Increased Enzyme Loading in PICsomes via Controlling Membrane Permeability Improves Enzyme Prodrug Cancer Therapy Outcome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Protein Expression and Purification
2.3. Material Characterization
2.4. Cell Lines and Animals
2.5. Preparation of CD@PICsomes
2.6. Preparation of Fluorescence Modified CD-Loaded PICsomes
2.7. Interaction of Pre-CL PICsomes and CD
2.8. Evaluation of Stability of CD@PICsome Based on Enzyme Activity Assay
2.9. Evaluation of In Vitro Cytotoxicity
2.10. Evaluation of Plasma Clearance and Biodistribution and Hepatotoxicity of CD@PICsome
2.11. Evaluation of In Vivo Therapeutic Effect
2.12. Statistical Analysis
3. Results and Discussion
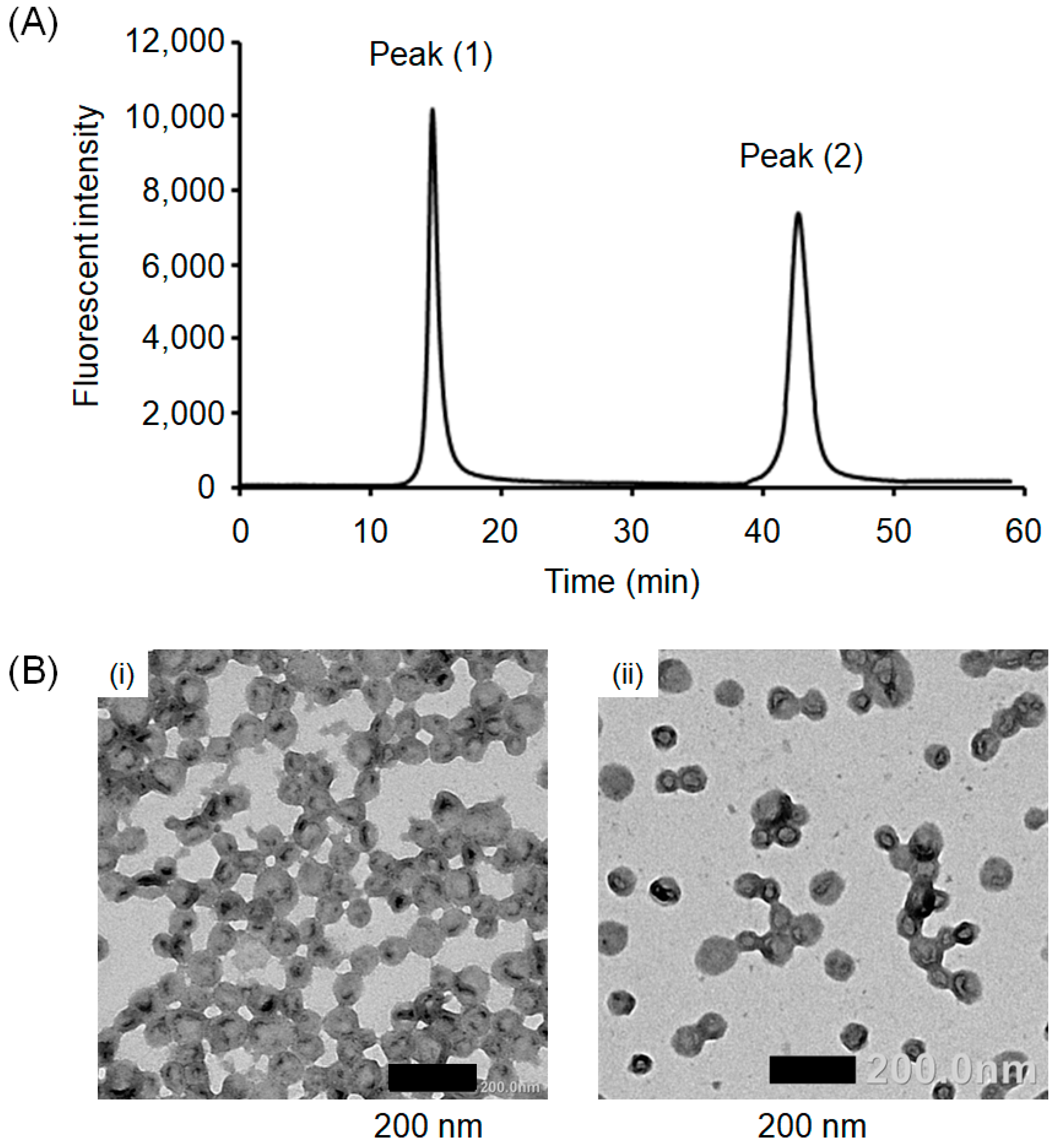
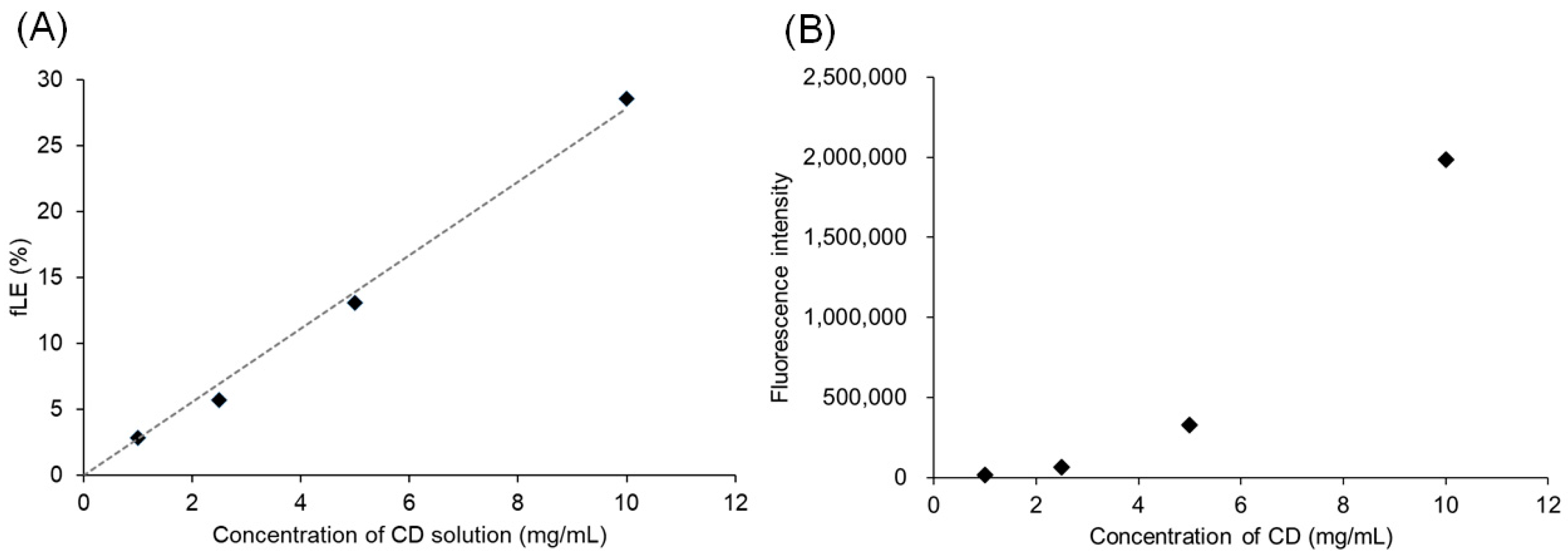
3.1. Preparation of Crosslinked PICsomes Loaded with CD (CD@PICsomes) by SWCL
3.2. Interaction of Pre-CL PICsomes and CD
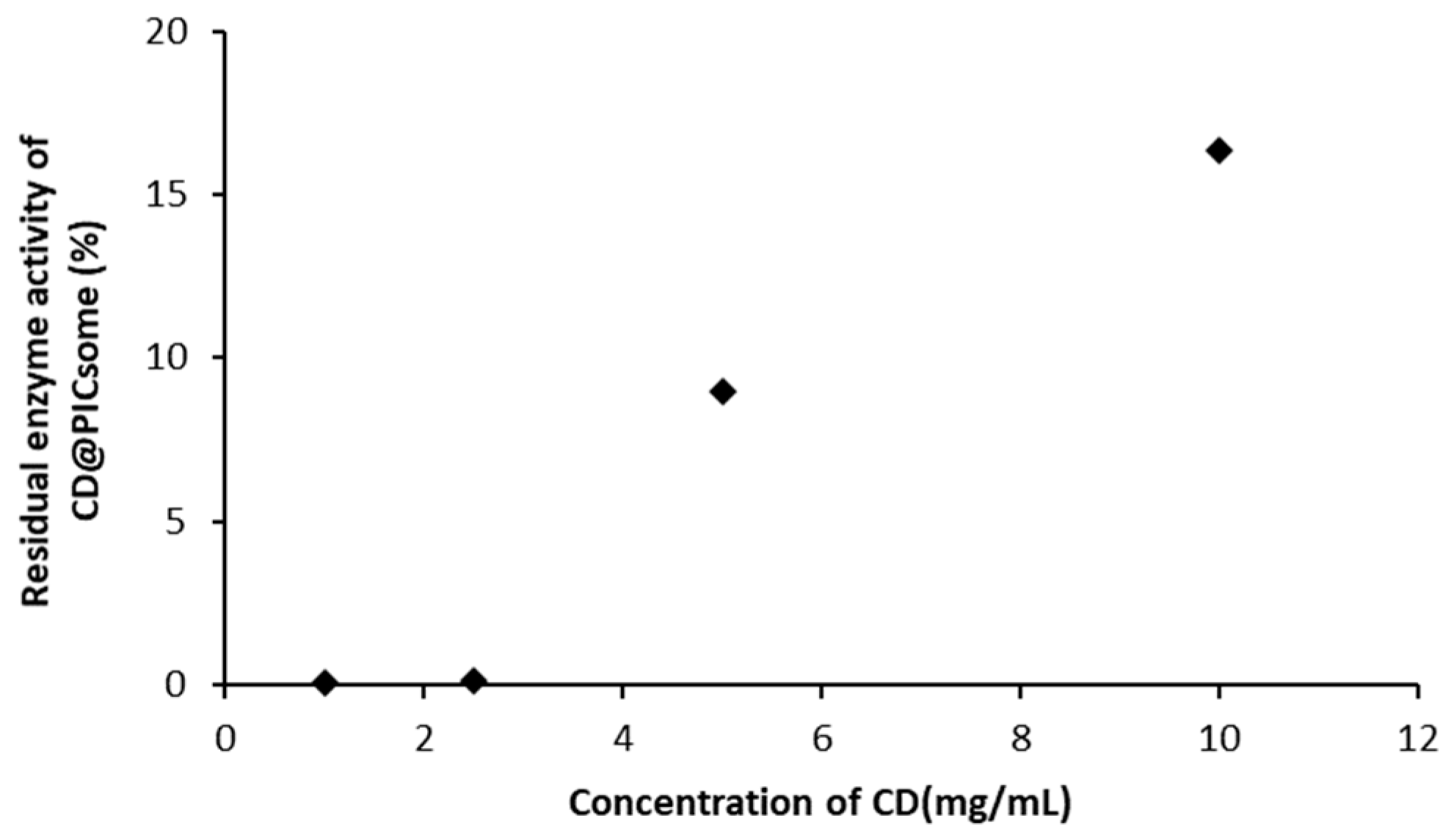
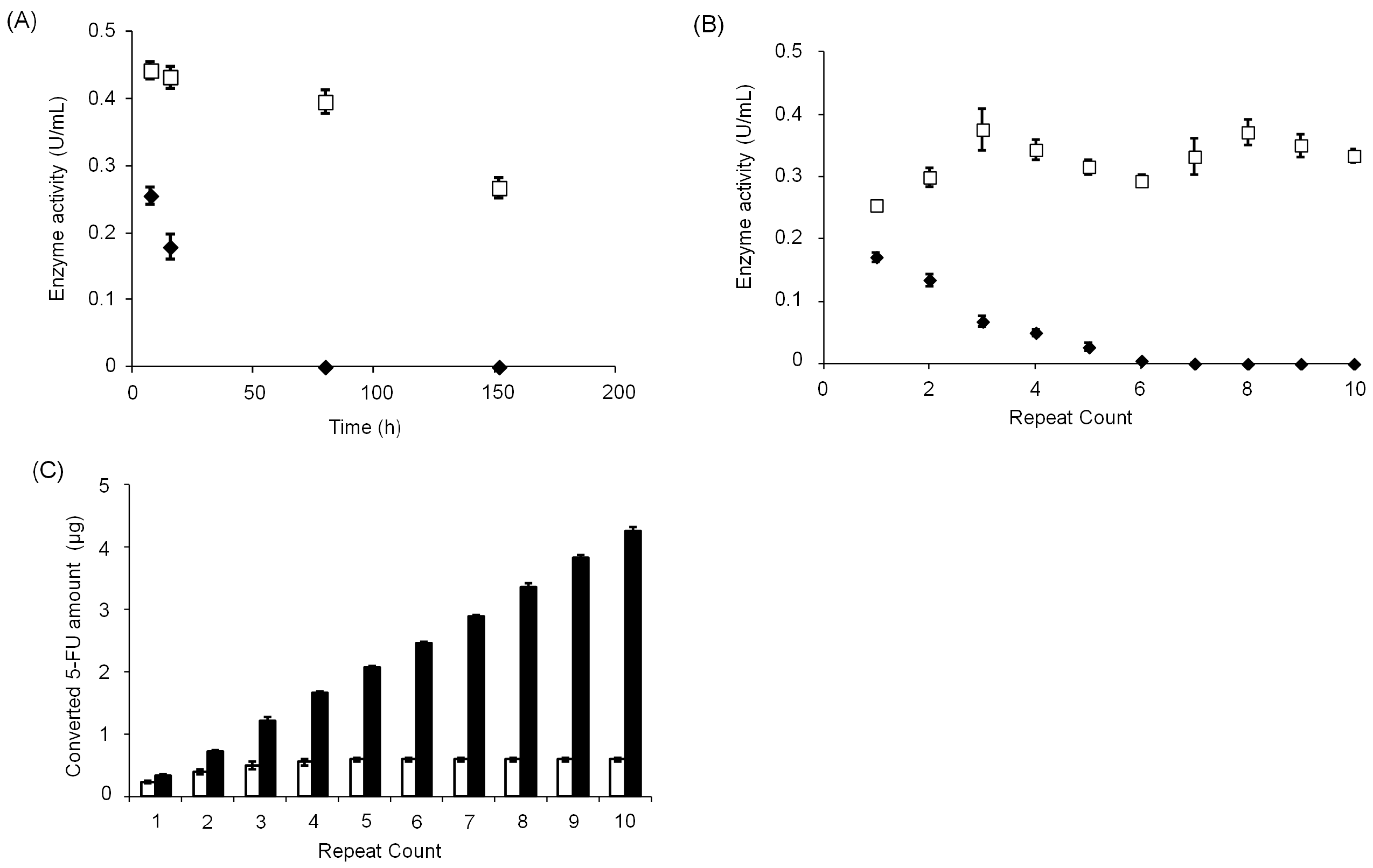
3.3. Stability of CD@PICsome Based on Enzyme Activity
3.4. Evaluation of Cytotoxicity of CD@PICsome
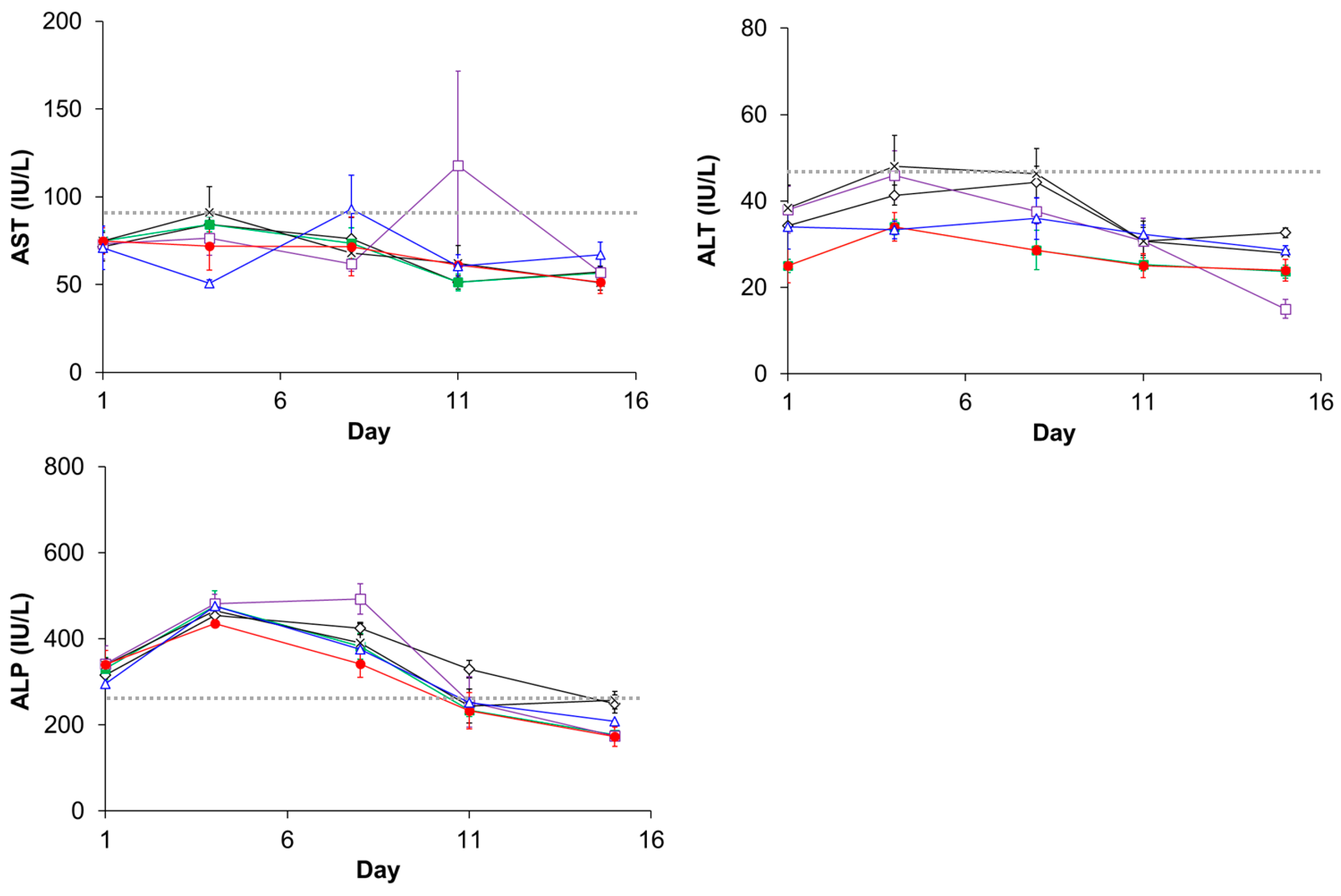
3.5. Evaluation of Plasma Clearance, Biodistribution, and Hepatotoxicity of CD@PICsome
