Bio-Polyethylene Composites Based on Sugar Cane and Curauá Fiber: An Experimental Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Curauá Fiber (CF)
2.3. Preparation of Biocomposites
2.4. Description
2.4.1. Fourier Transform Infrared Spectroscopy (FTIR-ATR)
2.4.2. X-ray Diffraction (XRD)
2.4.3. Thermal Analysis by Differential Scanning Calorimetry (DSC)
2.4.4. Thermogravimetry (TG and DTG)
2.4.5. Thermodynamic-Mechanical Analysis (DMA)
2.4.6. Analysis of Scanning Electron Microscopy (SEM)
2.4.7. Mechanical Properties
3. Results and Discussion
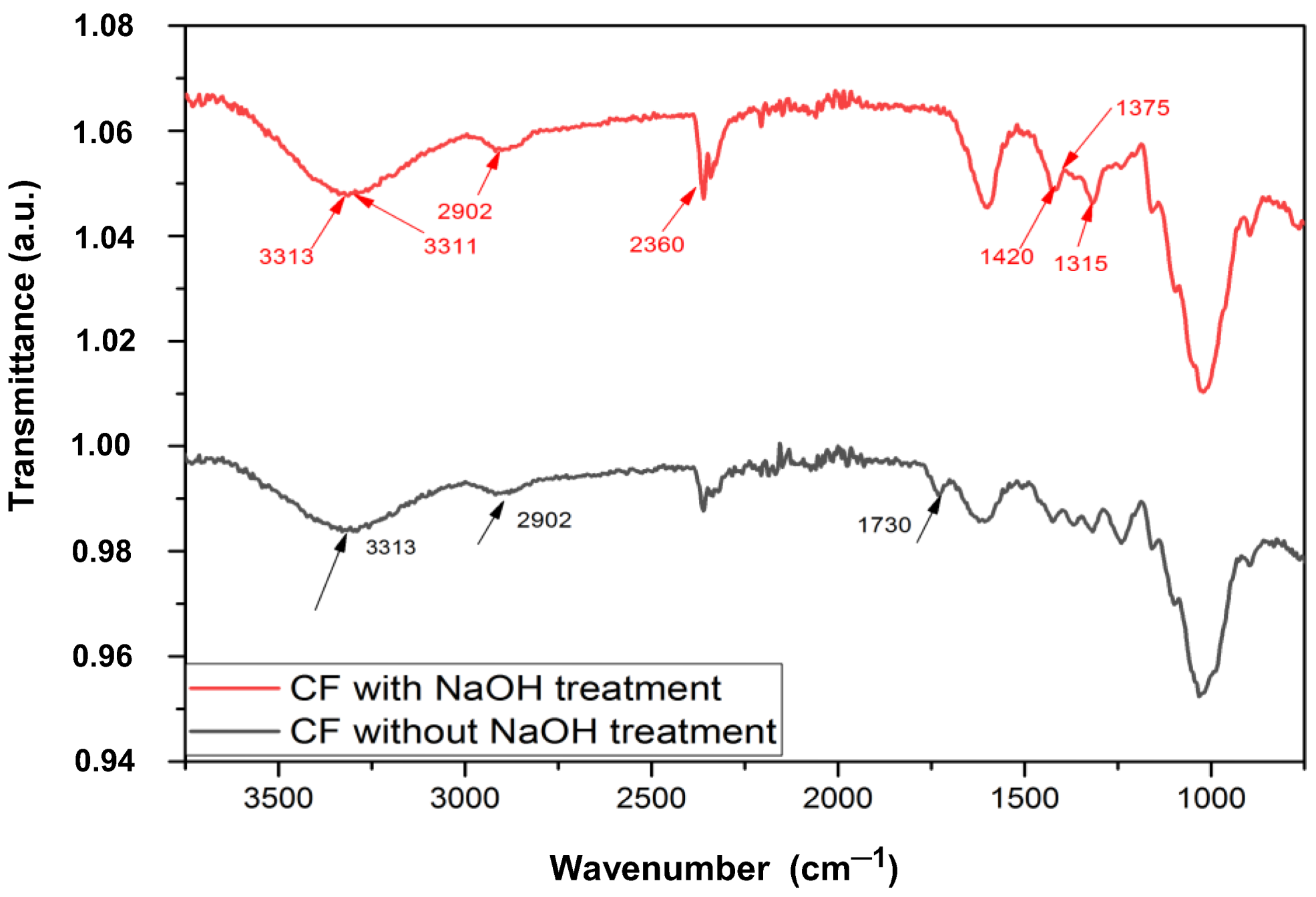
3.1. FTIR
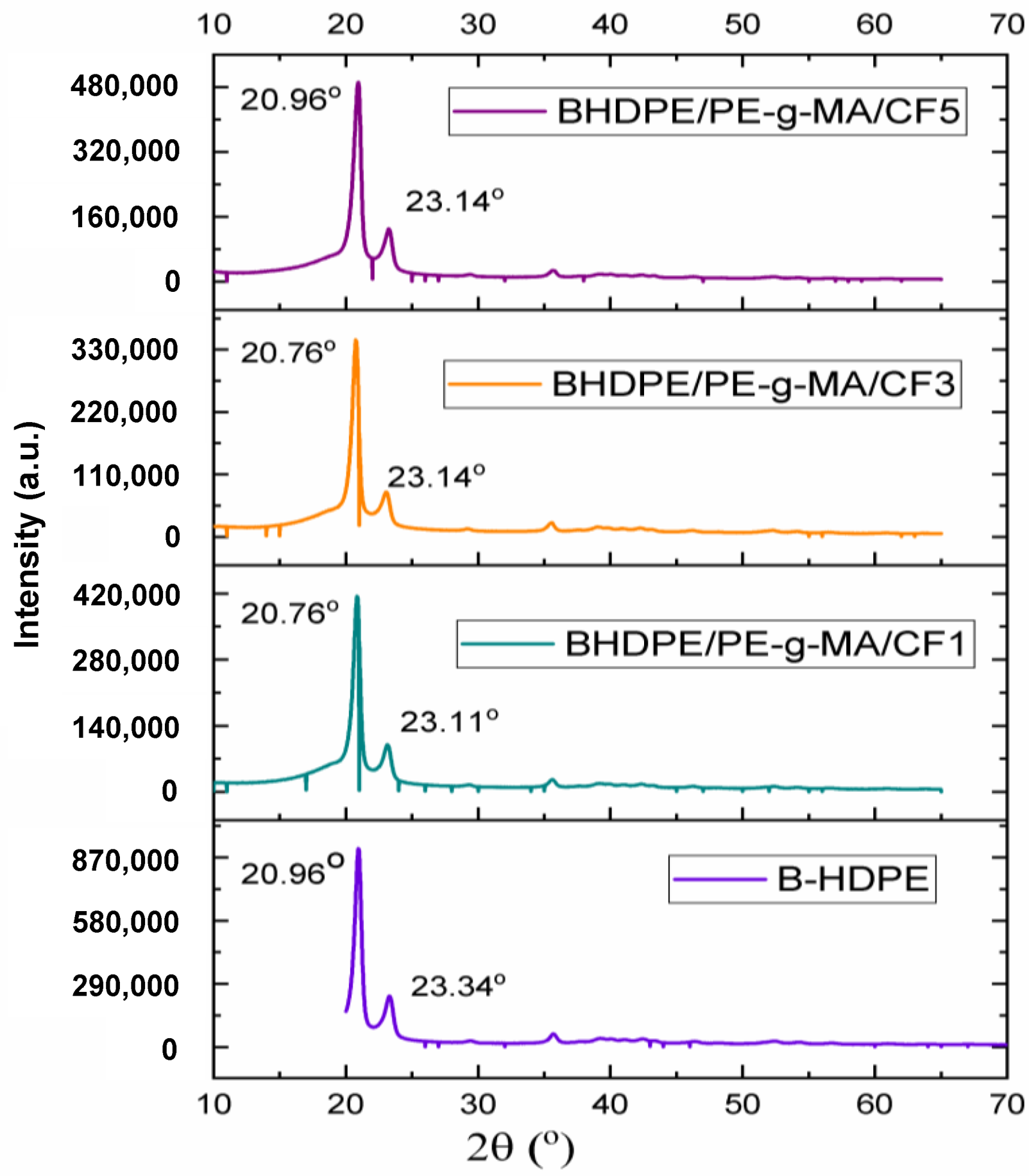
3.2. X-ray Diffraction (XRD)
3.3. Thermal Analysis by Differential Scanning Calorimetry (DSC)
3.4. Thermogravimetry
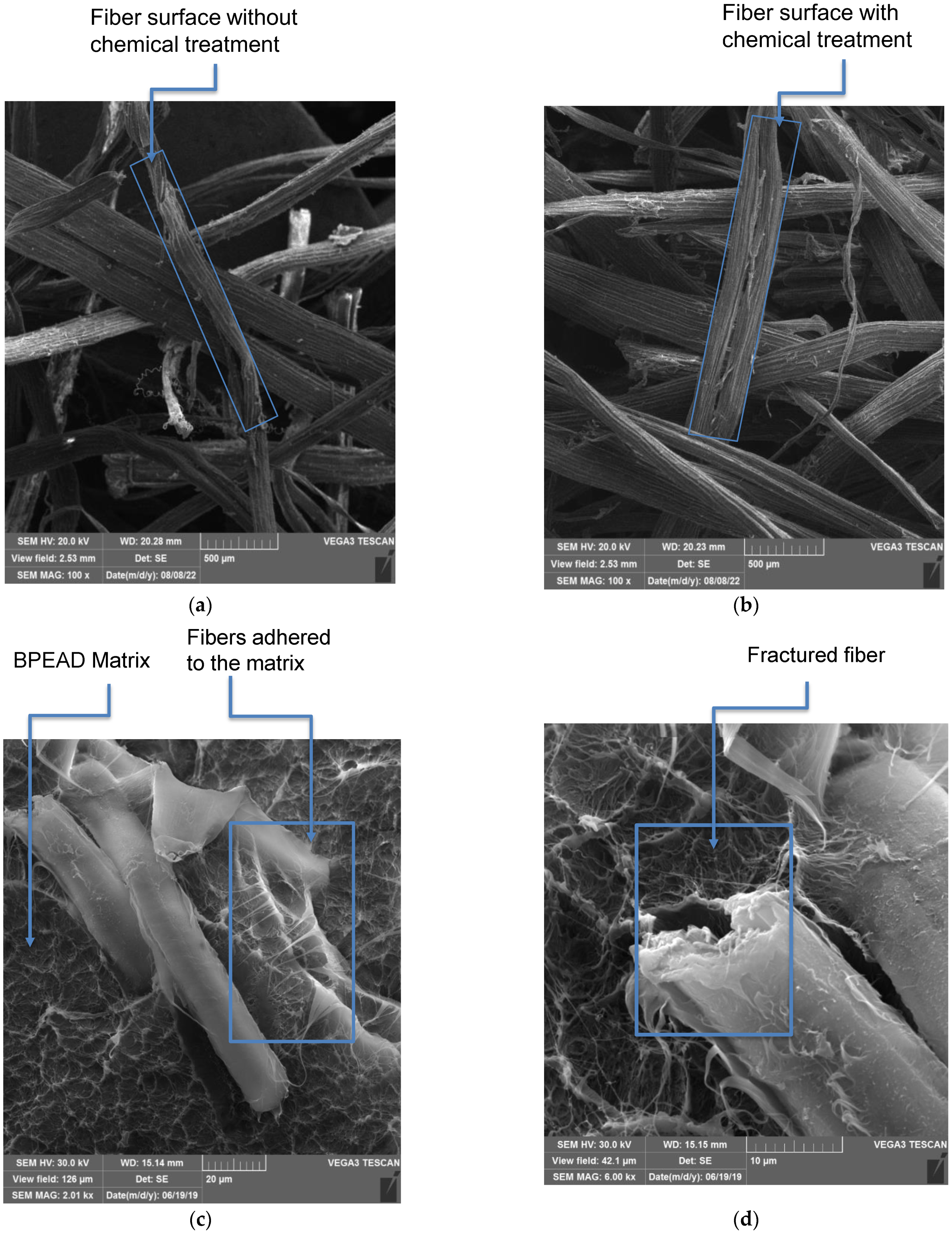
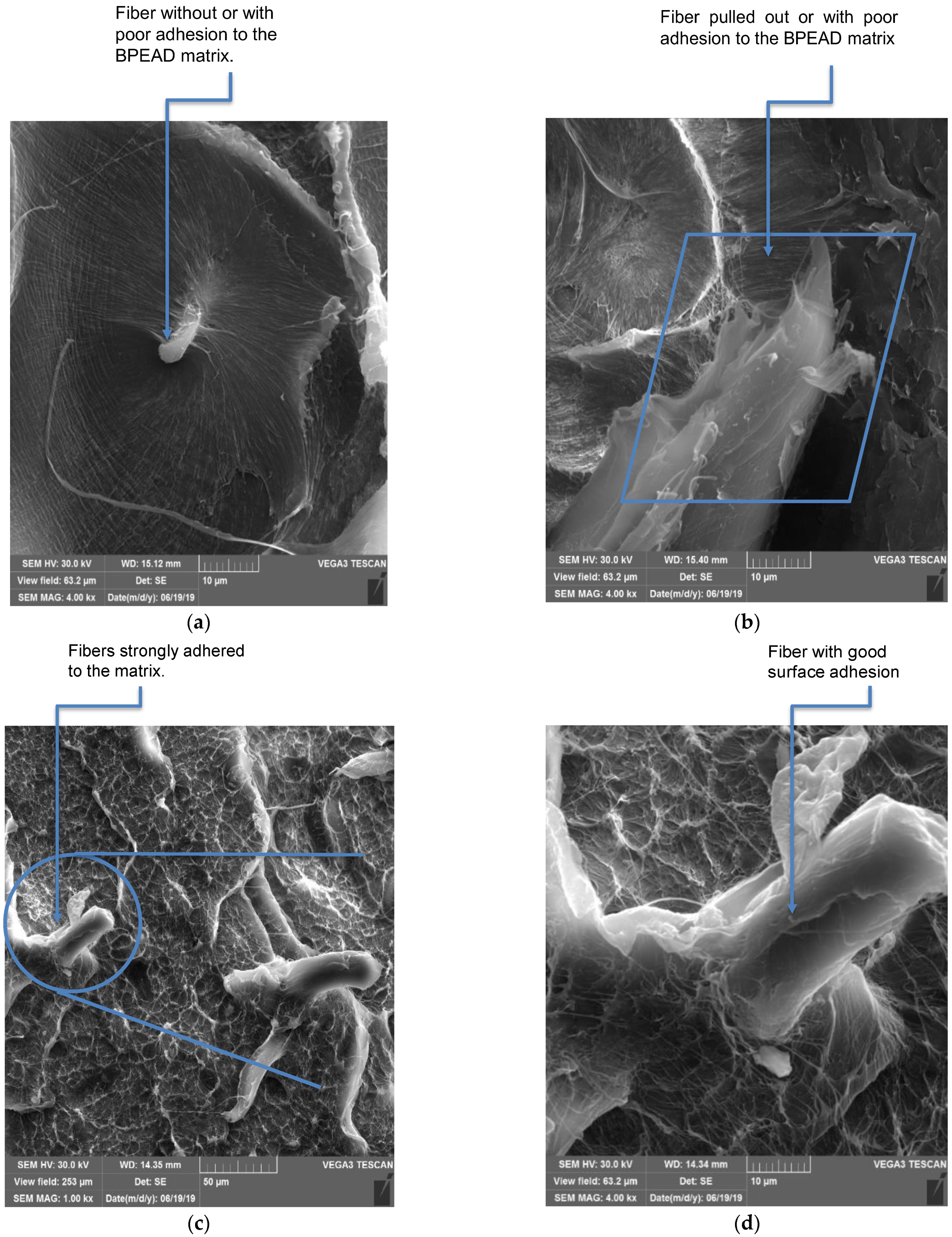
3.5. Analysis by Scanning Electron Microscopy (SEM)
