Preconcentration Factor | Detection Limit (µg L−1) | Ref. |
|---|---|---|---|---|
| BC-PEI | Pb: 148; Cu: 141 | - | - | [43] |
| APBC | Pb: 103; Cd: 76; Cu: 108 | Pb: 540; Cd: 540; Cu: 580 | Pb: 0.05; Cd: 0.04; Cu: 0.03 | [44] |
| BC/PVA/GO/APT | Pb: 218; Cu: 151 | Pb: 150; Cu: 150 | - | [45] |
| Cell-EDTA and Cell-CM | Pb: 41.2, 33.2; Cd: 63.4, 23.0 | - | - | [46] |
| CNP-EDTAPEI | Pb: 393; Cd: 202; Cu: 139 | Pb, Cd, Cu: 280 | Pb, Cd, Cu: 0.4 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsaeedi, H.; Ahmad, H.; Altowairqi, M.F.; Alhamed, A.A.; Alsalme, A. Covalently Functionalized Cellulose Nanoparticles for Simultaneous Enrichment of Pb(II), Cd(II) and Cu(II) Ions. Polymers 2023, 15, 532. https://doi.org/10.3390/polym15030532
Alsaeedi H, Ahmad H, Altowairqi MF, Alhamed AA, Alsalme A. Covalently Functionalized Cellulose Nanoparticles for Simultaneous Enrichment of Pb(II), Cd(II) and Cu(II) Ions. Polymers. 2023; 15(3):532. https://doi.org/10.3390/polym15030532
Chicago/Turabian StyleAlsaeedi, Huda, Hilal Ahmad, Malak Faisal Altowairqi, Afnan Abdullah Alhamed, and Ali Alsalme. 2023. "Covalently Functionalized Cellulose Nanoparticles for Simultaneous Enrichment of Pb(II), Cd(II) and Cu(II) Ions" Polymers 15, no. 3: 532. https://doi.org/10.3390/polym15030532