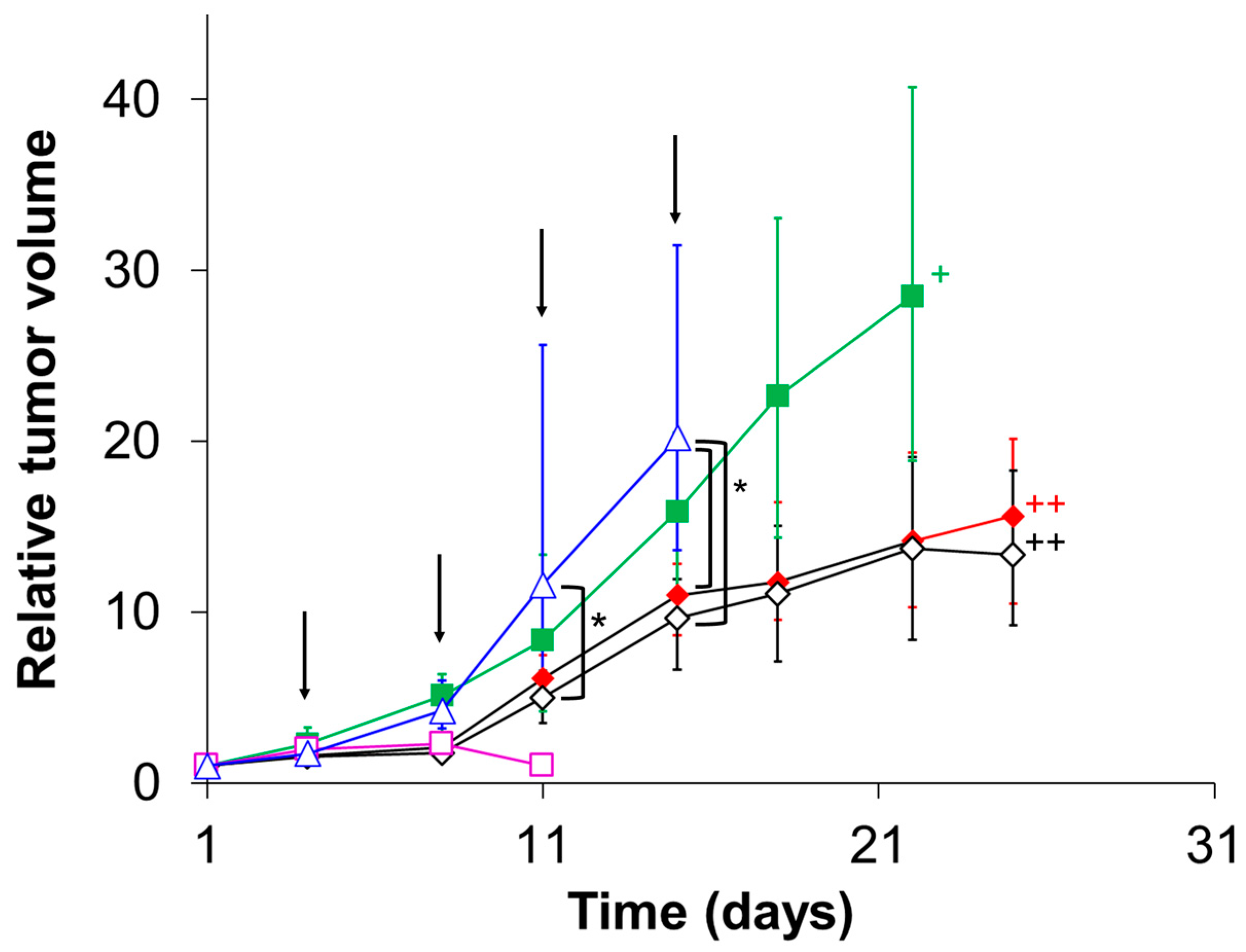
3.6. Evaluation of In Vivo Therapeutic Effect of CD@PICsome
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, J.S.; Feijen, J. Polymersomes for drug delivery: Design, formation and characterization. J. Control. Release 2012, 161, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Larrañaga, A.; Lomora, M.; Sarasua, J.R.; Palivan, C.G.; Pandit, A. Polymer capsules as micro-/nanoreactors for therapeutic applications: Current strategies to control membrane permeability. Prog. Mater. Sci. 2017, 90, 325–357. [Google Scholar] [CrossRef]
- Chuanoi, S.; Anraku, Y.; Hori, M.; Kishimura, A.; Kataoka, K. Fabrication of Polyion Complex Vesicles with Enhanced Salt and Temperature Resistance and Their Potential Applications as Enzymatic Nanoreactors. Biomacromolecules 2014, 15, 2389–2397. [Google Scholar] [CrossRef]
- Anraku, Y.; Kishimura, A.; Kamiya, M.; Tanaka, S.; Nomoto, T.; Toh, K.; Matsumoto, Y.; Fukushima, S.; Sueyoshi, D.; Kano, M.R.; et al. Systemically Injectable Enzyme-Loaded Polyion Complex Vesicles as In Vivo Nanoreactors Functioning in Tumors. Angew. Chem. Int. Ed. 2016, 55, 560–565. [Google Scholar] [CrossRef] [PubMed]