3.6. Dynamic Mechanical Analysis (DMA)
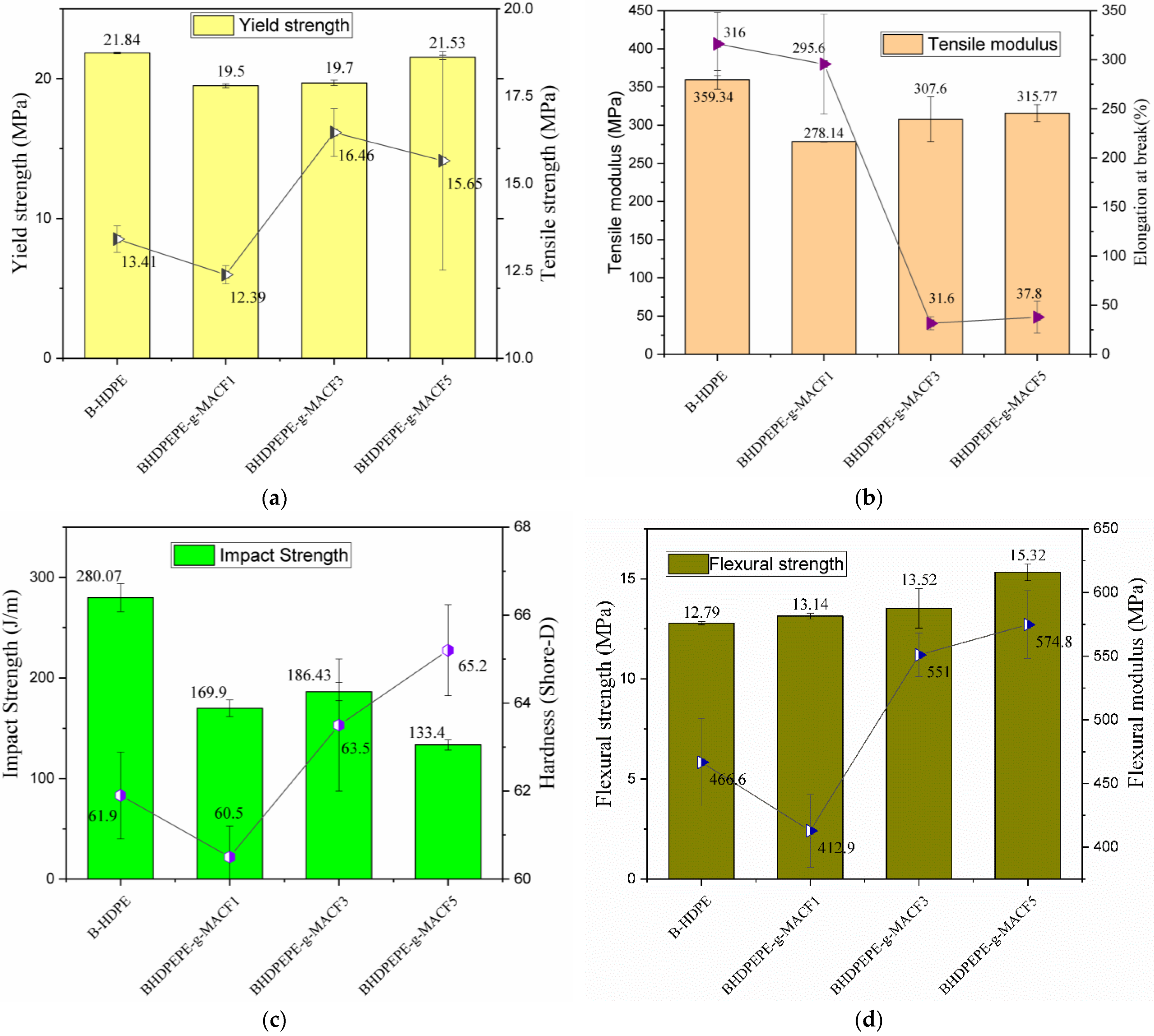
3.7. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vargas Mores, G.; Pauletto, C.; Finocchio, C.P.S.; Barichello, R.; Pedrozo, E.A. Sustainability and innovation in the Brazilian supply chain of green plastic. J. Clean. Prod. 2018, 177, 12–18. [Google Scholar] [CrossRef]
- Raquez, J.M.; Habibi, Y.; Murariu, M.; Dubois, P. Polylactide (PLA) based nanocomposites. Prog. Polym. Sci. 2013, 38, 1504–1542. [Google Scholar] [CrossRef]
- Zambanini, M.E.; Bresciani, L.P.; Silva Pereira, R.; de Souza, D.A.; Ortega, G. Sustainability and innovation: A study on green plastic. Rev. Agribus. Environ. 2014, 7, 429–453. (In Portuguese). Available online: https://periodicos.unicesumar.edu.br/index.php/rama/article/view/2854 (accessed on 20 February 2023). (In Portuguese).