- Sueyoshi, D.; Anraku, Y.; Komatsu, T.; Urano, Y.; Kataoka, K. Enzyme-Loaded Polyion Complex Vesicles as in vivo Nanoreactors Working Sustainably under the Blood Circulation: Characterization and Functional Evaluation. Biomacromolecules 2017, 18, 1189–1196. [Google Scholar] [CrossRef]
- Nishimura, T.; Sasaki, Y.; Akiyoshi, K. Biotransporting Self-Assembled Nanofactories Using Polymer Vesicles with Molecular Permeability for Enzyme Prodrug Cancer Therapy. Adv. Mater. 2017, 29, 1702406. [Google Scholar] [CrossRef]
- Ke, W.; Li, J.; Mohammed, F.; Wang, Y.; Tou, K.; Liu, X.; Wen, P.; Kinoh, H.; Anraku, Y.; Chen, H.; et al. Therapeutic Polymersome Nanoreactors with Tumor-Specific Activable Cascade Reactions for Cooperative Cancer Therapy. ACS Nano 2019, 13, 2357–2369. [Google Scholar] [CrossRef] [PubMed]
- Mukerabigwi, J.F.; Yin, W.; Zha, Z.; Ke, W.; Wang, Y.; Chen, W.; Japir, A.A.-W.M.M.; Wang, Y.; Ge, Z. Polymersome nanoreactors with tumor pH-triggered selective membrane permeability for prodrug delivery, activation, and combined oxidationchemotherapy. J. Control. Release 2019, 303, 209–222. [Google Scholar] [CrossRef]
- Varlas, S.; Foster, J.C.; Georgiou, P.G.; Keogh, R.; Husband, J.T.; Williams, D.S.; O’Reilly, R.K. Tuning the membrane permeability of polymersome nanoreactors developed by aqueous emulsion polymerization-induced self-assembly. Nanoscale 2019, 11, 12643–12654. [Google Scholar] [CrossRef] [Green Version]
- Anraku, Y.; Kishimura, A.; Kobayashi, A.; Oba, M.; Kataoka, K. Size-controlled long-circulating PICsome as a ruler to measure critical cut-off disposition size into normal and tumor tissues. Chem. Commun. 2011, 47, 6054–6056. [Google Scholar] [CrossRef]
- Anraku, Y.; Kishimura, A.; Oba, M.; Yamasaki, Y.; Kataoka, K. Spontaneous formation of nanosized unilamellar polyion complex vesicles with tunable size and properties. J. Am. Chem. Soc. 2010, 132, 1631–1636. [Google Scholar] [CrossRef]
- Tang, H.; Sakamura, Y.; Mori, T.; Katayama, Y.; Kishimura, A. Development of Enzyme Loaded Polyion Complex Vesicle (PICsome): Thermal Stability of Enzyme in PICsome Compartment and Effect of Coencapsulation of Dextran on Enzyme Activity. Macromol. Biosci. 2017, 17, 1600542. [Google Scholar] [CrossRef]
- Naoyama, K.; Mori, T.; Katayama, Y.; Kishimura, A. Fabrication of Dendrimer-Based Polyion Complex Submicrometer-Scaled Structures with Enhanced Stability under Physiological Conditions. Macromol. Rapid Commun. 2016, 37, 1087–1093. [Google Scholar] [CrossRef]