- Hassan, M.L.; Rowell, R.M.; Fadl, N.A.; Yacoub, S.F.; Christainsen, A.W. Preparation and characterization of esterified bagasse fibers. J. Appl. Polym. Sci. 2000, 76, 561–574. [Google Scholar] [CrossRef]
- Delgado, J.M.P.Q.; Lima, A.G.B.; Carvalho, L.H. Moisture Transport in Polymer Composites Materials: Computactional Modeling and Experiments, 1st ed.; Springer: Cham, Switzerland, 2022. [Google Scholar] [CrossRef]
- Brito, G.F.; Agrawal, P.; Araújo, E.M.; Mélo, T.J.A. Biopolymers, biodegradable polymers and green polymers. Rev. Eletrôn. Mater. Process. 2011, 6, 127–139. Available online: http://www2.ufcg.edu.br/revista-remap/index.php/REMAP/article/view/222 (accessed on 20 February 2023). (In Portuguese).
- Wong, S.; Shanks, R.; Hodzic, A. Interfacial improvements in poly (3 hydroxybutyrate)-flax fiber composites with hydrogen bonding additives. Compos. Sci. Technol. 2004, 64, 1321–1330. [Google Scholar] [CrossRef]
- Chen, L.J.; Wang, M. Production and evaluation of biodegradable composites based on PHB–PHV copolymer. Biomaterials 2002, 23, 2631–2639. [Google Scholar] [CrossRef]
- Monteiro, S.N.; Aquino, R.C.M.P.; Lopes, F.P.D. Performance of curaua fibers in pullout tests. J. Mater. Sci. 2008, 43, 489–493. [Google Scholar] [CrossRef]
- Rahman, M.R.; Hamdan, S.; Jayamani, E.; Kakar, A.; Bakri, M.K.B.; Yusof, F.A.B.M. Tert-butyl catechol/alkaline-treated kenaf/jute polyethylene hybrid composites: Impact on physico-mechanical, thermal and morphological properties. Polym. Bull. 2019, 76, 763–784. [Google Scholar] [CrossRef]
- Cruz, J.; Fangueiro, R. Surface modification of natural fibers: A review. Procedia Eng. 2016, 155, 285–288. [Google Scholar] [CrossRef] [Green Version]
- Satyanarayana, K.G.; Guimarães, J.L.; Wypych, F. Studies on lignocellulosic fibers of Brazil. Part I: Source, production, morphology, properties and applications. Compos. Part A Appl. Sci. Manuf. 2007, 38, 1694–1709. [Google Scholar] [CrossRef]
- Salazar, V.L.P.; Leão, A.L.; Rosa, D.S.; Gomez, J.G.C.; Alli, R.C.P. Biodegradation of Coir and Sisal Applied in the Automotive Industry. J. Polym. Environ. 2011, 9, 677–688. [Google Scholar] [CrossRef]
- Megiatto, J.; Jackson, D.; Hoareau, W.; Gardrat, C.; Frollini, E.; Castellan, A. Sisal fibers: Surface chemical modification using reagent obtained from a renewable source; characterization of hemicellulose and lignin as model study. J. Agric. Food Chem. 2007, 55, 8576–8584. [Google Scholar] [CrossRef]
- Satyanarayana, K.G.; Arizaga, G.G.C.; Wypych, F. Biodegradable composites based on lignocellulosic fibers-An overview. Prog. Polym. Sci. 2009, 34, 982–1021. [Google Scholar] [CrossRef]
- Terzopoulou, Z.N.; Papageorgiou, G.Z.; Papadopoulou, E.; Athanassiadou, E.; Alexopoulou, E.; Bikiaris, D.N. Green composites prepared from aliphatic polyesters and bast fibers. Ind. Crop. Prod. 2015, 68, 60–79. [Google Scholar] [CrossRef]
- Sever, K.; Erden, S.; Gülec, H.A.; Seki, Y.; Sarikanat, M. Oxygen plasma treatments of jute fibers in improving the mechanical properties of jute/HDPE composites. Mater. Chem. Phys. 2011, 129, 275–280. [Google Scholar] [CrossRef]
- Poletto, M.; Ornaghi, H.L., Jr.; Zattera, A.J. Thermal Decomposition of Natural Fibers: Kinetics and Degradation Mechanisms. React. Mech. Therm. Anal. Adv. Mater. 2015, 21, 515–545. [Google Scholar] [CrossRef]
- Bledzki, A.K.; Gassan, J. Composites reinforced with cellulose based fibres. Prog. Polym. Sci. 1999, 24, 221–274. [Google Scholar] [CrossRef]
- da Silva, F.S.; da Silva, W.A.; Luna, C.B.B.; Ferreira, E.S.B.; Araújo, E.M. Production of biopolyethylene/wood flour biocomposites compatible with PE-g-MA. Evaluation of maleic anhydride content in mechanical and thermomechanical properties. Res. Social Dev. 2021, 10, e23310716277. [Google Scholar] [CrossRef]