- Mutaf, O.F.; Anraku, Y.; Kishimura, A.; Kataoka, K. Unilamellar polyion complex vesicles (PICsomes) with tunable permeabilities for macromolecular solutes with different shapes and sizes. Polymer 2017, 133, 1–7. [Google Scholar] [CrossRef]
- Hori, M.; Cabral, H.; Toh, K.; Kishimura, A.; Kataoka, K. Robust polyion complex vesicles (PICsomes) under physiological condition reinforced by multiple hydrogen bond formation derived by guanidinium groups. Biomacromolecules 2018, 19, 4113–4121. [Google Scholar] [CrossRef]
- Austin, E.A.; Huber, B.E. A first step in the development of gene therapy for colorectal carcinoma: Cloning, sequencing, and expression of Escherichia coli cytosine deaminase. Mol. Pharmacol. 1993, 43, 380–387. [Google Scholar] [PubMed]
- Cheung, W.Y.; Fralick, R.A.; Cheng, S. The confused cancer patient: A case of 5-fluorouracil-induced encephalopathy. Curr. Oncol. 2008, 15, 234–236. [Google Scholar] [CrossRef] [Green Version]
- Phillips, T.A.; Howell, A.; Grieve, R.J.; Welling, P.G. Pharmacokinetics of oral and Intravenous fluorouracil in humans. J. Pharm. Sci. 1980, 69, 1428–1431. [Google Scholar] [CrossRef]
- Vermes, A.; Guchelaar, H.-J.; Dankert, J. Flucytosine: A review of its pharmacology, clinical indications, pharmacokinetics, toxicity, and drug interactions. J. Antimicrob. Chemother. 2000, 46, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Kishimura, A.; Koide, A.; Osada, K.; Yamasaki, Y.; Kataoka, K. Encapsulation of myoglobin in PEGylated polyion complex vesicles made from a pair of oppositely charged block ionomers: A physiologically available oxygen carrier. Angew. Chem. Int. Ed. 2007, 46, 6085–6088. [Google Scholar] [CrossRef]
- Anraku, Y.; Kishimura, A.; Yamasaki, Y.; Kataoka, K. Living Unimodal Growth of Polyion Complex Vesicles via Two-Dimensional Supramolecular Polymerization. J. Am. Chem. Soc. 2013, 135, 1423–1429. [Google Scholar] [CrossRef]
- Smith, M.J.; Haidar, I.A.; Striegel, A.M. Measuring the size of polymers with negative radii using MALS/QELS: An exploration of the thermodynamic radius. Analyst 2007, 132, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Malet-Martino, M.; Gilard, V.; Desmoulin, F.; Martino, R. Fluorine nuclear magnetic resonance spectroscopy of human biofluids in the field of metabolic studies of anticancer and antifungal fluoropyrimidine drugs. Clin. Chim. Acta 2006, 366, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Goto, A.; Yen, H.