- Rzayev, Z.M.O. Graft copolymers of maleic anhydride and its isostructural analogues: High performance engineering materials. arXiv 2011, arXiv:1105.1260. [Google Scholar] [CrossRef]
- Huang, H.; Dean, D. 3-D Printed Porous Cellulose acetate Tissue Scaffolds for Additive Manufacturing. Addit. Manuf. 2019, 31, 100927. [Google Scholar] [CrossRef]
- Syracuse, V.; Blanco, I. Bio-Polyethylene (Bio-PE), Bio-Polypropylene (Bio-PP) and Bio-Poly (ethylene terephthalate)(Bio-PET): Recent developments in bio-based polymers analogous to petroleum-derived ones for packaging and engineering applications. Polymers 2020, 12, 1641. [Google Scholar] [CrossRef] [PubMed]
- Mota, J.C.; Almeida, M.A.; Alencar, V.C.; Curi, W.F. Environmental, economic and social impacts and benefits of biofuels: A global view. Eng. Ambient. Espírito St. Pinhal 2009, 6, 220–242. (In Portuguese) [Google Scholar]
- Jagaba, A.H.; Kutty, S.R.M.; Baloo, L.; Hayder, G.; Birniwa, A.H.; Taha, A.T.B.; Mnzool, M.; Lawal, I.M. Waste Derived Biocomposite for Simultaneous Biosorption of Organic Matter and Nutrients from Green Straw Biorefinery Effluent in Continuous Mode Activated Sludge Systems. Processes 2022, 10, 2262. [Google Scholar] [CrossRef]
- Jagaba, A.H.; Kutty, S.R.M.; Abubakar, S.; Birniwa, A.H.; Lawal, I.M.; Umaru, I.; Usman, A.K.; Yaro, N.S.A.; Al-Zaqri, N.; Al-Maswari, B.M.; et al. Synthesis, Characterization, and Performance Evaluation of Hybrid Waste Sludge Biochar for COD and Color Removal from Agro-Industrial Effluent. Separations 2022, 9, 258. [Google Scholar] [CrossRef]
- Montanes, N.; Garcia-Sanoguera, D.; Segui, V.J.; Fenollar, O.; Boronat, T. Processing and characterization of environmentally friendly composites from biobased polyethylene and natural fillers from thyme herbs. J. Polym. Environ. 2018, 26, 1218–1230. [Google Scholar] [CrossRef]
- Beltrami, L.V.R.; Cristine Scienza, L.; Zattera, A.J. Effect of alkaline treatment of Curauá fibers on the properties of biodegradable matrix composites. Polímeros 2014, 24, 388–394. [Google Scholar] [CrossRef] [Green Version]
- Tomczak, F.; Satyanarayana, K.G.; Sydenstricker, T.H.D. Studies on lignocellulosic fibers of Brazil: Part III–Morphology and properties of Brazilian curauá fibers. Compos. Part A Appl. Sci. Manuf. 2007, 38, 2227–2236. [Google Scholar] [CrossRef]
- Marcovich, N.E.; Villar, M.A. Thermal and mechanical characterization of linear low-density polyethylene/wood flour composite. J. Appl. Polym. Sci. 2003, 90, 2775–2784. [Google Scholar] [CrossRef]
- Perez, I.S.B.; Manrich, S. Effect of adding different copolymers to post-consumer HDPE/HIPS blends: Phase morphology and thermal properties. Polymers 2008, 18, 207–214. [Google Scholar] [CrossRef]
- Ibrahim, N.A.; Hadithon, K.A.; Abdan, K. Effect of fiber treatment on mechanical properties of kenaf fiber-ecoflex composites. J. Reinf. Plast. Compos. 2010, 29, 2192–2198. [Google Scholar] [CrossRef]
- Hossain, M.K.; Dewan, M.W.; Hosur, M.; Jeelani, S. Mechanical performances of surface modified jute fiber reinforced biopol nanophased green composites. Compos. Part B Eng. 2011, 42, 1701–1707. [Google Scholar] [CrossRef]
- Hoareau, W.; Trindade, W.G.; Siegmund, B.; Castellan, A.; Frollini, E. Sugar cane bagasse and curaua lignins oxidized by chlorine dioxide and reacted with furfuryl alcohol: Characterization and stability. Polym. Degrad. Stab. 2004, 86, 567–576. [Google Scholar] [CrossRef]
- Lahor, A.; Nithitanakul, M.; Grady, G.P. Blends of low-density polyethylene with nylon compatibilized with a sodium-neutralized carboxylate ionomer. Eur. Polym. 2004, 40, 2409–2420. [Google Scholar] [CrossRef]
- Gutiérrez, M.C.; De Paoli, M.A.; Felisberti, M.I. Biocomposites based on cellulose acetate and short curauá fibers: Effect of plasticizers and chemical treatments of the fibers. Compos. Part A Appl. Sci. Manuf. 2012, 43, 1338–1346. [Google Scholar] [CrossRef]
- Boronat, T.; Fombuena, V.; Garcia-Sanoguera, D.; Sanchez-Nacher, L.; Balart, R. Development of a biocomposite based on green polyethylene biopolymer and eggshell. Mater. Des. 2015, 68, 177–185. [Google Scholar] [CrossRef]
- Castro, D.O.; Frollini, E.; Marini, J.; Ruvolo-Filho, A. Preparation and characterization of biocomposites based on curauá fiber, high-density biopolyethylene (BPEAD) and hydroxylated liquid polybutadiene (PBHL). Polymers 2013, 23, 65–73. [Google Scholar] [CrossRef] [Green Version]
- Moly, K.A.; Radusch, H.J.; Androsh, R.; Bhagawan, S.S.; Thomas, S. Nonisothermal crystallisation, melting behavior and wide angle X-ray scattering investigations on linear low density polyethylene (LLDPE)/ ethylene vinyl acetate (EVA) blends: Effects of compatibilization and dynamic crosslinking. Eur. Polym. J. 2005, 41, 1410–1419. [Google Scholar] [CrossRef]
- Castro, D.O.; Ruvolo-Filho, A.; Frollini, E. Materials prepared from biopolyethylene and curaua fibers: Composites from biomass. Polym. Test. 2004, 31, 880–888. [Google Scholar] [CrossRef]
- Castro, D.O.; Passador, F.; Ruvolo-Filho, A.; Frollini, E. Use of castor and canola oils in biopolyethylene curauá fiber. Compos. Part A Appl. Sci. 2017, 95, 22–30. [Google Scholar] [CrossRef]
- Barman, A.; Shrivastava, N.K.; Khatua, B.B.; Ray, B.C. Green composites based on high-density polyethylene and S accharum spontaneum: Effect of filler content on morphology, thermal, and mechanical properties. Polym. Compos. 2015, 36, 2157–2166. [Google Scholar] [CrossRef]
- Bos, H.L.; Müssig, J.; van den Oever, M. Mechanical properties of short-flax-fibre reinforced compounds. Compos. Part A Appl. Sci. 2006, 37, 1591–1604. [Google Scholar] [CrossRef]
- Thomason, J.L.; Vlug, M.A. Influence of fiber length and concentration on the properties of glass fibre-reinforced polypropylene: 4. Impact properties. Compos. Part A Appl. Sci. 1997, 28, 277–288. [Google Scholar] [CrossRef]
- Robledo-Ortíz, J.R.; González-López, M.E.; Rodrigue, D.; Gutiérrez-Ruiz, J.F.; Prezas-Lara, F.; Pérez-Fonseca, A.A. Improving the Compatibility and Mechanical Properties of Natural Fibers/Green Polyethylene Biocomposites Produced by Rotational Molding. J. Polym. Environ. 2020, 28, 1040–1049. [Google Scholar] [CrossRef]
- Zhang, S.; Ke, Y.; Cao, X.; Ma, Y.; Wang, F. Effect of Al2O3 fibers on the thermal conductivity and mechanical properties of high density polyethylene with the absence and presence of compatibilizer. J. Appl. Polym. Sci. 2012, 124, 4874–4881. [Google Scholar] [CrossRef]
- Bax, B.; Müssig, J. Impact and tensile properties of PLA/Cordenka and PLA/flax composites. Compos. Sci. Technol. 2008, 68, 1601–1607. [Google Scholar] [CrossRef] [Green Version]
- Sarkhel, G.; Choudhury, A. Dynamic mechanical and thermal properties of PE-EPDM based jute fiber composites. J. Appl. Polym. Sci. 2008, 108, 3442–3453. [Google Scholar] [CrossRef]
- Faulstich de Paiva, J.M.; Frollini, E. Unmodified and modified surface sisal fibers as reinforcement of phenolic and lignophenolic matrices composites: Thermal analysis of fibers and composites. Macromol. Mater. Eng. 2006, 291, 405–417. [Google Scholar] [CrossRef]
- Karger-Kocsis, J.; Mahmood, H.; Pegoretti, A. Recent advances in fiber/matrix interphase engineering for polymer composites. Prog. Mater. Sci. 2015, 73, 1–43. [Google Scholar] [CrossRef]
- Savas, L.A.; Tayfun, U.; Dogan, M. The use of polyethylene copolymers as compatibilizers in carbon fiber reinforced high density polyethylene composites. Compos. Part B Eng. 2016, 99, 188–195. [Google Scholar] [CrossRef]