-C.; Anraku, Y.; Fukushima, S.; Lai, P.-S.; Kato, M.; Kishimura, A.; Kataoka, K. Facile preparation of delivery platform of water-soluble low-molecular-weight drugs based on polyion complex vesicle (PICsome) encapsulating mesoporous silica nanoparticle. ACS Biomater. Sci. Eng. 2017, 3, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Speller, D.C.E. Antifungal Chemotherapy; John Wiley & Sons, Ltd.: Chichester, UK, 1980; pp. 271–344. [Google Scholar]
- Daneshmend, T.K.; Warnock, D.W. Clinical pharmacokinetics of systemic antifungal drugs. Clin. Pharmacokinet. 1983, 8, 17–42. [Google Scholar] [CrossRef] [PubMed]

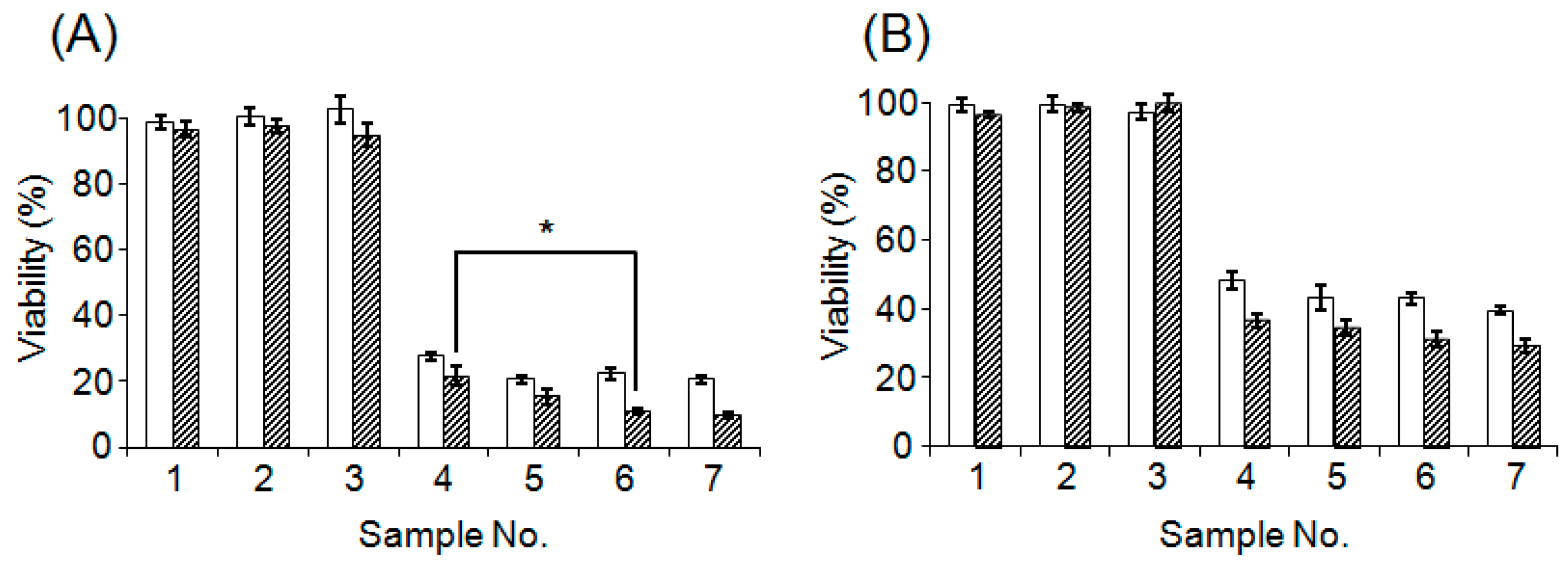

| Sample | Contents | ||
|---|---|---|---|
| CD@PICsome | 5-FC | 5-FU | |
| 1 | + | − | − |
| 2 | − | + (2 μg/mL) | − |
| 3 | − | + (4 μg/mL) | − |
| 4 | + | + (2 μg/mL) | − |
| 5 | + | + (4 μg/mL) | − |
| 6 | − | − | + (2 μg/mL) |
| 7 | − | − | + (4 μg/mL) |
| 8 | − | − | − |
| Group | Day | ||||
|---|---|---|---|---|---|
| 1 | 4 | 8 | 11 | 15 | |
| 1 | × | ♦ | ♦ | ♦ | ♦ |
| 2 | × | ◊ | ◊ | ◊ | ◊ |
| 3 | − | ■ | ■ | ■ | ■ |
| 4 | − | □ | □ | □ | □ |
| 5 | × | − | − | − | − |
| 6 | − | ♦ | ♦ | ♦ | ♦ |
| 7 | − | ◊ | ◊ | ◊ | ◊ |
| 8 | ∆ | ∆ | ∆ | ∆ | ∆ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goto, A.; Anraku, Y.; Fukushima, S.; Kishimura, A. Increased Enzyme Loading in PICsomes via Controlling Membrane Permeability Improves Enzyme Prodrug Cancer Therapy Outcome. Polymers 2023, 15, 1368. https://doi.org/10.3390/polym15061368
Goto A, Anraku Y, Fukushima S, Kishimura A. Increased Enzyme Loading in PICsomes via Controlling Membrane Permeability Improves Enzyme Prodrug Cancer Therapy Outcome. Polymers. 2023; 15(6):1368. https://doi.org/10.3390/polym15061368
Chicago/Turabian StyleGoto, Akinori, Yasutaka Anraku, Shigeto Fukushima, and Akihiro Kishimura. 2023. "Increased Enzyme Loading in PICsomes via Controlling Membrane Permeability Improves Enzyme Prodrug Cancer Therapy Outcome" Polymers 15, no. 6: 1368. https://doi.org/10.3390/polym15061368
APA StyleGoto, A., Anraku, Y., Fukushima, S., & Kishimura, A. (2023). Increased Enzyme Loading in PICsomes via Controlling Membrane Permeability Improves Enzyme Prodrug Cancer Therapy Outcome. Polymers, 15(6), 1368. https://doi.org/10.3390/polym15061368