- López-Manchado, M.A.; Biagitti, J.; Kenny, J.M. Comparative study of the effects of different fibers on the processing and properties of ternary composites based on PP-EPDM blends. Polym. Compos. 2002, 23, 779–789. [Google Scholar] [CrossRef]
- Palanivel, A.; Veerabathiran, A.; Duruvasalu, R.; Iyyanar, S.; Velumayil, R. Dynamic mechanical analysis and crystalline analysis of hemp fiber reinforced cellulose filled epoxy composite. Polímeros 2017, 27, 309–319. [Google Scholar] [CrossRef] [Green Version]
- de Oliveira Santos, R.P.; Castro, D.O.; Ruvolo-Filho, A.C.; Frollini, E. Processing and thermal properties of composites based on recycled PET, sisal fibers, and renewable plasticizers. J. Appl. Polym. Sci. 2014, 131, 40386. [Google Scholar] [CrossRef]
- Wang, M.; Joseph, R.; Bonfield, W. Hydroxyapatite-polyethylene composites for bone substitution: Effects of ceramic particle size and morphology. Biomaterials 1998, 19, 2357–2366. [Google Scholar] [CrossRef]
- Wells, J.K.; Beaumont, P.W.R. Debonding and pull-out processes in fibrous composites. J. Mater. Sci. 1985, 20, 1275–1284. [Google Scholar] [CrossRef]
- Joseph, S.; Sreekala, M.S.; Oommen, Z.; Koshy, P.; Thomas, S. A comparison of the mechanical properties of phenol formaldehyde composites reinforced with banana fibres and glass fibres. Compos. Sci. Technol. 2002, 62, 1857–1868. [Google Scholar] [CrossRef]
- Rahman, M.R.; Huque, M.M.; Islam, M.N.; Hasan, M. Mechanical properties of polypropylene composites reinforced with chemically treated abaca. Compos. Part A Appl. Sci. Manuf. 2009, 4, 511–517. [Google Scholar] [CrossRef]




| Designation | FC (%) | PE-g-MA (%) |
|---|---|---|
| B-HDPE | 0 | 0 |
| B-HDPE/PE-g-MA/CF1 | 1 | 10 |
| B-HDPE/PE-g-MA/CF3 | 3 | 10 |
| B-HDPE/PE-g-MA/CF5 | 5 | 10 |
| Samples | Tm (°C) | ΔHfa (J/kg) | Xc (%) |
|---|---|---|---|
| B-HDPE | 130.30 | 120.60 | 41.16 |
| B-HDPE/PE-g-MA/CF1 | 131.28 | 100.09 | 34.16 |
| B-HDPE/PE-g-MA/CF3 | 131.80 | 99.07 | 33.81 |
| B-HDPE/PE-g-MA/CF5 | 130.79 | 98.50 | 33.61 |
| B-HDPE | B-HDPE/PE-g-MA/CF1 | B-HDPE/PE-g-MA/CF3 | B-HDPE/PE-g-MA/CF5 | |
|---|---|---|---|---|
| Tonset (°C) | 391.64 | 419.36 | 405.81 | 413.95 |
| T(80) (°C) | 408.21 | 437.77 | 411.84 | 432.02 |
| T(50) (°C) | 436.18 | 460.23 | 449.37 | 460.98 |
| Tmax (°C) | 459.87 | 469.18 | 463.95 | 472.86 |
| Tendset (°C) | 483.91 | 491.27 | 490.31 | 490.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbalho, G.H.d.A.; Nascimento, J.J.d.S.; Silva, L.B.d.; Gomez, R.S.; Farias, D.O.d.; Diniz, D.D.S.; Santos, R.S.; Figueiredo, M.J.d.; Lima, A.G.B.d. Bio-Polyethylene Composites Based on Sugar Cane and Curauá Fiber: An Experimental Study. Polymers 2023, 15, 1369. https://doi.org/10.3390/polym15061369
Barbalho GHdA, Nascimento JJdS, Silva LBd, Gomez RS, Farias DOd, Diniz DDS, Santos RS, Figueiredo MJd, Lima AGBd. Bio-Polyethylene Composites Based on Sugar Cane and Curauá Fiber: An Experimental Study. Polymers. 2023; 15(6):1369. https://doi.org/10.3390/polym15061369
Chicago/Turabian StyleBarbalho, Gustavo Henrique de Almeida, José Jefferson da Silva Nascimento, Lucineide Balbino da Silva, Ricardo Soares Gomez, Daniel Oliveira de Farias, Diego David Silva Diniz, Rosilda Sousa Santos, Maria José de Figueiredo, and Antonio Gilson Barbosa de Lima. 2023. "Bio-Polyethylene Composites Based on Sugar Cane and Curauá Fiber: An Experimental Study" Polymers 15, no. 6: 1369. https://doi.org/10.3390/polym15061369
APA StyleBarbalho, G. H. d. A., Nascimento, J. J. d. S., Silva, L. B. d., Gomez, R. S., Farias, D. O. d., Diniz, D. D. S., Santos, R. S., Figueiredo, M. J. d., & Lima, A. G. B. d. (2023). Bio-Polyethylene Composites Based on Sugar Cane and Curauá Fiber: An Experimental Study. Polymers, 15(6), 1369. https://doi.org/10.3390/polym15061369









